Abstract
Antidepressants are widely prescribed for major depressive disorder and anxiety, yet their long-term use is associated with weight gain, affecting up to 55–65% of patients. This adverse effect contributes to treatment discontinuation, relapse, and worsened metabolic health outcomes, including increased risk for obesity and type 2 diabetes. This artic le presents a critical evaluation of the published reports on the mechanisms underlying antidepressant-induced weight gain, comparative effects across drug classes, and mitigation strategies. Weight gain varies significantly by antidepressant class. Tricyclic antidepressants, monoamine oxidase inhibitors, and a tetracyclic antidepressant, mirtazapine, are associated with the most substantial weight increases, while selective serotonin reuptake inhibitors typically induce weight gain after prolonged use. Mechanisms involve serotonergic and dopaminergic signaling, receptor desensitization, insulin resistance, and altered leptin and ghrelin levels. Genetic factors, including CYP2C19 metabolizer status, and lifestyle factors such as baseline body mass index and diet, further influence risk. Bupropion, a norepinephrine-dopamine reuptake inhibitor, is the only commonly prescribed antidepressant consistently associated with weight loss or neutrality. Mitigation strategies include switching medications, adding agents like metformin or GLP-1 receptor agonists, and incorporating behavioral interventions. Antidepressant-induced weight gain is a multifactorial issue requiring individualized management. Understanding pharmacologic mechanisms and patient-specific risk factors is essential for optimizing treatment efficacy while minimizing metabolic burden.
Keywords: Antidepressant, Bupropion, Dopaminergic pathway, GLP-1 receptor agonist, Metabolic effect, Monoamine oxidase inhibitor, Norepinephrine-dopamine reuptake inhibitor, Pharmacogenomics, Selective serotonin reuptake inhibitor (SSRI), Serotonergic pathway, Tetracyclic antidepressant, Tricyclic antidepressant, Weight gain
1. Introduction
Major depressive disorder (MDD) is a major global public health concern, affecting over 264 million individuals worldwide [1]. Currently, antidepressants are the most prescribed medications for psychiatric disorders and are the first-line pharmacologic treatment for MDD and anxiety disorders [2]. Additionally, they are used for various in-label and off-label indications, including insomnia, pain, migraines, and eating disorders [3–7]. While antidepressants have shown comparable efficacy across different classes, studies have shown a significant increase in discontinuation rates compared to placebo groups and one of the most common and concerning adverse effects of long-term antidepressant use is weight gain, which could affect as much as 55–65% of those on antidepressant therapy [8–10].
There are multiple classes of antidepressants, each with different mechanisms of action. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are typically considered first-line treatments for MDD. However, the selection of an antidepressant should be individualized based on prior treatment response, adverse effect profiles, and patient preference [8,11–12]. SSRIs, such as fluoxetine, sertraline, and citalopram, act by inhibiting the serotonin transporter (SERT), leading to increased serotonin levels in the synaptic cleft and enhanced serotonergic neurotransmission [13,14]. SNRIs, including venlafaxine and duloxetine, work similarly but also inhibit the norepinephrine transporter (NET), which increases both serotonin and norepinephrine levels [15,16].
Other classes of antidepressants include tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and atypical antidepressants. TCAs, such as amitriptyline and nortriptyline, inhibit both SERT and NET but also interact with histaminic, cholinergic, and alpha-adrenergic receptors, expanding their side effect profile [13,17]. Because of this, TCAs are typically reserved for treatment-resistant depression [18]. MAOIs, such as phenelzine, prevent the breakdown of serotonin, norepinephrine, and dopamine via inhibition of monoamine oxidase enzymes [19,20]. While effective, MAOIs, like TCAs, are generally used as a last resort due to dietary restrictions and potentially severe drug interactions [18]. Atypical antidepressants, such as bupropion and mirtazapine, have unique mechanisms. Bupropion is a norepinephrine-dopamine reuptake inhibitor (NDRI) with less effect on serotonin compared to other antidepressants. It is often prescribed to those experiencing sexual dysfunction with SSRIs or for smoking cessation [21,22]. Mirtazapine, a noradrenergic and specific serotonergic antidepressant (NaSSA), increases neurotransmission through presynaptic alpha-2-adrenergic receptor antagonism and is known for its sedative and appetite-stimulating properties [15,21,23].
Weight gain associated with antidepressant use is an important clinical consideration, as it can negatively impact treatment adherence and overall health outcomes [24,25]. Patients who experience weight gain may discontinue or avoid treatment, increasing the risk of relapse or worsening depressive symptoms [26,27]. Moreover, antidepressant-induced weight gain can exacerbate comorbid conditions such as obesity, diabetes, and cardiovascular disease [28,29]. Obesity is two to three times more prevalent in individuals with psychiatric disorders, and long-term antidepressant use has been linked to an increased risk of metabolic disorders [29,30]. Given that obesity is the second most common cause of preventable death after smoking, the need to monitor and mitigate antidepressant-induced weight gain is vital. Strategies such as lifestyle interventions and adjunct medications may provide ways that clinicians can manage this adverse effect.
The relationship between antidepressant use and weight gain is complex, as underlying psychiatric conditions such as depression could involve changes in appetite, physical activity, and energy metabolism. However, research suggests that antidepressants can contribute to weight gain through central mechanisms involving serotonergic and dopaminergic pathways [31].
In this article, a critical evaluation is presented on the mechanisms underlying antidepressant-induced weight gain. The major focus is on serotonergic and dopaminergic pathways, comparing weight gain across different classes of antidepressants, including SSRIs, SNRIs, TCAs, MAOIs, and atypical antidepressants. Furthermore, specific risk factors are identified for antidepressant-induced weight gain while evaluating the current available and emerging strategies for weight gain mitigation. The information presented in this article is a comprehensive overview on this subject to inform healthcare providers in their clinical decision-making and will guide future research aimed at balancing antidepressant therapy with metabolic health considerations.
2. Methods
A comprehensive literature review was conducted to evaluate the relationship between antidepressant use and weight gain, with a focus on serotonergic and dopaminergic mechanisms, comparative weight effects across medication classes, and strategies for mitigation. The databases PubMed and Google Scholar were utilized to identify relevant studies published primarily between 2000 and 2025, with foundational literature from earlier years included as needed. The following search terms were used alone and in combination: “antidepressant weight gain,” “serotonin and appetite regulation,” “dopamine and feeding behavior,” “SSRIs and metabolism,” “SNRIs and weight gain,” “tricyclic antidepressants and weight gain,” “monoamine oxidase inhibitors and weight change,” “mirtazapine weight gain,” “bupropion weight change,” “antidepressants insulin resistance,” “metabolic effects of antidepressants,” “pharmacogenomics antidepressants,” “gut microbiota and antidepressants,” “GLP-1 receptor agonists antidepressant weight gain,” “adjunctive therapy weight management,” and “lifestyle interventions antidepressants.”
Studies were filtered based on relevance to the review topic. Exclusion criteria included editorials, opinion pieces, and studies lacking detailed methodology or direct reference to weight-related outcomes of antidepressant therapy. Emphasis was placed on high-quality evidence, including randomized controlled trials, meta-analyses, large cohort studies, and systematic reviews.
3. Mechanisms Underlying Antidepressant-Induced Weight Gain
3.1. Serotonergic Pathways
3.1.1. Role of serotonin in appetite regulation and satiety
Serotonin (5-hydroxytryptamine [5-HT]) has a range of effects in central and peripheral nervous systems [31,32]. Interestingly, the highest concentration of serotonin is found in the GI tract’s enterochromaffin cells [32,33]. Here, serotonin functions to increase intestinal mobilization through stimulation of myenteric neurons, enhancing digestive processes while also reducing appetite [32,34]. Once released into portal circulation, serotonin is taken up into platelets and metabolized by the liver [35].
In the CNS, serotonin plays a role in mood, sleep, and appetite [31]. Beyond appetite regulation, serotonin in the CNS has a significant impact on mood. Its absence is associated with depression, anxiety, and manic episodes [34–36]. It is synthesized from the amino acid tryptophan, which is first converted into 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase (TPH) and then into serotonin via aromatic acid decarboxylase [37]. These reactions occur in the raphe nuclei of the brainstem, where serotonin is also released. The dorsal raphe nucleus (DRN) contains roughly 35% of serotonergic neurons in the CNS, while the median raphe nucleus (MRN), which contains approximately 8%, and several studies have demonstrated that GABA-A agonism in the MRN increases food intake [38,39].
Serotonin from the rostral nuclei of the serotonergic system regulates various functions, such as temperature, appetite, sleep cycles, emesis, and sexual behavior [36]. These functions are mediated by different serotonin receptors. There are seven serotonin receptor families, all of which act through G-coupled protein receptors except for 5-HT3, which is a ligand-gated ion channel [40]. Serotonin is a pan-agonist to these receptors and influences multiple CNS processes, including appetite regulation [40–42].
The DRN serotonergic neurons suppress appetite through multiple mechanisms, including innervation of the mediobasal hypothalamus [43]. Additionally, the DRN can decrease appetite via projections to the lateral hypothalamic area (LHA) and the bed nucleus of the stria terminalis (BNST) [44]. Serotonergic neurons also stimulate GABAergic neurons in the rostral zona incerta and paraventricular thalamus to inhibit appetite [45]. DRN serotonergic neurons maintain reciprocal connections with other brain regions, including the paraventricular nucleus of the hypothalamus (PVH), lateral hypothalamic area (LHA), arcuate nucleus (ARH), central amygdala (CeA), and parabrachial nucleus (PBN) [46]. A recent study demonstrated that activating the DRN serotonergic pathway to the ARH lead to a decrease of food intake via depolarization of anorexigenic proopiomelanocortin (POMC) neurons while simultaneously hyperpolarizing orexigenic agouti-related peptide (AgRP) neurons [47]. It was found that co-treating mice with fluoxetine and lipocalin 2, an anorexigenic hormone that is used to stimulate melanocortin 4 receptors, led to a normalization of feeding and weight [48].
Different serotonin receptor subtypes have varying effects on food intake. Activation of 5-HT2ARs and 5-HT2CR reduces food intake, whereas 5-HT2BR activation increases it [49–52]. 5-HT2CR agonists stimulate POMC neurons through phospholipase C (PLC) signaling [42,52]. This receptor is also found in the PVH, a region involved in appetite suppression [53,54].
Other, less studied serotonin receptors also influence appetite. 5-HT3R activation increases appetite and food intake in the nucleus accumbens (NAc) but has the opposite effect in the ventral tegmental area (VTA) and nucleus of the solitary tract (NTS) [55,56]. Activation of 5-HT4Rs in the NAc decreases food intake through increased expression of CART mRNA [57]. On the other hand, 5-HT6 increases food intake in the NAc, while 5-HT7 has the opposite effect [58,59].
3.1.2. Alterations due to SSRIs and other serotonergic agents
SSRIs are commonly associated with weight gain. They increase serotonin levels in the synaptic cleft by reducing its reuptake via serotonin transporters in the presynaptic neuron [60,61]. Given serotonin’s role in the CNS, it would be expected that SSRI use would lead to decreased appetite and food intake [62,63]. This is supported by studies in mice injected with fluoxetine, as well as observations in humans during the first few months of SSRI treatment, where some individuals experience weight loss [64–66].
However, more recent studies have shown that chronic SSRI use (≥1 year) is associated with weight gain [67–72]. This adverse effect can impair treatment adherence and have negative implications for overall health [69,70,72].
Several mechanisms have been proposed to explain SSRI-induced weight gain. One mechanism involves serotonin receptor modulation. Long-term fluoxetine (Prozac) use has been shown to downregulate brainstem serotonergic neurons through autoinhibitory signaling via 5-HT1RA. Over extended treatment periods, there is also a decrease in 5-HT2 receptor expression and activity, leading to reduced phosphorylation of CREB and STAT3, along with decreased POMC expression in hypothalamic neurons. This ultimately results in increased food intake and body weight [48]. Other SSRIs, such as paroxetine and citalopram, have been shown to induce weight gain via a similar mechanism [73–75].
Additionally, 5-HT2 receptor desensitization may contribute to weight gain. Studies have shown that pretreatment with 5-HT2 antagonists prevents the anorexic effects of d-fenfluramine (DFF), suggesting that SSRIs that desensitize or downregulate 5-HT2C over time could contribute to increased appetite and weight gain [76]. Supporting this, 5-HT2A receptor agonism promotes satiety, while inhibition leads to increased food intake and weight gain. Furthermore, 5-HT2C receptor antagonism has been associated with glucose intolerance [77], indicating that long-term SSRI or TCA use, which also affects these receptors, may contribute to insulin resistance and weight gain. Long-term SSRI use has also been linked to weight gain due to an increase in carbohydrate cravings [69,78].
Another proposed mechanism involves the inhibition of dopamine pathways in the striatum, which can lead to reduced energy expenditure and weight gain [79,80]. Additionally, activation of H1 histamine receptors via citalopram has been correlated with increased food intake [74,75] (Figure 1).
Figure 1:
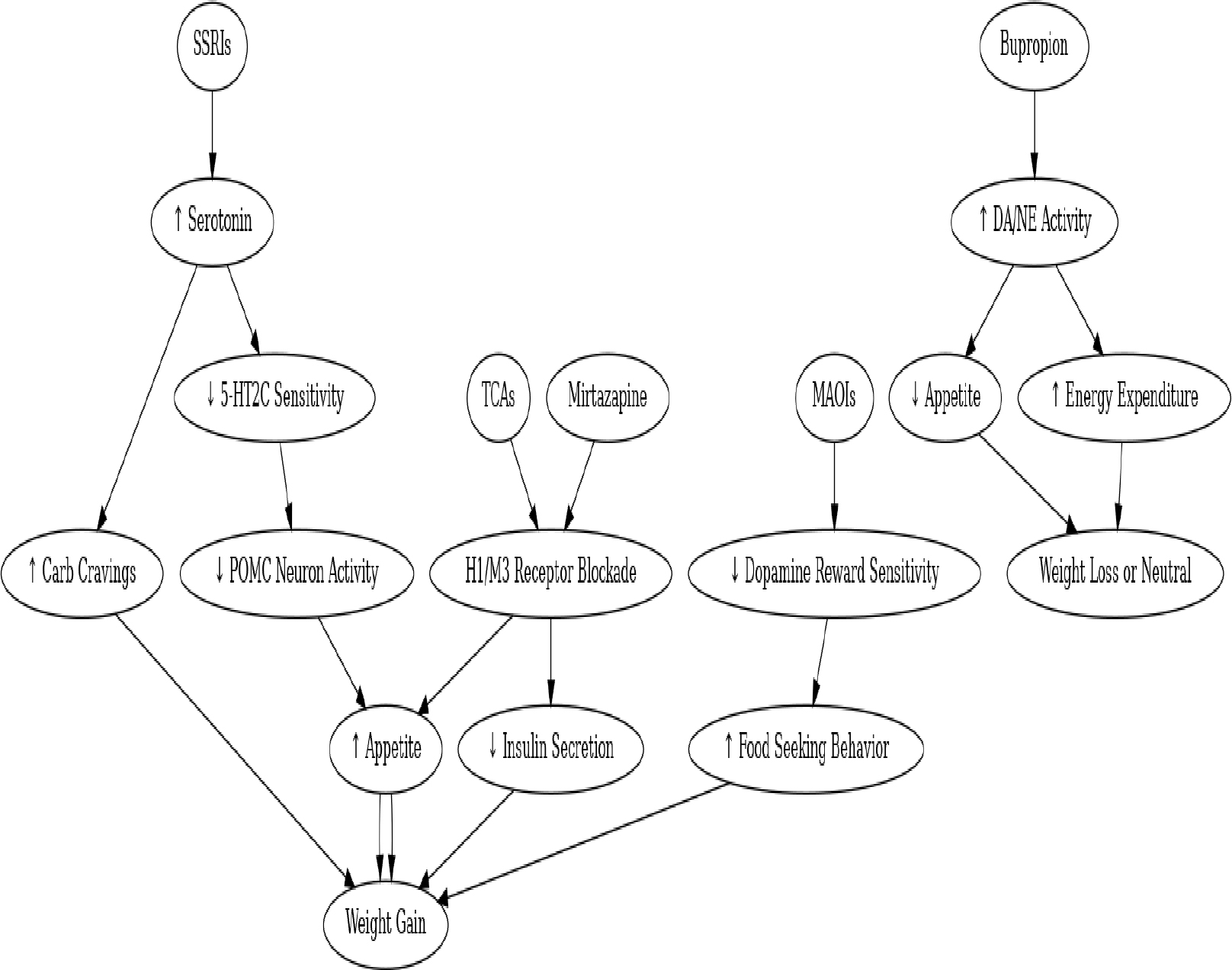
Neurochemical Pathways Linking Antidepressants to Weight Gain.
3.2. Dopaminergic Pathway
Influence on reward circuitry and food-seeking behavior
Food acquisition requires the recognition of rewarding stimuli, and in addition to the serotonergic pathway, the dopaminergic pathways have also been implicated in these processes [81]. Dopamine plays a key role in the reward aspects of feeding through dopaminergic projections from the ventral tegmental area (VTA) to the NAc. Alterations in this system can contribute to weight changes [82,83].
The dopaminergic mesolimbic pathways are essential for feeding behaviors, and disruptions in these pathways can lead to changes in food intake [81]. Dopamine release in the NAc has been observed during feeding, food anticipation, and in response to food-related stimuli [84–86]. Additionally, certain metabolic hormones, such as ghrelin and leptin, can act directly on the VTA to influence feeding behavior. Leptin administration in the VTA has been found to inhibit dopamine activity, leading to a decrease in food intake [87]. In contrast, ghrelin, which is secreted from the stomach and has receptors in the mesolimbic circuits, has the opposite effect—it enhances dopamine release and activity in the VTA, promoting food intake [88]. Furthermore, SSRIs were found to decrease ghrelin levels and alter GI motor activities through 5-HTCR2 receptors which can then lead to changes in feeding behaviors and weight gain [80].
TCAs, which primarily act on dopamine and histamine receptors, have also been associated with weight gain [80,89]. TCAs inhibit H1 histamine and muscarinic acetylcholine receptors, both of which have been linked to increased food intake and weight gain [90].
3.3. Hormonal and Metabolic Changes
Insulin sensitivity and glucose metabolism involving Leptin and ghrelin dysregulation
Psychotropic medications are associated not only with weight gain but also with metabolic changes such as diabetes and dyslipidemia [91]. Antidepressant use has been linked to changes in insulin resistance, with various antidepressants, including SSRIsm TCAs, and mirtazapine, implicated in the development of these metabolic disorders [91]. Many commonly used antidepressants can lead to insulin resistance (IR) in individuals with and without type 2 diabetes mellitus (T2DM) [92,93]. Specifically, treatment with SSRIs, TCAs, and mirtazapine has been shown to increase cortisol levels, which is associated with increased insulin resistance [94].
Long-term antidepressant use has correlated with an increased risk of T2DM. A case-control study in the United States found that antidepressant use greater than 24 months at moderate-to-high daily doses was associated with an increased risk of developing T2DM compared to non-users [72]. Similarly, a French cohort study with a six-year follow-up found a comparable increased risk of T2DM among antidepressant users, with no significant differences between SSRIs, TCAs, and mixed antidepressants [95].
A 2023 study analyzing the response to vortioxetine, an SSRI, found a significant increase in insulin resistance following treatment [96]. This study also indicated that increased IR contributed to treatment nonresponse and elevated C-reactive protein (CRP) levels, underscoring the metabolic effects of antidepressant therapy. Prior research has shown that CRP may serve as a predictor of antidepressant response [96,97].
TCAs may also influence insulin secretion through their inhibitory effects on M3 muscarinic receptors, which play a key role in insulin release [98]. Notably, antipsychotics that antagonize M3 receptors, such as clozapine, have been associated with the development of T2DM due to decreased insulin secretion [99]. Some TCAs, such as desipramine, have been linked to increased insulin resistance and hyperglycemia [91]. However, interestingly, in a study examining individuals with depression treated with TCAs, there was an observed improvement in IR, suggesting varied metabolic effects [100,101] (Figure 2).
Figure 2:
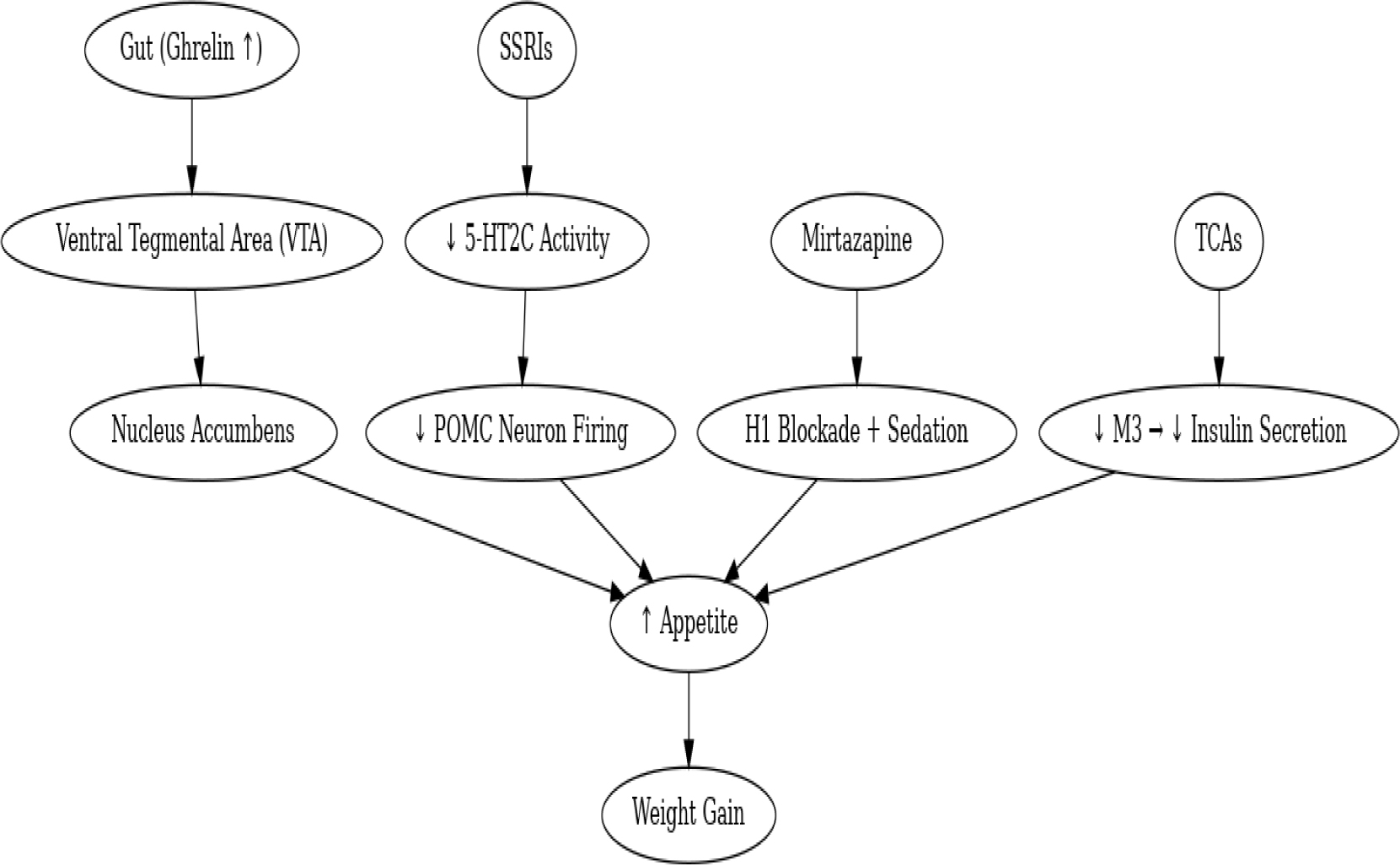
Neurohormonal Interaction Pathways.
4. Extent of Weight Gain Across Antidepressant Classes
Antidepressant-induced weight occurs through alterations in neurotransmitters, metabolic regulation, and behavioral changes. Meta-analysis by Alonso-Pedrero et al. [102] of 27 cohort studies with over 450,000 individuals found that the most significant weight gain was associated with TCAs, mirtazapine, and certain SSRIs. This study concluded that many individuals treated with antidepressants were at increased risk of gaining 5% of their baseline body weight, except for those treated with Bupropion [102]. Another study by Petimar et al. [103] included a target trail emulation study using EHR records from over 180,000 patients to compare weight changes across different antidepressant treatments.
4.1. Selective Serotonin Reuptake Inhibitors (SSRIs +SNRIs)
SSRIs are the most prescribed antidepressants but the effect they have on weight gain differs depending on the SSRI. Petimar et al. [103] found that escitalopram had the greatest weight gain over a 6-month period of +0.41kg. Compared to other SSRIs such as, paroxetine with +0.37kg, duloxetine +0.34kg, and venlafaxine +0.17kg. A previous research study by Gafoor et al. [104] found that paroxetine use was associated with a 21% increased risk of at least 5% weight over a 10-year period. Some SSRIs, such as fluoxetine had less of an association with weight changes and remained weight neutral [105].
4.2. Tricyclic Antidepressants (TCAs)
TCAs are associated with significant weight gain due to their effects on antihistaminergic and anticholinergic pathways. A study by Alonso-Pedrero et al. [102] found that short-term TCA use (4–12 weeks), specifically amitriptyline, mirtazapine, and nortriptyline had weight gains of +1.52 kg, +1.74 kg, and +2kg respectively. This coincides with other studies that have shown that TCA-associated weight gain has been demonstrated to be dose and time-dependent [106,107]. Specifically, amitriptyline use has been correlated to with continuing weight gain over 24 months and overall, a greater weight change compared to SSRI [107]. Due to the extent of short-term and long-term weight gain associated with TCAs, they are typically avoided in those who are overweight.
4.3. Monoamine Oxidase Inhibitors (MAOIs)
MAOIs are less commonly prescribed due to drug-drug interactions and dietary restrictions. They are also associated with weight gain, specifically phenelzine. Phenelzine has been associated with increased weight gain when compared to other classes of antidepressants and has been found to have an increase of 2–3kg over a 6-month course of treatment [102]. This significant increase in weight means that in treatment-resistant depression their consideration needs to be carefully evaluated, especially in patients with comorbidities such as obesity or metabolic syndrome [106].
4.4. Atypical Antidepressants
Atypical antidepressants have varying effects on body weight. Mirtazapine and Bupropion are on opposing sides of the weight gain spectrum. Mirtazapine is associated with weight gain and has been correlated with an average increase of +1.74kg in the first 12 weeks of treatment [102]. The mechanism of this weight gain is thought to be through antagonism of H1 and 5HTC receptors, which can lead to increased food intake [108]. On the other hand, Bupropion is associated with a net weight loss. Petimar et al. [103] found that when compared to sertraline there was a −0.22kg weight loss over 6 months [27]. Furthermore, those on Bupropion were found to have a 15% decreased risk of gaining 5% of baseline weight when compared to other SSRIs. Weight protective factors could be due to its unique mechanism of norepinephrine-dopamine reuptake inhibitor, which has been proposed to suppress appetite and increase energy expenditure [109] (Table 1).
Table 1:
Effect of Antidepressants on Weight Change and the potential underlying mechanisms.
| Class | Antidepressant | Weight Change (kg) | Risk of a 5% Weight Gain | Mechanism Contributing to Weight Change | Reference |
|---|---|---|---|---|---|
| SSRI | Paroxetine | +0.37 to +2.73 | 21% higher | Strong antihistaminergic effects, appetite stimulation | Petimar et al. [103]; Gafoor et al. [104] |
| SSRI | Fluoxetine | 0.07 to neutral | Neutral | Initial weight loss, potential stabilization | Petimar et al. [103]; Serretti and Mandelli [105] |
| TCA | Amitriptyline | +1.52 to +2 | High | Antihistaminergic and anticholinergic properties | Alonso-Pedrero et al. [102] |
| TCA | Nortriptyline | +1.52 to +2 | High | Antihistaminergic and anticholinergic properties | Alonso-Pedrero et al. [102] |
| SNRI | Duloxetine | +0.34 | 10–15% higher risk | Noradrenaline reuptake inhibition, possible metabolic impact | Petimar et al. [103] |
| SNRI | Venlafaxine | +0.17 | Moderate risk | Noradrenergic activity, moderate weight impact | Petimar et al. [103] |
| MAOI | Phenelzine | +2 to +3 | High | Metabolic changes, appetite regulation | Alonso-Pedrero et al. [102] |
| Atypical | Mirtazapine | +1.74 | High | Histamine (H1) and serotonin (5-HT2C) antagonism | Alonso-Pedrero et al. [102] |
| Atypical | Bupropion | −0.22 to −3.2 | 15% reduced | Dopamine and norepinephrine reuptake inhibition (appetite suppression) | Petimar et al. 2024 [103]; Aronne et al. [109] |
5. Individual Susceptibility Factors: Genetic predisposition, Lifestyle factors, and Baseline BMI
Certain risk factors such as genetic predisposition, lifestyle factors, and baseline BMI have been associated with antidepressant-induced weight gain and they highlight the need for close monitoring and potential early interventions in patients to prevent excessive weight gain.
A genome-wide association study by Sjaarda et al. [110] has identified four novel loci that were associated with weight gain during psychotropic treatment, including antidepressant treatment [110]. The loci were in proximity of genes that are involved with metabolic regulation, including MAN2A1 and SLCO3A1. Additionally, a retrospective cohort study by Ricardo-Silgado et al. [111] investigated the association between CYP metabolizer phenotypes and weight gain in patients prescribed SSRIs such as citalopram, paroxetine, sertraline, or fluoxetine. The study found that CYP2C19 poor/intermediate metabolizers prescribed citalopram gained significantly more weight (2.6% total body weight gain) compared to normal or rapid/ultra-rapid metabolizers (0.4% and −0.1%, respectively) at six months. These findings indicated that there are genetic predispositions that can influence weight changes in those undergoing antidepressant treatment.
In addition to genetic predisposition, BMI is an important predictor of weight gain during antidepressant use. A higher pretreatment BMI has been associated with a greater weight gain in those treated with psychotropic medications [112]. A study that used machine learning approaches confirmed that in addition to baseline BMI, factors such as age and waist circumference were also significant predictors of weight gain [112].
Lifestyle factors can also play a role in antidepressant-induced weight gain. A study by Simon et al. [113] found that certain lifestyle patterns such as emotional eating, cravings for fast food and sweets, and weight cycling were associated with a higher rate of obesity and metabolic syndrome in psychiatric patients [113]. A different study by Solmi et al. [28] highlighted the importance that lifestyle interventions play in mitigating weight gain associated with antidepressant treatment such as regular exercise and a healthy diet.
6. Strategies for Mitigating Antidepressant-Induced Weight Gain
6.1. Pharmacological Approaches
Pharmacological approaches to mitigating antidepressant-induced weight gain include switching to weight-neutral or antidepressants or using adjunctive medications targeted at weight control. The atypical antidepressant, bupropion, is consistently associated with the least weight gain among antidepressants and may even lead to weight loss.
Adjunctive medications can also be used for weight control. Metformin is the most used pharmacological treatment for preventing drug related weight gain and has been shown to be effective in reducing weight gain associated with antidepressant use [25,114]. Furthermore, glucagon-like-peptide-1 (GLP-1) receptor agonists, such as liraglutide and exenatide, have been shown to be effective in mitigating weight gain [25,115].
A combination therapy of naltrexone/bupropion (NB) as an adjunct to antidepressant therapy in those with obesity or who were overweight has shown that it can be effective in promoting weight loss with a mean adjusted weight of −6.3% compared to 4.3% in those in the placebo group [116].
The American College of Physicians recommends considering the potential for weight gain when initially selecting antidepressants and suggests switching to less weight-inducing options when necessary [117] (Table 2).
Table 2:
Pharmacological Mitigation Strategies.
| Intervention | Mechanism of Action | Evidence Strength | Common Side Effects | Remarks |
|---|---|---|---|---|
| Metformin | Insulin sensitizer | Moderate | GI upset | Good for insulin resistance |
| GLP-1 RAs | Appetite suppression | High | Nausea | Expensive, injectable |
| Bupropion | DA/NE reuptake inhibition | High | Insomnia | Useful for weight loss |
| Naltrexone/ Bupropion | Reward pathway modulation | Moderate | Nausea, headache | Combination product |
6.2. Behavioral and Lifestyle Interventions
In addition to dietary and exercising changes, adding cognitive-behavioral therapy (CBT) has been shown to be efficacious in managing both obesity and comorbid depression. Behavioral strategies should include but not limited to self-monitoring, goal setting, and problem solving to address barriers to weight loss [118].
Early weight gain can predict further weight gain, and regular monitoring of metabolic health at baseline and during follow-ups is important to help identify signs of weight gain to allow for interventions [28].
6.3. Emerging and Experimental Interventions: Targeting gut microbiota for weight management - Personalized medicine approaches (pharmacogenomics)
Targeting gut microbiota for weight management in patients with antidepressant-induced weight gain has shown some promising results, although the evidence is still emerging.
Minichino et al. [119] conducted a systematic review and meta-analysis that included studies on antidepressants and their effects on gut microbiota. They found significant changes in gut microbiota diversity metrics following treatment with antidepressants. Specifically, they reported a standard mean difference in alpha diversity of 0.12 (95% CI: 0.01–0.23; p = 0.04; I2: 14%) and significant changes in beta diversity (F = 15.59; R2 = 0.05; p < 0.001). These changes in gut microbiota composition were associated with differences in efficacy and tolerability of antidepressants, suggesting a potential role in managing weight gain [119].
Nikolova et al. [120] conducted a randomized controlled pilot trial where 49 people with major depressive disorder received either a multi-strain probiotic or placebo for 8 weeks. They found a significant increase in gut microbiota richness in the probiotic group (Chao1 bias-corrected, p = 0.04) and observed between-group differences in beta diversity at week 4 (p = 0.04). However, the study did not specifically measure weight changes [120].
A scoping review by Mötteli et al. [121] found that while probiotics alone did not significantly reduce pharmacologically induced weight gain, synbiotics (a combination of probiotics and prebiotics) included two studies that observed less weight gain in individuals receiving synbiotics compared to those who did not [121].
While the modulation of gut microbiota through synbiotics and probiotics shows potential in managing antidepressant-induced weight gain, more studies are needed to establish definitive clinical guidelines.
Pharmacogenomic treatment approaches to combat antidepressant-induced weight gain involve tailoring antidepressant therapy based on individual genetic profiles to minimize adverse effects such as weight gain.
The retrospective cohort study by Ricardo-Silgado et al. [111] mentioned earlier highlighted the association between CYP metabolizer phenotypes and weight gain in patients prescribed SSRIs such as citalopram, this suggests that pharmacogenomic testing for CYP2C19 could help identify patients at higher risk for weight gain with citalopram, allowing for alternative treatment strategies [111].
Additionally, a genome-wide interaction and enrichment analysis on weight gain during citalopram treatment identified molecular pathways, such as axon guidance and developmental biology, associated with weight gain. Variations in genes involved in collagen synthesis, thyroid hormone activity, energy metabolism, and adipocyte differentiation were implicated, suggesting that genetic profiling could predict weight gain risk and guide personalized treatment [122].
The U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline reviewed pharmacogenomic testing for antidepressant selection. They concluded that while there is interest in pharmacogenomic approaches, the evidence is currently insufficient to make a strong recommendation for its routine use due to mixed outcomes and low-quality evidence [123].
In summary, pharmacogenomic testing, particularly for CYP2C19, shows potential in predicting and managing antidepressant-induced weight gain, but further research is needed to establish its clinical utility comprehensively.
7. Conclusion
Antidepressant-induced weight gain is a significant clinical concern that can impact treatment adherence and overall patient well-being. The underlying mechanisms involve complex interactions between serotonergic, dopaminergic, and metabolic pathways, with different antidepressant classes contributing to varying degrees of weight gain. While antidepressant medications such as TCAs, mirtazapine, and MAOIs are more strongly associated with weight gain, bupropion remains a weight-neutral or weight-reducing option. Individual susceptibility factors can influence the extent of weight change.
Mitigation strategies include switching to weight-neutral antidepressants, implementing pharmacological interventions such as metformin and GLP-1 receptor agonists, and integrating behavioral and lifestyle modifications. Emerging research on pharmacogenomics and gut microbiota offers potential avenues for personalized treatment approaches. However, further longitudinal studies are needed to better understand the long-term metabolic consequences of antidepressant therapy to optimize treatment strategies in order to balance efficacy with metabolic health. Incorporating these insights into clinical practice would enable providers to make informed decisions in order to minimize the burden of weight gain from antidepressant treatment while also effectively managing depression and anxiety disorders.
8. Key Points.
Long-term antidepressant use is associated with clinically significant weight gain, impacting adherence and long-term health outcomes.
SSRIs are generally weight-neutral or cause mild weight loss in the short term but are linked to weight gain with prolonged use.
TCAs, MAOIs, and mirtazapine show the highest propensity for weight gain due to their effects on serotonergic, histaminergic, and dopaminergic systems.
Bupropion is consistently associated with weight neutrality or modest weight loss, making it a favorable option for patients concerned about weight gain.
Mechanisms of antidepressant-induced weight gain include 5-HT2 receptor desensitization, dopamine inhibition in reward pathways, increased carbohydrate craving, and hormonal dysregulation (leptin, ghrelin, insulin).
Genetic predispositions, including CYP2C19 metabolizer status, play a significant role in individual susceptibility to weight changes.
Higher baseline BMI, emotional eating, and sedentary lifestyle behaviors increase the risk of antidepressant-induced weight gain.
Metformin and GLP-1 receptor agonists have shown promise as adjunctive pharmacologic strategies to mitigate weight gain.
Lifestyle interventions such as regular physical activity, dietary modifications, and cognitive-behavioral therapy (CBT) are essential components of management.
Emerging approaches, including gut microbiota modulation and pharmacogenomic-guided therapy, offer future directions for personalized treatment plans.
Funding:
The research work of DKA is supported by the R25AI179582 and R01 HL147662 grants from the National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests: All authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication: All authors have read the manuscript and consented for publication.
References
- 1.Ritchie H, Roser M. Mental Health. Our World in Data; (2018). [Google Scholar]
- 2.Lunghi C, Dugas M, Leclerc J, et al. Global prevalence of antidepressant drug utilization in the community: protocol for a systematic review. BMJ open 12 (2022): e062197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother 10 (2018): 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy SH, Lam RW, McIntyre RS, et al. CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry 61 (2016): 540–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong J, Motulsky A, Eguale T, et al. Treatment Indications for Antidepressants Prescribed in Primary Care in Quebec, Canada, 2006–2015. JAMA 315 (2016): 2230–2. [DOI] [PubMed] [Google Scholar]
- 6.Schröder C, Dörks M, Kollhorst B, et al. Extent and risks of antidepressant off-label use in children and adolescents in Germany between 2004 and 2011. Pharmacoepidemiol Drug Saf 26 (2017): 1395–1402. [DOI] [PubMed] [Google Scholar]
- 7.Burch R Antidepressants for Preventive Treatment of Migraine. Curr Treat Options Neurol 21 (2019): 18. [DOI] [PubMed] [Google Scholar]
- 8.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. FOC 16 (2018): 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uguz F, Sahingoz M, Gungor B, et al. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry 37 (2015): 46–8. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient preference and adherence 28 (2016): 1401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade C Relative Efficacy and Acceptability of Antidepressant Drugs in Adults With Major Depressive Disorder: Commentary on a Network Meta-Analysis. J Clin Psychiatry 79 (2018): 18f12254. [DOI] [PubMed] [Google Scholar]
- 12.Kovich H, DeJong A. Common Questions About the Pharmacologic Management of Depression in Adults. Am Fam Physician 92 (2015): 94–100. [PubMed] [Google Scholar]
- 13.Stahl SM. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J Clin Psychiatry 59 (1998): 5–14. [PubMed] [Google Scholar]
- 14.Apparsundaram S, Stockdale DJ, Henningsen RA, et al. Antidepressants targeting the serotonin reuptake transporter act via a competitive mechanism. J Pharmacol Exp Ther 327 ( 2008): 982–90. [DOI] [PubMed] [Google Scholar]
- 15.Westenberg HG. Pharmacology of antidepressants: selectivity or multiplicity? J Clin Psychiatry 17 (1999): 4–8. [PubMed] [Google Scholar]
- 16.Andersen J, Kristensen AS, Bang-Andersen B, et al. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem Commun (Camb) 7 (2009): 3677–92. [DOI] [PubMed] [Google Scholar]
- 17.Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry 4 (1999): 4–11.. [PubMed] [Google Scholar]
- 18.Kishi T, Ikuta T, Sakuma K, et al. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis. Mol Psychiatry 28 (2023): 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay RR, Basile L, Maniquet A, et al. Parameters for Irreversible Inactivation of Monoamine Oxidase. Molecules 25 (2020): 5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duarte P, Cuadrado A, León R. Monoamine Oxidase Inhibitors: From Classic to New Clinical Approaches. Handb Exp Pharmacol 264 (2021): 229–259. [DOI] [PubMed] [Google Scholar]
- 21.Schwasinger-Schmidt TE, Macaluso M. Other Antidepressants. Handb Exp Pharmacol 250 (2019): 325–355. [DOI] [PubMed] [Google Scholar]
- 22.Arias HR. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int J Biochem Cell Biol 41 (2009): 2098–108. [DOI] [PubMed] [Google Scholar]
- 23.Organon LLC. (2012, February 17). REMERON (mirtazapine) tablet, film coated. DailyMed. National Library of Medicine. Retrieved March 4, 2025, from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8feda54f-a3fa-4108-bfc4-c15b0eab4396 [Google Scholar]
- 24.Ho SC, Chong HY, Chaiyakunapruk N, et al. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: A systematic review. J Affect Disord 193 (2016): 1–10. [DOI] [PubMed] [Google Scholar]
- 25.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 361 (2003): 653–61. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre RS, Kwan ATH, Rosenblat JD, et al. Psychotropic Drug-Related Weight Gain and Its Treatment. Am J Psychiatry 181 (2024): 26–38. [DOI] [PubMed] [Google Scholar]
- 27.Petimar J, Young JG, Yu H, et al. Medication-Induced Weight Change Across Common Antidepressant Treatments : A Target Trial Emulation Study. Ann Intern Med 177 (2024): 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solmi M, Miola A, Capone F, et al. ECNP Physical And meNtal Health Thematic Working Group (PAN-Health). Risk factors, prevention and treatment of weight gain associated with the use of antidepressants and antipsychotics: a state-of-the-art clinical review. Expert Opin Drug Saf 23 (2024): 1249–1269. [DOI] [PubMed] [Google Scholar]
- 29.Lassale C, Lugon G, Hernáez Á, et al. Trajectories of antidepressant use and 6-year change in body weight: a prospective population-based cohort study. Front Psychiatry 15 (2024): 1464898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harvard review of psychiatry 14 (2006): 212–22. [DOI] [PubMed] [Google Scholar]
- 31.Papakostas GI. Tolerability of modern antidepressants. J Clin Psychiatry 69 (2008): 8–13. [PubMed] [Google Scholar]
- 32.Smith C, Smith M, Cunningham R, et al. Recent Advances in Antiemetics: New Formulations of 5-HT3 Receptor Antagonists in Adults. Cancer Nurs 43 (2020): E217–E228. [DOI] [PubMed] [Google Scholar]
- 33.Bakshi A, Tadi P. Biochemistry, Serotonin. [Updated 2022 Oct 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Jan-. Available from: https://www-ncbi-nlm-nih-gov.proxy.westernu.edu/books/NBK560856/ [PubMed] [Google Scholar]
- 34.Kitson SL. 5-hydroxytryptamine (5-HT) receptor ligands. Curr Pharm Des 13 (2007): 2621–37. [DOI] [PubMed] [Google Scholar]
- 35.Sivolap YP. Antidepressants: the goals and possibilities of therapy. Zh Nevrol Psikhiatr Im S S Korsakova 118 (2018): 120–124. [DOI] [PubMed] [Google Scholar]
- 36.Coleman JA, Yang D, Zhao Z, et al. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299 (2003): 76. [DOI] [PubMed] [Google Scholar]
- 38.Ishimura K, Takeuchi Y, Fujiwara K, Tominaga M, Yoshioka H, Sawada T. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci Lett 91 (1988): 265–70. [DOI] [PubMed] [Google Scholar]
- 39.Klitenick MA, Wirtshafter D. Comparative studies of the ingestive behaviors produced by microinjections of muscimol into the midbrain raphe nuclei of the ventral tegmental area of the rat. Life Sci 42 (1988): 775–82. [DOI] [PubMed] [Google Scholar]
- 40.Xu P, Huang S, Zhang H, et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 592 (2021): 469–473. [DOI] [PubMed] [Google Scholar]
- 41.Shin Y, Kim S, Sohn JW. Serotonergic regulation of appetite and sodium appetite. J Neuroendocrinol 35 (2023): e13328. [DOI] [PubMed] [Google Scholar]
- 42.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci 36 (2013):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhave VM, Nectow AR. The dorsal raphe nucleus in the control of energy balance. Trends Neurosci 44 (2021): 946–960. [DOI] [PubMed] [Google Scholar]
- 44.Aklan I, Sayar-Atasoy N, Deng F, et al. Dorsal raphe serotonergic neurons suppress feeding through redundant forebrain circuits. Mol Metab 69 (2023): 101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Q, Nunez J, Zhang X. Raphe serotonin projections dynamically regulate feeding behavior through targeting inhibitory circuits from rostral zona incerta to paraventricular thalamus. Mol Metab 66 (2022): 101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardozo Pinto DF, Yang H, Pollak Dorocic I, et al. Characterization of transgenic mouse models targeting neuromodulatory systems reveals organizational principles of the dorsal raphe. Nat Commun 10 (2019): 4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, Cai X, Liu H, et al. 5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding. Mol Psychiatry 26 (2021): 7211–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortuño MJ, Schneeberger M, Ilanges A, et al. Melanocortin 4 receptor stimulation prevents antidepressant-associated weight gain in mice caused by long-term fluoxetine exposure. J Clin Invest 131 (2021): e151976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox MA, French HT, LaPorte JL, et al. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 212 (2010): 13–23. [DOI] [PubMed] [Google Scholar]
- 50.Kennett GA, Ainsworth K, Trail B, et al. BW 723C86, a 5-HT2B receptor agonist, causes hyperphagia and reduced grooming in rats. Neuropharmacology 36 (1997): 233–9. [DOI] [PubMed] [Google Scholar]
- 51.Martin CK, Redman LM, Zhang J, et al. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab 96 (2011): 837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y, Yao T, Deng Z, et al. TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons. Cell Rep 18 (2017): 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heisler LK, Pronchuk N, Nonogaki K, et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27 (2007): 6956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li MM, Madara JC, Steger JS, et al. The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron 102 (2019): 653–667.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483 (2012): 594–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pratt WE, Lin P, Pierce-Messick Z, et al. Contrasting effects of 5-HT3 receptor stimulation of the nucleus accumbens or ventral tegmentum on food intake in the rat. Behav Brain Res 323 (2017): 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jean A, Conductier G, Manrique C, et al. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A 104 (2007): 16335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pratt WE, Blackstone K, Connolly ME, et al. Selective serotonin receptor stimulation of the medial nucleus accumbens causes differential effects on food intake and locomotion. Behav Neurosci 123 (2009): 1046–57. [DOI] [PubMed] [Google Scholar]
- 59.Kotańska M, Lustyk K, Bucki A, et al. Idalopirdine, a selective 5-HT6 receptor antagonist, reduces food intake and body weight in a model of excessive eating. Metab Brain Dis 33 (2018): 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57 (1995): 411–41. [DOI] [PubMed] [Google Scholar]
- 61.Fox MA, Andrews AM, Wendland JR, et al. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 195 (2007): 147–66. [DOI] [PubMed] [Google Scholar]
- 62.Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51 (2006): 239–49. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Jones JE, Kohno D, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60 (2008): 582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav 58 (1997): 767–73. [DOI] [PubMed] [Google Scholar]
- 65.Voigt JP, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res 277 (2015): 14–31. [DOI] [PubMed] [Google Scholar]
- 66.Burke LK, Heisler LK. 5-hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol 27 (2015): 389–98. [DOI] [PubMed] [Google Scholar]
- 67.Jensen-Otsu E, Austin GL. Antidepressant Use is Associated with Increased Energy Intake and Similar Levels of Physical Activity. Nutrients 7 (2015): 9662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kivimäki M, Hamer M, Batty GD, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care 33 (2010): 2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SH, Paz-Filho G, Mastronardi C, et al. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl Psychiatry 6 (2016): e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carvalho AF, Sharma MS, Brunoni AR, et al. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother Psychosom 85 (2016): 270–88. [DOI] [PubMed] [Google Scholar]
- 71.Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ 361 (2018): k1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andersohn F, Schade R, Suissa S, et al. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 166 (2009): 591–8. [DOI] [PubMed] [Google Scholar]
- 73.Joubert AF, Gagiano CA, Joubert G. Antidepressants and weight: A comparative study of four antidepressants and their effect on weight [abstract]. Eur Neuropsychopharmacol 6 (1996): 143. [Google Scholar]
- 74.Bouwer CD, Harvey BH. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol 11 (1996): 273–8. [DOI] [PubMed] [Google Scholar]
- 75.Bouchard JM, Strub N, Nil R. Citalopram and viloxazine in the treatment of depression by means of slow drop infusion. A double blind comparative trial. J Affect Disorders 46 (1997): 51–8. [DOI] [PubMed] [Google Scholar]
- 76.Vickers SP, Clifton PG, Dourish CT. Behavioral evidence that d-fenfluramine-induced anorexia in the rat is not mediated by the 5HT1a receptor subtype. Psychopharmacol (Berl) 125 (1996): 168–75. [DOI] [PubMed] [Google Scholar]
- 77.Browne CJ, Fletcher PJ. Decreased incentive motivation following knockout or acute blockade of the serotonin transporter: role of the 5-HT2C receptor. Neuropsychopharmacology 41 (2016): 2566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwartz TL, Meszaros ZS, Khan R, et al. How to control weight gain when prescribing antidepressants. Current psychiatry 6 (2007): 43. [Google Scholar]
- 79.Arya DK. Extrapyramidal symptoms with selective serotonin reuptake inhibitors. Br J Psychiatry 165 (1994): 728–33. [DOI] [PubMed] [Google Scholar]
- 80.Fujitsuka N, Asakawa A, Hayashi M, et al. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry 65 (2009): 748–59. [DOI] [PubMed] [Google Scholar]
- 81.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Frontiers in Neuroendocrinology 31 (2010): 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volkow ND, Wise RA. How can drug addiction help us understand obesity?. Nature neuroscience 8 (2005): 555–60. [DOI] [PubMed] [Google Scholar]
- 83.Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society B: Biological Sciences 361 (2006): 1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Church WH, Justice JB Jr, Neill DB. Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain research 412 (1987): 397–9. [DOI] [PubMed] [Google Scholar]
- 85.Hernandez L, Hoebel BG. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Research Bulletin 25 (1990): 975–9. [DOI] [PubMed] [Google Scholar]
- 86.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89 (1999): 637–41. [DOI] [PubMed] [Google Scholar]
- 87.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51 (2006): 801–10. [DOI] [PubMed] [Google Scholar]
- 88.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. The Journal of Clinical Investigation 116 (2006): 3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beaumont G, Kasper S, O’Hanlon J, et al. Antidepressant side effects and adverse reactions. Depression 2 (1995): 138–44. [Google Scholar]
- 90.Gill H, Gill B, El-Halabi S, et al. Antidepressant medications and weight change: a narrative review. Obesity 28 (2020): 2064–72. [DOI] [PubMed] [Google Scholar]
- 91.Scheen AJ. Metabolic disorders induced by psychotropic drugs. In Annales d’Endocrinologie 84 (2023): 357–363. [DOI] [PubMed] [Google Scholar]
- 92.Fathallah N, Slim R, Larif S, et al. Drug-induced hyperglycaemia and diabetes. Drug safety 38 (2015): 1153–68. [DOI] [PubMed] [Google Scholar]
- 93.Fève B, Scheen AJ. When therapeutic drugs lead to diabetes. Diabetologia 65 (2022): 751–62. [DOI] [PubMed] [Google Scholar]
- 94.Kukucka T, Ferencova N, Visnovcova Z, et al. Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change. Int J Mol Sci (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khoza S, Barner JC, Bohman TM, et al. Use of antidepressant agents and the risk of type 2 diabetes. European Journal of Clinical Pharmacology 68 (2012): 1295–302. [DOI] [PubMed] [Google Scholar]
- 96.Rashidian H, Subramaniapillai M, Park C, et al. Changes in insulin resistance following antidepressant treatment mediate response in major depressive disorder. Journal of Psychopharmacology 37 (2023): 313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J, Yue Y, Thapa A, et al. Baseline serum C-reactive protein levels may predict antidepressant treatment responses in patients with major depressive disorder. J Affect Disord 50 (2019): 432–438. [DOI] [PubMed] [Google Scholar]
- 98.Jindal RD, Keshavan MS. Critical role of M3 muscarinic receptor in insulin secretion: implications for psychopharmacology. Journal of clinical psychopharmacology 26 (2006): 449–50. [DOI] [PubMed] [Google Scholar]
- 99.Weston-Green K, Huang XF, Deng C. Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor. CNS drugs 27 (2013): 1069–80. [DOI] [PubMed] [Google Scholar]
- 100.Okamura F, Tashiro A, Utumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: Minimal model analysis. Metabolism 49 (2000): 1255–1260. [DOI] [PubMed] [Google Scholar]
- 101.Pyykkönen A, Räikkönen K, Tuomi T, et al. Depressive symptoms, antidepressant medication use, and insulin resistance. Diabetes Care 34 (2011): 2545–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alonso-Pedrero L, Bes-Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: A systematic review. Obes Rev 20 (2019): 1680–1690. [DOI] [PubMed] [Google Scholar]
- 103.Petimar J, Young JG, Yu H, et al. Medication-Induced Weight Change Across Common Antidepressant Treatments: A Target Trial Emulation Study. Ann Intern Med 177 (2024): 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population-based cohort study. BMJ 361 (2018): k1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serretti A, Mandelli L, Laura M. Antidepressants and body weight: a comprehensive review and meta-analysis. The Journal of Clinical Psychiatry 71 (2010): 979. [DOI] [PubMed] [Google Scholar]
- 106.Blumenthal SR, Castro VM, Clements CC, et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA psychiatry 71 (2014): 889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arterburn D, Sofer T, Boudreau DM, et al. Long-term weight change after initiating second-generation antidepressants. Journal of Clinical Medicine 5 (2016): 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Demyttenaere K, Jaspers L. Bupropion and SSRI-induced side effects. Journal of Psychopharmacology 22 (2008): 792–804. [DOI] [PubMed] [Google Scholar]
- 109.Aronne LJ, Hall KD, M. Jakicic J, et al. Describing the weight-reduced state: physiology, behavior, and interventions. Obesity 29 (2021): S9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sjaarda J, Delacrétaz A, Dubath C, et al. Identification of four novel loci associated with psychotropic drug-induced weight gain in a Swiss psychiatric longitudinal study: A GWAS analysis. Mol Psychiatry 28 (2023): 2320–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ricardo-Silgado ML, Singh S, Cifuentes L, et al. Association between CYP metabolizer phenotypes and selective serotonin reuptake inhibitors induced weight gain: a retrospective cohort study. BMC Med 20 (2022): 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eder J, Glocker C, Barton B, et al. Who is at risk for weight gain after weight-gain associated treatment with antipsychotics, antidepressants, and mood stabilizers: A machine learning approach. Acta Psychiatr Scand 151 (2025): 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simon MS, Barton B, Zagler A, et al. Lifestyle behaviors, metabolic disturbances, and weight gain in psychiatric inpatients treated with weight gain-associated medication. Eur Arch Psychiatry Clin Neurosci 273 (2023): 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agarwal SM, Stogios N, Ahsan ZA, et al. Pharmacological interventions for prevention of weight gain in people with schizophrenia. Cochrane Database Syst Rev 10 (2022): CD013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Menon T, Lee S, Gong XY, et al. A systematic review on the efficacy of GLP-1 receptor agonists in mitigating psychotropic drug-related weight gain. CNS Spectr 25 (2024): 1–7. [DOI] [PubMed] [Google Scholar]
- 116.McIntyre RS, Paron E, Burrows M, et al. Psychiatric Safety and Weight Loss Efficacy of Naltrexone/bupropion as Add-on to Antidepressant Therapy in Patients with Obesity or Overweight. J Affect Disord 289 (2021): 167–176. [DOI] [PubMed] [Google Scholar]
- 117.Gartlehner G, Dobrescu A, Chapman A, et al. Nonpharmacologic and Pharmacologic Treatments of Adult Patients With Major Depressive Disorder: A Systematic Review and Network Meta-analysis for a Clinical Guideline by the American College of Physicians. Ann Intern Med 176 (2023): 196–211. [DOI] [PubMed] [Google Scholar]
- 118.Cao B, Xu J, Li R, et al. Interventions targeting comorbid depression and overweight/obesity: A systematic review. J Affect Disord 314 (2022): 222–232. [DOI] [PubMed] [Google Scholar]
- 119.Minichino A, Preston T, Fanshawe JB, et al. Psycho-Pharmacomicrobiomics: A Systematic Review and Meta-Analysis. Biol Psychiatry 95 (2024): 611–628. [DOI] [PubMed] [Google Scholar]
- 120.Nikolova VL, Cleare AJ, Young AH, et al. Exploring the mechanisms of action of probiotics in depression: Results from a randomized controlled pilot trial. J Affect Disord 376 (2025): 241–250. [DOI] [PubMed] [Google Scholar]
- 121.Mötteli S, Vetter S, Colla M, et al. Are probiotics effective in reducing the metabolic side effects of psychiatric medication? A scoping review of evidence from clinical studies. Transl Psychiatry 14 (2024): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corfitsen HT, Drago A. Insight gained from genome-wide interaction and enrichment analysis on weight gain during citalopram treatment. Neurosci Lett 637 (2017): 38–43. [DOI] [PubMed] [Google Scholar]
- 123.McQuaid JR, Buelt A, Capaldi V, et al. The Management of Major Depressive Disorder: Synopsis of the 2022 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med 175 (2022): 1440–1451. [DOI] [PubMed] [Google Scholar]


