ABSTRACT
Staphylococcus aureus is the causative agent of serious human health conditions, such as sepsis, endocarditis, and necrotizing pneumonia, as well as less severe clinical manifestations including epithelial and mucosal infections. This pathogen expresses a wide range of surface virulence factors, among which fibronectin‐binding proteins play a crucial role in both bacterial adhesion and infection of host cells. Fibronectin is utilized by S. aureus during the early stages of infection to form a protective coating that shields the bacterium from host defenses, facilitating adhesion to the extracellular matrix of host cells and promoting immune evasion and subsequent invasion. S. aureus protein A (SpA) is a key multi‐domain cell wall‐anchored and secreted molecule that functions to evade the human immune response by non‐specifically interacting with Fc and the Fab VH3 domains of immunoglobulins. Other known human ligands of SpA include the von Willebrand factor, the tumor necrosis factor receptor 1, and a platelet surface protein, all of which contribute to immune evasion and pathogenesis. The present study reveals that SpA can also bind human fibronectin with high affinity, adding a new function to this already multifunctional virulence factor. We show that the N‐terminus of fibronectin is involved in the interaction and demonstrate by carbene footprinting experiments that the SpA fibronectin binding site spans the interdomain linker region and helix 1 of the domains D, A, B, and C, partially overlapping with the Fc binding site. In the presence of fibronectin, SpA knock‐out mutant strains showed reduced adhesion to human endothelial cells compared to wild‐type bacteria, suggesting that this interaction may play a significant role in the attachment to host tissues by S. aureus .
Keywords: bacterial adhesion, fibronectin, monoclonal antibody, protein–protein interaction, SpA, Staphylococcus aureus
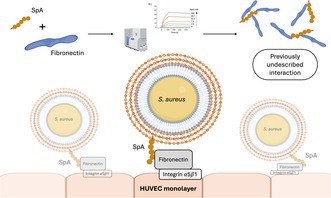
Through this study, we discovered and characterized a previously undescribed interaction between Staphylococcal protein A (SpA) and human fibronectin. We also present data that suggest a potential mechanism for SpA's contribution to bacterial adhesion to host cells, opening to potential new strategies to fight S. aureus infections. Image generated in Biorender (https://www.biorender.com).
1. Introduction
Staphylococcus aureus is both a commensal and an opportunistic human pathogen that has evolved to adapt to various environmental conditions in human and animal systems. As a commensal organism, S. aureus is typically found in the human skin and in the nasal cavity, but it is also the leading cause of a wide range of diseases, including skin and soft tissue infections, pneumonia, septicaemia, peritonitis, bacteraemia, and infective endocarditis [1, 2]. Adding to the growing concern of S. aureus as a significant threat to human health is the emergence of highly virulent strains, such as methicillin‐resistant (MRSA) and vancomycin‐resistant (VRSA) forms, which have developed in both community and hospital settings [3].
The high pathogenicity of S. aureus stems from its ability to express a wide array of virulence factors, among which are cell wall‐anchored (CWA) surface proteins that are critical for bacterial survival [4]. CWA proteins from Gram‐positive bacteria share a common cell wall‐anchoring mechanism wherein a C‐terminal sorting sequence is recognized by the transpeptidase sortase A [5, 6]. Among these proteins, the staphylococcal Protein A (SpA) plays a critical role in evading the host immune responses. SpA binds the Fcγ portion of immunoglobulin G (IgG) impairing its effector functions, and interacts with the Fab domain of the VH3‐clan of IgG and IgM B cell receptors, stimulating B lymphocyte proliferation and subsequent cell death [7, 8, 9, 10, 11].
SpA molecules contain four to five immunoglobulin‐binding domains (SpA‐D, SpA‐E, SpA‐A, SpA‐B, SpA‐C) each connected by linker peptides [12], followed by a C‐terminal repetitive octapeptide and a unique sequence domain [13] (Figure 1). Previous X‐ray data indicated that each single SpA domain can interact with two Ig molecules simultaneously via their Fcγ and Fab regions [14]. These interactions were investigated by Kim and colleagues [15, 16] who produced two mutant forms of SpA named SpA KK and SpA AA , lacking the ability to non‐specifically interact with antibodies. In particular, SpA KK contained a dipeptide substitution of 9Gln‐10Gln with two Lys residues in each domain that impaired Fcγ binding, while SpA AA had a 36Asp‐37Asp substitution with two Ala residues in each domain inhibiting VH3 binding (aminoacid residue numbering according to Uniprot sequence for SpA‐C domain, entry A0A0H3K686•SPA_STAAE).
FIGURE 1.

Schematic depiction of the SpA structure, highlighting its five Ig‐binding domains labeled by their respective letters. The Xr and Xc linker regions are also indicated: The Xr region exhibits variable length, while the Xc linker region contains motifs essential for bacterial cell wall binding. Additionally, the IgG‐binding regions within each domain are visualized, along with the mutants generated by knocking out these binding sites [14, 15]. The square brackets on top of the figure indicate the SpA wt region used for the experiments reported in this study.
SpA also binds the human von Willebrand factor (vWF) multimeric glycoprotein [17]. An additional interaction with gC1qR/p33 on immobilized platelets suggested a potential role of SpA in the pathogenesis of staphylococcal endocarditis [18, 19]. Other interactions modulating immune cell inflammatory responses include those with the tumor necrosis factor receptor 1 (TNFR1) [20] and the Epidermal Growth Factor Receptor (EGFR) [20, 21].
One of the key host molecules involved in S. aureus infections is fibronectin (Fn), a glycoprotein abundantly present in both the extracellular matrix (as insoluble filaments) and plasma (as soluble dimers) that plays a crucial role in processes such as development, organogenesis, cell adhesion, migration, and hemostasis [22, 23]. Fn is composed of three different types of homologous protein modules named FI, FII, and FIII [24], and can be proteolytically digested into conserved fragments [25, 26]. S. aureus interacts with Fn through specific members of the MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) family, among which are the Fn‐binding proteins FnBPA and FnBPB, Ebh, Eap, and Emp [27] and vWbp [28]. The recognized binding site for FnBPs is the N‐terminal domain of Fn [29]. These interactions enable Fn to coat the bacterial surface [30, 31], effectively hiding the bacterium from the immune system, protecting it, and promoting infection spread. Binding to Fn also represents a critical step for host cell adhesion [32], cellular invasion, and infection persistence [33].
In this study, we explored novel interactions of SpA with extracellular matrix (ECM) proteins and demonstrated that SpA is an additional member of the S. aureus repertoire of Fn‐binding proteins. We show that SpA exhibits high affinity for Fn and plays a significant role in Fn‐dependent adhesion to endothelial cells.
2. Material and Methods
2.1. Human Biological Samples (HBS) Compliance Statement
Human biological samples were sourced ethically, and their research use was in accordance with the terms of the informed consents under an IRB/REC‐approved protocol.
2.2. Bacterial Strains, Proteins and Antibodies
The Staphylococcus strains used in this study are reported in Table S1 and were grown in Brain Heart Infusion broth (BHI, VWR International Srl, Milan, Italy) at 37°C under shaking conditions.
Wild‐type Staphylococcal protein A (SpA wt ) and its mutant isoforms (SpA KK , SpA AA ) were produced as previously described [34]. Human Fn and its fragments (N29, GBF, 70 kDa, CBF, Hep2, and C‐ter) were obtained as previously reported [35]. Recombinant N29 modules (1–2FI, 2‐3FI, 3‐4FI, 4‐5FI) were kindly donated by Jennifer R. Potts, Dept. of Biology, University of York, UK. Human von Willebrand Factor was purchased from EMD Millipore (Burlington, MA).
Horseradish peroxidase (HRP)‐conjugated rabbit IgG was purchased from Dako Denmark (Glostrup Denmark). The anti‐SpA human monoclonal antibody mAb1 [34] and the non‐related mAb2 (derived from previously described MABp1 [36]) were internally produced in an IgG3 scaffold as reported [34].
2.3. Determination of SpA Interaction With Fn by ELISA
Microtiter wells were coated with 5 μg/well in 100 μL of different ECM molecules, that is, Fn, fibrinogen (Fbg), plasminogen (Plg), laminin (Lam), collagen type II (Coll II) and vitronectin (Vn), and incubated ON at 4°C. The following day, the wells were washed three times with 0.1% v/v Tween in Phosphate Buffer Saline (PBST) and incubated with 200 μL/w of 2% bovine serum albumin (BSA, Sigma Aldrich) in PBS for 1 h at room temperature (RT) with mild agitation (50 rpm) to block non‐specific binding sites. After incubation, BSA was discarded without washing, and 5 μg/w of SpA wt , SpA KK , and SpA AA were added to the wells. The plate was incubated for 1 h at RT with mild agitation. Wells were washed three times with PBST and incubated for 45 min at RT in mild agitation with a HRP‐conjugated rabbit IgG (Dako Denmark, Glostrup, Denmark). The interaction of the HRP‐IgG with SpA was revealed by incubation of the wells with ortho‐phenylenediamine dihydrochloride (OPD, Dako Denmark) and the absorbance was measured at 490 nm using a microplate reader (Model 680, BioRad, Richmond, CA). To determine the Fn fragments involved in SpA binding, Fn fragments N29, GBF, CBF, Hep2, and C‐term were immobilized on microtiter wells, and the procedure was repeated as already described. A minimum of three independent experiments were conducted. Statistical analysis was performed using Prism 9.0 (GraphPad). Groups were compared by the one‐way ANOVA test in combination with a post‐hoc test (Dunnett's multiple comparison test or Tukey's honest significant test). p values < 0.05 were considered statistically significant.
2.4. Determination of the Kinetic Parameters of SpA‐Fn Interaction by Surface Plasmon Resonance
Kinetic parameters for SpA‐Fn interactions were measured by (Surface Plasmon Resonance) employing a Biacore 8 K (Cytiva), at a constant flow rate of 30 μL/min. Using amine chemistry, 200 nM each of Fn, SpA wt , SpA KK , and SpA AA were immobilized on the flow cell (FC) 2 of a CM5 Sensor Chip (Cytiva), while the blank‐immobilized FC1 served as a reference. Soluble analytes were injected for 120 s and let to dissociate for 1800 s. Chip regeneration was achieved by injecting 3 M MgCl2 for 60 s. SpA wt , SpA KK , and SpA AA were used as analytes on immobilized Fn and vice versa. The stoichiometry of the interaction was maintained at a 1:1 M ratio. The calculation of kinetic parameters was performed by applying the Bivalent Analyte kinetic model, given the five domains composition of SpA, each one capable of interacting with SpA ligands.
Single SpA domains (SpA‐D, SpA‐E, SpA‐A, SpA‐B and SpA‐C) were used as analytes on the CM5 sensor chip previously described. SpA was used as a positive control for the analysis. Analyte concentration was maintained at 200 nM. The analysis was performed by injecting SpA single domains for 120 s, with 1800s dissociation time, maintaining 30 μL/min flow rate. Sensor chip regeneration after each cycle was obtained with 3 M MgCl2 for 60 s contact time. Kinetic parameters were not calculated for this analysis.
2.5. Luminex Assay to Assess Displacement of Fn Binding to SpA by Anti‐SpA mAbs
SpA‐coated magnetic beads prepared as previously described [34] were saturated with 306 μg/mL of biotinylated Fn (bFn) obtained by labeling Fn with EZ‐Link sulfo‐NHS‐LC‐biotin (Thermo Fisher Scientific). The bFn‐saturated SpA‐coated beads (1.25 × 106 beads/mL) were incubated for 30 min at RT with vigorous shaking with serial dilutions (ranging from 500 to 1 μg) of mAb1, mAb2, or PBS. After washing, the presence of bFn bound to SpA was revealed with 50 μL of a final concentration of 0.1 mg/mL PE‐conjugated streptavidin (Invitrogen), and the signal was acquired with FLEXMAP 3D (Luminex Corporation). Median fluorescence intensity (MFI) was subtracted from the background signal (SpA beads + bFn + streptavidin‐PE, in absence of mAb) and statistical analysis was performed in GraphPad Prism 10.0 using an unpaired parametric Welch's t test.
2.6. Carbene Footprinting to Detect the Fn Binding Site on the SpA‐C Domain
Similar to other footprinting techniques like Hydrogen Deuterium Exchange, chemical footprinting relies on the differential extent of labeling when a protein is either bound or unbound to its ligand [24, 37, 38]. To allow the formation of the complex, 10 μM SpA‐C were incubated for 25 min at RT with 12 μM N29 or with 5.4 μM of BSA as a negative control sample (CTRL). The sequences of the SpA‐C domain and of the N29 fragment are displayed in Table S2. Both CTRL and test samples were prepared as independent triplicates. BSA concentration was used in a 1:1 mass (μg) ratio with N29. After the first incubation, 5 mM 4‐(3‐Trifluoromethyl)‐3H‐Diazirin‐3yl) Benzoic Acid (TDBA, Sigma) was added to each vial; the final volume was 10 μL. The solutions were then incubated for 15 min at RT in the dark to achieve a homogeneous distribution of the diazirine label within the solution. After this second incubation, each vial was plunge frozen in liquid nitrogen and irradiated for 20″ with a UV laser (λ = 350 nm, 1000 Hz, single pulse power = 125–130 μJ).
Upon irradiation, samples were reduced/alkylated (15 min at 60°C) and digested with trypsin (iST 96x kit, PreOmics) for 2.5 h. at 37°C under shaking conditions (500 rpm). Following the PreOmics kit protocol, digested peptides were collected by centrifugation (3 min at 2200 g) in LoBind Eppendorf tubes and dried under vacuum for 2.5 h at 45°C (Concentrator plus, Eppendorf). The dried peptides were suspended in 30 μL LC‐LOAD buffer from the Preomics iST kit and transferred to low bind UPLC vials.
Samples were loaded onto a μPAC NEO HPLC Column 50 cm Nano LC (Thermo Scientific), with a set temperature of 50°C. The chromatographic run flow rate was set at 0.5 μL/min in buffer A (0.1% formic acid (H2O) + 3% DMSO), with a buffer B (0.1% formic acid (80% ACN) + 3% DMSO) gradient set in four steps (1.0%–2.0%; 2.0%–22.5%; 22.5%–45%; 45%–99%) over 68 min 12″. Mass Spectrometry analysis was performed on a Orbitrap Eclipse (Thermo Fisher) in OT/OT mode, equipped with a nanoflow electrospray ionization source. Spray voltage was set at 1900 V, with desolvation temperature at 275°C. Data were acquired in topN (2 s) Data‐Dependent Acquisition (DDA) mode. Peptide spectra were acquired in positive ion mode at a Orbitrap resolution of 120 000 over a mass range between 350 and 1200 m/z and a maximum injection time of 50 ms. To obtain MS/MS data of labeled peptides, ions were fragmented by high‐energy collisional dissociation (HCD) followed by detection at a nominal resolution setting of 15 000, the Normalized AGC Target % was 1000 and the maximum injection time was 500 ms. Identification and relative quantification of labeled peptides was performed in Mass Spec Studio (software version 2.4.0.3560) by using default parameters and mass tolerance set at 20 PPM. Further data processing was performed by a custom Knime workflow. The data processing entails grouping peptides based on start‐end positions, sequence and charge. The number of labeled Positive Spectral Matches (PSMs) from triplicates are summed and peptides with less than 3 PSMs are excluded. The fractional modification of labeled peptides is then calculated using the following equation: .
A protection factor per each peptide is then calculated as:
By permuting all the measured fractional modifications, we obtained a group of 9 possible ratios for each peptide. These groups were then compared using a two‐sample Wilcoxon nonparametric test.
2.7. Bacterial Adhesion to Fn
Microtiter wells were coated with 10 μg/well of Fn and incubated overnight (ON) at 4°C in 0.1 M sodium carbonate, pH 9.5. The plates were then washed three times with 0.5% (v/v) Tween 20 in PBS (PBST). To block non‐specific protein binding, wells were treated for 1 h at room temperature (RT) with 200 μL of 2% BSA in PBS. Bacterial adhesion was assessed by incubating 100 μL/well of bacterial cells (OD600nm = 1.0) in PBS for 1 h at 37°C. The plate was subsequently washed three times with PBS to remove unbound cells. Adhering bacteria were fixed by adding 200 μL/well of 4% formaldehyde (pH 6.9; Sigma) and incubating for 30 min at RT. After three washes with PBS, 100 μL/well of 10% Crystal Violet dye (Sigma) was added, and the plate was incubated for 15 min at RT. Following incubation, the plate was washed with tap water and 50 μL/well of 10% acetic acid (Sigma) was added. Adhering cells were quantified by measuring absorbance at 590 nm. A minimum of three independent experiments were conducted. Analyses were performed using Prism 9.0 (GraphPad). Groups were compared by the one‐way ANOVA test in combination with a post‐hoc test (Dunnett's multiple comparison test or Tukey's honest significant test). p values < 0.05 were considered statistically significant.
2.8. Bacterial Adhesion to Human Endothelial Cells
Human umbilical vein endothelial cells (HUVEC) from a single donor (Lonza, Spain) were kindly provided by the Neonatal Unit and Neonatal Intensive Care Unit, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy. HUVEC were selected due to the constitutive expression of integrin α5β1 [39] and cultured in T25 flasks (Becton, Dickinson, Germany) at 37°C in 5% CO2 with 100% humidity. Commercial basal medium supplemented with growth factors and cytokines (EGM BulletKit; Lonza) was used. Cells were seeded at 2 × 105 cell density per well in 24‐well tissue culture plates, cultured in medium supplemented with 10% Fn‐depleted fetal bovine serum (FBS) without antibiotics for 24 h and allowed to attach for 24 h at 37°C in 5% CO2. The effect of Fn on bacterial adhesion to monolayers was tested by pre‐incubating the cells with 10 μg/mL of Fn for 1 h prior to infection. S. aureus SH1000 wt and SH1000 Δspa cells grown to the late exponential phase and resuspended in PBS were used to infect the cell monolayers with a multiplicity of infection (MOI) of 10. After a 30 min incubation, monolayers were washed three times with PBS to remove the non‐adherent bacteria and lysed using 500 μL of cold 1% Triton X‐100; at this point serial dilutions of the cell lysates were plated on BHI‐agar and CFU (colony forming units) were counted after over‐night incubation at 37°C. Percent of adhesion of each strain was calculated as follows: where represents the number of CFU per plate, and represents the number of CFU of the initial inoculum. The same procedure was repeated for LAC USA300 and Newman strains, along with their spa KO derivatives. A minimum of three independent experiments were conducted. Analyses were performed using Prism 9.0 (GraphPad). Groups were compared by the one‐way ANOVA test in combination with a post‐hoc test (Dunnett's multiple comparison test or Tukey's honest significant test). p values < 0.05 were considered statistically significant.
3. Results
3.1. SpA Interacts With Fn With High Affinity
To investigate potential interactions of SpA with extracellular matrix (ECM) proteins, we first conducted an enzyme‐linked immunosorbent assay (ELISA) by immobilizing different human ECM proteins and measuring the binding of SpA using a horseradish peroxidase (HRP)‐conjugated rabbit IgG, previously tested for its non‐specific interaction with SpA. This initial experiment revealed that SpA interacted exclusively with Fn (Figure 2A) but not with other ECM proteins, that is, Fbg, Lam, Coll II, and Vn.
FIGURE 2.
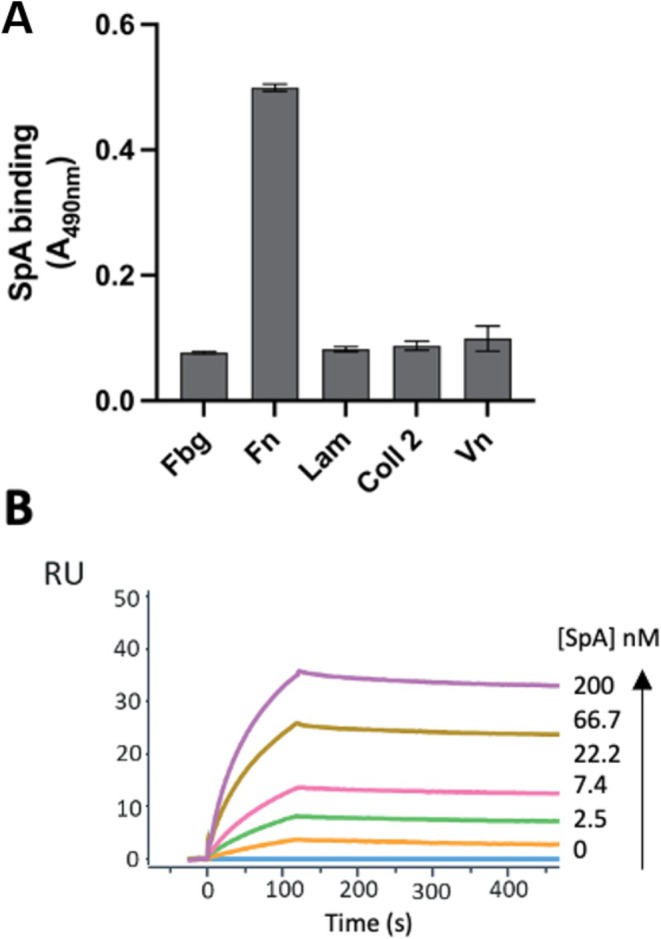
Specific binding of SpA to Fn with high affinity. (A) Binding of SpA to various immobilized ECM analyzed by ELISA. Complex formation was detected using HRP‐conjugated IgG bound by SpA. Data represent the means ± SD of three independent experiments, each performed in triplicate; statistically significant differences are indicated (***p < 0.001). (B) Representative sensorgrams from three different experiments showing the binding of increasing concentrations of SpA to immobilized Fn, analyzed by SPR. Fn was immobilized on a CM5 sensor chip and SpA was injected at increasing concentrations in the mobile phase.
The association rate constant (kon), dissociation rate constant (koff), and equilibrium dissociation constant (KD) of the interaction between SpA and Fn were investigated by Surface Plasmon Resonance (SPR) (Figure 2B). In this assay, Fn was covalently immobilized on a carboxymethylated dextran sensor chip (CM5) and increasing concentrations of SpA were injected into the mobile phase. Data analysis was carried out using a Bivalent Analyte Kinetic model to yield a KD for the Fn‐SpA interaction of 1.31 nM (Table 1). Importantly, only very little dissociation was observed over time once SpA was bound to immobilized Fn on the sensor surface, indicating an exceptionally high affinity and the formation of a highly stable complex.
TABLE 1.
Kinetic parameters for the interaction of SpA to immobilized Fn assessed by SPR and calculated using a Bivalent Analyte Kinetic model.
| Kinetic parameters | SpA |
|---|---|
| ka (1/Ms) | 4.10·104 |
| kd (1/s) | 5.38·10−5 |
| Rmax (RU) | 80,40 |
| KD (M) | 1.31·10−9 |
3.2. The fc Binding Site of SpA Is Also Involved in the Interaction With Fibronectin
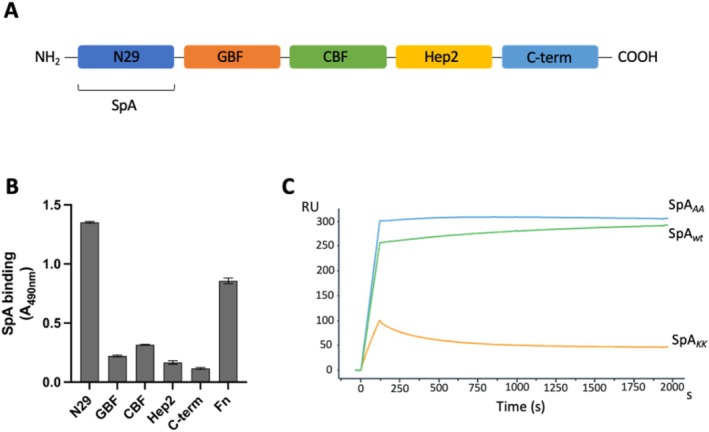
To determine which portion of SpA interacts with Fn, two mutant forms of the protein previously described by Kim et al. [27, 28] were employed (Figure 1), that is, SpA AA (which has an Ala‐Ala dipeptide substitution of residues 36Asp‐37Asp of SpA domain C and of the corresponding residues in each domain, inhibiting binding to IgG Fab VH3) and SpA KK (in which residues 9Gln‐10Gln in each domain were replaced with a Lys‐Lys dipeptide, impairing binding to IgG Fc). In this experiment, Fn was immobilized on microtiter wells and incubated with either wild‐type SpA (SpA wt ) or the mutant variants, all containing the five Ig EDABC domains. As shown in Figure 3, both SpA wt and SpA AA displayed similar binding with immobilized Fn, indicating that the interacting site is located within the multidomain region of SpA. Conversely, SpA KK showed a significantly reduced binding to Fn compared to both SpA wt and SpA AA , suggesting that the SpA Fc‐binding site was involved in the interaction with Fn.
FIGURE 3.

The Fc binding site of SpA is responsible for the SpA‐Fibronectin interaction. Binding of recombinant SpAwt or its mutant variants SpA AA and SpA KK to immobilized Fn analyzed by ELISA. Complex formation was detected using an HRP‐conjugated IgG. Data represent the means ± SD of three independent experiments, each performed in triplicate. Statistically significant differences are indicated (***p < 0.001).
To further validate these findings, SPR was employed to assess the binding of the two mutant forms of SpA. The analysis confirmed that SpA KK completely lost its ability to bind to Fn (Table 2). Conversely, SpA AA maintained a similar binding level to SpA wt , albeit presenting approximately five‐fold lower affinity (6.1 nM compared to 1.3 nM for SpA wt ) and an increased dissociation rate (1.54 × 10−3 1/s for SpA AA compared to 5.38 × 10−5 1/s for SpA wt ). This change was likely dependent on the charge perturbation caused by the mutation, where replacing two negatively charged amino acids (36Asp‐37Asp) with two neutral residues (Ala‐Ala) introduced a net positive charge that destabilizes the complex with Fn.
TABLE 2.
Kinetic parameters for the interaction of SpA AA and SpA KK to immobilized Fn assessed by SPR and calculated using a Bivalent Analyte Kinetic model.
| Kinetic parameters | SpAAA | SpAKK |
|---|---|---|
| ka (1/Ms) | 2.52·105 | 1.49·106 |
| kd (1/s) | 1.54·10−3 | 1.24·10−6 |
| Rmax (RU) | 138,9 | 18,7 |
| KD (M) | 6.10·10−9 | Not applicable |
3.3. The SpA Binding Site Is Located at the N‐Terminus of Fn
Fn consists of multiple domains that can be separated by proteolytic digestion (Figure 4A). To identify the specific Fn domain that interacts with SpA, full‐length Fn and its proteolytically generated fragments were immobilized on microtiter wells, and SpA binding was assessed by ELISA. The data revealed that SpA bound to the N29 fragment, which corresponds to the N‐terminal region of Fn (Figure 4B), similar to what was previously observed for other staphylococcal Fn‐binding proteins [30]. SPR experiments were performed with the N29 fragment of Fn immobilized on the chip, where the binding of SpA wt and its mutants, SpA AA and SpA KK , was evaluated at a single concentration (Figure 4C). Results confirmed that both SpA wt and SpA AA bound to the N29 fragment of Fn, while the SpA KK mutant did not, consistent with the data shown in Table 2.
FIGURE 4.
The SpA binding site is localized at the N‐terminus of Fn. (A) Schematic representation of Fn highlighting the N‐terminal domain (N29), gelatin‐binding domain (GBF), cell‐binding domain (CBF), heparin‐binding domain (Hep2), and C‐terminal domain (C‐term). Domains are named following established nomenclature [21]. The SpA binding site is indicated on the N‐terminal domain (NTD). (B) Binding of recombinant SpA wt to immobilized Fn or its fragments analyzed by ELISA. Complex formation was detected using a HRP‐conjugated anti‐IgG antibody. Data represent means ± SD of three independent experiments, each performed in triplicate. Statistically significant differences are indicated (***p < 0.001). (C) Representative sensograms from three independent experiments showing the binding of SpA wt and its mutants, SpA AA and SpA KK to immobilized N29 fragment, analyzed by SPR. N29 was immobilized on a CM5 sensor chip, and the recombinant protein was injected at a single concentration in the mobile phase.
3.4. The Fn Binding Site Extends Across the Inter‐Domain Linker Region and Helix 1 of SpA
To determine which of the five SpA domains were involved in the interaction with Fn, SPR was performed using immobilized N29 and recombinant soluble forms of each individual domain. The results revealed that domains D, A, B, and C interacted with N29, while the E domain did not participate in the binding and showed only a mild increase in the response units (RU) due to a bulk effect in the sensorgram (Figure 5A). SpA‐C exhibited the strongest binding and was selected to map more precisely its N29‐binding site using carbene footprinting.
FIGURE 5.
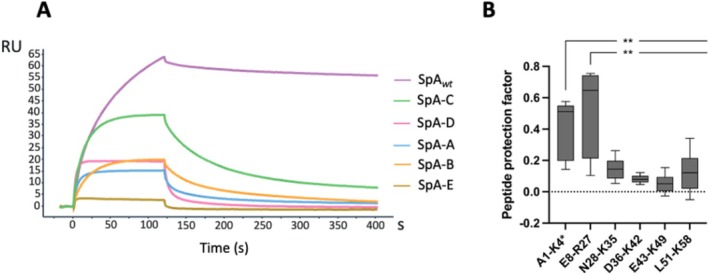
The Fn binding site spans the inter‐domain linker region and helix 1 of SpA. (A) Representative sensorgrams showing the binding of SpA wt and its individual domains (C‐D‐A‐B and E) to immobilized N29 fragment, analyzed by SPR. N29 was immobilized on a CM5 sensor chip, and each recombinant protein was injected at a single concentration in the mobile phase. (B) Box plot depicting the carbene footprinting‐derived protection factor for each of the analyzed peptides. Significance of the result was calculated via two‐samples Wilcoxon non‐parametric test. All peptides amino acid localization has been normalized on the Uniprot sequence for SpA‐C domain (entry A0A0H3K686 • SPA_STAAE) to account for the His‐tag insert used for affinity purification. Data derived from three independent experiments, each performed in triplicate and statistically significant differences are indicated (**p < 0.01).
The SpA‐C/N29 interaction was monitored with carbene footprinting employing TDBA diazirine, a labeling reagent that is converted into a reactive carbene upon exposure to a near‐UV laser. SpA‐C was incubated with N29 or BSA as control and, following the labeling, bound and unbound SpA were digested by trypsin. The resulting peptides were analyzed through Liquid Chromatography –Mass Spectrometry. The labeling extent was mapped for six peptides, covering 93.1% of the SpA‐C sequence, and a protection factor (corresponding to the level of labeling shielding provided by the interaction) was calculated for each of them. In the presence of N29, a reduction in labeling was observed for 2 out of the 6 SpA‐C peptides, specifically the peptides A1‐K4 and E8‐R27 which include the domain helix 1 and the inter‐domain linker region (Figure 5B). These data were in alignment with the observed impact of the Q9K‐Q10K mutations on SpA‐Fn interaction.
3.5. Anti‐SpA mAb1 Can Displace Fn From SpA
To investigate whether SpA‐specific antibodies could have the ability to interfere with the interaction between SpA and Fn, a previously described anti‐SpA monoclonal antibody (herein mAb1) [34] was tested for its ability to displace Fn bound to SpA‐coated magnetic beads under saturating conditions. A non‐SpA‐specific antibody (mAb2) was used as a negative control. The two monoclonal antibodies had their Fc region silenced to prevent binding to SpA as they were tested as IgG3, a subclass that does not interact with SpA [34]. The results of this assay are presented in Figure 6 and demonstrate that the tested mAb1, but not the mAb2 control, was able to efficiently displace SpA from its Fn interaction. The data suggested that mAb1 binds to a site that interferes with Fn binding, either directly or allosterically.
FIGURE 6.
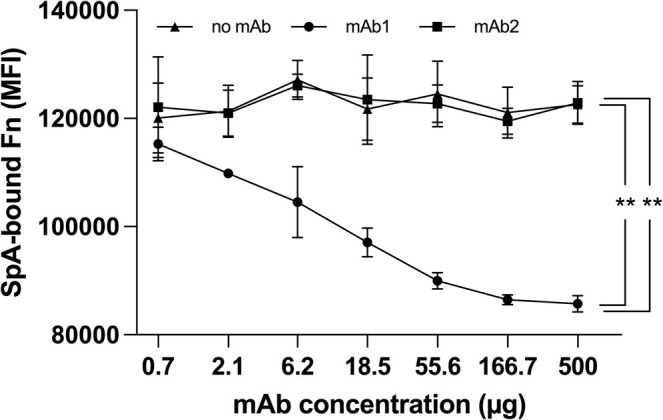
Luminex displacement of bFn from immobilized SpA by a SpA‐specific mAb. SpA‐coated magnetic beads were pre‐incubated with bFn under saturating conditions, followed by incubation with SpA‐specific mAb1 or non‐SpA‐specific mAb2 and subsequently with PE‐conjugated streptavidin. Data derived from three independent experiments, each performed in triplicate and statistically significant differences are indicated (**p < 0.01).
3.6. SpA Contributes to Fn‐Dependent Host Cell Adhesion by S. aureus
Given the role of Fn in S. aureus adhesion to host cells [33, 40], the impact of SpA‐Fn interaction on bacterial adhesion was examined using three different S. aureus strains (USA300, SH1000, and Newman) and their respective spa knockout (Δspa) derivatives. Binding of the strains to immobilized Fn in microtiter wells was assessed using crystal‐violet staining. As shown in Figure 7A, all Δspa strains exhibited a significant decrease in binding compared to their wild‐type counterparts, suggesting that SpA plays a crucial role in bacterial adhesion to Fn.
FIGURE 7.
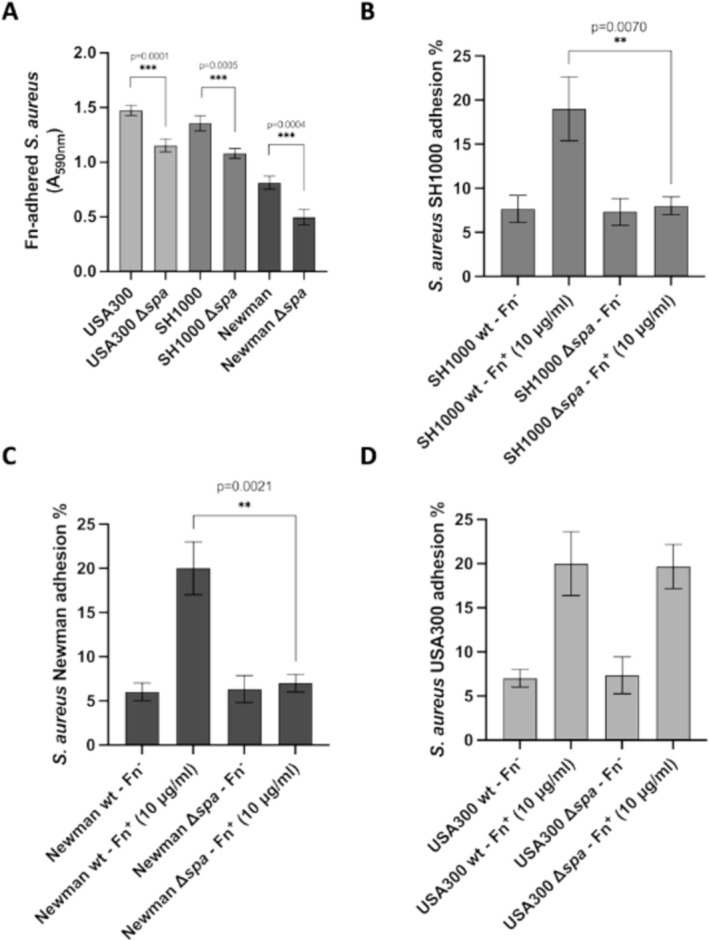
SpA mediates Fn‐dependent host cell adhesion. (A) Adhesion of the indicated S. aureus strains to immobilized Fn. Bound bacteria were detected by crystal‐violet staining. The data points are the means ± SD from three independent experiments, each performed in triplicate (***p < 0.001). B–D) Confluent HUVEC cell monolayers, grown in Fn‐free medium preincubated or not with exogenously added Fn, were infected with the indicated bacterial strains and SpA knockout mutants. The percentage of adhesion for each strain was calculated relative to the initial inoculum. The data points are the means ± SD from three independent experiments, each performed in triplicate. Statistically significant differences are indicated (**p < 0.01).
To examine the effect of Fn‐dependent bacterial adhesion to host cells, the same mutant strains were tested for adhesion to a HUVEC cell line both in the presence and absence of Fn. Consistent with the ELISA data, SH1000 Δspa and Newman Δspa showed significantly reduced adhesion to host cells in the presence of Fn, compared to their respective wild‐type strains (Figure 7). In contrast, no differences were observed between the USA300 wild‐type and its knockout mutant strain. In summary, the obtained data indicated that SpA is involved in Fn‐dependent adhesion to host cells, with the Δspa strains showing significantly reduced adhesion compared to wild‐type strains. However, the absence of a difference in cell adhesion between the USA300 wild‐type and knockout strains suggests potential strain‐specific variations in cell adhesion mechanisms among strains.
4. Discussion
The contribution of Protein A to S. aureus pathogenesis is well recognized since many years [9, 19, 41, 42]. In this study, we explored the interaction of SpA with human Fn, demonstrating that this virulence factor is also an important member of the family of surface proteins that bind extracellular matrix (ECM) components. Our data highlight that SpA binds to Fn with an equilibrium dissociation constant of 1.31 nM, indicative of a highly stable interaction. This KD value is in the range of those reported for similar interactions involving other bacterial Fn‐binding proteins like the Staphylococcal FnBPA and FnBPB [43], reinforcing the potential role of SpA in facilitating S. aureus cell adhesion. Moreover, studies involving other bacterial species like Streptococcus pyogenes show similar affinities for proteins like SfbI/F1 and F2 [44, 45], indicating a conserved strategy across different pathogens for Fn binding.
To dissect the molecular basis of the SpA‐Fn interaction, we analyzed the effect of specific SpA derivatives and observed that the SpA KK mutation, which disrupts SpA interaction with the IgG Fc region by introducing a positive charge to the molecule [14] also abolished Fn binding. Conversely, the SpA AA mutation that interferes with binding to the VH3 receptor only reduced the affinity for Fn by approximately six‐fold. The partial effect of this second mutation underscores the importance of the precise conformation of the interacting regions in stabilizing the SpA‐Fn complex. Together, these results suggest that both the Fc‐binding region and specific structural features are critical determinants of the interaction between SpA and Fn.
We identified the binding site of SpA within the N29 domain of Fn, a region also targeted by other ECM‐binding proteins of S. aureus, such as FnBP [43]. Carbene footprinting revealed that the inter‐domain linker and helix 1 of the SpA‐C domain were directly involved in Fn binding. These results confirm the importance of SpA conformation and suggest that subtle structural changes can modulate this interaction.
Finally, we show that the ability of SpA to bind Fn is significant for the adhesion of S. aureus to host cells. Indeed, knock‐out mutations in the SpA encoding gene significantly reduced the binding of S. aureus to Fn, as demonstrated by experiments comparing wild‐type strains with their respective Δspa variants. Notably, the Newman strain showed lower adherence to Fn than USA300 and SH1000, likely due to a mutation in the fnbA and fnbB genes which introduces a stop codon resulting in the truncation of FnBPs at the C‐terminal and impairing its interaction with immobilized Fn [46]. Furthermore, adhesion to human endothelial cells (HUVEC) in the presence of Fn was reduced when SpA was deleted, highlighting the role of SpA in Fn‐mediated adhesion. Interestingly, USA300 Δspa did not show impaired cell adhesion compared to wild‐type USA300, suggesting that in this strain other virulence factors or compensatory mechanisms such as FnBPs may influence adhesion.
The obtained results are consistent with previous studies showing that the ability to bind Fn is crucial for Staphylococcus adherence to host cells [30]. Several bacterial surface proteins bind Fn, each contributing differently to adhesion and virulence. For example, the Fn‐binding proteins (FnBPs) of S. aureus mediate attachment to host tissues [9]. Similarly, Streptococcus pyogenes utilizes proteins like SfbI and F2 to bind Fn, relying on mechanisms akin to those of SpA [44, 45]. Enterococcus faecalis employs AggA (aggregation substance) to adhere to Fn, forming biofilms that contribute to chronic infections [47]. SpA, however, exhibits a unique dual functionality, acting both as an immune evasion factor by binding immunoglobulins and several host innate immune proteins and as an adhesion mediator through ECM binding. This dual role distinguishes SpA from other Fn‐binding proteins and positions it as a versatile moonlighting factor in S. aureus virulence. While this study focuses on the SpA‐Fn interaction, previous research also highlighted SpA's ability to bind von Willebrand factor (vWF) under mechanical stress [48]. This distinct mechanism, facilitated by shear forces, may complement SpA's role in adhesion to ECM components like Fn, particularly in high‐shear environments such as blood flow. Although not directly addressed in this study, the SpA‐vWF interaction underscores the versatility of SpA in S. aureus pathogenesis and warrants further investigation.
Bear et al. (2023) [41] highlighted a role of SpA in immune evasion, suggesting that targeting SpA may enhance immune responses to Staphylococcal infections. Our study explored a potential strategy to disrupt SpA‐mediated interaction to Fn using specific antibodies. The tested anti‐SpA mAb1 was indeed able to effectively inhibit the SpA‐Fn interaction by displacing Fn from the SpA‐Fn complex, likely through direct or allosteric interference with the binding site. Boero and colleagues showed that mAb1 did not recognize the SpA AA mutant and suggested that the mAb1 target epitope could be located in proximity to the VH3 binding region of SpA [34]. We speculate an indirect effect of this antibody on SpA‐Fn interaction, possibly resulting from a conformational change caused by antibody binding to its target epitope outside of the Fc‐binding region. These findings are consistent with previous results by Chen et al. (2018) [49], who demonstrated that SpA‐targeting antibodies can decrease S. aureus colonization in a mouse infection model.
Therefore, targeting surface proteins like SpA could be an effective strategy to prevent or manage Staphylococcal infections [24, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55]. In addition, the results of this study open new avenues for the design of new compounds to effectively block the SpA‐Fn interaction. Such compounds could offer an alternative or complementary strategy to antibody‐based therapies, particularly relevant for addressing antibiotic‐resistant S. aureus strains.
In conclusion, the present study shows that SpA plays a central role in S. aureus adhesion to Fn and to endothelial cells, which are critical steps in the pathogenesis of staphylococcal infections. Targeting SpA interactions with ECM proteins like Fn could reduce bacterial adhesion and mitigate the progression of severe infections, such as infective endocarditis.
Author Contributions
S. Camaione, W. Pansegrau, F. Concetti, A.G.O. Manetti, M. Del Vecchio, and A. Pellegrini performed the experiments; I. Margarit, G. Pietrocola, S. Savino, L. Manzi, N. Norais, I. Ferlenghi, and L. Dello Iacono gave important feedback on the project and on results analysis. All authors reviewed the manuscript.
Conflicts of Interest
At the time of the study, all authors, excluding G. Pietrocola and A. Pellegrini , were employees of the GSK group of companies. S. Camaione was the recipient of a GSK fellowship from the PhD program of the University of Pavia. I. Margarit, N. Norais, I. Ferlenghi, and S. Savino hold shares of GSK.
Supporting information
Table S1. List of bacterial strains used in this study.
Table S2. Amino acid sequences of the recombinant SpA‐C fragment and the Fn N‐terminal domain (N29).
Funding: This work was supported by GlaxoSmithKline (GSK).
Contributor Information
I. Margarit, Email: immaculada.x.margarit-y-ros@gsk.com.
G. Pietrocola, Email: giampiero.pietrocola@unipv.it.
Data Availability Statement
Included in article.
References
- 1. Que Y. A., Haefliger J. A., Piroth L., et al., “Fibrinogen and Fibronectin Binding Cooperate for Valve Infection and Invasion in Staphylococcus aureus Experimental Endocarditis,” Journal of Experimental Medicine 201 (2005): 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Que Y. A. and Moreillon P., “Infective Endocarditis,” Nature Reviews. Cardiology 8, no. 6 (2011): 322–336, 10.1038/nrcardio.2011.43. [DOI] [PubMed] [Google Scholar]
- 3. DeLeo F. R. and Chambers H. F., “Reemergence of Antibiotic‐Resistant Staphylococcus aureus in the Genomics Era,” Journal of Clinical Investigation 119, no. 9 (2009): 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster T. J., Geoghegan J. A., Ganesh V. K., and Höök M., “Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus aureus ,” Nature Reviews. Microbiology 12, no. 1 (2014): 49–62, 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneewind O., Fowler A., and Faull K. F., “Structure of the Cell Wall Anchor of Surface Proteins in Staphylococcus aureus ,” Science 268, no. 5207 (1995): 103–106, 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 6. Schneewind O., Model P., and Fischetti V. A., “Sorting of Protein A to the Staphylococcal Cell Wall,” Cell 70, no. 2 (1992): 267–281, 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 7. Sasso E. H., Silverman G. J., and Mannik M., “Human IgM Molecules That Bind Staphylococcal Protein A Contain VHIII H Chains,” Journal of Immunology 142, no. 8 (1989): 2778–2783. [PubMed] [Google Scholar]
- 8. Lindmark R., Thorén‐Tolling K., and Sjöquist J., “Binding of Immunoglobulins to Protein A and Immunoglobulin Levels in Mammalian Sera,” Journal of Immunological Methods 62, no. 1 (1983): 1–13, 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 9. Forsgren A., Svedjelund A., and Wigzell H., “Lymphocyte Stimulation by Protein A of Staphylococcus aureus ,” European Journal of Immunology 6, no. 3 (1976): 207–213, 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- 10. Goodyear C. S. and Silverman G. J., “Staphylococcal Toxin Induced Preferential and Prolonged In Vivo Deletion of Innate‐Like B Lymphocytes,” Proceedings of the National Academy of Sciences of the United States of America 101, no. 31 (2004): 11392–11397, 10.1073/pnas.0404382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peterson P. K., Verhoef J., Sabath L. D., and Quie P. G., “Effect of Protein A on Staphylococcal Opsonization,” Infection and Immunity 15, no. 3 (1977): 760–764, 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sjodahl J., “Repetitive Sequences in Protein A From Staphylococcus Aureus. Arrangement of Five Regions Within the Protein, Four Being Highly Homologous and fc‐Binding,” European Journal of Biochemistry 73, no. 2 (1977): 343–351, 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 13. Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., and Sjödahl J., “Region X, the Cell‐Wall‐Attachment Part of Staphylococcal Protein A,” European Journal of Biochemistry 138, no. 2 (1984): 413–420, 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 14. Graille M., Stura E. A., Corper A. L., et al., “Crystal Structure of a Staphylococcus aureus Protein A Domain Complexed With the Fab Fragment of a Human IgM Antibody: Structural Basis for Recognition of B‐Cell Receptors and Superantigen Activity,” Proceedings of the National Academy of Sciences of the United States of America 97, no. 10 (2000): 5399–5404, 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim H. K., Cheng A. G., Kim H. Y., Missiakas D. M., and Schneewind O., “Nontoxigenic Protein A Vaccine for Methicillin‐Resistant Staphylococcus aureus ,” Journal of Experimental Medicine 207, no. 9 (2010): 1863–1870, 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H. K., Emolo C., DeDent A. C., Falugi F., Missiakas D. M., and Schneewind O., “Protein A‐Specific Monoclonal Antibodies and Prevention of Staphylococcus aureus Disease in Mice,” Infection and Immunity 80 (2012): 3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartleib J., Köhler N., Dickinson R. B., et al., “Protein A Is the Von Willebrand Factor Binding Protein on Staphylococcus aureus ,” Blood 96, no. 6 (2000): 2149–2156. [PubMed] [Google Scholar]
- 18. Nguyen T., Ghebrehiwet B., and Peerschke E. I., “ Staphylococcus Aureus Protein A Recognizes Platelet gC1qR/p33: A Novel Mechanism for Staphylococcal Interactions With Platelets,” Infection and Immunity 68, no. 4 (2000): 2061–2068, 10.1128/IAI.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullam P. M., Bayer A. S., Foss W. M., and Cheung A. L., “Diminished Platelet Binding In Vitro by Staphylococcus aureus Is Associated With Reduced Virulence in a Rabbit Model of Infective Endocarditis,” Infection and Immunity 64, no. 12 (1996): 4915–4921, 10.1128/iai.64.12.4915-4921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gómez M. I., Seaghdha M. O., and Prince A. S., “ Staphylococcus aureus Protein A Activates TACE Through EGFR‐Dependent Signaling,” EMBO Journal 26, no. 3 (2007): 701–709, 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu H., Liu S., Hon K., Psaltis A. J., Wormald P. J., and Vreugde S., “Staphylococcal Protein A Modulates Inflammation by Inducing Interferon Signaling in Human Nasal Epithelial Cells,” Inflammation Research 72, no. 2 (2023): 251–262, 10.1007/s00011-022-01656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. George E. L., Georges‐Labouesse E. N., Patel‐King R. S., Rayburn H., and Hynes R. O., “Defects in Mesoderm, Neural Tube and Vascular Development in Mouse Embryos Lacking Fibronectin,” Development 119, no. 4 (1993): 1079–1091. [DOI] [PubMed] [Google Scholar]
- 23. Ingham K. C., Brew S. A., and Erickson H. P., “Localization of a Cryptic Binding Site for Tenascin on Fibronectin,” Journal of Biological Chemistry 279, no. 27 (2004): 28132–28135. [DOI] [PubMed] [Google Scholar]
- 24. Jumper C. C. and Schriemer D. C., “Mass Spectrometry of Laser‐Initiated Carbene Reactions for Protein Topographic Analysis,” Analytical Chemistry 83, no. 8 (2011): 2913–2920, 10.1021/ac102655f. [DOI] [PubMed] [Google Scholar]
- 25. Pankov R. and Yamada K. M., “Fibronectin at a Glance,” Journal of Cell Science 115, no. Pt 20 (2002): 3861–3863. [DOI] [PubMed] [Google Scholar]
- 26. Maurer L. M., Ma W., and Mosher D. F., “Dynamic Structure of Plasma Fibronectin,” Critical Reviews in Biochemistry and Molecular Biology 51, no. 4 (2015): 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Speziale P., Arciola C. R., and Pietrocola G., “Fibronectin and Its Role in Human Infective Diseases,” Cells 8, no. 12 (2019): 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomer L., Schneewind O., and Missiakas D., “Multiple Ligands of Von Willebrand Factor‐Binding Protein (vWbp) Promote Staphylococcus aureus Clot Formation in Human Plasma,” Journal of Biological Chemistry 288, no. 39 (2013): 28283–28292, 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meenan N. A., Visai L., Valtulina V., et al., “The Tandem Beta‐Zipper Model Defines High Affinity Fibronectin‐Binding Repeats Within Staphylococcus aureus FnBPA,” Journal of Biological Chemistry 282, no. 35 (2007): 25893–25902, 10.1074/jbc.M703063200. [DOI] [PubMed] [Google Scholar]
- 30. Menzies B. E., “The Role of Fibronectin Binding Proteins in the Pathogenesis of Staphylococcus aureus Infections,” Current Opinion in Infectious Diseases 16, no. 3 (2003): 225–229, 10.1097/00001432-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 31. Valentin‐Weigand P., Timmis K. N., and Chhatwal G. S., “Role of Fibronectin in Staphylococcal Colonisation of Fibrin Thrombi and Plastic Surfaces,” Journal of Medical Microbiology 38, no. 2 (1993): 90–95, 10.1099/00222615-38-2-90. [DOI] [PubMed] [Google Scholar]
- 32. Massey R. C., Kantzanou M. N., Fowler T., et al., “Fibronectin‐Binding Protein A of Staphylococcus aureus has Multiple, Substituting, Binding Regions That Mediate Adherence to Fibronectin and Invasion of Endothelial Cells,” Cellular Microbiology 3, no. 12 (2001): 839–851, 10.1046/j.1462-5822.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- 33. Sinha B., François P. P., Nüsse O., et al., “Fibronectin‐Binding Protein Acts as Staphylococcus aureus Invasin via Fibronectin Bridging to Integrin alpha5beta1,” Cellular Microbiology 1, no. 2 (1999): 101–117, 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 34. Boero E., Cruz A. R., Pansegrau W., et al., “Natural Human Immunity Against Staphylococcal Protein A Relies on Effector Functions Triggered by IgG3,” Frontiers in Immunology 13 (2022): 834711, 10.3389/fimmu.2022.834711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pietrocola G., Rindi S., Nobile G., and Speziale P., “Purification of Human Plasma/Cellular Fibronectin and Fibronectin Fragments,” Methods in Molecular Biology 1627 (2017): 309–324, 10.1007/978-1-4939-7113-8_20. [DOI] [PubMed] [Google Scholar]
- 36. Hong D. S., Hui D., Bruera E., et al., “MABp1, a First‐In‐Class True Human Antibody Targeting Interleukin‐1α in Refractory Cancers: An Open‐Label, Phase 1 Dose‐Escalation and Expansion Study,” Lancet Oncology 15, no. 6 (2014): 656–666, 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 37. Jumper C. C., Bomgarden R., Rogers J., Etienne C., and Schriemer D. C., “High‐Resolution Mapping of Carbene‐Based Protein Footprints,” Analytical Chemistry 84, no. 10 (2012): 4411–4418, 10.1021/ac300120z. [DOI] [PubMed] [Google Scholar]
- 38. Manzi L., Barrow A. S., Scott D., et al., “Carbene Footprinting Accurately Maps Binding Sites in Protein‐Ligand and Protein‐Protein Interactions,” Nature Communications 7, no. 1 (2016): 13288, 10.1038/ncomms13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Claro T., Widaa A., O'Seaghdha M., et al., “ Staphylococcus aureus Protein A Binds to Osteoblasts and Triggers Signals That Weaken Bone in Osteomyelitis,” PLoS One 6, no. 4 (2011): e18748, 10.1371/journal.pone.0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hauck C. R. and Ohlsen K., “Sticky Connections: Extracellular Matrix Protein Recognition and Integrin‐Mediated Cellular Invasion by Staphylococcus aureus ,” Current Opinion in Microbiology 9, no. 1 (2006): 5–11, 10.1016/j.mib.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 41. Bear A., Locke T., Rowland‐Jones S., Pecetta S., Bagnoli F., and Darton T. C., “The Immune Evasion Roles of Staphylococcus aureus Protein A and Impact on Vaccine Development,” Frontiers in Cellular and Infection Microbiology 13 (2023): 1242702, 10.3389/fcimb.2023.1242702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jarp J., Mamo W., and Johne B., “Surface Properties of Staphylococcus aureus Isolated From Caprine Mastitis,” Acta Veterinaria Scandinavica 30, no. 3 (1989): 335–339, 10.1186/BF03548040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ingham K. C., Brew S., Vaz D., Sauder D. N., and McGavin M. J., “Interaction of Staphylococcus aureus Fibronectin‐Binding Protein With Fibronectin: Affinity, Stoichiometry, and Modular Requirements,” Journal of Biological Chemistry 279, no. 41 (2004): 42945–42953, 10.1074/jbc.M406984200. [DOI] [PubMed] [Google Scholar]
- 44. Kreikemeyer B., Oehmcke S., Nakata M., Hoffrogge R., and Podbielski A., “Streptococcus Pyogenes Fibronectin‐Binding Protein F2,” Journal of Biological Chemistry 279, no. 16 (2004): 15850–15859. [DOI] [PubMed] [Google Scholar]
- 45. Schwarz‐Linek U., Pilka E. S., Pickford A. R., et al., “High Affinity Streptococcal Binding to Human Fibronectin Requires Specific Recognition of Sequential F1 Modules,” Journal of Biological Chemistry 279, no. 37 (2004): 39017–39025. [DOI] [PubMed] [Google Scholar]
- 46. Grundmeier M., Hussain M., Becker P., Heilmann C., Peters G., and Sinha B., “Truncation of Fibronectin‐Binding Proteins in Staphylococcus aureus Strain Newman Leads to Deficient Adherence and Host Cell Invasion due to Loss of the Cell Wall Anchor Function,” Infection and Immunity 72 (2004): 7155, 10.1128/iai.72.12.7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rozdzinski E., Marre R., Susa M., Wirth R., and Muscholl‐Silberhorn A., “Aggregation Substance‐Mediated Adherence of Enterococcus faecalis to Immobilized Extracellular Matrix Proteins,” Microbial Pathogenesis 30, no. 4 (2001): 211–220, 10.1006/mpat.2000.0429. [DOI] [PubMed] [Google Scholar]
- 48. Viela F., Prystopiuk V., Leprince A., et al., “Binding of Staphylococcus aureus Protein A to Von Willebrand Factor Is Regulated by Mechanical Force,” MBio 10, no. 2 (2019): e00555‐19, 10.1128/mBio.00555-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen X., Sun Y., Missiakis D., and Schneewind O., “ Staphylococcus aureus Decolonization of Mice With Monoclonal Antibody Neutralizing Protein A,” Journal of Infectious Diseases 219, no. 6 (2018): 884–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baba T., Bae T., Schneewind O., Takeuchi F., and Hiramatsu K., “Genome Sequence of Staphylococcus aureus Strain Newman and Comparative Analysis of Staphylococcal Genomes: Polymorphism and Evolution of Two Major Pathogenicity Islands,” Journal of Bacteriology 190 (2008): 300–310, 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Halloran D. P., Wynne K., and Geoghegan J. A., “Protein A Is Released Into the Staphylococcus aureus Culture Supernatant With an Unprocessed Sorting Signal,” Infection and Immunity 83, no. 4 (2015): 1598–1609, 10.1128/IAI.03122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horsburgh M. J., Aish J. L., White I. J., Shaw L., Lithgow J. K., and Foster S. J., “sigmaB Modulates Virulence Determinant Expression and Stress Resistance: Characterization of a Functional rsbU Strain Derived From Staphylococcus Aureus 8325‐4,” Journal of Bacteriology 184, no. 19 (2002): 5457–5467, 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Brien L., Kerrigan S. W., Kaw G., et al., “Multiple Mechanisms for the Activation of Human Platelet Aggregation by Staphylococcus aureus: Roles for the Clumping Factors ClfA and ClfB, the Serine‐Aspartate Repeat Protein SdrE and Protein A,” Molecular Microbiology 44, no. 4 (2002): 1033–1044, 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 54. Aidoudi S., Bujakowska K., Kieffer N., and Bikfalvi A., “The CXC‐Chemokine CXCL4 Interacts With Integrins Implicated in Angiogenesis,” PLoS One 3, no. 7 (2008): e2657, 10.1371/journal.pone.0002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Speziale P. and Pietrocola G., “The Multivalent Role of Fibronectin‐Binding Proteins A and B (FnBPA and FnBPB) of Staphylococcus aureus in Host Infections,” Frontiers in Microbiology 11 (2020): 2054, 10.3389/fmicb.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of bacterial strains used in this study.
Table S2. Amino acid sequences of the recombinant SpA‐C fragment and the Fn N‐terminal domain (N29).
Data Availability Statement
Included in article.


