Abstract
Purpose of Review:
Once prostate cancer (PCa) bone metastases develop, the prognosis dramatically declines. The precise mechanisms regulating bone metastasis remain elusive. This review will explore recent findings related to cytokines and chemokines in the process of bone metastases.
Recent findings:
We discuss the role of cytokines in tumor growth, invasion, bone remodelling and angiogenesis and immune regulation in PCa skeletal metastases. Major advances in our understanding focus on immune evasion, immune checkpoint blockade, tumor-associated macrophages (TAMs), CAR-T cells, cytokine regulation of matrix metalloproteinases, cytokines including IL-10, IL-27, Interferon-γ, prostate transmembrane protein androgen induced 1 (Pmepa1), and regulation of RUNX2 transcription in supporting survival and growth of disseminated tumor cells (DTCs) and metastases development.
Summary:
The review highlights the complexity of cytokine actions in PCa bone metastases, suggesting potential therapeutic targets to disrupt interactions between cancer cells and their microenvironment.
Keywords: Cytokines, chemokines, prostate cancer, skeletal metastases, disseminated tumor cells
Introduction:
Metastatic prostate cancer (PCa) has a proclivity to grow in the bone marrow [1, 2]. Based upon data examining disseminated tumor cell (DTC) frequency in marrow, it appears that PCa cells leave the prostate early in the disease sequence and arrive at distant sites where they may remain dormant for extended periods of time [3]. There, intracellular signalling molecules including cytokines play a critical role in many of the facets of cell growth and invasion [4, 5], interactions with resident cells, angiogenesis and the inflammatory and immune response to DTCs [3, 6]. Cytokines were initially described as small proteins secreted by many cells in the immune system. They act as signalling molecules, playing a crucial role in cell communication, and coordinating the body’s response to infection, inflammation, and trauma [7, 8]. Cytokines are known to regulate the intensity and duration of immune responses by influencing the behavior of other cells, such as promoting cell growth, differentiation, and movement. They are essential for maintaining a balanced and effective immune system [9, 10]. While initially described as products of an activated immune system, cytokines are also produced by PCa and other host cells and have a much wider role in normal tissue physiology. This brief review will focus on the role that cytokines play in PCa skeletal metastases with particular emphasis on data generated over the last 3 years.
Tumor Growth and Invasion:
Many studies have demonstrated that cytokines produced by the tumor cells, as well as bone microenvironmental cells, regulate the processes of homing to marrow, invasion, and growth of PCa [11]. Amongst the most studied cytokines investigated in most recent publications continues to be work focused on IL-6 [12], IL-8 [13], tumor necrosis factor-alpha (TNF-α) [14, 15], and transforming growth factor-beta (TGF-β) [16-18] secreted by PCa or the microenvironment (Figure 1). These cytokines are known to both stimulate cancer cell proliferation and survival within the bone tissue and/or inhibit growth. For instance, IL-6 promotes PCa growth and resistance to cell death, contributing to the progression of skeletal metastasis− [19, 20]. Yet, ongoing work continues to demonstrate that the impact of cytokines is context dependent. For example, IL-6, IL-8, TNF-α and TGF-β drive or inhibit cancer cell proliferation and/or activate or limit immune surveillance / killing of the tumor cells themselves depending on context, how the ligand is presented and cell cycle status [21]. In other settings, the presence and/or absence of androgen receptor signalling has significant impact on proliferation and response to anti-IL-6 therapy [12]. Likewise, TGF-β can drive or limit tumor growth depending on what stage of disease is examined [18, 22].
Figure 1: The roles of cytokines and chemokines in prostate cancer (PCa) bone metastasis.
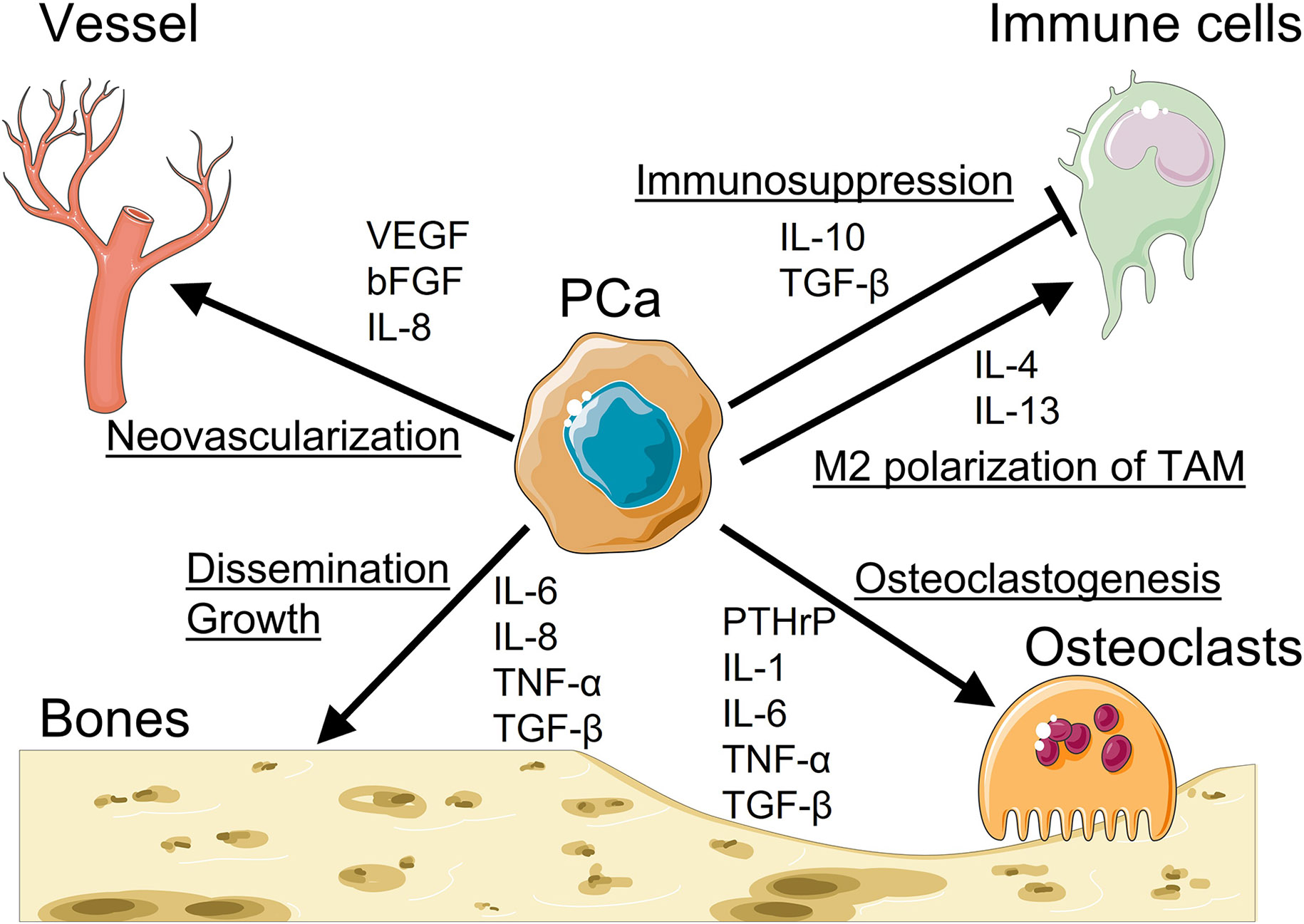
Studies regarding the roles of cytokines and chemokines in the context of PCa bone metastasis has yielded important insights into the crosstalk between bone metastatic PCa cells and bone marrow microenvironment. Cytokines and chemokines, produced by either PCa cells or bone marrow microenvironment, are known to regulate phenomena that are crucial for PCa bone metastatic development/progression. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and IL-8 induce neovascularization in the bone to support PCa growth in the bone. Moreover, IL-10 and TGF-β induce immunosuppressive effects and IL-4 and IL-13 promote M2 polarization of tumor-associate macrophages (TAM). Understanding of underlying mechanisms could help develop targeted therapies to disrupt the crosstalk between bone metastatic PCa cells and bone marrow microenvironment. Graphics adapted from Smart Servier Medical Art (https://smart.servier.com/).
Autocrine and paracrine secretion of cytokines by DTCs induces changes the expression of various growth factors, chemokines, and matrix metalloproteinases (MMPs) that support the survival and growth of DTCs while also affecting bone cells’ behavior [18, 23-25]. Furthermore, cytokines provide feedback which not only impacts PCa growth, conditions the microenvironment, but promotes a more aggressive phenotype by regulating an epithelial to mesenchymal transition (EMT) [26-28]. This process facilitates cancer cells’ ability to acquire migratory and invasive properties, and thus facilitates their movement from the primary tumor site to the bones, but it also aides in the adaption of DTC to the marrow microenvironment and impacts resistance to chemotherapy [18, 29, 30]. Perhaps this is why targeting cytokines in the context of bone metastases produces mixed results in preclinical and clinical trials [12, 13, 31-38]. Yet the impact of cytokines extends beyond their direct impact on PCa proliferation and/or migration. PCa cells exposed to cytokines either through autocrine production, or a paracrine secretion or membrane presentation are impacted by alterations of adhesion molecule expression, androgen receptor status, and interact the disruption of the normal balance of tissue physiology [39-41]. This imbalance further promotes the release of factors that attract cancer cells to the bone and create a favorable environment for their colonization and growth. [21, 42]
Thus far we have focused on only four cytokines. Major classes of cytokines can be distinguished predominantly based on their function. Pro-inflammatory cytokines like TNF, IL-6 also include IL-17, IL-22 and many others [43-46]. Anti-inflammatory cytokines, including TGFβ and IL-1 receptor antagonist (IL-1RA) help to resolve inflammation and bring tissues back into homeostatic states [47-49](Figure 1). IL-8 and CC-chemokine ligand 2 (CCL2), interferons, colony-stimulating factors (e.g. G-CSF, GM-CSF, M-CSF, SCF) activate immune and stem cell functions of resident cells [50, 51]. Others still regulate tissue metabolism at both the local and systemic levels. In 2007, Dinarello reported that “there were at least 33 cytokines known with over 100 separate genes coding for cytokine-like activities.” [8] The number probably extends well beyond that, but even so the ability to induce a wide spectrum of responses in a diverse range of cell subsets, activation of different signalling via receptor dimerization/oligomerization, and the elaborate crosstalk and shared usage of key components of cytokine signalling pathways, makes generalization of the role of cytokines in PCa skeletal metastases challenging [7].
Nevertheless, some generalizations possible; there are likely cytokines which drive dormancy, and those that drive proliferation. As alluded to previously, these activities are not necessarily mutually exclusive. For example, molecules known which regulate PCa dormancy include BMPs, growth arrest specific-6 (GAS6), SPARC, TGF-β, Wnt5a and likely others [52]. In the case of GAS6, there are at least three functional receptors (Tyro3, Axl, MerTK) which regulate a PCa response to the ligand. Furthermore, other proteins bind to the receptors including Protein S, Tubby and Tulp. Differential receptor expression, signalling and ligand binding significantly alter the proliferative response of PCa cell lines [53, 54]. In addition to proliferation, and/or down regulation of the cell cycle, cytokines in the context of bone regulate tissue remodelling, the inflammatory response, angiogenesis and immunity. In the following sections we will briefly outline recent developments in these fields.
Bone Remodeling: ‘Vicious cycle’ model.
In the context of bone and cytokines, a “vicious cycle” model has been proposed in which tumor secretion of cytokines activates osteoclastic (OC) bone resorption, which releases cytokines and growth factors trapped in bone matrix, which activates tumor proliferation [55]. When this model is applied to metastases it suggests that inhibiting OC activities would be effective as adjuvant therapy for PCa and breast cancer. However, bisphosphonates failed in PCa adjuvant therapy clinical trials and are not effective enough in breast cancer trials to become standard of care for most patients [56, 57]. Likewise, in non-metastatic (PSA only) castration resistant PCa, the osteoclast inhibitor denosumab delays the time to formation of gross metastases, but unfortunately has little impact on survival [58]. In fact, even patients treated with Radium-223 and TC99 which were designed to specifically target bone lesions experience tumor progression [59-61]. While not specifically targeting osteoclastic activity, the difficulty in treating bone lesions is apparent.
Bone Remodeling: Tumor-induced osteoclast activity.
While the precise mechanism (s) which tumors regulate OC activity remain unclear, several pathways have been elucidated. One of the most studied, is a protein produced at high levels by PCa related to parathyroid hormone: parathyroid hormone-related protein (PTHrP) [62, 63]. PTHrP stimulates OC differentiation. Additionally, PCa cells produce cytokines and growth factors, such as IL-6, and TGF-β that promote osteoclastogenesis, and may stimulate DTCs to express higher levels of the receptor (RANK) for RANKL, and disrupts the production of osteoprotegerin (OPG), the decoy receptor for RANKL that inhibits osteoclastogenesis [64]. In addition, as PCa activate OC activities stimulating the release of calcium ions and growth factors stored in the bone matrix believed to activate OCs and tumor growth [65, 66]. In fact, the interactions between DTCs and marrow elements create a pro-inflammatory milieu [67], where inflammation fosters osteoclastogenesis by producing cytokines like IL-1, IL-6 and TNF-α and others (Figure 1) [68-70].
Bone Remodeling: Tumor induced osteoblast activity.
Among the most critical publications in this context over the last three years was work focused on the use of nanoparticles (NPs) designed to localize in the tumor tissue in bone marrow. The treatment, encapsulating docetaxel, an anticancer drug (TXT-NPs), and denosumab, a monoclonal antibody that binds to RANKL showed promise in preventing tumor progression, prevented bone resorption and even went so far as to regress established lesions [71]. Similarly using a nanoparticle approach, nanoparticles carrying protein toxins with both bone-seeking and CD44-receptor-targeting and anti-RANKL antibodies demonstrated promise in preclinical animal models [72]. From a mechanistic perspective it was recently demonstrated that PCa expressing ADAM9 protein drives osteoblasts into PCa-associated osteoblasts (PCa-OBs). This leads to the induction of WISP-1 secretion providing feedback to promote PCa cell aggressiveness via epithelial-mesenchymal transition (EMT) activity in animal models [73]. Moreover, elevated levels of WISP-1 expression were detected in the serum of patients with PCa [73]. Together the study suggests that that the ADAM9/WISP-1 axis may regulate metastatic PCa progression, and therefore targeting of this axis may prove beneficial for the prevention of malignant phenotypes of PCa cells.
In the development of castration-resistant prostate cancer (CRPC) metastasizing to the bones, intratumoral steroidogenesis is a crucial factor. RUNX2, a transcription factor mainly associated with osteoblasts, significantly influences steroid production and gets activated within CRPC cells. Using immunohistochemistry of intratibial xenograft originating from CRPC cells, demonstrated elevated levels of RUNX2 expression and steroid-producing enzymes and receptors [74]. In lab-based experiments, osteoclasts were found to heighten RUNX2 expression in osteoblastic LNCaP-19 cells, but they had no impact on osteolytic PC-3 cells [74]. Silencing RUNX2 in LNCaP-19 cells co-cultured with osteoclasts led to the decrease in CYP11A1, CYP17A1, and HSD3B1, subsequently inhibiting KLK3 expression. Moreover, in PC-3 cells, osteoclasts promoted CYP11A1 while RUNX2 facilitated expression of AKR1C3, HSD17B3, and CYP19A1 [74]. These findings highlight how osteoclasts amplify RUNX2-controlled steroid-producing enzymes, influencing the activation of the androgen receptor in CRPC cells. Further investigation demonstrated that osteocytic CRPC cells are more responsive to osteoclast stimulation regarding gene expression including reduced apoptosis [75]. Exploring the potential of targeting RUNX2 to hinder the progression of skeletal metastases in CRPC is an avenue worth further investigation. [74, 75]
Using a different approach, a recent study focused on targeting the prostate transmembrane protein androgen induced 1 (Pmepa1) [76]. Pmepa1 regulates proliferation, migration, and metastasis of cancer cells. Inhibitors of bone resorption, such as alendronate, bafilomycin A1, and the PI3K inhibitor LY294002, suppressed the expression of Pmepa1 in osteoclasts. The study demonstrated that knockdown of Pmepa1 expression impaired bone resorption activity [76]. These promising results suggest that targeting Pmepa1 may prove useful as a therapeutic target in pathologic bone destruction. Also targeting osteoclastic activities, the role of 3-phosphoinositide-dependent protein kinase-1 (PDK1) in osteoclasts was explored in the context of PCa-induced bone damage [77]. Using mice lacking PDK1 in osteoclasts, it was demonstrated that reduced expression of PDK1 led to reduced bone resorption in response to PCa cells. The study demonstrated that PDK1 expression has a significant impact on the ability of PCa cells to activate osteoclastic activities and cancer-induced bone damage [77].
The “vicious cycle” model continues to guide our thinking as to the role that osteoclastic activities play in promoting the growth of malignant cells in the marrow [55]. The ability of osteoclasts directly stimulating DTC growth is an attractive concept, or indirectly by activating other host resident cells or releasing growth factors sequestered in the mineralized bone matrix surrounding the marrow. Yet given the overall disappointment of osteoclast targeting agents promoting survival, further investigation in this area is clearly needed. The recent work on novel delivery systems to target osteoclasts and the development of novel targets are welcome developments in the field. They provide promise that targeting these pathways may provide long-term benefit to mCRPC bone lesions.
Inflammatory Response: Immune Cell Responses.
As intercellular signals, cytokines are well known to stimulate an inflammatory response. Inflammation in the bone marrow is thought to promote the growth and survival of cancer cells and contribute to bone degradation. As described above, PCa cells secrete a vast array of cytokines including the many with known immune modulating function. These cytokines trigger a cascade of inflammatory responses that promote cancer cell survival, proliferation, and migration in the bone. Much of the work over the last 3 years in this area has focused on recruitment of immune cells into primary and metastatic lesions.
For example, focusing on primary lesions circulating IL-10 was shown to be associated with reduced risk of prostate cancer in a prospective cohort of elderly men [48]. IL-10 is predominantly described as an anti-inflammatory cytokine. The mechanism for how IL-10 levels lead to a reduced risk is unclear but presumably related to immune cell driving cancer progression (Figure 1). For skeletal lesions, one of the most interesting studies demonstrated that chemotherapies, such as cabozantinib, may have immune-priming effects which may sensitize tumors to immunotherapies [78]. Here the study focused on Interleukin-27 (IL-27) a multi-functional cytokine, which acts to recruitment immune cells into to tumors, while also promoting bone repair [79, 80]. The study demonstrated that a combination of chemotherapy (cabozantinib) and IL27 immunotherapy could be used to treat bone-metastatic prostate cancer and exert pro-osteogenic effects [78].
Inflammatory Response: Targeting the Immune Response.
From the perspective of bone metastasis and inflammation, it is the critical to understand how cytokines might play a role in regulating dormancy. Owen et al recently demonstrated immune signalling intrinsic to the tumor potentially may play a crucial role in maintaining cancer cell dormancy [81]. The study demonstrated that proliferating PCa cells in bone reduce intrinsic type I IFN expression, reducing their visibility to the immune system and hampering treatment response, while activating environmental bone cells to drive cancer progression [81]. Restoring IFN signalling using histone deacetylase HDAC inhibitors reduced PCa immune evasion, boosted long-term antitumor immunity, and halted cancer growth in the bone [81]. These findings were validated in patients, highlighting the loss of tumor specific IFN signalling and reduced immune response in bone metastases compared to primary tumors. This study strongly suggests a mechanism as to why current immunotherapies fail in bone metastatic PCa and suggests a therapeutic target to overcome immune-based therapy limitations in solid cancers [81].
It is likely in the future that with greater understanding of inflammatory cytokines and their pathways therapeutic avenues will emerge to manage the inflammatory responses induced by PCa skeletal metastasis. By interrupting the inflammatory microenvironment, there’s potential to hinder tumor growth and to alter complications related to bone involvement in those affected. Ongoing research into therapies targeting precise cytokines or their signalling pathways to curb cancer growth within the bone structure will be critical but has already started to bear fruit.
Cytokines and Angiogenesis:
Cytokines released by cancer cells can induce the formation of new blood vessels (angiogenesis) in the bone tissue. Angiogenesis brings needed glucose, nutrients, and oxygen to the growing tumor, facilitating its expansion in the bone. In PCa skeletal metastasis, cytokines contribute significantly to the process of angiogenesis. Amongst the most studied angiogenic cytokines are vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and IL-8 which are produced by PCa cells at high levels and are overall linked to poorer survival. Several reports focused on mechanisms related to angiogenic growth of PCa growth in bone (Figure 1).
Using samples from the CHAARTED trial, in which patients received androgen deprivation therapy (ADT) +/− chemotherapy (docetaxel) for metastatic hormone-sensitive prostate cancer (mHSPC), it was confirmed that higher levels of IL-8 were linked to poorer survival among patients starting ADT. Analysing 233 patient samples drawn within 28 days of starting ADT, higher IL-8 levels were consistently associated with shorter survival and quicker progression to castration-resistant prostate cancer (CRPC). These correlations held regardless of docetaxel use, disease extent, or timing of metastasis onset. The results emphasize the potential of targeting IL-8 to enhance outcomes in mHSPC. [13]
With a similar focus on angiogenesis, FOXA1 is a transcription factor which is known to PCa progression. In a recent study FOXA1’s impact on angiogenesis was examined in a xenograft model in nude mice. FOXA1 expression promoted endothelial cell proliferation, migration, and tube formation in vitro by increasing secretion of pro-angiogenic factors production, including EGF, Endothelin-1, and Endoglin [82]
It was also found that the microRNA miR-130b is significantly downregulated in human PCa tumors and cell lines [83]. Downregulating miR-130b enhanced proliferation, invasion, and tube formation in endothelial cells. Conversely, boosting miR-130b levels downregulated PCa angiogenesis in lab experiments and in mice [83]. Mechanistically, miR-130b directly regulated TNF-α, thereby dampening nuclear factor-κB (NF-κB) signalling and VEGFA. Elevated VEGFA, in turn, counteracted miR-130b’s anti-angiogenic impact, forming a feedback loop that fuels prostate cancer’s blood vessel growth [83].
Likewise, PCa cells expression of cell surface enolase-1 (ENO1), which serves as a plasminogen receptor to facilitate their migration via plasmin activation plays a significant role in angiogenesis [84]. Antibody targeting ENO1 reduced the growth of subcutaneous PC-3 xenograft, monocytes recruitment, and intratumoral angiogenesis. In a PC-3 intratibial implantation model, an antibody targeting ENOS1 reduced tumor growth and osteoclast activation in the bone [84]. The mechanism of action for the impact of antibody blockade of ENO1 appears to be reduced expression of VEGF-A [84].
An interesting finding from the perspective of treatment came from understanding ADT resistance mechanisms. Here enzalutamide induces treatment-induced hypoxia, and subsequent induction of angiogenesis, as novel mechanisms of relapse to enzalutamide, highlighting the importance of two hypoxia-regulated cytokines in underpinning relapse. Significantly, hypoxia increased androgen receptor expression and transcriptional activity in PCa cells [85]. Inhibition of both IL8 and VEGF-A restored tumor response in the presence of enzalutamide, confirming the functional importance of their elevated expression in enzalutamide-resistant models [85]. Interestingly, inhibition of IL8 and VEGF-A resulted in a durable sensitivity to enzalutamide. [85]
Comprehending the central role of cytokines and their signalling pathways in driving angiogenesis is crucial. This understanding is key to devising precise treatments that can interrupt this process, thereby restricting the blood supply to metastatic tumors and impeding their advancement within the bone environment. Therapeutic strategies focused on these angiogenic cytokines likely will show potential in effectively addressing skeletal metastasis in prostate cancer, potentially enhancing patient outcomes.
Modulating the Immune Response:
Within the bone microenvironment, immune cells are considered to play a significant role as contributors to metastatic progression by regulating metastatic homing, seeding, dormancy, and outgrowth in the bone [86]. The microenvironment contributes to disease progression through multiple mechanisms, including immune suppression mediated in part activation conversion of microenvironmental cells to release reservoirs of CCL2, CXCL12, TGF-β and VEGF to produce a positive feedback loop for progression [87, 88].
From a therapeutic approach, investigation has focused on promoting an anti-tumor immune response or to sufficiently activate the immune system to overcome the immune evasion by the cancer cells, allowing them to survive and proliferate in the bone microenvironment. As described previously, IL-10 and TGF-β are perhaps best recognized for their ability to exert immunosuppressive effects and therefore weaken anti-tumor activity of immune cells (Figure 1). Likewise, cancer cells may produce cytokines that downregulate major histocompatibility complex (MHC) molecules, impairing antigen presentation or induce immune tolerance and thereby reducing recognition by immune cells.
Interferon-γ (IFNγ), a cytokine with limited success in clinical trials thus far, holds promise in transforming immunologically “cold” prostate cancer (PCa), especially in metastatic castration-resistant prostate cancer (mCRPC), into lesions recognizable by the immune system. Recent research showcased that IFNγ treatment spurred the expression of major histocompatibility class-I (MHC-I) genes and PD-L1 while reducing E-cadherin expression in PCa cells in lab settings117. Additionally, IFNγ treatment not only decreased E-cadherin expression but also increased sensitivity to chemotherapy in vitro. Crucially, in a murine model of spontaneous metastatic prostate cancer, pre-treatment with systemic IFNγ elevated HLA-A expression and lowered E-cadherin levels in primary tumors and at metastatic sites. This led to heightened apoptosis and limited micrometastases upon paclitaxel treatment compared to controls117. Using a different approach, but a novel exosomal vaccine (interferon-γ [IFN-γ]-modified exosomal vaccine) in PCa induced an immune response that cleared prostate cancer cell-derived exosomes, thereby eliminating the regulatory effect of the exosomes [89]. These findings indicate that IFNγ may serve a valuable role in combined treatment approaches to enhance sensitivity to both immunotherapy and chemotherapy metastases of mCRPC.
Tumor-associated macrophages (TAMs) are a prevalent cell type within the tumor microenvironment including bone, which frequently promote lesion progression (Figure 1). Most TAMs display an M2-like phenotype that aids tumor growth, while protecting tumor cells from the immune system, thus facilitating metastasis and lesion growth. IL-4 and IL-13 are key cytokines directing macrophages towards this M2 subset, sharing a receptor known as IL-4 receptor alpha (IL-4R alpha). By treating human M2 macrophages and their precursors with an IL-4R alpha antagonist was recently shown to reduce M2-like features, altering both cell surface markers and gene expression [90]. In PCa animal models, inhibiting IL-4R alpha either through drugs or genetic methods in mice lacking IL-4R alpha led to decreased CD206 expression on TAMs [90]. Using a similar rationale to target M2-macrophages, targeting miRNA let-7-5p which is significantly augmented in M2 macrophages significantly alters the expression of IL-10, IL-12, IL-13, and TNFα. Therefore, targeting let-7b-5p may regulate M2 polarization, ultimately inhibiting the proliferation of PCa cells [91]. These findings underscore multiple and promising approaches to counter the pro-tumor M2-like macrophage phenotype, potentially offering a new avenue for cancer therapy.
Targeting a different arm of the immune system, the effectiveness of T cells modified with chimeric antigen receptors (CARs) has been proven in blood cancers, yet their impact on solid tumors remains limited due to factors like TGFβ suppressing CAR T cell activity. An interesting approach to overcome this limitation was recently developed using a switch receptor (SwR) by combining parts of the TGFβ receptor with elements from the IL-2/15 receptor [92]. Testing the SwR alongside two variants of a CAR targeting STEAP1, a protein found abundantly in PCa cells, was used to improve therapeutic performance in TGFβ-rich environments. After multiple rounds of stimulation, the modified cells maintained their strength and specificity against STEAP1 [92]. Conceptually, this SwR approach could significantly improve CAR T cell therapy in TGFβ-heavy settings such as bone and might be applicable as an additional element for CAR T cells or other types of cell-based therapies.
In a related development, immune checkpoint blockade has faced challenges in treating bone metastatic castrate-resistant prostate cancer (mCRPC). A recent study presented a novel strategy employing γδ-enriched chimeric antigen receptor (CAR) T cells in conjunction with zoledronate (ZOL), an FDA-approved bisphosphonate, for mCRPC treatment [93]. In a preclinical murine bone mCRPC model, γδ CAR-T cells targeting prostate stem cell antigen (PSCA) demonstrated rapid tumor regression, leading to increased survival and reduced cancer-associated bone disease. Preceding treatment with ZOL, activated γδ CAR-T cells boosting cytokine secretion and enhancing antitumor effectiveness.
Role of Chemokines in Skeletal Metastasis to the Craniofacial Region.
The skull is a relatively rare site for skeletal metastasis of PCa. In recent investigation, the prognostic value of skull metastases (SM) was evaluated for metastatic PCa patients receiving ADT [94]. Patients with SM (n = 26) had significantly higher Gleason scores, higher clinical T stage, higher prostate-specific antigen level at diagnosis, and were more likely to have high-burden metastasis and lymph node metastasis, compared with those without SM (n = 81) [94]. SM was significantly associated with shorter medium progression free survival (9.4 vs. 18.3 months, p < 0.001) and overall survival (22.2 vs. 58.2 months, p < 0.001) [94]. SM was significantly correlated with more aggressive disease and indicated poor prognosis in PCa patients with bone metastasis. The study is interesting in that it may provide useful reference for the risk stratification of PCa patients.
On the treatment side, PSMA-targeted radionuclide therapy is a promising treatment for PCa, but dose-limiting xerostomia can severely limit its clinical adaptation, especially when using alpha-emitting radionuclides. It remains unclear why relatively high expression of PSMA is expressed by the salivary glands. Preventing salivary gland uptake of PSMA-targeting radiopharmaceuticals is an clinical challenge a clinical unmet need, and different approaches were investigated in the past 29,130. External cooling orally administered monosodium glutamate, high-dose botulinum toxin injections, systemic administration of antisialagogues (e.g. atropine, scopolamine), may selectively decrease salivary uptake of systemically administered nucleotide without altering tumor uptake. [95, 96]
Finally, bisphosphonates and denosumab, a humanized human monoclonal antibody that specifically binds to the receptor activator of nuclear factor-κB ligand, which is key for osteoclast formation, function, and survival [97]. Osteonecrosis of the jaw (ONJ) is a significant and often painful development for those patients treated with bone-modifying agents, yet the true incidence is likely under reported. The mechanism which sensitize patients to developing ONJ in who use bone-modifying agents (BMAs) remains unclear [98].
One study which sought to explore the mechanism investigated macrophages (CD11b+Gr1hi) and γδ-T cells in zoledronic acid induced ONJ based on the IFN-γ involved osteoblast differentiation signaling pathway [99]. The study examined 20 patients with ONJ verse healthy controls as well as animals treated with zoledronic acid. The study found that the apoptotic rate of the macrophages and T-cells was significantly higher in the treated subjects, as well as expression of a number of other inflammatory cytokines including, IL-1β, IFN-γ, CCL3, CCL4, IL-12 and IL-13 TNF-α [99]. The investigators concluded that local proliferation of macrophages and γδ-T cells in response to zoledronic acid promotes inflammation resulting in ONJ [99].
Given the attractiveness of targeting chemokines and cytokines as therapeutic agents, a cautionary note should be considered when used in the context of skeletal metastases of PCa. Normal tissue homeostasis requires signalling by the pathways activated by chemokines and cytokines. A recent literature review examined the use of TNFα inhibitors as used as immunomodulators to manage inflammatory conditions such as rheumatoid arthritis and Crohn’s disease [100]. The limited data suggest that some patients will develop ONJ as a result of treatment with TNF-alpha inhibitors [100]. While the study has its limitations, it does suggest that as we develop targeted therapies to modulate chemokine secretion and signalling, we are likely to observe greater prevalence of OHJ, which can alter patient compliance to staying on therapy.
Finally, an area of investigation which is emerging is the utility of salivary diagnostics for the detection and monitoring of metastatic disease [101, 102]. By leveraging the unique properties of saliva as a biomarker, it may be possible to develop a non-invasive, accurate, and accessible diagnostic tool that can significantly improve patient care. We envision that if a metastatic signature of cytokines or chemokines can be identified, a point of care test could be developed to monitor disease stability, progression, and response to therapy.
Summary:
Metastatic PCa often takes root in the bone marrow. Studies focusing on intracellular signalling molecules, such as cytokines, have shown they play a key role in cell growth, invasion and angiogenesis (Figure 1). Cytokines, primarily secreted by immune system cells, can also be produced by tumor cells and other host cells, regulating immune responses and influencing normal tissue physiology. In the context of PCa, cytokines can stimulate cancer cell proliferation in the bone and influence their resistance to cell death. They also play a role in bone remodelling, influencing bone resorption and tumor proliferation. Nuclear protein/cytokine activation can promote angiogenesis, increasing the blood supply essential for tumor growth. Finally, cytokines have a significant role in regulating immune response, often suppressing the immune system, thereby favouring tumor growth and survival. Understanding these processes is crucial in developing targeted therapies to disrupt the interaction between cancer cells and their environment, potentially slowing down the progression of skeletal metastasis in PCa patients.
Funding:
This work was supported by the NIH/NCI (grant no. 3P01CA093900-018) (K.P., K.P., R.S.T), the Prostate Cancer Foundation (2014 Challenge Award) (K.P., R.S.T) and the Department of Defence (PC140665) (R.S.T.). In addition, the work was supported by R01CA238888 (Y.S.) and P30CA012197 to the Wake Forest Baptist Comprehensive Cancer Center (Y.S.).
Footnotes
Conflict of Interest Statement: The authors have no conflict of interests to declare.
Human and Animal Rights.
This review reports no primary studies using human or animal subjects.
References.
- 1.Shiozawa Y, Berry JE, Eber MR, Jung Y, Yumoto K, Cackowski FC, et al. The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer. Oncotarget. 2016. doi: 10.18632/oncotarget.9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cackowski FC, Taichman RS. Minimal Residual Disease in Prostate Cancer. Adv Exp Med Biol. 2018;1100:47–53. doi: 10.1007/978-3-319-97746-1_3. [DOI] [PubMed] [Google Scholar]
- *3. Cackowski FC, Wang Y, Decker JT, Sifuentes C, Weindorf S, Jung Y, et al. Detection and isolation of disseminated tumor cells in bone marrow of patients with clinically localized prostate cancer. Prostate. 2019;79(14):1715–27. doi: 10.1002/pros.23896. A rigorous investigation of the presence of DTCs in the marrow at the time of prostatectomy.
- 4.Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4(6):291–8. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 6.Jung Y, Wang J, Lee E, McGee S, Berry JE, Yumoto K, et al. Annexin 2-CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res. 2015;13(1):197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7. McFarlane A, Pohler E, Moraga I. Molecular and cellular factors determining the functional pleiotropy of cytokines. FEBS J. 2023;290(10):2525–52. doi: 10.1111/febs.16420. Discussion of ligand-receptor binding affinity and topology, signaling protein levels contribute to the modulation and diversification of cytokine responses.
- 8.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37 Suppl 1(Suppl 1):S34–45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. A seminal paper frequently cited exploring the causal relationship between inflammation, innate immunity and cancer probing the molecular and cellular mechanisms mediating this relationships.
- *10. Ihle JN. Cytokine receptor signalling. Nature. 1995;377(6550):591–4. doi: 10.1038/377591a0. Formative summary work outlining the relationships between cytokine receptor superfamilies, Janus kinases (JAKs) and signal transducers and activators of transcription (STATs). These signaling pathways diversify cytokine responses.
- 11.Pedersen EA, Shiozawa Y, Pienta KJ, Taichman RS. The prostate cancer bone marrow niche: more than just 'fertile soil'. Asian J Androl. 2012;14(3):423–7. doi: 10.1038/aja.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culig Z. Interleukin-6 Function and Targeting in Prostate Cancer. Adv Exp Med Biol. 2021;1290:1–8. doi: 10.1007/978-3-030-55617-4_1. [DOI] [PubMed] [Google Scholar]
- *13. Harshman LC, Wang VX, Hamid AA, Santone G, Drake CG, Carducci MA, et al. Impact of baseline serum IL-8 on metastatic hormone-sensitive prostate cancer outcomes in the Phase 3 CHAARTED trial (E3805). Prostate. 2020;80(16):1429–37. doi: 10.1002/pros.24074. This paper describes how elevated serum interleukin-8 (IL-8) at androgen deprivation therapy (ADT) start predicted poorer overall survival (OS) in metastatic hormone-sensitive prostate cancer (mHSPC) patients. Serum IL-8 levels were associated with shorter time to castration-resistant prostate cancer (CRPC). Targeting IL-8 may improve mHSPC outcomes, independent of docetaxel use or metastatic burden.
- 14.Olivas A, Price RS. Obesity, Inflammation, and Advanced Prostate Cancer. Nutr Cancer. 2021;73(11-12):2232–48. doi: 10.1080/01635581.2020.1856889. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Chang I, Jung Y, Kaplan Z, Hill EE, Taichman RS, et al. Mycoplasma hyorhinis infection promotes TNF-α signaling and SMAC mimetic-mediated apoptosis in human prostate cancer. Heliyon. 2023;9(10):e20655. doi: 10.1016/j.heliyon.2023.e20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zhang B, Xiang L, Xia S, Kucuk O, Deng X, et al. TGF-β causes Docetaxel resistance in Prostate Cancer via the induction of Bcl-2 by acetylated KLF5 and Protein Stabilization. Theranostics. 2020;10(17):7656–70. doi: 10.7150/thno.44567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito M, Fang C, Cook KC, Park N, Wei Y, Spadazzi C, et al. TGF-β-induced DACT1 biomolecular condensates repress Wnt signalling to promote bone metastasis. Nat Cell Biol. 2021;23(3):257–67. doi: 10.1038/s41556-021-00641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzaei S, Paskeh MDA, Saghari Y, Zarrabi A, Hamblin MR, Entezari M, et al. Transforming growth factor-beta (TGF-β) in prostate cancer: A dual function mediator? Int J Biol Macromol. 2022;206:435–52. doi: 10.1016/j.ijbiomac.2022.02.094. [DOI] [PubMed] [Google Scholar]
- 19.Archer M, Dogra N, Kyprianou N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers (Basel). 2020;12(10). doi: 10.3390/cancers12102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng K, Batbatan C, Jia Q. IL-6 Enhances the Viability and Invasion Ability of Prostate Cancer Cells. Stud Health Technol Inform. 2023;308:521–6. doi: 10.3233/shti230879. [DOI] [PubMed] [Google Scholar]
- 21.Deichaite I, Sears TJ, Sutton L, Rebibo D, Morgan K, Nelson T, et al. Differential regulation of TNFα and IL-6 expression contributes to immune evasion in prostate cancer. J Transl Med. 2022;20(1):527. doi: 10.1186/s12967-022-03731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu YM, Lou XL, Liu BZ, Sun L, Wan S, Wu L, et al. TGF-β1-regulated miR-3691-3p targets E2F3 and PRDM1 to inhibit prostate cancer progression. Asian J Androl. 2021;23(2):188–96. doi: 10.4103/aja.aja_60_20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Daouk R, Bahmad HF, Saleh E, Monzer A, Ballout F, Kadara H, et al. Genome-wide gene expression analysis of a murine model of prostate cancer progression: Deciphering the roles of IL-6 and p38 MAPK as potential therapeutic targets. PLoS One. 2020;15(8):e0237442. doi: 10.1371/journal.pone.0237442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganaie AA, Mansini AP, Hussain T, Rao A, Siddique HR, Shabaneh A, et al. Anti-S100A4 Antibody Therapy Is Efficient in Treating Aggressive Prostate Cancer and Reversing Immunosuppression: Serum and Biopsy S100A4 as a Clinical Predictor. Mol Cancer Ther. 2020;19(12):2598–611. doi: 10.1158/1535-7163.Mct-20-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZH, Liu MD, Yao K, Xu S, Yu DX, Xie DD, et al. Vitamin D deficiency aggravates growth and metastasis of prostate cancer through promoting EMT in two β-catenin-related mechanisms. J Nutr Biochem. 2023;111:109177. doi: 10.1016/j.jnutbio.2022.109177. [DOI] [PubMed] [Google Scholar]
- 26.Łabędź W, Przybyla A, Zimna A, Dąbrowski M, Kubaszewski Ł. The Role of Cytokines in the Metastasis of Solid Tumors to the Spine: Systematic Review. Int J Mol Sci. 2023;24(4). doi: 10.3390/ijms24043785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu C, et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat Commun. 2021;12(1):1714. doi: 10.1038/s41467-021-21976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadrkhanloo M, Paskeh MDA, Hashemi M, Raesi R, Motahhary M, Saghari S, et al. STAT3 signaling in prostate cancer progression and therapy resistance: An oncogenic pathway with diverse functions. Biomed Pharmacother. 2023;158:114168. doi: 10.1016/j.biopha.2022.114168. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong AJ, Nixon AB, Carmack A, Yang Q, Eisen T, Stadler WM, et al. Angiokines Associated with Targeted Therapy Outcomes in Patients with Non-Clear Cell Renal Cell Carcinoma. Clin Cancer Res. 2021;27(12):3317–28. doi: 10.1158/1078-0432.Ccr-20-4504. [DOI] [PubMed] [Google Scholar]
- 32.Macías M, García-Cortés Á, Torres M, Ancizu-Marckert J, Ignacio Pascual J, Díez-Caballero F, et al. Characterization of the perioperative changes of exosomal immune-related cytokines induced by prostatectomy in early-stage prostate cancer patients. Cytokine. 2021;141:155471. doi: 10.1016/j.cyto.2021.155471. [DOI] [PubMed] [Google Scholar]
- 33.Runcie KD, Dallos MC. Prostate Cancer Immunotherapy-Finally in From the Cold? Curr Oncol Rep. 2021;23(8):88. doi: 10.1007/s11912-021-01084-0. [DOI] [PubMed] [Google Scholar]
- 34.Stultz J, Fong L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021;24(3):697–717. doi: 10.1038/s41391-021-00340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28(4):724–34. doi: 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley JE, Pan S, Figg WD, Lopez-Bujanda ZA, Strope JD, Aggen DH, et al. Association between immunosuppressive cytokines and PSA progression in biochemically recurrent prostate cancer treated with intermittent hormonal therapy. Prostate. 2020;80(4):336–44. doi: 10.1002/pros.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. Jama. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djurhuus SS, Schauer T, Simonsen C, Toft BG, Jensen ARD, Erler JT, et al. Effects of acute exercise training on tumor outcomes in men with localized prostate cancer: A randomized controlled trial. Physiol Rep. 2022;10(19):e15408. doi: 10.14814/phy2.15408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39. Lasorsa F, di Meo NA, Rutigliano M, Ferro M, Terracciano D, Tataru OS, et al. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int J Mol Sci. 2023;24(2). doi: 10.3390/ijms24020910. Prostate cancer cells show increased production of fats and cholesterol. Studies have found higher levels of certain enzymes in these cells. Glutamine becomes crucial for prostate cancer cells, and targeting its metabolism could be a promising treatment.
- 40.Natani S, Sruthi KK, Asha SM, Khilar P, Lakshmi PSV, Ummanni R. Activation of TGF-β - SMAD2 signaling by IL-6 drives neuroendocrine differentiation of prostate cancer through p38MAPK. Cell Signal. 2022;91:110240. doi: 10.1016/j.cellsig.2021.110240. [DOI] [PubMed] [Google Scholar]
- 41.Neuwirt H, Bouchal J, Kharaishvili G, Ploner C, Jöhrer K, Pitterl F, et al. Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun Signal. 2020;18(1):11. doi: 10.1186/s12964-019-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao L, Wang L, Xu J, Zou J. The role of integrin family in bone metabolism and tumor bone metastasis. Cell Death Discov. 2023;9(1):119. doi: 10.1038/s41420-023-01417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell IJ, Chinni SR, Reddy SS, Zaslavsky A, Gavande N. Pro-inflammatory cytokines and chemokines initiate multiple prostate cancer biologic pathways of cellular proliferation, heterogeneity and metastasis in a racially diverse population and underlie the genetic/biologic mechanism of racial disparity: Update. Urol Oncol. 2021;39(1):34–40. doi: 10.1016/j.urolonc.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Puhr M, De Marzo A, Isaacs W, Lucia MS, Sfanos K, Yegnasubramanian S, et al. Inflammation, Microbiota, and Prostate Cancer. Eur Urol Focus. 2016;2(4):374–82. doi: 10.1016/j.euf.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Ravindranathan D, Alhalabi O, Rafei H, Shah AY, Bilen MA. Landscape of Immunotherapy in Genitourinary Malignancies. Adv Exp Med Biol. 2021;1342:143–92. doi: 10.1007/978-3-030-79308-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wautier JL, Wautier MP. Old and New Blood Markers in Human Colorectal Cancer. Int J Mol Sci. 2022;23(21). doi: 10.3390/ijms232112968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koerner J, Horvath D, Oliveri F, Li J, Basler M. Suppression of prostate cancer and amelioration of the immunosuppressive tumor microenvironment through selective immunoproteasome inhibition. Oncoimmunology. 2023;12(1):2156091. doi: 10.1080/2162402x.2022.2156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CE, Bauer DC, Yuan JM, Cauley JA. Circulating IL-10 is associated with reduced risk of prostate cancer in a prospective cohort of elderly men: the MrOS Study. Cancer Causes Control. 2023;34(1):59–68. doi: 10.1007/s10552-022-01639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Firdaus F, Kuchakulla M, Qureshi R, Dulce RA, Soni Y, Van Booven DJ, et al. S-nitrosylation of CSF1 receptor increases the efficacy of CSF1R blockage against prostate cancer. Cell Death Dis. 2022;13(10):859. doi: 10.1038/s41419-022-05289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu SY, Chen FH, Wang CC, Yu CF, Chiang CS, Hong JH. Role of Myeloid-Derived Suppressor Cells in High-Dose-Irradiated TRAMP-C1 Tumors: A Therapeutic Target and an Index for Assessing Tumor Microenvironment. Int J Radiat Oncol Biol Phys. 2021;109(5):1547–58. doi: 10.1016/j.ijrobp.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Munoz LE, Monterroza L, Bommireddy R, Shafizadeh Y, Pack CD, Ramachandiran S, et al. Dendritic Cells Pulsed with Cytokine-Adjuvanted Tumor Membrane Vesicles Inhibit Tumor Growth in HER2-Positive and Triple Negative Breast Cancer Models. Int J Mol Sci. 2021;22(16). doi: 10.3390/ijms22168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cackowski FC, Heath EI. Prostate cancer dormancy and recurrence. Cancer Lett. 2022;524:103–8. doi: 10.1016/j.canlet.2021.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche 33. Neoplasia. 2010;12(2):116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cackowski FC, Eber MR, Rhee J, Decker AM, Yumoto K, Berry JE, et al. Mer Tyrosine Kinase Regulates Disseminated Prostate Cancer Cellular Dormancy. J Cell Biochem. 2016. doi: 10.1002/jcb.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **55. Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2(6):570–2. This seminal work describes how cancer cells that spread to bone disrupt normal bone structure and mineral balance. Bone provides a favorable environment for cancer cells to grow, creating a cycle where cancer and bone cells stimulate each other’s growth. Targeted therapies are being developed to disrupt this cycle, but more effective treatments are needed. Understanding the biology of bone metastases is crucial for developing better therapies and preventing cancer-related bone problems.
- 56.Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open-label, randomised, phase 3 factorial trial. Lancet Oncol. 2014;15(10):1076–89. doi: 10.1016/S1470-2045(14)70328-6. [DOI] [PubMed] [Google Scholar]
- 57.Early Breast Cancer Trialists’ Collaborative G, Coleman R, Powles T, Paterson A, Gnant M, Anderson S, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–61. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 58.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isaacsson Velho P, Qazi F, Hassan S, Carducci MA, Denmeade SR, Markowski MC, et al. Efficacy of Radium-223 in Bone-metastatic Castration-resistant Prostate Cancer with and Without Homologous Repair Gene Defects. Eur Urol. 2019;76(2):170–6. doi: 10.1016/j.eururo.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heidenreich A, Gillessen S, Heinrich D, Keizman D, O’Sullivan JM, Carles J, et al. Radium-223 in asymptomatic patients with castration-resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer. 2019;19(1):12. doi: 10.1186/s12885-018-5203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-Pérez FO, Davanzo J, López-Buenrostro S, Santos-Cuevas C, Ferro-Flores G, Jímenez-Ríos MA, et al. Head to head comparison performance of (99m)Tc-EDDA/HYNIC-iPSMA SPECT/CT and (68)Ga-PSMA-11 PET/CT a prospective study in biochemical recurrence prostate cancer patients. Am J Nucl Med Mol Imaging. 2018;8(5):332–40. [PMC free article] [PubMed] [Google Scholar]
- 62.Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer. 2008;123(10):2267–78. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao J, McCauley LK. Skeletal metastasis: Established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev. 2006;25(4):559–71. [DOI] [PubMed] [Google Scholar]
- 64.Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther. 2014;141(2):222–33. doi: 10.1016/j.pharmthera.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keller ET, Zhang J, Cooper CR, Smith PC, McCauley LK, Pienta KJ, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20(3-4):333–49. [DOI] [PubMed] [Google Scholar]
- 66.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986;261(27):12665–74. [PubMed] [Google Scholar]
- 67.Brennen WN, Denmeade SR, Isaacs JT. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer. 2013;20(5):R269–90. doi: 10.1530/ERC-13-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steeve KT, Marc P, Sandrine T, Dominique H, Yannick F. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine & Growth Factor Reviews. 2004;15(1):49–60. [DOI] [PubMed] [Google Scholar]
- 69.Martin TJ, Romas E, Gillespie MT. Interleukins in the control of osteoclast differentiation. Critical Reviews in Eukaryotic Gene Expression. 1998;8(2):107–23. [DOI] [PubMed] [Google Scholar]
- 70.Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, et al. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. Journal of Immunology. 1999;163(1):434–42. [PubMed] [Google Scholar]
- *71. Rahman MT, Kaung Y, Shannon L, Androjna C, Sharifi N, Labhasetwar V. Nanoparticle-mediated synergistic drug combination for treating bone metastasis. J Control Release. 2023;357:498–510. doi: 10.1016/j.jconrel.2023.04.019. Bone metastasis in advanced solid tumors is often untreatable. A study using biodegradable nanoparticles targeted the tumor-bone marrow microenvironment in a prostate cancer model is describd using a combination treatment of docetaxel and Denosumab. This combination regressed tumors, preventing bone resorption and showing safety, efficacy, and synergy in modulating the tumor-bone microenvironment.
- 72.Niu Y, Yang H, Yu Z, Gao C, Ji S, Yan J, et al. Intervention with the Bone-Associated Tumor Vicious Cycle through Dual-Protein Therapeutics for Treatment of Skeletal-Related Events and Bone Metastases. ACS Nano. 2022;16(2):2209–23. doi: 10.1021/acsnano.1c08269. [DOI] [PubMed] [Google Scholar]
- 73.Chang AC, Lin LW, Chen YC, Chen PC, Liu SC, Tai HC, et al. The ADAM9/WISP-1 axis cooperates with osteoblasts to stimulate primary prostate tumor growth and metastasis. Int J Biol Sci. 2023;19(3):760–71. doi: 10.7150/ijbs.77495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74. Huang J, Hagberg Thulin M, Damber JE, Welen K. The roles of RUNX2 and osteoclasts in regulating expression of steroidogenic enzymes in castration-resistant prostate cancer cells. Mol Cell Endocrinol. 2021;535:111380. doi: 10.1016/j.mce.2021.111380. This study examined how osteoclasts affect the gene RUNX2, which is involved in steroid production. The authors reported that osteoclasts increase RUNX2 expression in prostate cancer cells, leading to the production of enzymes that promote cancer growth. Targeting RUNX2 could be a way to treat bone metastases in prostate cancer.
- 75.Huang J, Freyhult E, Buckland R, Josefsson A, Damber JE, Welen K. Osteoclasts directly influence castration-resistant prostate cancer cells. Clin Exp Metastasis. 2022;39(5):801–14. doi: 10.1007/s10585-022-10179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *76. Xu X, Hirata H, Shiraki M, Kamohara A, Nishioka K, Miyamoto H, et al. Prostate transmembrane protein androgen induced 1 is induced by activation of osteoclasts and regulates bone resorption. FASEB J. 2019;33(3):4365–75. doi: 10.1096/fj.201801573R. Osteoclasts, which break down bone, show increased expression of the protein Pmepa1 during bone resorption. Pmepa1 is crucial for osteoclast function, as its knockdown impairs bone resorption activity. This protein could serve as a marker for activated osteoclasts and a potential target for treating conditions involving pathological bone destruction.
- 77.Zhang Y, Nong H, Bai Y, Zhou Q, Zhang Q, Liu M, et al. Conditional knockout of PDK1 in osteoclasts suppressed osteoclastogenesis and ameliorated prostate cancer-induced osteolysis in murine model. Eur J Med Res. 2023;28(1):433. doi: 10.1186/s40001-023-01425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar S, Mulia GE, Figueiredo ML. Cabozantinib and IL-27 combinatorial therapy for bone-metastatic prostate cancer. Front Mol Biosci. 2023;10:1259336. doi: 10.3389/fmolb.2023.1259336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beizavi Z, Zohouri M, Asadipour M, Ghaderi A. IL-27, a pleiotropic cytokine for fine-tuning the immune response in cancer. Int Rev Immunol. 2021;40(5):319–29. doi: 10.1080/08830185.2020.1840565. [DOI] [PubMed] [Google Scholar]
- 80.Petrarca C, Frydas S, Donelan J, Boucher W, Papadopoulou N, Cao J, et al. Interleukin 27 (IL-27): A novel pleiotropic cytokine involved in T cell differentiation and T cell response modulation. Int J Immunopathol Pharmacol. 2005;18(2):191–4. doi: 10.1177/039463200501800201. [DOI] [PubMed] [Google Scholar]
- **81. Owen KL, Gearing LJ, Zanker DJ, Brockwell NK, Khoo WH, Roden DL, et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 2020;21(6):e50162. doi: 10.15252/embr.202050162. Castrate-resistant prostate cancer’s bone metastasis latency is linked to dormancy, where cancer cells remain inactive before lesion formation. Research using single-cell gene analysis and tissue testing revealed that immune signaling within the tumor maintains this dormancy. Restoring a lost immune signal with a specific drug increased cancer visibility to the immune system, enhancing long-term immune response and halting bone cancer growth, suggesting a new approach for more effective immune-based therapies in solid tumors.
- 82.Su Y, Zhang Y, Zhao J, Zhou W, Wang W, Han B, et al. FOXA1 promotes prostate cancer angiogenesis by inducing multiple pro-angiogenic factors expression. J Cancer Res Clin Oncol. 2021;147(11):3225–43. doi: 10.1007/s00432-021-03730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mu HQ, He YH, Wang SB, Yang S, Wang YJ, Nan CJ, et al. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin Transl Oncol. 2020;22(1):111–21. doi: 10.1007/s12094-019-02217-5. [DOI] [PubMed] [Google Scholar]
- 84.Chen ML, Yuan TT, Chuang CF, Huang YT, Chung IC, Huang WC. A Novel Enolase-1 Antibody Targets Multiple Interacting Players in the Tumor Microenvironment of Advanced Prostate Cancer. Mol Cancer Ther. 2022;21(8):1337–47. doi: 10.1158/1535-7163.Mct-21-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maxwell PJ, McKechnie M, Armstrong CW, Manley JM, Ong CW, Worthington J, et al. Attenuating Adaptive VEGF-A and IL8 Signaling Restores Durable Tumor Control in AR Antagonist-Treated Prostate Cancers. Mol Cancer Res. 2022;20(6):841–53. doi: 10.1158/1541-7786.Mcr-21-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng X, Wang Z. Immune Modulation of Metastatic Niche Formation in the Bone. Front Immunol. 2021;12:765994. doi: 10.3389/fimmu.2021.765994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brennen WN, DL JT, Jiang W, Krueger TE, Antony L, Denmeade SR, et al. Overcoming stromal barriers to immuno-oncological responses via fibroblast activation protein-targeted therapy. Immunotherapy. 2021;13(2):155–75. doi: 10.2217/imt-2020-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vickman RE, Broman MM, Lanman NA, Franco OE, Sudyanti PAG, Ni Y, et al. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. Prostate. 2020;80(2):173–85. doi: 10.1002/pros.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi X, Sun J, Li H, Lin H, Xie W, Li J, et al. Antitumor efficacy of interferon-γ-modified exosomal vaccine in prostate cancer. Prostate. 2020;80(11):811–23. doi: 10.1002/pros.23996. [DOI] [PubMed] [Google Scholar]
- 90.de Groot AE, Myers KV, Krueger TEG, Brennen WN, Amend SR, Pienta KJ. Targeting interleukin 4 receptor alpha on tumor-associated macrophages reduces the pro-tumor macrophage phenotype. Neoplasia. 2022;32:100830. doi: 10.1016/j.neo.2022.100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rong J, Xu L, Hu Y, Liu F, Yu Y, Guo H, et al. Inhibition of let-7b-5p contributes to an anti-tumorigenic macrophage phenotype through the SOCS1/STAT pathway in prostate cancer. Cancer Cell Int. 2020;20:470. doi: 10.1186/s12935-020-01563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beck C, Casey NP, Persiconi I, Moharrami NN, Sike A, Jin Y, et al. Development of a TGFβ-IL-2/15 Switch Receptor for Use in Adoptive Cell Therapy. Biomedicines. 2023;11(2). doi: 10.3390/biomedicines11020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **93. Frieling JS, Tordesillas L, Bustos XE, Ramello MC, Bishop RT, Cianne JE, et al. γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer. Sci Adv. 2023;9(18):eadf0108. doi: 10.1126/sciadv.adf0108. Combining γδ-enriched chimeric antigen receptor (CAR) T cells with zoledronate (ZOL) shows promise for treating bone metastatic castrate-resistant prostate cancer (mCRPC). In a mouse model, CAR-T cells targeting prostate stem cell antigen (PSCA) with ZOL pretreatment led to tumor regression and improved survival, suggesting a potential therapy for mCRPC.
- 94.Xiong T, Jiang M, Ye X, Zhu G, Cao F, Cui Y, et al. Skull metastasis is a poor prognostic factor for prostate cancer patients with bone metastasis: a retrospective study based on a Chinese population. BMC Urol. 2023;23(1):13. doi: 10.1186/s12894-023-01179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roy J, Warner BM, Basuli F, Zhang X, Zheng C, Goldsmith C, et al. Competitive blocking of salivary gland [(18)F]DCFPyL uptake via localized, retrograde ductal injection of non-radioactive DCFPyL: a preclinical study. EJNMMI Res. 2021;11(1):66. doi: 10.1186/s13550-021-00803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roy J, Warner BM, Basuli F, Zhang X, Wong K, Pranzatelli T, et al. Comparison of Prostate-Specific Membrane Antigen Expression Levels in Human Salivary Glands to Non-Human Primates and Rodents. Cancer Biother Radiopharm. 2020;35(4):284–91. doi: 10.1089/cbr.2019.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **97. Van Poznak CH, Unger JM, Darke AK, Moinpour C, Bagramian RA, Schubert MM, et al. Association of Osteonecrosis of the Jaw With Zoledronic Acid Treatment for Bone Metastases in Patients With Cancer. JAMA Oncol. 2021;7(2):246–54. doi: 10.1001/jamaoncol.2020.6353. Osteonecrosis of the jaw (ONJ) is a concern for cancer patients with bone metastases treated with zoledronic acid, but its true incidence is unclear. A study tracked 3491 patients receiving zoledronic acid for bone metastases from various cancers. They found a 2.8% cumulative incidence of ONJ at 3 years, with higher risk in patients with myeloma, fewer teeth, dentures, and current smokers. Adjusting dosing intervals may reduce risk.
- 98.Wichelmann TA, Ahdi HS, Pandravada S, Ehrenpreis ED. A Summary of the Rare Reports of Osteonecrosis of the Jaw Associated With Tumor Necrosis-α Inhibitors in the United States Food and Drug Administration’s Adverse Event Reporting System Database. J Oral Maxillofac Surg. 2023;81(10):1311–8. doi: 10.1016/j.joms.2023.06.015. [DOI] [PubMed] [Google Scholar]
- **99. Qu X, Wang Z, Zhou T, Shan L. Determination of the molecular mechanism by which macrophages and gammadelta-T cells contribute to ZOL-induced ONJ. Aging (Albany NY). 2020;12(20):20743–52. doi: 10.18632/aging.104006. This study investigates the role of macrophages and γδ-T cells in Zoledronic acid (ZOL)-induced osteonecrosis of the jaw (ONJ) through the IFN-γ signaling pathway. Results show increased CD11b+Gr1hi cells and γδ-T cells, along with elevated inflammatory markers in ONJ. Reduced expression of CTSK and FGFR3, and increased NF-κB and ERBB2IP, suggests a mechanism involving inflammation and bone remodeling.
- 100.Wichelmann TA, Ahdi HS, Pandravada S, Ehrenpreis ED. A Summary of the Rare Reports of Osteonecrosis of the Jaw Associated With Tumor Necrosis-alpha Inhibitors in the United States Food and Drug Administration’s Adverse Event Reporting System Database. J Oral Maxillofac Surg. 2023;81(10):1311–8. doi: 10.1016/j.joms.2023.06.015. [DOI] [PubMed] [Google Scholar]
- 101.Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47(6):699–707. doi: 10.1016/j.ijom.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 102.Redman RS, Bayley NC, Nylen ES. Salivary and serum biomarkers of inflammation in a man with metastatic medullary thyroid carcinoma and hyperreactive gingiva: a fourteen year odyssey. Biotech Histochem. 2019;94(6):389–97. doi: 10.1080/10520295.2019.1649463. [DOI] [PubMed] [Google Scholar]


