Abstract
Background & Objective
Presence of lupus nephritis is associated with a shorter time to death in systemic lupus erythematosus. The aim of this study was to determine the frequency of peritubular capillaritis (ptc) and its relationship with both active and chronic lesions in lupus nephritis.
Material-Methods
Specimens from 57 patients were re-evaluated. The degree of inflammation within the peritubular capillaries in each biopsy was assessed according to the Banff classification, and ptc scoring was performed. Additionally, patients were grouped based on high/low activity and chronicity index scores.
Results
Forty-five (78.9%) of the patients were female, with a mean age of 27.43 years. Ptc was identified in 45 (78.9%) patients, with ptc scores of 1, 2, and 3 observed in 20 (35.08%), 20 (35.08%), and 5 (8.7%) patients, respectively. Severe ptc (scores 2 or 3) was more commonly detected in patients with elevated serum creatinine (>1.20 mg/dL) compared to those with normal creatinine levels (92.3% vs 29.5%, p < 0.001). The severity of ptc was higher in Class 4 patients than in Class 3 patients (68.2% vs 21.7%, p = 0.005). The rate of high ptc scores was greater in patients with an activity index of 6 or above compared to those with an activity index below 6 (62.5% vs 30.3%, p = 0.032). No significant correlation was found between the chronicity index and ptc.
Conclusions
The findings of this study suggest that ptc is a frequently observed pathological feature in patients with lupus nephritis. Furthermore, the severity of ptc appears to be positively associated with elevated serum creatinine levels, and higher ptc scores are more commonly detected in cases with a high activity index.
Keywords: Lupus nephritis, peritubular capillaritis, banff classification, activity index, renal outcome
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystemic autoimmune disease that can affect any organ in the body, including the kidneys. The kidneys are the most frequently affected visceral organs in SLE. 1 Lupus nephritis (LN) is a type of glomerulonephritis that occurs in patients with SLE, affecting approximately 20%–65% of these patients during the course of the disease.2–4 It is the leading cause of morbidity and mortality in SLE patients. 5 Despite advancements in therapeutic modalities, around 10% of patients with LN progress to end-stage renal disease (ESRD). 6 Moreover, SLE patients with LN have a shorter life expectancy compared to those without LN.7,8
The International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification is the most widely used system for categorizing glomerular lesions in LN based on the location of immune complex deposition. In 2018, the ISN/RPS revised the classification for LN, clarifying certain definitions and proposing a modified National Institutes of Health (NIH) scoring system for all classes based on activity and chronicity indices. This revision replaced the earlier definitions for Class 3/4 lesions. According to this updated classification, LN is divided into non-proliferative (Classes 1, 2, and 5), proliferative (Classes 3 and 4), and advanced sclerotic LN (Class 6), based on glomerular pathology. 9
The Banff Classification is an internationally recognized standard for the histopathological evaluation of kidney transplants. It includes criteria for T cell-mediated rejection (TCMR), antibody-mediated rejection (AMR), and borderline changes. Histopathological findings in AMR include ptc, glomerulitis, and thrombotic microangiopathy (TMA).10–12
Ptc is associated with endothelial injury and is an important feature in the evaluation of rejection in transplant kidney biopsies. However, it is not exclusive to transplant cases and has also been detected in native kidney biopsies. Despite this, there remains insufficient research on the significance and frequency of ptc in native kidney disease. In this cross-sectional study, we re-examined renal biopsies from patients with LN to determine the frequency of ptc using the Banff scoring system. Additionally, we explored the association between the severity of ptc and various demographic, clinical, and histological characteristics of LN.
Materials and methods
Biopsy sections from 57 patients diagnosed with LN between 2015 and 2023 at our institution were obtained from the pathology department archive. The demographic and clinical characteristics at the time of renal biopsy were retrieved from the patients’ electronic health records. This study was approved by the Non-invasive Clinical Research Ethics Committee of Eskişehir Osmangazi University (E-25403353-050.99-2400052898).
Histopathologic evaluation
All biopsies were initially evaluated by light microscopy (H&E, Masson trichrome, Periodic acid–Schiff, Methenamine silver, and Congo red) and immunofluorescence microscopy (IgG, IgA, IgM, C3, C1q, κ, and λ). Pathological lesions in renal biopsies diagnosed with LN were redefined and reclassified according to the revised 2018 ISN/RPS classification. 9
The following histologic features were recorded: endocapillary hypercellularity, neutrophils/karyorrhexis, fibrinoid necrosis, wire loop lesions/hyaline deposits, cellular/fibrocellular crescents, interstitial inflammation, global and/or segmental sclerosis, fibrous crescents, tubular atrophy, interstitial fibrosis, and ptc.
Evaluation of ptc
The degree of inflammation within the peritubular capillaries in each biopsy was evaluated according to the Banff Classification and ptc scoring was performed. 12 Areas affected by acute pyelonephritis or necrosis, subcapsular cortex with nonspecific inflammation, inflammatory cells within veins and medullary capillaries (vasa recta), and longitudinally cut peritubulary capillaries were excluded from scoring. The scoring was as follows:
• ptc 0—Maximum number of leukocytes <3.
• ptc 1—At least 1 leukocyte cell in ≥10% of cortical peritubular capillaries, with 3-4 leukocytes in the most severely involved peritubulary capillaries.
• ptc 2—At least 1 leukocyte in ≥10% of cortical peritubular capillaries, with 5-10 leukocytes in the most severely involved peritubulary capillaries.
• ptc 3—At least 1 leukocyte in ≥10% of cortical peritubular capillaries, with >10 leukocytes in the most severely involved peritubulary capillaries.
Activity and chronicity assessment
The NIH activity and chronicity scoring system was applied to determine the activity and chronicity scores of biopsies. 9 This system is a modified version of the NIH activity and chronicity index in LN, based on the study by Austin et al 13 (Table 1).
Table 1.
The modified NIH activity and chronicity scoring system.
| Modified NIH activity index | Definition | Score |
|---|---|---|
| Endocapillary hypercellularity | Endocapillary hypercellularity in <25% (1+), 25%-50%, or >50% (3+) of glomeruli | 0-3 |
| Neutrophils/karyorrhexis | Neutrophils and/or karyorrhexis in <25% (1+), 25%-50%, or >50% (3+) of glomeruli | 0-3 |
| Fibrinoid necrosis | Fibrinoid necrosis in <25% (1+), 25%-50%, or >50% (3+) of glomeruli | (0-3) x 2 |
| Hyaline deposits | Wire loop lesions and/or hyaline thrombi in <25% (1+), 25%-50%, or >50% (3+) of glomeruli | 0-3 |
| Cellular/fibrocellular crescents | Cellular and/or fibrocellular crescents in <25% (1+), 25%-50%, or >50% (3+) of glomeruli | (0-3) x 2 |
| Interstitial inflammation | Interstitial leukocytes in <25% (1+), 25%-50%, or >50% (3+) of the cortex | 0-3 |
| Total | 0-24 |
Clinical evaluation
At the time of the biopsy and after the treatment, serum creatinine levels, serum albumin levels, the presence of hematuria (macroscopic and microscopic), quantitative value of proteinuria, and the presence of nephrotic syndrome were documented for all patients. Patients with serum creatinine levels greater than 1.20 mg/dL were categorized as having high creatinine, with clinically significant elevation. 14 Patients with 3 erythrocytes per high-power field (HPF) on urine microscopy analysis were considered to have microscopic hematuria. 15 Patients with visible blood in their urine were considered to have macroscopic hematuria. Patients with protein levels greater than 500 mg in a 24-h urine collection were considered to have proteinuria. 16 Additionally, post-biopsy treatment information, renal outcomes, and follow-up periods were recorded.
Statistical analysis
The normality of continuous variables was assessed using the Shapiro-Wilk normality test. Intergroup comparisons of continuous variables were performed using the Mann-Whitney U test and the Kruskal-Wallis test. Pearson’s chi-square, Yates’ chi-square, and Fisher’s exact tests were used to compare categorical variables between groups. A p-value of <0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics Version 25.
Results
A total of 57 newly diagnosed, biopsy-proven lupus nephritis (LN) cases were included in the study. The mean age of the participants was 29.4 years (range: 8–71). Nine (15.7%) patients were younger than 18 years of age. Forty-five patients (78.9%) were female and 12 (21.05%) were male. The median serum creatinine was 0.69 mg/dL (interquartile range [IQR]: 0.26–6.2 mg/dL). Thirteen patients (22.8%) had clinically significant elevated serum creatinine levels. Proteinuria was present in all patients, with a median value of 1619 mg/24h (range: 517–10,104 mg/24h). The median serum albumin level was 3.48 mg/dL (IQR: 1.70–4.50 mg/dL). Clinical manifestations consistent with nephrotic syndrome were observed in 15 (26.3%) patients. Hematuria was detected in 28 patients (49.1%).
Class 3 was the most common form of LN, accounting for 40.4% of cases, followed by Class 4 (38.6%), Class 5 (10.5%), and Class 2 (5.3%). Conversely, Classes 6 and 1 were the least frequently diagnosed, representing 1.7% and 3.5% of cases, respectively.
The median activity index score was 4.5 (range: 0–15), and the median chronicity index score was 2.2 (range: 0–9). Based on the activity index scores, patients were divided into low (0-5) and high (6-24) activity groups. Similarly, based on chronicity index scores, patients were categorized into low (0-2) and high (3-12) chronicity groups. A high activity index was identified in 42.1% (n = 24) of the biopsies, while 40.4% (n = 23) of the patients had a high chronicity index.
Ptc was detected in 45 (78.9%) patients, with ptc scores of 1, 2, and 3 observed in 20 (35.1%), 20 (35.1%), and 5 (8.8%) patients, respectively (Figures 1–3). The highest frequency of ptc was observed in Class 4 among proliferative LNs. Ptc was identified in 21 (96%) of the 22 patients with Class 4 LN. Of these, 6 (27%) had ptc score 1, 12 (55%) had ptc score 2, and 3 (14%) had ptc score 3. Conversely, ptc score 0 was found in 9 (39%) of the 23 patients with Class 3 LN. In total, ptc was observed in 14 (69%) Class 3 patients, including 9 patients with ptc score 1 and 5 patients with ptc score 2. No patients in Class 3 exhibited ptc score 3. Ptc score 1 was identified in all 3 biopsies diagnosed as Class 2.
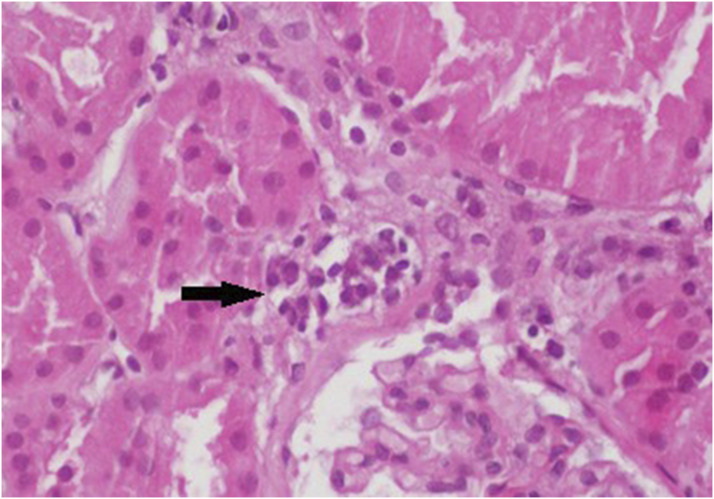
Figure 2.
Ptc with a score of 2 (indicated by the arrow). Representative ptc lesions are shown (H&E, ×400).
Figure 1.
Ptc with a score of 1 (indicated by the arrow). Representative ptc lesions are shown (H&E, ×400).
Figure 3.
Ptc with a score of 3 (indicated by the arrow). Representative ptc lesions are shown (H&E, ×400).
Among non-proliferative LN biopsies, ptc was observed in 5 (83.3%) of 6 Class 5 patients. Of these, 1 patient (16.6%) had ptc score 1, 3 patients (50%) had ptc score 2, and 1 patient (16.6%) had ptc score 3. Only 2 biopsy samples were diagnosed with Class 1 LN, with ptc scores of 0 and 1, respectively. The single biopsy classified as Class 6 had a ptc score of 3 (Figure 4).
Figure 4.
Distribution of the ptc scores according to the classes of the LN.
In this study, biopsies with ptc scores of 0 and 1 were categorized as low severity, while biopsies with ptc scores of 2 and 3 were categorized as high severity. Statistical analysis revealed a higher prevalence of ptc in Class 4 patients compared to Class 3 patients. High-severity ptc (scores 2-3) was observed in 68.2% (n = 15/22) of Class 4 biopsies, significantly higher than the 21.7% (n = 5/23) found in Class 3 biopsies (p = .005).
The detection rate of high-severity ptc (scores 2-3) was significantly higher in biopsy specimens with a high activity index compared to those with a low activity index (p = 0.032). Ptc scores 2-3 were recorded in 62.5% of cases with an activity index of 6 or greater, whereas only 30.3% of cases with a low activity index had ptc scores 2-3.
There was no significant difference between the severity of ptc in groups with low and high chronicity indices (p = 0.442).
A significantly higher incidence of ptc was observed in patients with clinically significant elevated serum creatinine levels compared to those with serum creatinine levels within the normal range (p < 0.001). High-severity ptc (scores 2-3) was found in 92.3% of patients with elevated serum creatinine levels.
Hematuria (either macroscopic or microscopic) was present in 28 patients (49.1%). High-severity ptc was observed in 41.4% of patients without hematuria and 46.4% of those with hematuria, with no significant difference between the two groups (p = 0.907).
The relationship between ptc severity and certain clinical and histopathological features is summarized in Table 2.
Table 2.
General characteristics of patient groups with ptc 0-1 (low score) and ptc 2-3 (high score).
| ptc 0-1 (n:32) | ptc 2-3 (n:25) | p value | |
|---|---|---|---|
| Gender (F/M), n | 24/8 | 21/4 | 0.617 |
| Age, mean ± SD | 27.72 ± 1.78 | 31.64 ± 2.93 | 0.741 |
| Hematuria, n (%) | 17 (58.6) | 12 (41.4) | 0.907 |
| Absent present | 15 (53.6) | 13 (46.4) | |
| Serum albumin value (mg/dl) mean ± SD | 3.53 ± 0.11 | 3.08 ± 0.13 | 0.008 |
| Serum creatinine value (mg/dl) mean ± SD | 0.65 ± 0.37 | 1.55 ± 0.26 | 0.001 |
| Serum creatinine level, n (%) | <0.001 | ||
| >1.2 mg/dl | 1 (7.7) | 12 (92.3) | |
| ≤1.2 mg/dl | 31 (70.5) | 13 (29.5) | |
| Proteinuria value (mg/24h) mean ± SD | 2266.4 ± 423.9 | 3509.9 ± 576.8 | 0.045 |
| Nephrotic syndrome, n (%) | 26 (61.9) | 16 (38.1) | 0.244 |
| Absent present | 6 (40) | 9 (60) | |
| Classes of lupus nephritis n (%) | |||
| Class 1 | 2 (100) | 0 (0) | 0.005 |
| Class 2 | 3 (100) | 0 (0) | |
| Class 3 | 18 (78.3) | 5 (21.7) | |
| Class 4 | 7 (31.8) | 15 (68.2) | |
| Class 5 | 2 (33.3) | 4 (66.7) | |
| Class 6 | 0 (0) | 1 (100) | |
| Activity index, mean ± SD | 4.09 ± 0.61 | 6.96 ± 0.81 | 0.005 |
| Activity index, n (%) | 0.032 | ||
| ≥6 | 9 (37.5) | 15 (62.5) | |
| <6 | 23 (69.7) | 10 (30.3) | |
| Chronicity index, mean ± SD | 2.18 ± 0.29 | 3.04 ± 0.46 | 0.213 |
| Chronicity index (≥3), n (%) | 0.442 | ||
| >2 | 11 (47.8) | 12 (52.2) | |
| ≤2 | 21 (61.8) | 13 (38.2) |
Clinical follow-up data were not available for 3 patients. The median follow-up period for the remaining 54 patients was 47.6 months (range: 4–105 months). In addition to steroids, combination therapies including cyclophosphamide, azathioprine, rituximab, and mycophenolate mofetil were administered to all patients. During the follow-up period, remission was achieved in 72.2% (39 of 54 patients) of patients, while partial remission and/or resistance occurred in 18.5% (10 of 54 patients). Chronic renal failure developed in 9.3% (5 of 54 patients) of patients, and 1 (1.8%) patient died. One patient underwent a renal transplant.
Proteinuria decreased in 76.4% of cases after treatment. This rate was 80.6% in patients with low ptc scores, while it was slightly lower in patients with high ptc scores (70.8%), though this difference was not statistically significant (p = 0.597). The median proteinuria level in patients with low ptc scores was 185 mg/24h (range: 53–5731 mg/24h), whereas it was 230 mg/24h (range: 67–4600 mg/24h) in patients with high ptc scores (p = 0.396). Serum creatinine levels decreased to below 1.2 mg/dL in 85.5% of cases. This rate was 90.3% in patients with low ptc scores, compared to 79.2% in patients with high ptc scores (p = 0.597). The median serum creatinine level in patients with low ptc scores was 0.63 mg/dL (range: 0.41–7.19 mg/dL), while it was 0.81 mg/dL (range: 0.37–7.16 mg/dL) in those with high ptc scores (p = 0.069).
During follow-up, remission was achieved in 80% (24 of 30 patients) of patients in the low ptc group, with partial remission and/or resistance in 20% (6 of 30 patients). No patients in the low ptc group progressed to chronic renal failure. In contrast, 62.5% (15 of 24 patients) of patients with high ptc scores achieved remission, 16.7% (4 of 24 patients) had partial remission and/or resistance, and 20.8% (5 of 24 patients) progressed to chronic renal failure (p = 0.032). The relationship between ptc group and renal outcomes is summarized in Table 3.
Table 3.
Clinical follow-up of patient groups with ptc 0-1 (low score) and ptc 2-3 (high score).
| ptc 0-1 (n:30) | ptc 2-3 (n:24) | Total (n:54) | p value | |
|---|---|---|---|---|
| Renal outcome, n (%) | ||||
| Remission | 24 (80) | 15 (62.5) | 39 (72.2) | 0.032 |
| Partial remission/Resistant | 6 (20) | 4 (16.7) | 10 (18.5) | |
| ESRD | 0 (0) | 5 (20.8) | 5 (9.3) | |
Discussion
One of the Banff lesion scores, ptc, assesses the degree of inflammation within the peritubular capillaries. Along with glomerulitis, ptc represents a form of microvascular inflammation characteristic of both acute AMR and chronic active AMR. 17 While ptc is commonly observed in transplant kidney biopsies, it can also be found in native kidney biopsies and has been documented in a variety of underlying kidney disease, including pauci-immune glomerulonephritis, 18 LN, 19 cryoglobulinemia,20,21 and viral infections. 22 It has also been observed in numerous other native kidney diseases.23,24
The most comprehensive study on the frequency of ptc in non-transplant kidney biopsies in the English literature was conducted by Sarıoğlu et al., who analyzed 169 native kidney biopsies and found ptc in 53.3% of cases. Among the 15 LN biopsies included in their study, ptc was present in 4%. 23 In contrast, the prevalence of ptc in our study was significantly higher, at 78.9%. Notably, 44% of LN patients exhibited high ptc severity.
In our study, no significant correlation was found between age, gender, hematuria, or nephrotic syndrome and the severity of ptc. However, high ptc severity was observed in 68% of Class 4 LN biopsies, while only 22% of Class 3 biopsies exhibited high ptc severity. The presence of proliferation in LN histopathology is widely accepted as a poor prognostic indicator, warranting more aggressive treatment for patients with Class 3 and 4 LN.25–27 Our results align with this notion, as ptc severity was significantly higher in Class 4 compared to Class 3 patients, suggesting that ptc may serve as an additional marker of disease severity.
A high serum creatinine level is a common finding in disorders affecting renal endothelial function. The patients diagnosed with LN, the baseline serum creatinine level was an effective predictor of the eventual outcome.28,29 Patients with high serum creatinine levels have a low remission rate, a poor overall long-term prognosis and an increased incidence of ESRD and mortality. Austin et al. reported that patients with a baseline serum creatinine level of ≥1.4 mg/dL had more than a 3.5-fold increased risk of progressing to ESRD over an eight-year follow-up period compared to patients with a serum creatinine level of <1.4 mg/dL. 30 Korbet et al. indicated that patients with an initial serum creatinine level of 1.0 mg/dL or lower had the most favorable prognosis. 28 In our study, we found that high ptc severity was significantly associated with elevated serum creatinine levels (>1.2 mg/dL), indicating a potential link between ptc and unfavorable renal outcomes in LN.
We conducted a retrospective study to evaluate the modified NIH activity and chronicity scores in patients with LN. The modified NIH scoring system provides more detailed information compared to the shorthand A, C, and A/C parameters previously used.9,13 In our study, we did not find an association between low (0-2) and high (3-12) chronicity index scores and the severity of ptc. Many studies have linked a higher chronicity index to poor renal outcomes.18,31–33 Increased glomerular and interstitial fibrosis may contribute to higher ptc severity. The limited number of Class 5 and Class 6 biopsies in the current study may have influenced the statistical results.
The results of previous studies have consistently shown a correlation between the activity index, its components, and renal outcomes. Austin et al. reported that the composite activity index, cellular crescents, and fibrinoid necrosis were highly predictive of renal failure and outcomes. 13 Rijnink et al. found that fibrinoid necrosis was associated with a higher risk for ESRD. 34 In their prediction tool for the development of ESRD in LN patients, Tang et al. identified cellular crescents and an activity index greater than 20 as significant factors. 35 Singh et al. also found a correlation between a higher activity index and a lower complete response to treatment. 36 In our study, a high activity index was identified in 42.1% of biopsy samples. This finding was not unexpected, given the relatively high incidence of Class 3 and 4 biopsies in the cohort. Additionally, the severity of ptc was statistically significantly higher in biopsy samples with an activity index of 6 or above.
Our study also found significant relationships between high ptc severity and elevated serum creatinine levels, higher proteinuria, and lower serum albumin levels (p = 0.001, p = 0.045, and p = 0.008, respectively). During follow-up, patients with high ptc scores had a lower remission rate (62.5% vs 80%) and a higher incidence of chronic renal insufficiency (20% vs 0%) compared to those with low ptc scores. These findings suggest that ptc severity may be a valuable prognostic marker in LN.
While there are few studies investigating ptc in native kidney biopsies, and even fewer that explore its prognostic significance, this study represents one of the first to address this issue. However, there are limitations. We were unable to compare pre- and post-treatment biopsy scores, as biopsies are typically not conducted immediately following treatment. Additionally, the follow-up period in our study was relatively short, limiting our ability to assess late-stage renal failure or long-term renal outcomes. Since biopsies were primarily conducted during disease flare-ups, the majority of patients in our study were classified as Class 3 and 4, limiting the ability to evaluate ptc in other classes. Furthermore, as a single-center study with a relatively small sample size, larger multi-center studies with longer follow-up are needed to validate these findings.
Conclusion
Despite recent advancements in treatment, LN remains a challenging condition, with approximately 10% of patients progressing to ESRD despite appropriate treatment. The modified NIH scoring system has proven prognostic value in predicting complete renal response, though the lesions comprising the system may evolve over time. In our study, we found a significant correlation between high ptc severity, elevated creatinine levels, and high activity index scores. These findings suggest that ptc severity may be an important marker of disease activity and severity in LN, potentially adding value to the NIH scoring system. Further studies with larger patient cohorts and longer follow-up are required to confirm the prognostic significance of ptc in LN.37–41
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement
Ethical approval
This study was approved by the Non-invasive Clinical Research Ethics Committee of Eskişehir Osmangazi University (E−25403353-050.99-2400052898) on February 27, 2024.
Informed consent
This is an retrospective study, patient consent was not required. Patient data will not be shared with third parties.
ORCID iDs
Emel Yaldır https://orcid.org/0000-0001-7297-9869
Balça Begüm Cengiz https://orcid.org/0000-0001-8878-7837
Data availability statement
Available to only corresponding author.*
References
- 1.Cojocaru M, Cojocaru IM, Silosi I, et al. Manifestations of systemic lupus erythematosus. Maedica (Buchar) 2011; 6: 330–336. [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcón GS. Multiethnic lupus cohorts: what have they taught us? Reumatol Clínica 2011; 7: 3–6. [DOI] [PubMed] [Google Scholar]
- 3.Bastian HM, Roseman JM, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002; 11: 152–160. [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Madr) 1999; 78: 167–175. [DOI] [PubMed] [Google Scholar]
- 5.Anders H-J, Saxena R, Zhao M, et al. Lupus nephritis, Nat Rev Dis Primers 2020; 6: 7. [DOI] [PubMed] [Google Scholar]
- 6.Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology 2011; 50: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 7.Yap DYH, Tang CSO, Ma MKM, et al. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 2012; 27: 3248–3254. [DOI] [PubMed] [Google Scholar]
- 8.Lerang K, Gilboe I-M, Steinar Thelle D, et al. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus 2014; 23: 1546–1552. [DOI] [PubMed] [Google Scholar]
- 9.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018; 93: 789–796. [DOI] [PubMed] [Google Scholar]
- 10.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am J Transplant 2020; 20: 2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loupy A, Haas M, Solez K, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 2017; 17: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018; 18: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin HA, Muenz LR, Joyce KM, et al. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984; 25: 689–695. [DOI] [PubMed] [Google Scholar]
- 14.ACCESS Trial Group . Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study. Arthritis Rheumatol 2014; 66: 3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barocas DA, Boorjian SA, Alvarez RD, et al. Microhematuria: AUA/SUFU guideline. J Urol 2020; 204: 778–786. [DOI] [PubMed] [Google Scholar]
- 16.Touma Z, Urowitz MB, Ibañez D, et al. Time to recovery from proteinuria in patients with lupus nephritis receiving standard treatment. J Rheumatol 2014; 41: 688–697. [DOI] [PubMed] [Google Scholar]
- 17.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation 2018; 102: 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol 1989; 135: 921–930. [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa S, Nakabayashi K, Karube M, et al. Tubulointerstitial immune complex nephritis in a patient with systemic lupus erythematosus: role of peritubular capillaritis with immune complex deposits in the pathogenesis of the tubulointerstitial nephritis. Clin Exp Nephrol 2006; 10: 146–151. [DOI] [PubMed] [Google Scholar]
- 20.Iwakura T, Namikawa A, Tsuji N, et al. Tubulointerstitial nephritis caused by peritubular capillaritis accompanied by cryoglobulinemia. Intern Med 2015; 54: 2885–2891. [DOI] [PubMed] [Google Scholar]
- 21.Troxell ML, Shackleton DV, Raguram P, et al. Acute kidney injury with cryoglobulinemic peritubular neutrophilic capillaritis. Clin Nephrol 2011; 76: 159–164. [DOI] [PubMed] [Google Scholar]
- 22.Gnemmi V, Verine J, Vrigneaud L, et al. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavirus nephropathy. Hum Pathol 2015; 46: 827–835. [DOI] [PubMed] [Google Scholar]
- 23.Sarioglu S, Tekin E, Unlu M, et al. Peritubular capillaritis in native kidney biopsies. Am J Clin Pathol 2022; 158: 389–394. [DOI] [PubMed] [Google Scholar]
- 24.Hakroush S, Tampe B. Incidence of arteritis and peritubular capillaritis in ANCA-associated vasculitis. J Am Soc Nephrol 2021; 32: 2974–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilori TO, Enofe N, Oommen A, et al. Comparison of outcomes between individuals with pure and mixed lupus nephritis: a retrospective study. PLoS One 2016; 11: e0157485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin DS, Lowenstein J, Rothfield NF, et al. The clinical course of the proliferative and membranous forms of lupus nephritis. Ann Intern Med 1970; 73: 929–942. [DOI] [PubMed] [Google Scholar]
- 27.Appel GB, Cohen DJ, Pirani CL, et al. Long-term follow-up of patients with lupus nephritis. A study based on the classification of the World Health Organization. Am J Med 1987; 83: 877–885. [DOI] [PubMed] [Google Scholar]
- 28.Collaborative Study Group. Korbet SM, Whittier WL, et al. The impact of baseline serum creatinine on complete remission rate and long-term outcome in patients with severe lupus nephritis. Nephron Extra 2016; 6: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farinha F, Barreira S, Couto M, et al. Risk of chronic kidney disease in 260 patients with lupus nephritis: analysis of a nationwide multicentre cohort with up to 35 years of follow-up. Rheumatology 2025; 64: 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin HA, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 1983; 75: 382–391. [DOI] [PubMed] [Google Scholar]
- 31.Umeda R, Ogata S, Hara S, et al. Comparison of the 2018 and 2003 International Society of Nephrology/Renal Pathology Society classification in terms of renal prognosis in patients of lupus nephritis: a retrospective cohort study. Arthritis Res Ther 2020; 22: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Choi S, Xu H, et al. Chronicity index, especially glomerular sclerosis, is the most powerful predictor of renal response following immunosuppressive treatment in patients with lupus nephritis. Int J Rheum Dis 2018; 21: 458–467. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S, Toyama T, Iwata Y, et al. The relationship between the modified National Institute of Health activity and chronicity scoring system, and the long-term prognosis for lupus nephritis: a retrospective single-center study. Lupus 2021; 30: 1739–1746. [DOI] [PubMed] [Google Scholar]
- 34.Rijnink EC, Teng YKO, Wilhelmus S, et al. Clinical and histopathologic characteristics associated with renal outcomes in lupus nephritis. Clin J Am Soc Nephrol 2017; 12: 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, Qin W, Peng W, et al. Development and validation of a prediction score system in lupus nephritis. Medicine (Baltim) 2017; 96: e8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh S, Sreenidhi HC. Prognostic value of modified National Institute of Health activity and chronicity scoring in determining complete renal response in newly diagnosed lupus nephritis: a retrospective single centre study. Int Urol Nephrol 2022; 54: 2075–2082. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa S, Nakabayashi K, Karube M, et al. Tubulointerstitial immune complex nephritis in a patient with systemic lupus erythematosus: role of peritubular capillaritis with immune complex deposits in the pathogenesis of the tubulointerstitial nephritis. Clin Exp Nephrol 2006; 10: 146–151. [DOI] [PubMed] [Google Scholar]
- 38.Iwakura T, Namikawa A, Tsuji N, et al. Tubulointerstitial nephritis caused by peritubular capillaritis accompanied by cryoglobulinemia. Intern Med 2015; 54: 2885–2891. [DOI] [PubMed] [Google Scholar]
- 39.Troxell ML, Shackleton DV, Raguram P, et al. Acute kidney injury with cryoglobulinemic peritubular neutrophilic capillaritis. Clin Nephrol 2011; 76: 159–164. [DOI] [PubMed] [Google Scholar]
- 40.Gnemmi V, Verine J, Vrigneaud L, et al. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavirus nephropathy. Hum Pathol 2015; 46: 827–835. [DOI] [PubMed] [Google Scholar]
- 41.Moroni G, Porata G, Raffiotta F, et al. Beyond ISN/RPS lupus nephritis classification: adding chronicity index to clinical variables predicts kidney survival. Kidney 2022; 3: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available to only corresponding author.*