Abstract
Liquid biopsy offers a minimally invasive method for detecting and monitoring cancer, with key biomarkers including circulating tumor cells (CTCs), cell-free DNA (cfDNA), and extracellular vesicles (EVs). Among these, CTCs provide dynamic insights into tumor heterogeneity, metastatic potential, and therapeutic resistance through molecular profiling and single-cell analysis. This review examines recent advancements in technologies such as microfluidics, nanotechnology, and next-generation sequencing (NGS), which have improved the accuracy and clinical use of CTC detection. A comparative analysis of liquid biopsy techniques highlights the strengths and limitations of key platforms, including NGS, digital PCR (ddPCR), and quantitative PCR (qPCR), providing insights into diagnostic accuracy and clinical significance. Combining genomic, transcriptomic, and proteomic analyses with artificial intelligence (AI) tools has enhanced tumor profiling and supports personalized treatment decisions in precision oncology. Despite notable progress, issues like assay standardization, sample variability, regulatory complexity, and data integration still limit widespread clinical use. Future directions emphasize interdisciplinary innovation, clinical validation, and robust bioinformatics frameworks to facilitate the seamless incorporation of CTC-based liquid biopsy into standard oncology practice. Overcoming these challenges may allow liquid biopsy to become a standard tool for early cancer detection, ongoing monitoring, and individualized treatment.
Keywords: Circulating tumor cells, Liquid biopsy, Cell-free DNA, Exosomes, Nanotechnology, Next-generation sequencing
1. Introduction
Circulating tumor cells (CTCs), released from both primary and metastatic tumors, are valuable indicators for monitoring tumor progression and treatment response [[1], [2], [3], [4]]. Since their initial discovery in the 19th century, CTCs have garnered significant attention due to their central role in hematogenous dissemination and their dynamic reflection of tumor heterogeneity [5]. Unlike conventional tissue biopsies, which offer a static and localized snapshot, CTC analysis allows continuous, non-invasive monitoring of tumor evolution, clonal selection, and the emergence of drug resistance [6], making CTC-based liquid biopsy a valuable tool in precision oncology. Recent improvements in techniques such as microfluidic capture, immunoaffinity enrichment, and label-free detection have enhanced the accuracy of CTC identification [[7], [8], [9], [10]]. Molecular analysis of CTCs provides insights into how tumors evolve, resist treatment, and spread [11]. Furthermore, the integration of next-generation sequencing (NGS) and single-cell transcriptomics has revolutionized CTC research, enabling high-resolution molecular characterization and refining the identification of actionable therapeutic targets for personalized interventions [12]. Despite these advancements, the clinical translation of CTC-based liquid biopsy continues to face critical challenges related to standardization of assays, pre-analytical variability, and regulatory approvals [13]. Addressing these barriers demands the incorporation of multi-omics approaches, artificial intelligence (AI)-driven analytics, and robust validation frameworks to enhance reliability, reproducibility, and clinical applicability [14].
While CTCs remain at the forefront of liquid biopsy innovation, their diagnostic and prognostic value is further enhanced when combined with other circulating biomarkers such as cell-free DNA (cfDNA) and extracellular vesicles (EVs) [15]. cfDNA analysis has demonstrated substantial potential in detecting tumor-specific genetic and epigenetic alterations and in monitoring minimal residual disease (MRD), complementing the phenotypic and functional information obtained from CTCs [16]. Similarly, EVs, including exosomes, mediate intercellular communication and carry molecular signatures that provide additional layers of biomarker discovery [17]. The synergistic integration of CTCs, cfDNA, and EVs within multi-analyte liquid biopsy platforms holds the promise of refining cancer diagnostics, improving therapeutic decision-making, and driving the development of personalized treatment strategies.
This review critically evaluates emerging technological innovations that enhance CTC detection, isolation, and molecular profiling, highlighting the transformative potential of liquid biopsy in tracking tumor heterogeneity, disease progression, and therapy resistance [1,5]. Despite notable progress, challenges related to assay standardization, data harmonization, and clinical validation continue to limit the widespread adoption of CTC-based liquid biopsy [13,16]. Bridging the gap between bench-side innovation and bedside application, the integration of CTC analysis into clinical workflows represents a paradigm shift in real-time tumor monitoring. Continued interdisciplinary advances will be pivotal in fully realizing the impact of CTC-based liquid biopsy in early cancer detection, precision therapeutic monitoring, and the next generation of personalized oncology.
2. Liquid biopsy: transforming cancer diagnostics and management with a focus on CTCs
Liquid biopsy has emerged as a transformative, non-invasive alternative to traditional tissue biopsies, providing real-time insights into tumor dynamics. Among various analytes, CTCs have been recognized as pivotal biomarkers, offering critical information on tumor progression, metastatic potential, and therapeutic response [18]. As cellular representatives of disease evolution, CTCs mirror tumor heterogeneity and therapy resistance mechanisms [19]. Despite their rarity in circulation, continuous advancements in detection and molecular characterization technologies have significantly expanded their clinical applicability.
2.1. Defining key components: CTCs, cfDNA, and EVs
Liquid biopsy leverages the detection and analysis of circulating biomarkers to monitor tumor biology dynamically. CTCs, shed from primary and metastatic tumors into the bloodstream, provide direct phenotypic and molecular insights into disease processes [4]. Their enumeration, morphological assessment, and molecular profiling have been extensively correlated with prognosis, therapeutic efficacy, and metastatic risk [16,18]. However, the low abundance of CTCs (approximately 1–10 cells/mL of blood) presents technical challenges [20], prompting the development of innovative microfluidic enrichment strategies and single-cell sequencing technologies that enable more precise characterization and identification of actionable targets [[7], [8], [9], [10]]. cfDNA comprises short DNA fragments released into circulation from apoptotic and necrotic tumor cells [21]. Analysis of cfDNA mutations, copy number variations, and methylation signatures using digital droplet PCR (ddPCR) and NGS platforms has allowed non-invasive tumor genotyping and the monitoring of minimal residual disease (MRD) with high specificity [22]. However, cfDNA lacks the cellular context necessary for assessing phenotypic plasticity or metastatic potential [23].
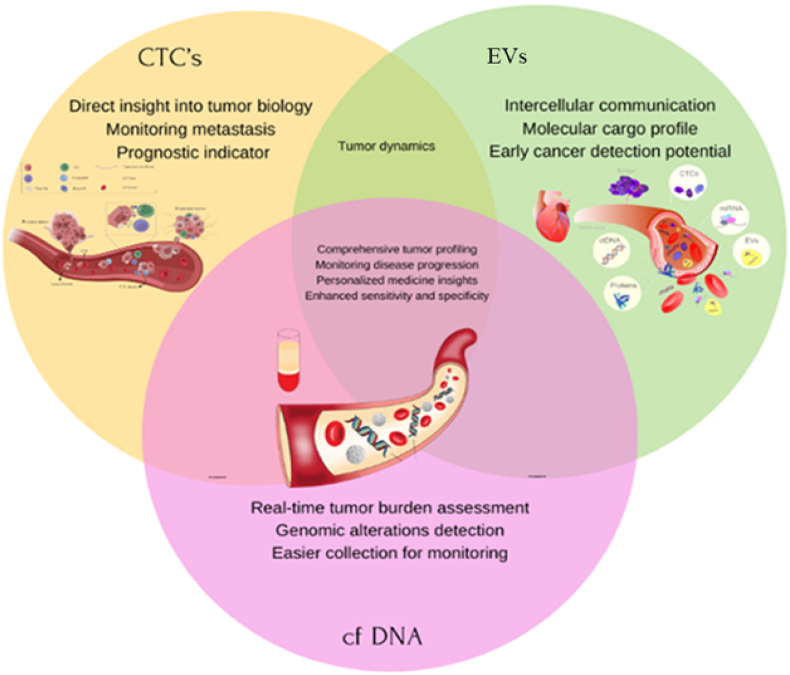
Conversely, EVs, including exosomes, are nano-sized vesicles secreted by tumor cells that carry a rich cargo of proteins, lipids, and nucleic acids [17]. EVs play critical roles in remodeling the tumor microenvironment, facilitating immune evasion, and promoting metastatic dissemination [24]. Advances in EV isolation techniques, such as ultracentrifugation, size-exclusion chromatography, and nanoparticle tracking, have greatly enhanced the clinical utility of EV profiling in cancer management [25]. Table 1 presents a comparative overview of the clinical relevance of CTCs, cfDNA, and EVs, emphasizing their respective contributions to early detection, prognosis, and therapeutic monitoring. The integration of these biomarkers within multi-omics frameworks further enhances the predictive precision of liquid biopsy. Fig. 1 illustrates the key components of liquid biopsy, highlighting their biological roles and clinical applications.
Table 1.
Key biomarkers (CTCs, cfDNA, exosomes) with their clinical significance and associated cancer types.
| Biomarker | Clinical significance | Associated cancer types | References |
|---|---|---|---|
| CTCs | Serve as non-invasive indicators of tumor presence, metastatic potential, and treatment efficacy. Quantification and molecular characterization of CTCs provide insights into tumor heterogeneity, drug resistance, and prognosis. CTC enumeration is an FDA-approved prognostic biomarker in metastatic breast, prostate, and colorectal cancers. | Breast, prostate, colorectal, lung, pancreatic, gastric, hepatocellular carcinoma, glioblastoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), malignant melanoma, renal cell carcinoma, head and neck squamous cell carcinoma (HNSCC), bladder cancer, esophageal cancer | [192], [193], [194] |
| cfDNA | Provides genetic information about tumor heterogeneity and clonal evolution. Useful for detecting actionable mutations (e.g., EGFR, KRAS, BRAF, TP53) and assessing minimal residual disease (MRD). Can be used for early cancer detection, treatment monitoring, and recurrence prediction. | Breast, lung (NSCLC, SCLC), colorectal, pancreatic, gastric, hepatocellular carcinoma, glioblastoma, meningioma, ovarian, endometrial, esophageal, prostate, bladder, renal cell carcinoma, head and neck cancers | [195], [196], [197] |
| EVs | Facilitate intercellular communication by transporting tumor-derived proteins, lipids, and nucleic acids (mRNA, miRNA, lncRNA, circRNA). Play key roles in tumor progression, immune modulation, drug resistance, and biomarker discovery. Potential therapeutic targets and delivery vehicles for cancer therapy. | Breast, ovarian, pancreatic, lung, gastric, hepatocellular carcinoma, colorectal, prostate, glioblastoma, melanoma, esophageal, bladder, head and neck cancers | 198,199 |
Fig. 1.
Unique contributions and overlapping roles of CTCs, cfDNA, and EVs in cancer diagnostics.
2.2. Advantages over conventional tissue biopsies
Liquid biopsy addresses many of the limitations of traditional tissue biopsies. [26]. It enables frequent, minimally invasive sampling for real-time monitoring of tumor dynamics, circumventing the need for repeated surgical interventions [27]. This approach not only reduces patient discomfort and procedural risks but also improves compliance with disease surveillance protocols. A major advantage of liquid biopsy, particularly CTC analysis, is its capacity to capture tumor heterogeneity and clonal evolution over time [28]. In contrast to static tissue biopsies that provide localized information, liquid biopsy offers a dynamic, real-time assessment of treatment-induced selection pressures and emerging resistance mechanisms [29]. Changes in CTC profiles can help adjust treatment before clinical signs of progression appear [30]. Additionally, liquid biopsy enhances early detection of recurrence by tracking CTC levels and cfDNA mutational changes, facilitating timely clinical intervention [31,32]. Its cost-effectiveness and scalability make it an attractive option for longitudinal monitoring across diverse patient populations. As summarized in Table 2, liquid biopsy provides key advantages over traditional tissue biopsy, including reduced invasiveness, faster turnaround, scalability, and compatibility with modern technologies. Clinical studies in advanced cancer have shown that CTCs can effectively guide treatment decisions. Although CTCs are scarce, recent advances in enrichment and detection methods have improved their reliability. Culturing CTCs in vitro can further enhance our understanding of metastasis and support the development of personalized therapies. Importantly, the integration of NGS, AI, and machine learning (ML) is expanding the clinical potential of liquid biopsy. These tools improve molecular profiling, enable early cancer detection, and support dynamic treatment monitoring, reinforcing the role of liquid biopsy in precision oncology.
Table 2.
| Comparison criteria | Traditional biopsy | Liquid biopsy | Comparative analysis |
|---|---|---|---|
| Invasiveness | Highly invasive; requires surgical or needle-based extraction, with procedural risks. | Minimally invasive; blood draw-based, reducing patient risk and discomfort. | Liquid biopsy significantly minimizes invasiveness, enhancing patient safety and compliance. |
| Turnaround Time | Longer (days to weeks) due to tissue processing and histological evaluation. | Faster (within days) due to direct molecular assays. | Liquid biopsy provides quicker results, enabling timely clinical decisions. |
| Cost | Higher costs associated with surgery, hospitalization, and complex tissue analysis. | Lower costs, mainly linked to blood sampling and molecular diagnostics. | Liquid biopsy is generally more cost-effective for longitudinal monitoring. |
| Accuracy | Gold standard for diagnosis but limited in representing tumor heterogeneity. | High sensitivity and specificity with evolving accuracy through multi-omics and AI integration. | Liquid biopsy enhances real-time tumor heterogeneity assessment, complementing traditional biopsy. |
| Sample representation | Captures only a specific tumor region at one time. | Reflects dynamic tumor evolution by analyzing circulating biomarkers. | Liquid biopsy offers broader and temporal tumor representation. |
| Monitoring capabilities | Requires repeated invasive procedures for follow-up. | Allows serial, non-invasive monitoring of MRD and therapy resistance. | Liquid biopsy facilitates safer and more frequent disease monitoring. |
| Technological advances | Based on imaging, histopathology, and immunohistochemistry (IHC). | Incorporates NGS, ddPCR, single-cell profiling, and AI-powered analytics. | Liquid biopsy leverages cutting-edge technologies for enhanced molecular insights. |
| Clinical utility | Essential for initial diagnosis, staging, and primary treatment guidance. | Emerging as a complementary tool for early detection, prognosis, and treatment adaptation. | Liquid biopsy is expanding its clinical utility across the cancer care continuum. |
| Challenges | Sampling bias, procedural complications, and limited ability to monitor tumor evolution. | Issues with assay standardization, pre-analytical variability, and clinical validation. | Both approaches have challenges; liquid biopsy requires further standardization for routine use. |
2.3. Technological innovations enhancing liquid biopsy functionality
Recent technological innovations have further refined the diagnostic capabilities of liquid biopsy, particularly through the integration of AI and advanced computational tools [33]. AI-based algorithms are revolutionizing the interpretation of vast multi-omics datasets by identifying subtle biomarker patterns and predicting therapeutic responses with remarkable accuracy [34]. These platforms enable rapid, automated analysis of CTC morphology, cfDNA mutation landscapes, and EV-derived molecular signatures, facilitating precise and data-driven clinical decision-making [35]. Beyond computational advancements, imaging technologies have also elevated liquid biopsy. High-content imaging systems and microfluidic chip-based capture platforms now allow real-time visualization of CTC clusters within circulation, which are implicated in metastasis and therapy resistance [36,37]. Furthermore, hybrid techniques integrating molecular imaging with liquid biopsy have enabled spatial mapping of tumor microenvironment interactions, offering unprecedented insights into metastatic biology [38].
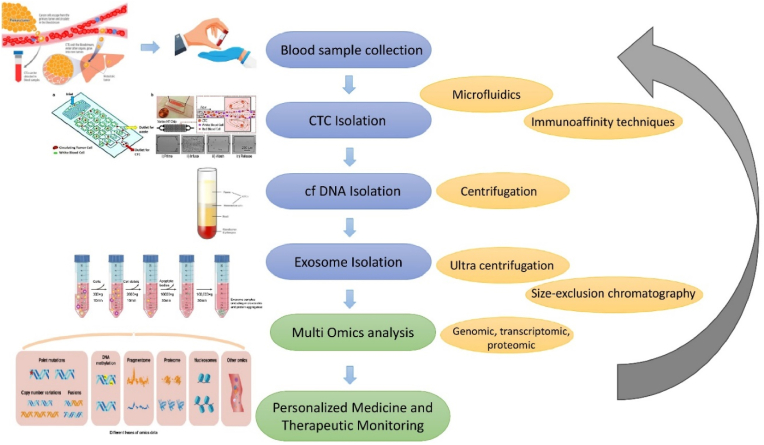
Fig. 2 outlines the workflow of integrated liquid biopsy platforms, demonstrating the collection, processing, and analysis of CTCs, cfDNA, and EVs for clinical applications [39]. The combined integration of multi-omics technologies and AI-powered analytics facilitates comprehensive tumor profiling, early detection of therapeutic resistance, and dynamic adaptation of treatment strategies [40]. These innovations collectively reinforce the role of liquid biopsy as a cornerstone of precision oncology [41]. By enabling non-invasive, high-resolution tumor monitoring in real time, liquid biopsy bridges the translational gap between research advancements and clinical application [42]. As the field evolves, future efforts must focus on addressing challenges related to assay standardization, regulatory validation, and clinical integration [43]. Emphasizing interdisciplinary collaboration and technological innovation will be critical to ensuring that liquid biopsy becomes an indispensable tool in the continuum of cancer care [44].
Fig. 2.
Comprehensive workflow of integrated liquid biopsy techniques for cancer diagnostics and monitoring.
3. Technological advancements in CTC profiling
Advances in technology have significantly enhanced the detection, isolation, and molecular characterization of CTCs, enabling real-time, high-precision insights into tumor biology [41]. Given their critical role in metastasis and therapy resistance, CTCs have emerged as cornerstone biomarkers within the liquid biopsy landscape [41]. Breakthroughs in microfluidics, nanotechnology, and NGS have dramatically improved the sensitivity, specificity, and throughput of CTC detection, thereby expanding their clinical applicability [45]. These technological innovations are reshaping cancer diagnostics, paving the way for early disease detection, dynamic monitoring, and personalized therapeutic strategies [46].
3.1. Microfluidic devices: enhancing precision in rare CTC isolation
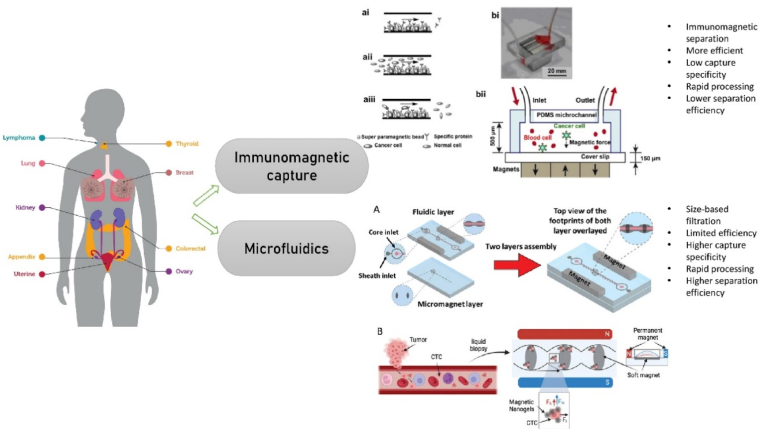
Microfluidic technologies have revolutionized CTC isolation by exploiting physical and biological properties such as size, deformability, and surface markers, outperforming traditional enrichment methods [47]. The rarity of CTCs in peripheral blood (∼1–10 cells/mL) demands highly efficient isolation platforms, and microfluidic devices offer superior specificity and recovery rates [48]. These devices utilize size-based filtration, deformability, and immunoaffinity principles to selectively isolate viable CTCs while minimizing contamination with hematologic components [49]. Size-based microfluidic systems are designed to allow smaller blood cells to pass through while retaining larger CTCs based on their distinct biophysical characteristics [50]. Deformability-based platforms leverage the mechanical differences between tumor cells and normal blood cells to achieve selective enrichment while keeping CTCs intact for further molecular analysis [48]. Immunoaffinity-based microfluidic devices use surface antigen-antibody interactions, targeting markers such as epithelial cell adhesion molecule (EpCAM) to achieve high-purity CTC capture [51]. Next-generation lab-on-a-chip systems further enhance CTC isolation by integrating multiple separation modalities, improving diagnostic accuracy, and enabling real-time disease monitoring [52,53]. Automation within microfluidic platforms also ensures standardized workflows, reproducibility, and scalability for clinical application. Table 3 provides a comparative overview of leading current technologies employed for CTC and cfDNA detection [54]. These advancements are crucial for early cancer detection and offer clinicians timely, actionable insights into disease progression [55].
Table 3.
Summary of current modern technologies for CTC and cfDNA detection.
| Technology | Type | Principle | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Microfluidics | CTCs | Utilizes fluid dynamics and microscale channels to isolate tumor cells based on size, deformability, surface markers, and affinity techniques. | High throughput; Requires minimal sample volume; Automation potential Capable of single-cell analysis. |
Requires precise control of flow dynamics; Potential for cell loss due to shear stress; Device clogging issues. |
[202,203] |
| Immunomagnetic Capture | CTCs | Uses magnetic beads coated with antibodies specific to tumor markers (e.g., EpCAM) to selectively isolate CTCs from blood samples. | High specificity; Suitable for rare cell detection; Compatible with downstream molecular analysis. |
Specificity depends on marker expression; Loss of antigen-negative CTCs; Requires additional purification steps. |
[204] |
| Size-Based Filtration | CTCs | Separates CTCs from normal blood cells based on size and deformability through microporous membranes or microfluidic devices. | Simple and cost-effective methodology; Label-free technique; Effective for larger CTCs. |
May miss smaller or highly deformable CTCs; Limited to size-dependent separation; Variability in cutoff size selection | [205] |
| Next-Generation Sequencing (NGS) | cfDNA | High-throughput sequencing technology enables comprehensive genomic analysis of cfDNA to detect somatic mutations, CNVs, and epigenetic modifications. | Highly sensitive and specific; Enables whole-genome/exome sequencing; Provides insights into tumor evolution and resistance mechanisms. | High cost; Requires specialized bioinformatics expertise; Data interpretation complexity | [206] |
| Digital PCR (dPCR) | cfDNA | Amplifies and partitions DNA into thousands of separate reactions, allowing precise quantification of mutations. | Extremely sensitive; Capable of absolute quantification of target DNA; Useful for detecting low-frequency mutations | Limited multiplexing capability; Higher reagent costs compared to qPCR; Requires droplet-based or chip-based platforms | [207] |
| Droplet-Based Technologies | cfDNA | Uses droplet microfluidics to partition cfDNA into nanoliter droplets for single-molecule analysis, enabling digital PCR and NGS applications. | High throughput; Efficient use of reagents; Compatible with single-cell sequencing |
Technical challenges in droplet generation and stability; Requires specialized microfluidic instrumentation. | [208] |
| Lateral Flow Assays (LFAs) | CTCs, cfDNA | Uses antibodies or nucleic acid probes immobilized on a strip to visually detect CTCs or cfDNA fragments in a rapid, point-of-care manner. | Fast and cost-effective; Portable and user-friendly; Minimal sample processing required. | Lower sensitivity compared to PCR and sequencing-based methods; Semi-quantitative results; May require secondary validation | [209] |
| Electrochemical biosensors | cfDNA | Uses electrochemical signals (e.g., impedance, voltammetry) to detect cfDNA based on hybridization with complementary probes. | Highly sensitive and real-time detection; Potential for point-of-care applications; - Low sample volume required | Requires probe optimization for specificity; Surface fouling may affect signal quality | [210] |
| CRISPR-based detection | CTCs, cfDNA | Utilizes CRISPR-Cas enzymes for sequence-specific detection of oncogenic mutations in cfDNA or CTC-derived RNA. | Ultra-high specificity; Minimal sample input; Potential for rapid, point-of-care testing | Requires precise guide RNA design; Emerging technology; clinical validation ongoing | [211] |
3.2. Nanotechnology-based approaches: boosting sensitivity and specificity
Nanotechnology has dramatically improved the sensitivity and specificity of CTC detection [56]. Functionalized nanoparticles, including gold, magnetic, and silica-based nanomaterials, enhance CTC capture efficiency by selectively binding to tumor-specific surface markers [57]. These nanoparticles are engineered to target markers such as EpCAM and tumor-associated antigens, ensuring high-affinity binding and improved detection [58]. The integration of nanotechnology with microfluidic platforms has led to the development of nano-enhanced assays, significantly lowering detection thresholds and enabling the identification of minimal residual disease (MRD) [59,60]. Detecting rare CTCs post-treatment offers critical insights into the risk of disease relapse and therapeutic resistance [61]. Beyond diagnostics, nanoparticle-based systems have enabled targeted drug delivery strategies, wherein therapeutic agents are conjugated to nanocarriers designed to selectively target and eliminate CTCs [62,63]. Nanotechnology thus plays dual roles, advancing both CTC detection and therapeutic targeting, making it a powerful tool in the arsenal of precision oncology [64]. Additionally, nano-enabled single-cell proteomics platforms have deepened our understanding of CTC heterogeneity, uncovering metastatic subpopulations and resistance phenotypes [65]. Collectively, these innovations underscore the transformative role of nanotechnology in expanding the frontiers of cancer diagnostics and management [66].
3.3. Next-generation sequencing (NGS): comprehensive molecular profiling of CTCs
NGS technologies have enabled comprehensive molecular profiling of CTCs, facilitating the detection of genetic mutations, epigenetic alterations, and markers of therapeutic resistance [67,68]. A major advancement lies in the application of single-cell sequencing to CTCs, offering unprecedented insights into intratumoral heterogeneity, clonal evolution, and epithelial-mesenchymal transition (EMT) processes [69]. Traditional bulk sequencing methods often obscure cellular diversity, whereas single-cell RNA sequencing (scRNA-seq) enables the resolution of distinct molecular subpopulations within the CTC pool [70]. These findings are key to understanding how tumors resist treatment and to designing personalized therapies [71]. The integration of NGS with AI-driven analytics has further enhanced CTC profiling capabilities by enabling automated pattern recognition, mutation detection, and predictive modeling [41,72]. Moreover, multi-omics integration—combining genomic, transcriptomic, and proteomic analyses of CTCs—offers a holistic view of tumor biology, bridging molecular discoveries with clinical application [73].
As the field of CTC-based liquid biopsy continues to advance, future research must focus on standardizing methodologies, expanding multicenter validation studies, and refining bioinformatics pipelines to enhance translational applicability [74]. The next frontier lies in developing multi-modal liquid biopsy platforms that seamlessly integrate CTC analysis with cfDNA sequencing, EV profiling, and AI-powered diagnostics, thereby providing a comprehensive and dynamic portrait of tumor evolution [75]. Collectively, these technological innovations establish CTCs as cornerstone biomarkers in precision oncology, unlocking new avenues for non-invasive cancer detection, real-time monitoring of therapeutic efficacy, and targeted intervention strategies [76]. By combining the precision of microfluidics, the sensitivity of nanotechnology, and the depth of NGS-powered molecular insights, liquid biopsy is poised to transform the future of cancer care, accelerating the clinical implementation of personalized medicine [77].
4. Integrated approaches for enhanced detection in liquid biopsy
Given the complexity of tumor biology, integrating multiple biomolecular platforms enables a comprehensive, dynamic, and high-resolution characterization of malignancies [78]. While liquid biopsy initially focused on individual biomarkers, recent advancements have transformed it into a multi-parametric diagnostic tool, providing real-time insights into tumor progression, metastasis, and therapeutic response [79]. Among circulating biomarkers, CTCs have emerged as particularly informative, offering both phenotypic and molecular perspectives on tumor evolution [80]. Analyzing CTCs, cfDNA, and EVs together within a liquid biopsy framework can improve diagnosis, prognosis, and real-time treatment monitoring [81].
4.1. Synergistic benefits of CTC and cfDNA dual analysis
The integration of CTC analysis with cfDNA sequencing has significantly advanced liquid biopsy applications, enhancing the ability to capture tumor heterogeneity, clonal evolution, and therapy resistance [82]. CTCs represent viable tumor cells actively disseminating into circulation, reflecting real-time biological processes, while cfDNA constitutes a molecular byproduct of apoptotic and necrotic tumor cells, harboring tumor-specific genetic alterations [83]. Together, this dual-analyte approach provides a more holistic and dynamic view of tumor evolution, facilitating early detection, therapy response assessment, and predictive modeling of disease progression [71]. CTC analysis offers insights into phenotypic plasticity, including epithelial-mesenchymal transition (EMT) status, metastatic potential, and resistance mechanisms [84], whereas cfDNA sequencing identifies actionable genomic alterations critical for refining targeted therapies and monitoring emerging resistant clones [85]. The application of advanced technologies such as single-cell RNA sequencing (scRNA-seq) and whole-genome sequencing (WGS) to CTCs and cfDNA further enhances the molecular resolution of liquid biopsy [86]. Detection platforms like digital droplet PCR (ddPCR), NGS, and CRISPR-based assays have improved sensitivity and specificity, enabling real-time monitoring and adaptive therapy adjustments [87]. Fig. 3 illustrates the detection capabilities of various CTC profiling techniques, emphasizing the superior diagnostic power of dual biomarker analysis. This combined approach marks a significant step forward in personalized oncology, enabling dynamic, real-time disease surveillance and improving clinical decision-making [88].
Fig. 3.
Comparative analysis of CTC detection methods: sensitivity, specificity, and clinical applicability.
4.2. Exosome profiling: insights into the tumor microenvironment and metastasis
Exosomes play a fundamental role in tumor communication with the microenvironment, immune evasion, and metastatic progression [89]. These nano-sized vesicles, actively secreted by tumor cells, encapsulate oncogenic proteins, lipids, mRNA, and miRNA, acting as molecular mediators that influence tumor behavior and therapy resistance [90]. Profiling exosomal cargo provides critical insights into tumor biology and serves as a promising source of novel prognostic and predictive biomarkers [91]. The combination of exosome profiling with CTC and cfDNA analysis substantially enhances the molecular landscape captured by liquid biopsy [92]. Exosomes contribute to the conditioning of the pre-metastatic niche and facilitate immune system modulation, both essential for successful metastatic colonization [93]. Their intrinsic stability in circulation and the advent of efficient isolation techniques such as ultracentrifugation, immunoaffinity capture, and nanoparticle tracking have significantly improved their clinical applicability [94]. Integrative approaches, such as combining exosomal RNA (exoRNA) analysis with CTC transcriptomics, offer deeper insights into the molecular pathways driving tumor progression, aiding in therapeutic targeting and precision treatment development [71,95]. Exosome profiling improves tumor classification and therapy monitoring, strengthening the overall value of liquid biopsy.
4.3. Multi-omics platforms: combining genomic, transcriptomic, and proteomic data
The emergence of multi-omics liquid biopsy platforms, combining genomic, transcriptomic, and proteomic analyses, has significantly advanced cancer diagnostics by enabling detailed molecular characterization of tumors [86]. Through the integration of CTC-derived single-cell transcriptomics, cfDNA mutation profiling, and exosomal proteomics, multi-omics approaches capture tumor heterogeneity at multiple biological levels, refining disease stratification and predictive modeling [96]. Single-cell transcriptomic analyses of CTCs provide unprecedented insights into clonal diversity, EMT progression, and metastatic dissemination patterns [97]. When combined with cfDNA-based mutation tracking and exosomal biomarker profiling, these multi-omics strategies substantially improve biomarker discovery and therapeutic targeting [98]. The application of artificial intelligence (AI) and machine learning (ML) algorithms has further propelled multi-omics integration, enabling the identification of complex biomarker signatures and enhancing predictive capabilities [99]. AI-driven analytics facilitate early diagnosis, treatment optimization, and the identification of emerging resistance mechanisms through sophisticated pattern recognition across large multi-modal datasets [100].
Table 4 summarizes the transformative potential of AI-enhanced multi-omics analyses, highlighting their impact on personalized oncology and biomarker-driven therapy selection.
Table 4.
AI-driven multi-omics integration in liquid biopsy.
| Multi-omics component | AI-driven enhancement | Clinical application | References |
|---|---|---|---|
| CTC single-cell transcriptomics | AI models identify clonal diversity, epithelial-mesenchymal transition (EMT) markers, and stemness signatures through deep learning-based clustering and feature selection. | Enables real-time monitoring of metastatic progression and treatment response. | 212 |
| cfDNA mutation profiling | Machine learning algorithms detect low-frequency mutations, tumor evolution patterns, and minimal residual disease (MRD) through error-correction methods. | Facilitates early resistance detection and personalized therapy adaptation. | 213 |
| Exosomal proteomics | AI-driven proteomic analysis deciphers key protein biomarkers associated with tumor heterogeneity, immune evasion, and therapy resistance. | Enhances therapy response prediction and biomarker discovery for targeted interventions. | 214 |
| AI-powered data integration | Correlates multi-omics data (CTCs, cfDNA, exosomes) using deep learning frameworks, enhancing feature selection and predictive modeling. | Advances precision oncology by improving diagnostic accuracy and personalized treatment plans. | 215 |
| Spatial imaging-liquid biopsy hybrids | AI-driven fusion of spatial transcriptomics with liquid biopsy-derived molecular data for integrative tumor profiling. | Enables tumor microenvironment characterization and identification of immune escape mechanisms. | 216 |
| Longitudinal biomarker tracking | AI-based longitudinal models analyze temporal biomarker changes to predict tumor evolution, resistance mechanisms, and relapse probability. | Supports adaptive treatment strategies and dynamic disease monitoring. | 217 |
Several cutting-edge advancements are accelerating the clinical translation of integrated liquid biopsy approaches. AI-driven predictive modeling enhances biomarker discovery by identifying high-confidence molecular signatures [101]; spatial imaging-liquid biopsy hybrids provide integrated molecular and spatial insights into tumor architecture [102]; and longitudinal biomarker tracking enables dynamic real-time monitoring of therapy-induced tumor evolution [103]. As liquid biopsy technologies continue to evolve, their seamless integration into routine oncology practice will facilitate precision-guided therapeutics, accelerate early detection strategies, and enable real-time tumor surveillance [104]. The convergence of CTCs, cfDNA, and exosomal biomarkers within an AI-powered multi-omics framework represents a significant milestone in cancer diagnostics, providing high-resolution, real-time molecular insights that drive personalized therapeutic interventions [105]. Through multi-analyte approaches, oncology is transitioning towards a truly individualized care paradigm, where therapeutic regimens are continuously optimized based on real-time molecular profiling [106].
Ultimately, the integration of CTCs, cfDNA, and exosomal biomarkers within liquid biopsy frameworks enhances the depth of tumor characterization, refines diagnostic accuracy, and improves clinical outcomes, ushering in a new era of precision cancer management [107,108].
5. Clinical translation of liquid biopsy techniques: opportunities and challenges
Recent advancements in liquid biopsy technologies have positioned CTCs as cornerstone biomarkers, offering a minimally invasive and dynamic approach to cancer detection and treatment monitoring [109]. By enabling real-time tracking of tumor evolution, liquid biopsy enhances early detection, therapeutic response evaluation, and metastasis prediction [110]. However, the widespread clinical adoption of CTC-based liquid biopsy still faces significant challenges, including assay standardization, analytical validation, and regulatory approvals [111]. Addressing these barriers is essential for realizing the full potential of liquid biopsy in precision oncology [112].
5.1. Applications in early detection and monitoring
Liquid biopsy has revolutionized early cancer detection by offering superior sensitivity and specificity compared to traditional diagnostic approaches [113]. The combined detection of CTCs and cfDNA enables the identification of malignancies at very early stages, often before the onset of clinical symptoms [114], a crucial advantage for improving patient survival and enabling timely interventions [115]. CTCs provide detailed phenotypic and molecular insights into tumor heterogeneity, metastatic potential, and therapy resistance [106], while cfDNA offers a complementary perspective by capturing tumor-specific genetic alterations [116]. Integration of CTC enumeration and molecular profiling with cfDNA mutation analysis enhances diagnostic precision, enabling the detection of actionable mutations and patient stratification for targeted therapies [117]. Table 5 summarizes key clinical trials supporting the efficacy of CTC-based liquid biopsy in disease monitoring and treatment planning. Moreover, CTC characterization aids in predicting metastatic progression through the analysis of morphological features, gene expression profiles, and epithelial-mesenchymal transition (EMT) markers [118,119]. The addition of exosome profiling further enriches tumor characterization, capturing biomolecular signatures reflective of the tumor microenvironment and malignancy progression [120]. This integrated multi-analyte approach strengthens precision oncology efforts by enabling individualized treatment strategies, recurrence surveillance, and adaptive therapy optimization [121].
Table 5.
Summary of clinical trials investigating liquid biopsy applications in cancer diagnostics and monitoring.
| Trial name | Cancer type | Biomarker | Objective | Key findings/Outcomes |
|---|---|---|---|---|
| NCT02868788 | Non-Small Cell Lung Cancer | cfDNA | To assess the efficacy of cfDNA in monitoring treatment response and detecting resistance mutations. | cfDNA dynamics correlated with imaging results; enabled earlier prediction of resistance mutations and treatment failure. |
| NCT03595960 | Breast Cancer | CTCs | Evaluate CTCs as predictors of response to targeted therapies. | Higher baseline CTC counts were linked to poorer prognosis; dynamic CTC changes reflected treatment effectiveness. |
| NCT03448716 | Colorectal Cancer | cfDNA and CTCs | To analyze the role of liquid biopsy in guiding clinical decisions during therapy. | Liquid biopsy-guided therapy adjustments improved patient outcomes; showed superior sensitivity for recurrence detection over standard imaging. |
| NCT04116567 | Ovarian Cancer | cfDNA Methylation Patterns | Investigate cfDNA methylation signatures for early detection and monitoring of disease recurrence. | Identified tumor-specific methylation markers; demonstrated high predictive value for relapse risk assessment. |
| NCT03612586 | Pancreatic Cancer | CTCs | Evaluate the role of CTCs in detecting metastatic progression and therapeutic response. | CTC presence strongly associated with disease progression; provided real-time insights into therapeutic efficacy. |
| NCT04212836 | Multiple Cancer Types | cfDNA and Exosomal Biomarkers | Explore multi-omics integration of cfDNA and exosome analysis for enhanced tumor monitoring. | AI-driven multi-omics analysis improved tumor heterogeneity characterization and personalized treatment response evaluation. |
| NCT05059444 | Prostate Cancer | CTCs and RNA Profiling | Assess RNA signatures in CTCs for monitoring androgen receptor signaling inhibitors. | RNA-based CTC profiling accurately predicted treatment resistance and disease progression. |
| NCT04717129 | Glioblastoma | cfDNA and Tumor-Derived EVs | Investigate extracellular vesicle (EV)-based cfDNA for non-invasive brain tumor monitoring. | Liquid biopsy enabled early detection of tumor progression and response to targeted therapy. |
5.2. Real-time assessment of therapeutic efficacy
CTC-based liquid biopsy provides dynamic monitoring of treatment efficacy, allowing real-time assessment of tumor adaptation under therapeutic pressure [122]. Molecular and functional profiling of CTCs reveals therapy-induced clonal evolution and emerging resistance mechanisms [123]. For instance, cfDNA-detected resistance mutations can preemptively indicate treatment failure, prompting therapeutic adjustments [131]. Changes in CTC counts correlate strongly with treatment responses: declining levels suggest therapeutic success, while rising counts indicate resistance or disease progression [124]. Single-cell sequencing of CTCs enables high-resolution molecular profiling, elucidating EMT dynamics, clonal diversification, and resistance mechanisms [125,126]. Integration of CTC transcriptomics with cfDNA mutational profiling refines predictive models of therapeutic response, facilitating adaptive treatment strategies that improve patient outcomes [127].
5.3. Challenges in clinical implementation
Despite its transformative potential, CTC-based liquid biopsy faces significant hurdles in clinical standardization and regulatory acceptance [128]. The heterogeneity of detection methodologies leads to variability in CTC isolation efficiency, purity, and downstream analyses [129], underscoring the urgent need for standardized protocols [130]. To gain regulatory approval, tests must be carefully validated for accuracy, reliability, and clinical relevance [131]. The complex regulatory landscape often delays clinical translation, necessitating harmonized frameworks and cross-institutional collaborations [132]. Table 6 outlines major regulatory considerations critical for integrating CTC-based diagnostics into routine oncology care. Another major challenge lies in integrating the high-dimensional datasets generated by multi-omics liquid biopsy approaches [133]. Effective interpretation requires sophisticated bioinformatics pipelines, AI-driven analytics, and interdisciplinary collaboration among oncologists, bioinformaticians, and molecular pathologists [134]. Developing robust, real-time, clinically actionable computational tools is key to unlocking the full potential of liquid biopsy [135,136]. Ongoing clinical trials and research collaborations are paving the way for broader clinical adoption [136,137]. As summarized in Table 7, efforts to standardize protocols, streamline regulatory pathways, and advance computational analytics are critical for establishing liquid biopsy as a mainstay in precision oncology. By overcoming these barriers, CTC-based liquid biopsy is poised to revolutionize cancer detection, monitoring, and individualized treatment planning [138,139].
Table 6.
| Regulatory challenge | Description | Potential solutions |
|---|---|---|
| Standardization of CTC Enumeration | Variability in detection methods affects reliability and reproducibility. | Establish global consensus guidelines and standardized protocols for CTC isolation and quantification. |
| Analytical Sensitivity & Specificity | Validation hurdles, including false positives/negatives, delay clinical adoption. | Implement harmonized regulatory frameworks with predefined performance metrics and inter-laboratory proficiency testing. |
| Data Integration Complexity | High-dimensional multi-omics datasets require advanced computational tools for meaningful interpretation. | Develop AI-driven bioinformatics pipelines and standardized data-sharing frameworks to enhance interoperability. |
| Cross-Institutional Variability | Differences in sample processing, CTC enrichment techniques, and analytical methods across laboratories hinder consistency. | Conduct multi-center validation studies with standardized methodologies and proficiency testing programs. |
| Clinical Utility Demonstration | Lack of large-scale, multi-phase clinical trials proving CTC-based liquid biopsy effectiveness in routine oncology care. | Expand real-world application studies, integrate CTC-based assays into precision oncology trials, and ensure post-market surveillance. |
| Regulatory Approval Pathways | Lack of clear regulatory pathways for novel CTC-based assays leads to prolonged approval times. | Advocate for adaptive regulatory frameworks that expedite approvals for validated CTC technologies with demonstrated clinical impact. |
| Reimbursement & Cost Barriers | High costs and lack of reimbursement policies limit clinical adoption. | Develop health-economic models demonstrating cost-effectiveness and advocate for insurance coverage policies. |
Table 7.
Strategies to overcome challenges in CTC-based liquid biopsy.
| Challenge | Current limitation | Proposed solution |
|---|---|---|
| CTC isolation & purity | Variability in detection methods and purity levels due to differences in isolation techniques. | Establish standardized isolation protocols integrating microfluidics, immunomagnetic capture, and size-based filtration. |
| Multi-Omics data interpretation | Complexity in integrating CTCs, cfDNA, and exosomal biomarkers for precision oncology. | AI- and ML-driven bioinformatics pipelines for multi-omics data harmonization and predictive modeling. |
| Regulatory approval hurdles | Stringent validation requirements and lengthy approval processes for clinical implementation. | Develop collaborative regulatory frameworks with adaptive approval pathways for novel liquid biopsy assays. |
| Clinical adoption | Limited physician awareness and reluctance to transition from traditional biopsies. | Implement comprehensive training programs and clinical guidelines to enhance physician familiarity and confidence. |
| Reproducibility | Variability in results across laboratories due to differences in sample processing and analysis. | Establish cross-laboratory standardization through multi-center validation studies and proficiency testing programs. |
| Real-time data utility | Delays in clinical decision-making due to manual data analysis and interpretation. | Integrate automated AI-powered reporting systems for real-time analysis and clinical decision support. |
| Cost and reimbursement issues | High costs of CTC-based assays and lack of insurance reimbursement limit accessibility. | Develop cost-effectiveness models and advocate for reimbursement policies through health economic studies. |
| Tumor heterogeneity representation | Single time-point CTC analysis may not capture real-time tumor evolution. | Implement longitudinal monitoring strategies using serial liquid biopsies for dynamic disease tracking. |
5.4. Clinical validation and real-world applications of CTC-based liquid biopsy
The clinical translation of CTC-based liquid biopsy has accelerated, driven by high-impact studies demonstrating its potential in early detection, real-time therapeutic monitoring, and prognostic assessment across cancer types [140]. Integration of CTC enumeration and molecular profiling into clinical workflows is reshaping personalized oncology strategies [141].
5.4.1. CTCs in breast cancer: monitoring treatment response and predicting outcomes
A landmark Phase III trial (SWOG S0500) validated CTC enumeration as a real-time predictor of treatment efficacy in metastatic breast cancer (MBC), showing that persistent high CTC levels after initial chemotherapy cycles correlated with poor progression-free survival (PFS) and overall survival (OS) [142,143]. This highlighted the utility of early therapeutic intervention based on CTC dynamics [144]. Recent HER2-positive breast cancer trials demonstrated that dynamic changes in CTC-HER2 status during therapy could guide switching between HER2-targeted treatments, as being evaluated in the ongoing PERSEVERE study [[145], [146], [147]].
5.4.2. CTC-based prognostic insights in colorectal cancer
In colorectal cancer (CRC), the VISNU-1 trial confirmed that high baseline CTC counts predict poor OS and PFS in patients receiving first-line chemotherapy [71,148,149]. Molecular profiling of CTCs has also revealed key mutations (e.g., KRAS, BRAF), enabling the early detection of treatment resistance and facilitating adaptive therapy strategies [150,151].
5.4.3. CTCs in lung cancer: early detection and therapy guidance
In lung cancer, the STIC CTC METABREAST study demonstrated that baseline CTC levels can predict treatment responses, guiding the choice between endocrine therapy and chemotherapy [134]. In NSCLC, CTC-derived PD-L1 expression has emerged as a promising biomarker to predict immunotherapy response, complementing tissue-based PD-L1 testing [133,[152], [153], [154]].
5.4.4. CTCs as a surrogate biomarker for MRD in prostate cancer
In prostate cancer, the PROPHECY trial demonstrated that AR-V7-positive CTCs predict poor response to androgen receptor inhibitors, influencing treatment decisions between hormonal therapy and chemotherapy [[155], [156], [157]]. The ongoing IMPACT trial explores CTC-based stratification for early therapeutic interventions in high-risk prostate cancer patients [158].
5.4.5. Clinical translation and future implications
The incorporation of CTC-based liquid biopsy into oncology practice is revolutionizing cancer monitoring and individualized treatment [159]. Real-time CTC profiling enables personalization of therapy based on tumor evolution and resistance emergence [160,161]. The detection of markers such as PD-L1 and AR-V7 within CTCs has introduced new standards for guiding immunotherapy and hormonal therapy selection [162]. Future research must focus on standardizing CTC detection protocols, integrating AI-driven analytics, and expanding clinical trials to establish universal guidelines [133,163]. CTC-based liquid biopsy is advancing precision oncology by enabling adaptive, personalized treatment strategies, reducing unnecessary interventions, and improving clinical outcomes. The clinical validation of CTC-based liquid biopsy underscores its transformative potential across multiple cancer types, setting the stage for its broader integration into precision oncology. While significant progress has been made in early detection, therapeutic monitoring, and prognostic modeling, the expanding applications of liquid biopsy extend beyond current clinical practice.
6. Potential applications of liquid biopsy with special focus on CTCs: advancements in cancer diagnostics and management
Liquid biopsy has emerged as a transformative platform in cancer diagnostics and therapeutic monitoring, offering a non-invasive, dynamic, and high-resolution approach for assessing tumor evolution in real time [164]. Among its various analytes, CTCs serve as central biomarkers, providing comprehensive insights into tumor heterogeneity, metastatic potential, and treatment resistance [165]. This section explores the diverse applications of CTC-based liquid biopsy across research, clinical practice, and pharmaceutical development, underscoring its growing impact on advancing precision oncology [166].
6.1. Role of liquid biopsy in cancer research and biomarker discovery
In oncological research, CTC analysis has revolutionized biomarker discovery and validation, facilitating the identification of tumor-specific genetic, epigenetic, and proteomic signatures critical for early cancer detection and targeted therapy development [167]. Unlike static tissue biopsies, CTC-based liquid biopsy enables real-time molecular profiling of live tumor cells, capturing dynamic tumor evolution and therapy-induced clonal selection [168]. The integration of advanced isolation techniques, such as microfluidic enrichment, immunoaffinity capture, and label-free detection platforms, has significantly improved CTC capture efficiency, enabling the study of rare metastatic subpopulations with unprecedented precision [169]. Furthermore, NGS and single-cell RNA sequencing (scRNA-seq) have facilitated high-resolution molecular characterization of CTCs, unveiling driver mutations, transcriptional alterations, and epithelial-mesenchymal transition (EMT)-associated gene expression patterns [170]. This helps improve early detection and supports the design of biomarker-based treatments [171]. Additionally, CTC-derived biomarkers offer great promise for patient stratification based on tumor biology, guiding personalized treatment plans [172]. By correlating CTC phenotypic and molecular characteristics with clinical outcomes, researchers are refining predictive models of disease progression and therapy response, paving the way for more effective targeted interventions [16]. Multi-omics profiling, integrating CTCs, cfDNA, and exosomes, further enhances the diagnostic power of liquid biopsy by offering a multidimensional view of tumor evolution [75].
6.2. Clinical applications: enhancing early detection, monitoring, and personalized treatment
In clinical practice, CTC-based liquid biopsy plays a pivotal role in real-time tumor monitoring, providing a minimally invasive alternative to tissue biopsies, particularly in cases involving anatomically challenging or surgically inaccessible tumors [173]. Serial tracking of CTC burden and molecular alterations enables early detection of disease recurrence, therapeutic resistance, and treatment efficacy [174]. Longitudinal CTC monitoring allows clinicians to detect emerging drug resistance mutations, phenotypic shifts, and clonal evolution, enabling proactive therapeutic adjustments [18]. For instance, an increase in mesenchymal-like CTCs often signals therapy resistance and metastatic progression, warranting shifts toward EMT-targeted therapies [175]. Conversely, a sustained decline in CTC counts following treatment correlates with favorable prognosis, reinforcing their clinical significance [176,177]. Moreover, the integration of CTC analysis with cfDNA mutation profiling and exosome-derived molecular signatures provides a comprehensive real-time assessment of tumor dynamics. This multi-analyte approach enables oncologists to design tailored therapeutic regimens, reduce unnecessary treatments, and ultimately improve patient survival outcomes.
6.3. Pharmaceutical applications: optimizing drug development and clinical trial design
The pharmaceutical industry is increasingly utilizing CTC-based liquid biopsy to optimize drug development and refine clinical trial design. Real-time monitoring of tumor evolution via CTC analysis enhances patient stratification for personalized treatments, improving therapeutic precision [178].
Pharmaceutical companies are employing CTC enumeration and molecular profiling to assess drug efficacy, predict resistance mechanisms, and optimize clinical trial inclusion criteria [179]. By identifying tumor-specific markers within CTC populations, researchers can tailor drug targeting strategies, maximizing therapeutic efficacy while minimizing adverse effects [180,181]. Additionally, CTC-based liquid biopsy serves as a valuable companion diagnostic tool, accelerating the development of biomarker-driven therapies [8]. One promising application involves tracking therapy-induced mutations and resistance pathways using CTC-derived RNA sequencing, which enables early intervention strategies such as combination therapy or second-line treatment adaptations [182]. This is particularly impactful in immuno-oncology, where immune checkpoint inhibitor resistance remains a significant challenge. Furthermore, CTC-based approaches are transforming clinical trial methodologies by enabling real-time patient monitoring, interim data-driven protocol adjustments, and more efficient regulatory approval processes. By continuously monitoring CTC dynamics and cfDNA alterations, researchers can optimize trial endpoints and reduce trial durations, thereby accelerating the clinical development of innovative therapies. As liquid biopsy technologies continue to advance, CTC-based approaches are poised to become indispensable in the evolving landscape of precision oncology. Future research must focus on standardizing CTC detection methods, optimizing bioinformatics pipelines, and advancing AI-driven biomarker discovery [183]. Key emerging trends include the development of CTC-derived organoids for functional drug testing, enabling patient-specific drug response modeling and truly personalized therapeutic selection; AI-powered predictive modeling of CTC transcriptomes to refine tumor evolution models and enhance therapy resistance prediction; and spatial mapping of CTCs within the bloodstream to deepen our understanding of metastatic dissemination pathways [184]. By addressing existing technical and clinical challenges, CTC-based liquid biopsy holds the potential to become a cornerstone of precision medicine, enabling earlier cancer detection, real-time monitoring, and biomarker-guided therapeutic interventions. The continuous refinement of CTC isolation, characterization technologies, and integration with multi-omics profiling will accelerate the transition toward an individualized oncology landscape, ultimately transforming cancer diagnosis, prognosis, and treatment paradigms.
7. Future directions and emerging trends in CTC-based liquid biopsy
As liquid biopsy technologies continue to advance, innovations in microfluidics, nanotechnology, artificial intelligence (AI), and multi-omics integration are driving improvements in the precision, sensitivity, and clinical applicability of CTC-based diagnostics [185]. CTCs are now seen as important real-time markers for tracking cancer, guiding treatment, and predicting spread, reinforcing their central role in precision oncology. However, their rarity in circulation presents significant technical challenges, necessitating the development of highly efficient isolation and molecular characterization platforms [186]. The integration of microfluidic systems has revolutionized CTC capture, enabling high-throughput, label-free enrichment with enhanced purity and cell viability, thereby facilitating downstream molecular analyses [50]. Concurrently, nanotechnology-driven approaches utilizing functionalized nanoparticles conjugated with CTC-specific biomarkers have enhanced the high-affinity capture and multi-modal characterization of CTCs across genomic, transcriptomic, and proteomic dimensions [56]. Advances in imaging methodologies, such as fluorescence-based high-content imaging and imaging mass cytometry, provide real-time tracking of CTCs in circulation, offering critical insights into metastatic progression, cellular heterogeneity, and treatment response [187].
AI and ML are transforming the landscape of CTC-based liquid biopsy by enhancing data interpretation, biomarker discovery, and clinical decision-making processes. AI-driven algorithms now analyze large-scale datasets derived from CTC morphology, single-cell transcriptomics, and multi-omics profiles to refine diagnosis and predict therapeutic responses [188]. Deep learning models facilitate image-based CTC classification and mutation profiling, improving detection accuracy, automating phenotypic analyses, and optimizing therapeutic targeting strategies [161]. The role of CTC analysis in personalized medicine continues to expand, particularly in the monitoring of minimal residual disease (MRD) and the early detection of disease relapse [189]. Unlike static tissue biopsies, which provide only a single snapshot of tumor biology, serial CTC profiling enables longitudinal monitoring of clonal evolution, therapy-induced resistance mechanisms, and tumor progression dynamics [190]. One of the most promising applications of CTC analysis is its capacity to guide therapeutic decisions based on the detection of dynamic molecular changes. Evaluation of CTC-derived transcriptomic and proteomic alterations enables the early identification of resistance markers, allowing for timely adjustment of targeted therapies or immunotherapy regimens [16].
Additionally, combining trends in CTC enumeration with cfDNA mutational profiling enhances patient stratification based on therapeutic response likelihood, thereby optimizing clinical outcomes [3]. The integration of CTC profiling with radiomic and imaging-based data further refines treatment response prediction, linking CTC phenotypic diversity to imaging-derived tumor characteristics and facilitating precision-guided therapeutic adaptations [191]. However, some important challenges still need to be addressed. Standardization of sample collection, processing, and data interpretation is critical for clinical implementation. Variability in isolation methodologies, discrepancies in molecular characterization protocols, and the absence of universally accepted clinical thresholds for CTC enumeration continue to impede widespread adoption. Establishing harmonized standard operating procedures (SOPs) across academic institutions, clinical laboratories, and industry stakeholders is essential to ensure reproducibility and clinical reliability. Regulatory approval remains another major hurdle. CTC-based diagnostics require extensive validation to demonstrate analytical performance, clinical sensitivity, and prognostic utility. Collaborative efforts among regulatory agencies, scientific consortia, and industry partners are needed to develop clear guidelines that facilitate the approval and commercialization of CTC-based assays.
Future research should prioritize the development of automated, AI-enhanced CTC detection platforms to improve standardization and reproducibility. Advancements in single-cell multi-omics profiling will be crucial for enabling detailed characterization of CTC heterogeneity and therapy resistance pathways. Additionally, integrating spatial and temporal CTC tracking with advanced imaging technologies will refine real-time, patient-specific therapeutic strategies.
As the field progresses, CTC-based diagnostics are poised to become integral components of next-generation cancer care, enabling ultra-sensitive early detection, adaptive therapeutic monitoring, and real-time precision medicine applications. Emerging trends in multi-omics integration, AI-driven biomarker discovery, and lab-on-a-chip microfluidic technologies will further establish CTCs as indispensable biomarkers within the liquid biopsy landscape. By overcoming current technical and regulatory barriers, CTC-based liquid biopsy is set to revolutionize cancer diagnostics, therapeutic decision-making, and clinical trial optimization. Over the next decade, the seamless integration of CTC analysis into routine oncology workflows is anticipated, ushering in an era of real-time molecular surveillance and patient-centric precision oncology.
8. Conclusion
This review highlights the pivotal role of liquid biopsy in advancing modern oncology, with a special emphasis on CTCs as central biomarkers for understanding tumor heterogeneity, disease progression, and therapeutic resistance. The integration of CTC analysis with complementary biomarkers such as cfDNA and exosomal profiling has significantly enhanced the real-time monitoring of tumor dynamics, offering a comprehensive and multidimensional molecular snapshot of cancer evolution. Technological advancements in microfluidics, nanotechnology, and NGS have substantially improved the sensitivity, specificity, and resolution of CTC isolation and characterization, enabling early detection, precise treatment stratification, and real-time assessment of therapeutic response. Emerging applications of AI and ML are revolutionizing the interpretation of complex multi-omics datasets, uncovering hidden biomarker patterns and refining predictive models for therapy resistance and disease progression. Nanotechnology-driven innovations, including the development of functionalized nanoparticles and the exploration of nanorobots for targeted therapeutic delivery, are further expanding the clinical utility of CTC-based liquid biopsy, facilitating real-time molecular surveillance and precision-targeted interventions. Together, these developments are helping establish liquid biopsy as a key tool in personalized cancer care by enabling non-invasive, real-time monitoring and adaptive therapeutic management. Despite these promising developments, several challenges must be addressed to achieve the widespread clinical adoption of CTC-based liquid biopsy. These include the standardization of sample collection and processing methodologies, the validation of predictive biomarkers across diverse patient populations, and the establishment of robust regulatory frameworks to ensure reproducibility, consistency, and clinical reliability. As the field continues to evolve, the convergence of multi-omics profiling, AI-driven analytics, and real-time liquid biopsy surveillance is poised to expand the applications of CTC-based technologies, solidifying their role in next-generation oncology diagnostics and therapeutics. With ongoing innovation and cooperation between researchers, clinicians, and regulators, liquid biopsy holds the potential to revolutionize cancer diagnosis, monitoring, and treatment—ushering in a new era of precision, personalization, and patient-centered oncology.
Credit author statement
Thanmayi Velpula: Investigation; Validation; Writing–original draft; Viswanath Buddolla: Concept, Resources; Supervision; Project administration; Reviewing & editing.
Ethical statement
This review forms part of a project approved by the Institutional Animal Ethics Committee (IAEC) of Dr. Buddolla's Institute of Life Sciences, Tirupati, India (DRBILS/IAEC/LS/2023/01).
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, we used LINER-AI to enhance the clarity, structure, and scientific presentation of the manuscript. After utilizing this tool, we thoroughly reviewed and edited the content to ensure its accuracy, originality, and alignment with the objectives of the publication. We take full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Dr. Buddolla's Research and Educational Society, India for their support and encouragement in successfully completing this manuscript.
References
- 1.Gerratana L., Davis A.A., Foffano L., Reduzzi C., Rossi T., Medford A.…Cristofanilli M. Integrating Machine Learning-Predicted Circulating Tumor Cells (CTCs) and circulating tumor DNA (ctDNA) in Metastatic Breast Cancer: a proof of principle study on endocrine resistance profiling. Cancer Lett. 2024 doi: 10.1016/j.canlet.2024.217325. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y., He J., Zhou Y., Yu Y., Hui H., Guo L., Yin H. Constructing a highly sensitive duplex immunoassay using AuNPs and AgNPs as nanolabels for investigating the epithelial-mesenchymal transition occurring on circulating tumor cells with lung cancer patients. Biosens Bioelectron. 2025;270 doi: 10.1016/j.bios.2024.116947. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto D.T., Sequist L.V., Lee R.J. Circulating tumour cells—monitoring treatment response in prostate cancer. Nat Rev Clin Oncol. 2014;11(7):401–412. doi: 10.1038/nrclinonc.2014.82. [DOI] [PubMed] [Google Scholar]
- 4.Viswanath B., Kim S. Recent insights into the development of nanotechnology to detect circulating tumor cells. TrAC, Trends Anal Chem. 2016;82:191–198. [Google Scholar]
- 5.Thery L., Meddis A., Cabel L., Proudhon C., Latouche A., Pierga J.Y., Bidard F.C. Circulating tumor cells in early breast cancer. JNCI Cancer Spectr. 2019;3(2) doi: 10.1093/jncics/pkz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L., Jiang H., Zeng B., Wang X., Bao Y., Chen C.…Yuan J. Liquid biopsy in lung cancer. Clin Chim Acta. 2024;554 doi: 10.1016/j.cca.2023.117757. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Xu J., Liu X., Li X., Xu Z. A microfluidic chip incorporating magnetic sorting and invasive separation for isolation, culture and telomerase analysis of circulating tumor cells. Talanta. 2025;285 doi: 10.1016/j.talanta.2024.127316. [DOI] [PubMed] [Google Scholar]
- 8.Gavioli V.D.A., Filho M.V.B., Castro G.R., Filho P.T.H., Ferrasi A.C., Pedrosa V.A. Rapid isolation of circulating tumor cells from glioblastoma patients using a lateral filter array microfluidic device. Chemosensors. 2025;13(2):64. [Google Scholar]
- 9.Gao S., Li X., Hu Z., Wang Z., Hao X. Dual targeting negative enrichment strategy for highly sensitive and purity detection of CTCs. Front Chem. 2024;12 doi: 10.3389/fchem.2024.1400988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lao Z., Ren X., Zhuang D., Xie L., Zhang Y., Li W.…Wang H. A phenotype-independent “label-capture-release” process for isolating viable circulating tumor cells in real-time drug susceptibility testing. Innovation. 2025 doi: 10.1016/j.xinn.2025.100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Q., Xie J., Mei W., Zeng C. Methylated circulating tumor DNA in hepatocellular carcinoma: a comprehensive analysis of biomarker potential and clinical implications. Cancer Treat Rev. 2024 doi: 10.1016/j.ctrv.2024.102763. [DOI] [PubMed] [Google Scholar]
- 12.Shang Z., Xiang C., Ding B., Zhu Q., Yu M., Han Y. Single-cell transcriptome analysis reveals 2 subtypes of tumor cells of sclerosing pneumocytoma with distinct molecular features and clinical implications. Mod Pathol. 2024;37(9) doi: 10.1016/j.modpat.2024.100560. [DOI] [PubMed] [Google Scholar]
- 13.Hariri A., Mirian M., Khosravi A., Zarepour A., Iravani S., Zarrabi A. Intersecting pathways: the role of hybrid E/M cells and circulating tumor cells in cancer metastasis and drug resistance. Drug Resist Updates. 2024 doi: 10.1016/j.drup.2024.101119. [DOI] [PubMed] [Google Scholar]
- 14.Hassane M. Artificial intelligence-driven precision medicine in cancer treatment. Science. 2024;1 [Google Scholar]
- 15.Rucci C., de Simone G., Salathia S., Casadidio C., Censi R., Bordoni L. Exploring mitochondrial DNA copy number in circulating cell-free DNA and extracellular vesicles across cardiovascular health status: a prospective case–control pilot study. FASEB J. 2024;38(10) doi: 10.1096/fj.202400463R. [DOI] [PubMed] [Google Scholar]
- 16.Kotsifaki A., Maroulaki S., Armakolas A. Exploring the immunological profile in breast cancer: recent advances in diagnosis and prognosis through circulating tumor cells. Int J Mol Sci. 2024;25(9):4832. doi: 10.3390/ijms25094832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar M.A., Baba S.K., Sadida H.Q., Marzooqi S.A., Jerobin J., Altemani F.H.…Bhat A.A. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Targeted Ther. 2024;9(1):27. doi: 10.1038/s41392-024-01735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X., Wei S., Lv X. Circulating tumor cells: from new biological insights to clinical practice. Signal Transduct Targeted Ther. 2024;9(1):226. doi: 10.1038/s41392-024-01938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat G.R., Sethi I., Sadida H.Q., Rah B., Mir R., Algehainy N.…Bhat A.A. Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024;43(1):197–228. doi: 10.1007/s10555-024-10172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Gao X., Ho Y.P., Liu M., Han Y., Li D.L.…Zhang C.Y. Controllable assembly of a quantum dot-based aptasensor guided by CRISPR/Cas12a for direct measurement of circulating tumor cells in human blood. Nano Lett. 2024;24(7):2360–2368. doi: 10.1021/acs.nanolett.3c04828. [DOI] [PubMed] [Google Scholar]
- 21.Jarmuzek P., Wawrzyniak-Gramacka E., Morawin B., Tylutka A., Zembron-Lacny A. Diagnostic and prognostic value of circulating DNA fragments in glioblastoma multiforme patients. Int J Mol Sci. 2024;25(8):4221. doi: 10.3390/ijms25084221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du S., Cao K., Yan Y., Wang Y., Wang Z., Lin D. Developments and current status of cell‐free DNA in the early detection and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 2024;39(2):231–244. doi: 10.1111/jgh.16416. [DOI] [PubMed] [Google Scholar]
- 23.Piana D., Iavarone F., De Paolis E., Daniele G., Parisella F., Minucci A.…Urbani A. Phenotyping tumor heterogeneity through proteogenomics: study models and challenges. Int J Mol Sci. 2024;25(16):8830. doi: 10.3390/ijms25168830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He D., Cui B., Lv H., Lu S., Zhu Y., Cheng Y.…Zhang H. Blood-derived extracellular vesicles as a promising liquid biopsy diagnostic tool for early cancer detection. Biomolecules. 2024;14(7):847. doi: 10.3390/biom14070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalali M., Lu Y., del Real Mata C., Rak J., Mahshid S. Nanoscopic technologies toward molecular profiling of single extracellular vesicles for cancer liquid biopsy. Appl Phys Rev. 2025;12(1) [Google Scholar]
- 26.Allen T.A. The role of circulating tumor cells as a liquid biopsy for cancer: advances, biology, technical challenges, and clinical relevance. Cancers. 2024;16(7):1377. doi: 10.3390/cancers16071377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zieren R.C., Zondervan P.J., Pienta K.J., Bex A., de Reijke T.M., Bins A.D. Diagnostic liquid biopsy biomarkers in renal cell cancer. Nat Rev Urol. 2024;21(3):133–157. doi: 10.1038/s41585-023-00818-y. [DOI] [PubMed] [Google Scholar]
- 28.Lu L., Wang Y., Ding Y., Wang Y., Zhu Z., Lu J.…Yang C. Profiling phenotypic heterogeneity of circulating tumor cells through spatially resolved immunocapture on nanoporous micropillar arrays. ACS Nano. 2024;18(45):31135–31147. doi: 10.1021/acsnano.4c08893. [DOI] [PubMed] [Google Scholar]
- 29.Thenuwara G., Javed B., Singh B., Tian F. Biosensor-enhanced organ-on-a-chip models for investigating glioblastoma tumor microenvironment dynamics. Sensors. 2024;24(9):2865. doi: 10.3390/s24092865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szostakowska-Rodzos M., Fabisiewicz A., Wakula M., Tabor S., Szafron L., Jagiello-Gruszfeld A., Grzybowska E.A. Longitudinal analysis of circulating tumor cell numbers improves tracking metastatic breast cancer progression. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-63679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayamajhi S., Sipes J., Tetlow A.L., Saha S., Bansal A., Godwin A.K. Extracellular vesicles as liquid biopsy biomarkers across the cancer journey: from early detection to recurrence. Clin Chem. 2024;70(1):206–219. doi: 10.1093/clinchem/hvad176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S.W., Tang C., Tan X., Srivastava S. Liquid biopsy for early cancer detection: technological revolutions and clinical dilemma. Expert Rev Mol Diagn. 2024;24(10):937–955. doi: 10.1080/14737159.2024.2408744. [DOI] [PubMed] [Google Scholar]
- 33.Foser S., Maiese K., Digumarthy S.R., Puig-Butille J.A., Rebhan C. Looking to the future of early detection in cancer: liquid biopsies, imaging, and artificial intelligence. Clin Chem. 2024;70(1):27–32. doi: 10.1093/clinchem/hvad196. [DOI] [PubMed] [Google Scholar]
- 34.Abbaker Namariq, et al. The future of artificial intelligence in thoracic surgery for non-small cell lung cancer treatment a narrative review. Front Oncol. 2024;14 doi: 10.3389/fonc.2024.1347464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald T.O., Cheng Y.C., Graser C., Nicol P.B., Temko D., Michor F. Computational approaches to modelling and optimizing cancer treatment. Nat Rev Bioeng. 2023;1(10):695–711. [Google Scholar]
- 36.Yang B., Dai X., Chen S., Li C., Yan B. Application of surface-enhanced Raman spectroscopy in head and neck cancer diagnosis. Anal Chem. 2025;97(7):3781–3798. doi: 10.1021/acs.analchem.4c02796. [DOI] [PubMed] [Google Scholar]
- 37.Lai Hung-Chih, et al. A preliminary analysis of circulating tumor microemboli from breast cancer patients during follow-up visits. Curr Oncol. 2024;31(9):5677–5693. doi: 10.3390/curroncol31090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafanan J., Ghani N., Kazemeini S., Nadeem-Tariq A., Shih R., Vida T.A. Modernizing neuro-oncology: the impact of imaging, liquid biopsies, and AI on diagnosis and treatment. Int J Mol Sci. 2025;26(3):917. doi: 10.3390/ijms26030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Zishan, et al. Precision medicine in colorectal cancer: leveraging multi-omics, spatial omics, and artificial intelligence. Clin Chim Acta. 2024 doi: 10.1016/j.cca.2024.119686. [DOI] [PubMed] [Google Scholar]
- 40.Jani Chinmay T., et al. Liquid biopsy for renal cell carcinoma: a comprehensive review of techniques, applications, and future prospects. Kidney Cancer. 2024;8(1):205–225. doi: 10.1177/24684570241303346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberca-del Arco F., Prieto-Cuadra D., Santos-Perez de la Blanca R., Sáez-Barranquero F., Matas-Rico E., Herrera-Imbroda B. New perspectives on the role of liquid biopsy in bladder cancer: applicability to precision medicine. Cancers. 2024;16(4):803. doi: 10.3390/cancers16040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya S., Bhirud D. Advancements in cancer research: exploring diagnostics and therapeutic breakthroughs. Bentham Science Publishers; 2025. Exploring the horizon of cancer research: pioneering breakthroughs in diagnostics and theranostics; pp. 1–16. [Google Scholar]
- 43.Bao Y., Zhang D., Guo H., Ma W. Beyond blood: advancing the frontiers of liquid biopsy in oncology and personalized medicine. Cancer Sci. 2024;115(4):1060–1072. doi: 10.1111/cas.16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradly Rodney. Research advances in tumor diagnosis and early detection. Asia-Pacific J. Oncol. 2024;5:55–65. [Google Scholar]
- 45.Fatima G., Siddiqui Z., Parvez S. 2024. AI and precision medicine: paving the way for future treatment. [Google Scholar]
- 46.Bagi M., Amjad F., Ghoreishian S.M., Sohrabi Shahsavari S., Huh Y.S., Moraveji M.K., Shimpalee S. Advances in technical assessment of spiral inertial microfluidic devices toward bioparticle separation and profiling: a critical review. BioChip J. 2024;18(1):45–67. [Google Scholar]
- 47.Shanehband N., Naghib S.M. Microfluidics-assisted tumor cell separation approaches for clinical applications: an overview on emerging devices. Comb Chem High Throughput Screen. 2025;28(2):202–225. doi: 10.2174/0113862073277130231110111933. [DOI] [PubMed] [Google Scholar]
- 48.Saghezi B.V., Mansouri K., Hallaj R. Microfluidic systems in cancer diagnosis: advancing exosome and CTC detection from laboratory research to clinical application. Nano Select. 2025 [Google Scholar]
- 49.Szewczyk K., Jiang L., Khawaja H., Miranti C.K., Zohar Y. Microfluidic applications in prostate cancer research. Micromachines. 2024;15(10):1195. doi: 10.3390/mi15101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansor M.A., Yang C., Chong K.L., Jamrus M.A., Liu K., Yu M.…Ren X. Label-free and rapid microfluidic design rules for circulating tumor cell enrichment and isolation: a review and simulation analysis. ACS Omega. 2025;10(7):6306–6322. doi: 10.1021/acsomega.4c08606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebrahimi A., Icoz K., Didarian R., Shih C.H., Tarim E.A., Nasseri B.…Avci H. Molecular separation by using active and passive microfluidic chip designs: a comprehensive review. Adv Mater Interfac. 2024;11(2) [Google Scholar]
- 52.Zhou J., Dong J., Hou H., Huang L., Li J. High-throughput microfluidic systems accelerated by artificial intelligence for biomedical applications. Lab Chip. 2024;24(5):1307–1326. doi: 10.1039/d3lc01012k. [DOI] [PubMed] [Google Scholar]
- 53.Brancato V., Esposito G., Coppola L., Cavaliere C., Mirabelli P., Scapicchio C.…Aiello M. Standardizing digital biobanks: integrating imaging, genomic, and clinical data for precision medicine. J Transl Med. 2024;22(1):136. doi: 10.1186/s12967-024-04891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alam M.A., Nabil A.R., Uddin M.M., Sarker M.T.H., Mahmud S. The role of predictive analytics in early disease detection: a data-driven approach to preventive healthcare. Front Appl Eng Technol. 2024;1(1):105–123. [Google Scholar]
- 55.Rad D.M., Nazari H., Naei V.Y., Lotfi M., Aref A.R., Warkiani M.E. Functionalized nanomaterials for cancer research. Academic Press; 2024. Cancer nanotechnology: a new approach to upgrade cancer diagnosis and therapy; pp. 37–62. [Google Scholar]
- 56.Yun Y., Kim S., Lee S.N., Cho H.Y., Choi J.W. Nanomaterial-based detection of circulating tumor cells and circulating cancer stem cells for cancer immunotherapy. Nano Convergence. 2024;11(1):1–27. doi: 10.1186/s40580-024-00466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan Q., Chen L., Wan M., Mao C., Shen J. Origin, metastasis, harm, recognition and capture, and elimination of circulating tumor cells. Adv Mater Technol. 2024;9(5) [Google Scholar]
- 58.Lotfabadi A.S., Abadi B., Rezaei N. Biomimetic nanotechnology for cancer immunotherapy: state of the art and future perspective. Int J Pharm. 2024 doi: 10.1016/j.ijpharm.2024.123923. [DOI] [PubMed] [Google Scholar]
- 59.Pantel K., Alix-Panabières C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat Rev Clin Oncol. 2024:1–13. doi: 10.1038/s41571-024-00967-y. [DOI] [PubMed] [Google Scholar]
- 60.Mahgoub E.O., Cho W.C., Sharifi M., Falahati M., Zeinabad H.A., Mare H.E., Hasan A. Role of functional genomics in identifying cancer drug resistance and overcoming cancer relapse. Heliyon. 2024;10(1) doi: 10.1016/j.heliyon.2023.e22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sv S., Augustine D., Hosmani J., Pagnoni F., Reda R., Testarelli L., Patil S. Nanoparticle-based biomolecules in cancer diagnosis, therapy, drug delivery and prognosis. Front Dent Med. 2024;5 doi: 10.3389/fdmed.2024.1482166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tinger L. 2024. Development of an integrated platform for personalized cancer treatment: diagnostic and therapeutic solutions based on biomarkers and nanotechnology. [Google Scholar]
- 63.Kemp J.A., Kwon Y.J. Cancer nanotechnology: current status and perspectives. Nano convergence. 2021;8(1):34. doi: 10.1186/s40580-021-00282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zalli D., Mai Z., Ferati E., Ramaj A., Bregu R., Pranjol M.Z.I. Advancing precision medicine: integrating next-generation sequencing and tumor markers for early cancer detection and personalized treatment. Handb Cancer Immunol. 2023:1–31. [Google Scholar]
- 65.Nam A.S., Chaligne R., Landau D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat Rev Genet. 2021;22(1):3–18. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smit D.J., Pantel K. Circulating tumor cells as liquid biopsy markers in cancer patients. Mol Aspect Med. 2024;96 doi: 10.1016/j.mam.2024.101258. [DOI] [PubMed] [Google Scholar]
- 67.Jovic D., Liang X., Zeng H., Lin L., Xu F., Luo Y. Single‐cell RNA sequencing technologies and applications: a brief overview. Clin Transl Med. 2022;12(3) doi: 10.1002/ctm2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morales-Durán N., León-Buitimea A., Morones-Ramírez J.R. Unraveling resistance mechanisms in combination therapy: a comprehensive review of recent advances and future directions. Heliyon. 2024;10(6) doi: 10.1016/j.heliyon.2024.e27984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaghoubi Naei V., Bordhan P., Mirakhorli F., Khorrami M., Shrestha J., Nazari H.…Ebrahimi Warkiani M. Advances in novel strategies for isolation, characterization, and analysis of CTCs and ctDNA. Ther Adv Med Oncol. 2023;15 doi: 10.1177/17588359231192401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aarif K.M., Hasan V.M.Y., Alam A., Ali K.S., Pakruddin B. Deep learning in genetics and genomics. Academic Press; 2025. Decoding DNA: deep learning's impact on genomic exploration; pp. 77–95. [Google Scholar]
- 71.Wang Q., Šabanović B., Awada A., Reina C., Aicher A., Tang J., Heeschen C. Single-cell omics: a new perspective for early detection of pancreatic cancer? Eur J Cancer. 2023;190 doi: 10.1016/j.ejca.2023.112940. [DOI] [PubMed] [Google Scholar]
- 72.Ghorbani A., Rostami M., Guzzi P.H. AI-enabled pipeline for virus detection, validation, and SNP discovery from next-generation sequencing data. Front Genet. 2024;15 doi: 10.3389/fgene.2024.1492752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Z., Lin T., Chen S., Zhang G., Xu Y., Zou H.…Liu Z. Omics-based molecular classifications empowering in precision oncology. Cell Oncol. 2024;47(3):759–777. doi: 10.1007/s13402-023-00912-8. [DOI] [PubMed] [Google Scholar]
- 74.Chaturvedi A., Garg J., Singh A.K. Frugal innovation in oncology: tracing the arc of microchip technology in early cancer detection and treatment. J Bus Chem. 2025;22(1):126. [Google Scholar]
- 75.Di Sario G., Rossella V., Famulari E.S., Maurizio A., Lazarevic D., Giannese F., Felici C. Enhancing clinical potential of liquid biopsy through a multi-omic approach: a systematic review. Front Genet. 2023;14 doi: 10.3389/fgene.2023.1152470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z., Wu Q. Advancements in non-invasive diagnosis of gastric cancer. World J Gastroenterol. 2025;31(6) doi: 10.3748/wjg.v31.i6.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Javanmard S.H., Rafiee L., Najafi M.B., Khorsandi D., Hasan A., Vaseghi G., Makvandi P. Microfluidic-based technologies in cancer liquid biopsy: unveiling the role of horizontal gene transfer (HGT) materials. Environ Res. 2023;238 doi: 10.1016/j.envres.2023.117083. [DOI] [PubMed] [Google Scholar]
- 78.Vasu S., Johnson V., Reddy K.A., Sukumar U.K. Circulating extracellular vesicles as promising biomarkers for precession diagnostics: a perspective on lung cancer. ACS Biomater Sci Eng. 2024;11(1):95–134. doi: 10.1021/acsbiomaterials.4c01323. [DOI] [PubMed] [Google Scholar]
- 79.Nikanjam M., Kato S., Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15(1):131. doi: 10.1186/s13045-022-01351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prkačin I., Mokos M., Ferara N., Šitum M. Melanoma's new frontier: exploring the latest advances in blood-based biomarkers for melanoma. Cancers. 2024;16(24):4219. doi: 10.3390/cancers16244219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asleh K., Dery V., Taylor C., Davey M., Djeungoue-Petga M.A., Ouellette R.J. Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology. Biomark Res. 2023;11(1):99. doi: 10.1186/s40364-023-00540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fridrichova I., Kalinkova L., Ciernikova S. Clinical relevancy of circulating tumor cells in breast cancer: epithelial or mesenchymal characteristics, single cells or clusters? Int J Mol Sci. 2022;23(20) doi: 10.3390/ijms232012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwan E.M., Wyatt A.W., Chi K.N. Towards clinical implementation of circulating tumor DNA in metastatic prostate cancer: opportunities for integration and pitfalls to interpretation. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1054497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dhar B.C. Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic. Anal Bioanal Chem. 2022;414(9):2903–2934. doi: 10.1007/s00216-022-03918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bronkhorst A.J., Holdenrieder S. The changing face of circulating tumor DNA (ctDNA) profiling: factors that shape the landscape of methodologies, technologies, and commercialization. Med Genet. 2023;35(4):201–235. doi: 10.1515/medgen-2023-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarkar M., Nguyen T., Gundre E., Ogunlusi O., El-Sobky M., Giri B., Sarkar T.R. Cancer-associated fibroblasts: the chief architect in the tumor microenvironment. Front Cell Dev Biol. 2023;11 doi: 10.3389/fcell.2023.1089068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye H., Hu X., Wen Y., Tu C., Hornicek F., Duan Z., Min L. Exosomes in the tumor microenvironment of sarcoma: from biological functions to clinical applications. J Nanobiotechnol. 2022;20(1):403. doi: 10.1186/s12951-022-01609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Preethi K.A., Selvakumar S.C., Ross K., Jayaraman S., Tusubira D., Sekar D. Liquid biopsy: exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol Cancer. 2022;21(1):54. doi: 10.1186/s12943-022-01525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren F., Fei Q., Qiu K., Zhang Y., Zhang H., Sun L. Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation. J Exp Clin Cancer Res. 2024;43(1):96. doi: 10.1186/s13046-024-03026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang R., Kang T., Chen S. The role of tumor-associated macrophages in tumor immune evasion. J Cancer Res Clin Oncol. 2024;150(5):238. doi: 10.1007/s00432-024-05777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang B., Xie D., Wang S., Li X., Wu G. Advances in early detection methods for solid tumors. Front Genet. 2023;14 doi: 10.3389/fgene.2023.1091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cammarata G., de Miguel-Perez D., Russo A., Peleg A., Dolo V., Rolfo C., Taverna S. Emerging noncoding RNAs contained in extracellular vesicles: rising stars as biomarkers in lung cancer liquid biopsy. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221131229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adhit K.K., Wanjari A., Menon S., Siddhaarth K. Liquid biopsy: an evolving paradigm for non-invasive disease diagnosis and monitoring in medicine. Cureus. 2023;15(12) doi: 10.7759/cureus.50176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hartl D., de Luca V., Kostikova A., Laramie J., Kennedy S., Ferrero E.…Roth A. Translational precision medicine: an industry perspective. J Transl Med. 2021;19(1):245. doi: 10.1186/s12967-021-02910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Addissouky T.A. Next generation of colorectal cancer management: integrating omics, targeted therapies, and smart technologies. Avicenna J Med Biochem. 2024;12(2):131–143. [Google Scholar]
- 96.Raikar G.V.S., Raikar A.S., Somnache S.N. Advancements in artificial intelligence and machine learning in revolutionising biomarker discovery. Brazilian J Pharmaceut Sci. 2023;59 [Google Scholar]
- 97.Gholami, A. Artificial intelligence and precision medicine: transforming the landscape of brain tumors treatment.
- 98.Arango-Argoty G., Bikiel D.E., Sun G.J., Kipkogei E., Smith K.M., Pro S.C., Jacob E. AI-driven predictive biomarker discovery with contrastive learning to improve clinical trial outcomes. medRxiv. 2024 doi: 10.1016/j.ccell.2025.03.029. 2024-01. [DOI] [PubMed] [Google Scholar]
- 99.Wang R., Hastings W.J., Saliba J.G., Bao D., Huang Y., Maity S.…Ning B. Applications of nanotechnology for spatial omics: biological structures and functions at nanoscale resolution. ACS Nano. 2024;19(1):73–100. doi: 10.1021/acsnano.4c11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heidrich I., Deitert B., Werner S., Pantel K. Liquid biopsy for monitoring of tumor dormancy and early detection of disease recurrence in solid tumors. Cancer Metastasis Rev. 2023;42(1):161–182. doi: 10.1007/s10555-022-10075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song B., Wang X., Qin L., Hussain S., Liang W. Brain gliomas: diagnostic and therapeutic issues and the prospects of drug-targeted nano-delivery technology. Pharmacol Res. 2024 doi: 10.1016/j.phrs.2024.107308. [DOI] [PubMed] [Google Scholar]
- 102.Wang W., Zheng Z., Lei J. CTC, ctDNA, and exosome in thyroid cancers: a review. Int J Mol Sci. 2023;24(18) doi: 10.3390/ijms241813767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sagawa T., Sato Y., Hirakawa M., Hamaguchi K., Tamura F., Nagashima H.…Takayama T. Case report: longitudinal monitoring of clonal evolution by circulating tumor DNA for resistance to anti-EGFR antibody in a case of metastatic colorectal cancer. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1203296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forood A.M.K., Osifuwa A.D., Idoko J.E., Oni O., Ajaelu C.S., Idoko F.A. Advancements in health information technology and their role in enhancing cancer care: innovations in early detection, treatment, and data privacy. GSC Adv Res Rev. 2024;21(1):228–241. [Google Scholar]
- 105.Paas-Oliveros E., Hernández-Lemus E., de Anda-Jáuregui G. Computational single cell oncology: state of the art. Front Genet. 2023;14 doi: 10.3389/fgene.2023.1256991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Osei-Poku P., Tritten L., Fordjour F., Kwarteng A. Cell-free DNA as a complementary diagnostic tool for neglected tropical diseases towards achieving the WHO NTDs elimination by 2030. J Liq Biopsy. 2025;7 doi: 10.1016/j.jlb.2024.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lone S.N., Nisar S., Masoodi T., Singh M., Rizwan A., Hashem S.…Macha M.A. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21(1):79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alix-Panabières C., Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 109.Forte V.A., Barrak D.K., Elhodaky M., Tung L., Snow A., Lang J.E. The potential for liquid biopsies in the precision medical treatment of breast cancer. Cancer Biol Med. 2016;13(1):19–40. doi: 10.28092/j.issn.2095-3941.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding Z., Wang N., Ji N., Chen Z.S. Proteomics technologies for cancer liquid biopsies. Mol Cancer. 2022;21(1):53. doi: 10.1186/s12943-022-01526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pashayan N., Antoniou A.C., Ivanus U., Esserman L.J., Easton D.F., French D.…Widschwendter M. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020;17(11):687–705. doi: 10.1038/s41571-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Capuozzo M., Ferrara F., Santorsola M., Zovi A., Ottaiano A. Circulating tumor cells as predictive and prognostic biomarkers in solid tumors. Cells. 2023;12(22):2590. doi: 10.3390/cells12222590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cristalli C., Scotlandi K. Targeting DNA methylation machinery in pediatric solid tumors. Cells. 2024;13(14):1209. doi: 10.3390/cells13141209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Addanki S., Meas S., Sarli V.N., Singh B., Lucci A. Applications of circulating tumor cells and circulating tumor DNA in precision oncology for breast cancers. Int J Mol Sci. 2022;23(14):7843. doi: 10.3390/ijms23147843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castro-Giner F., Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12(1):31. doi: 10.1186/s13073-020-00728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li K., Lin Y., Luo Y., Xiong X., Wang L., Durante K.…Zhang H. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer. 2022;21(1):21. doi: 10.1186/s12943-022-01499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wishart G., Templeman A., Hendry F., Miller K., Pailhes-Jimenez A.S. Molecular profiling of circulating tumour cells and circulating tumour DNA: complementary insights from a single blood sample utilising the Parsortix® system. Curr Issues Mol Biol. 2024;46(1):773–787. doi: 10.3390/cimb46010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pinzani P., D'Argenio V., Del Re M., Pellegrini C., Cucchiara F., Salvianti F., Galbiati S. Updates on liquid biopsy: current trends and future perspectives for clinical application in solid tumors. Clin Chem Lab Med. 2021;59(7):1181–1200. doi: 10.1515/cclm-2020-1685. [DOI] [PubMed] [Google Scholar]
- 119.Ring A., Nguyen-Sträuli B.D., Wicki A., Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023;23(2):95–111. doi: 10.1038/s41568-022-00536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Almodovar K., Iams W.T., Meador C.B., Zhao Z., York S., Horn L.…Lovly C.M. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol. 2018;13(1):112–123. doi: 10.1016/j.jtho.2017.09.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ko J.M.Y., Lam K.O., Kwong D.L.W., Wong I.Y.H., Chan F.S.Y., Wong C.L.Y.…Lung M.L. Circulating tumor cell enumeration for serial monitoring of treatment outcomes for locally advanced esophageal squamous cell carcinoma. Cancers. 2023;15(3):832. doi: 10.3390/cancers15030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tirosh I., Suva M.L. Cancer cell states: lessons from ten years of single-cell RNA-sequencing of human tumors. Cancer Cell. 2024;42(9):1497–1506. doi: 10.1016/j.ccell.2024.08.005. [DOI] [PubMed] [Google Scholar]
- 123.Labrie M., Brugge J.S., Mills G.B., Zervantonakis I.K. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat Rev Cancer. 2022;22(6):323–339. doi: 10.1038/s41568-022-00454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khan, M. A. I., & Zannat, N. A. Novel biomarkers and therapeutic avenues for precision oncology and effective treatment strategies.
- 125.Kurma K., Eslami-S Z., Alix-Panabières C., Cayrefourcq L. Liquid biopsy: paving a new avenue for cancer research. Cell Adhes Migrat. 2024;18(1):1–26. doi: 10.1080/19336918.2024.2395807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Descamps L., Le Roy D., Deman A.L. Microfluidic-based technologies for CTC isolation: a review of 10 years of intense efforts towards liquid biopsy. Int J Mol Sci. 2022;23(4):1981. doi: 10.3390/ijms23041981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kolinsky M.P., Stoecklein N., Lambros M., Gil V., Rodrigues D.N., Carreira S.…de Bono J.S. Genetic analysis of circulating tumour cells. Tumor liq biopsies. 2020:57–76. doi: 10.1007/978-3-030-26439-0_3. [DOI] [PubMed] [Google Scholar]
- 128.Parkinson D.R., Dracopoli N., Petty B.G., Compton C., Cristofanilli M., Deisseroth A.…Kelloff G.J. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:1–20. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moffitt L.R., Karimnia N., Wilson A.L., Stephens A.N., Ho G.Y., Bilandzic M. Challenges in implementing comprehensive precision medicine screening for ovarian cancer. Curr Oncol. 2024;31(12):8023–8038. doi: 10.3390/curroncol31120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen G., Zhang J., Fu Q., Taly V., Tan F. Integrative analysis of multi-omics data for liquid biopsy. Br J Cancer. 2023;128(4):505–518. doi: 10.1038/s41416-022-02048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tippur A. AI-powered precision oncology: computational insights redefining therapeutic landscapes. DHR Proc. 2023;3(S1):1–10. [Google Scholar]
- 132.Magro D., Venezia M., Balistreri C.R. The omics technologies and liquid biopsies: advantages, limitations, applications. Med Omics. 2024 [Google Scholar]
- 133.Reduzzi C., Singhal S., Venetis K., Ortega-Franco A., de Miguel-Perez D., Dipasquale A.…Cristofanilli M. Unveiling the impact of circulating tumor cells: two decades of discovery and clinical advancements in solid tumors. Crit Rev Oncol Hematol. 2024 doi: 10.1016/j.critrevonc.2024.104483. [DOI] [PubMed] [Google Scholar]
- 134.Reina C., Šabanović B., Lazzari C., Gregorc V., Heeschen C. Unlocking the future of cancer diagnosis–promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer. Transl Res. 2024;272:41–53. doi: 10.1016/j.trsl.2024.05.014. [DOI] [PubMed] [Google Scholar]
- 135.Dlamini Z., Francies F.Z., Hull R., Marima R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput Struct Biotechnol J. 2020;18:2300–2311. doi: 10.1016/j.csbj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh S., Miglione A., Raucci A., Numan A., Cinti S. Towards sense and sensitivity-based electrochemical biosensors for liquid biopsy-based breast cancer detection. TrAC, Trends Anal Chem. 2023;163 [Google Scholar]
- 137.Lockwood C.M., Merker J.D., Bain E., Compton C., Grossman R.L., Johann D.…Leiman L.C. Towards preanalytical best practices for liquid biopsy studies: a BLOODPAC landscape analysis. Clin Pharmacol Therapeut. 2025;117(1):28–33. doi: 10.1002/cpt.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jahan M., Rubab A., Ali M., Sultan A., Zuhair M., Zakria M., Hasan S.W. 2025. Circulating tumor cells and DNA in early diagnosis and prognosis of metastatic cancer. [Google Scholar]
- 139.Mazzeo R., Sears J., Palmero L., Bolzonello S., Davis A.A., Gerratana L., Puglisi F. Liquid biopsy in triple-negative breast cancer: unlocking the potential of precision oncology. ESMO Open. 2024;9(10) doi: 10.1016/j.esmoop.2024.103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smerage J.B. Circulating tumor cells. Springer New York; New York, NY: 2016. Prognostic implications of CTC in breast cancer; pp. 233–254. [Google Scholar]
- 141.Morris M.J., Autio K.A., Basch E.M., Danila D.C., Larson S., Scher H.I. Monitoring the clinical outcomes in advanced prostate cancer: what imaging modalities and other markers are reliable? Semin Oncol. 2013, June;40(3):375–392. doi: 10.1053/j.seminoncol.2013.04.008. WB Saunders. [DOI] [PubMed] [Google Scholar]
- 142.Di Cosimo S., De Marco C., Silvestri M., Busico A., Vingiani A., Pruneri G., Cappelletti V. Can we define breast cancer HER2 status by liquid biopsy? Int Rev Cell Mol Biol. 2023;381:23–56. doi: 10.1016/bs.ircmb.2023.07.003. [DOI] [PubMed] [Google Scholar]
- 143.Lowes L.E., Allan A.L. Recent advances in the molecular characterization of circulating tumor cells. Cancers. 2014;6(1):595–624. doi: 10.3390/cancers6010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bae S.Y., Kamalanathan K.J., Galeano-Garces C., Konety B.R., Antonarakis E.S., Parthasarathy J.…Drake J.M. Dissemination of circulating tumor cells in breast and prostate cancer: implications for early detection. Endocrinology. 2024;165(4) doi: 10.1210/endocr/bqae022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patelli G., Vaghi C., Tosi F., Mauri G., Amatu A., Massihnia D.…Sartore-Bianchi A. Liquid biopsy for prognosis and treatment in metastatic colorectal cancer: circulating tumor cells vs circulating tumor DNA. Target Oncol. 2021;16:309–324. doi: 10.1007/s11523-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan Y., Wu H. The significant prognostic value of circulating tumor cells in colorectal cancer: a systematic review and meta-analysis. Curr Probl Cancer. 2018;42(1):95–106. doi: 10.1016/j.currproblcancer.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Uen Y.H., Lu C.Y., Tsai H.L., Yu F.J., Huang M.Y., Cheng T.L.…Wang J.Y. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I–III colorectal cancer after curative resection. Ann Surg Oncol. 2008;15:2120–2128. doi: 10.1245/s10434-008-9961-7. [DOI] [PubMed] [Google Scholar]
- 148.Ronnekleiv-Kelly S.M., Burkhart R.A., Pawlik T.M. Molecular markers of prognosis and therapeutic targets in metastatic colorectal cancer. Surg Oncol. 2016;25(3):190–199. doi: 10.1016/j.suronc.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortés-Hernández L.E., Eslami-S Z., Alix-Panabières C. Liquid biopsy to detect circulating tumor cells: is it ready for a value proposition in laboratory medicine? J Applied Lab Med. 2020;5(5):1027–1037. doi: 10.1093/jalm/jfaa115. [DOI] [PubMed] [Google Scholar]
- 150.Salvianti F., Gelmini S., Mancini I., Pazzagli M., Pillozzi S., Giommoni E.…Antonuzzo L. Circulating tumour cells and cell-free DNA as a prognostic factor in metastatic colorectal cancer: the OMITERC prospective study. Br J Cancer. 2021;125(1):94–100. doi: 10.1038/s41416-021-01399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matikas A., Syrigos K.N., Agelaki S. Circulating biomarkers in non–small-cell lung cancer: current status and future challenges. Clin Lung Cancer. 2016;17(6):507–516. doi: 10.1016/j.cllc.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 152.Dall'Olio F.G., Gelsomino F., Conci N., Marcolin L., De Giglio A., Grilli G.…Ardizzoni A. PD-L1 expression in circulating tumor cells as a promising prognostic biomarker in advanced non–small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(5):423–431. doi: 10.1016/j.cllc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 153.Zhang T., Karsh L.I., Nissenblatt M.J., Canfield S.E. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2020;18(1):1–10. doi: 10.1016/j.clgc.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 154.Hu X., Gallo R., Stoyanova R., Breto A.L., Hara D., Shi J.…Tao W. Unraveling the complexities of metastasis. Academic Press; 2022. Diagnosis and treatment of metastatic prostate cancer: the role of imaging, liquid biopsies, and biomarkers for deciphering tumor heterogeneity; pp. 23–47. [Google Scholar]
- 155.Prasanth B.K., Alkhowaiter S., Sawarkar G., Dharshini B.D., Baskaran A.R., Prasanth K.…Baskaran A.R. Unlocking early cancer detection: exploring biomarkers, circulating DNA, and innovative technological approaches. Cureus. 2023;15(12) doi: 10.7759/cureus.51090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Selli C., Dixon J.M., Sims A.H. Accurate prediction of response to endocrine therapy in breast cancer patients: current and future biomarkers. Breast Cancer Res. 2016;18:1–10. doi: 10.1186/s13058-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Malone E.R., Oliva M., Sabatini P.J., Stockley T.L., Siu L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:1–19. doi: 10.1186/s13073-019-0703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cimadamore A., Aurilio G., Nolé F., Massari F., Scarpelli M., Santoni M.…Montironi R. Update on circulating tumor cells in genitourinary tumors with focus on prostate cancer. Cells. 2020;9(6):1495. doi: 10.3390/cells9061495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Riccio J., Presotto L., Doniza L., Inverso D., Nevo U., Chirico G. 2024. Predictive modeling and experimental control of macrophage pro-inflammatory dynamics. [Google Scholar]
- 160.Guo X., Lin F., Yi C., Song J., Sun D., Lin L.…Song J. Deep transfer learning enables lesion tracing of circulating tumor cells. Nat Commun. 2022;13(1):7687. doi: 10.1038/s41467-022-35296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dakal T.C., Dhakar R., Beura A., Moar K., Maurya P.K., Sharma N.K.…Kumar A. Emerging methods and techniques for cancer biomarker discovery. Pathol Res Pract. 2024 doi: 10.1016/j.prp.2024.155567. [DOI] [PubMed] [Google Scholar]
- 162.He S., Yu S., Wei J., Ding L., Yang X., Wu Y. New horizons in the identification of circulating tumor cells (CTCs): an emerging paradigm shift in cytosensors. Biosens Bioelectron. 2022;203 doi: 10.1016/j.bios.2022.114043. [DOI] [PubMed] [Google Scholar]
- 163.Gardner L., Kostarelos K., Mallick P., Dive C., Hadjidemetriou M. Nano-omics: nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. Nat Rev Clin Oncol. 2022;19(8):551–561. doi: 10.1038/s41571-022-00645-x. [DOI] [PubMed] [Google Scholar]
- 164.Heidrich I., Abdalla T.S., Reeh M., Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as a liquid biopsy marker in colorectal cancer. Cancers. 2021;13(18):4500. doi: 10.3390/cancers13184500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao M., Mi D., Ferdows B.E., Li Y., Wang R., Li J.…Tao W. State-of-the-art nanotechnologies for the detection, recovery, analysis and elimination of liquid biopsy components in cancer. Nano Today. 2022;42 [Google Scholar]
- 166.Lianidou E., Markou A., Strati A., Ntzifa A. Circulating tumor cells: advances in liquid biopsy technologies. Springer International Publishing; Cham: 2023. Development and validation of molecular assays for liquid biopsy applications; pp. 201–246. [Google Scholar]
- 167.Lawler M., Keeling P., Kholmanskikh O., Minnaard W., Moehlig-Zuttermeister H., Normanno N.…Verweij J. Empowering effective biomarker-driven precision oncology: a call to action. Eur J Cancer. 2024;209 doi: 10.1016/j.ejca.2024.114225. [DOI] [PubMed] [Google Scholar]
- 168.Lin D., Shen L., Luo M., Zhang K., Li J., Yang Q.…Zhou J. Circulating tumor cells: biology and clinical significance. Signal Transduct Targeted Ther. 2021;6(1):404. doi: 10.1038/s41392-021-00817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van de Sande B., Lee J.S., Mutasa-Gottgens E., Naughton B., Bacon W., Manning J.…Ferran E. Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov. 2023;22(6):496–520. doi: 10.1038/s41573-023-00688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Orrapin S., Thongkumkoon P., Udomruk S., Moonmuang S., Sutthitthasakul S., Yongpitakwattana P.…Chaiyawat P. Deciphering the biology of circulating tumor cells through single-cell RNA sequencing: implications for precision medicine in cancer. Int J Mol Sci. 2023;24(15) doi: 10.3390/ijms241512337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fonseca Teixeira A., Wu S., Luwor R., Zhu H.J. A new era of integration between multiomics and spatio-temporal analysis for the translation of EMT towards clinical applications in cancer. Cells. 2023;12(23):2740. doi: 10.3390/cells12232740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salu P., Reindl K.M. Advancements in circulating tumor cell research: bridging biology and clinical applications. Cancers. 2024;16(6):1213. doi: 10.3390/cancers16061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qureshi Z., Altaf F., Khanzada M., Safi A., Asghar Z., Warraich D., Shah S. Liquid biopsies for early detection and monitoring of cancer: advances, challenges, and future directions. Ann Med Surg. 2025:10–1097. [Google Scholar]
- 174.Majumder B., Nataraj N.B., Maitreyi L., Datta S. Mismatch repair-proficient tumor footprints in the sands of immune desert: mechanistic constraints and precision platforms. Front Immunol. 2024;15 doi: 10.3389/fimmu.2024.1414376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Greco L., Rubbino F., Morelli A., Gaiani F., Grizzi F., de'Angelis G.L.…Laghi L. Epithelial to mesenchymal transition: a challenging playground for translational research. Current models and focus on TWIST1 relevance and gastrointestinal cancers. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Atale N., Wells A. Statins as secondary preventive agent to limit breast cancer metastatic outgrowth. Int J Mol Sci. 2025;26(3):1300. doi: 10.3390/ijms26031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tsai Y.T., Schlom J., Donahue R.N. Blood-based biomarkers in patients with non-small cell lung cancer treated with immune checkpoint blockade. J Exp Clin Cancer Res. 2024;43(1):82. doi: 10.1186/s13046-024-02969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moon G.Y., Dalkiran B., Park H.S., Shin D., Son C., Choi J.H.…Bu J. Dual biomarker strategies for liquid biopsy: integrating circulating tumor cells and circulating tumor DNA for enhanced tumor monitoring. Biosensors. 2025;15(2):74. doi: 10.3390/bios15020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galsky M.D., Autio K.A., Cabanski C.R., Wentzel K., Graff J.N., Friedlander T.W.…Fong L. Clinical and translational results from PORTER, a multi-cohort Phase 1 platform trial of combination immunotherapy in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2025 doi: 10.1158/1078-0432.CCR-24-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.El-Tanani M., Rabbani S.A., Babiker R., Rangraze I., Kapre S., Palakurthi S.S.…Tambuwala M.M. Unraveling the tumor microenvironment: insights into cancer metastasis and therapeutic strategies. Cancer Lett. 2024 doi: 10.1016/j.canlet.2024.216894. [DOI] [PubMed] [Google Scholar]
- 181.Banerjee R., Maitra I., Bhattacharya T., Banerjee M., Ramanathan G., kumar Rayala S., Venkatraman G. Next-generation biomarkers for prognostic and potential therapeutic enhancement in triple negative breast cancer. Crit Rev Oncol-Hematol. 2024 doi: 10.1016/j.critrevonc.2024.104417. [DOI] [PubMed] [Google Scholar]
- 182.Yadav R., Singh A.V., Kushwaha S., Chauhan D.S. Emerging role of exosomes as a liquid biopsy tool for diagnosis, prognosis & monitoring treatment response of communicable & non-communicable diseases. Indian J Med Res. 2024;159(2):163–180. doi: 10.4103/ijmr.ijmr_2344_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xue R., Yang L., Yang M., Xue F., Li L., Liu M.…Zhao J. Circulating cell-free DNA sequencing for early detection of lung cancer. Expert Rev Mol Diagn. 2023;23(7):589–606. doi: 10.1080/14737159.2023.2224504. [DOI] [PubMed] [Google Scholar]
- 184.Khansari N. AI machine learning improves personalized cancer therapies. Australas Med J. 2024;17(2):1166–1173. [Google Scholar]
- 185.Song J., Ye X., Xiao H. Liquid biopsy entering clinical practice: past discoveries, current insights, and future innovations. Crit Rev Oncol Hematol. 2025 doi: 10.1016/j.critrevonc.2025.104613. [DOI] [PubMed] [Google Scholar]
- 186.Bashir S., Amn Zia M., Shoukat M., Kaleem I., Bashir S. Nanoparticles as a novel key driver for the isolation and detection of circulating tumour cells. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-67221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Keomanee-Dizon K., Shishido S.N., Kuhn P. Circulating tumor cells: high-throughput imaging of CTCs and bioinformatic analysis. Recent Results Cancer Res. 2020;215:89–104. doi: 10.1007/978-3-030-26439-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guha A., Sadeghi S.A., Kunhiraman H.H., Fang F., Wang Q., Rafieioskouei A.…Mahmoudi M. Ai-driven prediction of cardio-oncology biomarkers through protein corona analysis. Chem Eng J. 2025;509 doi: 10.1016/j.cej.2025.161134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Serafini M.S., Molteni E., Nicolò E., Gerratana L., Reduzzi C., Cristofanilli M. Cellular residual disease (CRD) in early breast cancer–Expanding the concept of minimal residual disease monitoring? J Liq Biopsy. 2024;3 doi: 10.1016/j.jlb.2023.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fabisiewicz A., Szostakowska-Rodzos M., Grzybowska E.A. Improving the prognostic and predictive value of circulating tumor cell enumeration: is longitudinal monitoring the answer? Int J Mol Sci. 2024;25(19) doi: 10.3390/ijms251910612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haag F., Hertel A., Tharmaseelan H., Kuru M., Haselmann V., Brochhausen C.…Froelich M.F. vol. 196. Georg Thieme Verlag KG; 2024. Imaging-based characterization of tumoral heterogeneity for personalized cancer treatment; pp. 262–272. (RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren). No. 03. [DOI] [PubMed] [Google Scholar]
- 192.Harigopal M., Kowalski D., Vosoughi A. Enumeration and molecular characterization of circulating tumor cells as an innovative tool for companion diagnostics in breast cancer. Expert Rev Mol Diagn. 2020;20(8):815–828. doi: 10.1080/14737159.2020.1784009. [DOI] [PubMed] [Google Scholar]
- 193.Thalgott M., Rack B., Eiber M., Souvatzoglou M., Heck M.M., Kronester C.…Nawroth R. Categorical versus continuous circulating tumor cell enumeration as early surrogate marker for therapy response and prognosis during docetaxel therapy in metastatic prostate cancer patients. BMC Cancer. 2015;15:1–10. doi: 10.1186/s12885-015-1478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Malkawi W., Lutfi A., Afghan M.K., Shah L.M., Costandy L., Ramirez A.B.…Kasi P.M. Circulating tumour cell enumeration, biomarker analyses, and kinetics in patients with colorectal cancer and other GI malignancies. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1305181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wan J.C., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C.…Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 196.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N.…Diaz Jr L.A. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci Transl Med. 2014;6(224) doi: 10.1126/scitranslmed.3007094. 224ra24-224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 198.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yáñez-Mó M., Siljander P.R.M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I.…De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1) doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esfahani M.S., Hamilton E.G., Mehrmohamadi M., Nabet B.Y., Alig S.K., King D.A.…Alizadeh A.A. Inferring gene expression from cell-free DNA fragmentation profiles. Nat Biotechnol. 2022;40(4):585–597. doi: 10.1038/s41587-022-01222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Faulkner L.G., Howells L., Lehman S., Cowley C., Sidat Z., Shaw J., Thomas A.L. Clinical validation of local versus commercial genomic testing in cancer: a comparison of tissue and plasma concordance. Cancer Invest. 2025:1–22. doi: 10.1080/07357907.2025.2464684. [DOI] [PubMed] [Google Scholar]
- 202.Mishra A., Huang S.B., Dubash T., Burr R., Edd J.F., Wittner B.S.…Toner M. Tumor cell-based liquid biopsy using high-throughput microfluidic enrichment of entire leukapheresis product. Nat Commun. 2025;16(1):32. doi: 10.1038/s41467-024-55140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ribeiro-Samy S., Oliveira M.I., Pereira-Veiga T., Muinelo-Romay L., Carvalho S., Gaspar J.…Diéguez L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal cancer patients. Sci Rep. 2019;9(1):8032. doi: 10.1038/s41598-019-44401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen H., Li Y., Zhang Z., Wang S. Immunomagnetic separation of circulating tumor cells with microfluidic chips and their clinical applications. Biomicrofluidics. 2020;14(4) doi: 10.1063/5.0005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Leitão T.P., Corredeira P., Kucharczak S., Rodrigues M., Piairo P., Rodrigues C.…Costa L. Clinical validation of a size-based microfluidic device for circulating tumor cell isolation and analysis in renal cell carcinoma. Int J Mol Sci. 2023;24(9):8404. doi: 10.3390/ijms24098404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma L., Li H., Wang D., Hu Y., Yu M., Zhang Q.…Wang J. Dynamic cfDNA analysis by NGS in EGFR T790M-positive advanced NSCLC patients failed to the first-generation EGFR-TKIs. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Coccaro N., Tota G., Anelli L., Zagaria A., Specchia G., Albano F. Digital PCR: a reliable tool for analyzing and monitoring hematologic malignancies. Int J Mol Sci. 2020;21(9):3141. doi: 10.3390/ijms21093141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shi J., Zhang Y., Fan Y., Liu Y., Yang M. Recent advances in droplet-based microfluidics in liquid biopsy for cancer diagnosis. Droplet. 2024;3(1) [Google Scholar]
- 209.Li G., Ge S., Niu P., Zhang J., Mao Y., Wang Y., Sun A. Simultaneous detection of circulating tumor DNAs using a SERS-based lateral flow assay biosensor for point-of-care diagnostics of head and neck cancer. Biomed Opt Express. 2022;13(8):4102–4117. doi: 10.1364/BOE.463612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim J., Jeong J., Ko S.H. Electrochemical biosensors for point-of-care testing. Bio-Design and Manufacturing. 2024;7(4):548–565. [Google Scholar]
- 211.Di Carlo E., Sorrentino C. State of the art CRISPR-based strategies for cancer diagnostics and treatment. Biomark Res. 2024;12(1):156. doi: 10.1186/s40364-024-00701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pastuszak K., Sieczczyński M., Dzięgielewska M., Wolniak R., Drewnowska A., Korpal M.…Żaczek A.J. Detection of circulating tumor cells by means of machine learning using Smart-Seq2 sequencing. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-61378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moser T., Kühberger S., Lazzeri I., Vlachos G., Heitzer E. Bridging biological cfDNA features and machine learning approaches. Trends Genet. 2023;39(4):285–307. doi: 10.1016/j.tig.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 214.Li B., Kugeratski F.G., Kalluri R. A novel machine learning algorithm selects proteome signature to specifically identify cancer exosomes. Elife. 2024;12 doi: 10.7554/eLife.90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bu J., Lee T.H., Poellmann M.J., Rawding P.A., Jeong W.J., Hong R.S., Hong S. 2021. Tri-modal liquid biopsy: combinational analysis of circulating tumor cells, exosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wolf J., Rasmussen D.K., Sun Y.J., Vu J.T., Wang E., Espinosa C., Mahajan V.B. Liquid-biopsy proteomics combined with AI identifies cellular drivers of eye aging and disease in vivo. Cell. 2023;186(22):4868–4884. doi: 10.1016/j.cell.2023.09.012. and cell-free DNA using machine learning algorithm. Clinical and translational medicine, 11(8), e499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li J., Li L., You P., Wei Y., Xu B. vol. 91. Academic Press; 2023. Towards artificial intelligence to multi-omics characterization of tumor heterogeneity in esophageal cancer; pp. 35–49. (Seminars in cancer biology). [DOI] [PubMed] [Google Scholar]
- 218.Goodsaid F.M. The labyrinth of product development and regulatory approvals in liquid biopsy diagnostics. Clinica transl sci. 2019;12(5):431–439. doi: 10.1111/cts.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]