Abstract
Background
Dietary patterns play a crucial role in osteoporosis prevention and management. Patients with osteoporosis need to select a dietary pattern for prevention. This meta-analysis aims to examine the influence of eight distinct dietary patterns on the risk of osteoporosis, including dietary inflammatory index (DII), Western/unhealthful dietary pattern, dietary approaches to stop hypertension (DASH), prudent/healthful dietary pattern, aquatic dietary pattern, plant-based diet index (PDI), healthful PDI, and unhealthful PDI.
Methods
Embase, Web of Science, PubMed, and Cochrane Library were systematically searched for observational studies up to April 10, 2025. Meta-analysis was conducted using random-effect models. Heterogeneity was evaluated by subgroup analyses and publication bias was assessed by Egger's test. If there was a risk of bias, the sensitivity analysis and trim-and-fill analysis were conducted. The odds ratios (ORs) and 95% confidence intervals (CIs) were combined to compare the lowest and highest dietary pattern categories.
Results
A total of 2,620 studies were retrieved, among which 2,600 were excluded. 20 observational studies, involving 8 dietary patterns were included, with 426,292 participants. The highest DII (OR: 1.82; 95% CI: 1.39, 2.37; P < 0.001) and the high adherence of unhealthful PDI (OR: 1.37; 95% CI: 1.11, 1.68; P = 0.003) were correlated with an increased risk of osteoporosis. Conversely, the highest category of the prudent/healthful dietary pattern (OR: 0.66; 95% CI: 0.53, 0.83; P < 0.001) presented a low osteoporosis risk. The Western/unhealthful dietary pattern, DASH, aquatic dietary pattern, and high adherence to PDI and healthy PDI dietary patterns were not associated with osteoporosis risk (All P > 0.05).
Conclusion
High DII or unhealthy PDI scores were associated with an increased risk of osteoporosis, while high adherence to prudent/healthy dietary patterns reduced the risk of osteoporosis.
Trial registration
This paper was registered with PROSPERO (CRD42024585588).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-025-05896-9.
Keywords: Bone mineral density, Dietary patterns, Meta-analysis, Osteoporosis
Introduction
Osteoporosis is characterized by the decline of bone mineral density (BMD) and bone mass, along with enhanced fragility of bones, thereby resulting in an elevated risk of fractures [1]. As a result of the growing aging population, the incidence of osteoporosis is rising rapidly. Osteoporotic fractures can lead to severe disabilities, reduce the quality of life, cause considerable medical costs, increase the risks of hospitalization and death, and impose a considerable burden on individuals, families, society, and the healthcare system [2]. Consequently, it has currently become a significant global public health issue [3]. The global prevalence of osteoporosis has amounted to 19.7% in 2022 [4]. The primary complication of osteoporosis is fragility fractures, among which hip and vertebral compression fractures are the most serious. It is estimated that by 2050, the global number of hip fractures will be roughly double the figure recorded in 2018. In the United Kingdom, the all-cause mortality rate following a hip fracture reaches 28.3% within one year. Due to the lower proportion of men using anti-osteoporosis drugs than women, men have a higher rate of all-cause mortality than women [5]. It is essential to implement new preventive measures to counteract these trends.
Osteoporosis is affected by diverse factors, including non-modifiable and modifiable factors that impair bone mass and health [6]. Non-modifiable factors encompass age, sex, hormonal profile, ethnicity, and genetics, while modifiable factors include dietary factors and lifestyles, such as smoking, alcohol consumption, and physical activity [6, 7]. Diet is essential in addressing osteoporosis, acting as a safe and modifiable risk factor. An adequate intake of vitamin D, calcium, and protein in the diet can improve bone quality [8, 9]. Nutrients in the daily diet, such as proteins, minerals, peptides, unsaturated fatty acids, phytoestrogens, and prebiotics, can modulate bone metabolism and relieve bone loss [6]. A diet rich in fruits and vegetables is beneficial to bone health by preventing oxidative stress and inflammation [10]. Therefore, healthy and balanced nutrition is imperative in the prevention and pathogenesis of osteoporosis.
Nonetheless, the impact of individual foods and nutrients is controversial, as it overlooks the close correlation among various nutrients. Many nutrients interact with one another, thereby affecting their bioavailability and absorption [11]. Therefore, an analysis focused solely on individual nutrients and foods cannot fully explain the interactions or changes of multiple nutrients and food components when consumed together [12]. For example, adequate calcium intake is crucial for preventing bone loss, but results on the association between dairy consumption and osteoporotic fractures are conflicting [13]. To figure out the synergistic and cumulative effects of the overall diet and address the confusion caused by other dietary factors, dietary pattern analysis is employed to ascertain these associations [14]. The findings from such analyses can guide dietary patterns to prevent chronic diseases [15].
Different epidemiological studies reveal inconsistent associations between various dietary patterns and osteoporosis risk. For example, a study from Australia found that there was no association between a high dietary inflammatory index (DII) score and BMD in old adults [16]. However, a meta-analysis discovered that a diet containing high pro-inflammatory components reduced BMD in the lumbar spine and total hip joint and increased the risk of osteoporosis. Nevertheless, this meta-analysis included a relatively small number of articles, and all of them utilized the food frequency questionnaire for dietary assessment [17]. The Mediterranean dietary pattern is associated with an increase in bone density [18]. Nevertheless, most studies on the Mediterranean diet pattern have been carried out in the Mediterranean region.
Current research indicates that a healthy dietary pattern is associated with a reduced risk of fractures, while a Western dietary pattern is not related to BMD or fractures. There is no meta-analysis on the associations of plant-based dietary patterns, dietary approaches to stop hypertension (DASH), and aquatic dietary patterns with the risk of osteoporosis [19]. Meta-analysis is capable of systematically summarizing the research outcomes of numerous articles, encompassing a wider population, and minimizing the impact of confounding factors, thereby precisely and objectively evaluating the effect size and compensating for the limitations of randomized controlled trials [20]. Therefore, this meta-analysis aims to collect the existing data and synthesize the evidence about the association between various dietary patterns and osteoporosis risk. Our study included recent research, involved other dietary assessment approaches, and performed subgroup analyses on regions and dietary assessment methods.
Methods
This paper was registered with PROSPERO (CRD42024585588) and followed the PRISMA guidelines [21].
Search strategy
Two researchers independently searched for studies in Embase, Web of Science, PubMed, and Cochrane Library until April 10, 2025, without language restrictions. The following search strategy (Table S1) was used: ("bone mineral density"or"osteoporosis"or"osteopenia") and ("dietary pattern"or"eating pattern"or"food pattern"). The bibliographic lists of all relevant studies were manually retrieved, and their accuracy was checked.
Eligibility criteria
Studies were enrolled based on the following criteria:
Population: adults ≥ 18 years;
Intervention/Exposure: dietary patterns;
Comparison: people with different dietary patterns who had the lower scores and the lower adherence were taken as the reference group;
Outcomes: osteoporosis;
Study design: observational studies, such as cohort, case–control, or cross-sectional studies.
Exclusion criteria
not reporting comprehensive data on effect sizes (hazard ratio [HR], relative risk [RR], or odds ratio [OR]), 95% confidence intervals (CIs), total cases, or total population;
Exposure was individual or food groups, not dietary patterns;
Animal studies, in vitro studies, and randomized controlled trials;
Systematic review or meta-analysis or umbrella review;
Case reports, conference papers, mendelian randomization studies, etc.
Data extraction
Two authors independently extracted and documented data, including first author, publication year, study design, country, population characteristics, BMD, osteoporosis site, dietary pattern, assessment measures, sample size, OR and corresponding 95%CI, and adjusted or matched variables. Any differences were tackled by reaching a consensus or via discussion with a third author.
Quality assessment
The Newcastle Ottawa Scale was leveraged to appraise the quality of case–control and cohort studies in 8 dimensions of 3 key areas (subject selection, comparability, and exposure). Five scores implied low quality, 6 or 7 scores indicated medium quality, and 8 or 9 scores suggested high quality [22]. Cross-sectional studies were appraised with the Joanna Briggs Institute Critical Assessment Checklist through sampling methods, size, diagnosis, measurement, and analysis [23]. Any discrepancies were tackled via discussion with a third author.
Data analyses
Osteoporosis data were integrated from diverse populations or disparate body sites to analyze the association between dietary patterns and osteoporosis risk of any site. In this meta-analysis, RR was regarded as equivalent to OR. Meta-analysis was performed using the Stata SE 15.0. P < 0.05 implied statistical significance. Firstly, OR and 95%CI were determined to calculate the effect size. I2 statistic was used to test heterogeneity. All of our analyses were conducted using random-effects models [24]. Secondly, Begg's and Egger's tests were employed to quantify publication bias [25, 26]. If publication bias was present, a trim-and-fill analysis was conducted. Sensitivity analysis was performed by excluding each study to assess the robustness of the results. Finally, considering the pronounced heterogeneity, subgroup analysis based on the geographical location of osteoporosis was conducted to ascertain the source of heterogeneity.
Results
Literature search and data sources
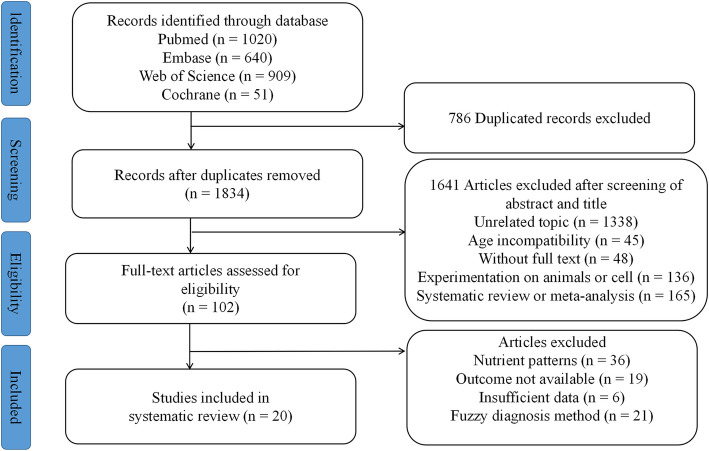
The screening process is displayed in Fig. 1. A total of 2620 studies were retrieved in PubMed (n = 1020), Web of Science (n = 909), Embase (n = 640), and Cochrane (n = 51). It is necessary to contact the author if the full text is not available. After duplicates and irrelevant articles were removed, a comprehensive assessment was conducted on 102 full-text studies. Ultimately, 20 studies were included [27–46].
Fig. 1.
Flow chart of literature selection
Characteristics and quality of the included studies
Baseline traits and quality scores are shown in Table 1. All the studies included were published from 2012 to 2025 with 426,292 participants. The involved countries encompassed Iran [28, 39], the United States [27, 31, 32, 34, 37, 40], South Korea [42, 43, 45, 46], the United Kingdom [30], China [29, 33, 35, 38], New Zealand [41], Mexico [44], and Jordan [36]. Among these 20 studies, three were cohort studies [30, 43, 46], one case–control study [36], and sixteen cross-sectional studies [27–29, 31–35, 37–42, 44, 45]. All included studies obtained food intake information through the food frequency questionnaire or 24-h meal recall. Four cohort and case–control studies were of high quality (with scores ≥ 7). Sixteen cross-sectional studies had quality scores of 15 to 19. The studies were mainly based on priori or established dietary patterns.
Table 1.
General characteristics of the studies included in the meta-analysis on dietary patterns and osteoporosis
| Author, year | Country | Study design | Sample size (case) | Population characteristics | Dietary pattern | Diet assessmentmethod | Osteoprosisdefinition method | Compare | Odd ratio (95%CI) | Adjustied confounding factors | Study quality | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al. 2025 [27] | US | Cross-sectional study | 7290(243) | Average age 50.6 years adults | DII | 24-h dietary recall interviews | DXA scan:BMD T score ≤ − 2.5 | Q4 vs Q1 | 1.88 (1.41–2.52) | Age, race, sex, education level, marital status, ratio of family income to poverty level, smoking, BMI, Calcium, ALT, AST, uric acid, ALP, BUN, phosphorus, hypertension, hyperlipidemia | 16 | |||
| Moham-adisima et al. 2024 [28] | Iran | Cross-sectional study | 232 (108) | Postmenopaus-al women | DII | 168-item FFQ | DXA scan:BMD T score ≤ − 2.5 | DII (categoricl) | 4.04 (1.79,9.10) | Age,BMI, post-menopausal years, parity, education, total energy intake, physical activity | 18 | |||
| Zheng et al. 2024 [29] | China | Cross-Sectional Study | 788 (130) | 55–65 years adults | hPDI | 12-item FFQ | DXA scan:BMDT score ≤ − 2.5 | Q5 vs Q1 | 1.83 (0.81, 4.10) | Age,sex,WHR, physical exercise, smoking status, alcohol consumption, tea consumption, history of diabetes, history of hypertension, history of dyslipidemia | 17 | |||
| PDI | 1.45 (0.63, 3.32) | |||||||||||||
| uPDI | 2.91 (1.27, 6.64) | |||||||||||||
| Zheng et al. 2024 [30] | UK | cohort study | 202,063 (4841) | Average age56 years adults | hPDI | 24-h dietary questionnaire | DXA scan:BMDT score ≤ − 2.5 | Q5 vs Q1 | 1.16 (1.05, 1.28) | Age, sex, ethnicity, BMI, Townsend deprivation index, education, physical activity, smoking status, alcohol consumption, energy intake, history of diabetes, history of hypertension, history of hip or spine fracture | 9 | |||
| uPDI |
1.15 (1.05, 1.26) |
|||||||||||||
| Meng et al. 2023 [31] | US | Cross-sectional study | 526 (133) | Average age 63.25 years adults | DII | 45-item FFQ | DXA scan:BMD T score ≤ − 2.5 | Q4 vs Q3 | 2.09 (1.05,4.23) | Age, gender, race, BMI group, smoking, steroids use, calcium intake, phosphorus intake, serum calcium, serum phosphorus, total 25-hydroxyvitamin D, albumin, estrogen, anti-osteoporosis drugs, physical activity | 18 | |||
| Li et al. 2023 [32] | US |
Cross- sectional study |
10,312 (-) | Median age 50.0 years adults | DII | 45-item FFQ |
DXA scan: BMD T score ≤ − 2.5 |
T3 vs T1 | 1.94 (1.02,3.69) |
Age, gender, race/ethnicity, smoker, BMI, eGFR, UACR, serum C-reactive protein, WBC count, NLR, serum calcium, arthritis, aspirin use, calcitonin use, biphosphonate use, DMARDs use, calcium intake, estrogen use |
19 | |||
| Shen et al. 2023 [33] | China |
Cross- sectional study |
839 (194) | Aged 50 years and older adults | DASH |
65-item FFQ |
DXA scan: BMD T score ≤ − 2.5 |
T3 vs T1 |
Men 0.96 (0.91, 1.02) |
Age, BMI, height, hypertension, diabetes, age at menarche and menopause for women, smoking status, alcohol use, calcium supplement intake, vitamin D supplement intake, total energy intake, physical activity | 17 | |||
|
Women 0.96 (0.91, 1.02) | ||||||||||||||
| Zheng et al. 2023 [34] | US | Cross-sectional study | 16,085 (1073) | ≥ 20 years adults | PDI | NHANES 24-h recall interviews |
DXA scan: BMD T score ≤ − 2.5 |
Q5 vs Q1 |
1.01 (0.68, 1.51) |
Age, sex, ethnicity, education, marital status, PIR, BMI, smoking status, physical exercise, hypertension, T2DM, CKD, cancer, history of fracture | 16 | |||
| uPDI |
1.48 (1.04, 2.11) |
|||||||||||||
| Hu et al. 2023 [35] | China | Cross-sectional study | 9613 (1848) | Adults over 60 years | hPDI | Simplifed FFQ |
DXA scan: BMD T score ≤ − 2.5 |
Q4 vs Q1 |
0.64 (0.56, 0.74) |
Age, gender, BMI, SMI, educational level, income, physical activity level, history of smoking, history of drinking, disease history | 15 | |||
| PDI |
0.80 (0.69, 0.93) |
|||||||||||||
| uPDI |
1.42 (1.23, 1.65) |
|||||||||||||
| Tayyem et al. 2023 [36] | Jordan | Case–control study | 200 (100) | Postmenopausal women | Unhealthy/Western | Arabic FFQ | NR | Q4 vs Q1 |
0.72 (0.29, 1.81) |
Age, BMI, physical activity level, total energy intake, number of pregnancies and lactation, health problems, smoking, education level, marital status, history of osteoporosis | 7 | |||
| Zhao et al. 2022 [37] | US | Cross-sectional study | 2206 (204) | Aged ≥ 50 years adults | DII | 45-item FFQ |
DXA scan: BMD T score ≤ − 2.5 |
Q4 vs Q1 |
Men 0.89 (0.20, 4.03) |
Age, race, BMI, martial status, smoking status, calcium | 15 | |||
|
Women 6.17(2.96, 12.84) | ||||||||||||||
| Zhao et al. 2021 [38] | China | Cross-sectional study | 476 (104) | Middle-aged and aged people | Aquatic food | Simplified FFQ |
DXA scan: BMD T score ≤ − 2.5 |
Q4 vs Q1 |
0.74 (0.43, 1.26) |
Gender, age, smoking | 17 | |||
| Shahriar-pour et al. 2020 [39] | Iran | Cross-sectional study | 151 (46) | Postmenopausal women | DASH | 168-item FFQ | DXA scan: BMD T score ≤ − 2.5 | T3 vs T1 | Lumbar spine 0.28 (0.09, 0.88) | Age, BMI, physical activity, age at menarche, age at menopause, parity, duration of lactation, energy intake, sunlight exposure, smoking, supplement intake, education | 16 | |||
| Noel et al. 2020 [40] | US | Cross-sectional study | 751 (-) | 59.9 ± 7.6 years adults | DASH | Semi-quantitative FFQ | DXA scan: BMD T score ≤ − 2.5 | Q5vs Q1 | Men 0.71 (0.40, 1.29) | Age, BMI, height, smoking status, season of bone mineral density measurement, osteoporosis medication use, calcium intake, serum vitamin D status | 16 | |||
| Women 0.54 (0.39, 0.75) | ||||||||||||||
| Ilesanmi-Oyelere et al. 2020 [41] | New Zealand | Cross-sectional study | 125 (65) | Postmenopausal Women | Dessert, cheese, red meat | 108-item FFQ | DXA scan: BMD T score ≤ − 2.5 | NR | 0.71 (0.44, 1.14) | Age, BMI, activity energy expenditure | 17 | |||
| Oily fish, sports drink, and seafood-rich | 0.86 (0.46, 1.60) | |||||||||||||
| Na et al. 2019 [42] | Korea | Cross-sectional clinical study | 2778 (1040) | Postmenopausal Women | DII | 41-item FFQ | DXA scan: BMD T score ≤ − 2.5 | T3 vs T1 | 1.27 (1.00,1.62) |
Age, BMI, vitamiD household income, smoking habits, physical activity, calcium intake, postmenopausal Period female-hormone use |
19 | |||
| Kim et al. 2018 [43] | Korea | cohort study | 159,846 (2572) | 40–79 years adults | DII | 106-item FFQ | NR | Q5vs Q1 | 1.33 (1.12,1.58) | Age, BMI, smoke, calcium intake, alcohol consumption, physical activity, energy intake | 8 | |||
| Denova-Gutiérrez et al. 2016 [44] | Mexico | Cross-sectional study | 6915 (-) | Adults aged 20–80 years | Prudent dietary pattern | 116-item FFQ | DXA scan: BMD T score ≤ − 2.5 | Q5 vs Q1 | 0.83 (0.63, 1.07) | Age, gender, BMI, height, multivitamin use,smoking status, physical activity, energy intake, estrogen use, age of menarche, parity, menopause | 18 | |||
| Westernized dietary pattern |
1.74 (1.10, 2.76) |
|||||||||||||
| Shin et al. 2013 [45] | Korea | Cross-sectional study | 3735 (1959) | Postmenopausal Women | Dairy and fruit | Standardised questionnaire and a 24-h recall method |
DXA scan: BMD T score ≤ − 2.5 |
Q5 vs Q1 |
Femoral neck 0.80 (0.54, 1.19); Lumbar spine 0.47 (0.34, 0.65) |
Age, BMI,energy intake, parathyroid hormone serum 25-hydroxyvitamin D, smoking, alcohol intake, moderate physical activity, supplement use,oral contraceptive use | 15 | |||
| Meat, alcohol and sugar |
Femoral neck 0.89 (0.60, 1.31); Lumbar spine 0.78 (0.57, 1.07) |
|||||||||||||
| Park et al. 2012 [46] | Korea | Cohort study | 1464 (429) | Postmenopausal women | Dairy | 103-item FFQ |
Ultrasound bone densitometer, speed of sound (m/s) BMD: T score ≤ −2.5 |
Q5 vs Q1 |
Radius 0.63 (0.42, 0.93); Tibia 0.56 (0.35, 0.90) |
Age, residual area, exercise, passive smoking | 9 | |||
| Western |
Radius 0.46 (1.02, 2.10); Tibia 1.46 (0.91, 2.33) |
|||||||||||||
DII Dietary Inflammatory Index, FFQ food frenquency questionnaire, DASH dietary approaches to stop hypertension, BMD bone mineral density, BMI body mass index, PDI plant-based diet index, hPDI healthy plant-based diet index, uPDI unhealthy plant-based diet index, NHANEX National Health and Nutrition Examination Survey, T2DM type 2 diabetes mellitus, CKD, chronickidney disease, eGFR estimated glomerular filtration rate, UACR urine albumin-to-creatinine ratio, BUN blood urea nitrogen, WBC white blood cell, NLR neutrophil to lymphocyte ratio, DMARDs disease-modifying antirheumatic drugs, PIR poverty income ratio, WHR Waist-to-Hip Ratio, SMI skeletalmuscle mass index, NR not reported, CI confidence interval, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase
DII and osteoporosis risk
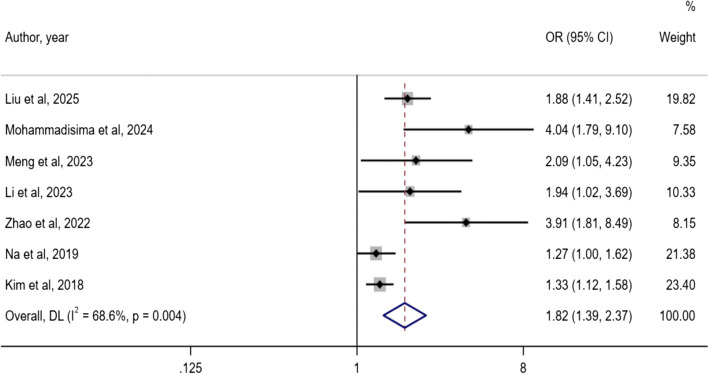
As shown in the forest plot in Fig. 2, seven studies concerned DII and osteoporosis risk [27, 28, 31, 32, 37, 42, 43]. Heterogeneity was present (I2 = 68.6%, P < 0.001), and a random-effects model was selected to combine the effect size. A dietary pattern with high DII was associated with an increased risk of osteoporosis. (OR: 1.82; 95% CI: 1.39, 2.37). Egger's test suggested the existence of publication bias (P = 0.012).
Fig. 2.
Forest plot of the association between DII with osteoporosis risk
Western/unhealthful dietary pattern and osteoporosis risk
As shown in the forest plot in Fig. 3, five studies reported the Western/unhealthful dietary pattern and osteoporosis risk [36, 41, 44–46]. Heterogeneity existed (I2 = 75.8%, P = 0.002), and a random-effects model was selected. There was no pronounced difference in the risk of osteoporosis between the lowest intake category and the highest intake category (OR: 1.12; 95% CI: 0.78, 1.62). Egger's test implied no publication bias (P = 0.727).
Fig. 3.
Forest plot of the association between Western/unhealthy dietary patterns and osteoporosis risk
DASH, prudent/healthful dietary pattern, and aquatic dietary pattern and osteoporosis risk
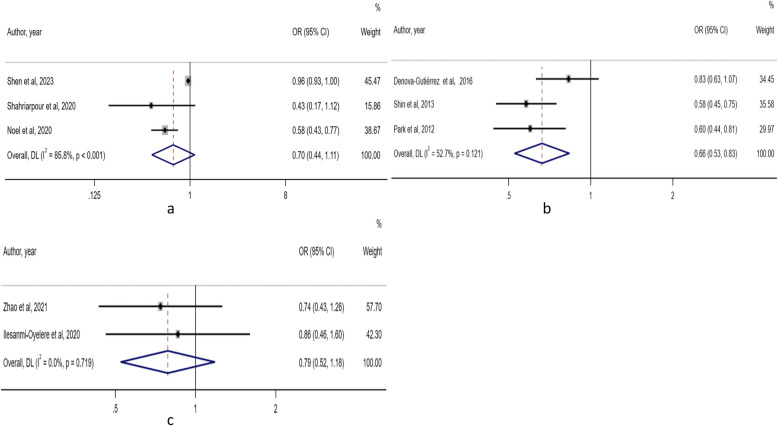
Three studies focused on DASH and osteoporosis risk [33, 39, 40]. Heterogeneity existed (I2 = 85.8%, P < 0.001), and a random-effects model was utilized. There was no considerable difference in the risk of osteoporosis between the lowest intake category and the highest intake category (OR: 0.70; 95% CI: 0.44, 1.11). Egger's test implied no publication bias (P = 0.240) (Fig. 4a).
Fig. 4.
Forest plot of the association between three dietary patterns and osteoporosis risk. a: DASH; b: Prudent/Healthful dietary pattern; c: Aquatic dietary pattern
Three studies focused on Prudent/Healthful dietary patterns and osteoporosis risk [44–46]. Heterogeneity was presented (I2 = 52.7%, P = 0.121), and a random-effects model was utilized. Compared with the category with the lowest intake, the highest intake category had a reduced risk of osteoporosis (OR: 0.66; 95% CI: 0.53, 0.83). The Egger's test implied no publication bias (P = 0.885) (Fig. 4b).
Two studies focused on the aquatic dietary pattern and osteoporosis risk [38, 41]. There was no heterogeneity (I2 = 0%, P = 0.719). The random-effects model was used. There was no marked difference in the risk of osteoporosis between the lowest intake category and the highest intake category (OR: 0.79; 95% CI: 0.52, 1.18). Due to the small number of studies included, Egger's test was not performed (Fig. 4c).
Healthful plant-based diet index (PDI), unhealthful PDI, overall PDI and osteoporosis risk
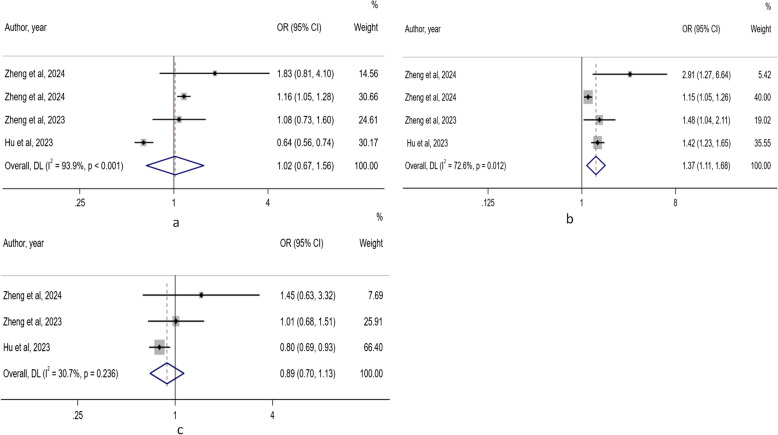
Four studies focused on the healthful PDI and osteoporosis risk [29, 30, 34, 35]. Heterogeneity existed (I2 = 93.9%, P < 0.001), and a random-effects model was utilized. There was no pronounced difference in the risk of osteoporosis between the lowest category and the highest category of healthful PDI (OR: 1.02; 95% CI: 0.67, 1.56). Egger's test implied no publication bias (P = 0.981) (Fig. 5a).
Fig. 5.
Forest plots of the association between three plant-based diet indexes and osteoporosis risk. a: healthful PDI; b: unhealthful PDI; c: overall PDI
Four studies focused on the unhealthful PDI and osteoporosis risk [29, 30, 34, 35]. Heterogeneity was presented (I2 = 72.6%, P = 0.012), and a random-effects model was utilized. Compared with the lowest category, the highest category was associated with an increased risk of osteoporosis (OR: 1.37; 95% CI: 1.11, 1.68). Egger's test implied no publication bias (P = 0.146) (Fig. 5b).
Three studies focused on the overall PDI and osteoporosis [29, 34, 35]. There was no heterogeneity (I2 = 30.7%, P = 0.236). Compared with the lowest category, the highest category was not associated with the risk of osteoporosis (OR: 0.89; 95% CI: 0.70, 1.13). Egger's test suggested the existence of publication bias (P = 0.022) (Fig. 5c).
Subgroup analysis
To investigate the sources of heterogeneity, we carried out subgroup analyses. Owing to data constraints, we were only able to conduct subgroup analyses based on geographical regions and diet assessment methods (Tables 2 and 3). In two Chinese studies regarding healthful PDI (OR: 1.00, 95% CI: 0.36, 2.77; I2 = 84.0%, P-heterogeneity = 0.01), healthful PDI was not associated with osteoporosis risk. However, in the other two countries (US, UK) (OR: 1.16, 95%CI: 1.05, 1.27; I2 = 0.00%, P-heterogeneity = 0.729), the healthy PDI was associated with osteoporosis risk. In two Chinese studies on unhealthy PDI (OR: 1.80, 95% CI: 0.93, 3.50; I2 = 64.3%, P-heterogeneity = 0.09), unhealthful PDI was not associated with osteoporosis risk. In the other two countries (US, UK), unhealthful PDI was also not associated with osteoporosis risk (OR: 1.23, 95% CI: 0.99, 1.53; I2 = 45.4%, P-heterogeneity = 0.176). Table 3 presents the results of different subgroups based on the diet assessment method, which are the same as those in Table 2. There was no visible difference among other subgroups.
Table 2.
Subgroup analysis of dietary pattern and osteoporosis risk by country
| Dietary patterns | Geographic location | Number of studies | Pooled Odd ratio (95% CI) |
Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| P value | I2 (%) | P valuea | Model | ||||
| DII | Korea | 2 | 1.31 (1.14, 1.51) | < 0.001 | 0.00 | 0.760 | Random |
| US | 4 | 2.0 5 (1.61, 2.61) | < 0.001 | 1.8 | 0.383 | Random | |
| hPDI | China | 2 | 1.00 (0.36, 2.77) | 1.000 | 84.0 | 0.010 | Random |
| Others | 2 | 1.16 (1.05, 1.27) | 0.003 | 0.00 | 0.729 | Random | |
| PDI | China | 2 | 0.93 (0.56, 1.55) | 0.781 | 47.5 | 0.167 | Random |
| uPDI | China | 2 | 1.80 (0.93, 3.50) | 0.081 | 64.3 | 0.090 | Random |
| Others | 2 | 1.23 (0.99, 1.53) | 0.063 | 45.4 | 0.176 | Random | |
| Western/Unhealthy | Korea | 2 | 1.25 (0.84, 1.88) | 0.766 | 88.9 | 0.003 | Random |
| Others | 3 | 1.17 (0.61, 2.23) | 0.638 | 72.5 | 0.026 | Random | |
| Prudent/Healthy | Korea | 2 | 0.59 (0.48, 0.72) | < 0.001 | 0.00 | 0.870 | Random |
DII Dietary Inflammatory Index, PDI plant-based diet index, hPDI healthy plant-based diet index, uPDI unhealthy plant-based diet index, CI, confidence interval
aP value for heterogeneity within each subgroup
Table 3.
Subgroup analysis of dietary pattern and osteoporosis risk by diet assessment method
| Dietary patterns | Diet assessment method | Number of studies | Pooled Hazard ratio (95% CI) | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| P value | I2 (%) | P valuea | Model | ||||
| hPDI | FFQ | 2 | 1.16 (1.05, 1.27) | 0.003 | 0.00 | 0.729 | Random |
| 24-h dietary recall | 2 | 1.00 (0.36, 2.77) | 1.000 | 84.0 | 0.012 | Random | |
| PDI | FFQ | 2 | 0.93 (0.56, 1.55) | 0.781 | 47.5 | 0.167 | Random |
| uPDI | FFQ | 2 | 1.80 (0.93, 3.50) | 0.081 | 64.3 | 0.090 | Random |
| 24-h dietary recall | 2 | 1.23 (0.99, 1.53) | 0.063 | 45.4 | 0.176 | Random | |
| Western/Unhealthy | FFQ | 4 | 1.25 (0.84, 1.88) | 0.272 | 64.9 | 0.036 | Random |
| Prudent/Healthy | FFQ | 2 | 0.71 (0.52, 0.98) | 0.036 | 59.6 | 0.115 | Random |
PDI plant-based diet index, hPDI healthy plant-based diet index, uPDI unhealthy plant-based diet index, FFQ food frenquency questionnaire, CI confidence interval
aP value for heterogeneity within each subgroup
Sensitivity analysis and publication bias
Owing to the limited quantity of incorporated studies (fewer than 10 for each outcome), the funnel plot symmetry test was not carried out. Publication bias was observed in the association of dietary patterns of DII and PDI with the risk of osteoporosis. Hence, sensitivity analyses (Figs. 6 and 7) and trim-and-fill analyses (Tables 4 and 5) were conducted. A sensitivity analysis was performed by omitting one study each time. The results demonstrated that the association between DII and the risk of osteoporosis remained, while the association between PDI and the risk of osteoporosis was not in line with the pooled results. 6.710
Fig. 6.
Sensitivity analysis chart of DII and osteoporosis risk
Fig. 7.
Sensitivity analysis chart of PDI and osteoporosis risk
Table 4.
The trim-and-fill analysis of DII and the risk of osteoporosis
| Pooled | 95% CI | Asymptotic | No. of | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Est | Lower | Upper | z_value | p_value | studies | Q_value | p_value | |
| Meta-analysis | Fixed | 0.408 | 0.289 | 0.527 | 6.710 | 0.000 | 7 | 19.106 | 0.004 |
| Random | 0.597 | 0.331 | 0.863 | 4.395 | 0.000 | ||||
|
Filled Meta-analysis |
Fixed | 0.350 | 0.236 | 0.465 | 5.96 9 | 0.000 | 10 | 34.148 | 0.000 |
| Random | 0.391 | 0.111 | 0.672 | 2.738 | 0.006 | ||||
DII Dietary Inflammatory Index, CI confidence interval
Table 5.
The trim-and-fill analysis of PDI and the risk of osteoporosis
| Pooled | 95% CI | Asymptotic | No. of | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Est | Lower | Upper | z_value | p_value | studies | Q_value | p_value | |
| Meta-analysis | Fixed | −0.179 | −0.317 | −0.041 | −2.544 | 0.011 | 3 | 2.884 | 0.236 |
| Random | −0.117 | −0.358 | 0.124 | −0.951 | 0.342 | ||||
|
Filled Meta-analysis |
Fixed | −0.223 | −0.352 | −0.094 | −3.398 | 0.001 | 10 | 6.558 | 0.161 |
| Random | −0.223 | −0.456 | 0.010 | −1.878 | 0.060 | ||||
PDI plant-based diet index, CI confidence interval
Discussion
This is the first meta-analysis exploring the association between diverse dietary patterns and the risk of osteoporosis. Among the 20 articles included, eight distinct dietary patterns were assessed and different dietary patterns showed diverse risks of osteoporosis.
In our analysis of 183,087 participants from seven studies, the research results indicated that a higher DII score was associated with an increased risk of osteoporosis, consistent with previous reports. In a previous meta-analysis of 11 articles and 127,769 subjects, continuous DII was negatively associated with lumbar BMD. The highest DII score was associated with a 31% elevated risk of osteoporosis [17]. In another systematic review of 13 studies with 211,938 subjects, the average BMD value was greatly lowered in the highest DII category [47]. At present, diet may be crucial in regulating systemic inflammation. DII is an epidemiological survey tool to assess the inflammatory potential of diets. It can quantify the impact of nutrients and foods on inflammation. A higher DII score denotes a more powerful effect of the diet on inflammation. In contrast, a lower DII score denotes the stronger anti-inflammatory effect of the diet [48]. Osteoporosis is significantly affected by inflammation and dietary inflammatory patterns. Existing studies illustrated that multiple pro-inflammatory indexes (IL-6, IL-1β, IL-17, and TNF-α) could trigger RANK-RANKL signaling and facilitate osteoclast absorption and bone loss [49]. These inflammatory factors could also induce osteoclast differentiation and activation independent of the typical RANKL pathway, ultimately leading to bone loss [50]. However, Egger's test suggested the presence of publication bias. A trim-and-fill analysis suggested that the results did not change significantly and were stable. The sensitivity analysis further confirmed our findings.
In our analysis of 12,114 participants from three studies, high adherence of prudent/healthy dietary patterns can reduce the risk of osteoporosis, consistent with previous reports. A meta-analysis noted that the prudent/healthful dietary pattern was associated with a low risk of low BMD and fractures [51]. In another systematic review of 175,060 participants, a higher intake of the prudent/healthy dietary pattern was negatively associated with the risk of low BMD, and male participants with a higher intake had a lower risk of fractures [52]. A prudent/healthful dietary pattern involves considerable intake of vegetables, fruits, berries, vegetable oils, whole-grain goods, and low-fat dairy products [53]. Such dietary patterns are rich in diverse essential nutrients, like calcium, potassium, magnesium, vitamins, proteins, omega-3 polyunsaturated fatty acids, fibers, and monounsaturated fatty acids [54]. These nutritional elements yield significant anti-inflammatory effects, which could enhance bone metabolism [17, 55]. The dietary pattern constituted by fruits and vegetables comprises two alkaloids, which facilitate bone formation, restrain bone resorption by lowering oxidative stress and inflammation, and notably impact the volume, number, and thickness of trabecular bone [56]. High concentrations of magnesium inhibited the activity of osteoblasts and alkaline phosphatase that were engaged in bone formation [57]. Vitamin C and carotenoids might improve bone metabolism via antioxidant-related mechanisms [58].
Plant-based diets constitute diverse dietary patterns for flexitarians and vegetarians, with an emphasis on diminishing the intake of animal-based foods and elevating the intake of plant-based foods [59, 60]. Clinical studies have yielded varying results on the association between different qualities of plant-based diets and diseases [60, 61]. Hence, three versions of PDI have been established. Among them, overall PDI reflects the intake of all plant-based foods while minimizing the intake of animal-based foods. Healthful PDI underlines the intake of healthful plant-based foods, like whole grains, nuts, fruits, vegetables, legumes, and vegetable oils. The unhealthful PDI emphasizes the intake of unhealthful plant-based foods, including juices, potatoes, sugary beverages, refined grains, and confections/desserts [59]. In our analysis of 26,486 participants from three studies, we found that the PDI score was not associated with the risk of osteoporosis. Egger's test indicated the presence of publication bias. Sensitivity analysis revealed the instability of the results, suggesting that the results should be interpreted with caution. This phenomenon may be related to the limited number of included studies and participants, the study population, and the adjusted confounding factors. Additionally, in our analysis of 228,549 participants from four studies, higher unhealthy PDI scores were associated with an increased risk of osteoporosis, consistent with previous reports. In a previous meta-analysis of 10 studies involving 14,247 individuals [62], a greater intake of vegetables was related to a lower risk of osteoporosis. Additionally, a prospective observational study from the Careggi Hospital in Florence included 200 women (aged 30–80) and indicated that a Mediterranean dietary pattern dominated by plant-based foods increased vitamin D levels and offered significant protection against osteoporosis [8]. A case–control study of 262 postmenopausal women from Iran linked an unhealthful plant-based diet with enhanced abnormal bone density in the femoral neck and lumbar spine [63]. Nevertheless, our analysis of 228,549 participants from four studies discovered that a high healthful PDI score was not associated with osteoporosis, inconsistent with previous reports [63]. In our subgroup analysis, the high healthy PDI score was associated with the risk of osteoporosis in European and American populations, while no such correlation was identified in China. This is mainly ascribed to the differences in the number of participants, dietary structures, genetic and metabolic variations, and environmental factors. As plant-based foods are rich in potassium, vitamins C and K, magnesium, zinc, dietary fibers, and multiple phytochemicals, with lower levels of saturated fat and cholesterol [63, 64], they could reduce the acid load in the diet, lower bone resorption, and enhance BMD [65]. Additionally, the phytoestrogens they contain could protect bones by blocking bone resorption and facilitating bone formation [66]. However, some vegetarians may have difficulty getting enough protein to maintain optimal bone health, resulting in deficiencies of vitamin D, long-chain omega-3 PUFAs, iron, and calcium, which are mainly presented in animal-based foods or are less bioavailable in plant-based foods [67]. An increase in unhealthful PDI can decrease osteocalcin levels [68], and osteocalcin is significant in regulating bone mineralization and osteoblast/osteoclast activities [69]. Therefore, not all plant-based foods are beneficial.
In this study, the Western/unhealthful dietary pattern, DASH dietary pattern, and aquatic dietary pattern were not correlated with osteoporosis. In a previous systematic review of 175,060 participants, compared with the lowest intake group, the risk of fractures increased by 10% in the highest intake group of the Western/unhealthful dietary pattern [52]. In a cross-sectional study of 1,092 men from China, consuming fish could reduce the risk of osteoporosis [70]. The inconsistent conclusions may mainly lie in the following aspects. First, the sample size in previous studies was relatively small; second, most previous studies were cross-sectional ones, which failed to consider the possible changes in diet over time and could not rule out residual confounding factors; third, during the freezing/thawing and frozen storage processes of aquatic products, protein may be oxidated, thereby influencing the quality and nutritional value of the food [71].
Our study had some strengths. Firstly, this was the first meta-analysis to comprehensively search for existing research and examine the association between various dietary patterns and osteoporosis risk. Secondly, a considerable number of studies were incorporated, not restricted by population, region, bone density measurement method, and covariate. Thirdly, the literature included was of medium-to-high quality, with active control and adjustment of covariates, which ensured the high reliability of the research results. Fourth, previous studies have revealed that dietary patterns can be utilized for osteoporosis prevention [51, 52]. However, only common Western and healthy dietary patterns were examined. Our research demonstrates that different dietary patterns have disparate effects on the risk of osteoporosis. The dietary patterns analyzed in our study are more comprehensive, offering reference values for clinical practice. Finally, subgroup analyses were implemented to ascertain the sources of heterogeneity, and publication bias tests proved the stability of the main results. Nevertheless, this meta-analysis also had several limitations. Firstly, the subjects in individual studies were restricted to certain specific populations, which may exert specific influences on the results and may not be suitable for the general population. Secondly, although the food frequency questionnaire is a standard tool to obtain dietary information, participants may not precisely recall their food intake. High heterogeneity was found in some dietary patterns (DII, DASH, and health PDI), possibly due to population characteristics, study design, population, location, and adjusted covariates. Therefore, these findings need to be interpreted with caution. Thirdly, the number of studies on some dietary patterns was limited. Further large-sample prospective studies are warranted for verification.
Conclusions
This meta-analysis indicates that dietary patterns with high pro-inflammatory factors or high unhealthful PDI scores may increase the risk of osteoporosis. High adherence to prudent/healthful dietary patterns could decrease the risk of osteoporosis. The geographical areas should be expanded to further clarify the influence of other diets on osteoporosis, providing evidence-based references for different ethnic groups and regions.
Supplementary Information
Acknowledgements
Not applicable
Abbreviations
- CIs
Confidence intervals
- BMD
Bone mineral density abbreviations
- DII
Dietary inflammatory index
- HRs
Hazard ratios
- PDI
Plant-based diet index
Authors’ contributions
All authors contributed to the study conception and design. Writing - original draft preparation: [Bing Tan]; Writing - review and editing: [Min Liang]; Conceptualization: [Hong Wei Su]; Methodology: [Lan Ya Wei]; Formal analysis and investigation: [Bing Tan]; Funding acquisition: [Min Liang]; Resources: [Hong Wei Su]; Supervision: [Min Liang], and all authors commented on previous versions of the manuscript. All authors read and approved of the final manuscript.
Funding
None.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, Shamliyan T, Cooney TG, Cross JT Jr, Fitterman N, Lin JS, Maroto M, Obley AJ, Tice JA, Tufte JE. Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline From the American College of Physicians. Ann Intern Med. 2023;176(2):224–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marques ALÓ, da Silva JA. The burden of osteoporotic hip fractures in Portugal: costs, health related quality of life and mortality. Osteoporos Int. 2015;26(11):2623–30. [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. [DOI] [PMC free article] [PubMed]
- 4.Xiao PL, Cui AY, Hsu CJ, Peng R, Jiang N, Xu XH, Ma YG, Liu D, Lu HD. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. 2022;33(10):2137–53. [DOI] [PubMed] [Google Scholar]
- 5.Sing CW, Lin TC, Bartholomew S, Bell JS, Bennett C, Beyene K, Bosco-Levy P, Bradbury BD, Chan AHY, Chandran M, Cooper C, de Ridder M, Doyon CY, Droz-Perroteau C, Ganesan G, Hartikainen S, Ilomaki J, Jeong HE, Kiel DP, Kubota K, Lai EC, Lange JL, Lewiecki EM, Lin J, Liu J, Maskell J, de Abreu MM, O'Kelly J, Ooba N, Pedersen AB, Prats-Uribe A, Prieto-Alhambra D, Qin SX, Shin JY, Sørensen HT, Tan KB, Thomas T, Tolppanen AM, Verhamme KMC, Wang GH, Watcharathanakij S, Wood SJ, Cheung CL, Wong ICK. Global Epidemiology of Hip Fractures: Secular Trends in Incidence Rate, Post-Fracture Treatment, and All-Cause Mortality. J Bone Miner Res. 2023;38(8):1064–75. [DOI] [PubMed]
- 6.Guo D, Zhao M, Xu W, He H, Li B, Hou T. Dietary interventions for better management of osteoporosis: An overview. Crit Rev Food Sci Nutr. 2023;63(1):125–44. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela-Martínez S, Ramírez-Expósito MJ, Carrera-González MP, Martínez-Martos JM. Physiopathology of Osteoporosis: Nursing Involvement and Management. Biomedicines. 2023;11(4):1220. [DOI] [PMC free article] [PubMed]
- 8.Quattrini S, Pampaloni B, Gronchi G, Giusti F, Brandi ML. The Mediterranean Diet in Osteoporosis Prevention: An Insight in a Peri- and Post-Menopausal Population. Nutrients. 2021;13(2):531. [DOI] [PMC free article] [PubMed]
- 9.Feng W, Wang X, Huang D, Lu A. Role of diet in osteoporosis incidence: Umbrella review of meta-analyses of prospective observational studies. Crit Rev Food Sci Nutr. 2023;63(19):3420–9. [DOI] [PubMed] [Google Scholar]
- 10.Kajarabille N, Díaz-Castro J, Hijano S, López-Frías M, López-Aliaga I, Ochoa JJ. A new insight to bone turnover: role of ω-3 polyunsaturated fatty acids. ScientificWorldJournal. 2013;2013: 589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Avgerinou C. Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review. Nutrients. 2023;15(19):4255. [DOI] [PMC free article] [PubMed]
- 12.Keykhaei F, Norouzy S, Froughipour M, Nematy M, Saeidi M, Jarahi L, Amiri F, Malek Ahmadi M, Norouzy A. Adherence to healthy dietary pattern is associated with lower risk of multiple sclerosis. J Cent Nerv Syst Dis. 2022;14:11795735221092516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malmir H, Larijani B, Esmaillzadeh A. Consumption of milk and dairy products and risk of osteoporosis and hip fracture: a systematic review and Meta-analysis. Crit Rev Food Sci Nutr. 2020;60(10):1722–37. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Li Z, Gao Q, Zhao H, Chen S, Huang L, Wang W, Wang T. A review of statistical methods for dietary pattern analysis. Nutr J. 2021;20(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Song M, Eliassen AH, Wang M, Fung TT, Clinton SK, Rimm EB, Hu FB, Willett WC, Tabung FK, Giovannucci EL. Optimal dietary patterns for prevention of chronic disease. Nat Med. 2023;29(3):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervo MMC, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Handelsman DJ, Ribeiro RV, Waite LM, Shivappa N, Hebert JR, Hirani V. Proinflammatory Diet Increases Circulating Inflammatory Biomarkers and Falls Risk in Community-Dwelling Older Men. J Nutr. 2020;150(2):373–81. [DOI] [PubMed] [Google Scholar]
- 17.Fang Y, Zhu J, Fan J, Sun L, Cai S, Fan C, Zhong Y, Li Y. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2021;32(4):633–43. [DOI] [PubMed] [Google Scholar]
- 18.Noori M, Jayedi A, Khan TA, Moradi S, Shab-Bidar S. Mediterranean dietary pattern and bone mineral density: a systematic review and dose-response meta-analysis of observational studies. Eur J Clin Nutr. 2022;76(12):1657–64. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen HH, Wu F, Makin JK, Oddy WH, Wills K, Jones G, Winzenberg T. Associations of dietary patterns with bone density and fractures in adults: A systematic review and meta-analysis. Aust J Gen Pract. 2021;50(6):394–401. [DOI] [PubMed] [Google Scholar]
- 20.Mathew JL. Systematic Reviews and Meta-Analysis: A Guide for Beginners. Indian Pediatr. 2022;59(4):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 23. Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z. Manual for Evidence Synthesis. JBI: Miami, FL, USA. 2018.
- 24.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27(3):317–21. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 27.Liu Z, Jian H, Peng Z, Xiong S, Zhang Z. Association between dietary inflammatory index and osteoporosis in the US population: evidence from NHANES 2003–2010. Front Nutr. 2025;12:1508127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadisima N, Farshbaf-Khalili A, Ostadrahimi A, Pourmoradian S. Positive relation between dietary inflammatory index and osteoporosis in postmenopausal women. Int J Vitam Nutr Res. 2024;94(2):86–94. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Gao N, Li Y, Fan M, Tian W, Jiang Y, Wang Y, Cui M, Suo C, Zhang T, Jin L, Xu K, Chen X. Unraveling the role of serum metabolites in the relationship between plant-based diets and bone health in community-dwelling older adults. Current Research in Food Science. 2024;8. [DOI] [PMC free article] [PubMed]
- 30.Zheng Y, Wang J, Li Y, Wang Y, Suo C, Jiang Y, Jin L, Xu K, Chen X. Unraveling the role of BMI and blood markers in the relationship between plant-based diets and osteoporosis: A prospective cohort study. Prev Med. 2024;187: 108103. [DOI] [PubMed] [Google Scholar]
- 31.Meng X, Sha W, Lou X, Chen J. The relationship between dietary inflammatory index and osteoporosis among chronic kidney disease population. Sci Rep. 2023;13(1):22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SY, Zeng MR. The association between dietary inflammation index and bone mineral density: results from the United States National Health and nutrition examination surveys. Renal Failure. 2023;45(1):2209200. [DOI] [PMC free article] [PubMed]
- 33.Shen J, Yang L, Li X, Li X, Tian X, Xiao H, Dai J. Adherence to the dietary approaches to stop hypertension and bone health in the Chinese elderly. J Bone Miner Metab. 2023;41(6):844–53. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y, Wang J, Wang Y, Xu K, Chen X. The Hidden Dangers of Plant-Based Diets Affecting Bone Health: A Cross-Sectional Study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005–2018. Nutrients. 2023;15(7):1794. [DOI] [PMC free article] [PubMed]
- 35.Hu J, Li Y, Wang Z, Li X, Hou T, Ning Z, Huang R, Ma C, Yuan X, Wang D. Association of plant-based dietary patterns with the risk of osteoporosis in community-dwelling adults over 60 years: a cross-sectional study. Osteoporos Int. 2023;34(5):915–23. [DOI] [PubMed] [Google Scholar]
- 36.Tayyem RF, Ajeen R, Al-Khammash A. Dietary patterns associated with the risk of osteoporosis in postmenopausal women. Food Production Processing and Nutrition. 2023;5(1):19.
- 37.Zhao S, Gao W, Li J, Sun M, Fang J, Tong L, He Y, Wang Y, Zhang Y, Xu Y, Yang S, Jin L. Dietary inflammatory index and osteoporosis: the National Health and Nutrition Examination Survey, 2017–2018. Endocrine. 2022;78(3):587–96.3 [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Li J, Yuan Z, Li X, Gu H, Jiao C, Zhang Z. The Relationship between Dietary Patterns and Bone Mineral Density of 476 Middle-Aged and Aged People. Iran J Public Health. 2021;50(10):2010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahriarpour Z, Nasrabadi B, Shariati-Bafghi SE, Karamati M, Rashidkhani B. Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women. Osteoporos Int. 2020;31(11):2179–88. [DOI] [PubMed] [Google Scholar]
- 40.Noel SE, Mangano KM, Mattei J, Griffith JL, Dawson-Hughes B, Bigornia S, Tucker KL. Dietary Approaches to Stop Hypertension, Mediterranean, and Alternative Healthy Eating indices are associated with bone health among Puerto Rican adults from the Boston Puerto Rican Osteoporosis Study. Am J Clin Nutr. 2020;111(6):1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilesanmi-Oyelere BL, Coad J, Roy NC, Kruger MC. Dietary Patterns, Body Composition, and Bone Health in New Zealand Postmenopausal Women. Front Nutr. 2020;7: 563689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Na W, Park S, Shivappa N, Hébert JR, Kim MK, Sohn C. Association between inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: Data from fourth and fifth Korea national health and nutrition examination surveys. Nutrients. 2019;11(4):885. [DOI] [PMC free article] [PubMed]
- 43.Kim HS, Sohn C, Kwon M, Na W, Shivappa N, Hébert JR, Kim MK. Positive Association between Dietary Inflammatory Index and the Risk of Osteoporosis: Results from the KoGES_Health Examinee (HEXA) Cohort Study. Nutrients. 2018;10(12):1999. [DOI] [PMC free article] [PubMed]
- 44.Denova-Gutiérrez E, Clark P, Tucker KL, Muñoz-Aguirre P, Salmerón J. Dietary patterns are associated with bone mineral density in an urban Mexican adult population. Osteoporos Int. 2016;27(10):3033–40. [DOI] [PubMed] [Google Scholar]
- 45.Shin S, Joung H. A dairy and fruit dietary pattern is associated with a reduced likelihood of osteoporosis in Korean postmenopausal women. Br J Nutr. 2013;110(10):1926–33. [DOI] [PubMed] [Google Scholar]
- 46.Park SJ, Joo SE, Min H, Park JK, Kim Y, Kim SS, Ahn Y. Dietary Patterns and Osteoporosis Risk in Postmenopausal Korean Women. Osong Public Health and Research Perspectives. 2012;3(4):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taheri E, Mahdavi-Gorabi A, Moludi J, Asayesh H, Qorbani M. A meta-analysis of dietary inflammatory index and bone health status. J Diabetes Metab Disord. 2022;21(1):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu L, Liu R, Zhang L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int Immunopharmacol. 2022;111: 109095. [DOI] [PubMed] [Google Scholar]
- 50.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–50. [DOI] [PubMed] [Google Scholar]
- 51.Fabiani R, Naldini G, Chiavarini M. Dietary Patterns in Relation to Low Bone Mineral Density and Fracture Risk: A Systematic Review and Meta-Analysis. Adv Nutr. 2019;10(2):219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denova-Gutiérrez E, Méndez-Sánchez L, Muñoz-Aguirre P, Tucker KL, Clark P. Dietary patterns, bone mineral density, and risk of fractures: A systematic review and meta-analysis. Nutrients. 2018;10(12):1922. [DOI] [PMC free article] [PubMed]
- 53.Chen X, Maguire B, Brodaty H, O’Leary F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J Alzheimers Dis. 2019;67(2):583–619. [DOI] [PubMed] [Google Scholar]
- 54.Hess JM, Comeau ME, Casperson S, Slavin JL, Johnson GH, Messina M, Raatz S, Scheett AJ, Bodensteiner A, Palmer DG. Dietary Guidelines Meet NOVA: Developing a Menu for A Healthy Dietary Pattern Using Ultra-Processed Foods. J Nutr. 2023;153(8):2472–81. [DOI] [PubMed] [Google Scholar]
- 55.Ha U, Herpich C, Norman K. Anti-Inflammatory Diets and Fatigue. Nutrients. 2019;11(10):2315. [DOI] [PMC free article] [PubMed]
- 56.Perna S, Avanzato I, Nichetti M, D'Antona G, Negro M, Rondanelli M. Association between Dietary Patterns of Meat and Fish Consumption with Bone Mineral Density or Fracture Risk: A Systematic Literature. Nutrients. 2017;9(9):1029. [DOI] [PMC free article] [PubMed]
- 57.Leidi M, Dellera F, Mariotti M, Maier JA. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res. 2011;24(1):1–6. [DOI] [PubMed] [Google Scholar]
- 58.Rondanelli M, Faliva MA, Barrile GC, Cavioni A, Mansueto F, Mazzola G, Oberto L, Patelli Z, Pirola M, Tartara A, Riva A, Petrangolini G, Peroni G. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients. 2021;14(1):74. [DOI] [PMC free article] [PubMed]
- 59.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016;13(6): e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trautwein EA, McKay S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients. 2020;12(9):2671. [DOI] [PMC free article] [PubMed]
- 61.Craig WJ, Mangels AR, Fresán U, Marsh K, Miles FL, Saunders AV, Haddad EH, Heskey CE, Johnston P, Larson-Meyer E, Orlich M. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients. 2021;13(11):4144. [DOI] [PMC free article] [PubMed]
- 62.Zeng LF, Yang WY, Liang GH, Luo MH, Cao Y, Chen HY, Pan JK, Huang HT, Han YH, Zhao D, Lin JT, Hou SR, Ou AH, Guan ZT, Wang Q, Liu J. Can increasing the prevalence of vegetable-based diets lower the risk of osteoporosis in postmenopausal subjects? A systematic review with meta-analysis of the literature. Complement Ther Med. 2019;42:302–11. [DOI] [PubMed] [Google Scholar]
- 63.Ghadiri M, Cheshmazar E, Shateri Z, Gerami S, Nouri M, Gargari BP. Healthy plant-based diet index as a determinant of bone mineral density in osteoporotic postmenopausal women: A case-control study. Front Nutr. 2022;9:1083685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chuang TL, Lin CH, Wang YF. Effects of vegetarian diet on bone mineral density. Tzu Chi Med J. 2021;33(2):128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burckhardt P. The role of low acid load in vegetarian diet on bone health: a narrative review. Swiss Med Wkly. 2016;146: w14277. [DOI] [PubMed] [Google Scholar]
- 66.Kimball JS, Johnson JP, Carlson DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg Am. 2021;103(15):1451–61. [DOI] [PubMed] [Google Scholar]
- 67.Galchenko A, Gapparova K, Sidorova E. The influence of vegetarian and vegan diets on the state of bone mineral density in humans. Crit Rev Food Sci Nutr. 2023;63(7):845–61. [DOI] [PubMed] [Google Scholar]
- 68.Shahinfar H, Amini MR, Payandeh N, Naghshi S, Sheikhhossein F, Djafarian K, Shab-Bidar S. The link between plant-based diet indices with biochemical markers of bone turn over, inflammation, and insulin in Iranian older adults. Food Sci Nutr. 2021;9(6):3000–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komori T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int J Mol Sci. 2020;21(20):7513. [DOI] [PMC free article] [PubMed]
- 70.Li X, Lei T, Tang Z, Dong J. Analyzing the association between fish consumption and osteoporosis in a sample of Chinese men. J Health Popul Nutr. 2017;36(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bao Y, Ertbjerg P, Estévez M, Yuan L, Gao R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr Rev Food Sci Food Saf. 2021;20(6):5548–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].