Abstract
Objective
Abexinostat, an novel pan-histone deacetylase inhibitor, induces tumor apoptosis and demonstrates therapeutic potential in B cell non-Hodgkin lymphoma (NHL). This phase 1 study investigate the safety, pharmacokinetics (PK), and efficacy of abexinostat in Chinese patients with relapsed/refractory (r/r) B cell NHL.
Methods
Patients with r/r B cell NHL received abexinostat orally at escalating doses of 40 mg twice daily (bis in die, BID), 60 mg BID, and 80 mg BID with a 4-h interval, for seven days followed by a 7-day drug-free interval. Patients took abexinostat once on 3 days before day 1 (D-3) of the first cycle in single dose period. If no dose limiting toxicity (DLT) occurred from D-3 to C1D1, the continuous dose period was started from C1D1, abexinostat was given BID. The Primary endpoints were safety and PK.
Results
From April 13, 2020 to November 30, 2023, 12 r/r B cell NHL patients were enrolled, including 6 follicular lymphoma (FL), 5 diffuse large B cell lymphoma (DLBCL) and 1 mantle cell lymphoma (MCL). 11 patients received at least one dose abexinostat included in the safety set. No DLT were observed, 80 mg BID was the recommended phase 2 dose (RP2D). Most treatment emergent adverse events (TEAEs) were grade 1 or 2, and grade 3 TEAEs included thrombocytopenia (2/11, 18.2%) and hypertriglyceridemia (3/11, 27.3%). The median time to maximum concentration (Tmax) was 0.5–1.0 h and the median terminal elimination half-life (T1/2) was 2.56–8.31 h. Ten patients were included in full analysis set. The objective response rate (ORR) was 40.0% (4/10, 95% CI: 12.2–73.8), including 1 complete response and 3 partial response. The ORR was 50.0% (3/6, 95% CI: 11.8–88.2) of FL patients. The median progression-free survival and duration of response of FL were 8.38 months (95% CI: 1.05-NE) and 7.82 months(95% CI: 7.33-NE), respectively. The OS was not reached.
Conclusions
Abexinostat showed favorable tolerability with no DLT in Chinese patients with r/r B cell NHL. The RP2D was 80 mg BID. The plasma concentration was dose-proportional manner. The PK result demonstrated that BID “one week on, one week off” administration is reasonable. Promising anti-tumor activity were seen in these patients population. This result support further investigation.
Trial registration
ClinicalTrials.gov (NCT04024696). Date of registration: 18 July 2019.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14370-y.
Keywords: Abexinostat, B cell non-Hodgkin lymphoma, Phase 1 study
Introduction
Lymphoma is a heterogeneous group of lymphoproliferative diseases, comprise Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). The most common types of B cell NHL include diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) [1]. There were 553,010 new cases as well as 250,475 deaths of NHL in GLOBOCAN 2022 [2]. In China, 97,788 new cases as well as 57,929 deaths of NHL in 2022 [3]. As the second leading lymphoma type, FL is account for 12% (13,960/112,380) of mature NHL in the United States in 2016 [4]. In China, FL constitutes 2.5–6.6% of NHL cases [5, 6].
Current frontline treatment regimens for FL include cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or CHOP–like regimens combined with CD20 monoclonal antibodies, such as rituximab or obinutuzumab, rituximab plus lenalidomide (R2) and rituximab/obinutuzumab plus bendamustine (R/GB) regimens [7–10]. Rituximab maintenance after chemoimmunotherapy induction showed significantly higher 5-year PFS rate and OS rate than observation [11, 12]. Advanced stages FL is incurable, ~ 20% of patients experience disease progression within 2 years (POD24) of first-line treatment [13]. For relapsed or refractory (r/r) FL patients with POD24, may experience a shorter duration of response (DoR) to subsequent treatments as reflected by a reduction in progression-free survival (PFS) from 6.6 years after first-line treatment to 1.5 years and 0.83 years after the second and third lines treatment, respectively [14]. Therefore, novel therapies are urgently needed for these patients’ population.
Histone deacetylases (HDACs) regulate the expression and activity of numerous proteins involved in both cancer initiation and progression [15]. Several HDAC inhibitor (HDACi) have been developed and approved for the treatment of T cell lymphomas, including vorinostat [16], belinostat [17] and chidamide [18, 19]. Vorinostat had an objective response rate (ORR) of 49% (19/39, 95% confidence interval [CI]: 32·4–65·2) in patients with r/r FL and a median PFS of 20 months (95% CI: 11.2–29.7) [20]. Belinostat demonstrated an ORR of 10.5% (2/19, 95%CI: 1.3–33.1) in patients with DLBCL [21]. Abexinostat has shown therapeutic potential in previous studies for r/r B cell NHL [22, 23]. All patients in above studies didn’t enroll Chinese patients.
Abexinostat is an novel hydroxamic acid-derived pan-HDACi developed by Xynomic Pharmaceuticals (Nanjing) Co., Ltd, Nanjing, China. Since 2009, the efficacy and safety of abexinostat has been investigated in patients with different types of r/r solid tumors or hematological malignancies in 16 company-sponsored clinical studies, which recruited more than 700 patients (NCT00473577, NCT00562224, NCT00724984, NCT01149668, EudraCT-2009–013691-47, EudraCT-2011–002161-38, etc.). In these studies, abexinostat demonstrated anti-tumor activity on mantle cell lymphoma (MCL), FL, and DLBCL [22, 23]. In this phase 1 study, we investigated the safety, pharmacokinetics (PK) and efficacy of abexinostat for the treatment of Chinese patients with r/r B cell NHL.
Material and methods
Study design
This multi-center, phase 1 study was conducted at 4 hospitals across China. Abexinostat (Xynomic Pharmaceuticals [Nanjing] Co., Ltd, China) was 20 mg/tablet. Dose escalation followed a 3 + 3 design. The dose levels of abexinostat were orally 40 mg twice daily (bis in die, BID), 60 mg BID, and 80 mg BID at least half an hour before or 2 h after meals, with a 4-h interval, for 7 consecutive days followed by a 7-day discontinuation period, each cycle was 28 days [22, 23]. Each dose group was divided into two period, including single dose period and continuous dose period. The single dose period started from 3 days before day 1 (D-3) of the first cycle (C1D1) to 1 day before the C1D1 (D-1). Patients took abexinostat once on D-3. If no dose limiting toxicity (DLT) occurred from D-3 to C1D1, the continuous dose period was started from C1D1. At the continuous dose period, abexinostat was given BID. The DLTs were assessed after cycle 1. If no DLT were occurred during cycle 1 in this dose group, the study will start upper dose group. Treatment was continued until disease progression, death, unacceptable toxicity, withdrawal of consent, unsuitability to continue per the investigator’s discretion, loss to follow-up, or study termination. The primary endpoints were safety and PK. The secondary endpoint was efficacy.
Patients
All patients in this study had histologically confirmed B cell NHL who have failed or cannot tolerate previous standard treatment. Patient inclusion criteria were: age 18–65 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–1; life expectancy ≥ 3 months; white blood cell count ≥ 3 × 109/L, absolute neutrophil count ≥ 1.5 × 109/L; hemoglobin ≥ 90 g/L; platelet count ≥ 90 × 109/L; serum creatinine ≤ 1.5 × upper limit of normal (ULN); total bilirubin ≤ 1.5 × ULN; alanine aminotransferase and aspartate aminotransferase ≤ 2.5 × ULN. Patient exclusion criteria were: surgery or chemotherapy within 4 weeks or radiotherapy within 2 weeks before enrollment; central nervous system involvement of lymphoma; active infection; other severe internal disease; serologic evidence of hepatitis C virus or human immunodeficiency virus infection. Patients who tested positive for hepatitis B virus surface antigen or core antibody and negative for serum hepatitis B viral DNA were eligible for inclusion. The detail inclusion and exclusion criteria could be seen in supplementary material.
The study protocol was approved by the ethics committees of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (19–115/1899). The study was conducted in accordance with the Declaration of Helsinki, the principles of the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice Guidelines, and the local regulations. Written informed consent was obtained from all patients before enrollment. This study was registered at ClinicalTrials.gov (NCT04024696).
Safety
Safety evaluations were conducted at all study visits. Adverse events (AEs) were assessed by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. DLTs included grade 4 thrombocytopenia; ≥ grade 3 thrombocytopenia with obvious bleeding tendency; grade 4 neutropenia; ≥ grade 3 neutropenia with fever (≥ 38.5 °C); ≥ grade 4 other hematological toxicity; and any ≥ grade 3 non-hematological toxicity.
Pharmacokinetics
During the single dosing period (D-3), blood samples were collected at the following time points: 5–30 min before abexinostat administration, 10 min, 20 min, 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, and 24 h after abexinostat administration. During the continuous dose period, blood samples were collected at the following time points: 5–30 min before first dose of abexinostat administration, 30 min, 1 h, 2 h, 3 h, and 4 h after first dose of abexinostat administration, 30 min, 1 h, 2 h, 4 h, 6 h, and 20 h after second dose abexinostat administration on C1D1; 5–30 min before first dose of abexinostat administration on C1D4, C1D5, and C1D6; 5–30 min before first dose of abexinostat administration, 30 min, 1 h, 2 h, 3 h, and 4 h after first dose of abexinostat administration, 30 min, 1 h, 2 h, 4 h, 6 h, and 20 h after second dose of abexinostat administration on C1D7; 5–30 min before first dose of abexinostat administration on C1D15.
A validated high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC–MS/MS) method was used to measure plasma abexinostat concentrations. PK parameters were estimated using noncompartmental methods with Phoenix WinNonlin Version 8.0 (Pharsight Corporation Mountain View, CA, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Efficacy
Efficacy was evaluated based on computed tomography examination. Tumor responses were assessed using the Lugano 2014 criteria at 4 weeks ± 3 days after C1D1 and every 8 weeks ± 7 days thereafter until disease progression, unacceptable toxicity, initiation of a new anti-tumor therapy, withdrawal of consent, loss to follow-up, death, or study termination.
Statistical analysis
No formal hypothesis testing was performed in this study. The safety set (SS) included patients who received at least one dose of abexinostat treatment and have at least one safety evaluation for safety analysis. The pharmacokinetics set (PKS) included all patients who received at least one dose of abexinostat treatment and completed at least once blood sample testing for PK analysis. The full analysis set (FAS), which comprised all patients who received at least one dose of abexinostat treatment and had at least one efficacy evaluation for efficacy analysis. Demographic data were collected in this study and statistically summarized as the number of patients in each cohort and the total number of patients.
Results
Patients baseline characteristics
Between April 13, 2020 and November 30,2023, 18 patients were screened, 12 patients were enrolled in this study (Figure S1). One patient in the 60 mg BID group diagnosed with MCL was withdrawn from the study due to unexplained sudden death and did not receive any abexinostat treatment. Of the 11 patients enrolled, the median age was 56 years (range, 32–67), 7 (63.6%) were male. Six were FL and 5 were DLBCL. Nine were Ann Arbor stage of III or IV. All patients previously received rituximab-containing chemotherapy (Table 1).
Table 1.
Patients baseline characteristics in the SS
| Baseline characteristics | N = 11 |
|---|---|
| Gender, n | |
| Male | 7 |
| Female | 4 |
| Age, median (range), years | 56 (32–67) |
| ≤ 60 | 9 |
| > 60 | 2 |
| ECOG PS score, n | |
| 0 | 9 |
| 1 | 2 |
| Histology, n | |
| FL | 6 |
| DLBCL | 5 |
| Ann Arbor stage at enrollment, n | |
| I/II | 2 |
| III/IV | 9 |
| Number of prior chemotherapy regimens | |
| Median (range) | 3(1–8) |
| Prior rituximab, n | 11 |
SS Safety set, ECOG Eastern Cooperative Oncology Group, PS Performance status, FL Follicular lymphoma, DLBCL Diffuse large B cell lymphoma
Eleven patients received at least one dose of abexinostat treatment and underwent least one safety evaluation were included in the SS. These patients completed at least one blood sample test were included in PKS (N = 11). Among them, 4 patients were in the 40 mg BID group, 3 patients were in the 60 mg BID group, and 4 patients were in the 80 mg BID group. One patient with DLBCL receiving 40 mg BID abexinostat withdrew from the study on C1D4 due to personal reason and didn’t undergo efficacy evaluation. Therefore, 10 patients were enrolled in the FAS (Figure S1).
Safety
No DLT events were observed, 80 mg BID was recommended phase 2 dose (RP2D). The most common any grade treatment-emergent adverse events (TEAEs) were hypertriglyceridemia (6/11, 54.5%), neutropenia (5/11, 45.5%), hypoalbuminemia (5/11,45.5%), hypoproteinemia(5/11, 45.5%), thrombocytopenia (5/11, 45.5%), fatigue (4/11, 36.4%), abnormal T wave on electrocardiogram (4/11, 36.4%), leukopenia (4/11, 36.4%), diarrhea (4/11, 36.4%) and anemia (4/11, 36.4%). TEAEs of any grade occurring in at least 20% of patients are summarized in Table 2. Dose reduction due to TEAEs occurred in one patient each in the 60 mg BID (thrombocytopenia) and 80 mg BID group (QTc interval prolongation). Dose interruption occurred in one patient each in the 40 mg BID and 60 mg BID group, both due to thrombocytopenia. One patient diagnosed with DLBCL in the 40 mg BID group voluntarily withdrew from the study due to personal reason on C1D4. The most common ≥ grade 3 hematological AE was thrombocytopenia (2/11, 18.2%), the most common ≥ grade 3 non-hematological AE was hypertriglyceridemia (3/11, 27.3%). The majority of AE recovered or remained stable after treatment suspension.
Table 2.
TEAE(≥ 20%) in the SS
| TEAEs, n (%) | 40 mg BID (n = 4) | 60 mg BID (n = 3) | 80 mg BID (n = 4) | Total (n = 11) | ||||
|---|---|---|---|---|---|---|---|---|
| Any grade | ≥ Grade 3 | Any grade | ≥ Grade 3 | Any grade | ≥ Grade 3 | Any grade | ≥ Grade 3 | |
| Any TEAEs | 4 (100) | 1 (25.0) | 3 (100) | 2 (66.7) | 4 (100) | 2 (50.0) | 11 (100) | 5 (45.5) |
| Hypertriglyceridemia | 1 (25.0) | 2 (66.7) | 1 (33.3) | 3 (75.0) | 2 (50.0) | 6 (54.5) | 3 (27.3) | |
| Neutropenia | 1 (25.0) | 1 (33.3) | 3 (75.0) | 5 (45.5) | ||||
| Hypoalbuminemia | 2 (50.0) | 2 (66.7) | 1 (25.0) | 5 (45.5) | ||||
| Hypoproteinemia | 1 (25.0) | 2 (66.7) | 2 (50.0) | 5 (45.5) | ||||
| Thrombocytopenia | 2 (50.0) | 1 (25.0) | 2 (66.7) | 1 (33.3) | 1 (25.0) | 5 (45.5) | 2 (18.2) | |
| Abnormal T-wave on electrocardiogram | 0 | 1 (33.3) | 3 (75.0) | 4 (36.4) | ||||
| Leukopenia | 0 | 1 (33.3) | 3 (75.0) | 4 (36.4) | ||||
| Diarrhea | 0 | 1 (33.3) | 3 (75.0) | 4 (36.4) | ||||
| Fatigue | 1 (25.0) | 1 (33.3) | 2 (50.0) | 4 (36.4) | ||||
| Anemia | 1 (25.0) | 1 (33.3) | 2 (50.0) | 4 (36.4) | ||||
| Elevated α-hydroxybutyrate dehydrogenase | 1 (25.0) | 1 (33.3) | 1 (25.0) | 3 (27.3) | ||||
| Elevated low-density lipoprotein | 1 (25.0) | 2 (66.7) | 0 | 3 (27.3) | ||||
| Urinary white blood cell positivity | 1 (25.0) | 1 (33.3) | 1 (25.0) | 3 (27.3) | ||||
| Abnormal ST segment on electrocardiogram | 0 | 1 (33.3) | 2 (50.0) | 3 (27.3) | ||||
| Nausea | 0 | 1 (33.3) | 2 (50.0) | 3 (27.3) | ||||
| Lymphopenia | 0 | 1 (33.3) | 2 (50.0) | 3 (27.3) | ||||
| Hyperuricemia | 1 (25.0) | 1 (33.3) | 1 (25.0) | 3 (27.3) | ||||
| Hypercholesterolemia | 1 (25.0) | 1 (33.3) | 1 (25.0) | 3 (27.3) | ||||
| Hyperglycemia | 1 (25.0) | 0 | 2 (50.0) | 1 (25.0) | 3 (27.3) | 1 (9.1) | ||
Pharmacokinetics
Abexinostat single dose administration on D-3
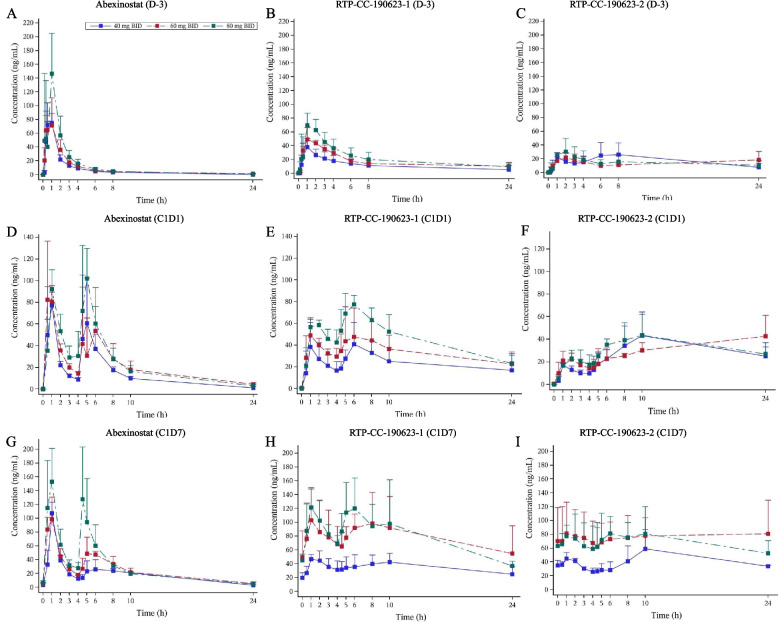
The median time to maximum concentration (Tmax) was 0.5–1.0 h, the median terminal elimination half-life (T1/2) was 2.56–8.31 h in single dose of abexinostat administration on D-3. RTP-CC-190123-1 (PCI-27789/S 78730) and RTP-CC-190123-2 (PCI-27887/S 78731) are the two main metabolites of abexinostat [24]. For RTP-CC-190123-1, the median Tmax was 1.0–1.49 h, the median T1/2 was 5.86–9.82 h. For RTP-CC-190123-2, the median Tmax was 1.0–6.97 h, the median T1/2 could not be calculated. PK of abexinostat prototype, RTP-CC-190623-1 and RTP-CC-190623-2 are presented in Table 3. The plasma concentration changes of abexinostat, RTP-CC-190623-1 and RTP-CC-190623-2 over time under different dose levels are presented in Fig. 1A-C. In the dose range of 40–80 mg, the dose slope and 90% CI of the area under the concentration–time curve from time zero to time t (AUC0-t) for the prototype, RTP-CC-190123-1 and RTP-CC-190123-2 after natural logarithmic transformation were 0.86 [0.53, 1.19], 0.97 [0.35, 1.59], and −0.19 [−0.78, 0.40], respectively. The dose slope and 90% CI of maximum concentration (Cmax) for abexinostat prototype, RTP-CC-190123-1 and RTP-CC-190123-2 after natural logarithmic transformation were 0.64 [−0.02, 1.31], 1.10 [0.62, 1.58] and −0.01 [−0.75, 0.74], respectively. The 90% CI of the dose slope of AUC0-t and Cmax for abexinostat prototype and RTP-CC-190123-1 included 1. In the dose range of 40–80 mg BID, the exposure to abexinostat, RTP-CC-190123-1 and RTP-CC-190123-2 increased in an approximately dose-proportional manner.
Table 3.
Pharmacokinetics parameters of abexinostat prototype, two main metabolites RTP-CC-190623-1 and RTP-CC-190623-2
| AUC0-t (h·ng/mL), Mean ± SD | AUC0-∞ (ng·h/mL), Mean ± SD | Cmax (ng/mL), Mean ± SD | Tmax (h), Median | T1/2 (h), Median | MRTlast (h), Median | |
|---|---|---|---|---|---|---|
| Abexinostat single dose administration on D-3 | ||||||
| Abexinostat prototype | ||||||
| 40 mg (n = 4) | 159 ± 12.1 | 171 ± 12.7 | 94.9 ± 11.4 | 0.500 | 2.56 | 2.80 |
| 60 mg (n = 3) | 225 ± 47.2 | 242 ± 41.0 | 89.8 ± 22.3 | 1.00 | 8.31 | 6.97 |
| 80 mg (n = 4) | 294 ± 76.5 | 309 ± 76.3 | 164 ± 60.7 | 0.990 | 4.81 | 3.47 |
| RTP-CC-190623-1 | ||||||
| 40 mg (n = 4) | 286 ± 119 | 268 ± 104 | 38.0 ± 12.4 | 1.00 | 7.75 | 9.92 |
| 60 mg (n = 3) | 422 ± 108 | 426 ± NC | 53.1 ± 10.7 | 1.00 | 9.82 | 11.6 |
| 80 mg (n = 4) | 533 ± 163 | 462 ± NC | 78.6 ± 10.8 | 1.49 | 5.86 | 6.74 |
| RTP-CC-190123-2 | ||||||
| 40 mg (n = 4) | 421 ± 175 | 33.1 ± 12.5 | 6.97 | |||
| 60 mg (n = 3) | 352 ± 83.4 | 26.0 ± 7.35 | 2.00 | |||
| 80 mg (n = 4) | 360 ± 92.1 | 373 ± NC | 34.6 ± 15.4 | 1.00 | 7.03 | 8.68 |
| Abexinostat continuous administration on C1D1 | ||||||
| 40 mg (n = 4) | 339 ± 90.9 | 361 ± 88.3 | 98.1 ± 27.6 | 1.00 | 3.69 | |
| 60 mg (n = 3) | 521 ± 60.6 | 563 ± 69.6 | 115 ± 24.2 | 4.55# | 6.28 | |
| 80 mg (n = 4) | 603 ± 104 | 625 ± 116 | 128 ± 25.3 | 4.78& | 5.13 | |
| RTP-CC-190623-1 | ||||||
| 40 mg (n = 4) | 523 ± 292 | 560 ± NC | 44.5 ± 18.0 | 5.59 | 7.07 | |
| 60 mg (n = 3) | 803 ± 186 | 902 ± NC | 68.3 ± 9.23 | 5.05 | 9.41 | |
| 80 mg (n = 4) | 1090 ± 229 | 1390 ± NC | 80.3 ± 10.5 | 6.02 | 7.68 | |
| RTP-CC-190123-2 | ||||||
| 40 mg (n = 4) | 586 ± 344 | 44.7 ± 19.3 | 10.0 | |||
| 60 mg (n = 3) | 718 ± 168 | 46.1 ± 13.3 | 23.9 | |||
| 80 mg (n = 4) | 766 ± 144 | 44.7 ± 17.7 | 6.03 | |||
| Abexinostat continuous administration on C1D7 | ||||||
| 40 mg (n = 4) | 463 ± 32.5 | 491 ± 43.7 | 107 ± 16.3 | 1.00 | 4.84 | |
| 60 mg (n = 3) | 600 ± 53.8 | 656 ± 72.0 | 104 ± 26.9 | 0.480 | 6.53 | |
| 80 mg (n = 4) | 767 ± 140 | 744 ± 132 | 184 ± 27.0 | 2.74 | 5.03 | |
| RTP-CC-190623-1 | ||||||
| 40 mg (n = 4) | 837 ± 263 | 50.6 ± 5.45 | 1.97 | |||
| 60 mg (n = 3) | 1870 ± 874 | 113 ± 41.7 | 0.980 | |||
| 80 mg (n = 4) | 1910 ± 604 | 151 ± 29.6 | 2.99 | |||
| RTP-CC-190123-2 | ||||||
| 40 mg (n = 4) | 1010 ± 287 | 62.6 ± 21.5 | 9.92 | |||
| 60 mg (n = 3) | 1820 ± 668 | 98.3 ± 42.7 | 23.9 | |||
| 80 mg (n = 4) | 1650 ± 503 | 105 ± 21.0 | 6.02 | |||
Missing data indicates that there were no patient pharmacokinetics parameters available to calculate the corresponding data
AUC0-t Area under the concentration–time curve from time zero to time t, AUC0-∞ area under the concentration–time curve from time zero to infinite time, Cmax maximum concentration, Tmax time to peak drug concentration, T1/2 elimilation half-life, MRTlast residence time from the time of dosing to the time of the last measurable concentration, SD standard deviation
# 0.5 h after second dose of abexinostat; & 0.97 h after second dose of abexinostat
Fig. 1.
Mean concentration–time curves for abexinostat (A), RTP-CC-190123-1 (B), and RTP-CC-190123-2 (C) on D-3 after a single dose of 40 mg, 60 mg, and 80 mg; abexinostat (D), RTP-CC-190123-1 (E), and RTP-CC-190123-2 (F) on C1D1 after continuous dose at 40 mg, 60 mg, and 80 mg; abexinostat (G), RTP-CC-190123-1 (H), and RTP-CC-190123-2 (I) on C1D7 after continuous dose at 40 mg, 60 mg, and 80 mg. The data was Mean ± SD. SD: standard deviation
Abexinostat continuous administration on C1D1
After being administered twice daily, the peak concentration was 0.55–1.0 h of abexinostat prototype. The PK of abexinostat prototype, RTP-CC-190623-1 and RTP-CC-190623-2 are presented in Table 3. The plasma concentration changes of abexinostat, RTP-CC-190623-1 and RTP-CC-190623-2 over time under different dose levels are presented in Fig. 1D-F. Within the dose range of 40–80 mg BID, the dose slope and 90% CI of AUC0-t for the prototype, RTP-CC-190123-1, and RTP-CC-190123-2 after natural logarithmic transformation were 0.87 [0.428 1.27], 1.31 [0.41, 2.20], and 0.62 [−0.28, 1.51], respectively.
Abexinostat continuous administration on C1D7
After being administrated twice daily for 7 consecutive days, the peak concentration was 0.48–2.74 h of abexinostat prototype. The PK of abexinostat prototype, RTP-CC-190623-1 and RTP-CC-190623-2 are presented in Table 3. The plasma concentration changes of abexinostat, RTP-CC-190623-1 and RTP-CC-190623-2 over time under different dose levels are presented in Fig. 1G-I. Within the dose range of 40–80 mg BID, the dose slope and 90% CI of AUC0-t for the abexinostat prototype, RTP-CC-190123-1, and RTP-CC-190123-2 after natural logarithmic transformation were 0.78 [0.26, 1.30], 1.56 [1.07, 2.06] and 0.81 [0.01, 1.62], respectively. Compared to the PK characteristics of the C1D1 of abexinostat continuous administration, the prototype, RTP-CC-190123-1 and RTP-CC-190123-2 at 40–80 mg BID of abexinostat had average accumulation ratios for the AUC (RA-AUC) of 1.16–1.52, 1.28–2.45, and 1.47–2.33, respectively, and average accumulation ratios for Cmax (RA-Cmax) of 0.94–1.48, 1.04–1.90, and 1.37–2.72, respectively.
Efficacy
At the data cutoff date on November 30, 2023, among 10 patients were included in the FAS, one patient with DLBCL achieved complete response (CR). Three patients with FL achieved partial response (PR, Figs. 2A-C, Table S1). The ORR was 40.0% (4/10, 95% CI: 12.2–73.8) and the disease control rate (DCR) was 60% (6/10) in the FAS. The ORR was 50% (3/6, 95% CI: 11.8–88.2) and the DCR was 83.3% (5/6) in patients with FL. The median progression-free survival (PFS) was 5.01 months (95% CI: 0.99–12.02) in the FAS. The duration of response (DoR) was not reached (95%CI:7.33-NE). The 12-month and 30-month PFS rate were 30.0% (95% CI:7.1–57.8) and 20.0% (95% CI: 3.1–47.5), respectively. The median PFS and DoR of patients with FL were 8.38 months (95% CI: 1.05-NE) and 7.82 months (95% CI: 7.33-NE), respectively. The median overall survival (OS) was not reached (95%CI:8.08-NE), and the 39-month OS rate was 64.8% (95% CI: 25.3–87.2) (Fig. 2C). Seven patients are still alive.
Fig. 2.
Waterfall plot of best percentage change from baseline in total sum of target lesion diameters in the FAS (A). Spider plot of percentage change from baseline in total sum of target lesion diameters in the FAS (B). Swimmer plot of patient in the FAS (C). All dose groups were given abexinostat treatment BID. FAS: full analysis set; SPD: sum of the product of the perpendicular diameters for multiple lesions; PR: partial response; SD: stable disease; PD: progressive disease; BID: bis in die
Discussion
This phase 1 study demonstrates that abexinostat is well-tolerated and has encouraging efficacy in Chinese patients with r/r B cell NHL. No DLT and no unexpected safety concerns were observed in this study. The 80 mg BID was RP2D, consistent with previous studies [22, 23]. The most common ≥ grade 3 TEAEs were hypertriglyceridemia (3/11, 27.3%), which did not cause dose reduction or treatment discontinuation, and thrombocytopenia (2/11, 27.3%), which caused dose interruption, but not treatment discontinuation. The thrombocytopenia was also the most common ≥ grade 3 AEs in both in PCYC-0401 and PCYC-0403 studies [22, 23]. The hypertriglyceridemia was a frequent AE in the present study, but was not observed in the PCYC-0403 and PCYC-1401 studies [22, 23]. Thrombocytopenia is a common AE associated with HDACis, which involves impaired platelet production by megakaryocytes, suggested that abexinostat shares the same mechanism as other HDACis [25]. Patients who experienced hypertriglyceridemia in this study had a history of hypertriglyceridemia, further studies are wanted.
Abexinostat was rapidly absorbed, with a median T1/2 of the first administration of 2.56–8.31 h. The exposure of abexinostat, its two main metabolites RTP-CC-190623-1 and RTP-CC-190623-2 increased in an approximately dose-proportional manner. Abexinostat, its metabolites RTP-CC-190623-1 and RTP-CC-190623-2 showed slight accumulation on C1D7. Abexinostat was given on a “7 days on, 7 days off” schedule in the PCYC-0403 study [22], “14 days on, 7 days off” schedule in the PCYC-1401 study [23]. In this study, PK parameters has demonstrated that the “one week on, one week off” schedule for abexinostat was reasonable in Chinese patients with r/r B cell NHL.
Abexinostat has shown favorable treatment efficacy in B cell NHL, particularly in r/r FL patients [22, 23]. In the PCYC-0403 study, abexinostat demonstrated an ORR of 64.3% (9/14) in r/r FL patients, including 1 CR and 8 PR. The DCR was 85.7% (12/14). A median PFS was 20.5 months. For mantle cell lymphoma, the ORR was 27.3% (3/11), median PFS was 3.9 months [22]. In the PCYC-0401 study, all patients with r/r B cell NHL or chronic lymphocytic leukemia received abexinostat treatment, the ORR in patients with FL and DLBCL were 56% (9/16) and 31% (5/16), respectively. The Median PFS was 10.2 months (95%CI: 2.8-NA) for FL, 2.8 months (95%CI: 1.4–3.7) for DLBCL. The median OS was not reached for FL (95%CI: 18.4-NA), 10.2 months (95%CI: 4.4-NA) for DLBCL [23]. In the FAS of this study, the ORR was 50% (3/6) and DCR was 83.3% (5/6) in FL patients, abexinostat demonstrated primary efficacy in this patients’ population. For r/r DLBCL patients in this study, the ORR and DCR were both 25% (1/4). The patient achieved CR is still on the treatment (40 mg BID) with PFS over 30 months. The median PFS in the FAS was 5.01 months (95%CI: 0.99–12.02). This discrepancy with previous studies may be attributed to the small sample size of this phase 1 study. The pivotal phase 2 study of abexinostat in Chinese patients with r/r FL (NCT03934567) is completed with 90 patients enrolled, and we look forward to the results of that study.
Abexinostat combination therapy could also serve as a potential option for patients with r/r solid tumors. Abexinostat combined with pazopanib had ORR of 21% (9/43) for all advanced solid tumor. The ORR of renal cell carcinoma (RCC) patients was 27% (6/22). The DoR was 9.1 months, with manageable safety profile [26]. In follow-up of above study, one patient with RCC remains on treatment for > 11 years. The median duration of treatment in exceptional responders measured 44.1 (39.8–133 +) months [27]. Abexinostat combined with doxorubicin demonstrated modest anti-tumor activity in patients with metastatic sarcoma, 1 PR and 9 SD among 17 evaluable patients [28].
Under the same treatment landscape, several PI3K inhibitors have previously been approved by the U.S. Food and Drug Administration (FDA) including idelalisib, copanlisib, and duvelisib for the treatment of r/r FL. However, the U.S. FDA recently concluded that PI3K inhibitors fail to demonstrate survival benefits in this patient population, with an additional concern regarding their safety profile [29]. As a result, the companies withdrew their approved indications. Further study will provide the evident whether abexinostat could provide survival benefit of r/r FL.
Abexinostat not only demonstrated its anti-tumor effect as a single agent but also showed potential for combination with other agents. For example, chimeric antigen receptor T cell therapy is being investigated for FL patients and has demonstrated promising efficacy [30, 31]. However, certain scholars recommended that this treatment modality should be reserved for patients with a pronounced medical necessity, in order to justify the cost and treatment-related toxicity [30]. As an epigenetic agent, HDACis can modulate T cell function [31]. Several studies have shown that HDACis can improve the expression of CD20 in B cell lymphoma [32, 33]. Furthermore, in other studies, abexinostat showed positive treatment efficacy when combined with anti-vascular endothelial growth factor tyrosine kinase inhibitor therapy [26], Bruton tyrosine kinase inhibitor therapy (NCT03939182), radiotherapy [34], and chemotherapies [34–37].
This study was limited by the relatively small patient number, restricting the ability to perform detailed subgroup analyses. Future studies should include a larger population of Chinese B cell NHL patients to validate the therapeutic efficacy and safety of abexinostat.
Conclusion
This study demonstrated that abexinostat has favorable tolerability with no DLT. The RP2D was 80 mg BID. The plasma concentration was dose-proportional manner. The PK parameters demonstrated that BID “one week on, one week off” administration is reasonable. Promising anti-tumor activity were seen in Chinese patients with r/r B cell NHL. This result support further investigation for this patients’ population.
Supplementary Information
Supplementary Material 1. Figure S1. Study flow chart. This patient withdrawn from the study due to unexplained sudden death before treatment initiation.# This patient voluntarily withdrew from the study on C1D4 due to personal reason. Therefore, didn’t undergo efficacy evaluation. FAS: full analysis set; SS: safety set; PKS: pharmacokinetics set; C: cycle; D: day.
Acknowledgements
The authors thank the patients, their families, the investigators, and the teams who participated in this study.
Abbreviations
- AEs
Adverse events
- AUC0-t
Area under the concentration–time curve from time zero to time t
- AUC0-∞
Area under the concentration–time curve from time zero to infinite time
- BID
Bis in die
- CI
Confidence interval
- Cmax
Maximum concentration
- CR
Complete response
- DCR
Disease control rate
- DLBCL
Diffuse large B cell lymphoma
- DLT
Dose limiting toxicity
- DoR
Duration of response
- FAS
Full analysis set
- FDA
Food and Drug Administration
- FL
Follicular lymphoma
- HDAC
Histone deacetylase
- HL
Hodgkin lymphoma
- HPLC–MS/MS
High-performance liquid chromatography coupled with tandem mass spectrometry
- MCL
Mantle cell lymphoma
- MRTlast
Residence time from the time of dosing to the time of the last measurable concentration
- NHL
Non-Hodgkin lymphoma
- ORR
Overall response rate
- OS
Overall survival
- PFS
Progression-free survival
- PD
Progressive disease
- PR
Partial response
- RP2D
Recommended phase 2 dose
- SD
Stable disease
- SPD
Sum of the product of the perpendicular diameters for multiple lesions
- SS
Safety set
- TEAEs
Treatment emergent adverse events
- Tmax
Time to peak drug concentration
- T1/2
Elimilation half-life
- ULN
Upper limit of normal
Authors’ contributions
YS is the principal investigator of this study, contributed to the design, conceived, supervising, patients recruitment and management, data analysis of the study, writing, revising and editing of the manuscript. LG contributed to study design, patients recruitment and management, data collection and analysis, manuscript writing, revising and editing. RT contributed to data analysis, manuscript writing, revising and editing. ZX and XC Chen contributed to patients recruitment and mangement, data collection, data analysis, manuscript writing, revising and editing. All other authors contributed to patients recruitment and management, data collection and analysis.
Funding
The study was fully supported by Xynomic Pharmaceuticals (Nanjing) Co., Ltd, Nanjing, China.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committees of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (19–115/1899). The study was conducted in accordance with the Declaration of Helsinki, the principles of the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice Guidelines, and the local regulations. Written informed consent was obtained from all patients before enrollment. This study was registered at ClinicalTrials.gov (NCT04024696).
Consent for publication
Not applicable.
Competing interests
Ran Tao is an employee of Xynomic Pharmaceuticals (Nanjing) Co., Ltd. Other authors have no conflicts of interest to declare.
Other authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 3.Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–59. [DOI] [PubMed] [Google Scholar]
- 5.Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, Bi CF, Zuo Z, Wang XQ, Huang J, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XM, Bassig BA, Wen JJ, Li GD, Liu ZB, Yao WX, Hu W, Wang Y, Li JM, Wang XD, et al. Clinical analysis of 1629 newly diagnosed malignant lymphomas in current residents of Sichuan province. China Hematol Oncol. 2016;34(4):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luminari S, Ferrari A, Manni M, Dondi A, Chiarenza A, Merli F, Rusconi C, Tarantino V, Tucci A, Vitolo U, et al. Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the Initial Treatment of Patients With Advanced-Stage Symptomatic Follicular Lymphoma. J Clin Oncol. 2018;36(7):689–96. [DOI] [PubMed] [Google Scholar]
- 8.Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, Fruchart C, Libby EN, Casasnovas RO, Flinn IW, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med. 2018;379(10):934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377(14):1331–44. [DOI] [PubMed] [Google Scholar]
- 11.Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Qin Y, He X, Liu P, Yang J, Zhou L, Zhou S, Gui L, Yang S, Zhang C, et al. Long-term survival and prognostic analysis of advanced stage follicular lymphoma in the rituximab era: A China single-center retrospective study. Asia Pac J Clin Oncol. 2021;17(3):289–99. [DOI] [PubMed] [Google Scholar]
- 13.Casulo C, Nastoupil L, Fowler NH, Friedberg JW, Flowers CR. Unmet needs in the first-line treatment of follicular lymphoma. Ann Oncol. 2017;28(9):2094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Link BK, Day BM, Zhou X, Zelenetz AD, Dawson KL, Cerhan JR, Flowers CR, Friedberg JW. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019;184(4):660–3. [DOI] [PubMed] [Google Scholar]
- 15.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. [DOI] [PubMed] [Google Scholar]
- 16.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–15. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766–71. [DOI] [PubMed] [Google Scholar]
- 19.Rai S, Kim WS, Ando K, Choi I, Izutsu K, Tsukamoto N, Yokoyama M, Tsukasaki K, Kuroda J, Ando J, et al. Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results. Haematologica. 2023;108(3):811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogura M, Ando K, Suzuki T, Ishizawa K, Oh SY, Itoh K, Yamamoto K, Au WY, Tien HF, Matsuno Y, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2014;165(6):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puvvada SD, Li H, Rimsza LM, Bernstein SH, Fisher RI, LeBlanc M, Schmelz M, Glinsmann-Gibson B, Miller TP, Maddox AM, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk Lymphoma. 2016;57(10):2359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evens AM, Balasubramanian S, Vose JM, Harb W, Gordon LI, Langdon R, Sprague J, Sirisawad M, Mani C, Yue J, et al. A Phase I/II Multicenter, Open-Label Study of the Oral Histone Deacetylase Inhibitor Abexinostat in Relapsed/Refractory Lymphoma. Clin Cancer Res. 2016;22(5):1059–66. [DOI] [PubMed] [Google Scholar]
- 23.Ribrag V, Kim WS, Bouabdallah R, Lim ST, Coiffier B, Illes A, Lemieux B, Dyer MJS, Offner F, Felloussi Z, et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica. 2017;102(5):903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buggy JJ, Cao ZA, Bass KE, Verner E, Balasubramanian S, Liu L, Schultz BE, Young PR, Dalrymple SA. CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther. 2006;5(5):1309–17. [DOI] [PubMed] [Google Scholar]
- 25.Bishton MJ, Harrison SJ, Martin BP, McLaughlin N, James C, Josefsson EC, Henley KJ, Kile BT, Prince HM, Johnstone RW. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood. 2011;117(13):3658–68. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal R, Thomas S, Pawlowska N, Bartelink I, Grabowsky J, Jahan T, Cripps A, Harb A, Leng J, Reinert A, et al. Inhibiting Histone Deacetylase as a Means to Reverse Resistance to Angiogenesis Inhibitors: Phase I Study of Abexinostat Plus Pazopanib in Advanced Solid Tumor Malignancies. J Clin Oncol. 2017;35(11):1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang ES, Aggarwal RR, Bergsland EK, Calabrese S, Rozie A, Chaudhuri S, Dhawan MS, Pawlowska N, Grabowsky J, Thomas S, et al. Updated Survival Follow-Up for Phase Ib Trial of the Histone Deacetylase Inhibitor Abexinostat With Pazopanib in Patients With Solid Tumor Malignancies. JCO Precis Oncol. 2024;8: e2400328. [DOI] [PubMed] [Google Scholar]
- 28.Choy E, Flamand Y, Balasubramanian S, Butrynski JE, Harmon DC, George S, Cote GM, Wagner AJ, Morgan JA, Sirisawad M, et al. Phase 1 study of oral abexinostat, a histone deacetylase inhibitor, in combination with doxorubicin in patients with metastatic sarcoma. Cancer. 2015;121(8):1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oncologic Drugs Advisory Committee Meeting: Phosphatidylinositol 3-Kinase (PI3K) Inhibitors in Hematologic Malignancies. April 21, 2022:Available online: https://fda.report/media/157762/ODAC-20220421-Backgrounder-FDA.pdf.
- 30.Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, López-Guillermo A, Fitzgibbon J. Follicular lymphoma. Nat Rev Dis Primers. 2019;5(1):83. [DOI] [PubMed] [Google Scholar]
- 31.Akbari B, Ghahri-Saremi N, Soltantoyeh T, Hadjati J, Ghassemi S, Mirzaei HR. Epigenetic strategies to boost CAR T cell therapy. Mol Ther. 2021;29(9):2640–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu R, Kikuchi J, Wada T, Ozawa K, Kano Y, Furukawa Y. HDAC inhibitors augment cytotoxic activity of rituximab by upregulating CD20 expression on lymphoma cells. Leukemia. 2010;24(10):1760–8. [DOI] [PubMed] [Google Scholar]
- 33.Bobrowicz M, Dwojak M, Pyrzynska B, Stachura J, Muchowicz A, Berthel E, Dalla-Venezia N, Kozikowski M, Siernicka M, Miazek N, et al. HDAC6 inhibition upregulates CD20 levels and increases the efficacy of anti-CD20 monoclonal antibodies. Blood. 2017;130(14):1628–38. [DOI] [PubMed] [Google Scholar]
- 34.Deutsch E, Moyal EC, Gregorc V, Zucali PA, Menard J, Soria JC, Kloos I, Hsu J, Luan Y, Liu E, et al. A phase 1 dose-escalation study of the oral histone deacetylase inhibitor abexinostat in combination with standard hypofractionated radiotherapy in advanced solid tumors. Oncotarget. 2017;8(34):56199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He WL, Li YH, Hou WJ, Ke ZF, Chen XL, Lu LY, Cai SR, Song W, Zhang CH, He YL. RAD51 potentiates synergistic effects of chemotherapy with PCI-24781 and cis-diamminedichloroplatinum on gastric cancer. World J Gastroenterol. 2014;20(29):10094–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X, Mankin H, Duan Z. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother Pharmacol. 2011;67(2):439–46. [DOI] [PubMed] [Google Scholar]
- 37.Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, Zhu QS, Bornmann WG, McConkey DJ, Pollock RE, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15(10):3472–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Figure S1. Study flow chart. This patient withdrawn from the study due to unexplained sudden death before treatment initiation.# This patient voluntarily withdrew from the study on C1D4 due to personal reason. Therefore, didn’t undergo efficacy evaluation. FAS: full analysis set; SS: safety set; PKS: pharmacokinetics set; C: cycle; D: day.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.