This cohort study investigates the implications of sex, age, socioeconomic status, cardiovascular disease (CVD) diagnosis, cardiothoracic surgery, and comorbidity for the association between cardiac rehabilitation participation and all-cause mortality.
Key Points
Question
What is the patient-specific, disease-specific, and comorbidity-specific association of cardiac rehabilitation participation with all-cause mortality?
Findings
In this cohort study, 31% of 83 687 eligible patients with cardiovascular disease participated in a multidisciplinary outpatient cardiac rehabilitation program. Cardiac rehabilitation participation was associated with a 32% lower risk of all-cause mortality compared with nonparticipation, which was independent of patient-related and comorbidity-related characteristics.
Meaning
Cardiac rehabilitation participation is associated with a lower mortality risk compared with nonparticipation; however, cardiac rehabilitation remains underused, especially in older adults with chronic diseases or multimorbidity.
Abstract
Importance
Cardiac rehabilitation (CR) is an effective strategy to improve clinical outcomes, but it remains underused in some subgroups of patients with cardiovascular disease (CVD).
Objective
To investigate the implications of sex, age, socioeconomic status, CVD diagnosis, cardiothoracic surgery, and comorbidity for the association between CR participation and all-cause mortality.
Design, Setting, and Participants
Observational cohort study with patient enrollment between July 1, 2012, and December 31, 2017, and a follow-up to March 19, 2020. The dates of analysis were March to May 2020. This study was performed among Dutch patients with CVD with a multidisciplinary outpatient CR program indication and who were insured at Coöperatie Volksgezondheidszorg, one of the largest health insurance companies in the Netherlands. Among 4.1 million beneficiaries, patients with CVD with an acute coronary event (myocardial infarction or unstable angina pectoris), stable angina pectoris, chronic heart failure, or cardiothoracic surgery (coronary artery bypass grafting, valve replacement, or percutaneous coronary intervention) were identified by inpatient diagnosis codes and included in the study.
Main Outcomes and Measures
Cox proportional hazards models were used to evaluate the association between CR participation and all-cause mortality. Stabilized inverse propensity score weighting was used to account for patient and disease characteristics associated with CR participation.
Results
Among 83 687 eligible patients with CVD (mean [SD] age, 67 [12] years; 60.4% [n = 50 512] men), only 31.3% (n = 26 171) participated in CR, with large variation across different subgroups (range, 5.1%-73.0%). During a mean (SD) of 4.7 (1.8) years of follow-up, 1966 CR participants (7.5%) and 13 443 CR nonparticipants (23.4%) died. After multivariable adjustment, CR participation was associated with a 32% lower risk of all-cause mortality (adjusted hazard ratio, 0.68; 95% CI, 0.65-0.71) compared with nonparticipation. Sex, age, socioeconomic status, and comorbidity did not alter risk reduction after CR participation, but a statistically significant interaction association was found across categories of CVD diagnosis and cardiothoracic surgery. Larger reductions in risk estimates for all-cause mortality were found after CR participation for STEMI (adjusted HR, 0.59; 95% CI, 0.52-0.68 vs 0.72; 95% CI, 0.65-0.79; P < .001), NSTEMI (adjusted HR, 0.64; 95% CI, 0.58-0.70 vs 0.72; 95% CI, 0.65-0.79; P < .001), and stable AP (adjusted HR, 0.69; 95% CI, 0.63-0.76 vs 0.72; 95% CI, 0.65-0.79; P < .001) compared with patients with chronic heart failure, whereas unstable AP had a smaller risk reduction (adjusted HR, 0.75; 95% CI, 0.67-0.85 vs 0.72; 95% CI, 0.65-0.79; P < .001).
Conclusions and Relevance
In this cohort study, CR participation was associated with a 32% risk reduction in all-cause mortality, and this benefit was independent of sex, age, socioeconomic status, and comorbidity. These findings reinforce the importance of CR participation in secondary prevention and highlight the possibility that CR should be prescribed more widely to vulnerable patients with CVD, such as older adults with chronic diseases or multimorbidity.
Introduction
Cardiac rehabilitation (CR) is a key component of secondary prevention strategies for patients with cardiovascular disease (CVD).1 Risk reduction for all-cause mortality, CVD mortality, unplanned hospitalization, and reinfarction has been reported in CR participants vs nonparticipants in multiple meta-analyses.2,3,4,5,6 Therefore, the American Heart Association, American College of Cardiology, and European Society of Cardiology have included CR referral as a Class IA recommendation for stable patients after an acute coronary syndrome or heart failure diagnosis.7,8,9,10 Despite these strong recommendations, participation rates of patients with CVD among hospital-based CR programs remain low, varying between 10% and 35% of eligible patients.11,12 Demographic and clinical factors, such as female sex, advanced age, socioeconomic status (SES), recent cardiothoracic surgery, and existing comorbidity, are known to reduce CR referral and participation,13,14 but evidence to refute these referral biases is scarce.
The present study aimed to investigate the implications of sex, age, SES, CVD diagnosis, cardiothoracic surgery, and comorbidity for the association between participation in a multidisciplinary outpatient CR program and all-cause mortality risk reduction. The study analyzed health insurance claims data from a nationwide cohort of 4.1 million individuals in the Netherlands. We hypothesized that participation in CR would be associated with a lower mortality risk for patients with CVD compared with nonparticipation, and we expected the risk reduction would vary across subgroups with different risk profiles.
Methods
Cohort Characteristics and Patient Selection
In the Netherlands, it is mandatory to have health insurance. For the present study, health insurance claims data were used from Dutch patients with CVD with a multidisciplinary outpatient CR program indication and who were insured at Coöperatie Volksgezondheidszorg (VGZ), one of the largest health insurance companies in the Netherlands. The VGZ cohort consists of 4.1 million beneficiaries, representing 24% of the Dutch population,15 with nationwide coverage and a representative sample of individuals from all age categories, SES, and both rural and urban areas (eFigure 1 in the Supplement). Individuals eligible for the present study were patients with a new diagnosis of CVD with an indication for CR according to Dutch guidelines,16 including an acute coronary event (myocardial infarction [MI] or unstable angina pectoris [AP]), stable AP, chronic heart failure, or cardiothoracic surgery (coronary artery bypass grafting [CABG], valve replacement, or percutaneous coronary intervention [PCI]). Reimbursement for outpatient CR is provided on the condition that the patient is referred by a cardiologist. Health insurance claims data were used to assess the type and date of diagnosis, and patients were enrolled between July 1, 2012, and December 31, 2017, with follow-up to March 19, 2020. The dates of analysis were March to May 2020. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Radboud University Medical Center Institutional Review Board deemed that this observational cohort study was exempt from informed consent because it involved retrospective analysis of an anonymized data set.
CR in the Netherlands
The Dutch Society of Cardiology released a multidisciplinary CR guideline,16 which is consistent with international recommendations.17,18 Group-based outpatient CR programs consist of the following 4 modules: (1) supervised exercise training, (2) mental health and stress relief, (3) social health, and (4) cardiovascular risk management. The multidisciplinary CR program typically lasts 6 to 12 weeks; on average, 85% of the patients receive exercise training, 39% receive relaxation therapy, 17% receive lifestyle modification therapy, and 75% receive education.19 Hence, the content of the contemporary Dutch CR program is largely comparable to that of CR programs in other countries. Accessibility to CR in the Netherlands is excellent because most CR facilities are located near the residence of patients (<30 km).
Study Population and Analytic Cohort
A total of 101 940 patients met the initial selection criteria. Consistent with previous observational studies,20,21 patients were classified as having received CR if a claim was filed for at least one of the group-based outpatient treatments. Excluded from further analysis were patients who (1) were insured less than 365 days before or less than 180 days after their diagnosis of CVD, (2) began CR more than 90 days after their diagnosis, or (3) died within 180 days after their diagnosis. Patients with multiple CVD-related insurance claims within 30 days after the initial diagnosis were reclassified according to the most severe diagnosis using the following rankings for CVD diagnosis and cardiothoracic surgery.20 For CVD diagnosis, the ranking was (1) ST-segment elevation MI (STEMI), (2) non-STEMI (NSTEMI), (3) unstable AP,(4) stable AP, and (5) chronic heart failure. For cardiothoracic surgery, the ranking was (1) CABG, (2) valve replacement, (3) acute PCI, and (4) elective PCI. Accordingly, 83 687 eligible patients with CVD were available for analysis (eFigure 2 in the Supplement).
Primary Outcome
Survival status of study participants was obtained from the Dutch Population Register, and the date of death was extracted if applicable. All-cause mortality was the primary outcome, and survival time in days was calculated from the date of the initial diagnosis of CVD. The maximum follow-up in the cohort was 7.7 years (92 months). Patients were censored if they switched to another health insurance company or if they were still alive during the last survival check.
Patient Characteristics
Sex and age were extracted from the VGZ health insurance database. Income and SES were obtained from the national statistical office (Statistics Netherlands) using postal code information for the patient’s residence. Socioeconomic status was classified using the SES score from Statistics Netherlands as low (bottom 40%), moderate (middle 30%), or high (upper 30%) (eFigure 1C in the Supplement).
Comorbidity
Comorbidity was identified using diagnosis codes from the Dutch Business Intelligence Center for Healthcare (Vektis). Insurance claims data for pharmaceutical agents were used to evaluate the association of diabetes, cancer, gout, Parkinson disease, respiratory diseases, thyroid diseases, and dementia as binary variables with CR outcomes.22 We evaluated the consequences of multimorbidity using the Charlson Comorbidity Index (CCI). As a continuous variable, the CCI was calculated as the sum of comorbid conditions23 as follows. One point was given for MI, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, ulcer disease, mild liver disease, and diabetes. Two points were given for diabetes with end-organ damage, any tumor, leukemia, and lymphoma. Three points were given for moderate to severe liver disease. Six points were given for metastatic solid tumors and AIDS. We compared the association of CR with all-cause mortality across patients with CCI scores of 1 or less, 2, 3, 4, and 5 or greater.
Statistical Analysis
Baseline characteristics were summarized as mean (SD) or median (interquartile range) for continuous variables and as number (percentage) for categorical variables. Differences in individual and disease characteristics between CR participants and nonparticipants were assessed using t test for continuous variables and χ2 test for categorical variables. Kaplan-Meier curves and a log-rank test were used to assess the difference in all-cause mortality between CR participants and nonparticipants.
Information about patient characteristics, income, SES, distance to CR facility, disease characteristics, comorbidity, pharmaceutical agents in the year of diagnosis of CVD, and health care expenses was used to calculate the inverse propensity score. The crude hazard ratio (HR) (with 95% CI) was calculated by univariable Cox proportional hazards models. Subsequently, we corrected for sex, age, category of CVD diagnosis, cardiothoracic surgery, Charlson Comorbidity Index, and cardiac medication. Stabilized inverse propensity score weighting was applied to the fully adjusted multivariable Cox proportional hazards models to account for differences between CR participants and nonparticipants.24,25,26 We evaluated the robustness of the main outcomes via sensitivity analyses. For this purpose, the period between the initial event date and the minimum follow-up period was changed from 180 days to 1 to 6 years.
The presence of effect modification was tested for using an interaction term to assess the implications of sex (reference group, men), age (reference group, <50 years), SES (reference group, low SES), CVD diagnosis (reference group, chronic heart failure), cardiothoracic surgery (reference group, elective PCI), and comorbidity (reference group, CCI≤1) for the association between CR participation and all-cause mortality risk. Two-sided P < .05 was considered statistically significant. All statistical analyses were performed in SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
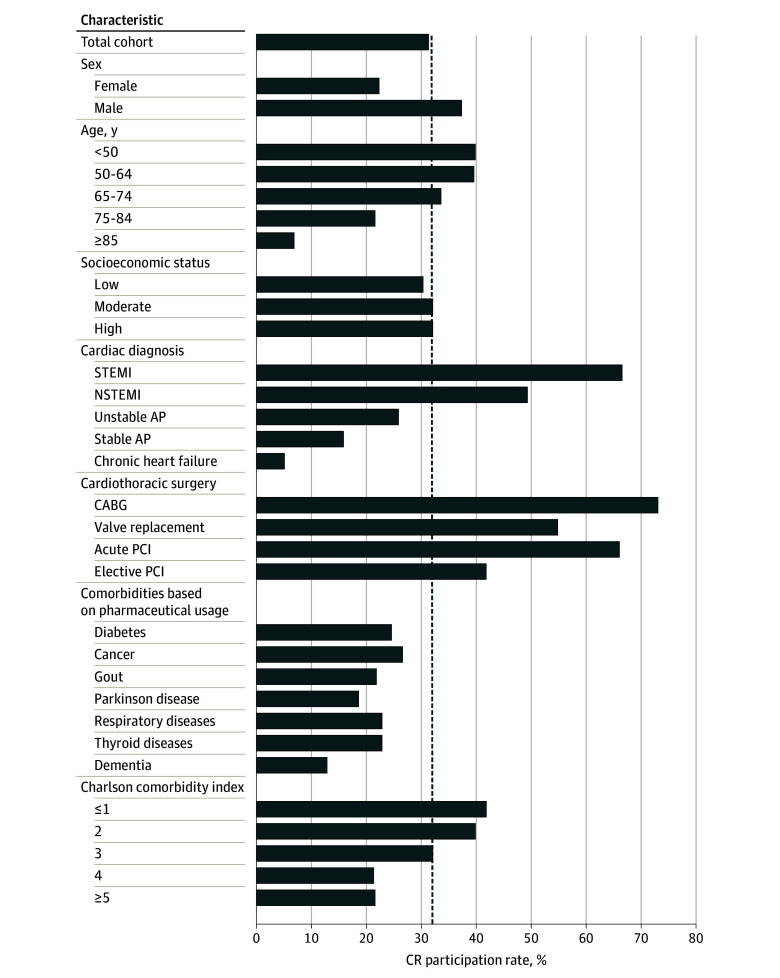
In this cohort of 83 687 patients with CVD (mean [SD] age, 67 [12] years; 60.4% [n = 50 512] men), only 31.3% (n = 26 171) participated in CR at 89 CR facilities in the Netherlands. The CR participation rate increased across calendar years from 25.7% in 2012 to 38.6% in 2017 (eTable 1 in the Supplement). Distance to the CR facility was greater for CR participants than for nonparticipants (mean [SD], 15.4 [19.3] vs 14.7 [20.2] km; P < .001). Compared with nonparticipants, CR participants were younger, included more male patients, had a lower CCI score, and had a higher income (Table 1). Cardiac medication use was lower in the year before CVD diagnosis among CR participants compared with nonparticipants, but this association largely reversed in the year after CVD diagnosis. Cardiac rehabilitation participation rates were higher among men (37.2%), patients younger than 75 years (33.5% to 39.8%), those with a diagnosis of STEMI (66.6%) or NSTEMI (49.2%), those with a CCI score of 3 or less (32.0% to 41.7%), and those undergoing CABG (73.0%), valve replacement (54.8%), or acute (66.0%) or elective (41.8%) PCI. In contrast, CR was underused in women (22.2%), patients 75 years or older (6.7% to 21.5%), those with comorbidity (12.7% to 26.5%), and those having a CVD diagnosis of unstable (25.9%) or stable (15.9%) AP or chronic heart failure (5.1%) (Figure 1).
Table 1. Comparison of Patient Characteristics Between Cardiac Rehabilitation (CR) Participants and Nonparticipants.
| Variable | No. (%) | P value | |
|---|---|---|---|
| CR participants (n = 26 171) | Nonparticipants (n = 57 516) | ||
| Male sex | 18 790 (71.8) | 31 722 (55.2) | <.001 |
| Age, mean (SD), y | 64 (11) | 69 (12) | <.001 |
| CCI in the year before the study, mean (SD) | 3.1 (3.8) | 4.4 (6.6) | <.001 |
| Income, mean (SD), € | 36 000 (7000)a | 35 500 (7200)b | <.001 |
| SES | <.001 | ||
| Low | 11 188 (42.7) | 25 656 (44.6) | |
| Moderate | 8233 (31.5) | 17 526 (30.5) | |
| High | 6750 (25.8) | 14 334 (24.9) | |
| Distance to CR facility, km | 15.4 (19.3) | 14.7 (20.2) | <.001 |
| CVD diagnosis | |||
| STEMI | 8169 (31.2) | 4096 (7.1) | <.001 |
| NSTEMI | 7881 (30.2) | 8123 (14.2) | |
| Unstable AP | 3571 (13.6) | 10 242 (17.8) | |
| Stable AP | 3897 (14.9) | 20 652 (35.9) | |
| Chronic heart failure | 495 (1.9) | 9285 (16.1) | |
| Only cardiothoracic surgery | 2158 (8.2) | 5118 (8.9) | |
| Cardiothoracic surgery | |||
| CABG | 4078 (15.6) | 1511 (2.6) | <.001 |
| Valve replacement | 1327 (5.1) | 1095 (1.9) | |
| Acute PCI | 10 396 (39.7) | 5350 (9.3) | |
| Elective PCI | 5908 (22.6) | 8235 (14.3) | |
| No cardiothoracic surgery | 4462 (17.0) | 41 325 (71.9) | |
| CCI score | |||
| ≤1 | 7210 (27.5) | 10 078 (17.5) | <.001 |
| 2 | 6720 (25.7) | 10 195 (17.7) | |
| 3 | 4976 (19.0) | 10 580 (18.4) | |
| 4 | 2516 (9.6) | 9315 (16.2) | |
| ≥5 | 4749 (18.1) | 17 348 (30.2) | |
| Cardiac medication use in the year before CVD diagnosis | |||
| ACE inhibitors | 5553 (21.1) | 17 577 (30.6) | <.001 |
| Angiotensin II receptor blockers | 4648 (17.8) | 13 303 (23.1) | <.001 |
| Antithrombotic drugs | 11 654 (44.5) | 37 830 (65.8) | <.001 |
| β-Blockers | 9879 (37.7) | 31 610 (55.0) | <.001 |
| Calcium channel blockers | 4939 (18.9) | 14 607 (25.4) | <.001 |
| Diuretics | 11 151 (42.6) | 31 429 (54.6) | <.001 |
| Lipid-lowering drugs | 4789 (18.3) | 20 155 (35.0) | <.001 |
| Other antihypertensive agents | 313 (1.2) | 1172 (2.0) | <.001 |
| Cardiac medication in the year after CVD diagnosis | |||
| ACE inhibitors | 17 625 (67.3) | 25 772 (44.8) | <.001 |
| Angiotensin II receptor blockers | 6308 (24.1) | 14 423 (25.1) | .003 |
| Antithrombotic drugs | 26 061 (99.6) | 51 156 (88.9) | <.001 |
| β-Blockers | 23 747 (90.7) | 44 186 (76.8) | <.001 |
| Calcium channel blockers | 7559 (28.9) | 18 184 (31.6) | <.001 |
| Diuretics | 7975 (30.5) | 25 206 (43.8) | <.001 |
| Lipid-lowering drugs | 24 896 (95.1) | 42 994 (74.8) | <.001 |
| Other antihypertensive agents | 411 (1.6) | 1424 (2.5) | <.001 |
Abbreviations: ACE, angiotensin-converting enzyme; AP, angina pectoris; CABG, coronary artery bypass grafting; CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SES, socioeconomic status; STEMI, ST-segment elevation myocardial infarction.
US $40 475 ($7870).
US $39 914 ($8095).
Figure 1. Cardiac Rehabilitation (CR) Participation Rates Across Strata of Patient and Disease Characteristics.
The dashed line represents the average CR participation rate (31.3%) of the analytic cohort (N = 83 687). AP indicates angina pectoris; CABG, coronary artery bypass grafting; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, ST-segment elevation myocardial infarction.
Clinical Outcomes
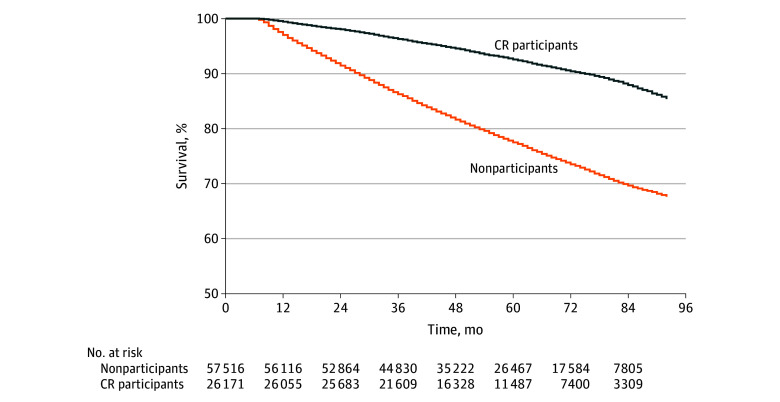
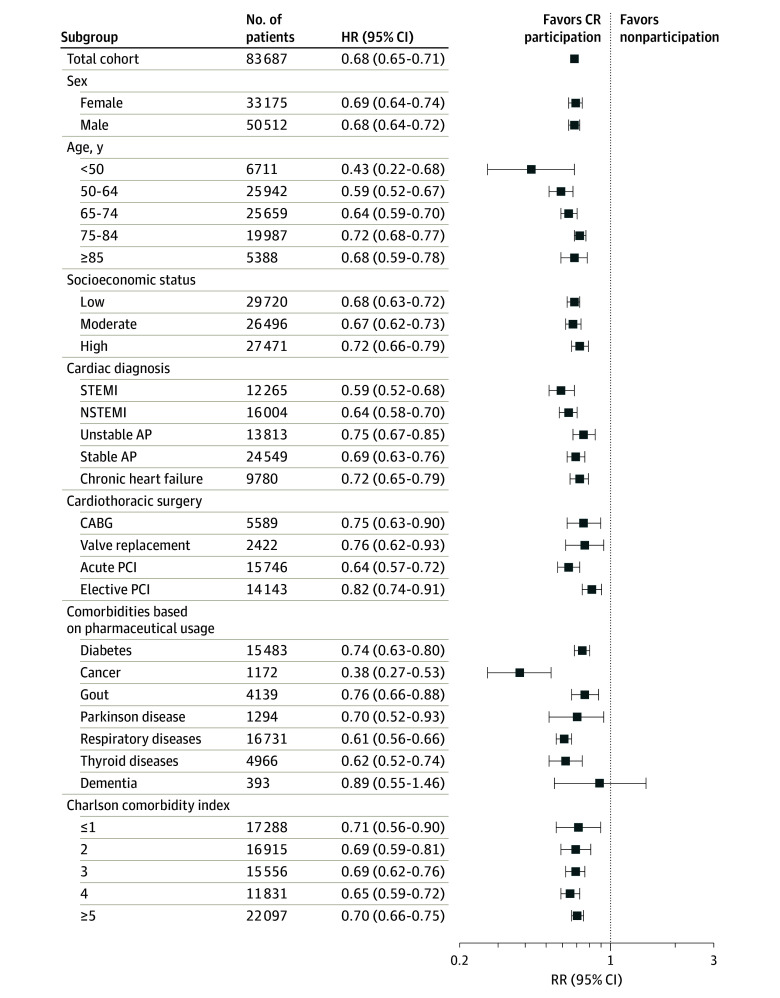
During a mean (SD) of 4.7 (1.8) years of follow-up (56 [22] months), 1966 CR participants (7.5%) and 13 443 nonparticipants (23.4%) died (P < .001) (Table 2). Kaplan-Meier analysis revealed that CR participation was associated with better event-free survival (Figure 2). Cox proportional hazards models showed a 68% lower risk of all-cause mortality (crude HR, 0.32; 95% CI, 0.30-0.33) in CR participants compared with nonparticipants (Table 2). Mortality risk remained lower in CR participants after stepwise correction for confounders (adjusted HR, 0.68; 95% CI, 0.65-0.71) (eTable 2 in the Supplement). After multivariable adjustment, CR participation was associated with a 32% lower risk of all-cause mortality (adjusted HR, 0.68; 95% CI, 0.65-0.71) compared with nonparticipation. Mortality rates during follow-up were variable across subgroups (Table 2), ranging from 1.3% to 63.9%. Cardiac rehabilitation participation was associated with improved event-free survival and a statistically significantly lower risk of all-cause mortality among all subgroups, except for patients with dementia (Figure 3). Sex, age, SES, and comorbidity did not alter risk reduction after CR participation, with no effect modification observed. However, a statistically significant interaction association was found across categories of CVD diagnosis and cardiothoracic surgery. Larger reductions in risk estimates for all-cause mortality were found after CR participation for STEMI (adjusted HR, 0.59; 95% CI, 0.52-0.68 vs 0.72; 95% CI, 0.65-0.79; P < .001), NSTEMI (adjusted HR, 0.64; 95% CI, 0.58-0.70 vs 0.72; 95% CI, 0.65-0.79; P < .001), and stable AP (adjusted HR, 0.69; 95% CI, 0.63-0.76 vs 0.72; 95% CI, 0.65-0.79; P < .001) compared with patients with chronic heart failure, whereas unstable AP had a smaller risk reduction (adjusted HR, 0.75; 95% CI, 0.67-0.85 vs 0.72; 95% CI, 0.65-0.79; P < .001). Effect modification was also present for cardiothoracic surgery, with acute PCI having a larger reduction in the risk estimate for all-cause mortality after CR participation (adjusted HR, 0.64; 95% CI, 0.57-0.72 vs 0.82; 95% CI, 0.74-0.91; P < .001) compared with elective PCI, whereas no difference in risk reduction was found for CABG (adjusted HR, 0.75; 95% CI, 0.63-0.90 vs 0.82; 95% CI, 0.74-0.91; P = .53) or valve replacement (adjusted HR, 0.76; 95% CI, 0.62-0.93 vs 0.82; 95% CI, 0.74-0.91; P = .53).
Table 2. Mortality Rates and Crude Hazard Ratios (HR) for the Total Cohort and Subgroups.
| Variable | Total cohort, No. | CR participants | Nonparticipants | Crude HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Deaths, No. (%) | Deaths per 1000 person-years | No. | Deaths, No. (%) | Deaths per 1000 person-years | |||
| Total cohort | 83 687 | 26 171 | 1966 (7.5) | 15.9 | 57 516 | 13 443 (23.4) | 50.3 | 0.32 (0.30-0.33) |
| Sex | ||||||||
| Female | 33 175 | 7381 | 541 (7.3) | 15.6 | 25 794 | 5903 (22.9) | 49.4 | 0.32 (0.29-0.34) |
| Male | 50 512 | 18 790 | 1425 (7.6) | 16.0 | 31 722 | 7540 (23.8) | 51.1 | 0.31 (0.30-0.33) |
| Age, y | ||||||||
| <50 | 6711 | 2668 | 36 (1.3) | 2.7 | 4043 | 135 (3.3) | 6.3 | 0.43 (0.30-0.63) |
| 50-64 | 25 942 | 10 243 | 357 (3.5) | 7.2 | 15 699 | 1201 (7.7) | 14.9 | 0.49 (0.43-0.55) |
| 65-74 | 25 659 | 8605 | 667 (7.8) | 16.4 | 17 054 | 2986 (17.5) | 36.5 | 0.45 (0.42-0.49) |
| 75-84 | 19 987 | 4294 | 788 (18.4) | 41.5 | 15 693 | 5907 (37.6) | 89.1 | 0.46 (0.43-0.50) |
| ≥85 | 5388 | 361 | 118 (32.7) | 84.6 | 5027 | 3214 (63.9) | 193.1 | 0.42 (0.35-0.51) |
| SES | ||||||||
| Low | 36 844 | 11 188 | 900 (8.0) | 17.0 | 25 656 | 6383 (24.9) | 54.1 | 0.31 (0.29-0.34) |
| Moderate | 25 759 | 8233 | 622 (7.6) | 16.0 | 17 526 | 4086 (23.3) | 50.2 | 0.32 (0.29-0.35) |
| High | 21 084 | 6750 | 444 (6.6) | 13.9 | 14 334 | 2974 (20.7) | 43.9 | 0.32 (0.29-0.35) |
| CVD diagnosis | ||||||||
| STEMI | 12 265 | 8169 | 488 (6.0) | 12.6 | 4096 | 917 (22.4) | 47.8 | 0.26 (0.24-0.29) |
| NSTEMI | 16 004 | 7881 | 595 (7.5) | 16.4 | 8123 | 2460 (30.3) | 70.2 | 0.23 (0.21-0.25) |
| Unstable AP | 13 813 | 3571 | 284 (8.0) | 16.1 | 10 242 | 1819 (17.8) | 35.1 | 0.46 (0.41-0.52) |
| Stable AP | 24 549 | 3897 | 265 (6.8) | 14.3 | 20 652 | 2506 (12.1) | 24.4 | 0.60 (0.53-0.68) |
| Chronic heart failure | 9780 | 495 | 97 (19.6) | 46.2 | 9285 | 4263 (45.9) | 119.7 | 0.38 (0.31-0.47) |
| Cardiothoracic surgery | ||||||||
| CABG | 5589 | 4078 | 313 (7.7) | 15.6 | 1511 | 232 (15.4) | 30.0 | 0.53 (0.45-0.63) |
| Valve replacement | 2422 | 1327 | 164 (12.4) | 25.7 | 1095 | 276 (25.2) | 54.1 | 0.48 (0.39-0.57) |
| Acute PCI | 15 746 | 10 396 | 606 (5.8) | 12.5 | 5350 | 1065 (19.9) | 42.6 | 0.29 (0.27-0.33) |
| Elective PCI | 14 143 | 5908 | 465 (7.9) | 16.8 | 8235 | 1367 (16.6) | 34.7 | 0.49 (0.44-0.54) |
| Comorbidity based on pharmaceutical agent use | ||||||||
| Diabetes | 15 483 | 3788 | 511 (13.5) | 29.5 | 11 695 | 3970 (33.9) | 78.7 | 0.37 (0.34-0.41) |
| Cancer | 1172 | 311 | 39 (12.5) | 28.3 | 861 | 290 (33.7) | 81.7 | 0.34 (0.25-0.48) |
| Gout | 4139 | 901 | 142 (15.8) | 34.9 | 3238 | 1326 (41.0) | 100.9 | 0.34 (0.29-0.41) |
| Parkinson disease | 1294 | 240 | 33 (13.8) | 29.2 | 1054 | 396 (37.6) | 87.9 | 0.33 (0.23-0.47) |
| Respiratory diseases | 16 731 | 3807 | 431 (11.3) | 24.5 | 12 924 | 4430 (34.3) | 79.9 | 0.30 (0.28-0.34) |
| Thyroid diseases | 4966 | 1131 | 109 (9.6) | 20.6 | 3835 | 1055 (27.5) | 61.4 | 0.33 (0.27-0.41) |
| Dementia | 393 | 50 | 10 (20.0) | 46.4 | 343 | 204 (59.5) | 153.2 | 0.30 (0.16-0.56) |
| CCI | ||||||||
| ≤1 | 17 288 | 7210 | 144 (2.0) | 4.1 | 10 078 | 293 (2.9) | 5.5 | 0.75 (0.62-0.72) |
| 2 | 16 915 | 6720 | 243 (3.6) | 7.5 | 10 195 | 688 (6.7) | 13.1 | 0.58 (0.50-0.67) |
| 3 | 15 556 | 4976 | 415 (8.3) | 17.8 | 10 580 | 1876 (17.7) | 36.1 | 0.51 (0.45-0.56) |
| 4 | 11 831 | 2516 | 363 (14.4) | 31.3 | 9315 | 3409 (36.6) | 84.8 | 0.36 (0.33-0.41) |
| ≥5 | 22 097 | 4749 | 801 (16.9) | 38.5 | 17 348 | 7177 (41.4) | 104.1 | 0.37 (0.34-0.39) |
Abbreviations: AP, angina pectoris; CABG, coronary artery bypass grafting; CCI, Charlson Comorbidity Index; CR, cardiac rehabilitation; CVD, cardiovascular disease; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SES, socioeconomic status; STEMI, ST-segment elevation myocardial infarction.
Figure 2. Kaplan-Meier Curves of All-Cause Mortality During Follow-up.
Cardiac rehabilitation (CR) participation was associated with statistically significantly better event-free survival compared with nonparticipation (log-rank test comparing curves, P < .001 for overall survival).
Figure 3. Subgroup-Specific Adjusted Hazard Ratios (HR) for All-Cause Mortality Risk Among Patients With Cardiovascular Disease Eligible to Enroll in a Cardiac Rehabilitation (CR) Program.
Data are presented on a logarithmic scale. A 32% lower risk of all-cause mortality was found for the total cohort, and adjusted HRs between 0.38 (95% CI, 0.27-0.53) and 0.89 (95% CI, 0.55-1.46) were observed across subgroups. Participation in CR is favored for all subgroups because risk estimates were all less than 1. However, a nonstatistically significant risk reduction was observed for patients with dementia likely because of the small sample size, contributing to a wide 95% CI. AP indicates angina pectoris; CABG, coronary artery bypass grafting; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, non–ST-segment elevation myocardial infarction.
To evaluate the robustness of the main outcomes, the adjusted HRs of CR participation on all-cause mortality were recalculated. For this purpose, mortality risk reduction for different durations during follow-up was calculated. These sensitivity analyses showed risk reduction for all-cause mortality similar to the initial findings (adjusted HR, 0.68-0.86) (eTable 3 in the Supplement).
Discussion
Cardiac rehabilitation programs aim to improve prognosis and quality of life via a multifaceted intervention. The present study demonstrated that CR participation was associated with a 32% lower risk of all-cause mortality compared with nonparticipation after adjustment for confounding factors. The association between CR participation and all-cause mortality was independent of sex, age, socioeconomic status, and comorbidity, but the magnitude of risk reduction after CR differed across categories of CVD diagnosis and type of cardiothoracic surgery. On average, the CR participation rate was 31.3%, but it increased from 25.7% in 2012 to 38.6% in 2017. Large variation in CR use was observed (range, 5.1%-73.0%) across different subgroups. Findings from this Dutch nationwide observational cohort study underscore the health benefit of CR and emphasize the need to better use CR, such as improving referral and encouragement by clinicians, particularly in vulnerable patients with CVD (eg, older individuals or those with comorbidity or multimorbidity).
All-Cause Mortality
The crude risk reduction in all-cause mortality after CR participation was 68%, which was reduced to 32% after adjustment for confounding factors. The quantification of mortality risk reduction in the present study is substantially larger compared with outcomes of previously published meta-analyses (−1% to 13%).2,5,27,28 A potential explanation for the discrepant outcomes may relate to the study design. We used an observational population-based cohort to study the health benefit of CR in a real-world setting, yielding high external validity. This design is in contrast to meta-analyses, which use data from randomized clinical trials (RCTs). Although RCTs may reduce the risk of confounding because of randomization, vulnerable patients (eg, older individuals or those with comorbidity or multimorbidity) are typically excluded. Hence, RCTs have high internal validity but typically have lower external validity.29 The health benefit of CR may be larger in patients who are underrepresented in or excluded from RCTs. Alternatively, the content of the CR program may alter the outcomes.17 Our patients were enrolled in a multidisciplinary program, including supervised exercise training, mental health and stress relief, social health, and cardiovascular risk management,16 whereas most RCTs compared exercise-based CR with a control condition.5 Hence, mortality risk reduction may have been larger in our cohort because of the multifaceted treatment that patients received.30 Indeed, our findings align with other large observational population studies20,31,32 reporting a 33% to 35% risk reduction in CR participants. Independent of the risk reduction of the present study vs data from RCTs, these joint outcomes further reinforce the Class IA recommendation for CR participation in current and future clinical guidelines of professional associations.7,8,9,10
Risk Reduction Across Subgroups
A unique aspect of the present study is the assessment of mortality risk across different subgroups of patients eligible for CR. Better survival was found for all subgroups participating in CR. The magnitude of risk reduction after CR differed statistically significantly across categories of CVD diagnosis and type of cardiothoracic surgery, but risk estimates and their 95% CIs were less than 1 for every subgroup (Figure 3), indicating that CR participation provides a better outcome than nonparticipation. This finding is notable because the level of evidence of CR health benefits is weak for some subgroups. Few studies on CR report sex-specific outcomes,33 but risk reduction after CR differs between men and women.20 Although CR use is lower in women than in men, we observed a similar mortality risk reduction with CR participation in men (32.1%) and women (31.3%) (Figure 3), suggesting that both sexes benefit equally from CR.
Older patients are often underrepresented in CR programs, despite their higher disease prevalence.34 Hence, recommendations for treatment of older patients (≥75 years) are needed that are typical of those individuals encountered in routine clinical practice.35 Age did not alter the association between CR participation and better mortality outcomes. In fact, CR was associated with large reductions in all-cause mortality in patients aged 75 to 84 years (27.8%) and 85 years or older (32.0%) (Figure 3). These findings suggest that CR improves survival even in older age groups, which have mortality rates of up to 63.9%.
The implications of CR for mortality in patients with stable AP remain unclear. In a recent systematic review and meta-analysis28 (including 7 studies and 581 patients), risk estimates could not be calculated because of insufficient power. Among 24 549 patients with stable AP in our study, CR participation was associated with a 31% mortality risk reduction (adjusted HR, 0.69; 95% CI, 0.63-0.77) (Figure 3). Similarly, among 13 813 patients with unstable AP, CR participation was associated with a 25% mortality risk reduction (adjusted HR, 0.75; 95% CI, 0.67-0.85). Our observations underscore the mortality benefit of CR for patients with stable AP and patients with unstable AP.
Multiple comorbid conditions are increasingly prevalent among patients with CVD.36 Patients with multimorbidity have a worse prognosis37 and a lower CR participation rate,38 and current clinical practice is mainly targeted toward care of patients with a single disease. We found that CR participants with diabetes, cancer, gout, Parkinson disease, respiratory diseases, and thyroid diseases had better survival rates compared with nonparticipants. Patients with CVD with dementia did not demonstrate a statistically significant risk reduction after CR, but the risk estimate indicated a 10.8% reduction in all-cause mortality (adjusted HR, 0.89; 95% CI, 0.55-1.46) (Figure 3). The lack of statistical significance is likely because of the small sample size (n = 393), which contributed to a wide 95% CI (−45% to 46%). Cardiac rehabilitation participation among patients with CVD with the highest CCI scores was associated with notable risk reduction (≥30%). These findings emphasize that CR has substantial health value for patients with CVD with comorbidity and should lead clinicians to encourage their patients to participate in CR.
CR Use
Findings from the present study not only reinforce the health benefit of CR but also demonstrate the underuse of this powerful intervention. Only 31.3% of eligible Dutch patients participated in CR, which is high compared with US data11,12 but is similar to values reported in European studies.39,40 However, more notable is the increase in CR use from 25.7% in 2012 to 38.6% in 2017. Future studies are warranted to elucidate the reasons for this substantial improvement as well as to understand temporal changes in predictors of participation. Outcomes from such analyses can be used to develop strategies to further increase CR use across the globe.
Large variability in CR participation rates across different subgroups was also observed. Participation rates were high for medical conditions with clear guidelines and strong recommendations, such as STEMI9 (66.6%), CABG7 (73.0%), valve replacement7 (54.8%), and acute (66.0%) or elective (41.8%) PCI.41 Participation rates were low in subgroups with chronic conditions, such as older patients and those with stable AP, chronic heart failure, comorbidities (ie, diabetes, cancer, gout, etc), and multimorbidity (CCI≥4) (Figure 1). The lower participation rates likely relate to the frailty of these patients, a lack of physician referral, or to practical barriers, such as a lack of transportation.42 Alternative strategies to deliver CR, such as home-based CR,43,44 telerehabilitation,45 or eHealth interventions,46 may enable patients with CVD to profit from CR.
Limitations
This study has some limitations. The main limitation is its observational design. Analyses based on CR components were not possible to perform because of a change in the medical claims registry system during the study period and an insufficient level of detail regarding the number of sessions and the combination of components. The use of health insurance claims data did not allow us to control for all potential confounding factors, such as cardiovascular risk factors, disease severity, lifestyle (eg, physical activity, smoking, and diet), and patient motivation. Nevertheless, we were able to extract data regarding patient characteristics, CVD diagnosis, type of cardiothoracic surgery, hospital stay, pharmaceutical agent use, comorbidity, and health care costs to correct for differences between CR participants and nonparticipants. Stabilized inverse propensity score weighting was used to create a pseudopopulation and subsequently adjust for the covariates in the Cox proportional hazards models to minimize potential group differences. Matched-pairs analysis (n = 32 780) confirmed the findings of our primary analysis (data not shown). Race was not available, but most of the Dutch population is of white race/ethnicity (approximately 87.7%).47 Residual confounding may have occurred because unmeasured variables could explain some of the observed mortality differences between CR participants and nonparticipants. However, results from the present study demonstrated a consistent mortality risk reduction among all subgroups of CR participants, and the health benefit of CR was further reinforced in the sensitivity analyses. Finally, the inclusion of a large and heterogeneous patient population in combination with the high accuracy of health insurance data48 supports the value of the present study beyond previously published RCTs and meta-analyses.
Conclusions
After multivariable adjustment, participation in a multidisciplinary CR program was associated with 32% lower risk of all-cause mortality (adjusted HR, 0.68; 95% CI, 0.65-0.71) compared with nonparticipation during a mean (SD) of 4.7 (1.8) years of follow-up. The survival benefit associated with CR participation was observed in virtually all subgroups of patients with CVD (except for patients with dementia), including risk estimates specific to sex, age, SES, CVD diagnosis, cardiothoracic surgery, and comorbidity. Findings from the present study further reinforce current recommendations of the American Heart Association, American College of Cardiology, and European Society of Cardiology and suggest that CR should be prescribed more widely to vulnerable patients with CVD, such as older adults with chronic diseases or multimorbidity. Cardiac rehabilitation participation rates remain low in these at-risk subgroups, whereas the benefits of cardiac rehabilitation are similar to those of low-risk subgroups.
eTable 1. Yearly Inclusion Rate of Patients Eligible for CR Participation Among the VGZ Cohort
eTable 2. Crude and Multivariable Adjusted Hazard Ratios for All-Cause Mortality Among CR Participants
eTable 3. Sensitivity Analyses for Different Thresholds of Minimal Follow-up Time
eFigure 1. The VGZ Cohort Is a Valid Representation of the Dutch Population
eFigure 2. Flowchart of Patient Enrollment From the VGZ Health Insurance Database
References
- 1.Eijsvogels TM, Molossi S, Lee DC, Emery MS, Thompson PD. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67(3):316-329. doi: 10.1016/j.jacc.2015.11.034 [DOI] [PubMed] [Google Scholar]
- 2.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. doi: 10.1002/14651858.CD001800.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162(4):571-584.e2. doi: 10.1016/j.ahj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 4.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4(4):CD003331. doi: 10.1002/14651858.CD003331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1-12. doi: 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 6.Lewinter C, Doherty P, Gale CP, et al. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol. 2015;22(12):1504-1512. doi: 10.1177/2047487314559853 [DOI] [PubMed] [Google Scholar]
- 7.Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amsterdam EA, Wenger NK, Brindis RG, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;130(25):e433-e434]. Circulation. 2014;130(25):e344-e426. [DOI] [PubMed] [Google Scholar]
- 9.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425. doi: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 10.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons [published correction appears in Circulation. 2014;129(16):e462]. Circulation. 2012;126(25):3097-3137. doi: 10.1161/CIR.0b013e3182776f83 [DOI] [PubMed] [Google Scholar]
- 11.Golwala H, Pandey A, Ju C, et al. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from Get With the Guidelines–Heart Failure registry. J Am Coll Cardiol. 2015;66(8):917-926. doi: 10.1016/j.jacc.2015.06.1089 [DOI] [PubMed] [Google Scholar]
- 12.Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in Medicare and Veterans Affairs populations: opportunity for improvement. Circulation. 2018;137(18):1899-1908. doi: 10.1161/CIRCULATIONAHA.117.029471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med. 2015;175(10):1700-1702. doi: 10.1001/jamainternmed.2015.3819 [DOI] [PubMed] [Google Scholar]
- 14.Sukul D, Seth M, Barnes GD, et al. Cardiac rehabilitation use after percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(24):3148-3152. doi: 10.1016/j.jacc.2019.03.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Netherlands . Population counter. Published May 25, 2020. Accessed June 16, 2020. https://www.cbs.nl/en-gb/visualisaties/population-counter
- 16.Revalidatiecommissie Nederlandse Vereniging Voor Cardiologie/Nederlandse Hartstichting (Richtlijn Hartrevalidatie 2004), Projectgroep PAAHR (gedeeltelijke herziening 2011). Multidisciplinary Cardiac Rehabilitation Guideline [in Dutch]. Nederlandse Vereniging Voor Cardiologie; 2011. [Google Scholar]
- 17.Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol. 2015;65(4):389-395. doi: 10.1016/j.jacc.2014.10.059 [DOI] [PubMed] [Google Scholar]
- 18.Balady GJ, Williams MA, Ades PA, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation . Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(20):2675-2682. doi: 10.1161/CIRCULATIONAHA.106.180945 [DOI] [PubMed] [Google Scholar]
- 19.Goud R, de Keizer NF, ter Riet G, et al. Effect of guideline based computerised decision support on decision making of multidisciplinary teams: cluster randomised trial in cardiac rehabilitation. BMJ. 2009;338:b1440. doi: 10.1136/bmj.b1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries H, Kemps HM, van Engen-Verheul MM, Kraaijenhagen RA, Peek N. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J. 2015;36(24):1519-1528. doi: 10.1093/eurheartj/ehv111 [DOI] [PubMed] [Google Scholar]
- 21.Martin BJ, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126(6):677-687. doi: 10.1161/CIRCULATIONAHA.111.066738 [DOI] [PubMed] [Google Scholar]
- 22.Huber CA, Szucs TD, Rapold R, Reich O. Identifying patients with chronic conditions using pharmacy data in Switzerland: an updated mapping approach to the classification of medications. BMC Public Health. 2013;13:1030. doi: 10.1186/1471-2458-13-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 24.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277. doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoli GN, Sanders RD, Myles P. Demystifying propensity scores. Br J Anaesth. 2014;112(1):13-15. doi: 10.1093/bja/aet290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698-709. doi: 10.1002/pds.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. doi: 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long L, Anderson L, Dewhirst AM, et al. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev. 2018;2:CD012786. doi: 10.1002/14651858.CD012786.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slack MK, Draugalis JR. Establishing the internal and external validity of experimental studies. Am J Health Syst Pharm. 2001;58(22):2173-2181. doi: 10.1093/ajhp/58.22.2173 [DOI] [PubMed] [Google Scholar]
- 30.Müller-Riemenschneider F, Meinhard C, Damm K, et al. Effectiveness of nonpharmacological secondary prevention of coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2010;17(6):688-700. doi: 10.1097/HJR.0b013e32833a1c95 [DOI] [PubMed] [Google Scholar]
- 31.Patel DK, Duncan MS, Shah AS, et al. Association of cardiac rehabilitation with decreased hospitalization and mortality risk after cardiac valve surgery. JAMA Cardiol. 2019;4(12):1250-1259. doi: 10.1001/jamacardio.2019.4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beatty AL, Doll JA, Schopfer DW, et al. Cardiac rehabilitation participation and mortality after percutaneous coronary intervention: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. J Am Heart Assoc. 2018;7(19):e010010. doi: 10.1161/JAHA.118.010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghisi GLM, Chaves GSDS, Bennett A, Lavie CJ, Grace SL. The effects of cardiac rehabilitation on mortality and morbidity in women: a meta-analysis attempt. J Cardiopulm Rehabil Prev. 2019;39(1):39-42. doi: 10.1097/HCR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 34.Deaton C. Addressing the paradox of age and participation in cardiac rehabilitation. Eur J Prev Cardiol. 2019;26(10):1050-1051. doi: 10.1177/2047487319839258 [DOI] [PubMed] [Google Scholar]
- 35.Rich MW, Chyun DA, Skolnick AH, et al. ; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society . Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133(21):2103-2122. doi: 10.1161/CIR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 36.Forman DE, Maurer MS, Boyd C, et al. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149-2161. doi: 10.1016/j.jacc.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canivell S, Muller O, Gencer B, et al. Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PLoS One. 2018;13(4):e0195174. doi: 10.1371/journal.pone.0195174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown TM, Hernandez AF, Bittner V, et al. ; American Heart Association Get With the Guidelines Investigators . Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association’s Get With the Guidelines program. J Am Coll Cardiol. 2009;54(6):515-521. doi: 10.1016/j.jacc.2009.02.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotseva K, Wood D, De Bacquer D; EUROASPIRE Investigators . Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol. 2018;25(12):1242-1251. doi: 10.1177/2047487318781359 [DOI] [PubMed] [Google Scholar]
- 40.Kotseva K, Wood D, De Backer G, De Bacquer D; EUROASPIRE III Study Group . Use and effects of cardiac rehabilitation in patients with coronary heart disease: results from the EUROASPIRE III survey. Eur J Prev Cardiol. 2013;20(5):817-826. doi: 10.1177/2047487312449591 [DOI] [PubMed] [Google Scholar]
- 41.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization [published correction appears in Eur Heart J. 2019;40(37):3096]. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 42.Schopfer DW, Forman DE. Cardiac rehabilitation in older adults. Can J Cardiol. 2016;32(9):1088-1096. doi: 10.1016/j.cjca.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:CD007130. doi: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140(1):e69-e89. doi: 10.1161/CIR.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 45.Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015;21(1):45-53. doi: 10.1177/1357633X14562732 [DOI] [PubMed] [Google Scholar]
- 46.Saner H, van der Velde E. eHealth in cardiovascular medicine: a clinical update. Eur J Prev Cardiol. 2016;23(2)(suppl):5-12. doi: 10.1177/2047487316670256 [DOI] [PubMed] [Google Scholar]
- 47.Statistics Netherlands . Ethnic groups in the Netherlands. Bevolking naar migratieachtergrond. Published November 21, 2016. Accessed June 16, 2020. https://www.cbs.nl/nl-nl/achtergrond/2016/47/bevolking-naar-migratieachtergrond
- 48.Smeets HM, de Wit NJ, Hoes AW. Routine health insurance data for scientific research: potential and limitations of the Agis Health Database. J Clin Epidemiol. 2011;64(4):424-430. doi: 10.1016/j.jclinepi.2010.04.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Yearly Inclusion Rate of Patients Eligible for CR Participation Among the VGZ Cohort
eTable 2. Crude and Multivariable Adjusted Hazard Ratios for All-Cause Mortality Among CR Participants
eTable 3. Sensitivity Analyses for Different Thresholds of Minimal Follow-up Time
eFigure 1. The VGZ Cohort Is a Valid Representation of the Dutch Population
eFigure 2. Flowchart of Patient Enrollment From the VGZ Health Insurance Database