Abstract
Mason-Pfizer monkey virus (M-PMV) encodes a transmembrane glycoprotein with a 38-amino-acid-long cytoplasmic tail. After the release of the immature virus, a viral protease-mediated cleavage of the cytoplasmic tail (CT) results in the loss of 17 amino acids from the carboxy terminus and renders the envelope protein fusion competent. To investigate the role of individual amino acid residues in the CT in fusion, a series of mutations was introduced, and the effects of these mutations on glycoprotein biosynthesis and fusion were examined. Most of the alanine-scanning mutations in the CT had little effect on fusion activity. However, four amino acid substitutions (threonine 4, lysine 7, glutamine 9, and isoleucine 10) resulted in substantially increased fusogenicity, while six (leucine 2, phenylalanine 5, isoleucine 13, lysine 16, proline 17, and glycine 31) resulted in much-reduced fusion. Interestingly, the bulk of these mutations are located upstream of the CT cleavage site in a region that has the potential to form a coiled-coil in the Env trimer. Substitutions at glutamine 9 and isoleucine 10 with alanine had the most dramatic positive effect and resulted in the formation of large syncytia. Taken together, these data demonstrate that individual residues within the cytoplasmic domain of M-PMV Env can modulate, in both a positive and negative manner, biological functions that are associated with the extracellular domains of the glycoprotein complex.
The glycoprotein complex of retroviruses consists of two polypeptides: the external, highly glycosylated, hydrophilic, surface (SU) glycoprotein and the transmembrane or TM glycoprotein. The glycoproteins are initially translated as a polyprotein precursor from a spliced envelope (env) gene-specific mRNA. The polyprotein precursor Env is proteolytically cleaved during its transport through the Golgi complex to the plasma membrane of the cell (19, 28, 30, 58, 75). While Env proteins are not required for the formation of virus particles, they play an important role in the virus replication cycle. The SU glycoprotein is responsible for receptor binding for the virus, whereas the TM glycoprotein is responsible for anchoring the SU protein at the surface of the infected cell or the virus membrane (30, 41, 48, 61). The TM glycoprotein also mediates virus-cell membrane fusion during virus entry as well as cell-cell fusion via a fusion peptide and heptad repeat motifs that are located in the extracellular domain (15, 64, 72). The two heptad repeat regions located in the TM proteins of viruses such as paramyxovirus (6, 9, 33, 53), influenza virus (73), coronavirus (13, 39), and retroviruses (23, 24) have been shown to play an essential role in viral fusion and infectivity (10, 15, 25, 59, 64, 70).
The glycoproteins of different retroviruses have structural similarity while their sizes and amino acid compositions differ. The amino terminus of the Env precursors contains the signal peptide, a short stretch of hydrophobic amino acids that directs the entry of the newly synthesized env gene protein into the secretory pathway. At the C terminus is a hydrophobic region that stops the process of translocation of the precursor glycoprotein through the endoplasmic reticulum membrane, anchors the protein in the membrane, and causes it to span the lipid bilayer. This region is located within the TM glycoprotein. The glycoprotein is thus oriented with its N terminus outside and its C terminus in the cytoplasm, a type I glycoprotein. A third hydrophobic domain, the fusion peptide, is located at the N terminus of the TM glycoprotein (22). N-terminal to this domain is a conserved basic amino acid sequence (Arg-X-Lys-Arg) which directs proteolytic cleavage of the precursor protein by a cellular endopeptidase located in the Golgi apparatus (30).
To date, the bulk of evidence has identified regions within the extracellular domain of Env as playing key roles in fusion. The process is triggered either by receptor binding of the SU protein or by mildly acidic pH during receptor-mediated endocytosis (26, 43). Conformational changes induced by these interactions allow the fusion peptide to insert into target cell membrane (21, 29, 54). Additional conformational changes involve a transition to a six-helix bundle structure in which the N-terminal heptad repeats create an extended, triple-stranded, coiled-coil into which the C-terminal heptad repeats are closely packed. The formation of this structure is hypothesized to bring the viral and cellular membranes in apposition, allowing lipid mixing to occur (17, 42, 54).
The membrane-spanning domain is followed by a stretch of amino acids known as the cytoplasmic tail (CT). This region is located in the cytoplasm of the infected cell and in the interior of the virions. The CT of retrovirus Envs is generally quite short, ranging from 20 to 40 amino acids, but the TM proteins of lentiviruses have unusually long cytoplasmic tails (16, 32) and in simian immunodeficiency virus and human immunodeficiency virus (HIV) they exceed 150 amino acids. The CT of the TM protein for most retroviruses determines the rate of endocytosis of the glycoprotein at the plasma membrane through well-conserved tyrosine-based endocytosis motifs (3, 14, 47, 57, 63), mediates interactions with assembled Gag precursors (12, 20, 46), and plays a role in incorporation of glycoprotein into budding virions (4, 50, 56, 63, 71). Unlike lentiviral Env proteins, the cytoplasmic tail of Mason-Pfizer monkey virus (M-PMV) is only 38 amino acids long and, following virus release, is cleaved by the virus-encoded protease (5, 62, 63). This results in conversion of gp22 into gp20 in the virus particle. Proteolytic cleavage of the cytoplasmic tail during virus maturation has also been observed in murine leukemia virus (MuLV) and equine infectious anemia virus (2, 11, 27, 52).
In M-PMV, maturational cleavage of the CT occurs between a histidine at position 21 and a tyrosine at position 22 and results in the loss of 17 amino acids from the carboxyl terminus. We have demonstrated previously that truncation of the cytoplasmic tail to amino acid residue 22 enhanced fusogenicity dramatically and blocked incorporation of the mutant glycoprotein into virions, thereby abrogating virus infectivity (63). These results suggested that postassembly cleavage of the cytoplasmic tail removes a necessary incorporation signal and activates fusion activity. Thus, for M-PMV the CT constitutes a regulatory switch for the viral life cycle (49, 55). In this study, to further investigate the biological roles of cytoplasmic tail on the fusion activity of the glycoprotein, we introduced a series of mutations into the cytoplasmic tail and determined the effect of these mutations on the biosynthesis, transport, and cell fusion activity of the M-PMV glycoprotein. The results of this study indicate that single amino acids at several positions within the CT can play critical roles in modulating the fusion activity of the intact Env protein.
MATERIALS AND METHODS
Cell and transfections.
African green monkey kidney cells (COS-1) were obtained from the American Type Culture Collection. The HOS-CD4/LTR-hGFP (GHOST) cell line, which expresses green fluorescent protein (GFP) under the control of the HIV type 2 (HIV-2) long terminal repeat (LTR), was obtained though the AIDS Reference and Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, and was originally contributed by Vineet N. KewalRamani and Dan R. Littman (7, 44). Cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Sigma), 10 U of penicillin G sodium/ml, and 10 μg of streptomycin sulfate/ml (Pen-Strep; GibcoBRL). The HOS-CD4/LTR-hGFP cells were additionally maintained in medium containing hygromycin and G418 (Geneticin) as recommended by the contributor. Each mutant DNA was transfected into COS-1 cells using FuGENE6 (Roche Molecular Biochemicals).
Plasmid construction and site-directed mutagenesis.
The pTMT plasmid is a vector that expresses M-PMV Env and HIV-1 Tat as described previously (64). In order to introduce alanine-scanning, substitution, and nonsense mutations at specific amino acid residues within the cytoplasmic tail (residues 2 to 33), site-directed mutagenesis was carried out using the mega primer PCR method. A 2.596-kb EcoRI fragment encompassing the entire env gene and the 3′ LTR regions from pTMT was purified and used as a template for PCR mutagenesis. In the first PCR step, a reverse primer (5′-GCCAAGACATCATCCG), consisting of nucleotides 7844 to 7830 within the 3′ LTR region (65), was used in combination with mutagenic primers containing the desired mutation for each corresponding site in order to amplify the DNA sequence. After the first PCR step, all the PCR products were purified. For the second PCR step, a primer (5′-CAGAGCAGGGAGGTATC) consisting of nucleotides 7251 to 7267 within the gp22 coding sequence was used in combination with the first PCR products as a reverse primer. The second-round PCR products were digested with BlpI (nucleotide 7270) and BspEI (nucleotide 7813) followed by ligation into the glycoprotein expression vector pTMT. The nucleotide sequence of the mutated region of each cytoplasmic tail construct was determined to confirm that only a single amino acid change was introduced. Constructs were designated pTMT followed by the position of the mutation outlined above. For example, pTMT K16A is a mutated TM protein in which the lysine at position 16 of the cytoplasmic domain has been replaced by alanine.
Protein expression, radioactive labeling, and immunoprecipitation.
COS-1 cells in 60-mm-diameter plates were transfected with 2 μg of pTMT plasmids containing either wild-type or mutant M-PMV env genes by the FuGENE6 method (Roche Molecular Biochemicals). At 24 h posttransfection, cells were divided into two sets of 60-mm-diameter plates, one for pulse and the other for pulse-chase labeling. At 48 h posttransfection, the cells were starved in leucine-free DMEM for 90 min and then pulse-labeled in leucine-free DMEM supplemented with [3H]leucine (1 mCi/ml, 0.25 ml/plate; Perkin-Elmer NEN) for 30 min. At this point one set of pulse-labeled cells was lysed with lysis buffer A on ice (1% Triton X-100, 15% sodium deoxycholate, 0.15 M NaCl, 0.05 M Tris, pH 7.5). The remaining set was then chased in complete DMEM for an additional 4 h prior to lysis of the cells. Using a goat anti-M-PMV antibody, viral proteins were immunoprecipitated from the cell lysates and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Biotinylation of cell surface proteins.
Cell surface proteins were detected by a surface biotinylation assay based on the method of Lisanti et al. (38). Following pulse-chase radiolabeling, as described above, the transfected COS-1 cells were placed on ice and washed three times with ice-cold PBS-C/M (phosphate-buffered saline containing 0.1 mM CaCl2 and 1 mM MgCl2), prior to incubation with PBS-C/M containing 0.5 mg/ml of sulfo-NHS-LC-biotin (Pierce) for 30 min on ice. The cells were washed twice with ice-cold PBS-C/M containing 50 mM NH4Cl, once with ice-cold PBS-C/M, and then lysed in lysis buffer A and divided into two equal aliquots. Both aliquots were immunoprecipitated using goat anti-M-PMV antiserum as described above. One aliquot was further treated with 10 μl of 10% SDS and heated at 95°C for 5 min to release bound glycoproteins. The dissociated proteins were added to 1 ml of lysis buffer A and incubated with 30 μl of streptavidin-agarose beads (Pierce) at 4°C overnight. The bound biotinylated samples were washed two times with lysis buffer B (lysis buffer A with 0.1% SDS), once with 20 mM Tris-HCl (pH 6.8), and then heated at 95°C for 5 min in the presence of gel loading buffer before comparing with the immunoprecipitated aliquot by analysis on 12% SDS-PAGE.
Fusion assays of wild-type and mutant M-MPV Env proteins.
The fusion activity of M-PMV Env proteins was determined as described previously (64). Briefly, the glycoprotein expression vector pTMT containing either wild-type or mutant M-PMV env genes was transfected into COS-1 cells by the FuGENE6 method (Roche Molecular Biochemicals) for analysis in the cell fusion assay. At 24 h posttransfection, the COS-1 cells were trypsinized, mixed at a 1:2 ratio with untransfected HOS-CD4/LTRhGFP indicator cells, and replated in a six-well plate. After 36 h of incubation, cells were washed twice with deficient PBS (PBS lacking 0.1 mM CaCl2 and 1 mM MgCl2) and then 500 μl of PBS-1 mM EDTA was added to dissociate the cells. The resuspended cells were analyzed for GFP expression by flow cytometry.
Fluorescence dye redistribution fusion assay.
COS-1 cells were plated to 50% confluence in six-well plates and then cotransfected with the glycoprotein expression vector pTMT containing either wild-type or mutant M-PMV env genes and pIRES2-EGFP by using FuGENE6 as described above. JC53-BL target cells were trypsinized and washed in PBS and then loaded with the cytoplasmic dye CellTracker Blue (Molecular Probes), at a 1 μM final concentration in PBS, for 90 min at 37°C. Fast DiI (Molecular Probes) was added (0.4 μM final concentration), and cells were incubated for 10 min at 37°C. Cells were collected by centrifugation and washed three times with PBS and then added to transfected COS-1 cells at a 5:1 ratio in 1 ml DMEM plus 10% fetal bovine serum. The cells were incubated together for 2 h at 37°C and then washed once with PBS, trypsinized, washed two times with PBS, and finally fixed in 4% paraformaldehyde. After fixation, they were analyzed by flow cytometry. Briefly, cells were gated on the GFP-positive population (marker for cells expressing envelope) and then analyzed for the percentage of Fast DiI- and CellTracker Blue-positive cells. Cells that were positive for GFP, Fast DiI, and CellTracker Blue were determined to be fused cells.
RESULTS
Construction of mutants in the cytoplasmic tail of the env gene.
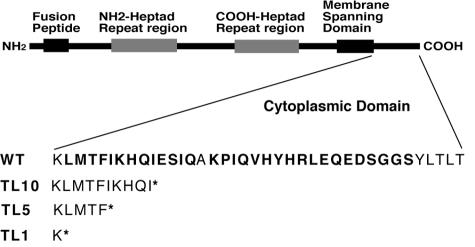
We demonstrated previously that introduction of truncation mutations into the CT of M-PMV TM increased membrane fusion activity, indicating that this region of the CT can inhibit membrane fusion activity. To further investigate the mechanism of this regulatory effect of the CT, we introduced a series of mutations, individual alanine-scanning and truncation mutations, into specific residues of the cytoplasmic domain of the TM protein from leucine at position 2 to serine at position 33 (Fig. 1). This segment of the cytoplasmic domain is conserved among D-type retroviruses and spans a region that previous truncation analyses had implicated in both mediating viral glycoprotein incorporation and regulating fusogenicity. All of the mutations were introduced by PCR mutagenesis, as described in Materials and Methods, and sequence analysis confirmed that no additional mutations had occurred during the PCR amplification. The mutant env genes were subsequently cloned into an M-PMV glycoprotein expression vector, pTMT.
FIG. 1.
Schematic diagram of M-PMV TM protein organization. The amino acid sequence of the cytoplasmic tail is shown. Amino acid residues that were substituted with alanines in this study (position 2 to position 33 of the cytoplasmic domain of the TM protein) are shown in bold. The designation of the truncation mutants is given on the left. The * indicates a stop codon introduced on that site to generate a truncation mutation.
Envelope protein synthesis and processing of the cytoplasmic tail mutants.
To study the effects of the substitution mutations on the biosynthesis and processing of the M-PMV glycoprotein, the mutant env genes were inserted into the glycoprotein expression vector pTMT. This vector has been shown to direct the synthesis of high levels of glycoprotein when transfected into COS-1 cells. Two days after transfection of the wild-type or mutant env expression vectors, the transfected cells were starved in leucine-free medium for 90 min, labeled for 30 min with [3H]leucine, and then further incubated for 4 h in the presence of complete medium. The Env proteins were immunoprecipitated from cell lysates with goat anti-M-PMV polyclonal antibody followed by SDS-PAGE analysis.
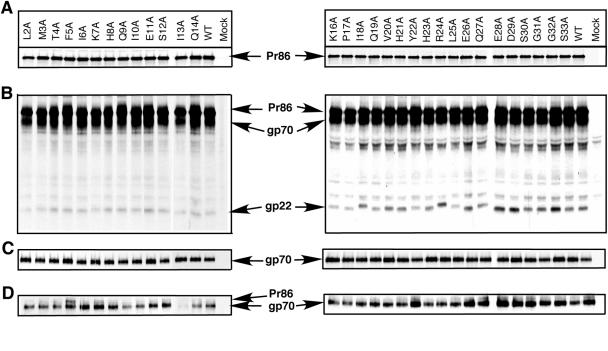
The results of a pulse-chase experiment in COS-1 cells transfected with pTMT, as well as with 31 other constructs containing cytoplasmic domain mutations, are shown in Fig. 2. The Env precursor Pr86 was translated during the 30-min pulse, and similar levels of expression were observed for each mutant and the wild type (Fig. 2A). During the chase, the wild-type and mutated env gene precursor proteins were similarly processed to the mature proteins, gp70 and gp22 (Fig. 2B). The gp22 of the I18A, Y22A, R24A, L25A, Q27A, and S30A mutants migrated more slowly than the wild-type TM protein, which presumably reflects conformational or charge changes that are reflected in SDS-PAGE. Since the cleavage of the glycoprotein precursor into gp70 and gp22 subunits occurs late in the Golgi complex, none of the mutations appear to alter intracellular transport of the glycoprotein at least through the Golgi complex.
FIG. 2.
Synthesis and processing of mutant and wild-type glycoproteins. (A) COS-1 cells were transfected with the pTMT expression vector containing either the wild-type or mutant env genes; at 48 h posttransfection, the cells were labeled with [3H]leucine for 30 min and immunoprecipitated with goat anti-M-PMV serum as described in the text. The mutant designation is shown above each lane, and the position of the precursor glycoprotein, Pr86, is indicated in the center. (B) Processing of the Env polyprotein precursor protein. Following a 4-h chase in unlabeled medium, Env proteins were immunoprecipitated with goat anti-M-PMV serum and analyzed by 12% SDS-PAGE. The positions of the precursor glycoprotein, Pr86, and the cleavage products, gp70 and gp22, are shown. (C) Secretion of SU into culture media. Following pulse-chase, culture media were collected, immunoprecipitated with goat anti-M-PMV serum, and analyzed for the released SU proteins. (D) Surface biotinylation of envelope glycoproteins. Transfected cells expressing radiolabeled glycoprotein were biotinylated and immunoprecipitated with goat anti-M-PMV serum. The immunoprecipitated samples were boiled in SDS, and streptavidin-agarose beads were added to isolate biotinylated Env proteins. The biotinylated Env proteins were then analyzed by SDS-PAGE. Mutations are designated by the standard one-letter code of the wild-type amino acid followed by its numerical position within the cytoplasmic domain and then the resulting mutant amino acids. The mutant designation is shown above each lane, and the positions of the viral bands are indicated in the middle.
A small fraction of the gp70 SU protein of M-PMV is secreted into the culture medium when Env is expressed in the COS-1 cells. Labeled gp70 was therefore immunoprecipitated from the culture medium after a 4-h chase to determine whether any of the mutations affected the stability of interactions between gp70 and gp22. A similar level of gp70 was secreted for each mutant and wild type (Fig. 2C). These data indicate that the alanine mutations we introduced into the cytoplasmic tail do not affect the normal expression, processing, and transport of the M-PMV glycoproteins.
To investigate whether the mutations in the cytoplasmic tail affect the ability of the Env complex to be transported to and expressed on the plasma membrane, a surface biotinylation assay was performed. COS-1 cells were transfected with pTMT containing either wild-type or mutant env genes, and at 48 h posttransfection, the cells were labeled with [3H]leucine for 30 min and then chased for 4 h. After the chase, labeled glycoprotein that had been transported to and was resident in the plasma membrane was tagged with the membrane-impermeable sulfo-NHS-LC-biotin as described in Materials and Methods. The entire complement of labeled viral proteins within the lysates of biotinylated cells was first immunoprecipitated with goat anti-M-PMV antibody, and then biotinylated proteins present in one aliquot of the immunoprecipitate were selected following reprecipitation with streptavidin-agarose beads (Fig. 2D).
Similar levels of surface-expressed Env were observed for each of the mutant and wild-type Envs, although the Q9A and I13A mutants exhibited consistently low levels of SU glycoprotein on the plasma membrane surface compared to the wild type. Interestingly, in cells expressing the F5A mutant the precursor glycoprotein, Pr86, was always detected along with processed SU protein in this surface biotinylation assay.
Effects of mutations in the cytoplasmic tail on cell fusion activity of the M-PMV Env protein.
To test whether the alanine substitutions in the CT affected the fusogenicity of the M-PMV Env protein, COS-1 cells were transfected with the Env expression vector pTMT, which encodes the wild-type or mutant env genes and HIV Tat. As a negative control, COS-1 cells were transfected with a pTMTΔEnv vector that encodes only HIV-1 Tat. GHOST cells that have the cellular receptor for M-PMV glycoprotein and encode a GFP gene under the control of the HIV-2 LTR promoter were used as indicator cells. The pTMT vector can express high levels of both the HIV-1 Tat protein under the control of the simian virus 40 early promoter and M-PMV Env in the transfected COS-1 cells. Thus, fusion of COS-1 cells transfected with the pTMT vector with a GHOST cell results in activation of GFP expression.
At 24 h posttransfection, the COS-1 cells were trypsinized and mixed with GHOST cells at a ratio 1:2. After 36 h, the cells were resuspended and analyzed for the number of the GFP-positive fused cells by gating on larger-than-single cells using flow cytometry. This allowed a quantitative analysis of fusion for each of the mutants.
The relative fusion efficiencies of each of the mutants compared to wild type were averaged from three independent experiments and are shown in Fig. 3. For these calculations, background fusion observed with the negative (pTMTΔEnv) control was deducted from each experimental value. In each experiment, this represented less than 5% of the wild-type value (data not shown). Surprisingly, a majority of the alanine substitutions resulted in decreased fusogenicity. The bulk of these mutants mediated fusion with an efficiency 50 to 70% that of wild type. A dramatic decrease in fusion activity was observed for six mutants. In five of these the mutations (L2A, F5A, I13A, K16A, and P17A) were located upstream of the maturation cleavage site, while the other (G31A) was located downstream. The I6A, I18A, and Y22A mutants showed a small increase in fusion activity, but four of the mutant Envs (T4A, K7A, Q9A, and I10A) had significantly higher fusion activities compared to that of the wild type. All of these mutations were located upstream of the maturation cleavage site. The Q9A and I10A mutations not only had increased levels of fusogenicity in the GFP-based fluorescence-activated cell sorter (FACS) analysis but also induced extensive syncytium formation in the cell-cell fusion assay (Fig. 4, Q9A and I10A). It is important to note that this high level of fusion occurred in the absence of the maturational cleavage normally mediated by the viral protease. The Q9A mutant glycoprotein in particular mediated syncytium formation comparable to that of the TL 15 mutants described previously (4). Because all the mutants have levels of Env surface expression equivalent to the wild type (and in the case of Q9A, lower than wild type), the difference in fusogenicity must be due to a functional change of the TM proteins caused by the mutations introduced into the cytoplasmic domain.
FIG. 3.
Cell-cell fusion assay of the alanine-scanning mutants. COS-1 cells were transfected with the pTMT expression vector encoding either the mutant or wild-type env genes. COS-1 cells expressing glycoproteins were mixed at a 1:2 ratio with HOS-CD4/LTR-hGFP cells and replated. The cells were analyzed for expression of GFP and quantitated by FACS 36 h later as described in Materials and Methods. The * indicates syncytium formation in the context of full-length glycoprotein. The mean percentage (± the standard deviation) of GFP-expressing cells relative to wild type from three independent experiments is shown for each of the mutants.
FIG. 4.
Fusion of COS-1 cells with GHOST cells by Q9 mutant envelope proteins. COS-1 cells were transfected as described for protein expression. At 24 h posttransfection, they were mixed with GHOST cells. Cells were fixed and then stained with Giemsa stain. Cells were photographed 24 h later.
Previously our lab demonstrated that a series of truncation mutations introduced into the M-PMV glycoprotein CT increase fusogenicity and allow Env-mediated syncytium formation (4). We have expanded this approach further upstream toward the membrane-spanning domain to investigate whether there is any relationship between the enhanced fusion observed with Q9A and I10A and that observed following truncation. For this purpose we introduced three additional truncation mutations (TL10, TL5, and TL1) into the CT. Similar levels of glycoprotein were synthesized, processed, and transported to the surface of the plasma membrane, compared to wild type, for each of the three truncation mutants (data not shown).
We tested the fusogenicity of the truncation mutations using the quantitative analysis method described above. As reported previously, truncation of the CT toward the membrane-spanning domain increased fusogenicity and syncytium formation, and this continued with TL10 (Fig. 4 and 5). However, truncation mutations upstream of CT residue 10 reversed the enhancing effect on the fusion activity of the glycoprotein, with TL5 fusing cells at a level similar to wild type and TL1 exhibiting dramatically decreased fusion activity (Fig. 5). Furthermore, neither of the more severely truncated mutants induced any syncytia in cell-cell fusion assays.
FIG. 5.
Cell-cell fusion assay of the truncation mutants. COS-1 cells were transfected with the pTMT expression vector encoding either the truncation mutant or wild-type env genes. COS-1 cells expressing glycoproteins were mixed at a 1:2 ratio with HOS-CD4/LTR-hGFP cells and replated. The cells were analyzed for expression of GFP and quantitated by FACS 24 h later as described in Materials and Methods. The * indicates syncytium formation in the context of glycoprotein.
Effect of additional substitutions for glutamine 9 on cell fusion activity of the Env protein.
To investigate the biological role of the glutamine at position 9 in the CT of the M-PMV glycoprotein in more detail, we introduced additional hydrophilic (threonine), hydrophobic (leucine), charged (glutamate or lysine), or polar (asparagine) amino acid substitutions in place of the glutamine using PCR-based mutagenesis as described in Materials and Methods. These mutant env genes were also cloned into the glycoprotein expression vector pTMT.
The results of pulse-chase and surface biotinylation experiments employing COS-1 cells transfected with wild-type or mutant env constructs containing additional substitutions for glutamine 9 are shown in Fig. 6. Env precursor Pr86 was translated during the 30-min pulse, and similar levels of expression were observed for each mutant and the wild type (Fig. 6A). During the chase, the wild-type and mutated env gene precursor proteins (with the exception of Q9E) were similarly processed to the mature proteins, gp70 and gp22 (Fig. 6B). Variable levels of gp70 protein were released into the culture medium, with the Q9L and Q9N mutants releasing less and Q9K releasing more than that observed for the wild type (Fig. 6C). Surface biotinylation showed that similar levels of surface-expressed Env proteins were observed for three of the mutants (Q9T, Q9L, and Q9N) and the wild type (Fig. 6D). In contrast, the Q9K mutant showed lower surface levels of gp70 and higher levels of released gp70, while the Q9E mutant showed both gp70 and Pr86 on the plasma membrane, similar to the F5A mutant.
FIG. 6.

Expression of Q9 mutant envelope proteins. Proteins were expressed and labeled with 3H as described in Materials and Methods. (A) Pulse; (B) pulse-chase; (C) supernatants; (D) surface biotinylation. The mutant designation is shown above each lane, and the positions of the viral bands are indicated on the left.
We tested the effects of these mutations on the fusion kinetics of the glycoprotein in time course experiments. In order to determine the fusion kinetics at early time points, we used a fluorescent dye redistribution assay. In this assay, COS-1 cells were plated to 50% confluence in six-well plates and then cotransfected with the glycoprotein expression vector pTMT containing either wild-type or mutant M-PMV env genes and the GFP expression vector pIRES2-EGFP. JC53-BL cells that express M-PMV receptor on the plasma membrane were used as target cells. The target cells were loaded with the cytoplasmic dye CellTracker Blue and the lipid dye Fast DiI as described in Materials and Methods. After loading with dyes, the JC53-BL cells were collected and washed with PBS and then added to the cotransfected COS-1 cells at a 5:1 ratio. Cells were incubated for 2 h at 37°C and then washed with PBS, trypsinized, and fixed in 4% paraformaldehyde. After fixation, cells were analyzed by flow cytometry. Cells were first gated on the GFP-positive population to identify Env-expressing cells, and then they were analyzed for the percentage of Fast DiI- and CellTracker Blue-positive cells. Cells that were positive for GFP, Fast DiI, and CellTracker Blue were counted as fused cells.
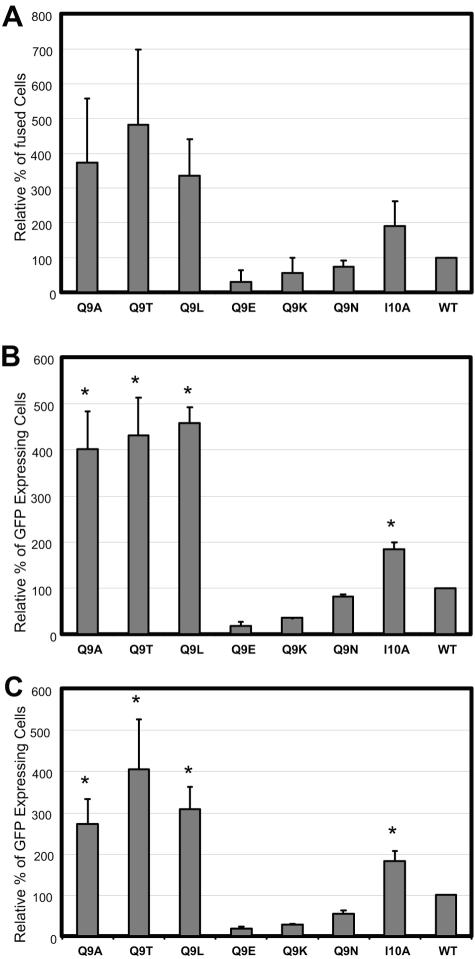
After 2 h of incubation of transfected COS-1 cells with JC53-BC target cells, two distinctly different results were observed. Q9A, Q9T, and Q9L mutants had three to five times greater fusion activities than the wild type, indicating that even at 2 h after mixing, these mutants already showed increased levels of fusogenicity. In contrast, substitution mutations with either charged or polar amino acids (Q9E, Q9K, or Q9N) mediated fusion at levels 20 to 70% that of the wild type, with Q9E exhibiting the lowest activity. The I10A mutant showed an approximate twofold increase in fusogenicity compared to the wild type (Fig. 7A).
FIG. 7.
Effects of mutations on the fusion kinetics of the glycoprotein in time course experiments. (A) Fusion activities of Q9 envelope proteins monitored by fluorescent dye transfer. COS-1 cells expressing both GFP and the M-PMV Env constructs were used as effector cells, and target JC-53BL cells were labeled with the cytoplasmic dye CellTracker Blue and the lipid dye Fast DiI. Doubly labeled JC-53BL cells were overlaid on the COS-1 cells, incubated for 2 h at 37°C, and then fixed in 4% paraformaldehyde. After fixation, cells were analyzed by flow cytometry for the percentage of Fast DiI- and CellTracker Blue-positive cells. Cells that were positive for GFP, Fast DiI, and CellTracker Blue were determined to be fused cells. (B and C) COS-1 cells were transfected with the pTMT expression vector encoding either the mutant or wild-type env genes. COS-1 cells expressing Q9 mutants were mixed with GHOST cells and replated. The cells were analyzed for expression of GFP and quantitated by FACS 24 h later (B) or 48 h later (C) as described in Materials and Methods. The * indicates syncytium formation in the context of the Q9 mutants. The mean percentage (± the standard deviation) of GFP-expressing cells relative to wild type from three independent experiments is shown for each of the mutants.
For longer incubation times, we employed the GFP-based fusion assay described previously. After a 24-h incubation of transfected COS-1 cells, expressing either wild-type or mutant env genes, with indicator GHOST cells, it was clear that the Q9A, Q9T, and Q9L mutants exhibited at least fourfold-higher fusogenicity than the wild type. Consistent with the dye redistribution assay, the Q9E, Q9K, and Q9N mutants had reduced levels of GFP-positive cells compared to the wild type and no syncytia were observed in these cultures (Fig. 7B).
Even after 48 h of incubation of the transfected COS-1 cells with the GHOST cells, the number of GFP-positive cells observed for the Q9A, Q9T, and Q9L mutants remained significantly higher than the wild type. At this time point extensive syncytium formation was observed for these mutants (Fig. 4, for example), and the number of nuclei per syncytium for these mutants was comparable to the TL10-induced syncytia. The I10A mutant consistently exhibited a twofold increase in GFP-positive cells at both time points, although the induced syncytia were smaller than for the most actively fusing mutants (Fig. 4).
Taken together these data indicate that the mutations at the Q9 position within the cytoplasmic tail of M-PMV glycoprotein modulate both the fusogenicity and the syncytium formation capacity of the TM either in a positive or a negative manner, depending on the specific amino acids substituted into the site.
Predicted probability of the membrane-proximal region of the cytoplasmic domain forming a coiled-coil and fusogenicity.
In order to investigate whether the cytoplasmic domain of the M-PMV Env could adopt a conformation that might modulate the fusogenicity of the Env trimer, we subjected the wild-type and mutant gp22 amino acid sequences to the structure prediction program COILS (http://www.ch.embnet.org/software/COILS_form.html) (40), which calculates the probability that a sequence will adopt a coiled-coil conformation. For each of the sequences both the N-terminal and the C-terminal heptad repeats were strongly predicted to form coiled-coils (94% and 99%, respectively [data not shown]). In addition, a third region with a low (3.6% in wild type) but consistent probability to form a coiled-coil was predicted to extend through the first 16 amino acids of the cytoplasmic domain (Fig. 8). Mutations I10A, Q9A, and K7A, which increased the fusogenicity of the M-PMV Env 1.7-, 2.45-, and 2.75-fold, respectively, decreased this predicted probability to 2, 1.1, and 0.8%, respectively (Fig. 8). In contrast, mutations such as 9QE and I13A, which decreased fusogenicity to 30% and 20% of that of the wild type, exhibited predicted coiled-coil probabilities of 5.6% and 9.3% (Fig. 8). Mutation P17A, which decreases fusion to 12% of wild type, increased the probability even higher to 13% and extended the predicted coiled-coil by more than 10 amino acids. Although a majority of the mutants exhibited phenotypes consistent with the predicted probability of a coiled-coil structure, the correlation was not perfect: three mutants, E11A, Q14A, and K16A, with reduced fusogenicities had low predicted coiled-coil probabilities (0.9 to 17%), while the slightly more fusogenic I6A exhibited a probability twice that of wild type.
FIG. 8.
Predicted probability for coiled-coil formation in the cytoplasmic domain of M-PMV Env. The amino acid sequences of the membrane-spanning domain and cytoplasmic domain (residues 520 to 586 of Env) for the wild-type and mutant proteins were used for analysis by COILS (40). The output for the region spanning the first 30 amino acids of the cytoplasmic domain is shown.
DISCUSSION
During the normal viral life cycle, the M-PMV glycoprotein undergoes an additional cleavage that truncates the cytoplasmic tail. This cleavage of the M-PMV Env CT occurs during a virus maturation step by an activated viral protease (5). It takes place between amino acids H21 and Y22 and results in the loss of 17 amino acids from the carboxy terminus of the CT. We previously demonstrated that this C-terminal truncation removes a necessary incorporation signal and enhances the fusogenicity of the viral Env protein (4, 63).
Several lines of evidence indicate that the cytoplasmic domain of viral glycoproteins can regulate fusogenicity (32, 34, 69). In MuLV and equine infectious anemia virus, like that described for M-PMV, the cytoplasmic tail of the TM glycoprotein is further processed by the viral protease after virus particle release, and this process activates Env fusion activity (31, 36, 37, 52, 58, 76). Truncation of the cytoplasmic tail of lentiviral Env proteins can occur under certain culture conditions (8, 35, 60). In HIV-1, HIV-2, and simian immunodeficiency virus this truncation of the cytoplasmic tail of the TM glycoprotein also increases fusion activity (16, 45, 66, 67, 74, 77).
In this study, we have investigated the effect of mutations in the cytoplasmic tail of the M-PMV Env protein on the regulation of membrane fusion. Using site-directed PCR mutagenesis, we have introduced a series of mutations into the cytoplasmic tail from L2 to S33. This region of the cytoplasmic tail is well conserved among D-type retroviruses and also encompasses a region that previous truncation and substitution mutation analyses had implicated in mediating viral glycoprotein incorporation and in regulating fusogenicity.
In order to investigate the effects of each mutation on the synthesis, plasma membrane expression, and the biological function of the TM, each mutated env gene was cloned into the pTMT envelope glycoprotein expression vector. The results of these experiments showed that except for the F5A and I13A mutations, all alanine-scanning mutants exhibited levels of synthesis, processing, and transport to the plasma membrane similar to the wild type. Interestingly, while the F5A, Q9E, and I13A mutant Env precursors were expressed at a level comparable to that of the wild type in the pulse-chase experiment, the F5A and Q9E mutants reproducibly exhibited equal amounts of gp70 and Pr86 on the plasma membrane surface, and the plasma membrane level of the I13A mutant Env proteins was dramatically reduced. In case of F5A and Q9E, it is difficult to understand why single amino acid changes in the CT would influence accessibility of the ectodomain cleavage site. However, truncation of the HIV Env CT results in increased exposure of conserved epitopes in gp120 (18), and it is possible that similar ectodomain changes induced by the F5A and Q9E point mutations in the M-PMV Env occlude the cleavage site. Alternatively, it is possible that these mutations interfere with intracellular transport signals in such a way that the Env precursor now either inefficiently traverses the endoprotease-containing region of the Golgi or traverses so rapidly that cleavage is inefficient—the conservation of the phenylalanine residue at position 5 in both M-PMV and MuLV Envs would be consistent with the presence of a targeting sequence in this region.
Assays of cell fusion activity have provided new insights into the influence that each amino acid residue within the cytoplasmic domain can exert on the biological activity of the Env protein. Moreover, these results also point to a distinct conformation for the cytoplasmic domain prior to and after maturational cleavage. In this study, we have employed two different fusion assays that enable us to accurately quantitate the effects of the mutations on the fusion kinetics and fusogenicity of the mutant Env proteins. Cell fusion studies showed that six of these mutations (L2A, F5A, I13A, K16A, P17A, and G31A) nearly abrogated the fusogenicity of the TM protein. A majority of these mutations are located upstream of the maturational cleavage site and do not decrease surface glycoprotein expression. Four of the mutations (T4A, K7A, Q9A, and I10A), all located upstream of the maturational cleavage site, significantly increased fusion activity compared to that of the wild type. Surprisingly, both the Q9A and I10A mutations induced extensive syncytium formation in the context of the full-length TM protein, with the average size of the syncytia formed by the Q9A mutant glycoprotein being comparable to that of the TL15 and TL10 mutants. Additional substitutions into Q9 demonstrated that Q9T and Q9L, along with Q9A, could increase fusogenicity of the TM protein dramatically as well as induce very extensive syncytium formation. By using a fluorescent dye redistribution assay, we demonstrated that these mutations (Q9A, Q9T, and Q9L) induce fusion faster and mediate more massive fusion activity of the TM protein at an early time point. In contrast, the Q9E and Q9K mutations decreased fusion activity significantly. It is possible that Q9 and I10 are located in a critical fusion regulatory region of the CT, since mutation of K7 also results in a more fusogenic full-length Env. This is somewhat unexpected, since truncation mutations point to additional negative-regulatory sequences downstream of I10. Because all the mutants have levels of Env surface expression comparable to the wild type, the differences in fusogenicity must be due to a functional change in the TM proteins that is caused by the mutations introduced into the cytoplasmic domain. Thus, single amino acid changes at Q9 within the cytoplasmic domain of the TM protein can modulate in a positive manner a process that is primarily dependent on conformational changes in the ectodomain of the glycoprotein complex. We therefore hypothesize that substitutions into Q9 induce changes in the structure of the glycoprotein that facilitate conformational changes occurring in the TM ectodomain that are necessary to bring membranes together and initiate lipid bilayer fusion. Truncation of the Env cytoplasmic tail leads to differences in conformation-specific monoclonal antibody reactivity for both HIV-1 and MuLV Env proteins (1, 18), showing that a conformational change in the cytoplasmic tail could be transmitted to the ectodomain. Viruses such as M-PMV may have adopted this additional maturational cleavage step to regulate Env fusogenicity and to prevent the induction of cell death (51, 52). Our preliminary data have indicated that the expression of the Q9A, Q9T, or Q9L mutant glycoproteins in 293T cells does in fact induce cell death (data not shown). Investigations are currently under way to determine the detailed molecular mechanisms of this cytotoxic effect.
We and others have predicted that the M-PMV membrane-spanning and cytoplasmic domain sequences would preferentially fold into an alpha-helical structure (63, 68), and we show here that the first 16 amino acids of the cytoplasmic domain have the potential to interact in a coiled-coil with the same region of the other members of the Env trimer. Such interactions could stabilize the trimer and restrict conformational changes in the ectodomain that are necessary for fusion to proceed. The fact that the substitution of alanines for residues in the center of this region (mutants K7A, Q9A, and I10A) decreases the potential for coiled-coil formation and concomitantly increases Env-mediated fusion would be consistent with this hypothesis. In contrast, substitution of a charged residue (glutamate for glutamine) at position 9 increases the probability of coiled-coil formation and results in reduced fusion. Interestingly, the proline at position 17 appears to terminate the predicted coiled-coil in the wild-type protein, and substitution of this residue with alanine results in a stronger prediction for a coiled-coil region that now extends to residue 28 of the cytoplasmic domain. Consistent with our hypothesis, this mutant now exhibits only 12% of the fusion observed for the wild-type protein. Insertional mutations in the cytoplasmic tail of the MuLV glycoprotein that suppress the inhibitory effect of the “R” peptide and allow fusion may also break the rigidity of an alpha-helical structure in the cytoplasmic domain (37). Thus, it is tempting to speculate that the overall flexibility of Env oligomers might be regulated by alpha-helical interactions in the cytoplasmic domain and that the Q9 residue (and nearby residues) plays a pivotal role in maintaining this critical structure.
The studies presented here, taken together with our previous observations that truncation and substitution mutations in the cytoplasmic domain of the M-PMV TM protein can modulate the fusogenicity of the glycoprotein, inhibit incorporation in the virus particle, and play a role in endocytosis, argue strongly for a multifunctional role for the cytoplasmic domain. These data also have implications for the overall mechanism of activation of retroviral fusion proteins. Our identification of the glutamine residue at position 9 as a key element for Env fusion regulation in the M-PMV TM protein suggests that activation of the fusion process that occurs following cytoplasmic domain cleavage may result from destabilizing coiled-coil interactions between cytoplasmic tail residues in the Env trimer.
Acknowledgments
We thank Susan Dubay for her valuable advice on experiments and Tshana Thomas for excellent technical assistance.
This work was supported by grant CA-27834 from the National Institutes of Health to E.H. FACS analysis was performed at the Flow Cytometry Core Facilities of the University of Alabama at Birmingham Center for AIDS Research (supported by NIH grant P30-AI-27767).
REFERENCES
- 1.Aguilar, H. C., W. F. Anderson, and P. M. Cannon. 2003. Cytoplasmic tail of Moloney murine leukemia virus envelope protein influences the conformation of the extracellular domain: implications for mechanism of action of the R peptide. J. Virol. 77:1281-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobkova, M., J. Stitz, M. Engelstadter, K. Cichutek, and C. J. Buchholz. 2002. Identification of R-peptides in envelope proteins of C-type retroviruses. J. Gen. Virol. 83:2241-2246. [DOI] [PubMed] [Google Scholar]
- 3.Bowers, K., A. Pelchen-Matthews, S. Honing, P. J. Vance, L. Creary, B. S. Haggarty, J. Romano, W. Ballensiefen, J. A. Hoxie, and M. Marsh. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1:661-674. [DOI] [PubMed] [Google Scholar]
- 4.Brody, B. A., S. S. Rhee, and E. Hunter. 1994. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J. Virol. 68:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody, B. A., S. S. Rhee, M. A. Sommerfelt, and E. Hunter. 1992. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc. Natl. Acad. Sci. USA 89:3443-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckland, R., E. Malvoisin, P. Beauverger, and F. Wild. 1992. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J. Gen. Virol. 73:1703-1707. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, L., M. Emerman, P. Tiollais, and P. Sonigo. 1989. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J. Virol. 63:4395-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers, P., C. R. Pringle, and A. J. Easton. 1990. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J. Gen. Virol. 71:3075-3080. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S. S., S. F. Lee, H. J. Hao, and C. K. Chuang. 1998. Mutations in the leucine zipper-like heptad repeat sequence of human immunodeficiency virus type 1 gp41 dominantly interfere with wild-type virus infectivity. J. Virol. 72:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christodoulopoulos, I., and P. M. Cannon. 2001. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J. Virol. 75:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 13.de Groot, R. J., W. Luytjes, M. C. Horzinek, B. A. van der Zeijst, W. J. Spaan, and J. A. Lenstra. 1987. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J. Mol. Biol. 196:963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubay, J. W., S. J. Roberts, B. Brody, and E. Hunter. 1992. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J. Virol. 66:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 18.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 76:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einfeld, D., and E. Hunter. 1988. Oligomeric structure of a prototype retrovirus glycoprotein. Proc. Natl. Acad. Sci. USA 85:8688-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 22.Gallaher, W. R. 1987. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell 50:327-328. [DOI] [PubMed] [Google Scholar]
- 23.Gallaher, W. R., J. M. Ball, R. F. Garry, M. C. Griffin, and R. C. Montelaro. 1989. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res. Hum. Retrovir. 5:431-440. [DOI] [PubMed] [Google Scholar]
- 24.Gallaher, W. R., C. DiSimone, and M. J. Buchmeier. 2001. The viral transmembrane superfamily: possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 1:1. [Online.] doi: 10.1186/1471-2180-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh, J. K., and Y. Shai. 1999. Direct evidence that the N-terminal heptad repeat of Sendai virus fusion protein participates in membrane fusion. J. Mol. Biol. 292:531-546. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons, D. L., M. C. Vaney, A. Roussel, A. Vigouroux, B. Reilly, J. Lepault, M. Kielian, and F. A. Rey. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 427:320-325. [DOI] [PubMed] [Google Scholar]
- 27.Green, N., T. M. Shinnick, O. Witte, A. Ponticelli, J. G. Sutcliffe, and R. A. Lerner. 1981. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc. Natl. Acad. Sci. USA 78:6023-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallenberger, S., V. Bosch, H. Angliker, E. Shaw, H. D. Klenk, and W. Garten. 1992. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360:358-361. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 30.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 31.Januszeski, M. M., P. M. Cannon, D. Chen, Y. Rozenberg, and W. F. Anderson. 1997. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J. Virol. 71:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston, P. B., J. W. Dubay, and E. Hunter. 1993. Truncations of the simian immunodeficiency virus transmembrane protein confer expanded virus host range by removing a block to virus entry into cells. J. Virol. 67:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi, S. B., R. E. Dutch, and R. A. Lamb. 1998. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology 248:20-34. [DOI] [PubMed] [Google Scholar]
- 34.Kim, F. J., N. Manel, Y. Boublik, J. L. Battini, and M. Sitbon. 2003. Human T-cell leukemia virus type 1 envelope-mediated syncytium formation can be activated in resistant mammalian cell lines by a carboxy-terminal truncation of the envelope cytoplasmic domain. J. Virol. 77:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodama, T., D. P. Wooley, Y. M. Naidu, H. W. Kestler III, M. D. Daniel, Y. Li, and R. C. Desrosiers. 1989. Significance of premature stop codons in env of simian immunodeficiency virus. J. Virol. 63:4709-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubo, Y., and H. Amanuma. 2003. Mutational analysis of the R peptide cleavage site of Moloney murine leukaemia virus envelope protein. J. Gen. Virol. 84:2253-2257. [DOI] [PubMed] [Google Scholar]
- 37.Li, M., C. Yang, and R. W. Compans. 2001. Mutations in the cytoplasmic tail of murine leukemia virus envelope protein suppress fusion inhibition by R peptide. J. Virol. 75:2337-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisanti, M. P., I. W. Caras, T. Gilbert, D. Hanzel, and E. Rodriguez-Boulan. 1990. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc. Natl. Acad. Sci. USA 87:7419-7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo, Z., A. M. Matthews, and S. R. Weiss. 1999. Amino acid substitutions within the leucine zipper domain of the murine coronavirus spike protein cause defects in oligomerization and the ability to induce cell-to-cell fusion. J. Virol. 73:8152-8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 41.McDougal, J. S., J. K. Nicholson, G. D. Cross, S. P. Cort, M. S. Kennedy, and A. C. Mawle. 1986. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J. Immunol. 137:2937-2944. [PubMed] [Google Scholar]
- 42.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 44.Morner, A., A. Bjorndal, J. Albert, V. N. Kewalramani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulligan, M. J., G. V. Yamshchikov, G. D. Ritter, Jr., F. Gao, M. J. Jin, C. D. Nail, C. P. Spies, B. H. Hahn, and R. W. Compans. 1992. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J. Virol. 66:3971-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochsenbauer, C., S. R. Dubay, and E. Hunter. 2000. The Rous sarcoma virus Env glycoprotein contains a highly conserved motif homologous to tyrosine-based endocytosis signals and displays an unusual internalization phenotype. Mol. Cell. Biol. 20:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ott, D., and A. Rein. 1992. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J. Virol. 66:4632-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson, R. G., C. J. Russell, and R. A. Lamb. 2000. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology 270:17-30. [DOI] [PubMed] [Google Scholar]
- 50.Piller, S. C., J. W. Dubay, C. A. Derdeyn, and E. Hunter. 2000. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J. Virol. 74:11717-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragheb, J. A., and W. F. Anderson. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reitter, J. N., T. Sergel, and T. G. Morrison. 1995. Mutational analysis of the leucine zipper motif in the Newcastle disease virus fusion protein. J. Virol. 69:5995-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell, C. J., K. L. Kantor, T. S. Jardetzky, and R. A. Lamb. 2003. A dual-functional paramyxovirus F protein regulatory switch segment: activation and membrane fusion. J. Cell Biol. 163:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salzwedel, K., J. T. West, and E. Hunter. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schultz, A., and A. Rein. 1985. Maturation of murine leukemia virus env proteins in the absence of other viral proteins. Virology 145:335-339. [DOI] [PubMed] [Google Scholar]
- 59.Sergel-Germano, T., C. McQuain, and T. Morrison. 1994. Mutations in the fusion peptide and heptad repeat regions of the Newcastle disease virus fusion protein block fusion. J. Virol. 68:7654-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu, H., F. Hasebe, H. Tsuchie, S. Morikawa, H. Ushijima, and T. Kitamura. 1992. Analysis of a human immunodeficiency virus type 1 isolate carrying a truncated transmembrane glycoprotein. Virology 189:534-546. [DOI] [PubMed] [Google Scholar]
- 61.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 62.Sommerfelt, M. A., S. R. Petteway, Jr., G. B. Dreyer, and E. Hunter. 1992. Effect of retroviral proteinase inhibitors on Mason-Pfizer monkey virus maturation and transmembrane glycoprotein cleavage. J. Virol. 66:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song, C., S. R. Dubay, and E. Hunter. 2003. A tyrosine motif in the cytoplasmic domain of Mason-Pfizer monkey virus is essential for the incorporation of glycoprotein into virions. J. Virol. 77:5192-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song, C., and E. Hunter. 2003. Variable sensitivity to substitutions in the N-terminal heptad repeat of Mason-Pfizer monkey virus transmembrane protein. J. Virol. 77:7779-7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sonigo, P., C. Barker, E. Hunter, and S. Wain-Hobson. 1986. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell 45:375-385. [DOI] [PubMed] [Google Scholar]
- 66.Spies, C. P., and R. W. Compans. 1994. Effects of cytoplasmic domain length on cell surface expression and syncytium-forming capacity of the simian immunodeficiency virus envelope glycoprotein. Virology 203:8-19. [DOI] [PubMed] [Google Scholar]
- 67.Spies, C. P., G. D. Ritter, Jr., M. J. Mulligan, and R. W. Compans. 1994. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein alters the conformation of the external domain. J. Virol. 68:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor, G. M., and D. A. Sanders. 2003. Structural criteria for regulation of membrane fusion and virion incorporation by the murine leukemia virus TM cytoplasmic domain. Virology 312:295-305. [DOI] [PubMed] [Google Scholar]
- 69.Waning, D. L., C. J. Russell, T. S. Jardetzky, and R. A. Lamb. 2004. Activation of a paramyxovirus fusion protein is modulated by inside-out signaling from the cytoplasmic tail. Proc. Natl. Acad. Sci. USA 101:9217-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng, Y., and C. D. Weiss. 1998. Mutational analysis of residues in the coiled-coil domain of human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 72:9676-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West, J. T., P. B. Johnston, S. R. Dubay, and E. Hunter. 2001. Mutations within the putative membrane-spanning domain of the simian immunodeficiency virus transmembrane glycoprotein define the minimal requirements for fusion, incorporation, and infectivity. J. Virol. 75:9601-9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wild, C., J. W. Dubay, T. Greenwell, T. Baird, Jr., T. G. Oas, C. McDanal, E. Hunter, and T. Matthews. 1994. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc. Natl. Acad. Sci. USA 91:12676-12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiley, D. C., and J. J. Skehel. 1987. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 56:365-394. [DOI] [PubMed] [Google Scholar]
- 74.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 75.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang, C., and R. W. Compans. 1997. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J. Virol. 71:8490-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]