Abstract
Infectious bursal disease (IBD), caused by the infectious bursal disease virus (IBDV), threatens the global poultry industry with acute immunosuppression. The non-lethal novel variant IBDV (nVarIBDV) has been identified as the primary cause of a new outbreak of atypical IBD in China. However, since 2023, several poultry farms in China have continuously reported the detection of vvIBDV in chicken flocks exhibiting atypical IBD characteristics. To analyze this abnormal problem, the prevalence of IBDV in major poultry farming areas in China from 2023 to 2024 was monitored. Our epidemiological survey revealed four coexisting IBDV strains in China, nVarIBDV (69.9 %, 65/93) ranked the first, followed by vvIBDV (16.1 %, 15/93), attIBDV (8.6 %, 8/93), and cIBDV (5.4 %, 5/93). Notably, all these 15 vvIBDV strains detected in this study harbored single or combination mutations in the VP2 hypervariable region (HVR) including residue 279: D279N (seven strains), G254D/I256L/D279N (one strain), or A222T/G254D/I256L/D279N (seven strains). Furthermore, one representative strain of IBDV-NMG23-1701 which contained the D279N mutation was isolated. In the phylogenetic tree constructed based on the IBDV genome, IBDV-NMG23-1701 was located in the vvIBDV branch. However, IBDV-NMG23-1701 did not kill chickens directly but induced severe subclinical symptoms of atypical IBD. We named these strains mutated vvIBDV (mvvIBDV), which genotypically belonged to vvIBDV but exhibited atypical IBD symptoms. The mvvIBDV has been detected in several major poultry farming areas in China. This is the first study to identify mvvIBDV as a new pathogen causing atypical IBD, providing a scientific basis for optimizing prevention and control strategies.
Keywords: Atypical IBD, Novel variant IBDV (nVarIBDV), Mutated very virulent IBDV (mvvIBDV), Pathogenicity, Subclinical symptoms
Introduction
Infectious bursal disease (IBD), also known as Gumboro disease, is an important immunosuppressive and contagious disease affecting the global poultry industry. It causes immunosuppression and acute mortality in chicks, leading to substantial financial losses (Müller et al., 2003). The pathogen of IBD is the infectious bursal disease virus (IBDV), a double-stranded RNA virus belonging to the genus Avibirnavirus of the family Birnaviridae, characterized by a non-enveloped icosahedral structure (Bao et al., 2022; Jackwood, 2017). The IBDV genome comprises segments A and B (Brown and Skinner, 1996). Segment A (approximately 3600 bp) contains two overlapping open reading frames (ORFs). The smaller ORF (438 bp) encodes the non-structural protein VP5, whereas the larger ORF (3039 bp) encodes the polyprotein (pVP2-VP4-VP3), which is autocatalytically processed into VP2, VP4, and VP3 (Chevalier et al., 2002; Raja et al., 2016). VP2 is the principal component of the viral capsid and the primary protective antigen of IBDV (Jackwood et al., 2008). Mutations in the VP2 protein are most likely to occur within the region spanning amino acids (aa) 206–350, which is known as the HVR of IBDV (Jackwood, 2017; Letzel et al., 2007). The HVR, located in the outermost part of the virion, contains four loop structures: PBC (aa 204–236), PDE (aa 240–265), PFG (aa 270–293), and PHI (aa 305–337) (Bao et al., 2022; Coulibaly et al., 2005). The HVR is closely associated with viral virulence, antigenic variation, and cell tropism. Key amino acid mutations in the HVR may lead to alterations in viral pathogenicity and antigenicity (Fan et al., 2022; Jackwood et al., 2008; Jiang et al., 2023; Qi et al., 2009). Segment B (approximately 2800 bp) encodes the RNA-dependent RNA polymerase VP1, which is crucial for viral replication and genetic evolution (Escaffre et al., 2013; Gao et al., 2014; Yu et al., 2013). The VP1 B-marker (aa 110–252) represents the characteristic feature of the segment B. The VP2 HVR and the B-marker have been extensively applied in the phylogenomic studies of IBDV (Alfonso-Morales et al., 2015; Wang et al., 2021).
There are two serotypes of IBDV, I and II. While serotype II is non-pathogenic to both turkey and chicken, serotype I exhibits pathogenicity in chicken (Mahgoub et al., 2012). IBDV is an RNA virus with a segmented genome, making it prone to genetic variation due to factors such as natural selection pressure and vaccination (Gao et al., 2023). Genetic mutations (Wang et al., 2020), segment reassortment (Pikula et al., 2020), and homologous recombination (Wu et al., 2020) are fundamental mechanisms involved in the genetic evolution of IBDV. Since the first report of classic IBDV (cIBDV) in Gumboro (USA) in 1957 (Cosgrove, 1962), IBDV has undergone three major genetic variations. In the early 1980s, the United States witnessed the emergence of the variant IBDV (varIBDV), which is antigenically distinct from cIBDV (Jackwood and Saif, 1987). Subsequently, in the late 1980s, the very virulent IBDV (vvIBDV), known for its high mortality rates, was first identified in Europe, later spread globally and caused considerable economic detriment to the poultry industry (Chettle et al., 1989). Recent genotype classification based on both genome segments delineated IBDV into genotypes A1B1 (cIBDV), A2B1 (varIBDV), A3B2 / A3B3 (vvIBDV), and A8B1 (attIBDV) (Wang et al., 2021). This genotyping system also revealed that there are two genotypes of vvIBDV prevalent in China. In addition to the traditional vvIBDV (A3B2) originating in Europe, the HLJ0504-like vvIBDV (A3B3) has posed a significant challenge to the sustainable growth of the China poultry industry for the last three decades (Zhang et al., 2022). In recent years, atypical IBD, characterized by low or no mortality but causing severe immune suppression and immune escape, has become a significant feature of the global IBD prevalence (Fan et al., 2020; Mató T et al., 2020). Since 2017, the novel variant IBDV (nVarIBDV, A2dB1), which is genetically distinct from the early varIBDV (A2aB1, A2bB1, and A2cB1) in the USA, has been reported for the first time in China and identified as a primary cause of the new outbreak of atypical IBD (Fan et al., 2019).
However, most recently, some poultry farms in China have continuously reported the detection of “vvIBDV” in chicken flocks exhibiting atypical IBD characteristics. To analyze this abnormal problem, the prevalence of IBDV in major poultry farming areas in China from 2023 to 2024 was detected and monitored. This study described the latest epidemic situation of IBDV in major poultry farming areas in China; for the first time, it identified the mutated vvIBDV (mvvIBDV), which has the genotype of vvIBDV but is not lethal to chickens, as an important cause of atypical IBD.
Materials and methods
Clinical samples
Between May 2023 and May 2024, 525 bursa samples were collected in nine major poultry farming regions in eastern China, including Neimenggu, Liaoning, Hebei, Shandong, Shanxi, Henan, Anhui, Fujian, and Guangdong provinces. These samples were sourced from 34 chicken flocks with clinical signs of weight loss and depression that were suspected to have IBDV infection. These chickens were 23–35 days old. All samples were marked with collection dates and regional locations, then transported at 4°C to the Avian Immunosuppressive Disease Division Harbin Veterinary Research Institute (HVRI), the Chinese Academy of Agricultural Sciences (CAAS) (hereinafter referred to as our laboratory) for testing.
Viral RNA extraction and RT-PCR
Bursa samples were minced under sterile conditions. Tissue homogenates were prepared using a tissue grinder, with approximately 1 g of minced bursa tissue transferred into a 2 mL EP tube containing 1 mL of phosphate-buffered saline (pH 7.2). After three freeze-thaw cycles, the homogenate was centrifuged at 4°C for 10 min at 3000 × g. The RNAiso Plus reagent (Takara Biotechnology Co., Ltd., Dalian, China) was employed to extract total RNA from 0.2 mL of supernatant, adhering to the manufacturer's protocol. The RNA was reverse transcribed into complementary DNA (cDNA) using the M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Subsequently, cDNA was used as a template for the polymerase chain reaction (PCR) to amplify the characteristic fragments of genomic segments A and B of IBDV. For segment A, a pair of primers, IBDV 2 U (5′-CCTCAGCTTACCCACATC-3′) and IBDV 2 L (5′-CCTTCCCCAATTGCATGG-3′), was deployed to amplify a 929 bp fragment encompassing the HVR of the VP2 gene. For segment B, a distinct set of primers, B293U (5′-TTTTGCAGCCGCGGTCTCT-3′) and B1008L (5′-GTTTGACCCCTTTGTCCCTGC-3′), was utilized to amplify a 716 bp fragment that contains the B-marker region within the VP1 gene. The PCR products were analyzed by 1 % agarose gel electrophoresis. Positive RT-PCR products were sent to Ruibiotech Corporation (Harbin, China) for Sanger sequencing. The obtained nucleotide sequences of the VP2 HVR and the VP1 B-marker were deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/).
Sequence analysis
The Nucleotide sequences of the VP2 HVR and VP1 B-marker of IBDV were aligned with reference strains retrieved from the GenBank database using the DNASTAR software (Madison, WI, USA). Maximum likelihood (ML) trees were built with MEGA7 (ASU, Phoenix, AZ, USA) using the Kimura 2-parameter model and 1,000 bootstrap replicates, and then visualized using Interactive Tree Of Life (iTOL). MegAlign (MegAlign, DNASTAR Inc., Madison, WI, USA) was used to perform genetic similarity calculations and amino acid comparisons. The nucleotide sequences of the reference strain’s VP2 HVR and VP1 B-marker were documented in Tables S1 and S2, respectively.
Chickens and virus
Specific-pathogen-free (SPF) chickens were provided by the National Poultry Laboratory Animal Resource Center of HVRI, CAAS. These chickens were housed in negative pressure-filtered air isolators. All animal experiments were approved by the HVRI of the CAAS and were performed according to the animal ethics guidelines and approved protocols. The vvIBDV representative strain HuB-1 (Gao et al., 2014) was identified and preserved by our laboratory.
Mutated vvIBDV isolation
A representative strain of mutated vvIBDV (abbreviated as mvvIBDV), IBDV-NMG23-1701, was isolated by infecting five 3-week-old SPF chickens via the oculonasal route with original tissue supernatant. To evaluate the isolation of IBDV-NMG23-1701, bursas were harvested from the infected chickens at 7 days post-infection (d.p.i.), then processed and tested using RT-PCR as previously described. For the determination of IBDV isolate purity, a suite of diagnostic assays was employed as described previously (Wang et al., 2021): RT-PCR was utilized to detect Avian reovirus (ARV) and Avian metapneumovirus (aMPV); PCR was applied to detect Avian leukosis virus (ALV), Chicken anemia virus (CAV), Marek’s disease virus (MDV), Reticuloendotheliosis virus (REV), Fowl adenovirus Serotype 4 (FAdV-4) and Mycoplasma; Hemagglutination assay was performed to detect viruses with hemagglutination activity including avian influenza virus (AIV) and Newcastle disease virus (NDV); Bacteria were assessed by LB culture for 24 h.
Genome cloning and analysis
As previously described (Wang et al., 2020), four pairs of specific primers (AU/A1542L and A1421U2/AL2 for segment A; BU/B1344L and B1344U/BL for segment B) were used to synthesize the genome of IBDV-NMG23-1701 strain by RT-PCR. PCR products of segments A and B were individually cloned into the pMD18-T vector (Takara, Dalian, China). The recombinant plasmids were sent to Ruibo Corporation (Harbin, China) for Sanger sequencing. The nucleotide sequences of the polyprotein and the VP1 of the IBDV-NMG23-1701 strain were aligned with reference strains retrieved from the GenBank database. ML trees were assembled with the Kimura 2-parameter model in MEGA 7.0 and performed 1,000 bootstrap replicates. Genetic similarity calculations were performed using MegAlign. The nucleotide sequences of reference strains were detailed in Tables S1 and S2, respectively.
Pathogenicity evaluation
In this experiment, twenty-five 21-day-old SPF chickens were randomly divided into three groups. The first and second groups, each consisting of ten chickens, were respectively infected with IBDV-NMG23-1701 strain and the control vvIBDV strain HuB-1 through ocular and nasal routes at a dose of 2.5 × 105 copies/200 µL per bird. The third group, serving as the negative control, comprised five chickens that were administered 200 µL of PBS. Clinical symptoms were evaluated over seven days using the mean symptom index (MSI), which ranges from 0 to 3, with 0 indicating asymptomatic and 3 indicating the most severe symptoms. Survival curves for each group were recorded and plotted throughout the experimental period. Seven d.p.i., all chickens were euthanized and necropsied. The weights of the bursa and body of the chickens were recorded to calculate the bursa:body weight index (BBIX). A portion of the bursa tissue was fixed in 10 % formalin and stained with hematoxylin and eosin (H&E) for histopathological examination.
Statistical analyses
GraphPad Prism software 10.0 (GraphPad Software, Inc., San Diego, CA, USA) was employed for the analysis of all data. Unpaired t-tests were used to determine the statistical significance among the groups. Differences with P < 0.05 were considered significant.
Results
Detection of IBDV
Molecular detection revealed that IBDV was present in chicken flocks across seven of the nine provinces surveyed: Neimenggu, Liaoning, Hebei, Shandong, Henan, Fujian, and Guangdong. The proportion of positive flocks was 70.5 % (24/34) and the proportion of positive samples was 52.8 % (277/525). From these positive samples, 93 representative strains of IBDV were selected for sequencing, yielding a 435 bp fragment of the VP2 HVR (bp 746–1180, aa 206–350) and a 429 bp fragment of the VP1 B-marker (bp 439–867, aa 110–252). These sequences were deposited in GenBank, with the accession numbers listed in Table 1.
Table 1.
The IBDV strains identified in this study.
| No. | Stains | Phenotypea | Genotype | Source | Breed | Age (d) | Sampling date | GenBank accession no. |
|
|---|---|---|---|---|---|---|---|---|---|
| VP1 | VP2 | ||||||||
| 1 | IBDV-NMG23-0709 | nVar | A2dB1 | Neimenggu | Broiler | 25 | 202306 | PQ683409 | PQ673683 |
| 2 | IBDV-NMG23-0712 | nVar | A2dB1 | Neimenggu | Broiler | 25 | 202306 | PQ683410 | PQ673684 |
| 3 | IBDV-SD23-1201 | nVar | A2dB1 | Shandong | Broiler | 35 | 202308 | PQ683411 | PQ673685 |
| 4 | IBDV-SD23-1202 | nVar | A2dB1 | Shandong | Broiler | 35 | 202308 | PQ683412 | PQ673686 |
| 5 | IBDV-SD23-1223 | nVar | A2dB1 | Shandong | Broiler | 35 | 202308 | PQ683413 | PQ673687 |
| 6 | IBDV-SD23-1407 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683414 | PQ673688 |
| 7 | IBDV-SD23-1409 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683415 | PQ673689 |
| 8 | IBDV-SD23-1410 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683416 | PQ673690 |
| 9 | IBDV-SD23-1413 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683417 | PQ673691 |
| 10 | IBDV-SD23-1414 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683418 | PQ673692 |
| 11 | IBDV-SD23-1423 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683419 | PQ673693 |
| 12 | IBDV-SD23-1424 | nVar | A2dB1 | Shandong | Broiler | 30 | 202308 | PQ683420 | PQ673694 |
| 13 | IBDV-SD23-1505 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683421 | PQ673695 |
| 14 | IBDV-SD23-1506 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683422 | PQ673696 |
| 15 | IBDV-SD23-1508 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683423 | PQ673697 |
| 16 | IBDV-SD23-1509 | c | A1B1 | Shandong | Broiler | 30 | 202309 | PQ683424 | PQ673698 |
| 17 | IBDV-SD23-1510 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683425 | PQ673699 |
| 18 | IBDV-SD23-1511 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683426 | PQ673700 |
| 19 | IBDV-SD23-1514 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683427 | PQ673701 |
| 20 | IBDV-SD23-1516 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683428 | PQ673702 |
| 21 | IBDV-SD23-1517 | nVar | A2dB1 | Shandong | Broiler | 30 | 202309 | PQ683429 | PQ673703 |
| 22 | IBDV-FJ23-1601 | att | A8B1 | Fujian | Local | 28 | 202309 | PQ683430 | PQ673704 |
| 23 | IBDV-FJ23-1603 | att | A8B1 | Fujian | Local | 28 | 202309 | PQ683431 | PQ673705 |
| 24 | IBDV-NMG23-1701 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ673682 | PQ673681 |
| 25 | IBDV-NMG23-1704 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683432 | PQ673706 |
| 26 | IBDV-NMG23-1706 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683433 | PQ673707 |
| 27 | IBDV-NMG23-1707 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683434 | PQ673708 |
| 28 | IBDV-NMG23-1717 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683435 | PQ673709 |
| 29 | IBDV-NMG23-1718 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683436 | PQ673710 |
| 30 | IBDV-NMG23-1719 | vv | A3B2 | Neimenggu | Broiler | 35 | 202309 | PQ683437 | PQ673711 |
| 31 | IBDV-SD23-1907 | nVar | A2dB1 | Shandong | Broiler | 32 | 202310 | PQ683438 | PQ673712 |
| 32 | IBDV-HeB23-2109 | nVar | A2dB1 | Hebei | Broiler | 30 | 202310 | PQ683439 | PQ673713 |
| 33 | IBDV-HeB23-2111 | nVar | A2dB1 | Hebei | Broiler | 30 | 202310 | PQ683440 | PQ673714 |
| 34 | IBDV-SD23-2213 | nVar | A2dB1 | Shandong | Broiler | 28 | 202311 | PQ683441 | PQ673715 |
| 35 | IBDV-SD23-2214 | nVar | A2dB1 | Shandong | Broiler | 28 | 202311 | PQ683442 | PQ673716 |
| 36 | IBDV-SD23-2215 | nVar | A2dB1 | Shandong | Broiler | 28 | 202311 | PQ683443 | PQ673717 |
| 37 | IBDV-SD23-2216 | nVar | A2dB1 | Shandong | Broiler | 28 | 202311 | PQ683444 | PQ673718 |
| 38 | IBDV-LN24-0130 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683445 | PQ673719 |
| 39 | IBDV-LN24-0162 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683446 | PQ673720 |
| 40 | IBDV-LN24-0163 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683447 | PQ673721 |
| 41 | IBDV-LN24-0168 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683448 | PQ673722 |
| 42 | IBDV-LN24-0169 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683449 | PQ673723 |
| 43 | IBDV-LN24-0172 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683450 | PQ673724 |
| 44 | IBDV-LN24-0173 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683451 | PQ673725 |
| 45 | IBDV-LN24-0175 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683452 | PQ673726 |
| 46 | IBDV-LN24-0176 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683453 | PQ673727 |
| 47 | IBDV-LN24-0179 | nVar | A2dB1 | Liaoning | Broiler | 25 | 202401 | PQ683454 | PQ673728 |
| 48 | IBDV-HeN24-0203 | nVar | A2dB1 | Henan | Broiler | 24 | 202401 | PQ683455 | PQ673729 |
| 49 | IBDV-HeN24-0206 | vv | A3B3 | Henan | Broiler | 26 | 202401 | PQ683456 | PQ673730 |
| 50 | IBDV-HeN24-0207 | vv | A3B3 | Henan | Broiler | 26 | 202401 | PQ683457 | PQ673731 |
| 51 | IBDV-HeN24-0209 | vv | A3B3 | Henan | Broiler | 26 | 202401 | PQ683458 | PQ673732 |
| 52 | IBDV-HeN24-0215 | vv | A3B3 | Henan | Broiler | 25 | 202401 | PQ683459 | PQ673733 |
| 53 | IBDV-HeN24-0249 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683460 | PQ673734 |
| 54 | IBDV-HeN24-0251 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683461 | PQ673735 |
| 55 | IBDV-HeN24-0253 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683462 | PQ673736 |
| 56 | IBDV-HeN24-0254 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683463 | PQ673737 |
| 57 | IBDV-HeN24-0255 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683464 | PQ673738 |
| 58 | IBDV-HeN24-0256 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683465 | PQ673739 |
| 59 | IBDV-HeN24-0259 | nVar | A2dB1 | Henan | Broiler | 27 | 202401 | PQ683466 | PQ673740 |
| 60 | IBDV-HeN24-0261 | nVar | A2dB1 | Henan | Broiler | 29 | 202401 | PQ683467 | PQ673741 |
| 61 | IBDV-HeN24-0263 | c | A1B1 | Henan | Broiler | 23 | 202401 | PQ683468 | PQ673742 |
| 62 | IBDV-HeN24-0268 | nVar | A2dB1 | Henan | Broiler | 23 | 202401 | PQ683469 | PQ673743 |
| 63 | IBDV-HeN24-0310 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683470 | PQ673744 |
| 64 | IBDV-HeN24-0316 | nVar | A2dB1 | Henan | Broiler | 30 | 202401 | PQ683471 | PQ673745 |
| 65 | IBDV-HeN24-0317 | nVar | A2dB1 | Henan | Broiler | 30 | 202401 | PQ683472 | PQ673746 |
| 66 | IBDV-HeN24-0318 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683473 | PQ673747 |
| 67 | IBDV-HeN24-0319 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683474 | PQ673748 |
| 68 | IBDV-HeN24-0320 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683475 | PQ673749 |
| 69 | IBDV-HeN24-0321 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683476 | PQ673750 |
| 70 | IBDV-HeN24-0323 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683477 | PQ673751 |
| 71 | IBDV-HeN24-0325 | nVar | A2dB1 | Henan | Broiler | 31 | 202401 | PQ683478 | PQ673752 |
| 72 | IBDV-HeN24-0328 | nVar | A2dB1 | Henan | Broiler | 32 | 202401 | PQ683479 | PQ673753 |
| 73 | IBDV-HeN24-0336 | nVar | A2dB1 | Henan | Broiler | 32 | 202401 | PQ683480 | PQ673754 |
| 74 | IBDV-HeN24-0337 | nVar | A2dB1 | Henan | Broiler | 32 | 202401 | PQ683481 | PQ673755 |
| 75 | IBDV-HeN24-0340 | nVar | A2dB1 | Henan | Broiler | 32 | 202401 | PQ683482 | PQ673756 |
| 76 | IBDV-HeN24-0342 | nVar | A2dB1 | Henan | Broiler | 24 | 202401 | PQ683483 | PQ673757 |
| 77 | IBDV-HeN24-0343 | nVar | A2dB1 | Henan | Broiler | 24 | 202401 | PQ683484 | PQ673758 |
| 78 | IBDV-HeN24-0345 | nVar | A2dB1 | Henan | Broiler | 24 | 202401 | PQ683485 | PQ673759 |
| 79 | IBDV-FJ24-0614 | att | A8B1 | Fujian | Local | 27 | 202403 | PQ683486 | PQ673760 |
| 80 | IBDV-FJ24-0802 | att | A8B1 | Fujian | Local | 27 | 202403 | PQ683487 | PQ673761 |
| 81 | IBDV-FJ24-0803 | att | A8B1 | Fujian | Local | 27 | 202403 | PQ683488 | PQ673762 |
| 82 | IBDV-GD24-0905 | att | A8B1 | Guangdong | Broiler | 27 | 202403 | PQ683489 | PQ673763 |
| 83 | IBDV-GD24-0907 | att | A8B1 | Guangdong | Broiler | 27 | 202403 | PQ683490 | PQ673764 |
| 84 | IBDV-GD24-0909 | nVar | A2dB1 | Guangdong | Broiler | 27 | 202403 | PQ683491 | PQ673765 |
| 85 | IBDV-GD24-0915 | nVar | A2dB1 | Guangdong | Broiler | 27 | 202403 | PQ683492 | PQ673766 |
| 86 | IBDV-FJ24-1006 | att | A8B1 | Fujian | Broiler | 27 | 202403 | PQ683493 | PQ673767 |
| 87 | IBDV-HeB24-1203 | vv | A3B3 | Hebei | Broiler | 27 | 202404 | PQ683494 | PQ673768 |
| 88 | IBDV-LN24-1307 | vv | A3B3 | Liaoning | Broiler | 27 | 202404 | PQ683495 | PQ673769 |
| 89 | IBDV-LN24-1309 | vv | A3B3 | Liaoning | Broiler | 27 | 202404 | PQ683496 | PQ673770 |
| 90 | IBDV-SD24-1401 | c | A1B1 | Shandong | Broiler | 27 | 202404 | PQ683497 | PQ673771 |
| 91 | IBDV-SD24-1402 | c | A1B1 | Shandong | Broiler | 27 | 202404 | PQ683498 | PQ673772 |
| 92 | IBDV-SD24-1403 | vv | A3B3 | Shandong | Broiler | 27 | 202404 | PQ683499 | PQ673773 |
| 93 | IBDV-SD24-1404 | c | A1B1 | Shandong | Broiler | 27 | 202404 | PQ683500 | PQ673774 |
VV, very virulent strain; nVar, novel variant strain; att, attenuated strain; c, classical strain.
Phylogenetic analysis
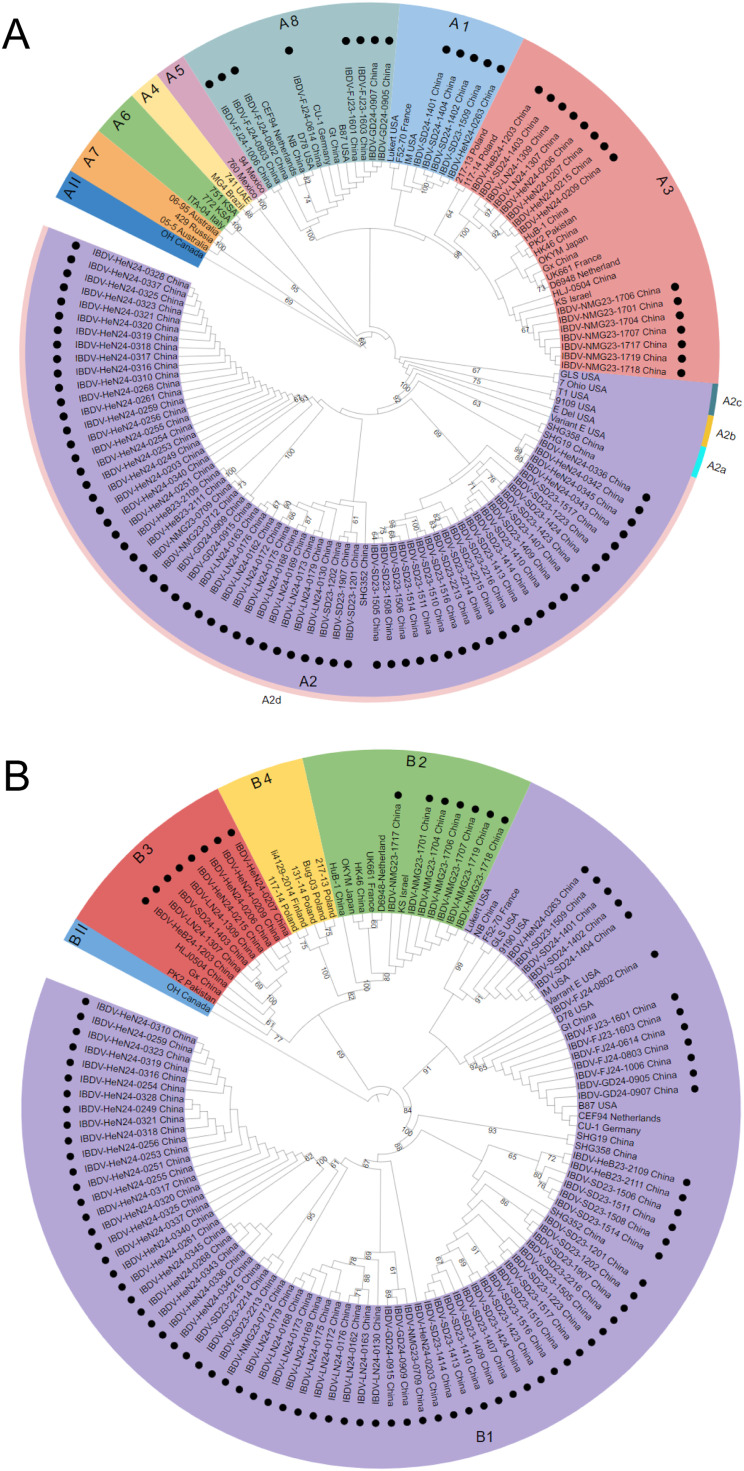
Following the recent classification scheme for IBDV genotyping (Wang et al., 2021), the genogroup was identified based on a single segment, whereas the genotype was determined using a combination of both segments. The phylogenetic tree based on the VP2 HVR nucleotide sequences of IBDV categorized serotype I strains into eight distinct genogroups (A1–A8), while serotype II strains were assigned to a single genogroup, AII. Additionally, genogroup A2 was further differentiated into four lineages: A2a, A2b, A2c, and A2d (Fig. 1A). Within the 93 IBDV strains identified in this study, 65 strains were classified under genogroup A2d, which is associated with the nVarIBDV. Nucleotide sequence homology analysis of the VP2 HVR revealed that the 65 strains had a high degree of similarity with the A2 genogroup strains (88.2–97.9 %), particularly with the A2d genogroup strains (94.9–97.9 %), which was considerably higher than that of other genogroup strains (83.2–90.8 %). Additionally, 15 strains clustered within the vvIBDV genogroup A3, showing the highest homology with A3 genogroup strains (91.3–98.6 %), and lower homology with other strains (82.7–94.7 %). Eight strains clustered with the attIBDV in genogroup A8, with high similarity (98.9–99.5 %) compared to other genogroup strains (86.8–97.0 %). Furthermore, five strains grouped together with the cIBDV in genogroup A1, demonstrating extremely high similarity (95.0–100 %) compared to other strains (87.5–95.6 %).
Fig. 1.
Phylogenetic trees of the hypervariable region (HVR) of VP2 gene (A) in segment A and the B-marker of VP1 gene (B) in segment B of IBDV. Bootstrap values are shown for branches that have a support above 60 %. The phylogenetic analysis of the VP2 HVR involved 40 reference nucleotide sequences, while the VP1 B-marker analysis involved 30 reference nucleotide sequences. Both phylogenetic trees include 93 IBDV strains detected in this study, represented by solid circles (●). Nine genotypes are marked based on the HVR of the VP2 gene, and five genotypes are classified according to the B-marker of VP1 gene. Each genotype is depicted in a different color.
The phylogenetic tree constructed from the B-marker of VP1 divided the serotype I strains into four evolutionary branches, B1–B4 (Fig. 1B). Among the 93 IBDV strains isolated here, 78 strains were clustered under the B1 branch, exhibiting the highest nucleotide sequence homology with B1 genogroup strains (90.9–100 %) and lower homology with other strains (86.0–90.2 %). Eight strains clustered with the HLJ0504-like vvIBDV within the B3 genogroup, with homology ranging from 90.9 % to 93.2 %, which was higher than that of other genogroup reference strains (88.0–90.7 %). Moreover, seven strains were clustered within the B2 branch of vvIBDV, showing high homology with this group (98.6–99.3 %) and lower with others (85.4–88.8 %).
The phylogenetic analysis of the representative gene fragments of segments A (VP2 HVR) and B (B-marker) revealed that the 93 IBDV strains isolated in this study encompass five genotypes: A2dB1 (65/93, 69.9 %), A3B3 (8/93, 8.6 %), A3B2 (7/93, 7.5 %), A8B1 (8/93, 8.6 %), and A1B1 (5/93, 5.4 %). Among them, the phenotypes of nVarIBDV (A2dB1) accounted for the largest proportion (69.9 %), vvIBDV (A3B3 and A3B2) accounted for a relatively small proportion, representing 16.1 % of the total, whereas attIBDV (A8B1) and cIBDV (A1B1) were the least prevalent, with proportions of 8.6 % and 5.4 %, respectively.
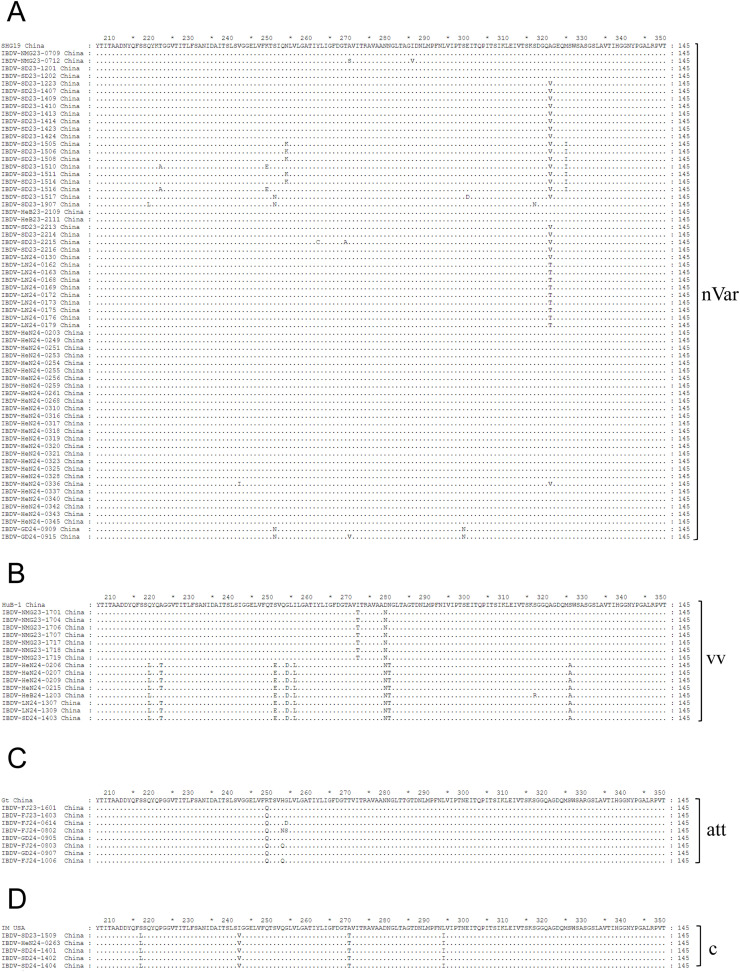
Alignment of key amino acid residues
Alignment of the characteristic amino acids in the VP2 HVR revealed that the 65 strains within genogroup A2d contained amino acids 213 N, 318D, and 323E (characteristic of varIBDV), as well as the amino acids 221 K and 252I (unique to nVarIBDV) (Fig. 2A). Fifteen strains clustered in genogroup A3 possessed the typical amino acids 242I, 294I, and 299S of vvIBDV, but they had nine main mutations at Q219L, A222T, S251E, G254D, I256L, I272T, D279N, N280T, and S326N compared to vvIBDV, of which A222T/G254D/I256L/D279N have been reported to be associated with virulence and antigenic changes (Letzel et al., 2007; Jiang et al., 2023; Wang et al., 2024). Among the 15 strains in genogroup A3, seven strains exhibited four mutations at A222T/G254D/I256L/D279N, one strain had three mutations at G254D/I256L/D279N, and seven strains (including IBDV-NMG23-1701) had one mutation at D279N. Notably, 100 % (15/15) of genogroup A3 isolates had the mutation D279N (Fig. 2B). Eight strains in the genogroup A8 displayed characteristic amino acids consistent with attIBDV: 279 N, 253H, 284T, and 330R (Fig. 2C). Five strains in genogroup A1 possessed characteristic amino acids consistent with cIBDV: 256 V, 279D, 284A, and 330S (Fig. 2D). All 15 strains in the A3 branch detected in this study had mutations, including D279N, which may have affected the pathogenicity and antigenicity of these strains. The VP1 of these 15 strains contained the characteristic residues 145TDN147 (genogroup B2) or 145TEG147 (genogroup B3) of vvIBDV.
Fig. 2.
Amino acid substitutions in the VP2 HVR of the 93 strains isolated in this study and their corresponding genotypic reference strains. (A) The 65 strains of varied detected aligned with the SHG19 strain (GenBank accession number: MH879092). (B) The 15 “vvIBDV” strains align with the HuB-1 strain (GenBank accession number: KF569805). (C) The 8 strains of attIBDV compared with the Gt strain (GenBank accession number: DQ403248). (D) The 5 strains of cIBDV align with the IM strain (GenBank accession number: AY029166). Identical residues in aligned sequences are marked with dots, while distinct residues are denoted by individual letters. nVar, novel variant strain; vv, very virulent strain; att, attenuated strain; c, classical strain.
Isolation and genome sequencing of one mutated vvIBDV (mvvIBDV)
The RT-PCR detection results targeting the VP2 HVR and VP1 B-marker genes were both positive, indicating that the representative mvvIBDV strain of IBDV-NMG23-1701 was successfully isolated. The isolated strain was verified as pure, with no detection of other pathogens, including ARV, aMPV, ALV, CAV, MDV, REV, FAdV-4, AIV, NDV, Mycoplasma, or bacteria. The genome sequencing results revealed that the segment A of IBDV-NMG23-1701 (3260 bp) consists of a large ORF (3039 bp) encoding the polyprotein (pVP2-VP4-VP3), a small ORF encoding VP5 (438 bp), a 5′ non-coding region (NCR, 96 bp), and a 3′ NCR (91 bp). Segment B (2827 bp) consists of an ORF encoding VP1 (2646 bp), a 5′ NCR (111 bp), and a 3′ NCR (70 bp). The complete genomic sequence of IBDV-NMG23-1701 was deposited to the GenBank, with accession numbers PQ673681 for Segment A and PQ673682 for Segment B, respectively.
Sequence analysis of mvvIBDV genome
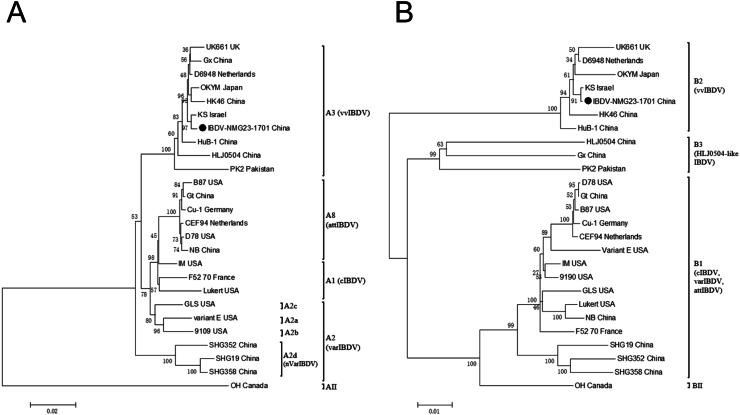
The phylogenetic tree constructed from the nucleotide sequences of the polyprotein (pVP2-VP4-VP3) in segment A displayed that the serotype I strains consisted of four previously described genogroups: A1 (cIBDV), A2 (varIBDV), A3 (vvIBDV), and A8 (attIBDV). Furthermore, genogroup A2 included the subgroups A2a/b/c (variant IBDV) and A2d (nVarIBDV). The representative mvvIBDV strain of IBDV-NMG23-1701 strain was classified within the vvIBDV genogroup A3 branch (Fig. 3A)). Homology analysis of the polyprotein nucleotide sequences revealed that the IBDV-NMG23-1701 strain exhibited a high degree of homology with A3 genogroup IBDV strains (97.9–99.6 %), which was higher than with other genogroup strains (94.0–96.2 %). The phylogenetic tree constructed from the nucleotide sequences of VP1 in segment B showed that the serotype I strains were categorized into three genogroups: B1, B2, and B3. The IBDV-NMG23-1701 strain was located within the B2 branch of the vvIBDV genogroup (Fig. 3B). The VP1 nucleotide sequence shared the highest homology with B2 genogroup strains (98.4–99.9 %), in contrast to lower homology with other strains (89.3–90.2 %). In general, the results of the whole-genome analysis were consistent with the analysis of the representative genomic fragments mentioned above, indicating that the mvvIBDV strain IBDV-NMG23-1701 belongs to the A3B2 genotype of vvIBDV.
Fig. 3.
Phylogenetic analysis of the nucleotide sequences encoding the polyprotein (A) and VP1 (B). The trees are proportionally rendered, with branch lengths quantified based on the frequency of mutations per position. The genogroup and corresponding phenotype for each branch are labeled. The mvvIBDV strain IBDV-NMG23-1701 isolated in this study is distinguished with a filled circle (●).
Pathogenicity evaluation of mvvIBDV
To evaluate the pathogenicity of the representative mvvIBDV strain of IBDV-NMG23-1701, an animal experiment was performed using SPF chickens. MSI indicated that the HuB-1 group began to exhibit severe clinical symptoms from 2 d.p.i. and reached a peak value at 3–5 d.p.i. (Fig. 4A). These symptoms include disheveled feathers, loss of appetite, lethargy, diarrhea, immobility, and even death. In contrast, neither the IBDV-NMG23-1701 group nor the negative control group displayed obvious clinical symptoms. Survival curves revealed that the HuB-1 group experienced mortality at 3, 4, and 5 d.p.i., with cumulative mortality rates of 30 % (3/10), 70 % (7/10), and 100 % (10/10) respectively (Fig. 4B). Throughout the experimental period, no deaths were observed in the IBDV-NMG23-1701 or negative control groups. At 7 d.p.i., necropsy findings revealed that the bursa in the IBDV-NMG23-1701 infected group exhibited severe atrophy (Fig. 4D) and inflammatory exudate, and the BBIX was below 0.7 (Fig. 4C). Histopathological examination further revealed severe histopathological changes in the bursa caused by the IBDV-NMG23-1701 strain, including the atrophy of lymphoid follicles, depletion of lymphocytes, and proliferation of fibrous connective tissue around the follicles. In contrast, no lesions were observed in the negative control group (Fig. 4E). These results suggest that the representative mvvIBDV strain IBDV-NMG23-1701 with mutations, including D279N in the VP2 HVR, differs significantly in pathogenicity from vvIBDV.
Fig. 4.
Pathogenicity evaluation of the mvvIBDV strain IBDV-NMG23-1701. (A) Mean symptom index (MSI) of chickens infected with IBDV-NMG23-1701 or HuB-1 with a dose of 2.5 × 105 viral RNA copies/200 μL. (B) Survival curve of infected chickens. (C) The bursa:body weight index (BBIX) at 7 d.p.i. (*** p < 0.001); (D) The bursa appearance at 7 d.p.i. (scale bar, 1 cm). (E) Hematoxylin-eosin (H&E) staining of bursa tissues harvested at 7 d.p.i. (scale bar, 100 μm).
Discussion
Since its initial appearance in 1992, vvIBDV has rapidly spread throughout China (Li and Wu, 1991), posing a significant threat to the poultry industry owing to its high mortality and severe immunosuppression (Jiang et al., 2021; Zhang et al., 2022). Although subsequent vaccinations and improvements in breeding management have controlled the acute epidemic (Hou et al., 2022), atypical IBD has occurred in many chicken farms across China since 2017 (Fan et al., 2019; Hou et al., 2022). Atypical IBD is not characterized by obvious clinical symptoms or mortality, but causes severe damage to the central immune organs in chickens, leading to significant immunosuppression and a decline in production performance (Fan et al., 2022). Researchers have identified nVarIBDV as a key pathogen of atypical IBD (Fan et al., 2019). Notably, since 2023, many farms have reported atypical IBD, but the preliminary molecular biological tests showed that the pathogen is “vvIBDV” rather than nVarIBDV. Further research and analyses are required to elucidate this phenomenon.
In this study, we identified four distinct phenotypes of coexisting IBDV strains in an epidemiological survey conducted from 2023 to 2024 in the main poultry farming areas of eastern China. Among these, nVarIBDV (A2dB1) accounted for the highest proportion (69.9 %), indicating that it remains the main pathogen causing atypical IBD in China. In addition, vvIBDV (A3B3 and A3B2), attIBDV (A8B1), and cIBDV (A1B1) were detected, highlighting the complexity of IBDV prevalence. In response to the atypical IBD caused by “vvIBDV” in chicken farms since last year, this study thoroughly analyzed the detected “vvIBDV” strains. The VP2 protein is a key determinant of IBDV virulence (Coulibaly et al., 2005; Eterradossi et al., 1998). Therefore, we compared the key amino acid sites in the VP2 HVR of 15 “vvIBDV” strains with vvIBDV reference strains and found that all these “vvIBDV” strains exhibited single mutation or combination mutations of characteristic amino acids including residue 279: D279N (seven strains), G254D/I256L/D279N (one strain), or A222T/G254D/I256L/D279N (seven strains).
To further investigate the biological characteristics of these “vvIBDV”, one representative strain IBDV-NMG23-1701 containing D279N was isolated and its full-length genome was analyzed. In the phylogenetic tree based on the whole genome, the IBDV-NMG23-1701 strain was located in the vvIBDV branch (A3B2 genotype). The pathogenicity of IBDV-NMG23-1701 strain was evaluated using an infection model of SPF chickens. Compared to the vvIBDV reference strain HuB-1, IBDV-NMG23-1701 did not cause obvious clinical symptoms, and the mortality rate was 0 %. However, IBDV-NMG23-1701 strain caused severe bursa lesion with lymphoid follicles atrophy and lymphocytes depletion. These results indicated that the IBDV-NMG23-1701 strain induced severe subclinical symptoms of atypical IBD, which is consistent with field observations. We named these strains mutated vvIBDV (mvvIBDV), which genotypically belong to vvIBDV but exhibited atypical IBD symptoms.
Genome sequence analysis confirmed the mutation D279N of the VP2 HVR in the mvvIBDV strain IBDV-NMG23-1701 compared to that in the vvIBDV reference strain HuB-1. Residue 279 is located in the PFG Loop of VP2 and is associated with virulence. Our earlier studies showed that D279N can considerably attenuate vvIBDV, with lower viremia, lower inflammatory effects, and reduced mortality from 70 % to 0 %. These data suggest that residue mutation of D279N of VP2 plays a central role in atypical IBD caused by mvvIBDV. Research has also shown that residue 279 is a key amino acid affecting viral antigenicity. The D279N mutation can reduce the antibody-antigen binding ability and allows the virus to escape the neutralization by vvIBDV antiserum (Jiang et al., 2023). These molecular characteristics explain the underlying molecular mechanisms that allow the mvvIBDV strain IBDV-NMG23-1701 to cause atypical IBD without killing the chickens.
Among the mvvIBDV strains identified in this study, besides D279N, other cooperative mutations including A222T, G254D, and I256L of VP2 were also detected. These mutations may induce the immune escape of IBDV. Residue 222, located in the PFG loop of the VP2 HVR, is closely related to viral antigenicity. Mutations at position 222 (P222T or P222S) have been shown to significantly alter the reactivity of the corresponding epitope with MAb 67 (Letzel et al., 2007). Additionally, a naturally occurring T222A mutation in VP2 of the IBDV Del-E strain has been reported to mediate immune escape (Jackwood and Sommer-Wagner, 2011). The reverse mutation A222T observed in mvvIBDV may also modify viral antigenicity, thereby mediating immune escape. Mutations at positions 254 and 256 are also closely related to the antigenicity of IBDV. Studies have shown that mutations V252I, G254N, and I256V in the PDE region can individually or synergistically reduce antigen-antibody affinity and interfere with the neutralization of antiserum (Wang et al., 2024). Although the G254D/I256L mutation in mvvIBDV is not completely consistent with the reported G254N/I256Vmutation in terms of amino acid substitution, the tertiary structure modeling of viral VP2 suggests that both alter the physicochemical properties of the amino acid sites. Similarly to the G254N mutation, the G254D mutation changes the microenvironment at position 254 from non-polar to polar. The I256L mutation significantly modifies the side chain topology from a γ-branched structure to a straight-chain structure. Likewise, the I256V mutation also alters the side-chain topology from a γ-branched structure to a β-branched structure. These changes may disrupt the charge complementarity and spatial fit at the interface between VP2 and neutralizing antibodies. Therefore, we speculate that the G254D and I256L mutations could also affect antigen-antibody binding, potentially leading to antigenic drift. These findings suggest that mvvIBDV may overcome the protection provided by existing vaccines through antigenic drift, thereby contributing to its widespread prevalence. Certainly, this hypothesis requires further validation through serum cross-neutralization assays and virus challenge protection experiments.
Since 2023, mvvIBDV has been detected in large breeding provinces with continuously increasing momentum at least including Neimenggu, Liaoning, Shandong, and Henan. Recently, atypical IBD has become an important disease threatening the poultry industry in Europe (Legnardi et al., 2023; Mató T et al., 2020; Pikuła and Lisowska, 2022; Pikuła et al., 2023; Reddy et al., 2024) and the Middle East (Legnardi et al., 2024), the main pathogen of which is reportedly the segment reassortment IBDV strain of genotype A3B1. Interestingly, most A3B1 strains in Europe possess key amino acid mutations (Q219L, Y220F, Q221K, G254D, D279N, and N280T) in VP2 compared to vvIBDV (Mató T et al., 2020; Pikuła and Lisowska, 2022; Pikuła et al., 2023; Reddy et al., 2024), which are similar to segment A of mvvIBDV in China. Further research on the industrial hazards, epidemic patterns, pathogenic mechanisms, and prevention and control measures for mvvIBDV and its related strains is urgently needed.
Under the pressures of immune system attacks and vaccination, viruses that evade the immunity are more likely to survive and spread (Chen et al., 2023). IBDV relies on an RNA polymerase for replication (Brown and Skinner, 1996), which has weak error-correction ability and is prone to generate and retain gene mutations during the process (Vignuzzi et al., 2006). Site mutations and segment reassortment are important evolutionary mechanisms in IBDV (Campbell et al., 2020; Pikuła et al., 2020; Wei et al., 2008). Widespread use of antigen-matched vaccines can reduce viral transmission and population size, consequently reducing the opportunity for viral evolution through genetic mutations and segment reassortment (Cobey et al., 2021). Antigenic variations in mvvIBDV may enable it to escape the protection conferred by traditional vvIBDV vaccines. Given the rapid variation characteristics and the complex epidemiological situation of IBDV, it is necessary to strengthen epidemiological monitoring and early warning research, reveal the laws and mechanisms of viral genetic variation, and develop antigen-matched broad-spectrum vaccines.
Conclusion
For the first time, this study identified mvvIBDV as a new pathogen causing atypical IBD. With the extremely high homology of gene sequences, mvvIBDV is easily mistaken as vvIBDV in molecular testing. However, due to mutations in key amino acids related to pathogenicity, mvvIBDV does not directly kill chickens but can cause acute damage to the central immune organs of the bursa and B lymphocytes, leading to atypical IBD. These findings demonstrate the complexity of IBDV prevalence and provide a scientific basis for optimizing preventive and control strategies.
Ethical Approval
All animal experiments were approved by the Animal Experimental Welfare Ethics Committee of HVRI. The approval number is 240418-02-GR. All procedures were performed following the approved protocols for animal welfare.
CRediT authorship contribution statement
Hangbo Yu: Investigation, Methodology, Formal analysis, Writing – original draft. Guodong Wang: Methodology, Investigation. Wenying Zhang: Methodology, Formal analysis. Ziwen Wu: Methodology, Investigation. Xinxin Niu: Methodology, Investigation. Mengmeng Huang: Methodology, Investigation. Yulong Zhang: Methodology, Investigation. Runhang Liu: Methodology, Investigation. Jinze Han: Methodology, Investigation. Mengmeng Xu: Methodology, Investigation. Jingzhe Han: Methodology, Investigation. Dan Ling: Methodology, Investigation. Erjing Ke: Methodology, Investigation. Suyan Wang: Methodology. Hongyu Cui: Methodology. Yanping Zhang: Methodology. Yuntong Chen: Methodology. Yongzhen Liu: Methodology. Yulu Duan: Methodology. Yulong Gao: Funding acquisition, Resources, Supervision. Xiaole Qi: Funding acquisition, Supervision, Resources, Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32473000, 32072852, U20A2061), the National Key Research and Development Program of China (2022YFD1800300), the Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-CSLPDCP-202402), and the China Agriculture Research System (CARS-41-G15).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105195.
Contributor Information
Yulong Gao, Email: gaoyulong@caas.cn.
Xiaole Qi, Email: qixiaole@caas.cn.
Appendix. Supplementary materials
References
- Alfonso-Morales A., Rios L., Martínez-Pérez O., Dolz R., Valle R., Perera C.L., Bertran K., Frías M.T., Ganges L., Díaz de Arce H., Majó N., Núñez J.I., Pérez L.J. Evaluation of a phylogenetic marker based on genomic segment B of infectious bursal disease virus: facilitating a feasible incorporation of this segment to the molecular epidemiology studies for this viral agent. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao K., Qi X., Li Y., Gong M., Wang X., Zhu P. Cryo-EM structures of infectious bursal disease viruses with different virulences provide insights into their assembly and invasion. Sci. Bull. 2022;67:646–654. doi: 10.1016/j.scib.2021.12.009. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Skinner M.A. Coding sequences of both genome segments of a European 'very virulent' infectious bursal disease virus. Virus Res. 1996;40:1–15. doi: 10.1016/0168-1702(95)01253-2. [DOI] [PubMed] [Google Scholar]
- Campbell E.A., Reddy V.R.A.P., Gray A.G., Wells J., Simpson J., Skinner M.A., Hawes P.C., Broadbent A.J. Discrete virus factories form in the cytoplasm of cells coinfected with two replication-competent tagged reporter birnaviruses that subsequently coalesce over time. J. Virol. 2020;94(13) doi: 10.1128/JVI.02107-19. e02107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Huang Z., Guo Y., Guo H., Jian L., Xiao J., Yao X., Yu H., Cheng T., Zhang Y., Guan M., Mao R., Zhang J., Xia N., Yuan Q. Evolving spike mutations in SARS-CoV-2 Omicron variants facilitate evasion from breakthrough infection-acquired antibodies. Cell. Discov. 2023;9:86. doi: 10.1038/s41421-023-00584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettle N., Stuart J.C., Wyeth P.J. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989;125:271–272. doi: 10.1136/vr.125.10.271. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Lepault J., Erk I., Da Costa B., Delmas B. The maturation process of pVP2 requires assembly of infectious bursal disease virus capsids. J. Virol. 2002;76:2384–2392. doi: 10.1128/jvi.76.5.2384-2392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobey S., Larremore D.B., Grad Y.H., Lipsitch M. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat. Rev. Immunol. 2021;21:330–335. doi: 10.1038/s41577-021-00544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove A.S. An apparently new disease of chickens: avian nephrosis. Avian Dis. 1962;6:385–389. [Google Scholar]
- Coulibaly F., Chevalier C., Gutsche I., Pous J., Navaza J., Bressanelli S., Delmas B., Rey F.A. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell. 2005;120:761–772. doi: 10.1016/j.cell.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Escaffre O., Le Nouën C., Amelot M., Ambroggio X., Ogden K.M., Guionie O., Toquin D., Müller H., Islam M.R., Eterradossi N. Both genome segments contribute to the pathogenicity of very virulent infectious bursal disease virus. J. Virol. 2013;87:2767–2780. doi: 10.1128/JVI.02360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eterradossi N., Arnauld C., Toquin D., Rivallan G. Critical amino acid changes in VP2 variable domain are associated with typical and atypical antigenicity in very virulent infectious bursal disease viruses. Arch. Virol. 1998;143:1627–1636. doi: 10.1007/s007050050404. [DOI] [PubMed] [Google Scholar]
- Fan L., Wang Y., Jiang N., Gao Y., Niu X., Zhang W., Huang M., Bao K., Liu A., Wang S., Gao L., Li K., Cui H., Pan Q., Liu C., Zhang Y., Wang X., Qi X. Residues 318 and 323 in capsid protein are involved in immune circumvention of the atypical epizootic infection of infectious bursal disease virus. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.909252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Wu T., Hussain A., Gao Y., Zeng X., Wang Y., Gao L., Li K., Wang Y., Liu C., Cui H., Pan Q., Zhang Y., Liu Y., He H., Wang X., Qi X. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019;230:212–220. doi: 10.1016/j.vetmic.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Fan L., Wu T., Wang Y., Hussain A., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y., Wang X., Qi X. Novel variants of infectious bursal disease virus can severely damage the bursa of fabricius of immunized chickens. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108507. [DOI] [PubMed] [Google Scholar]
- Gao H., Wang Y., Gao L., Zheng S.J. Genetic insight into the interaction of IBDV with host: a clue to the development of novel IBDV vaccines. Int. J. Mol. Sci. 2023;24:8255. doi: 10.3390/ijms24098255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Li K., Qi X., Gao H., Gao Y., Qin L., Wang Y., Shen N., Kong X., Wang X. Triplet amino acids located at positions 145/146/147 of the RNA polymerase of very virulent infectious bursal disease virus contribute to viral virulence. J. Gen. Virol. 2014;95:888–897. doi: 10.1099/vir.0.060194-0. [DOI] [PubMed] [Google Scholar]
- Hou B., Wang C.Y., Luo Z.B., Shao G.Q. Commercial vaccines used in China do not protect against a novel infectious bursal disease virus variant isolated in Fujian. Vet. Rec. 2022;191:e1840. doi: 10.1002/vetr.1840. [DOI] [PubMed] [Google Scholar]
- Jackwood D.H., Saif Y.M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987;31:766–770. [PubMed] [Google Scholar]
- Jackwood D.J. Advances in vaccine research against economically important viral diseases of food animals: infectious bursal disease virus. Vet. Microbiol. 2017;206:121–125. doi: 10.1016/j.vetmic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Sommer-Wagner S.E. Amino acids contributing to antigenic drift in the infectious bursal disease Birnavirus (IBDV) Virology. 2011;409:33–37. doi: 10.1016/j.virol.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Sreedevi B., LeFever L.J., Sommer-Wagner S.E. Studies on naturally occurring infectious bursal disease viruses suggest that a single amino acid substitution at position 253 in VP2 increases pathogenicity. Virology. 2008;377:110–116. doi: 10.1016/j.virol.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Jiang N., Wang G., Zhang W., Wang Y., Niu X., Huang M., Gao L., Li K., Cui H., Liu C., Zhang Y., Bao K., Wang S., Chen Y., Wang X., Gao Y., Qi X. A single mutation of VP2 is responsible for the lethality and antigenicity differences between novel variant and very virulent IBDV strains. Transbound. Emerg. Dis. 2023;2023(1) doi: 10.1155/2023/6684304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Wang Y., Zhang W., Niu X., Huang M., Gao Y., Liu A., Gao L., Li K., Pan Q., Liu C., Zhang Y., Cui H., Wang X., Qi X. Genotyping and molecular characterization of infectious bursal disease virus identified in important poultry-raising areas of China during 2019 and 2020. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.759861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnardi M., Franzo G., Tucciarone C.M., Koutoulis K., Cecchinato M. Infectious bursal disease virus in Western Europe: the rise of reassortant strains as the dominant field threat. Avian Pathol. 2023;52:25–35. doi: 10.1080/03079457.2022.2130172. [DOI] [PubMed] [Google Scholar]
- Legnardi M., Poletto F., Alam S., Cherfane A., Le-Tallec B., Franzo G., Tucciarone C.M., Lupini C., Pasotto D., Cecchinato M. Molecular epidemiology of infectious bursal disease virus in the Near East and Persian Gulf regions. Avian Pathol. 2024;53:56–67. doi: 10.1080/03079457.2023.2270531. [DOI] [PubMed] [Google Scholar]
- Letzel T., Coulibaly F., Rey F.A., Delmas B., Jagt E., van Loon A.A.M.W., Mundt E. Molecular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. J. Virol. 2007;81:12827–12835. doi: 10.1128/JVI.01501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Wu Z. Isolation and preliminary identification of a supervirulent strain of infectious bursal disease virus. Chin. J. Prev. Vet. Med. 1991;6:3–6. [Google Scholar]
- Mahgoub H.A., Bailey M., Kaiser P. An overview of infectious bursal disease. Arch. Virol. 2012;157:2047–2057. doi: 10.1007/s00705-012-1377-9. [DOI] [PubMed] [Google Scholar]
- Mató T., Tatár-Kis T., Felföldi B., Jansson D.S., Homonnay Z., Bányai K., Palya V. Occurrence and spread of a reassortant very virulent genotype of infectious bursal disease virus with altered VP2 amino acid profile and pathogenicity in some European countries. Vet. Microbiol. 2020;245 doi: 10.1016/j.vetmic.2020.108663. [DOI] [PubMed] [Google Scholar]
- Müller H., Islam M.R., Raue R. Research on infectious bursal disease—The past, the present and the future. Vet. Microbiol. 2003;97:153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Pikuła A., Lisowska A. Genetics and pathogenicity of natural reassortant of infectious bursal disease virus emerging in Latvia. Pathogens. 2022;11:1081. doi: 10.3390/pathogens11101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuła A., Lisowska A., Domańska-Blicharz K. Epidemiology of infectious Bursal Disease Virus in Poland during 2016-2022. Viruses. 2023;15:289. doi: 10.3390/v15020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikula A., Smietanka K., Perez L.J. Emergence and expansion of novel pathogenic reassortant strains of infectious bursal disease virus causing acute outbreaks of the disease in Europe. Transbound. Emerg. Dis. 2020;67:1739–1744. doi: 10.1111/tbed.13510. [DOI] [PubMed] [Google Scholar]
- Qi X., Gao H., Gao Y., Qin L., Wang Y., Gao L., Wang X. Naturally occurring mutations at residues 253 and 284 in VP2 contribute to the cell tropism and virulence of very virulent infectious bursal disease virus. Antiviral Res. 2009;84:225–233. doi: 10.1016/j.antiviral.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Raja P., Senthilkumar T.M., Parthiban M., Thangavelu A., Gowri A.M., Palanisammi A., Kumanan K. Complete genome sequence analysis of a naturally reassorted infectious bursal disease virus from India. Genome Announc. 2016;4(4) doi: 10.1128/genomeA.00709-16. e00709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V.R.A.P., Bianco C., Poulos C., Egana-Labrin S.C., Brodrick A.J., Nazki S., Schock A., Broadbent A.J. Molecular characterization of reassortant infectious bursal disease virus (IBDV) strains of genogroup A3B1 detected in some areas of Britain between 2020 and 2021. Virology. 2024;600 doi: 10.1016/j.virol.2024.110269. [DOI] [PubMed] [Google Scholar]
- Vignuzzi M., Stone J.K., Arnold J.J., Cameron C.E., Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Jiang N., Yu H., Niu X., Huang M., Zhang Y., Zhang W., Han J., Xu M., Liu R., Wu Z., Han J., Wang S., Gao L., Cui H., Zhang Y., Chen Y., Gao Y., Qi X. Loop PDE of viral capsid protein is involved in immune escape of the emerging novel variant infectious bursal disease virus. Vet. Microbiol. 2024;293 doi: 10.1016/j.vetmic.2024.110094. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Fan L., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y.-P., Wang X., Qi X. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agric. 2021;20:1372–1381. [Google Scholar]
- Wang Y., Fan L., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y., Wang X., Qi X. Naturally occurring cell-adapted classic strain of infectious bursal disease virus. Vet. Microbiol. 2020;243 doi: 10.1016/j.vetmic.2020.108620. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang N., Fan L., Niu X., Zhang W., Huang M., Gao L., Li K., Gao Y., Liu C., Cui H., Liu A., Pan Q., Zhang Y., Wang X., Qi X. Identification and pathogenicity evaluation of a novel reassortant infectious bursal disease virus (Genotype A2dB3) Viruses. 2021;13:1682. doi: 10.3390/v13091682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Yu X., Zheng J., Chu W., Xu H., Yu X., Yu L. Reassortant infectious bursal disease virus isolated in China. Virus Res. 2008;131:279–282. doi: 10.1016/j.virusres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Wu T., Wang Y., Li H., Fan L., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y., Wang X., Qi X. Naturally occurring homologous recombination between novel variant infectious bursal disease virus and intermediate vaccine strain. Vet. Microbiol. 2020;245 doi: 10.1016/j.vetmic.2020.108700. [DOI] [PubMed] [Google Scholar]
- Yu F., Ren X., Wang Y., Qi X., Song J., Gao Y., Qin L., Gao H., Wang X. A single amino acid V4I substitution in VP1 attenuates virulence of very virulent infectious bursal disease virus (vvIBDV) in SPF chickens and increases replication in CEF cells. Virology. 2013;440:204–209. doi: 10.1016/j.virol.2013.02.026. [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang X., Gao Y., Qi X. The over-40-years-epidemic of infectious bursal disease virus in China. Viruses. 2022;14:2253. doi: 10.3390/v14102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.