Abstract
Coronary artery disease (CAD) is a leading cause of morbidity and mortality globally, necessitating innovative therapeutic strategies. Panax ginseng Meyer, esteemed in traditional Chinese medicine as the “King of Herbs,” has garnered scientific attention for its cardiovascular benefits. Among its bioactive components, ginsenoside Rg1 stands out as a potent therapeutic candidate. This review consolidates current research on Rg1's multifaceted mechanisms in CAD management, including its roles in enhancing endothelial function, inhibiting smooth muscle cell proliferation, modulating inflammation, regulating lipid metabolism, preventing thrombosis, and promoting angiogenesis. Furthermore, Rg1 exhibits cardioprotective properties by mitigating ischemic myocardial injury, reducing myocardial hypertrophy, and preventing fibrosis. Despite promising preclinical findings, the clinical translation of Rg1 is hindered by challenges such as poor bioavailability, potential drug interactions, and limited clinical trial data. This review underscores the need for rigorous clinical studies to validate Rg1's efficacy and safety, paving the way for its incorporation into CAD therapeutic regimens.
Keywords: Cardioprotection, Coronary artery disease, Ginsenoside Rg1
Graphical abstract
1. Introduction
Coronary artery disease (CAD) remains the leading cause of mortality worldwide, with an alarming incidence of nearly 7 million fatalities and 129 million disabilities annually, predominantly in low- and middle-income countries [1]. Despite significant advancements in secondary prevention and revascularization techniques like coronary artery bypass grafting and percutaneous coronary interventions, the long-term incidence of adverse cardiovascular events remains high [2]. This underscores the urgent need for novel cardioprotective agents.
Panax ginseng Meyer (P. ginseng), a cornerstone of traditional Chinese medicine (TCM), is derived from perennial plants of the Panax genus within the Araliaceae family. Documented in the ancient medical text “Shennong Bencao Jing” for its restorative properties, P. ginseng is utilized based on the principle that “qi and blood are interconnected; qi governs blood movement while blood nurtures qi.” Modern pharmacological research has identified various bioactive constituents in P. ginseng, including polysaccharides, ginsenosides, peptides, and fatty acids [3]. Among these, ginsenosides receive particular attention due to their therapeutic potential.
Ginsenoside Rg1, a prominent tetracyclic triterpenoid of the 20(S)-protopanaxatriol type [4], is recognized for its abundance and bioactivity in P. ginseng, contributing significantly to its pharmacological effects [5]. Accumulating evidence [[6], [7], [8], [9], [10]] indicates that Rg1 exerts protective effects against CAD through anti-atherosclerotic properties and cardiomyocyte protection against ischemic injury. This review explores the mechanisms and therapeutic potential of Rg1 in CAD, providing a basis for developing Rg1-based interventions to enhance CAD management and patient outcomes.
2. Mechanism of cardioprotective role in anti-atherosclerosis
Arteriosclerosis is the main pathological process underlying CAD, involving endothelial dysfunction, smooth muscle cell (SMC) proliferation and migration, monocyte recruitment, lipid infiltration, inflammation, and foam cell formation [11]. These processes culminate in plaque formation and lumen narrowing, impairing blood supply. Plaque rupture triggers platelet aggregation and thrombus formation, further exacerbating cardiac function.
Recent advancements in atherosclerosis treatment include medications like evolocumab [12], which reduces lipid accumulation, colchicine, targeting inflammation [13], and ticagrelor, inhibiting platelet aggregation [14]. Ginsenoside Rg1 has shown potential in addressing various aspects of atherosclerosis formation, including endothelial cell damage, SMC proliferation, monocyte recruitment, lipid deposition, inflammation, and thrombus formation, highlighting its prospect for clinical application. Here, we provide a comprehensive review of the mechanisms by which Rg1 exerts its anti-atherosclerotic effects (Table 1; Fig. 1).
Table 1.
Summarized the effects and mechanisms of ginsenoside Rg1 on anti-atherosclerosis.
| Primary Action | Experimental models | Specific Effects | Mechanisms | Ref |
|---|---|---|---|---|
| Enhance endothelial function | STZ-induced diabetic rats; HG-induced HAECs |
Improve endothelial function, preserve mitochondrial structure and function, reduced oxidative stress | Inhibit calpain-1/ROS/PKC-β axis | [20] |
| CIH-induced HCAECs | Suppress mitoROS, preserve mitochondrial function, increase the release of NO | Inhibit calpain-1/PP2A/eNOS axis | [6] | |
| PM2.5-induced HUVECs | Decrease ROS and MDA content | Upregulate the expression of HO-1 and Nrf2 | [21] | |
| Ox-LDL-induced HUVECs | Increase SOD activity, reduce the production of reactive species | Stimulate AMPK phosphorylation, increase SIRT3 expression, reduce p53 level. | [8] | |
| Serum-depleted medium-cultured PAECs | Boost eNOS phosphorylation, stimulate L-arginine transport into ECs | Activate PI3K/Akt pathway, increase CAT-1 expression | [24] | |
| Shear stress-induced HUVECs | Decrease MCP-1 expression | Inhibit MAPK pathway include ERK, p38, JNK phosphorylation | [26] | |
| Inhibit SMC proliferation | Carotid balloon injury-induced rats | Inhibit VSMCs proliferation | Inhibit ERK2 pathway, elavate MKP-1 expression, reduce c-fos and SMα-actin mRNA expression | [7] |
| TNF-α-induced HASMCs | Inhibit HASMCs proliferation | Inhibit ERK and PI3K/PKB, downregulate cyclin D1, upregulate p53, p21 and p27 levels | [28] | |
| TNF-α-induced VSMC | Prevent VSMCs proliferation cycle progression from G1 to S phase | Decrease PKC-ζ, GRK and N-ras protein, increase p21 expression | [30] | |
| Anti-inflammation | LPS-induced macrophages; mouse peritoneal macrophages | Decrease TNF-α and IL-6 mRNA levels, increase TNF-α protein expression | Reduce the phosphorylation of IκB and NF-κB, upregulate the PI3K/Akt/mTORC1 signaling pathway | [34] |
| Hypoxia-induced PAH rats; hypoxia-induced HPAECs | Reduce TNF-α and IL-1β levels | Inhibit the activation of the NF-κB/TGF-β pathway through upregulating CCN1 | [35] | |
| STZ-induced diabetic rats | Decrease the expression levels of IL-1β, IL-6,TNF-α, NF-κB, NLRP3, TLR4, and ASC | Stimulate AMPK phosphorylation, increase Nrf2 and HO-1expression | [36] | |
| Regulate lipid metabolism | High-sugar and high-fat diets-raised rats | Reduce the levels of TC and TG, inhibit the secretion of IL-1, IL-6, IL-8, IL-18, and TNF-α | Promote the expression of PPAR-α, CPT1A, CPT2, and CYP-7A, inhibit the expression of SREBP-1C | [39] |
| High-fat diet-induced NAFLD rats; | Reduce TC, TG, LDL-C, free fatty acids, and increase HDL-C | Promote fatty acid oxidation via PPARα activation | [40,41] | |
| Male db/db mice | ||||
| 3T3-L1 cells; | Reduce TC and TG levels, decrease lipid droplets accumulation in 3T3-L1 cells | Activate AMPK pathway, reduce the expression of PPARγ, CCAAT/C/EBP and SREBP, suppress ACC activity | [42] | |
| High fat diet-raised obese mice | ||||
| Anti-thrombotic effects | Human blood from healthy subjects; | Inhibit platelet aggregation and spreading, decrease platelet adhesion on collagen-coated surface in human blood, reduce platelet adhesion and thrombus formation | Inhibit PKC-MAPK pathway | [47] |
| 10 % FeCl3- induced mice mesenteric arterial thrombosis | ||||
| Promote angiogenesis | Diabetes and myocardial infarction rat | Increase arteriolar density | Inhibit the expression of PKCδ, stimulate the activation of the VEGF/PI3K-Akt/eNOS pathway | [55] |
| HUVECs | Increase tube-like structure number | Upregulate GR and enhance cooperation between GR and FGFR-1, thereby activating the PI3K/Akt pathway, consequently enhance eNOS, GSK3β and HIF-α expression | [[56], [57], [58]] | |
| HUVECs | Promote eNOS expression, enhance VEGF-A production, enhance angiogenic capacity | Downregulate miR-23a and miR-214, upregulate MET protein | [[59], [60], [61]] |
Fig. 1.
Mechanisms of the anti-atherosclerotic effects of ginsenoside Rg1.
Ginsenoside Rg1 (Rg1) exerts multiple protective effects against atherosclerosis by targeting key cellular components involved in the pathogenesis of the disease. A. Endothelial cells (ECs): Rg1 enhances endothelial function by reducing oxidative stress, promoting the dissociation of the Keap1/Nrf2 complex, and facilitating nuclear translocation of Nrf2. This activation leads to the upregulation of antioxidant enzymes such as SOD and GSH, and increased NO production while reducing ROS and MDA levels. Rg1 also stimulates NO release by increasing ETB receptor expression, facilitating L-arginine transport, and enhancing eNOS phosphorylation. Additionally, Rg1 suppresses the expression of adhesion molecules such as VCAM-1 and ICAM-1, thereby reducing monocyte adhesion and clustering. B. Smooth muscle cells (SMCs): Rg1 inhibits SMC proliferation by modulating the expression of genes through the ERK, PI3K, and PKC pathways, thereby exerting an inhibitory effect on cell proliferation and preventing neointimal hyperplasia.
Abbreviations: ETB, endothelin receptor type B; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; Keap1, kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; AMPK, AMP-activated protein kinase; SIRT3, sirtuin-3; PKC, protein kinase C; PP2A, protein phosphatase 2A; CAT-1, cationic amino acid transporter 1; PI3K, phosphoinositide 3-kinase; Akt/PKB, protein kinase B; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; NO, nitric oxide; eNOS-P, endothelial nitric oxide synthase phosphorylation; mitoROS, mitochondrial reactive oxygen species; ERK, extracellular signal-regulated kinase; MKP-1, mitogen-activated protein kinase phosphatase-1; c-fos, cellular oncogene fos; SM-α actin, smooth muscle alpha-actin.
2.1. Enhancing endothelial function
The vascular endothelium serves as the initial barrier for blood components and plays a fundamental role in regulating vascular homeostasis, tone, and integrity [15]. Endothelial injury is a primary step in atherosclerotic plaque formation [16]. Rg1 resolves endothelial dysfunction by promoting vasodilation, reducing oxidative stress, and inhibiting leukocyte adhesion.
2.1.1. Improving oxidative stress
Oxidative stress is a critical factor in endothelial dysfunction, resulting from an imbalance between reactive oxygen species (ROS) production and the body's antioxidant defenses [17]. Rg1 has been extensively studied for its antioxidative properties and its potential to mitigate endothelial oxidative stress.
In rat models of carotid artery balloon injury, Rg1 administered intraperitoneally at doses of 4, 8, and 16 mg/kg daily for 14 days significantly increased superoxide dismutase (SOD) activity and decreased malondialdehyde (MDA) levels in the vascular intima, indicating reduced oxidative damage [18]. Similarly, In H2O2-induced human umbilical vein endothelial cells (HUVECs), Rg1 (4, 8, and 16 μmol/L for 24 h) enhanced cell viability in a dose-dependent manner [19], confirming its protective effects against oxidative stress.
Mechanistically, Rg1 activates several antioxidative pathways. By using streptozotocin (STZ)-induced diabetic mice, Lu et al. found that Rg1 at doses of 10 and 20 mg/kg for 8 weeks reduced oxidative stress, preserved mitochondrial structure and function, and improved endothelial function. Similar effects were observed in high-glucose-induced human aortic endothelial cells (HAECs), where pretreatment with Rg1 (10, 20 μM) inhibited the calpain-1/ROS/protein kinase C-beta (PKC-β) axis [20]. Furthermore, Rg1 has been demonstrated to preserve mitochondrial function and suppress mitochondrial ROS production in models of chronic intermittent hypoxia (CIH) and human coronary artery endothelial cells (HCAECs). This involves inhibiting the calpain-1 pathway, highlighting Rg1's role in maintaining mitochondrial integrity [6].
Further studies in PM2.5-stimulated HUVECs revealed that preincubation with Rg1 (2.5, 10, 40 μg/mL) increased cell viability, decreased ROS and MDA levels, and upregulated heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) expression [21]. These effects are mediated through the Nrf2-Keap1 pathway activation, indicating that Rg1 enhances the cellular antioxidant defense system. In HUVECs activated by oxidized low-density lipoprotein (ox-LDL), Rg1 treatment (1, 5, or 10 μM for 24 h) improved cell survival by reducing reactive species production and increasing SOD activity. This was achieved through the stimulation of AMP-activated protein kinase (AMPK) phosphorylation and upregulation of sirtuin 3 (SIRT3), leading to decreased tumor protein p53 expression [8].
Collectively, these findings underscore the beneficial role of Rg1 in mitigating oxidative stress through multiple signaling pathways, including calpain-1, Nrf2/HO-1, and AMPK/SIRT3/p53. These mechanisms highlight the therapeutic potential of Rg1 in managing endothelial dysfunction and oxidative stress associated with cardiovascular diseases.
2.1.2. Promoting nitric oxide (NO) release
NO is a critical signaling molecule in vascular homeostasis, involved in modulating vascular tone, inhibiting platelet aggregation, and reducing inflammation. Impaired NO synthesis contributes to endothelial dysfunction and atherosclerosis progression [22].
Rg1 has been shown to significantly increase NO production by upregulating endothelial nitric oxide synthase (eNOS) expression and activity. In rat models of carotid artery balloon injury, Rg1 treatment increased eNOS mRNA expression and NO levels, demonstrating its ability to enhance endothelial function under oxidative stress conditions [18]. Similarly, in H2O2-induced HUVECs, Rg1 enhanced NO production, underscoring its protective effects against oxidative damage [19]. Further studies revealed that pretreatment with Rg1 in tumor necrosis factor (TNF)-induced HUVECs leads to an upregulation of eNOS mRNA and NO levels via increased endothelin type-B (ETB) receptor expression [23]. This suggests that Rg1 enhances NO synthesis via receptor-mediated pathways.
Additionally, in spontaneously hypertensive rats, Rg1 promoted eNOS phosphorylation and increased NO content, improving endothelium-dependent vasodilation. The mechanistic insights were further elucidated using porcine aortic endothelial cells (PAECs). Rg1 was found to boost eNOS phosphorylation by activating the phosphoinositide 3-kinase/protein kinase B (PI3K/PKB) pathway and stimulating L-arginine transport into endothelial cells via increased cationic amino acid transporter-1 (CAT-1) expression [24]. This indicates that Rg1 facilitates NO production through multiple signaling pathways. Moreover, Zhao et al. [6] observed elevated levels of eNOS protein, phosphorylated-eNOS, and NO in both in vivo and in vitro endothelial injury models treated with Rg1. This effect was achieved by inhibiting the calpain-1/protein phosphatase 2A (PP2A) pathway, resulting in increased eNOS phosphorylation at Ser1177. In CIH-induced diabetic rats and high glucose-stimulated HAECs, Rg1 increased eNOS protein and NO levels by inhibiting the calpain-1/ROS/PKC-β axis [20].
In summary, Rg1 significantly enhances NO production by upregulating ETB and CAT-1 expression and modulating key signaling pathways, including PI3K/Akt, calpain-1/PP2A/eNOS, and calpain-1/ROS/PKC-β. These mechanisms highlight Rg1's potential in improving endothelial function and preventing atherosclerosis progression through enhanced NO release.
2.1.3. Inhibiting monocyte aggregation
Monocytes play a critical role in atherosclerosis development by adhering to the endothelium, migrating into the intimal layer, and differentiating into macrophages, which contribute to atherosclerotic plaque formation. This process involves multiple steps, including capture, slow rolling, adhesion strengthening, spreading, intravascular crawling, and transmigration, all mediated by chemokines, selectins, integrins, and adhesion molecules. Once within the intima, monocytes differentiate and exacerbate plaque formation, creating a necrotic core that further destabilizes the plaque [25].
Vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) are crucial adhesion molecules expressed on the surface of endothelial cells and leukocytes, facilitating monocyte adhesion to the vascular wall. A study by Lv et al. [23] demonstrated that Rg1 pretreatment significantly reduces the expression of VCAM-1, ICAM-1, and p-selectin in tumor necrosis factor-alpha (TNF-α)-induced HUVECs. Furthermore, in shear stress-induced HUVECs, Rg1 pretreatment led to the inhibition of monocyte chemoattractant protein-1 (MCP-1) gene expression and protein secretion, subsequently reducing monocyte adhesion. This inhibitory effect is attributed to the suppression of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase phosphorylation within the MAPK signaling pathway [26].
These findings suggest that Rg1 effectively attenuates monocyte aggregation by downregulating key adhesion molecules and chemokines, mitigating the inflammatory cascade associated with atherosclerotic progression. This highlights the therapeutic potential of Rg1 in preventing the early stages of atherosclerosis through targeted inhibition of monocyte-endothelial interactions.
2.2. Inhibiting smooth muscle cell proliferation
Vascular smooth muscle cells (VSMCs) are pivotal in atherosclerosis progression, particularly through their shift from a contractile to synthetic phenotype when exposed to stimuli like hypoxia and inflammation [27]. This change in phenotype results in increased proliferation, migration, and secretion of extracellular matrix components, ultimately contributing to neointimal hyperplasia and plaque formation.
Rg1 has demonstrated significant potential in inhibiting VSMC proliferation. In carotid artery balloon injury rat models, administering Rg1 (4, 8, and 16 mg/kg/day) for 2 weeks effectively reduced neointimal hyperplasia. This effect was mediated by the inhibition of ERK2 signaling, increased expression of MAPK phosphatase-1 (MKP-1), and decreased expression of cellular oncogene fos and smooth muscle α-actin mRNA [7]. Further studies have explored the effects of Rg1 on human arterial vascular smooth muscle cells (HASMCs) induced by TNF-α. Incubation with various concentrations of Rg1 resulted in a dose-dependent inhibition of HASMC proliferation. This was achieved through the downregulation of cyclin D1 levels and the upregulation of p53, p21, and p27 levels, indicating the involvement of ERK and PI3K/PKB pathways [28].
Rg1 also inhibits VSMC proliferation induced by platelet-derived growth factor-BB (PDGF-BB) by restricting cell cycle progression from the G0/G1 to S phase. This mechanism is linked to eNOS mRNA expression promotion, resulting in increased NO and cyclic guanosine monophosphate (cGMP) production [29]. Additionally, Rg1 treatment (10–160 mg/L) prevented VSMCs from progressing from the G1 to S phase and decreased the expression of G-protein coupled receptor kinase (GRK), protein kinase C-ζ (PKC-ζ), and N-ras protein. Concurrently, it increased the levels of cell cycle-related protein p21 in TNF-α-induced VSMCs, suggesting that Rg1 inhibits VSMC proliferation via PKC-ζ and p21 pathway regulation [30].
These findings collectively indicate that Rg1 inhibits VSMC proliferation primarily by interfering with cell cycle progression, enhancing NO production, and modulating key signaling pathways such as ERK, PI3K/PKB, and PKC-ζ. These mechanisms underscore the potential of Rg1 as a therapeutic agent for atherosclerosis by targeting smooth muscle cell proliferation and stabilizing vascular plaques.
2.3. Anti-inflammation
Inflammation is a key component in atherosclerosis progression and a significant therapeutic target in CAD management. Rg1 has demonstrated significant anti-inflammatory effects in various models, indicating its potential as a therapeutic agent for CAD [31].
In vivo, studies in naturally aging rats showed that Rg1 at a dose of 0.1 mg/kg/day alleviated age-related inflammation by decreasing TNF-α and increasing interleukin-10 (IL-10) levels [32]. Similarly, in vitro studies reported that Rg1 at concentrations of 12.5, 20, and 50 μM for 6 h significantly reduced TNF-α secretion in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [33].
However, another study using a similar LPS-stimulation model in RAW 264.7 macrophages and mouse peritoneal macrophages showed that Rg1 treatment (5 and 10 μM for 1 h) significantly decreased TNF-α and interleukin-6 (IL-6) mRNA levels by reducing inhibitor of κB (IκB) and nuclear factor-kappa B (NF-κB) phosphorylation, while paradoxically increasing TNF-α protein expression through PI3K/Akt/mTORC1 signaling pathway upregulation [34]. This inconsistency in TNF-α protein expression is thought to be associated with the concentration of LPS used.
LYU et al. [8] demonstrated that Rg1 therapy (1, 5, or 10 μM for 24 h) reduces IL-6, interleukin-1 beta (IL-1β), and TNF-α levels in ox-LDL activated HUVECs by regulating the AMPK/SIRT3/p53 signaling pathway. Furthermore, Tang et al. [35] found that Rg1 reduces TNF-α and IL-1β expression levels in the lung tissue and serum of hypoxia-induced pulmonary arterial hypertension rats, as well as in human pulmonary artery endothelial cells exposed to hypoxia. The study demonstrated that Rg1 downregulates the expression of p-NF-κB, p65, transforming growth factor-beta 1 (TGF-β1), and p-Smad2/3 while upregulating cellular communication network factor 1 (CCN1). This suggests that the anti-inflammatory effects of Rg1 are achieved by inhibiting the NF-κB/TGF-β pathway activation through CCN1 upregulation.
In a study with STZ-induced diabetic rats, Qin et al. [36] reported that administering Rg1 (20 mg/kg/day for 8 weeks) markedly enhanced cardiac function and reduced inflammation. It also lowered the expression levels of IL-1β, IL-6, TNF-α, NF-κB, NOD-like receptor protein 3 (NLRP3) inflammasome, toll-like receptor 4 (TLR4), and apoptosis-associated speck-like protein containing a caspase recruitment domain. These effects were achieved by promoting AMPK phosphorylation and boosting Nrf2 and HO-1 expression.
Collectively, these findings demonstrate that Rg1 exerts its anti-atherosclerotic effects by decreasing the secretion of inflammatory factors, thereby alleviating inflammation. These mechanisms highlight the potential of Rg1 as a therapeutic agent in managing inflammation-related cardiovascular diseases.
2.4. Regulating lipid metabolism
Low-density lipoprotein (LDL) plays a central role in the initiation and progression of atherosclerosis by depositing in the arterial walls and becoming retained within the intimal layer. Therefore, lipid-lowering therapy remains foundational in managing CAD [37,38].
Recent studies indicate that Rg1 significantly modulates lipid metabolism. Hou et al. [39] demonstrated that administering Rg1 orally (30, 60 mg/kg/day) to rats on a high-sugar and high-fat diet for 4 or 8 weeks reduced total cholesterol (TC) and triglyceride (TG) levels. This effect is attributed to the upregulation of carnitine palmitoyl transferase 1α (CPT1A), carnitine palmitoyl transferase 2 (CPT2), and cholesterol 7α-hydroxylase (CYP-7A), alongside downregulation of sterol regulatory element-binding proteins-1C (SREBP-1C) via increased peroxisome proliferator-activated receptor-α (PPAR-α) expression.
In nonalcoholic fatty liver disease (NAFLD) mice, Rg1 (20 or 40 mg/kg/day for 1 month) decreased TG, TC, high-density lipoprotein cholesterol (HDL-C), and LDL-C levels, improving lipid peroxidation through PPAR-α upregulation [40]. In male db/db mice, Rg1 (10 mg/kg for 15 days) significantly reduced plasma TG and free fatty acids while increasing HDL-C concentrations by promoting fatty acid oxidation via PPAR-α activation [41].
Rg1 also reduced TC and TG levels in obese mice and minimized lipid droplet accumulation in 3T3-L1 cells. Furthermore, Rg1 lowered PPARγ, CCAAT/enhancer-binding protein (C/EBP), and SREBP expression; activated the AMPK pathway; and suppressed acetyl-CoA carboxylase (ACC) activity, enhancing lipolysis and reducing lipogenesis both in vivo and in vitro [42]. In high-fat diet-induced NAFLD rats, Rg1 (5, 10, 20 mg/kg/day for 8 weeks) decreased TC, TG, and LDL-C levels while increasing HDL-C in plasma. Additionally, it promoted hepatic acyl-CoA synthetase 1 (CoASH1), carnitine acyltransferase (CAT1), and acyl-CoA oxidase 1 (ACOX1) expression, indicating enhanced fatty acid oxidation [43].
2.5. Antithrombotic effects
Thrombosis is crucial in the pathogenesis of atherosclerosis-related thrombotic events involving platelet activation, coagulation factor activation, and fibrinolytic system imbalance [44]. Rg1 exhibits potential antithrombotic effects through both antiplatelet and anticoagulant mechanisms.
Rg1 inhibits platelet aggregation in blood samples from healthy individuals and ADP-induced platelet aggregation in rat blood. Zhou et al. [45] reported that both PNS and its component, Rg1 (100 μM), significantly inhibited platelet aggregation and spreading and reduced platelet adhesion on a collagen-coated surface in human blood samples. Additionally, Rg1 at concentrations of 1.5, 2.5, 3, and 4 mmol/L was found to inhibit platelet aggregation in rat blood in vitro [46]. The in vivo effect of Rg1 was further confirmed in a mouse mesenteric arterial thrombosis model, where Rg1 (10 mg/kg, bolus injection) markedly attenuated vessel injury-induced platelet adhesion and thrombus formation. These antiplatelet effects are likely achieved by interference with the platelet PKC-MAPK pathway, as evidenced by decreased ERK and PKC substrate phosphorylation [47]. Despite these promising findings, the precise antiplatelet effects of Rg1 remain controversial, the current in vitro experiments utilize Rg1 treatment concentrations that are significantly higher than the reported peak plasma concentration (Cmax ≈ 1134.4 ± 562.80 ng/mL) observed in pharmacokinetic studies [48], indicating a substantial discrepancy between experimental models and physiological conditions. The use of such supraphysiological concentrations may result in deviations from the true biological effects of Rg1, thereby weakening the relevance of experimental findings to its in vivo functionality. Therefore, further research is needed to clarify its role in antiplatelet, particularly at physiological concentrations.
Additionally, Rg1 exhibits anticoagulant effects in rat blood, prolonging prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) while reducing fibrinogen concentrations [49]. Similar effects were observed in human blood, where Rg1 extended PT, APTT, and TT [50]. Lv et al. [23] also suggested that Rg1 may exert antithrombotic effects by downregulating plasminogen activator inhibitor 1 (PAI-1), which regulates intravascular fibrinolysis and tissue proteolysis.
2.6. Promoting angiogenesis
Angiogenesis, the formation of new blood vessels from preexisting ones, is crucial in both normal physiology and pathological conditions, offering a promising therapeutic strategy for improving ischemia [51,52]. Rg1 has been shown to stimulate angiogenesis effectively both in vivo and in vitro [53].
Sengupta et al. [54] found that Rg1 promotes functional neovascularization in vivo and tube-like structure formation in vitro by upregulating the PI3K/Akt/eNOS pathway. Similarly, Pan et al. [55] showed that Panax Quinquefolium Saponins, which include Rg1, inhibit PKCδ expression and facilitate the activation of the vascular endothelial growth factor (VEGF)/PI3K-Akt/eNOS pathway. Cheung et al. [56] demonstrated that Rg1 increases tube formation and activates the PI3K/Akt/eNOS pathway in PAECs by upregulating glucocorticoid receptor (GR) and fibroblast growth factor receptor-1 (FGFR-1), enhancing synergy between these receptors.
In HUVECs, Rg1 increased β-catenin levels and enhanced nuclear accumulation, promoting VEGF expression and tube formation via the PI3K/Akt pathway and glycogen synthase kinase 3β (GSK3β) phosphorylation [57]. Additionally, Rg1 increased VEGF expression through the PI3K/Akt/p70 pathway mediated by GR, ultimately enhancing hypoxia-inducible factor-α (HIF-α) expression [58]. Moreover, microRNAs (miRNAs) have emerged as important regulators of angiogenesis. A study by Wu et al. found that Rg1 promotes new blood vessel formation by inhibiting miR-23a, increasing Runt-related transcription factor 2 (RUNX2) expression, and enhancing VEGF-A production in HUVECs [59]. Kwok et al. [60] found that Rg1 activates GR, reduces miR-23a expression, and upregulates hepatocyte growth factor receptor protein, promoting angiogenesis. Chan et al. [61] reported that Rg1 downregulates miR-214 in HUVECs, increasing eNOS expression and tube formation. Xiong et al. [62] revealed that Rg1 stimulates endothelial progenitor cells to release exosomes containing angiogenesis-related miRNAs such as miRNA-126–5p, miRNA-146a-5p, miRNA-210, and miRNA-214–5p.
In summary, Rg1 promotes angiogenesis through multiple pathways involving GR and miRNAs. It enhances cooperation between GR and FGFR-1, activates the PI3K/Akt pathway, and modulates the expression of key angiogenic factors such as eNOS, β-catenin, HIF-α, RUNX2, and VEGF.
3. Mechanism of cardioprotective action in myocardium
The progressive formation of atherosclerotic plaques within coronary arteries results in diminished blood supply, leading to insufficient oxygen and nutrient delivery to myocardial cells within the ischemic environment. Consequently, myocardial cells undergo compensatory hypertrophy to sustain normal cardiac function [63]. Prolonged ischemia, however, causes myocardial fibrosis, significantly impairing the heart's elasticity and contractility, thereby affecting its pumping function. Hence, protecting cardiomyocytes is crucial for enhancing CAD progression and prognosis [64]. Rg1 has demonstrated potential in ameliorating ischemic myocardial injury, inhibiting cardiac hypertrophy and fibrosis, thus decelerating CAD progression (Table 2).
Table 2.
Summarized the effects and mechanisms of ginsenoside Rg1 Protective effect on ischemic cardiomyocytes.
| Primary Action | Experimental models | Specific Effects | Mechanisms | Ref |
|---|---|---|---|---|
| Improve myocardial injury | ISO-induced AMI rats | Improve oxidative stress and inflammation, inhibit cardiomyocyte apoptosis | Inhibit PTEN expression thereby activating PI3K/Akt pathway | [69] |
| I/R-induced H9c2 cells | Reduce ROS and MDA production, increase antioxidant enzyme levels | Activate Nrf2 and HO-1 | [71] | |
| LPS or H/R-induced H9C2 cells | Inhibit mitochondrial Ca2+ overload, increase MMP, reduce ROS levels | Activate Akt/GSK-3β pathway | [72] | |
| I/R-induced myocardial injury rats | Inhibit cardiomyocytes apoptosis | Decrease Bax-2/Bcl-2 ratio | [76] | |
| I/R-induced myocardial injury rats | Modulate energy metabolism, improve myocardial viability | Inhibit RhoA/ROCK pathway, enhance the activity and expression of mitochondrial respiratory chain complexes | [77] | |
| Nutritional stress-induced H9c2 cell | regulate mitochondrial autophagy and inhibite apoptosis | Activate AMPK and PINK1, dissociate of Bcl-2-Beclin1 complex | [78,82] | |
| H/R-induced H9c2 cells | Inhibit endoplasmic reticulum stress, reduce cardiomyocyte damage and apoptosis | Activate the PI3K/Akt signaling pathway by phosphorylating Akt | [79] | |
| H/R-induced H9c2 cells | Increase cellular ATP content, inhibit autophagosomal formation and apoptosis | Prevent AMPK activation, promote mTOR activation | [81] | |
| Myocardial I/R-induced diabetes rats | Inhibit cardiomyocytes apoptosis | Activate HIF-1α/ERK pathway, decrease Bax-2/Bcl-2 ratio, reduce caspase-3 and caspase-9 activity | [80] | |
| Hypoxia-induced H9c2 cells | Increase cell viability, decrease cell apoptosis and autophagy | Activate PI3K/AKT/mTOR pathway, upregulate Bcl-2 and p62, downregulate caspase-9, caspase-3, LC3-II/LC3-I, and Beclin-1 | [83] | |
| I/R-induced myocardial injury rats | Decrease infarct size, reduce cardiomyocyte apoptosis, lower levels of TNF-α, IL-1β, and p65 protein. | Inhibit NF-κB pathway, Increase IκB expression | [85] | |
| LPS-induced cardiac dysfunction mice; | Suppress inflammation, restore cardiac function | Inhibit TLR4/NF-κB/NLRP3 pathway, downregulate TLR4, NF-κB, and NLRP3 | [86] | |
| LPS-induced NRCMs | ||||
| I/R-induced rats; | Reduce IL-1β and TNF-α mRNA expression, maintain stable IL-10 expression, reduces cardiac inflammation and fibrosis | Promote macrophage M2 polarization | [87] | |
| BMDMs | ||||
| Inhibit cardiac hypertrophy | Angiotensin II and isoproterenol-stimulated neonatal rat cardiomyocytes | Mitigate myocardial hypertrophy, downregulate ANP | Upregulate SIRT1 and PGC-1α expression | [10] |
| AAC-induced cardiac hypertrophy rats | Alleviate cardiac hypertrophy, improve cardiac hemodynamic function | Inhibit TNF-α/NF-kB pathway | [90] | |
| AAC-induced left ventricular hypertrophy rats | Ameliorate left ventricular hypertrophy | Inhibit CaN/MAPK pathway | [91] | |
| ISO-induced H9c2 cells | Inhibit cardiomyocyte hypertrophy | Inhibit Ca 2+/calpain-1 pathway | [92] | |
| TAC-induced left ventricular hypertrophy rats | Ameliorate left ventricular hypertrophy, improve cardiac dysfunction | Regulate expression of SIRT1 and PGC-1α, activate Akt, inhibit TNF-α/NF-κB and p38 MAPK signaling pathways | [93] | |
| Improve myocardial fibrosis | LAD ligation-induced MI mice; | Enhance cell viability, reduce apoptosis, attenuate fibrotic remodeling | Activate SIRT1/PINK1/Parkin pathway | [95] |
| H2O2-induced H9C2 cells | ||||
| AAC-induced rats | Alleviate cardiac fibrosis, improve cardiac decompensation | Inhibit [Ca2+]i increase, activate the CaN/NFAT3 pathway | [97] |
3.1. Modulating myocardial injury
Myocardial injury results from restricted blood flow, leading to an imbalance between myocardial oxygen supply and demand. This imbalance is exacerbated by oxidative stress, inflammation, and myocardial necrosis and apoptosis, ultimately leading to cardiac dysfunction [65] (Fig. 2).
Fig. 2.
Mechanisms of ginsenoside Rg1 in protecting ischemic myocardium.
Ginsenoside Rg1 (Rg1) offers significant cardioprotection in ischemic myocardium through multiple mechanisms. A. Anti-apoptotic effects: Rg1 prevents apoptosis in cardiomyocytes by promoting the dissociation of Bcl-2 and Beclin-2, regulating the HIF-α/ERK and PI3K/Akt/mTOR pathways, as well as inhibiting intracellular ATP depletion to suppress AMPK pathway activation. This leads to increased expression of anti-apoptotic proteins Bcl-2 and Beclin-2, and decreased expression of pro-apoptotic proteins Bax, caspase-3, and caspase-9. B. Antioxidant activity: Rg1 enhances oxidative stress response by regulating intracellular Ca2+ equilibrium, reducing ROS, and boosting mitochondrial membrane potential, thus preserving mitochondrial structure and function. Key pathways include inhibition of the RhoA/ROCK pathway, suppression of GSTP-1 to facilitate OPA-1 expression, and activation of the Akt/GSK-3β pathway through P2X7 expression inhibition. C. Anti-inflammatory effects: Rg1 attenuates inflammatory responses in myocardial cells by inhibiting the NF-κB/p65 pathway through TLR4 receptor suppression, stimulating the AMPK/Nrf2/HO-1 pathway, preventing the activation of the NLRP-3 inflammasome, and promoting macrophage M2 polarization. Collectively, these mechanisms reduce the secretion of inflammatory cytokines, thereby alleviating inflammation.
Abbreviations: HIF-α, hypoxia-inducible factor alpha; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; Bcl-2, B-cell lymphoma 2; Bax, bcl-2-associated X protein; RhoA, ras homolog gene family member A; ROCK, Rho-associated protein kinase; GSTP-1, glutathione S-transferase pi-1; OPA-1, optic atrophy 1; GSK-3β, glycogen synthase kinase 3 beta; P2X7, purinergic receptor P2X ligand-gated ion channel 7; MMP, mitochondrial membrane potential; NF-κB, nuclear factor kappa B; NLRP-3, NOD-like receptor protein 3; TLR4, toll-like receptor 4; AMPK, AMP-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1.
3.1.1. Ameliorating oxidative stress
Oxidative stress, characterized by an imbalance between ROS production and antioxidant defenses, is a critical factor in myocardial injury. Excessive ROS generation during ischemia induces cell apoptosis and degrades the extracellular matrix, causing significant myocardial damage [66]. Rg1 has demonstrated considerable potential in mitigating oxidative stress and protecting myocardial cells.
In a rat model of acute myocardial ischemia (AMI), Rg1 was found to reduce myocardial infarction size and tissue damage significantly. This was accompanied by increased activities of SOD and glutathione peroxidase (GSH-Px) alongside decreased levels of MDA, indicating reduced oxidative damage [67,68]. Similarly, in rats with isoproterenol (ISO)-induced AMI, Rg1 pretreatment (20, 30, and 40 mg/kg for 14 days) alleviated myocardial injury, increased SOD activity, and decreased myocardial MDA levels [69].
The protective effects of Rg1 against oxidative stress are closely related to its ability to preserve mitochondrial function. Zhu et al. [70] demonstrated that Rg1 effectively suppressed ROS accumulation and increased GSH levels in primary neonatal rat ventricular myocytes (NRVMs). The mechanism involves Rg1 binding to glutathione S-transferase P1 (GSTP1), inhibiting its kinase activity and maintaining the interaction between optic atrophy 1 (OPA1) and mitofilin by preventing glutathionylation. This action protects the mitochondria from myocardial damage. Li et al. found that Rg1 protects H9c2 cells from simulated ischemia/reperfusion (I/R) injury by activating antioxidant defenses via Nrf2 and HO-1, reducing ROS and MDA production and increasing antioxidant enzyme levels. This also improved mitochondrial function, as evidenced by increased mitochondrial membrane potential (MMP) [71].
Furthermore, Liu et al. [72] showed that Rg1 activates the Akt/GSK-3β signaling pathway via the purinergic 2X7 (P2X7) receptor in cardiomyocytes. This activation inhibits mitochondrial Ca2+ overload, increases MMP, reduces ROS levels, and alleviates myocardial dysfunction in a hypoxia/reoxygenation (H/R) model of H9C2 cells. Treatment with Rg1 (3.125, 6.25, 12.5 μM for 12 h) post-H/R in H9C2 cells enhanced mitochondrial viability, reduced cardiac toxicity, increased ATP production, and decreased ROS production in a dose-dependent manner. These effects are attributed to mitochondrial dynamics balance maintenance through the mitofusin 2 (MFN2)-mediated pathway [73].
A recent study employed a combination of network pharmacology, ligand-based protein docking, proteomics, and Western blot to analyze protein expression changes in H9c2 cells damaged by H2O2 and pretreated with Rg1. The study suggests that Rg1 effectively alleviates oxidative stress damage in cardiomyocytes by acting on key proteins such as MAPK1 and adenosine kinase and inhibiting the oxidative phosphorylation pathway [74].
In summary, Rg1 ameliorates oxidative stress and protects myocardial cells through multiple mechanisms. These include enhancing antioxidant enzyme activity, preserving mitochondrial function, and modulating key signaling pathways. The ability of Rg1 to manage oxidative stress and myocardial injury highlights its potential as a cardioprotective agent.
3.1.2. Inhibiting myocardial apoptosis
Protecting cardiomyocytes from necrosis and apoptosis following ischemia is a key therapeutic target in CAD treatment [75]. Several studies suggest that Rg1 significantly inhibits myocardial apoptosis, thus preserving cardiac function.
In an AMI model induced by H/R, pretreatment with Rg1 demonstrated a marked protective effect on myocardial structure and function. This effect was associated with a significant reduction in myocardial apoptosis, as evidenced by decreased infarct size and improved myocardial viability [68]. Shen et al. reported that Rg1 reduced the infarct area in rats subjected to I/R injury. Rg1 effectively inhibited I/R-induced cardiomyocyte apoptosis by increasing Bcl-2 mRNA expression and decreasing Bax mRNA expression. Additionally, Cleaved-Poly (ADP-ribose) Polymerase (PARP), caspase-9, and caspase-3 protein expressions were significantly downregulated [76]. Another study in I/R rats showed that Rg1 treatment improved cardiomyocyte survival by regulating energy metabolism and enhancing the activity and expression of mitochondrial respiratory chain complexes via the RhoA/ROCK signaling pathway [77].
In vitro studies further elucidate the mechanisms underlying Rg1's cardioprotective effects. Pretreatment with Rg1 (50 mg/kg, 7 days) enhanced the expression of aldose reductase, AMPK, and PTEN-induced kinase 1 (PINK1) in fasting mice hearts, promoting mitochondrial autophagy. In vitro, Rg1 improved H9c2 cell viability under nutrient deprivation, reduced apoptosis, and maintained mitochondrial stability by upregulating aldose reductase, activating AMPK and PINK1, and regulating mitochondrial autophagy, ultimately inhibiting apoptosis [78]. Liu et al. established an in vitro ischemia-hypoxia model using H9c2 cells and found that under ischemia-hypoxia conditions, Rg1 activated the PI3K/Akt signaling pathway by phosphorylating Akt, leading to upregulation of HIF-1α and its downstream protective factors. This activation played a protective role in cardiomyocytes. Furthermore, HIF-1α upregulation inhibited the expression of C/EBP Homologous Protein (CHOP), a pro-apoptotic factor mediated by endoplasmic reticulum stress, thereby reducing cardiomyocyte damage and apoptosis [79]. Additionally, Rg1 was found to modulate the expression of apoptosis-related proteins in diabetic myocardial infarction models. Specifically, Rg1 treatment decreased the bcl-2-associated X protein/B-cell lymphoma 2 (Bax-2/Bcl-2) ratio and reduced caspase-3 and caspase-9 activity by activating the HIF-1α/ERK signaling pathway [80].
In cardiomyocytes, autophagy plays a dual role, exhibiting either protective or detrimental effects under varying pathological conditions. In H9c2 cardiomyocytes subjected to H/R, treatment with Rg1 (100 μmol/L for 24 h) significantly inhibited both autophagy and apoptosis. This protective effect was achieved by maintaining cellular ATP levels, preventing AMPK activation, and promoting mTOR activation, as evidenced by the reduced expression of autophagy markers LC3BII and Beclin-1 [81]. Conversely, another study demonstrated that Rg1 induces autophagy in nutrient-deprived H9c2 cells by facilitating the dissociation of Beclin-1 from Bcl-2, thereby increasing free Beclin-1 and Bcl-2 levels in the cytoplasm. This mechanism led to a reduction in apoptosis in a dose- and time-dependent manner [82]. These findings indicate that Rg1 exerts protective effects on cardiomyocytes by modulating autophagy differently, depending on the specific pathological conditions.
Moreover, in an ISO-induced AMI rat model, Rg1 reduced myocardial apoptosis by regulating the PI3K/PTEN/Akt pathway [69]. This regulation resulted in lower Bax-2 levels and higher Bcl-2 levels, indicating a shift toward cell survival. In both in vivo and in vitro models, Rg1's protective effects against myocardial infarction were associated with the upregulation of Bcl-2 and p62, as well as the downregulation of caspase-9, caspase-3, LC3-II/LC3-I. These findings further support its role in activating the PI3K/Akt/mTOR pathway [83].
In conclusion, Rg1 exerts its cardioprotective effects through multiple mechanisms, including modulating apoptosis-related signaling pathways, regulating autophagy, preserving mitochondrial function, and maintaining energy metabolism. These findings highlight Rg1's potential as a therapeutic agent in protecting against myocardial apoptosis and improving cardiac outcomes in patients with CAD.
3.1.3. Alleviating inflammation
Ischemia triggers a potent inflammatory response that significantly contributes to myocardial dysfunction [84]. Studies using ISO-induced AMI models demonstrate that Rg1 pretreatment mitigates this inflammatory response, reducing levels of creatine kinase-MB, lactate dehydrogenase (LDH), IL-6, and TNF-α [69].
In I/R injury models, Rg1 pretreatment (10 mg/kg for 15 days) decreased infarct size, reduced cardiomyocyte apoptosis, and lowered levels of IL-1β, TNF-α, and p65 protein. Notably, increased IκB expression indicates that Rg1 ameliorates myocardial injury by inhibiting the NF-κB pathway [85].
In neonatal rat cardiomyocytes (NRCMs), Rg1 mitigated LPS-induced cardiac dysfunction by reducing the expression of inflammatory cytokines and reversing the upregulation of NF-κB, TLR4, and NLRP3. These findings suggest that Rg1 suppresses inflammation and restores cardiac function by modulating the TLR4/NF-κB/NLRP3 pathway [86]. Additionally, Rg1 treatment decreased TNF-α, IL-6, and IL-1β levels in the myocardium of STZ-induced diabetic rats. This anti-inflammatory effect is linked to inhibiting NF-κB and NLRP3 while activating the AMPK/Nrf2/HO-1 pathway [36].
Another study conducted by Xu et al. reported that Rg1 alleviates myocardial I/R injury in SD rats by reducing IL-1β and TNF-α mRNA expression and stabilizing IL-10 expression. In vitro, Rg1 reverses necrotic DNA-induced changes in macrophage inflammatory protein expression, decreases iNOS, and increases Arginase 1 expression, indicating that Rg1 reduces cardiac inflammation and fibrosis by promoting macrophage M2 polarization [87].
In summary, Rg1 alleviates myocardial inflammation by inhibiting key inflammatory pathways, activating protective signaling cascades, and modulating macrophage polarization. These actions reduce inflammatory cytokine production and improve cardiac function, highlighting Rg1's potential as a therapeutic agent for inflammatory cardiovascular conditions.
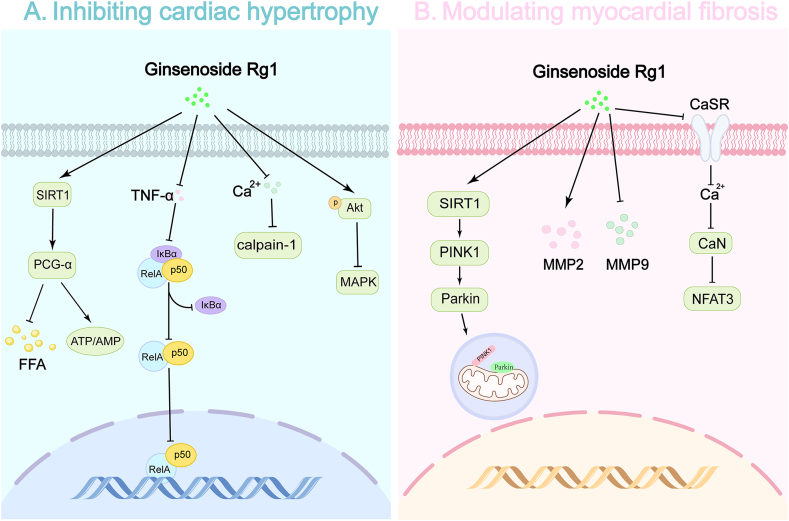
3.2. Inhibiting cardiac hypertrophy
Cardiac hypertrophy often develops as a response to increased workload caused by conditions like hypertension and myocardial infarction from CAD [88]. Research indicates that Rg1 effectively inhibits cardiac hypertrophy, thereby preserving cardiac function.
Sun et al. [10] demonstrated that Rg1 significantly reduced cardiomyocyte volume and downregulated atrial natriuretic peptide (ANP) expression in angiotensin II and ISO-stimulated neonatal rat cardiomyocytes. Notably, Rg1 increased the ATP/AMP ratio and elevated the expression of PPAR-gamma coactivator 1-alpha (PGC-1α) and sirtuin-1 (SIRT1). These effects were partly inhibited by a SIRT1 blocker, suggesting that Rg1 mitigates myocardial hypertrophy by upregulating PGC-1α and SIRT1 expression.
Wang et al. [89] found that Rg1 may enhance cellular energy metabolism by regulating PGC-1α expression in hypertrophic cardiomyocytes. In rats with abdominal aortic coarctation (AAC)-induced cardiac hypertrophy, Rg1 administration dose-dependently alleviated cardiac hypertrophy and enhanced cardiac hemodynamic function, possibly by inhibiting the TNF-α/NF-kB pathway [90]. Further in vitro experiments using TNF-induced NRVMs confirmed these findings.
In another AAC-induced rat model, Rg1 (3.75, 7.5, and 15 mg/kg/day for 3 weeks) ameliorated left ventricular hypertrophy in a dose-dependent manner, primarily through inhibition of the calcineurin (CaN) and MAPK signaling pathways [91]. Pretreatment of H9c2 cells subjected to ISO-induced injury with Rg1 showed a comparable inhibitory effect on cardiomyocyte hypertrophy to propranolol. Rg1 also reduced calpain-1 protein expression and intracellular calcium levels, implicating the Ca2+/calpain-1 pathway in its protective effects [92]. Additionally, Zhang et al. showed that Rg1 had a protective effect against transverse aortic constriction (TAC)-induced left ventricular hypertrophy and cardiac dysfunction, linked to Akt activation and p38 MAPK pathway inhibition [93].
Collectively, these studies indicate that Rg1 alleviates cardiac hypertrophy by regulating PGC-1α and SIRT1 expression, activating Akt, and inhibiting the TNF-α/NF-κB, Ca2+/calpain-1, and p38 MAPK signaling pathways. This highlights the potential of Rg1 as a therapeutic agent for cardiac hypertrophy in patients with CAD (Fig. 3).
Fig. 3.
Mechanisms of ginsenoside Rg1 in modulating myocardial hypertrophy and fibrosis.
Ginsenoside Rg1 (Rg1) inhibits myocardial hypertrophy and fibrosis through Various signaling pathways. A. Inhibiting cardiac hypertrophy: Rg1 inhibits cardiac hypertrophy by activating SIRT1/PGC-1α to regulate FFA and energy metabolism in cardiomyocytes, inhibiting the TNF-α/NF-κB and Ca2+/Calpain-1 signaling pathways, and promoting Akt phosphorylation to suppress the MAPK signaling pathway. B. Modulating myocardial fibrosis: Rg1 regulates myocardial fibrosis by activating the SIRT1/PINK1/Parkin pathway, inhibiting the Ca2+/CaN/NFAT3 pathway, promoting MMP2 secretion, and reducing MMP9 secretion.
Abbreviations: SIRT1, Sirtuin-1; PGC-1α, PPAR-gamma coactivator 1-alpha; FFA, free fatty acid; NF-κB, nuclear factor kappa B; MAPK, Mitogen-activated protein kinase; PINK1, PTEN-induced kinase 1; CaN, Calcineurin; NFAT3, Nuclear factor of activated T-cells 3.
3.3. Modulating myocardial fibrosis
Chronic ischemia damages myocardial tissue, leading to its replacement with fibrous scar tissue. This process reduces the myocardium's contractile capacity, exacerbating cardiac dysfunction. Rg1 has shown potential in mitigating myocardial fibrosis, thereby preserving cardiac function [94].
In studies involving H9c2 cells, Rg1 (0–80 μM) significantly reduced apoptosis, enhanced cell viability, and attenuated fibrotic remodeling induced by H2O2. These effects were mediated through mitophagy modulation via activation of the SIRT1/PINK1/Parkin axis [95]. In a rat model of chronic thromboembolic pulmonary hypertension, Rg1 (100 mg/kg/d for 4 weeks) reduced myocardial fibrosis, increased MMP-2 expression, and decreased MMP-9 expression. There was a negative correlation between the protein expressions of MMP-2, MMP-9, and collagen-I, indicating that Rg1 improved ventricular remodeling through matrix metalloproteinase regulation [96]. In another study, Rg1 alleviated cardiac fibrosis and improved cardiac decompensation induced by AAC in rats by inhibiting [Ca2+]i increase and the CaN/NFAT3 pathway activation, both mediated by the calcium-sensing receptor (CaSR) [97].
In summary, Rg1 exerts anti-fibrotic effects by regulating mitophagy, modulating matrix metalloproteinases, and inhibiting calcium-sensing pathways. These mechanisms collectively reduce myocardial fibrosis and improve cardiac outcomes (Fig. 3).
4. Conclusion
In recent years, numerous preclinical studies have demonstrated the promising potential of ginsenosides for treating cardiovascular diseases. Among these, the ginsenoside monomers Rb1, Rd, Rg1, and Rg3 have emerged as research hotspots. These monomers exhibit cardioprotective properties, including anti-inflammatory, antioxidative stress, and anti-cardiomyocyte apoptosis effects [[98], [99], [100], [101]]. Through a summary and synthesis of existing literature, we found that among these ginsenoside monomers, Rg1 exhibits a broader range of pharmacological activities. In addition to the established cardioprotective effects, Rg1 inhibits VSMC proliferation, reduces lipid levels, suppresses platelet aggregation, and promotes angiogenesis, exerting protective effects at various stages of CAD occurrence and development [28,39,45,54]. Based on this, we focused on the protective effects of ginsenoside Rg1 in CAD treatment. This review offers the first systematic summary of the protective effects and potential mechanisms of Rg1 from the perspective of CAD's pathological progression, providing new insights and a theoretical basis for its prevention and treatment.
In atherosclerosis, Rg1 exerts its protective effects by enhancing endothelial function through modulation of Nrf2/HO-1, AMPK/SIRT3/p53, PI3K/Akt, and calpain-1 pathways, thereby improving vasodilation, reducing oxidative stress, and inhibiting leukocyte adhesion. Concurrently, it effectively inhibits VSMC proliferation by interfering with cell cycle progression. The anti-inflammatory properties of Rg1 are primarily mediated through AMPK activation and NF-κB inhibition, resulting in reduced levels of pro-inflammatory factors like IL-1β, IL-6, and TNF-α. Furthermore, Rg1 optimizes lipid metabolism by regulating the expression of specific proteins and genes, while its antithrombotic properties are achieved by downregulating PAI-1 expression. Rg1 also exhibits pro-angiogenic effects via activation of the PI3K/Akt pathway and modulation of key angiogenic factors.
Beyond its anti-atherosclerotic effects, Rg1 demonstrates significant cardioprotective properties through multiple mechanisms. It ameliorates oxidative stress in ischemic myocardium by enhancing antioxidant enzyme activity and preserving mitochondrial function, particularly through the GSTP1, Akt/GSK-3β, and MFN2-mediated pathways. Rg1 inhibits myocardial apoptosis by modulating the RhoA/ROCK, PI3K/Akt/mTOR, HIF-1α/ERK, and AMPK/PINK1 signaling pathways while simultaneously alleviating inflammation by suppressing the NF-κB and TLR4/NF-κB/NLRP3 pathways, activating the AMPK/Nrf2/HO-1 pathway, and regulating macrophage polarization. Moreover, Rg1 mitigates cardiac hypertrophy by regulating SIRT1/PGC-1α expression and inhibiting TNF-α/NF-κB, calpain-1, and Akt/MAPK signaling. Additionally, it improves myocardial fibrosis through the SIRT1/PINK1/Parkin axis and modulation of matrix metalloproteinases. These diverse actions collectively contribute to Rg1's potential as a therapeutic agent in inhibiting atherosclerotic plaque formation and improving overall cardiac function in CAD.
The therapeutic potential of Rg1 in CAD is compelling, yet the journey from “bench to bedside” is fraught with challenges. Bioavailability remains a significant hurdle, as Rg1 exhibits poor oral absorption [102]. Innovative drug delivery systems, such as nanoparticles or liposomes, could enhance its bioavailability and therapeutic efficacy. Additionally, potential interactions between Rg1 and standard cardiovascular medications must be thoroughly investigated to ensure safe and effective combination therapies.
Despite extensive preclinical evidence supporting Rg1's cardioprotective effects, clinical data remain limited. Well-designed clinical trials are imperative to validate the findings from basic research and establish Rg1 as a viable therapeutic option for CAD. These studies should focus on dosing strategies, long-term efficacy, and safety profiles to provide a comprehensive understanding of Rg1's clinical potential. Moreover, the molecular mechanisms underlying Rg1's actions warrant deeper exploration. While current studies highlight its various effects in CAD, the intricate signaling networks involved need further elucidation. Advanced techniques such as proteomics, metabolomics, and CRISPR-based gene editing could offer new insights into Rg1's multifaceted networks.
In conclusion, ginsenoside Rg1 shows significant promise as a therapeutic agent for CAD. Its diverse mechanisms of action address key aspects of atherosclerosis and myocardial protection, positioning it as a multifaceted intervention in cardiovascular disease management. The path forward requires rigorous research, innovative approaches to drug delivery, and comprehensive clinical validation. With continued exploration and collaboration, Rg1 may soon emerge as a cornerstone in the treatment of CAD.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the project of the National Natural Science Foundation of China (No. 82274345), Fundamental Research Funds for the Central public welfare research institutes Grant (No. ZZ13-YQ-016 and No. ZZ13-YQ-016-C1), National High Level Hospital Clinical Research Funding and Elite Medical Professionals Project of China-Japan Friendship Hospital (NO. ZRJY2023-GG22).
Contributor Information
Lin Xu, Email: 723731711@qq.com.
Ming Guo, Email: mguo@xycacms.ac.cn.
Abbreviations
- AAC
Abdominal aortic coarctation
- ACC
Acetyl-CoA carboxylase
- AMI
Acute myocardial ischemia
- AMPK
AMP-activated protein kinase
- APTT
Activated partial thromboplastin time
- Bax-2
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- CAD
Coronary artery disease
- CaN
Calcineurin
- CAT-1
Cationic amino acid transporter-1
- CCN1
Cellular communication network factor 1
- CHOP
C/EBP Homologous Protein
- CIH
Chronic intermittent hypoxia
- ERK
Extracellular signal-regulated kinase
- ETB
Endothelin receptor type B
- FGFR-1
Fibroblast growth factor receptor-1
- GR
Glucocorticoid receptor
- GRK
G-protein coupled receptor kinase
- GSTP1
Glutathione S-transferase P1
- H/R
Hypoxia/reoxygenation
- HAECs
Human aortic endothelial cells
- HASMCs
Human arterial vascular smooth muscle cells
- HCAECs
Human coronary artery endothelial cells
- HDL-C
High-density lipoprotein cholesterol
- HIF-α
Hypoxia-inducible factor-α
- HO-1
Heme oxygenase-1
- HUVECs
Human umbilical vein endothelial cells
- I/R
Ischemia/reperfusion
- ICAM
Intercellular adhesion molecule
- IL-10
Interleukin 10
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- IκB
Inhibitor of κB
- ISO
Isoproterenol
- LDH
Lactate dehydrogenase
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MDA
Malondialdehyde
- MKP-1
Mitogen-activated protein kinase phosphatase-1
- MMP
Mitochondrial membrane potential
- NAFLD
Nonalcoholic fatty liver disease
- NFAT3
Nuclear factor of activated T-cells 3
- NF-κB
Nuclear factor kappa B
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NLRP3
NOD-like receptor protein 3
- NO
Nitric oxide
- NRVMs
Neonatal rat ventricular myocytes
- OPA1
Optic atrophy 1
- P. ginseng
Panax ginseng Meyer
- PAECs
Porcine aortic endothelial cells
- PARP
Poly (ADP-ribose) Polymerase
- PDGF-BB
Platelet-derived growth factor-BB
- PGC-1α
PPAR-gamma coactivator 1-alpha
- PI3K
Phosphoinositide 3-kinase
- PINK1
PTEN-induced kinase 1
- PKC-β
Protein kinase C-beta
- PP2A
Protein phosphatase 2A
- PT
Prothrombin time
- ROS
Reactive oxygen species
- RUNX2
Runt-related transcription factor 2
- SIRT1
Sirtuin-1
- SIRT3
Sirtuin-3
- SMCs
Smooth muscle cells
- SOD
Superoxide dismutase
- STZ
Streptozotocin
- TAC
Transverse aortic constriction
- TC
Total cholesterol
- TG
Triglyceride
- TGF-β1
Transforming growth factor-beta 1
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-alpha
- TT
Thrombin time
- VCAM
Vascular cell adhesion molecule
- VEGF
Vascular endothelial growth factor
- VSMCs
Vascular smooth muscle cells
- cGMP
Cyclic guanosine monophosphate
- eNOS
Endothelial nitric oxide synthase
- miRNAs
MicroRNAs
References
- 1.Ralapanawa U., Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11:169–177. doi: 10.2991/jegh.k.201217.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone G.W., Kappetein A.P., Sabik J.F., Pocock S.J., Morice M.C., Puskas J., Kandzari D.E., Karmpaliotis D., Brown W.M., 3rd, Lembo N.J., et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 3.Zhou G., Wang C.Z., Mohammadi S., Sawadogo W.R., Ma Q., Yuan C.S. Pharmacological effects of ginseng: multiple constituents and multiple actions on humans. Am J Chin Med. 2023;51:1085–1104. doi: 10.1142/S0192415X23500507. [DOI] [PubMed] [Google Scholar]
- 4.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W., Balan P., Popovich D.G. Comparison of ginsenoside components of various tissues of New Zealand forest-grown asian ginseng (Panax ginseng) and American ginseng (Panax Quinquefolium L.) Biomolecules. 2020;10 doi: 10.3390/biom10030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao F., Lu M.L., Wang H.X. Ginsenoside Rg1 ameliorates chronic intermittent hypoxia-induced vascular endothelial dysfunction by suppressing the formation of mitochondrial reactive oxygen species through the calpain-1 pathway. Journal of Ginseng Research. 2023;47:144–154. doi: 10.1016/j.jgr.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y., Deng J., Yu X.F., Yang D.L., Gong Q.H., Huang X.N. Ginsenoside Rg1 inhibits vascular intimal hyperplasia in balloon-injured rat carotid artery by down-regulation of extracellular signal-regulated kinase 2. J Ethnopharmacol. 2011;138:472–478. doi: 10.1016/j.jep.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Lyu T.J., Zhang Z.X., Chen J., Liu Z.J. Ginsenoside Rg1 ameliorates apoptosis, senescence and oxidative stress in ox-LDL-induced vascular endothelial cells via the AMPK/SIRT3/p53 signaling pathway. Exp Ther Med. 2022;24:545. doi: 10.3892/etm.2022.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan L.S., Yue P.Y., Wong Y.Y., Wong R.N. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem Pharmacol. 2013;86:392–400. doi: 10.1016/j.bcp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Sun N., Wang H., Lu M. Ginsenoside Rg1 inhibits hypertrophy induced by AngⅡ in cardiac myocytes of neonatal rats via SIRT1/PGC-1alpha signal pathway. Pharmacology and Clinics of Chinese Materia Medica. 2019;35:69–73. [Google Scholar]
- 11.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 12.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., Kuder J.F., Wang H., Liu T., Wasserman S.M., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 13.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 14.Carbonell-Prat B., Flores-Umanzor E., Brugaletta S. Ticagrelor or prasugrel in acute coronary syndromes. N Engl J Med. 2020;382:486. doi: 10.1056/NEJMc1915621. [DOI] [PubMed] [Google Scholar]
- 15.Xu S., Ilyas I., Little P.J., Li H., Kamato D., Zheng X., Luo S., Li Z., Liu P., Han J., et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73:924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- 16.Verma S., Anderson T.J. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 17.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgözoğlu L., Lewis E.F. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y., Wu Q., Yang D-l, Deng J., Huang X-n. Inhibitory effect of ginsenoside Rg1 on vascular neointimal hyperplasia induced by balloon-injury in rats and the relation to its anti-oxidant action and up-regulating eNOS expression. Chin Pharmacol Bull. 2012;28:388–392. [Google Scholar]
- 19.Huang G-d, Mao J., Ji Z. Evaluation of ginsenoside Rg1 as a potential antioxidant for preventing or ameliorating progression of atherosclerosis. Trop J Pharmaceut Res. 2013;12:941–948. [Google Scholar]
- 20.Lu M., Zhao F., Ran C., Xu Y., Zhang J., Wang H. Ginsenoside Rg1 attenuates diabetic vascular endothelial dysfunction by inhibiting the calpain-1/ROS/PKC-β axis. Life Sci. 2023;329 doi: 10.1016/j.lfs.2023.121972. [DOI] [PubMed] [Google Scholar]
- 21.Li C.P., Qin G., Shi R.Z., Zhang M.S., Lv J.Y. Ginsenoside Rg1 reduces toxicity of PM(2.5) on human umbilical vein endothelial cells by upregulating intracellular antioxidative state. Environ Toxicol Pharmacol. 2013;35:21–29. doi: 10.1016/j.etap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Tousoulis D., Kampoli A.M., Tentolouris C., Papageorgiou N., Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 23.Lü J.P., Ma Z.C., Yang J., Huang J., Wang S.R., Wang S.Q. Ginsenoside Rg1-induced alterations in gene expression in TNF-alpha stimulated endothelial cells. Chin Med J (Engl) 2004;117:871–876. [PubMed] [Google Scholar]
- 24.Pan C., Huo Y., An X., Singh G., Chen M., Yang Z., Pu J., Li J. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vascul Pharmacol. 2012;56:150–158. doi: 10.1016/j.vph.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Kanter J.E. Monocyte recruitment versus macrophage proliferation in atherosclerosis. Circ Res. 2017;121:1109–1110. doi: 10.1161/CIRCRESAHA.117.311973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J., Li Y.-L. Ginsenoside Rg1 downregulates the shear stress induced MCP-1 expression by inhibiting MAPK signaling pathway. Am J Chin Med. 2015;43:305–317. doi: 10.1142/S0192415X15500202. [DOI] [PubMed] [Google Scholar]
- 27.Wen T., Wang R.N., Zhang X.Y., Sun J.X., Zhang X.X., Han H., Yan X.C. Recent advances of phenotypic switch of vascular smooth muscle cells in vascular diseases. Chinese Heart Journal. 2023;35:337–343. Chinese. [Google Scholar]
- 28.Zhang H.S., Wang S.Q. Ginsenoside Rg1 inhibits tumor necrosis factor-alpha (TNF-alpha)-induced human arterial smooth muscle cells (HASMCs) proliferation. J Cell Biochem. 2006;98:1471–1481. doi: 10.1002/jcb.20799. [DOI] [PubMed] [Google Scholar]
- 29.Huang J., Li L.S., Yang D.L., Gong Q.H., Deng J., Huang X.N. Inhibitory effect of ginsenoside Rg1 on vascular smooth muscle cell proliferation induced by PDGF-BB is involved in nitric oxide formation. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/314395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Z.C., Gao Y., Wang Y.G., Tan H.L., Xiao C.R., Wang S.Q. Ginsenoside Rg1 inhibits proliferation of vascular smooth muscle cells stimulated by tumor necrosis factor-alpha. Acta Pharmacol Sin. 2006;27:1000–1006. doi: 10.1111/j.1745-7254.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 31.Bentzon J.F. Targeting inflammation in atherosclerosis. J Am Coll Cardiol. 2016;68:2794–2796. doi: 10.1016/j.jacc.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh C.-C., Chang C.-Y., Yar Lee T.X., Wu J., Saovieng S., Hsieh Y.-W., Zhu M., Huang C.-Y., Kuo C.-H. Longevity, tumor, and physical vitality in rats consuming ginsenoside Rg1. Journal of Ginseng Research. 2023;47:210–217. doi: 10.1016/j.jgr.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song Y., Zhao F., Zhang L., Du Y., Wang T., Fu F. Ginsenoside Rg1 exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia. 2013;91:173–179. doi: 10.1016/j.fitote.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Liu Y., Zhang X.-Y., Xu L.-H., Ouyang D.-Y., Liu K.-P., Pan H., He J., He X.-H. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-κB and PI3K/Akt/mTOR pathways. Int Immunopharmacol. 2014;23:77–84. doi: 10.1016/j.intimp.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Tang B.L., Liu Y., Zhang J.L., Lu M.L., Wang H.X. Ginsenoside Rg1 ameliorates hypoxia-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition and inflammation by regulating CCN1. Biomed Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114920. [DOI] [PubMed] [Google Scholar]
- 36.Qin Q., Lin N., Huang H., Zhang X., Cao X., Wang Y., Li P. Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats. Diabetes Metab Syndr Obes. 2019;12:1091–1103. doi: 10.2147/DMSO.S208989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ference B.A., Kastelein J.J.P., Catapano A.L. Lipids and lipoproteins in 2020. JAMA. 2020;324:595–596. doi: 10.1001/jama.2020.5685. [DOI] [PubMed] [Google Scholar]
- 39.Hou Y., Gu D., Peng J., Jiang K., Li Z., Shi J., Yang S., Li S., Fan X. Ginsenoside Rg1 regulates liver lipid factor metabolism in NAFLD model rats. ACS Omega. 2020;5:10878–10890. doi: 10.1021/acsomega.0c00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawase A., Yamada A., Gamou Y., Tahara C., Takeshita F., Murata K., Matsuda H., Samukawa K., Iwaki M. Increased effects of ginsenosides on the expression of cholesterol 7α-hydroxylase but not the bile salt export pump are involved in cholesterol metabolism. J Nat Med. 2013;67:545–553. doi: 10.1007/s11418-012-0713-4. [DOI] [PubMed] [Google Scholar]
- 41.Jae Hong P., Jiyoun L., Jiyoung Y., Jeong Su N., Myeong Ho J. Antihyperlipidemic effect of ginsenoside Rg1 in type 2 diabetic mice. Korean Journal of Life Science. 2011;21:932–938. [Google Scholar]
- 42.Liu H., Wang J., Liu M., Zhao H., Yaqoob S., Zheng M., Cai D., Liu J. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. 2018;10 doi: 10.3390/nu10070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng X., Huang D., Yan M., Peng S. Ginsenoside Rg1 improves liver function by regulating fat metabolism in rats with non-alcoholic fatty liver disease. Chin J Pathophysiol. 2015;31:864–870. [Google Scholar]
- 44.Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 45.Dandan W., Linyan L., Yue X., Kai J., Feng C., Jie Q., Ming C., Guanping L., Yaozu X. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111287. [DOI] [PubMed] [Google Scholar]
- 46.Hua S.Y., Qu F., Chen L.P., Kang L.Y., Zhang B.L. The effect of rat platelet aggregation and platelet cyclic AMP metabolism by Ginsenoside Rg1. Journal of Tianjin University of Traditional Chinese Medicine. 2012;31:31–33. Chinese. [Google Scholar]
- 47.Zhou Q., Jiang L., Xu C., Luo D., Zeng C., Liu P., Yue M., Liu Y., Hu X., Hu H. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb Res. 2014;133:57–65. doi: 10.1016/j.thromres.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Feng L., Wang L., Hu C., Jiang X. Pharmacokinetics, tissue distribution, metabolism, and excretion of ginsenoside Rg1 in rats. Arch Pharm Res (Seoul) 2010;33(12):1975–1984. doi: 10.1007/s12272-010-1213-2. [DOI] [PubMed] [Google Scholar]
- 49.Cui Z.Y., Liu C.L., Li D.D., Wang Y.Z., Xu F.R. Anticoagulant activity analysis and origin identification of Panax notoginseng using HPLC and ATR-FTIR spectroscopy. Phytochem Anal. 2022;33:971–981. doi: 10.1002/pca.3152. [DOI] [PubMed] [Google Scholar]
- 50.Li C.T., Wang H.B., Xu B.J. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharm Biol. 2013;51:1077–1080. doi: 10.3109/13880209.2013.775164. [DOI] [PubMed] [Google Scholar]
- 51.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 52.Henry T.D. Therapeutic angiogenesis. Bmj. 1999;318:1536–1539. doi: 10.1136/bmj.318.7197.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu L.C., Chen S.C., Chang W.C., Huang Y.C., Lin K.M., Lai P.H., Sung H.W. Stability of angiogenic agents, ginsenoside Rg1 and Re, isolated from Panax ginseng: in vitro and in vivo studies. Int J Pharm. 2007;328:168–176. doi: 10.1016/j.ijpharm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Sengupta S., Toh S.A., Sellers L.A., Skepper J.N., Koolwijk P., Leung H.W., Yeung H.W., Wong R.N., Sasisekharan R., Fan T.P. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- 55.Pan D., Xu L., Chen P., Miao L., Tian Y., Shi D., Guo M. Panax Quinquefolium Saponins enhances angiogenesis in rats with diabetes and myocardial infarction. J Ethnopharmacol. 2024;319 doi: 10.1016/j.jep.2023.117252. [DOI] [PubMed] [Google Scholar]
- 56.Cheung L.W., Leung K.W., Wong C.K., Wong R.N., Wong A.S. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res. 2011;89:419–425. doi: 10.1093/cvr/cvq300. [DOI] [PubMed] [Google Scholar]
- 57.Leung K.W., Pon Y.L., Wong R.N.S., Wong A.S.T. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and β-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 58.Leung K.W., Ng H.M., Tang M.K., Wong C.C., Wong R.N., Wong A.S. Ginsenoside-Rg1 mediates a hypoxia-independent upregulation of hypoxia-inducible factor-1α to promote angiogenesis. Angiogenesis. 2011;14:515–522. doi: 10.1007/s10456-011-9235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X.D., Guo T., Liu L., et al. MiR-23a targets RUNX2 and suppresses ginsenoside Rg1-induced angiogenesis in endothelial cells. Oncotarget. 2017;8:58072–58085. doi: 10.18632/oncotarget.19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwok H.H., Chan L.S., Poon P.Y., Yue P.Y., Wong R.N. Ginsenoside-Rg1 induces angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression through miR-23a. Toxicol Appl Pharmacol. 2015;287:276–283. doi: 10.1016/j.taap.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Chan L.S., Yue P.Y., Mak N.K., Wong R.N. Role of microRNA-214 in ginsenoside-Rg1-induced angiogenesis. Eur J Pharm Sci. 2009;38:370–377. doi: 10.1016/j.ejps.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Xiong W., Tan M.X., Zou X.L., Bai X., Zhu H.C., Liang Y., Zhang L.Y., Zhang X. Ginsenoside Rg1 regulates the secretion of exosomes and the expression of angiogenesis-related miRNAs by endothelial progenitor cells. Lishizhen Medicine and Materia Medica Research. 2022;33:277–280. Chinese. [Google Scholar]
- 63.Xiang Q., Yi X., Zhu X.H., Wei X., Jiang D.S. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol Metab. 2024;35:219–234. doi: 10.1016/j.tem.2023.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 65.Frangogiannis N.G. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 66.Wang W., Kang P.M. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants. 2020;9 doi: 10.3390/antiox9121292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Q., Chen Y., Liu F., Chen X. Effect of ginsenosides-rg1 on acute myocardial ischemic anti-ox idative injury indexes and ultrastructure in experimental rats. Chinese Circulation Journal. 2015;30:164–167. [Google Scholar]
- 68.Yang C., Jiang G., Xing Y. Protective effect of ginsenosides Rg1 on ischemic injury of cardiomyocytes after acute myocardial infarction. Cardiovasc Toxicol. 2022;22:910–915. doi: 10.1007/s12012-022-09767-1. [DOI] [PubMed] [Google Scholar]
- 69.Jin G., Ma J. Protective effect of ginsenoside Rg1 on isoproterenol-induced acute myocardial ischemia in rats. Int J Clin Exp Med. 2017;10:4100–4106. [Google Scholar]
- 70.Zhu H., Yan C., Yao P., Li P., Li Y., Yang H. Ginsenoside Rg1 protects cardiac mitochondrial function via targeting GSTP1 to block S-glutathionylation of optic atrophy 1. Free Radic Biol Med. 2023;204:54–67. doi: 10.1016/j.freeradbiomed.2023.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Li Q., Xiang Y., Chen Y., Tang Y., Zhang Y. Ginsenoside Rg1 protects cardiomyocytes against hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling and inhibition of JNK. Cell Physiol Biochem. 2017;44:21–37. doi: 10.1159/000484578. [DOI] [PubMed] [Google Scholar]
- 72.Liu Z., Pan H., Zhang Y., Zheng Z., Xiao W., Hong X., Chen F., Peng X., Pei Y., Rong J., et al. Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β signaling pathway. J Biochem Mol Toxicol. 2022;36 doi: 10.1002/jbt.22885. [DOI] [PubMed] [Google Scholar]
- 73.Dong G., Chen T., Ren X., Zhang Z., Huang W., Liu L., Luo P., Zhou H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion. 2016;26:7–18. doi: 10.1016/j.mito.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Xie R., Li C., Zhong C., et al. Integration of virtual screening and proteomics reveals potential targets and pathways for ginsenoside Rg1 against myocardial ischemia. J Ginseng Res. 2024;48:395–404. doi: 10.1016/j.jgr.2024.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jose Corbalan J., Vatner D.E., Vatner S.F. Myocardial apoptosis in heart disease: does the emperor have clothes? Basic Res Cardiol. 2016;111:31. doi: 10.1007/s00395-016-0549-2. [DOI] [PubMed] [Google Scholar]
- 76.Shen W.Y., Li Y.D., Yang S.Z. Effects of Ginsenoside Rg1 on arrhythmias induced by ischemic/reperfusion injury in rats. Journal of Clinical Cardiology. 2017;33:465–469. Chinese. [Google Scholar]
- 77.Li L., Pan C.-S., Yan L., Cui Y.-C., Liu Y.-Y., Mu H.-N., He K., Hu B.-H., Chang X., Sun K., et al. Ginsenoside Rg1 ameliorates rat myocardial ischemia-reperfusion injury by modulating energy metabolism pathways. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Z., Li C., Liu Q., Yang H., Li P. Ginsenoside Rg1 protects H9c2 cells against nutritional stress-induced injury via aldolase/AMPK/PINK1 signalling. J Cell Biochem. 2019;120:18388–18397. doi: 10.1002/jcb.29150. [DOI] [PubMed] [Google Scholar]
- 79.Liu R., Song R., Yuan L. Effect of ginsenoside-rg1 on rat's cardiomyocytes with its mechanism of signal pathway in vitro. Journal of clinical cardiology. Chinese Circulation Journal. 2015;30:1096–1100. Chinese. [Google Scholar]
- 80.Yuan C., Wang H., Yuan Z. Ginsenoside Rg1 inhibits myocardial ischaemia and reperfusion injury via HIF-1α-ERK signalling pathways in a diabetic rat model. Pharmazie. 2019;74:157–162. doi: 10.1691/ph.2019.8858. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z.L., Fan Y., Liu M.L. Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol Cell Biochem. 2012;365:243–250. doi: 10.1007/s11010-012-1265-3. [DOI] [PubMed] [Google Scholar]
- 82.Li D., Wang J., Hou J., et al. Ginsenoside Rg1 protects starving H9c2 cells by dissociation of Bcl-2-Beclin1 complex. BMC Complement Altern Med. 2016;16:146. doi: 10.1186/s12906-016-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin L., Fan S., Jia R., Liu Y. Ginsenoside Rg1 protects cardiomyocytes from hypoxia-induced injury through the PI3K/AKT/mTOR pathway. Pharmazie. 2018;73:349–355. doi: 10.1691/ph.2018.8329. [DOI] [PubMed] [Google Scholar]
- 84.Marchant D.J., Boyd J.H., Lin D.C., Granville D.J., Garmaroudi F.S., McManus B.M. Inflammation in myocardial diseases. Circ Res. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 85.He X., Zhang Y. Ginsenoside Rg1 attenuates myocardial ischemia reperfusion injury through NF-kappaB signaling pathway. Pharmacology and Clinics of Chinese Materia Medica. 2015;31:16–19. [Google Scholar]
- 86.Luo M., Yan D., Sun Q., Tao J., Xu L., Sun H., Zhao H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121:2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 87.Xu X., Wu Q., Pei K., et al. Ginsenoside Rg1 reduces cardiac inflammation against myocardial ischemia/reperfusion injury by inhibiting macrophage polarization. J Ginseng Res. 2024;48:570–580. doi: 10.1016/j.jgr.2024.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maillet M., van Berlo J.H., Molkentin J.D. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H., Gao H. Ginsenoside Rg1 protect cardiac hypertrophy induced by ISO via PGC-1alpha signaling pathway. Pharmacology and Clinics of Chinese Materia Medica. 2015;31:55–59. [Google Scholar]
- 90.Tang F., Lu M., Yu L., Wang Q., Mei M., Xu C., Han R., Hu J., Wang H., Zhang Y. Inhibition of TNF-α-mediated NF-κB activation by ginsenoside Rg1 contributes the attenuation of cardiac hypertrophy induced by abdominal aorta coarctation. J Cardiovasc Pharmacol. 2016;68:257–264. doi: 10.1097/FJC.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 91.Deng J., Lv X.T., Wu Q., Huang X.N. Ginsenoside Rg(1) inhibits rat left ventricular hypertrophy induced by abdominal aorta coarctation: involvement of calcineurin and mitogen-activated protein kinase signalings. Eur J Pharmacol. 2009;608:42–47. doi: 10.1016/j.ejphar.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y.Y., Xiao Y.Q., Yuan X. Ginsenoside Rg1 attenuates the apoptosis of hypertrophic cardiomyocyte via calpain-1 signaling pathway. Pharmacology and Clinics of Chinese Materia Medica. 2017;33:17–20. Chinese. [Google Scholar]
- 93.Zhang Y.-J., Zhang X.-L., Li M.-H., Iqbal J., Bourantas C.V., Li J.-J., Su X.-Y., Muramatsu T., Tian N.-L., Chen S.-L. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol. 2013;62:50–57. doi: 10.1097/FJC.0b013e31828f8d45. [DOI] [PubMed] [Google Scholar]
- 94.Ibáñez B., Heusch G., Ovize M., Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 95.Guan S., Xin Y., Ding Y., Zhang Q., Han W. Ginsenoside Rg1 protects against cardiac remodeling in heart failure via SIRT1/PINK1/parkin-mediated mitophagy. Chem Biodivers. 2023;20 doi: 10.1002/cbdv.202200730. [DOI] [PubMed] [Google Scholar]
- 96.Li C.Y., Deng W., Liao X.Q., Deng J., Zhang Y.K., Wang D.X. The effects and mechanism of ginsenoside Rg1 on myocardial remodeling in an animal model of chronic thromboembolic pulmonary hypertension. Eur J Med Res. 2013;18:16. doi: 10.1186/2047-783X-18-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu M.-L., Wang J., Sun Y., Li C., Sun T.-R., Hou X.-W., Wang H.-X. Ginsenoside Rg1 attenuates mechanical stress-induced cardiac injury via calcium sensing receptor-related pathway. Journal of Ginseng Research. 2021;45:683–694. doi: 10.1016/j.jgr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan W., Huang Y., Zheng H., et al. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: pharmacology and mechanisms. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110915. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Zeng L., Zhang Y., et al. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.975784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin Z., Xie R., Zhong C., Huang J., Shi P., Yao H. Recent progress (2015-2020) in the investigation of the pharmacological effects and mechanisms of ginsenoside Rb1, a main active ingredient in Panax ginseng Meyer. J Ginseng Res. 2022;46(1):39–53. doi: 10.1016/j.jgr.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng Q., Ling L., Yuan H., Guo Z., Ma J., Ginsenoside Rd. A promising target for ischemia-reperfusion injury therapy (A mini review) Biomed Pharmacother. 2024;171 doi: 10.1016/j.biopha.2023.116111. [DOI] [PubMed] [Google Scholar]
- 102.He C., Feng R., Sun Y., Chu S., Chen J., Ma C., Fu J., Zhao Z., Huang M., Shou J., et al. Simultaneous quantification of ginsenoside Rg1 and its metabolites by HPLC-MS/MS: rg1 excretion in rat bile, urine and feces. Acta Pharm Sin B. 2016;6:593–599. doi: 10.1016/j.apsb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]