Abstract
Background
Trihexyphenidyl (THP), an anticholinergic drug used to manage Parkinson’s disease and dystonia, has been associated with oxidative stress and metabolic disturbances, particularly affecting pulmonary function. Long-term exposure to THP may induce lung toxicity through increased oxidative stress, mitochondrial dysfunction, and apoptosis. Coenzyme Q10 (CoQ10), a lipid-soluble antioxidant and mitochondrial cofactor, has been shown to protect against oxidative damage and apoptosis in various models of toxicity. However, its role in mitigating THP-induced pulmonary toxicity remains unexplored. This study investigated the protective effects of CoQ10 against THP-induced pulmonary toxicity in male Wistar rats.
Methods
Thirty-two adult male Wistar rats (180–200 g) were randomly assigned to four groups (n = 8 per group): (i) Control (vehicle-treated), (ii) THP (1.5 mg/kg), (iii) CoQ10 (10 mg/kg), and (iv) THP + CoQ10. Treatments were administered orally once daily for 21 days. Body weight was recorded at baseline and endpoint. At the end of treatment, rats were euthanized, and lungs were excised, weighed, and processed for biochemical and histological analyses. Oxidative stress markers were assessed, including catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), reduced glutathione (GSH), and malondialdehyde (MDA). Metabolic enzymes such as lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH) were measured. Angiotensin-converting enzyme (ACE) activity was evaluated to assess vascular function, while caspase-3 levels were determined as an apoptotic marker. Histopathological examination of lung tissues was performed using hematoxylin and eosin staining.
Results
THP administration resulted in significant weight loss, increased lung weight, oxidative stress (decreased CAT, GPx, SOD, and GSH; increased MDA), and metabolic alterations (elevated LDH, PDH, lactate, and pyruvate). ACE activity was reduced, and caspase-3 was elevated, indicating apoptosis. CoQ10 co-administration mitigated these effects, restoring antioxidant enzyme activity, metabolic balance, and ACE levels while reducing MDA and caspase-3 expression. Histological analysis confirmed that CoQ10 ameliorated THP-induced pulmonary damage.
Conclusion
CoQ10 demonstrated significant protective effects against THP-induced oxidative stress, metabolic disturbances, and apoptosis, likely due to its antioxidant and anti-inflammatory properties. These findings suggest CoQ10 as a potential therapeutic agent for THP-induced pulmonary toxicity, warranting further research.
Clinical trial number
Not applicable.
Keywords: Coenzyme Q10, Trihexyphenidyl, Oxidative stress, Lung toxicity, Antioxidant enzymes, Lactate dehydrogenase, Pyruvate metabolism, Caspase-3
Background
The increasing prevalence of neurodegenerative disorders such as Parkinson’s disease (PD) has led to widespread use of pharmacological agents aimed at managing its symptoms [1–3]. Among these medications, trihexyphenidyl, an anticholinergic agent, remains an essential component of PD therapy due to its ability to alleviate tremors, muscle rigidity, and bradykinesia [4, 5]. By antagonizing muscarinic acetylcholine receptors, trihexyphenidyl effectively reduces excessive cholinergic activity in the central nervous system, thereby improving motor function [6, 7]. However, despite its therapeutic benefits, long-term use of trihexyphenidyl has been associated with adverse effects, including cognitive dysfunction, dry mouth, blurred vision, and most notably, oxidative stress [4, 8]. Oxidative stress results from an imbalance between reactive oxygen species (ROS) production and the body’s antioxidant defense mechanisms, leading to cellular damage [9]. While previous studies have explored oxidative stress as a consequence of trihexyphenidyl treatment in neurological and hepatic tissues [8], its potential impact on pulmonary oxidative stress remains largely underexplored. Given the vital role of the lungs in oxygen exchange and their susceptibility to oxidative injury, investigating pulmonary oxidative stress markers in trihexyphenidyl-exposed models is crucial for understanding its broader systemic effects.
Pulmonary oxidative stress, characterized by excessive ROS production, can contribute to respiratory dysfunction by impairing cellular integrity and promoting inflammatory responses [10]. The lungs are particularly vulnerable to oxidative damage due to their continuous exposure to environmental toxins and high oxygen content [11]. When oxidative stress overwhelms the antioxidant defense system, this can trigger a cascade of deleterious effects, including lipid peroxidation, protein oxidation, and DNA damage, ultimately culminating in pulmonary tissue damage and dysfunction. In the context of trihexyphenidyl exposure, understanding whether the drug induces oxidative damage in lung tissues is essential, as it may have significant implications for individuals undergoing long-term PD treatment [10]. Despite the well-established link between oxidative stress and neurodegenerative diseases, the potential for oxidative stress-induced pulmonary dysfunction in trihexyphenidyl users remains an open question.
Co-enzyme Q10 (CoQ10), a naturally occurring antioxidant, has been widely studied for its protective effects against oxidative stress-related damage in various tissues [12–15]. As a vital cofactor in the electron transport chain, CoQ10 is essential for maintaining optimal mitochondrial energy production and bioenergetic function, facilitating ATP synthesis while simultaneously serving as a potent neutralizer of reactive oxygen species (ROS). Its ability to stabilize mitochondrial function and prevent lipid peroxidation has made it a promising therapeutic agent for conditions characterized by oxidative damage [16, 17]. While CoQ10 has been widely studied for its potential to protect against neurodegenerative disorders, its potential in mitigating oxidative stress-induced pulmonary damage remains relatively unexplored. Given the possibility that trihexyphenidyl induces oxidative stress in the lungs, evaluating the efficacy of CoQ10 as a protective agent could offer valuable insights into novel therapeutic strategies [18–20].
In addition to oxidative stress markers, trihexyphenidyl exposure may also affect key metabolic enzymes involved in cellular respiration and energy production [21]. Lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH) are critical enzymes in metabolic pathways, playing essential roles in glycolysis and the tricarboxylic acid (TCA) cycle, respectively [22, 23]. Disruptions in their activity could indicate metabolic stress and potential mitochondrial dysfunction in pulmonary tissues. Elevated LDH activity is often associated with tissue damage and hypoxia, while alterations in PDH activity can reflect impaired glucose metabolism and mitochondrial respiration [24, 25]. Similarly, lactate and pyruvate levels serve as indicators of metabolic efficiency, with imbalances suggesting potential shifts in energy homeostasis. Investigating how trihexyphenidyl exposure modulates these metabolic markers will provide a deeper understanding of its systemic impact beyond its neurological effects [26–28].
Furthermore, the role of inflammatory responses and programmed cell death in trihexyphenidyl-induced oxidative stress warrants further investigation. Angiotensin-converting enzyme (ACE) is not only central to blood pressure regulation but also implicated in inflammatory responses within the pulmonary system [29]. Increased ACE activity has been associated with oxidative stress and inflammatory lung diseases, suggesting that trihexyphenidyl-induced oxidative damage may involve inflammatory pathways [30]. Additionally, caspase-3, a key executioner of apoptosis, serves as a crucial indicator of programmed cell death in response to oxidative stress. Elevated caspase-3 expression in pulmonary tissues could signify increased apoptotic activity, potentially leading to cellular loss and compromised lung function [31, 32].
This study examines the effects of trihexyphenidyl on pulmonary oxidative stress, metabolic dysfunction, and inflammatory pathways, while evaluating the protective role of CoQ10 in mitigating these effects. By integrating oxidative stress markers, metabolic enzymes, and apoptotic mediators, the research aims to elucidate the mechanisms underlying trihexyphenidyl-induced pulmonary damage and assess the therapeutic potential of CoQ10 in restoring metabolic balance. The findings could bridge knowledge gaps regarding the systemic effects of trihexyphenidyl, highlight the role of antioxidant interventions in neurodegenerative disease management, and facilitate the translation of preclinical findings into clinical practice, ultimately enhancing patient outcomes.
Methods
Chemicals and drugs
Trihexyphenidyl (THP; Pfizer, USA) and Coenzyme Q10 (CoQ10; Sigma-Aldrich, USA) were dissolved in distilled water containing 0.1% dimethyl sulfoxide (DMSO) to ensure solubility. Fresh solutions were prepared weekly and stored at 4 °C in amber vials to prevent photodegradation.
Experimental animals
This study was conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Twenty adult male Wistar rats were used in the study; they are between 12 and 14 weeks, weighing between 160 g and 180 g. Wistar rats were procured from the animal house of the Department of Physiology, Ladoke Akintola University of Technology. The animals were housed in well-ventilated cages lined with dried wood shavings as bedding at the laboratory animal house of the Anchor Biomedical Research Institute, Ogbomoso, Oyo State, where the experiment was conducted. Prior to the study, the rats underwent a two-week acclimatization period, during which they were monitored twice daily. Throughout the study, the animals received a standard laboratory pellet diet and had access to water ad libitum.
Ethical considerations and care of experimental animals
The handling and care of the experimental animals complied with the ethical guidelines outlined by the National Medical Research Council and the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences (National Institute of Health Publication no. 80 − 23, revised 1978). This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the review committee of the Faculty of Basic Medical Sciences Ladoke Akintola University of Techno logy. Ogbomoso, Nigeria with reference number ERCFBMSLAUTECH: 032/05/2014.
Experimental design
The researchers were not blinded to the group assignments, as the study design did not require blinding. The 20 male Wistar rats were randomly divided into four groups of five animals each (n = 5). The treatment groups were as follows:
Experimental design
| Group | Treatment & Dose | Label | Reference |
|---|---|---|---|
| 1 | Distilled water (0.5 mL) | Control | |
| 2 | Trihexyphenidyl (1.5 mg/kg BW) | THP | [33] |
| 3 | CoQ10 (10 mg/kg BW) | CoQ10 | [34] |
| 4 | Trihexyphenidyl (1.5 mg/kg BW) + CoQ10 (10 mg/kg BW) | THP + CoQ10 |
Drugs were administered daily via oral gavage between 8–9 AM
THP dose (1.5 mg/kg): Based on prior studies showing oxidative stress induction at this dose without mortality [33]
CoQ10 dose (10 mg/kg): Selected from literature demonstrating antioxidant efficacy in rodent models [34]
Duration (9 weeks): Reflects chronic THP use in Parkinson’s patients and allows observation of cumulative pulmonary effects
Experimental protocol
At the end of the ninth week of treatment, the animals were euthanized. Euthanasia was performed via intraperitoneal injection after intraperitoneal administration of ketamine (40 mg/kg) and xylazine (4 mg/kg), followed by dissection after confirmation of loss of consciousness. Loss of consciousness was confirmed by absence of pedal and corneal reflexes before proceeding with dissection. Blood samples were collected through cardiac puncture and centrifuged at 3500 rpm for 20 min to obtain serum. The lungs were harvested, with one portion fixed in fixed in 10% neutral-buffered formalin (pH 7.0) for 48 h, then transferred to 70% ethanol and 0.1 M phosphate buffer solution (pH 7.0) for histological analysis, and another portion for biochemistry stored in a freezer until homogenization (10% w/v in PBS, pH 7.4).
Evaluation of body and organ morphometry
Body weight measurement
Body weight measurements were recorded weekly using a digital weighing scale. The final body weight of each animal was recorded before ketamine administration. The change in body weight was calculated using the formula:
Body Weight Change (g) = Final Body Weight - Initial Body Weight
Lung weight measurement
After dissection, the lungs were carefully removed and weighed using a sensitive weighing scale. The relative lung weight was calculated using the formula:
Relative Lung Weight (%) = (Lung Weight (g) / Final Body Weight (g)) × 100
Biochemical assays
All assays were performed in triplicate using fresh supernatants (centrifuged at 3,500 rpm for 20 min at 4 °C). The following biochemical parameters were assessed using standard assay kits according to the manufacturers’ protocols.
Caspase-3 assay
Caspase-3 levels in pulmonary tissue were determined using an ELISA kit (Elabscience, catalog number E-EL-R0160). The assay employed a sandwich ELISA principle. A microplate pre-coated with anti-human caspase-3 antibody was used. Standards and samples were incubated with detection antibody and Avidin-HRP conjugate, followed by substrate addition. The optical density (OD) was measured at a wavelength of 450 nm.
Lactate assay
Lactate concentration was measured using a standard kit from Fortress Diagnostics Ltd, UK (product code BXC0621). The reaction involved lactate oxidation to pyruvate with concomitant formation of hydrogen peroxide, which reacted with chromogenic agents to produce a colored product measured at 540 nm.
Pyruvate assay
Pyruvate concentration was determined using an Elabscience kit. The assay was based on the reaction of pyruvic acid with a chromogenic agent, producing a reddish-brown color in an alkaline solution. The intensity of the color was directly proportional to the pyruvate concentration, measured spectrophotometrically at 505 nm.
Angiotensin-converting enzyme (ACE) assay
Pulmonary ACE levels were determined using an ACE assay kit (Fortress Diagnostics Ltd, UK, product code BXC0176A). The reaction involved the addition of ACE reagent to the sample, incubation, and absorbance measurement at 340 nm.
Superoxide dismutase (SOD) assay
Pulmonary SOD activity was measured based on the inhibition of pyrogallol autoxidation, as described by [35, 36]. Absorbance was read at 420 nm.
Malondialdehyde (MDA) assay
Pulmonary malondialdehyde (MDA) levels were determined using the Thio barbituric acid reactive substances (TBARS) assay. The MDA-Thio barbituric acid complex formed a pink chromophore, which was quantified spectrophotometrically at 532 nm.
Glutathione peroxidase (GPx) assay
Pulmonary glutathione peroxidase (GPx) activity was assessed by measuring the oxidation of reduced glutathione in the presence of cumene hydroperoxide. The reaction involved the oxidation of NADPH to NADP+, which was quantified by measuring the decrease in absorbance at 340 nm.
Reduced glutathione (GSH) assay
The pulmonary reduced glutathione (GSH) concentration was determined using Ellman’s method (1959). This assay relies on the reduction of 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB), resulting in the formation of a yellow chromophore, which was quantified spectrophotometrically at 412 nm.
Catalase (CAT) assay
Pulmonary catalase activity was evaluated by measuring the rate of hydrogen peroxide decomposition. The reaction was terminated with ammonium molybdate, resulting in the formation of a yellow complex that was quantified spectrophotometrically at 405 nm.
Histological preparation of lung tissue
Lung tissue samples were processed for histopathological examination using standard protocols. Samples were fixed in 10% neutral-buffered formalin (pH 7.0) for 48 h., then transferred to 70% ethanol, dehydrated, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin (H&E) according to [37]’s method. Histopathological alterations were examined under a light microscope.
Statistical analysis
Data are mean ± SEM. Normality (Shapiro-Wilk test) and homogeneity (Levene’s test) were confirmed. One-way ANOVA with Tukey’s post-hoc test (GraphPad Prism 10.0) was used; p < 0.05 was significant.
Results
Effect of co-enzyme Q10 on body and lungs weight in male Wistar rats treated with trihexyphenidyl
Body weight change
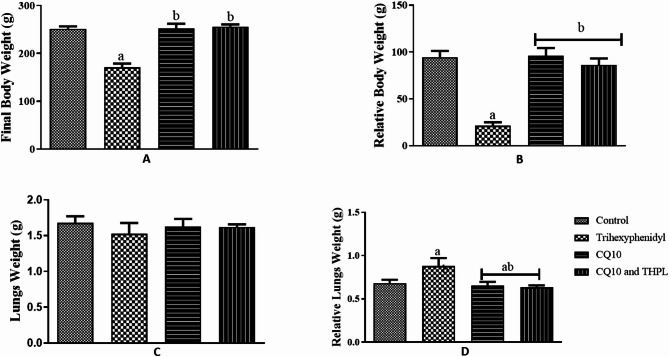
Administration of Trihexyphenidyl (THP) significantly reduced the final body weight of THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated rats (p = 0.0001; f = 44.63). In contrast, CoQ10 administration increased body weight in treated rats compared to THP-treated and control groups. Notably, co-administration of CoQ10 and THP resulted in a significant increase in body weight gain compared to THP treatment alone (Fig. 1A and B).
Fig. 1.
Effect of Co-enzyme Q10 on body weight of male Wistar rats treated with trihexyphenidyl. Values are means of five replicates measurements ± SEM; bars carrying a = significantly different from control; b = significantly different from THP group; c = significantly different from CQ10 group
Relative lungs weight
Exposure to Trihexyphenidyl (THP) significantly increased the relative lung weight of THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated rats (p = 0.0290; F = 3.893). In contrast, CoQ10 administration caused a significant reduction in relative lung weight compared to the control. Notably, co-administration of CoQ10 and THP resulted in a significant increase in relative lung weight gain compared to the control and THP-treated groups (Fig. 1C and D).
Effect of co-enzyme Q10 on oxidative activities of male Wistar rats treated with trihexyphenidyl
Catalase concentration in the lungs
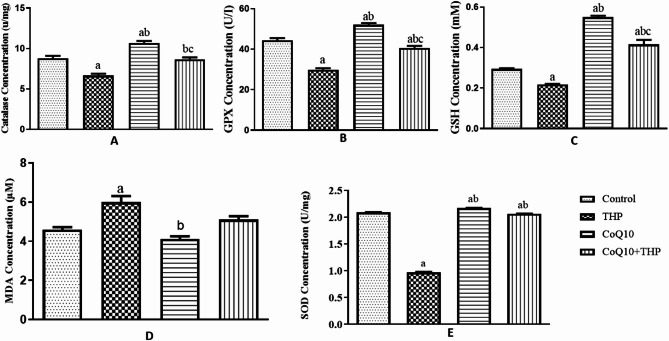
Exposure to Trihexyphenidyl (THP) significantly decreased catalase activity in THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated rats (p = 0.0001, f = 40.22). Conversely, CoQ10 administration significantly increased catalase activity in the lungs of treated rats compared to control and THP-treated groups. Notably, co-administration of CoQ10 and THP resulted in a significant increase in catalase activity compared to THP treatment alone (Fig. 2A).
Fig. 2.
Effect of Co-enzyme Q10 on body weight of male Wistar rats treated with trihexyphenidyl. Values are means of five replicates measurements ± SEM; bars carrying a = significantly different from control; b = significantly different from THP group; c = significantly different from CQ10 group
GPX concentration in the lungs
Exposure to Trihexyphenidyl (THP) significantly decreased glutathione peroxidase (GPX) activity in THP-treated rats compared to control (p = 0.0001, f = 102.9). In contrast, Co-enzyme Q10 administration significantly increased GPX activity in treated rats compared to THP-treated and control groups. However, co-administration of Co-enzyme Q10 and THP resulted in a significant decrease in GPX activity compared to the THP group alone (Fig. 2B).
GSH concentration in the lungs
Exposure to Trihexyphenidyl (THP) significantly decreased reduced glutathione (GSH) concentration in THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated rats (p = 0.0001, f = 147.6). Conversely, CoQ10 administration significantly increased GSH concentration in treated rats compared to THP-treated and control groups. Moreover, co-administration of CoQ10 and THP resulted in a significant increase in GSH concentration compared to control and THP-treated groups (Fig. 2C).
MDA concentration on the lungs
Exposure to Trihexyphenidyl (THP) significantly increased malondialdehyde (MDA) concentration in the lungs of THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated rats (p = 0.001, f = 16.48). In contrast, CoQ10 administration reduced MDA concentration in treated rats compared to THP-treated and control groups. Furthermore, co-administration of CoQ10 and THP resulted in a decrease in MDA concentration compared to THP-treated and CoQ10-treated groups (Fig. 2D).
SOD concentration on the lungs
Exposure to Trihexyphenidyl (THP) significantly decreased superoxide dismutase (SOD) concentration in the lungs of treated rats compared to control (p = 0.001, f = 11542). Conversely, Co-enzyme Q10 administration significantly increased SOD concentration in the lungs of treated rats compared to THP-treated and control groups. Moreover, co-administration of Co-enzyme Q10 and THP resulted in a significant increase in SOD concentration compared to THP treatment alone (Fig. 2E).
Effect of co-enzyme Q10 on lactate and pyruvate of male Wistar rats treated with trihexyphenidyl
Lactate concentration in the serum
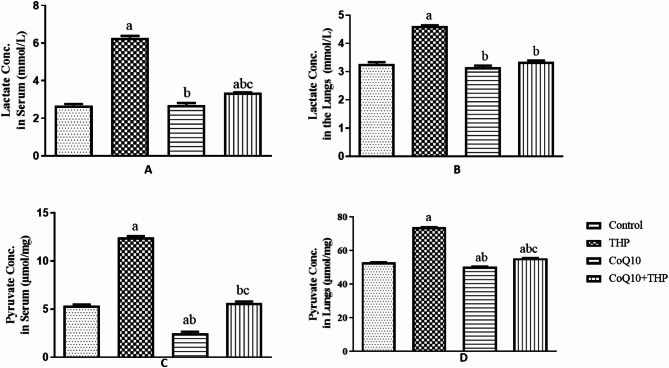
Exposure to Trihexyphenidyl (THP) significantly elevated serum lactate levels in treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated groups (p = 0.0001, f = 348.6). CoQ10 administration altered serum lactate levels in treated rats compared to control and THP-treated groups. Notably, co-administration of CoQ10 and THP resulted in a significant increase in serum lactate levels compared to control and THP-treated groups (Fig. 3A).
Fig. 3.
Effect of Co-enzyme Q10 on body weight of male Wistar rats treated with trihexyphenidyl. Values are means of five replicates measurements ± SEM; bars carrying a = significantly different from control; b = significantly different from THP group; c = significantly different from CQ10 group
Lactate concentration on the lungs
Exposure to Trihexyphenidyl (THP) significantly increased lactate concentration in the lungs of THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated groups (p = 0.0001, f = 184.4). Conversely, CoQ10 administration reduced lactate concentration in the lungs of treated rats compared to THP-treated groups. Moreover, co-administration of CoQ10 and THP resulted in a significant decrease in lactate concentration compared to THP treatment alone (Fig. 3B).
Pyruvate concentration in the serum
Exposure to Trihexyphenidyl (THP) significantly elevated serum pyruvate levels in THP-treated rats compared to control and Co-enzyme Q10 (CoQ10)-treated groups (p = 0.0001, f = 904.6). Conversely, CoQ10 administration reduced serum pyruvate levels in treated rats compared to control. Moreover, co-administration of CoQ10 and THP resulted in a significant decrease in serum pyruvate levels compared to THP treatment alone (Fig. 3C).
Pyruvate concentration in the lungs
Administration of Trihexyphenidyl (THP) significantly increased pyruvate activity in the lungs of THP-treated rats compared to the control group (p = 0.0001, f = 4515). In contrast, Coenzyme Q10 (CoQ10) treatment slightly reduced pyruvate activity compared to the control group, although this difference was not significant. Notably, co-administration of CoQ10 and THP had no significant effect on pyruvate activity compared to the control group (Fig. 3D).
Effect of co-enzyme Q10 on lactate and pyruvate dehydrogenases of male Wistar rats treated with trihexyphenidyl
Effect of coenzyme Q10 in serum lactate dehydrogenase in Trihexyphenidyl-Exposed rats
Administration of Trihexyphenidyl (THP) resulted in a significant increase in serum lactate dehydrogenase (LDH) activity in THP-treated rats compared to control (p = 0.0001, f = 348.6). In contrast, Coenzyme Q10 (CoQ10) treatment showed no significant difference in LDH activity compared to control. However, co-administration of CoQ10 and THP caused a modest increase in LDH activity compared to control. These findings indicate that THP significantly altered LDH levels, while CoQ10 had a minimal effect (Fig. 4A).
Fig. 4.
Effect of Co-enzyme Q10 on body weight of male Wistar rats treated with trihexyphenidyl. Values are means of five replicates measurements ± SEM; bars carrying a = significantly different from control; b = significantly different from THP group; c = significantly different from CQ10 group
Effect of coenzyme Q10 lactate dehydrogenase in lungs homogenate in trihexyphenidyl-exposed rats
Administration of Trihexyphenidyl (THP) significantly increased lactate dehydrogenase (LDH) activity in the lungs of THP-treated rats compared to the control group (p = 0.0001, f = 184.4). In contrast, Coenzyme Q10 (CoQ10) treatment alone showed no significant difference in LDH activity compared to control. However, co-administration of CoQ10 and THP resulted in a modest increase in LDH activity, although this was not significantly different from the control group. These findings indicate that THP altered LDH levels, while CoQ10 had a minimal effect, both alone and in combination with THP (Fig. 4B).
Effect of coenzyme Q10 on serum pyruvate dehydrogenase in trihexyphenidyl-exposed rats
Administration of Trihexyphenidyl (THP) significantly increased serum pyruvate dehydrogenase activity (p = 0.0001, f = 904.6) compared to the control group. Conversely, Coenzyme Q10 (CoQ10) treatment resulted in a substantial decrease in pyruvate dehydrogenase activity compared to control. Notably, co-administration of CoQ10 and THP showed no significant difference in pyruvate dehydrogenase activity compared to control. These findings indicate that THP significantly altered pyruvate dehydrogenase levels, whereas CoQ10 caused a marked decline, and its combination with THP did not differ significantly from the control group (Fig. 4C).
Effect of coenzyme Q10 on pyruvate dehydrogenase in lung homogenate of trihexyphenidyl-exposed rats
Figure 4D shows that Trihexyphenidyl (THP) administration significantly increased pyruvate dehydrogenase activity in the lungs (p = 0.0001, f = 4515) compared to the control group. In contrast, Coenzyme Q10 (CoQ10) treatment resulted in a slight, but significant, decrease in pyruvate dehydrogenase activity compared to control. Notably, co-administration of CoQ10 and THP showed no significant difference in pyruvate dehydrogenase activity compared to control.
Effect of coenzyme Q10 on angiotensin converting enzyme in trihexyphenidyl-exposed rats
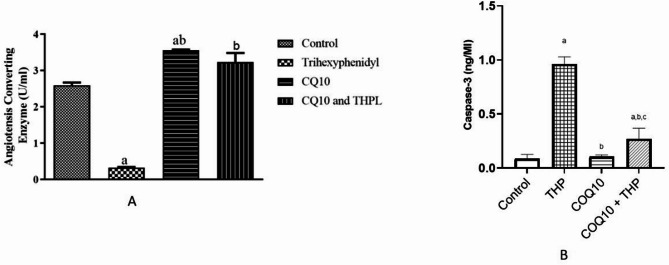
Figure 5a illustrates that Trihexyphenidyl (THP) administration significantly decreased angiotensin-converting enzyme (ACE) activity compared to control (p = 0.0001, f = 124.8). In contrast, Coenzyme Q10 (CoQ10) treatment resulted in a substantial increase in ACE activity compared to control and THP-treated groups. Notably, co-administration of CoQ10 and THP showed a moderate increase in ACE activity compared to control, whereas CoQ10 alone exhibited a marked elevation.
Fig. 5.
Effect of Coenzyme-Q10 administration on ACE and Cascape-3 in trihexyphenidyl exposed rats. Values are means of five replicates measurements ± SEM; bars carrying a = significantly different from control; b = significantly different from THP group; c = significantly different from CQ10 group
Effect of coenzyme Q10 on caspase-3 enzyme in trihexyphenidyl-exposed rats
Figure 5b 6 showed that there is a significant increase in lung Caspase-3 level of rats in THP exposed group and CoQ10 + THP group when compared to the control groups. However, there is a significant decrease in CoQ10 and THP + CoQ10 groups compared to THP group, while, there is a significant increase in caspase-3 level of rats in THP + CoQ10 group compared to CoQ10 group.
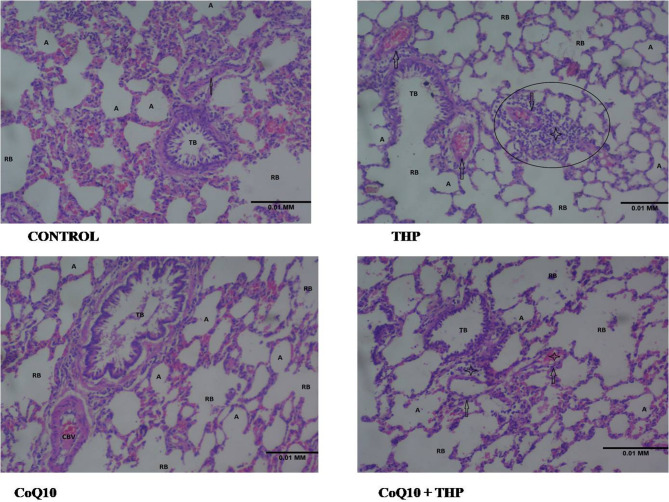
Effect of co-enzyme Q10 on the histology of the lungs of male Wistar rats treated with trihexyphenidyl
The control group displays normal lung architecture, with preserved alveoli (A) lined by typical type I and type II pneumocytes, as well as intact alveolar spaces and interalveolar septa. The branches of the bronchi, including terminal bronchi (TB) and respiratory bronchi (RB), are lined by normal epithelium. The pulmonary vessels (arrow) show no notable abnormalities.
In the THP group, the photomicrograph of lung tissue shows alveoli (A) lined by sloughing type I and type II pneumocytes. The alveolar septa appear thin, resulting in widened alveolar spaces. The bronchiolar branches, including terminal bronchioles (TB) and respiratory bronchioles (RB), are lined by respiratory epithelium. The pulmonary vessels (arrow) generally appear congested, with significant perivascular inflammatory cell infiltration (circled).
The CoQ10 group photomicrograph shows the characteristic fine lace-like appearance of the lung tissue, consisting of well-preserved alveoli (A) lined by unremarkable type I and type II pneumocytes. The interalveolar septa are thin, and the alveolar spaces appear wide. The branches of the bronchi, including terminal bronchioles (TB) and respiratory bronchioles (RB), are lined by respiratory epithelium. A congested blood vessel (CBV) is also observed.
The CoQ10 + THP group shows the characteristic fine lace appearance of the lung tissue, with preserved alveoli (A) lined by unremarkable type I and type II pneumocytes and mild narrowing of interalveolar septa. The branches of the respiratory bronchi (RB) are lined by regular epithelium. The branches of the pulmonary vessels (arrow) appear mildly congested (CV), with moderate perivascular inflammatory cell infiltration (star) (Fig. 6).
Fig. 6.
Representative photomicrograph of a lung section showing the effect of Co-enzyme Q10 on the histology of the lungs of male Wistar rats treated with trihexyphenidyl (H&E ×100)
Discussion
This research examined the potential of Coenzyme Q10 (CoQ10) to counteract the adverse effects of trihexyphenidyl (THP) on the lungs of male Wistar rats, focusing on oxidative stress, metabolic disturbances, and cell death. Various biomarkers, including caspase-3 activity, lactate and pyruvate levels, angiotensin-converting enzyme activity, and oxidative stress indicators, were assessed to determine CoQ10’s protective potential. The results indicate that CoQ10 may safeguard against THP-induced lung damage by leveraging its antioxidant, anti-inflammatory, and mitochondrial energy-boosting properties.
Body weight and lung weight alterations
Contrary to expectations, THP exposure did not significantly affect body weight and relative lung weight compared to the control group, a finding consistent with previous studies [4, 38], which reported that neither THP nor CoQ10 had a significant impact on weight gain, respectively. However, a notable finding in this study is the increase in body weight observed in the CoQ10-treated groups, particularly in the CoQ10 + THP co-administration group. This weight increase suggests that CoQ10 may play a role in energy metabolism and weight maintenance, possibly due to its function in mitochondrial energy production and reduction of oxidative stress, which may contribute to metabolic dysfunction [39].
Interestingly, the THP group showed an increase in relative lung weight, suggesting pulmonary inflammation and edema, potentially caused by oxidative stress and apoptosis. However, the anti-inflammatory and antioxidant properties of CoQ10 appeared to mitigate these effects, as indicated by reduced lung weight in the CoQ10-treated groups. Previous studies have demonstrated that CoQ10 modulates inflammatory pathways, reduces tissue damage, and scavenges reactive oxygen species (ROS), thereby preventing oxidative damage in lung tissue [40].
Pulmonary apoptotic marker (Caspase-3 activity)
One of the significant findings of this study is the elevated caspase-3 activity in THP-exposed rats, indicating increased apoptosis in lung tissue. This result is consistent with previous research by [41], which demonstrated that THP exposure triggers apoptotic pathways through oxidative stress-induced mitochondrial dysfunction. While apoptosis is essential for maintaining cellular homeostasis, excessive apoptotic activity in lung tissues can contribute to pulmonary diseases and compromised lung function [41].
Administration of CoQ10 significantly reduced caspase-3 activity in the THP-exposed group, indicating its protective role against THP-induced apoptosis. This protective effect is likely attributed to CoQ10’s ability to stabilize mitochondrial membranes and enhance electron transport chain (ETC) function. By improving mitochondrial respiration, CoQ10 reduces cytochrome c leakage and subsequent caspase-3 activation, thereby inhibiting apoptotic cell death [19, 20, 42]. Furthermore, CoQ10 has been reported to modulate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, which plays a crucial role in regulating apoptosis and inflammatory responses in lung tissue [39].
ACE activity and pulmonary inflammation
The decreased ACE activity in THP-exposed rats raises concerns about potential cardiovascular and pulmonary implications of THP administration. ACE plays a critical role in the renin-angiotensin system (RAS) by converting angiotensin I to angiotensin II, a potent vasoconstrictor involved in blood pressure regulation and inflammatory responses. The reduction in ACE activity suggests that THP may exert an ACE-inhibitory effect, disrupting RAS homeostasis. This finding aligns with previous research linking ACE inhibition to reduced inflammatory responses in lung tissues, as observed in models of lung injury and pulmonary fibrosis [42].
However, CoQ10 administration significantly restored ACE activity, indicating a compensatory mechanism that helps maintain RAS homeostasis. This restoration may be attributed to CoQ10’s role in modulating mitochondrial bioenergetics, reducing oxidative stress, and preserving ACE function. Additionally, CoQ10’s ability to upregulate endothelial nitric oxide synthase (eNOS) activity may contribute to vascular homeostasis and anti-inflammatory effects in lung tissue [22]. These findings highlight CoQ10 as a potential therapeutic agent for pulmonary conditions associated with ACE dysregulation and oxidative stress.
Lactate and pyruvate metabolism
Lactate and pyruvate concentrations were evaluated as indicators of metabolic function and cellular energy homeostasis. Typically, the lungs produce minimal lactate, as it is primarily formed through pyruvate reduction under anaerobic conditions. However, increased lactate production is often observed in pulmonary diseases, such as acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD) [43].
This study found that THP-exposed rats exhibited significant increases in lactate and pyruvate concentrations, indicating metabolic dysregulation and impaired mitochondrial function. These findings align with studies on metformin, which increases lactate levels by inhibiting mitochondrial respiratory complex I, leading to a shift toward anaerobic glycolysis [44, 45]. The increased lactate production in THP-exposed rats may indicate mitochondrial dysfunction and a shift toward glycolytic metabolism, often observed in conditions of oxidative stress and inflammation.
Administration of CoQ10 significantly reduced lactate and pyruvate concentrations, suggesting its role in restoring mitochondrial function and oxidative phosphorylation. By enhancing pyruvate dehydrogenase activity and facilitating pyruvate conversion to acetyl-CoA, CoQ10 maintains aerobic respiration and reduces lactate accumulation [46]. This metabolic shift may contribute to improved lung function and reduced pulmonary toxicity in THP-exposed rats.
Oxidative stress and antioxidant enzyme activity
Oxidative stress is a major contributor to pulmonary toxicity and inflammation. THP exposure significantly increased malondialdehyde (MDA) levels, a biomarker of lipid peroxidation, while reducing the activities of key antioxidant enzymes, including catalase, glutathione peroxidase (GPX), superoxide dismutase (SOD), and glutathione (GSH). These findings suggest that THP induces oxidative damage by generating excessive reactive oxygen species (ROS), which overwhelm the antioxidant defense system [47].
CoQ10 administration effectively mitigated oxidative stress by reducing MDA levels and restoring antioxidant enzyme activity. CoQ10’s potent antioxidant properties enable it to neutralize ROS and prevent lipid peroxidation, thereby protecting cellular membranes from oxidative damage [13, 48]. Additionally, CoQ10 enhances mitochondrial function, reducing superoxide radical production and improving cellular redox balance [19]. These findings support the potential role of CoQ10 as a therapeutic agent for oxidative stress-related pulmonary disorders.
Histology
Histopathological analysis of lung tissue further corroborated the biochemical findings. The THP-exposed group exhibited mild congestion of pulmonary vessels and inflammatory cell infiltration, indicative of lung tissue damage. These histological changes align with the biochemical evidence of oxidative stress and apoptosis, suggesting that THP-induced pulmonary toxicity is associated with inflammatory and degenerative changes in lung tissue.
CoQ10 treatment significantly ameliorated these histopathological alterations, preserving lung tissue integrity and reducing inflammation. The observed protective effect may be attributed to CoQ10’s ability to modulate inflammatory signaling pathways, enhance mitochondrial function, and reduce oxidative stress, as previously demonstrated [49, 50].
Conclusion
This study provides evidence for the protective role of Coenzyme Q10 (CoQ10) against trihexyphenidyl (THP)-induced pulmonary toxicity in male Wistar rats. CoQ10 administration attenuated oxidative stress markers, reduced caspase-3-mediated apoptotic activity, modulated angiotensin-converting enzyme (ACE) levels, and partially restored metabolic balance, as indicated by decreased lactate and pyruvate accumulation. Histopathological observations further supported these findings, showing reduced inflammation and preserved lung tissue architecture in CoQ10-treated groups. Collectively, these results suggest that CoQ10’s antioxidant, anti-inflammatory, and mitochondrial-stabilizing properties may help counteract THP-associated pulmonary toxicity. However, further mechanistic studies are needed to elucidate the precise pathways involved, and clinical investigations are warranted to assess the translational potential of CoQ10 in patients receiving THP therapy.
Acknowledgements
The authors acknowledge the technical assistance provided by the laboratory staff of the Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria. We also appreciate the contributions of colleagues who provided insights during the research process.
Abbreviations
- ACE
Angiotensin-Converting Enzyme
- ARRIVE
Animal Research: Reporting of In Vivo Experiments
- CAT
Catalase
- CoQ10
Coenzyme Q10
- COPD
Chronic Obstructive Pulmonary Disease
- DTNB
5,5’-Dithiobis-(2-nitrobenzoic acid)
- ELISA
Enzyme-Linked Immunosorbent Assay
- ETC
Electron Transport Chain
- GPx
Glutathione Peroxidase
- GSH
Reduced Glutathione
- H&E
Hematoxylin and Eosin
- LDH
Lactate Dehydrogenase
- MDA
Malondialdehyde
- NF-κB
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- PD
Parkinson’s Disease
- PDH
Pyruvate Dehydrogenase
- RAS
Renin-Angiotensin System
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- TBARS
Thio barbituric Acid Reactive Substances
- THP
Trihexyphenidyl
- TCA
Tricarboxylic Acid (Cycle)
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Joseph Gbenga Omole, Lydia Oluwatoyin Ajayi, Itunuoluwa Rachael Ajewole, Teniola Osholonge, Oyedayo Phillips Akano, and Ayodeji Folorunsho Ajayi. The first draft of the manuscript was written by Ayodeji Folorunsho Ajayi, Joseph Gbenga Omole and Oyedayo Phillips Akano, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The handling and care of the experimental animals complied with the ethical guidelines outlined by the National Medical Research Council and the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences (National Institute of Health Publication no. 80 − 23, revised 1978). This study was performed in accordance with the principles of the Declaration of Helsinki. Ethical approval was granted by the review committee of the Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria, with reference number ERCFBMSLAUTECH: 032/05/2014.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gadhave DG, Sugandhi VV, Jha SK, Nangare SN, Gupta G, Singh SK, et al. Neurodegenerative disorders: mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res Rev. 2024;99:102357. [DOI] [PubMed] [Google Scholar]
- 2.Kouli A, Torsney KM, Kuan WL. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK536722/ [PubMed]
- 3.Zahoor I, Shafi A, Haq E. Pharmacological Treatment of Parkinson’s Disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK536726/
- 4.Jilani TN, Sabir S, Patel P, Sharma S. Trihexyphenidyl. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK519488/ [PubMed]
- 5.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of parkinson´s disease. Cochrane Database Syst Rev. 2002;2002(3):CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naji A, Gatling JW. Muscarinic Antagonists. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557541/ [PubMed]
- 7.Szczurowska E, Szánti-Pintér E, Chetverikov N, Randáková A, Kudová E, Jakubík J. Modulation of muscarinic signalling in the central nervous system by steroid hormones and neurosteroids. Int J Mol Sci. 2022;24(1):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baslam A, Azraida H, Aboufatima R, Ait-El-Mokhtar M, Dilagui I, Boussaa S, et al. Trihexyphenidyl alters its host’s metabolism, neurobehavioral patterns, and gut Microbiome feedback Loop—The modulating role of Anacyclus pyrethrum. Antioxidants. 2023;13(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzal S, Abdul Manap AS, Attiq A, Albokhadaim I, Kandeel M, Alhojaily SM. From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol. 2023;14:1269581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezerra FS, Lanzetti M, Nesi RT, Nagato AC, Silva CP, e, Kennedy-Feitosa E, et al. Oxidative stress and inflammation in acute and chronic lung injuries. Antioxidants. 2023;12(3):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers LK, Cismowski MJ. Oxidative stress in the Lung – The essential paradox. Curr Opin Toxicol. 2018;7:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AL-Megrin WA, Soliman D, Kassab RB, Metwally DM, Ahmed E, Abdel Moneim, El-Khadragy MF, Coenzyme Q. 10 Activates the Antioxidant Machinery and Inhibits the Inflammatory and Apoptotic Cascades Against Lead Acetate-Induced Renal Injury in Rats. Front Physiol [Internet]. 2020 Feb 7 [cited 2025 Feb 11];11. Available from: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2020.00064/full [DOI] [PMC free article] [PubMed]
- 13.Silva SV e, Gallia MC, da Luz JRD, de Rezende AA, Bongiovanni GA, Araujo-Silva G, et al. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients. 2022;14(16):3265. [DOI] [PMC free article] [PubMed]
- 14.Ulla A, Mohamed MK, Sikder B, Rahman AT, Sumi FA, Hossain M, et al. Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol Toxicol. 2017;18(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Huang X, Liu N, Liu M, Sun C, Qi B, et al. Discovering the potential value of coenzyme Q10 in oxidative stress: enlightenment from a synthesis of clinical evidence based on various population. Front Pharmacol. 2022;13:936233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallotti F, Bergamini C, Lamperti C, Fato R. The roles of coenzyme Q in disease: direct and indirect involvement in cellular functions. Int J Mol Sci. 2021;23(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suárez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I, Munuera-Cabeza M, et al. Coenzyme Q10 analogues: benefits and challenges for therapeutics. Antioxidants. 2021;10(2):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagheri S, Haddadi R, Saki S, Kourosh-Arami M, Rashno M, Mojaver A, et al. Neuroprotective effects of coenzyme Q10 on neurological diseases: a review article. Front Neurosci [Internet]. 2023 Jun 23 [cited 2025 Feb 11];17. Available from: https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2023.1188839/full [DOI] [PMC free article] [PubMed]
- 19.Gutierrez-Mariscal FM, Arenas-de Larriva AP, Limia-Perez L, Romero-Cabrera JL, Yubero-Serrano EM, López-Miranda J. Coenzyme Q10 supplementation for the reduction of oxidative stress: clinical implications in the treatment of chronic diseases. Int J Mol Sci. 2020;21(21):7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young AJ, Johnson S, Steffens DC, Doraiswamy PM. Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr. 2007;12(1):62–8. [DOI] [PubMed] [Google Scholar]
- 21.Dolgacheva LP, Zinchenko VP, Goncharov NV. Molecular and cellular interactions in pathogenesis of sporadic Parkinson disease. Int J Mol Sci. 2022;23(21):13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farhana A, Lappin SL, Biochemistry. Lactate Dehydrogenase. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557536/ [PubMed]
- 23.Shakespear MR, Iyer A, Cheng CY, Das Gupta K, Singhal A, Fairlie DP, et al. Lysine deacetylases and regulated Glycolysis in macrophages. Trends Immunol. 2018;39(6):473–88. [DOI] [PubMed] [Google Scholar]
- 24.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71(14):2577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in Cancer cells. J Biol Chem. 2008;283(33):22700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian X, Wang Q, Wang Y, Lou S. The function of previously unappreciated exerkines secreted by muscle in regulation of neurodegenerative diseases. Front Mol Neurosci [Internet]. 2024 Jan 5 [cited 2025 Feb 11];16. Available from: https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2023.1305208/full [DOI] [PMC free article] [PubMed]
- 27.Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng S, Wang Y, Ying M, Jin C, Ying C, Wang D, et al. Elucidating the kinetic and thermodynamic insight into regulation of glycolysis by lactate dehydrogenase and its impact on tricarboxylic acid cycle and oxidative phosphorylation in cancer cells. eLife [Internet]. 2024 Sep 25 [cited 2025 Feb 11];13. Available from: https://elifesciences.org/reviewed-preprints/99576
- 29.Herman LL, Padala SA, Ahmed I, Bashir K. Angiotensin-Converting Enzyme Inhibitors (ACEI). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Feb 11]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK431051/ [PubMed]
- 30.Fang Y, Gao F, Liu Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM Mon J Assoc Physicians. 2019;112(12):914–24. [DOI] [PubMed] [Google Scholar]
- 31.Elmore S, Apoptosis. A review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119(1):3–19. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh WA. Prophylactic use of trihexyphenidyl (Artane) in schizophrenia and psychosis: A critical review of literature to guide for evidence based practice in Zambia. Med J Zambia. 2019;46(2):133–9. [Google Scholar]
- 34.El-khadragy M, Al-Megrin WA, AlSadhan NA, Metwally DM, El-Hennamy RE, Salem FEH, et al. Impact of coenzyme Q10 administration on lead Acetate-Induced testicular damage in rats. Oxid Med Cell Longev. 2020;2020(1):4981386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SJ, Han D, Moon KD, Rhee JS. Measurement of superoxide dismutase-like activity of natural antioxidants. Biosci Biotechnol Biochem. 1995;59(5):822–6. [DOI] [PubMed] [Google Scholar]
- 36.Magnani L, Gaydou EM, Hubaud JC. Spectrophotometric measurement of antioxidant properties of flavones and flavonols against superoxide anion. Anal Chim Acta. 2000;411(1):209–16. [Google Scholar]
- 37.Humason GL. Animal tissue techniques. San Franc WH Freeman. 1962;468.
- 38.Saboori S, Rad EY, Mardani M, Khosroshahi MZ, Nouri Y, Falahi E. Effect of Q10 supplementation on body weight and body mass index: A systematic review and meta-analysis of randomized controlled clinical trials. Diabetes Metab Syndr. 2019;13(2):1179–85. [DOI] [PubMed] [Google Scholar]
- 39.Mantle D, Hargreaves IP, Domingo JC, Castro-Marrero J. Mitochondrial dysfunction and coenzyme Q10 supplementation in Post-Viral fatigue syndrome: an overview. Int J Mol Sci. 2024;25(1):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Liu S, Jiang J, Zhang Y, Luo Y, Zhao J, et al. CoQ10 enhances the efficacy of airway basal stem cell transplantation on bleomycin-induced idiopathic pulmonary fibrosis in mice. Respir Res. 2022;23(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iscra F, Gullo A, Biolo G. Bench-to-bedside review: lactate and the lung. Crit Care Lond Engl. 2002;6(4):327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65(2):20–9. [DOI] [PubMed] [Google Scholar]
- 45.Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005;20(5):255–71. [DOI] [PubMed] [Google Scholar]
- 46.Bossardi Ramos R, Adam AP. Molecular mechanisms of vascular damage during lung injury. Adv Exp Med Biol. 2021;1304:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awang Daud DM, Ahmedy F, Baharuddin DMP, Zakaria ZA. Oxidative stress and antioxidant enzymes activity after cycling at different intensity and duration. Appl Sci. 2022;12(18):9161. [Google Scholar]
- 48.Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78(8):803–11. [DOI] [PubMed] [Google Scholar]
- 49.Farsi F, Heshmati J, Keshtkar A, Irandoost P, Alamdari NM, Akbari A, et al. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;148:104290. [DOI] [PubMed] [Google Scholar]
- 50.Sifuentes-Franco S, Sánchez-Macías DC, Carrillo-Ibarra S, Rivera-Valdés JJ, Zuñiga LY. Sánchez-López VA. Antioxidant and Anti-Inflammatory effects of coenzyme Q10 supplementation on infectious diseases. Healthcare. 2022;10(3):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.