Abstract
Individuals infected with human immunodeficiency virus type 1 (HIV-1) harbor a mixture of viral variants with different sequences and in some instances with different phenotypic properties. Major and rapid fluctuations in the proportion of viral variants coexisting in an infected individual can be observed under strong pharmacological and immune selective pressure. Because of the short half-life of HIV-infected cells and of HIV virions in the blood, plasma virus populations are highly relevant to HIV evolution in the face of these selective pressures. Here we analyzed the sensitivity to antibody-mediated neutralization of viral variants coexisting in the plasma virus populations of two infected patients. For each patient, several replication-competent viral clones were constructed that carry primary envelope gene sequences obtained from a single plasma sample. Viral clones differed in their tropism and replicative capacity and in the number and positions of glycosylation sites in the envelope glycoproteins. Viruses were tested against heterologous and autologous sera obtained at different time points. Interestingly, we found that viral variants coexisting in each plasma sample were highly heterogeneous in terms of sensitivity to neutralization. The order of sensitivity depended on the serum used and was not associated with virus tropism. The neutralization potency of sera increased with the duration of the infection for both autologous and heterologous neutralization.
Antibody-mediated neutralization is an essential mechanism of protection against several pathogens, but its role in protecting and limiting the spread of human immunodeficiency virus type 1 (HIV-1) infection is unclear (2, 17, 31, 32, 35, 37, 38). Two lines of evidence support the idea of the ability of humoral immunity to influence the outcome of retroviral infection: (i) effective protection was obtained by passive transfer of antibodies before experimental exposure of macaques to pathogenic strains of simian/human immunodeficiency viruses (3, 29, 34, 52), and (ii) higher levels of neutralizing antibodies are found in patients who are long-term nonprogressors compared to individuals with more-rapid disease progression (8, 33, 37). Progression of HIV pathogenesis in spite of the presence of neutralizing antibodies could result from inefficient neutralization, delayed antibody production, and rapid virus adaptation.
Recent longitudinal studies precisely measured both the increase in the potency of the antibody response and the evolution of the susceptibility of viruses to neutralization during the course of infection (1, 16, 41, 54). Interestingly, sexually transmitted viral variants appear to be particularly sensitive to neutralization by antibodies (14), suggesting that reduced sensitivity to antibodies comes at a cost in terms of virus replicative capacity. Potent antibody response is mounted very early in HIV infection in a percentage of patients (41), and the lack of control of virus spread is currently attributed to the continual selection of escape variants (41, 54). A close competition is thus engaged early in the course of infection between the capacity of the virus to modify its antigenic determinants and the ability of the immune response to adapt to these changes. In this context, HIV genetic variability and rapid viral turnover confer sufficient advantage to the virus to grant virus persistence and spread. The mechanisms involved in viral escape from neutralizing antibodies include accumulation and shuffling of glycosylation sites (4, 10, 39, 48, 54) as well as conformational masking of receptor binding sites (22).
In view of the rapid replication dynamics of HIV in vivo (27, 53, 55), one would predict that at any given time point, viral variants coexisting in an infected individual might be relatively homogeneous in terms of susceptibility to neutralization, since the more sensitive strains should be rapidly cleared. Here we analyzed the sensitivity to antibody-mediated neutralization of viral variants coexisting in the plasma virus populations of two infected patients. Plasma was chosen as the virus source, because fluctuations in the virus population due to changes in the selective pressure can be detected very early in this compartment (6, 27, 53, 55). For each patient, several replication-competent viral clones were constructed that carry primary envelope sequences obtained from a single plasma sample. The two treatment-naïve patients studied here were previously characterized as carrying virus populations capable of using both CCR5 and CXCR4 chemokine receptors (47), allowing the comparison of levels of sensitivity to neutralization as a function of virus tropism. Viral clones were characterized for their chemokine receptor usage, viral replicative capacity, and sensitivity to antibody-mediated neutralization by use of both autologous and heterologous sera. Interestingly, we found that viral variants coexisting in each plasma sample were highly heterogeneous in terms of sensitivity to neutralization. The level and the order of sensitivity of different clones depended on the serum used, and neutralization sensitivity was not associated with chemokine receptor usage or with the replicative capacity of virions.
MATERIALS AND METHODS
Cell culture.
293-T cells were cultivated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and antibiotics (50 IU/ml penicillin and 50 μg/ml streptomycin) (complete DMEM). U373MG-CD4 cells stably transfected with an expression vector for the chemokine receptor CCR5 or CXCR4 (23) were cultured in complete DMEM in the presence of 10 μg of puromycin/ml and 100 μg of hygromycin B to maintain the expression of the receptors. The HeLa-derived P4C5 cell line, characterized by constitutive expression of CXCR4 and stably transfected with an expression vector for CD4 and CCR5 (26), was maintained in complete DMEM containing hygromycin B (100 μg/ml).
Amplification and cloning of patient-derived envelope sequences.
We amplified viral envelope sequences from two symptomatic, untreated patients, patients T28 and T5, characterized by 30 and 13 CD4+ T lymphocytes per mm3, respectively, and 105 and 106 viral RNA copies/ml of plasma. Viral RNA was isolated from frozen plasma samples by use of a Roche Amplicor kit (Roche Diagnostics). An initial reverse transcriptase PCR (RT-PCR) amplification was carried out using the following primers: E00, 5′GAAAGAGCAGAAGACAGTGGCAATGA3′ (nucleotides [nt] 6196 to 6224 of pNL4-3) (43), and E01−, 5′TCCAGTCCCCCCTTTTCTTTTAAAAA3′ (nt 9054 to 9079 of pNL4-3) (43). An aliquot of the RT-PCR product was then used in a nested PCR with the following primers: E10, 5′TTGTGGGTCACAGTCTATTATGGGGT3′ (nt 6320 to 6345 of pNL4-3) (43), and FuB, 5′GGTGGTAGCTGAAGAGGCACAGG3′ (nt 8503 to 8522 of pNL4-3) (23). By this approach, a 2,200-bp product spanning the gp120 and most of the gp41 region was obtained (23). This PCR product was column purified (QIAGEN) and cloned using a Topo-TA cloning kit (Invitrogen) according to the manufacturer's instructions.
Primary envelope sequences of independent clones were then transferred in a variant of pNL4-3, pNL4-3XC-MS2, which contains an MluI site in the C1 domain of gp120 (nt 6435) in addition to the natural BamHI site in the cytoplasmic domain of gp41 (nt 8465). To allow cloning, the MluI and BamHI restriction sites were introduced in the subcloned envelope sequences (BamHI was sometimes present in the primary sequences) by use of a QuikChange site-directed mutagenesis kit (Stratagene) and the following primers: NBam(+) (5′CGATTAGTGAACGGATCCTTAGCACTTATCTGGG3′) and NBam(−) (5′CCCAGATAAGTGCTAAGGATCCGTTCACTAATCG3′) for patient T28 or NBamT5(+) (5′GATCCGGGACATTAGTGGATGGATCCTTAGCAATTTTCTGG3′) and NBamT5(−) (5′CCAGAAAATTGCTAAGGATCCATCCACTAATGTCCCGGATC3′) for patient T5 and MluV(+) (5′GGGCCACACACGCGTGTGTACCCACAG3′) and MluV(−) (5′CTGTGGGTACACACGCGTGTGTGGCCC3′). The MluI/BamHI fragment was then excised from the pCR2.1 plasmid and transferred in pNL4-3XC-MS2.
Sequence analysis.
Nucleotide sequences of primary envelope subclones were analyzed using Sequence Navigator and were manually corrected and aligned using the Clustal X program (49) followed by visual inspection and manual adjustment. Based on these sequences, distance-matrix based trees were built using the neighbor-joining method (42) and the Kimura's two-parameter model of nucleotide substitution (20), after gap stripping. The reliability of the branching order was estimated by performing 100 bootstrap replicates. Amino-acid sequences were inferred from nucleotide sequences.
Transfection of proviral clones and production of viral stocks.
Transfection of 293-T cells with proviral constructs was carried out using polyfectamine (QIAGEN) according to the manufacturer's instructions. Briefly, 12 μg of the proviral DNA in a final volume of 450 μl of DMEM were mixed with 120 μl of polyfectamine and incubated for 5 to 10 min at room temperature, and 3 ml of complete DMEM was added and used to transfect subconfluent 293-T cells in a 10 ml final volume, in 75-cm2 flasks. At 12 to 18 h after transfection, cells were washed with phosphate-buffered saline (PBS) and fresh medium was added. Viral supernatants were harvested every 24 h during 3 days, centrifuged, and frozen (−80°C).
Determination of viral infectivity.
After determination of p24 antigen concentrations of viral stocks (p24 detection kit; NEN), serial dilutions of supernatants were used to infect P4C5 cells in presence of 20 μg/ml DEAE dextran. After an incubation period of 12 h, medium was replaced by fresh medium containing 1 μM zidovudine to guarantee a single cycle of viral infection. After 48 h, infection of target cells was determined in a CPRG (chlorophenolred-β-d-galactopyranoside) assay (51) or by blue staining using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Briefly, cells were washed with PBS and fixed using 0.5% glutaraldehyde in PBS. A total of 60 μl of an X-Gal solution (4 mM potassium ferricyanide, 4 mM potassium ferrocyanide, 0.4 mg X-Gal, 2 mM MgCl2 in 1× PBS) was added. After 3 h at 37°C, the reaction was stopped by replacing the X-Gal solution with PBS, and blue cells were counted using a light microscope. The dilution of supernatant in which infection of target cells was observed in 50% of the wells (50% tissue culture infectious dose [TCID50]) was determined for each virus.
Neutralization assay.
Viral stocks were diluted to obtain 400 TCID50/ml in complete medium containing 40 μg/ml DEAE dextran; a volume of 60 μl was then incubated for 1 h at 37°C with 60 μl of serial dilutions of decomplemented serum in complete medium. Aliquots of 100 μl of the virus/serum mixture, corresponding to 20 TCID50/well, were used to infect P4C5 target cells (26) (10,000 cells/well seeded in 96-well microtiter plates 24 h before infection). As for the infectivity assay, medium was replaced by fresh complete DMEM containing 1 μM zidovudine 12 h after infection. After 48 h of incubation, a CPRG assay was carried out. Optical density values were measured 30 min to 8 h after addition of substrate.
Determination of the effective concentration for 50% inhibition (EC50 values).
The optical density (OD) value obtained in absence of human serum was taken as 100% infectivity. In each experiment, a pool of sera from HIV-negative donors was used as a control. To calculate the percentage of residual infectivity in the presence of different serum dilutions, the ratio between the OD value measured for the sample serum and the OD value in presence of negative serum at the same dilution was calculated and multiplied by 100. Serum dilutions required to reduce 50% of infectious events (EC50) were extrapolated in a nonlinear regression using Prism (GraphPad Software), by plotting the residual infectivity as a function of the serum dilutions. Values of EC50 (and not EC90) were used because they can be more precisely evaluated, being derived from the linear portion of the sigmoid curve.
Nucleotide sequence accession numbers.
Sequences corresponding to the V1-to-V3 region of 25 clones from patient T5 and 19 clones from patient T28 have been deposited in the EMBL database under the following accession numbers: AJ888821 to AJ888864. Full-length envelope sequences of five clones from patient T28 and five clones from patient T5 have been deposited in the EMBL database under the following accession numbers: AJ810475.2 to AJ810484.2
RESULTS
Analysis of plasma virus sequence variability.
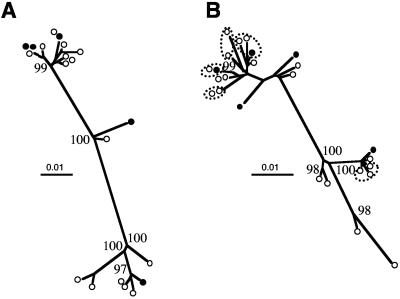
Envelope sequences spanning the entire gp120 domain and most of gp41 were RT-PCR amplified from plasma samples and subcloned into Topo pCR2.1 plasmid. Phylogenetic trees were drawn using the neighbor-joining method (42), after sequencing the V1-V3 region of the envelope gene from 19 clones isolated from patient T28 (Fig. 1A) and 25 clones from patient T5 (Fig. 1B).
FIG. 1.
Phylogenetic trees of the V1-V3 viral envelope sequences from plasma virus populations isolated from patient T28 (A) and T5 (B). Trees were drawn using Clustal X after gap stripping, using 705 nucleotides for patient T28 and 687 nucleotides for patient T5. Clones from patient T5 sharing identical amino acid sequences are grouped within dashed ellipses. Filled circles represent clones selected for further infectivity and neutralization analyses. Numbers at each branch point indicate the bootstrap values derived from 100 bootstrap resamplings. Bootstrap values greater than 90 are indicated.
On the basis of the nucleotide sequences we calculated genetic distances using the Kimura two-parameter model (20). The average genetic distance of clones from patient T28 was 5.1% (range, 0.1 to 9.7%) while for clones from patient T5 it was 3.1% (range, 0.0 to 7.5%). When the amino acid sequences were compared, all clones from patient T28 were found to be unique, while for patient T5, five sequences were found in two or three different clones (clones with identical amino acid sequences are highlighted in Fig. 1B).
Construction and characterization of viral clones carrying patient-derived envelope sequences.
We then transferred the gp120 and most of gp41 envelope domains of independent clones in a variant of the HIV molecular clone pNL4-3, as described in the Materials and Methods section. Five replication-competent clones per patient were obtained. For patient T5, two of these clones, T5-R5-1 and T5-R5-2, harbored amino acid sequences that were represented three and two times in the viral population, respectively. We determined the chemokine receptor usage of these patient-derived clones by transfection of 293-T cells and infection of the U373-MG-CD4-CCR5 and U373-MG-CD4-CXCR4 reporter cell lines (23). Infection of these cell lines with R5- and X4-tropic HIV-1 strains, respectively, results in the induction of β-galactosidase, which can be conveniently detected by a colorimetric assay (51). For patient T28, two viral clones were found to use CCR5 (T28-R5-1 and T28-R5-2) and three clones used CXCR4 (T28-X4-1, T28-X4-2, and T28-X4-3), whereas for patient T5, three clones were R5 (T5-R5-1, T5-R5-2, and T5-R5-3) and two were able to use both CCR5 and CXCR4 (T5-R5X4-1 and T5-R5X4-2).
To determine the replicative capacity of the viral clones independently of virus tropism, limiting serial dilutions of viral supernatants from transfected 293-T cells were used to infect a reporter cell line (P4C5) that can be infected by both CCR5- and CXCR4-tropic viruses (26). Tissue culture infectious doses (TCID50) were measured as a function of p24 antigen concentration. Interestingly, viral clones isolated from each patient were characterized by marked differences in their replicative capacities. Clones obtained from patient T28 displayed values ranging from 0.02 to 11 TCID50 per ng of p24 antigen. These two extreme values were found for the two R5 clones from this patient (T28-R5-1 being the virus with the highest replicative capacity). Similarly, for clones obtained from patient T5 the replicative capacity values ranged from 0.03 to 4 TCID50 per ng of p24. These values were observed for a dual-tropic (T5-R5X4-1) and an R5 (T5-R5-3) clone, respectively. Thus, chemokine receptor usage does not appear to be a major determinant of replicative capacity, since R5 and X4 viruses displayed similar replicative capacity values, while the highest and the lowest replicative capacities were measured for two R5 viruses from the same patient.
Sensitivity to neutralization by heterologous sera.
The analysis of the sensitivity to neutralization by heterologous sera provides an estimation of the intrinsic sensitivity of viruses to neutralization and can be useful to compare viruses obtained from different patients. On the other hand, autologous neutralization provides information on virus evolution and on the specificity of the humoral response.
The sensitivity of different clones to neutralization was measured on P4C5 target cells, because these cells allow the direct comparison of viruses with different chemokine receptor usage. Dilutions of control serum (a pool of five HIV-negative sera) were performed for all experiments, and no significant inhibition was measured. The inhibitory effect of each positive serum was calculated by comparing infectivity in the presence of the same dilution of control sera. To draw inhibition curves, infection in the absence of human serum was taken as 100% infectivity.
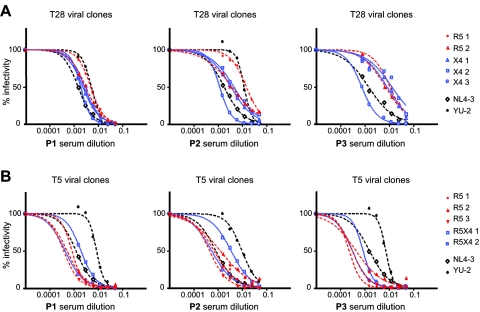
We initially used three pools of sera, each composed of serum from three HIV-infected patients. The samples corresponded to the time when HIV infection was diagnosed, and these individuals were asymptomatic. The sensitivity of two prototypic viral clones was measured and used as reference in all experiments: NL4-3, a tissue culture-adapted X4 virus, and YU-2, an R5 isolate. As previously shown (48), NL4-3 was found to be highly sensitive to neutralization, while YU-2 was relatively resistant (Fig. 2A and 2B).
FIG. 2.
Titration curves of plasma-derived viral clones with different tropism against the three serum pools (P1, P2, and P3) from HIV-1-positive asymptomatic individuals. Percentages of infection are plotted against the serum dilutions. R5 tropic viral clones are indicated in red, and viruses using CXCR4 exclusively (X4) or in addition to CCR5 (R5X4 or dual tropic) are indicated in blue. The reference viruses NL4-3 and YU-2 are indicated in black. Values represent the mean of triplicate wells. The graphic is representative of at least two independent experiments. (A) Neutralization curves of viral clones from patient T28. (B) Neutralization curves of viral clones from patient T5.
Interestingly, a wide range of neutralization sensitivities was observed for the viral clones isolated from patient T28 (Fig. 2A). This disparity was particularly evident when the serum pools P2 and P3 were used. The clone T28-X4-3 was consistently the most susceptible clone, while R5-1 was the least susceptible. The order of sensitivity of the other clones depended on the serum pool used. R5 clones from this patient appeared less susceptible to neutralization than X4 clones when serum pool P1 was used, but this was not the case with the other sera.
Similar variability in the level of sensitivity to neutralization was also observed for the clones isolated from patient T5 (Fig. 2B), although these clones were overall more sensitive to neutralization than clones obtained from patient T28. Most of the clones from patient T5 were as sensitive as NL4-3 when tested with serum pools P1 and P3. Again, sensitivity to neutralization did not appear to correlate with virus tropism.
The EC50 values of each virus and serum pairing are reported in a matrix format in Table 1. Although less informative than actual neutralization curves, this representation has the advantage of allowing the quantification of the differences observed in neutralization sensitivity. The diversity of EC50 values obtained with different clones from patient T28 amounted to a 17-fold difference (serum pool P3), and a difference of 7.5-fold was observed for viral clones from patient T5 (when tested with serum pool P2).
TABLE 1.
Antibody neutralization titers (EC50) for paired virus/serum samples
| Serum | Virus
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NL4-3 | YU-2 | T28
|
T5
|
|||||||||
| R5-1 | R5-2 | X4-1 | X4-2 | X4-3 | R5-1 | R5-2 | R5-3 | R5X4-1 | R5X4-2 | |||
| P1 | 0.00124 | 0.00884 | 0.00505 | 0.00313 | 0.00238 | 0.00279 | 0.00194 | 0.00085 | 0.00053 | 0.00039 | 0.00199 | 0.00044 |
| P2 | 0.00082 | 0.00991 | 0.01506 | 0.00378 | 0.00340 | 0.00451 | 0.00119 | 0.00069 | 0.00123 | 0.00046 | 0.00347 | 0.00055 |
| P3 | 0.00159 | 0.00756 | 0.01096 | 0.00711 | 0.00816 | 0.01298 | 0.00076 | 0.00023 | 0.00045 | 0.00032 | 0.00087 | 0.00032 |
| DRO | 0.00643 | 0.01138 | 0.00839 | 0.00718 | 0.02199 | 0.01413 | 0.00085 | |||||
| CAB | 0.00197 | 0.00409 | 0.00247 | 0.00344 | 0.00651 | 0.00528 | 0.00105 | |||||
| JAN | 0.00163 | 0.01705 | 0.00263 | 0.00147 | 0.00723 | 0.00408 | 0.00051 | |||||
| DRO*a | 0.01552 | NDb | 0.00017 | 0.00083 | 0.00009 | 0.00030 | 0.00016 | |||||
| CAB*a | 0.00493 | 0.02378 | 0.00056 | 0.00240 | 0.00046 | 0.00700 | 0.00078 | |||||
| JAN*a | ND | ND | 0.00019 | 0.00016 | 0.00010 | 0.00039 | 0.00013 | |||||
| Late T28 | 0.03517 | >0.05 | 0.00810 | 0.00255 | 0.00684 | 0.00263 | 0.00409 | |||||
| Late T5 | 0.00831 | >0.05 | >0.05 | 0.01083 | 0.00013 | 0.00011 | 0.00009 | 0.00018 | 0.00014 | |||
| Early T5 | >0.05 | >0.05 | 0.00549 | 0.00428 | ND | >0.05 | 0.00553 | |||||
Serum samples DRO*, CAB*, and JAN*, used to neutralize viral clones from patient T5, were collected at an earlier time point than those used to neutralize viral clones from patient T28.
ND, not determined.
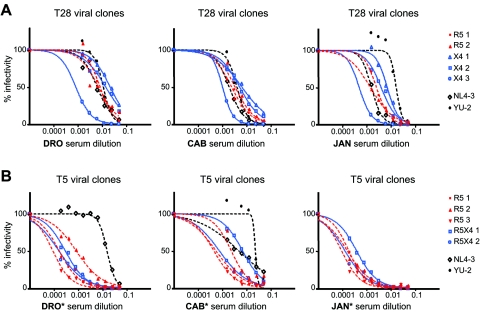
In a separate set of experiments, heterologous neutralization was measured using three different heterologous sera from single patients (patients DRO, CAB, and JAN; designations for patients were randomly selected and are not patients’ initials) whose inhibitory capacities were previously characterized as weak, average, and strong, respectively, using peripheral blood mononuclear cell-derived primary viruses of different subtypes (5). These sera were obtained from treatment-naïve long-term-infected patients, with viral loads ranging from 3.3 to 4.5 log10 RNA copies/ml.
Neutralization curves of reference viruses and viral clones from patient T28 are shown in Fig. 3A. As expected, NL4-3 was easily neutralized by all sera, whereas YU-2 displayed higher resistance to neutralization, the extent of which depended on the serum used. As for the assay conducted with the serum pools, the clone T28-X4-3 was particularly sensitive to neutralization. The wide range of neutralization sensitivity of viruses carrying envelope sequences isolated from patient T28 was particularly evident when tested with sera from DRO and JAN (Fig. 3A), with differences in the EC50 values of 26- and 14-fold, respectively (Table 1).
FIG. 3.
Titration curves of plasma-derived viral clones against three previously characterized sera (DRO, CAB, and JAN). Percentages of infection are plotted against the serum dilutions. R5-tropic viral clones are indicated in red; viruses using CXCR4 exclusively (X4) or in addition to CCR5 (R5X4 or dual-tropic) are indicated in blue. The reference viruses NL4-3 and YU-2 are indicated in black. Values represent the means of triplicate wells. The graphic is representative of at least two independent experiments. (A) Neutralization curves of viral clones from patient T28. (B) Neutralization curves of viral clones from patient T5. Asterisks indicate that serum samples DRO*, CAB*, and JAN*, used to neutralize viral clones from patient T5, were collected at an earlier time point than those used to neutralize viral clones from patient T28.
Due to the limited quantity of serum available from patients CAB, DRO, and JAN, the sera of these patients used for the neutralization of clones from patient T5 corresponded to time points that were earlier (more than 2 years) than those used for patient T28. The lower neutralization potency of these earlier sera, indicated as DRO*, CAB*, and JAN* in Fig. 3B, can be appreciated by comparing the neutralization profiles of reference strains NL4-3 and YU-2 in Fig. 3A and 3B. Despite the relatively low neutralization potency of these sera, we could confirm the higher neutralization sensitivity of the clones obtained from patient T5 compared to those obtained from patient T28. The EC50 of these sera on some T5 clones was lower than 1/5,120, which corresponded to the highest serum dilution tested here. With serum from CAB*, 15-fold differences in EC50 values were measured (Table 1).
To assess that differences in neutralization sensitivity were not the result of mutations inserted by the amplification or mutagenesis procedure, NL4-3 viral particles were diluted in donor plasma and subjected to the same procedure used to obtain patient-derived clones. Several independent reconstructed NL4-3 clones were analyzed with serum pool 2, and their neutralization curves were virtually indistinguishable (data not shown).
Sensitivity to neutralization by autologous sera.
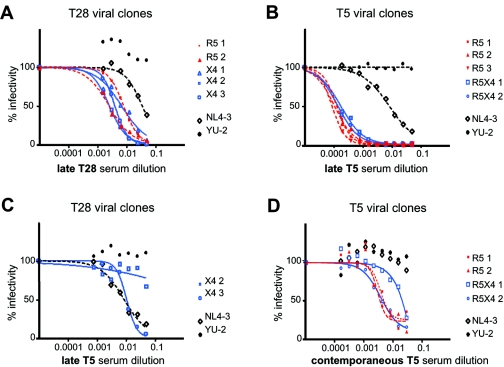
In order to test the sensitivity of different viral clones to autologous neutralization, we used the sera of patient T28 and T5 collected 35 and 21 months, respectively, after the plasma sample from which viral clones were reconstructed. For both patients, these “late” sera samples corresponded to an advanced symptomatic phase of infection, following a first-line treatment failure.
The neutralization curves of the reference strains show that these sera were characterized by a weak neutralizing activity, since YU-2 was not inhibited even at high serum concentrations (Fig. 4A and 4B). They also indicate that reverse transcriptase inhibitors, supposedly present in the plasma of these treated patients, did not exert a measurable inhibitory effect.
FIG. 4.
Neutralization of plasma-derived viral clones by autologous sera. Percentages of infection are plotted against the serum dilutions. R5-tropic viral clones are indicated in red; viruses using CXCR4 exclusively (X4) or in addition to CCR5 (R5X4 or dual-tropic) are indicated in blue. The reference viruses NL4-3 and YU-2 are indicated in black. Values represent the means of triplicate wells. The graphic is representative of at least two independent experiments. (A) Neutralization of viral clones from patient T28 by its autologous late serum, collected 35 months after the sample from which viral clones were isolated. (B) Neutralization of viral clones from patient T5 by the autologous late (21 months) serum. (C) Heterologous neutralization of viral clones from patient T28 by the late T5 serum. (D) Neutralization of viral clones from patient T5 by its autologous contemporaneous serum.
Analysis of viral clones from patient T28 showed a modest degree of variability (3.2-fold differences in EC50) (Table 1). Of note, we did not observe a correlation between virus chemokine receptor usage and sensitivity to autologous neutralization (Fig. 4A). Interestingly, the clone T28-X4-3, which was the most sensitive clone in the heterologous neutralization analyses, showed higher resistance to neutralization than clones T28-R5-2 and T28-X4-2 when tested with the autologous serum, indicating that neutralization sensitivity is not an intrinsic property of this virus but depends on the serum used.
Clones isolated from patient T5 showed a remarkable sensitivity to neutralization by the autologous serum, with all viruses being neutralized more than 50% in the presence of serum diluted 1/5,120. The neutralization curves for the clones from patient T5 were very similar, and only a twofold difference was observed among their EC50 values (Table 1).
It is worth mentioning that for both T28 and T5 sera, the specificity of the immune response against autologous viruses becomes evident by comparing the neutralization curves of reference viruses, which are poorly neutralized, and patient-derived clones, which are strongly inhibited. In this respect, we also tested two X4 clones from patient T28 against the late serum from patient T5 (Fig. 4C). This serum only partially inhibited the heterologous viruses from patient T28, and the neutralizing profiles of these clones clearly differed from those from patient T5 (Fig. 4B).
Finally, for patient T5, we could test the viral clones against the “autologous contemporaneous” serum, a serum sample obtained at the same time point from which viral envelope sequences were amplified. The neutralizing activity of this serum was clearly lower than that of the late serum from this patient, not only for reference strains, which were not inhibited by this serum, but also for autologous viruses (Fig. 4D). Inhibition of 50% of virus infectivity was measured at serum dilutions between 1/20 and 1/320. This stands in contrast to the results obtained with the late serum, for which the EC50 values were below a 1/5,120 dilution. These results underline the increase in the potency of the immune response with time. In contrast to autologous neutralization by the late sera, EC50 values of individual clones differed up to 12-fold by use of the autologous contemporaneous serum (Table 1), showing that viruses with clearly different sensitivities to antibody-mediated neutralization coexisted in the plasma virus population of this patient.
Envelope sequence analyses of viral clones.
The PCR-amplified and cloned envelope sequences were entirely sequenced for all clones by use of the primers E20, J53Y, and FuB (Fig. 5). Sequence analysis showed that all clones were different. In addition, the genotypic prediction of virus tropism, based on the charge of residues 11 and 25 of the V3 loop and on the overall charge of this region (19, 40), was in agreement with the phenotypic determination of chemokine receptor usage. Of note, genotypic predictions do not distinguish between X4- and dual-tropic variants. Accordingly, the two clones able to use both CCR5 and CXCR4 (from patient T5) were identified as X4 variants.
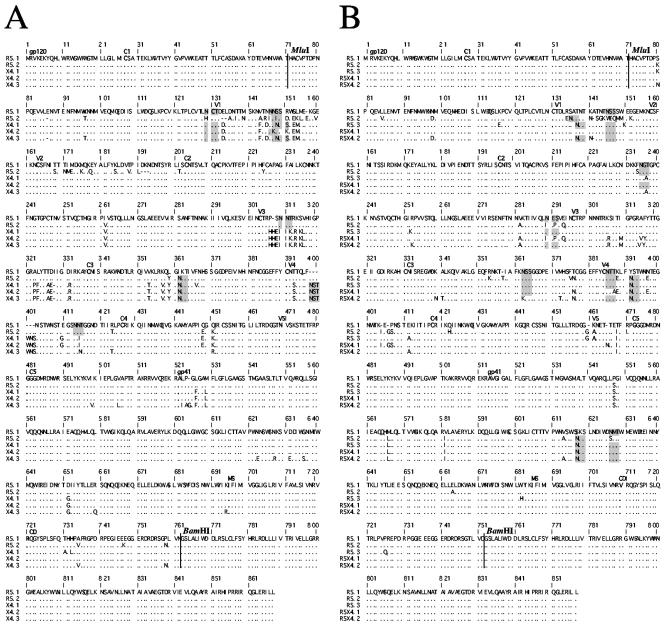
FIG. 5.
Full-length envelope sequences of viral clones from patient T28 (A) and patient T5 (B) used in this study. The N-terminal 71 residues of the envelope glycoproteins (upstream of the MluI restriction site) as well as the C-terminal 106 residues (downstream of the BamHI restriction site) are derived from NL4-3. Amino acid identity (·), insertions/deletions (−), or substitutions are indicated. The first amino acid of each variable (V) and conserved (C) region is indicated in boldface. The potential N-linked glycosylation sites that differed in clones from the same patient are shown as shaded boxes.
Sequence analysis allows the determination of the number and position of potential N-linked glycosylation sites, which were previously shown to modulate the neutralization sensitivity of primary viruses (4, 10, 39, 48, 54). We observed that several positions of potential glycosylation sites differed in the clones from each patient, whereas their overall number was relatively constant, with 30 to 31 sites per clone for patient T28 (variations in V1, V3, and V4) and 25 to 28 sites for the clones of patient T5 (variations in V1, C2, C3, V4, and gp41). Some of the sites that were present in some and absent in other clones corresponded to positions at the beginning of the V4 domain (Fig. 5) that were previously observed to evolve in longitudinal follow-up of patients (54). Note, however, that in our analysis, these differences correspond to sequence variability of coexisting viruses and not of sequential isolates. We observed additional differences in potential glycosylation sites (Fig. 5), in particular at positions 130, 147, 310, and 362 for patient T28 and 136, 146, 235, 290, 363, 386, and 392 as well as 618 and 627 in gp41 for patient T5. No single site appears to determine the neutralization sensitivity. However, the lower overall number of potential glycosylation sites in clones from patient T5 correlates with the higher sensitivity to neutralization of these clones compared to those of patient T28.
DISCUSSION
Analysis of the viral genomes present in the plasma of HIV-infected patients provides insight into the potency and the nature of the selective forces that shape plasma virus populations throughout the course of disease progression and during treatment. Treatment of patients with effective antiretroviral therapy results in a rapid and dramatic reduction of viremia. With time, in a percentage of patients, viral variants harboring changes in the viral enzymes targeted by the treatment are selected and expand with kinetics that depend on the selective advantage conferred by the mutations. The impact of the immune pressure on plasma virus population has been more difficult to establish. Data from infected patients as well as from animal models allowed identification of the HIV-specific cytotoxic-T-lymphocyte response as a key determinant of the set point of viremia following primary HIV infection (6, 21). Despite remarkable research efforts in this field, the role of the humoral immune response has remained more elusive (2, 3, 17, 25, 28, 29, 31, 32, 34-38, 46). Recent studies, facilitated by improvements in the techniques used to reconstruct viruses carrying plasma-derived envelope sequences, have permitted a better estimate of the kinetics of development of neutralizing antibodies in recently infected patients (41, 54). These studies document the production of neutralizing antibody in a percentage of patients only weeks after HIV infection. In these patients, rapid selection of virus variants with reduced sensitivity to neutralization initiates a cycle in which the immune system needs to keep a constant adaptive pace. Selection of viral variants able to resist neutralization is probably even faster than the selection of drug-resistant variants, because the targets of neutralization, the viral envelope glycoproteins, are naturally more variable and polymorphic than viral enzymes targeted by current regimens.
In this setting, coexisting viral variants are expected to be characterized by similar neutralization sensitivities. In the study by Wei and colleagues, this was indeed the case for viruses carrying envelope sequences obtained from three contemporaneous variants, obtained 16 days after the onset of symptoms of acute HIV infection (54). When two variants obtained at a later time point (day 212) were compared, however, a 10-fold difference in their sensitivities to neutralization was observed (54). Both variants were more resistant (10 and 100 times) than the earlier-time-point variants. The difference between the homogeneity of the clones from day 16 and the variability of clones from day 212 could be due to the fact that the virus population is quite homogeneous early in infection compared to later time points (13, 45). Analysis of early time points in infected patients may have underestimated the extent and the impact of the variability in neutralization sensitivity among coexisting viral variants. Interestingly, a recent study in which viruses present in donor-recipient couples were analyzed showed that recently transmitted viruses were more sensitive to neutralization than those of the donors and exhibited a narrower range of sensitivity to neutralization (14).
In our study, we analyzed coexisting viral variants isolated from patients with a longer history of infection. Both patients were symptomatic at the time when the plasma samples used here were collected, with low CD4 counts (below 50 per mm3) and relatively high viremia (equal to or above 105 copies/ml). As previously shown, plasma samples from these patients harbored both CCR5- and CXCR4-tropic viral variants (47), a feature that permits the comparison of the neutralization sensitivities of viruses with different chemokine receptor utilization characteristics. We analyzed neutralization by both autologous and heterologous sera. Neutralization by heterologous sera reveals the general sensitivity of a virus to neutralization. We found that individual viral clones were characterized by up to 25-fold differences in their sensitivities to heterologous neutralization. This could be due to the fact that the neutralization by heterologous sera relies on the cross-recognition of epitopes, the efficacy of which depends on the conservation of the epitope in different viruses from different patients. Widely neutralizing antibodies, which would provide more-homogeneous neutralization profiles, were probably absent at least from the pooled sera, given that they were obtained from asymptomatic patients. Interestingly, the order of sensitivity to neutralization of viral clones was dependent on the sera used, suggesting that different epitopes were indeed recognized by each serum.
In some instances, heterologous neutralization provided homogeneous neutralization curves for the clones obtained from one patient (e.g., pool P1 against viruses for patient T28 or serum JAN* against viruses for patient T5). This observation suggests that neutralizing antibodies, targeting epitopes that are conserved among different clones from one patient, were present in these sera. Interestingly, the neutralizing titers of these sera were comparable to those of the autologous late sera, confirming that heterologous neutralization can be relatively potent. Neutralization by the autologous sera, obtained 21 and 35 months (for patient T5 and T28, respectively) after the plasma sample from which viral clones were isolated, showed that a strain-restricted neutralizing response was mounted and persisted in both patients. Neither patient appeared to have developed broadly neutralizing antibody, since a reference virus known to be highly sensitive to neutralization (NL4-3) was inefficiently neutralized by these sera. Sensitivity to neutralization by the autologous sera was relatively homogeneous among clones from each patient (two to threefold differences), supporting the idea of the impact of the selection by antibodies in determining the composition of plasma virus population.
The most puzzling observation, however, was that highly variable neutralizing titers were observed when viral clones from patient T5 were challenged by the autologous contemporaneous serum. This finding shows that viral variants coexisting in the plasma population differed in their sensitivity to neutralization not only by heterologous sera but also by the antibodies circulating in the bloodstream of this patient at the very same time. In view of the rapid clearance of susceptible viral variants and the continuous change in the specificity of the antibody response reported in recent studies, one would expect a rather homogeneous sensitivity to neutralization by the autologous contemporaneous serum. Of note, a dual-tropic clone from this patient (T5-R5X4-1) appeared to be more resistant to the autologous contemporaneous serum than the other clones. Interestingly, the plasma virus population of patient T5 at this time point was composed predominantly of dual-tropic variants, which persisted for at least 21 months (47). These observations suggest that a more-resistant variant was recently selected and emerged in the virus population. Alternatively, the observed variability may suggest that different selective pressures contribute to define the composition of the plasma virus population at any given time point. Antibody-sensitive variants may still be advantaged in terms of replicative capacity in vivo, sensitivity to chemokines, or T-cell lysis, among other mechanisms. On the other hand, detection of neutralization-sensitive variants in the plasma virus population could also reflect virus replication taking place in tissue compartments where the selective pressure by antibodies is lower.
One additional feature of our study was the possibility of analyzing neutralization sensitivity of primary viruses as a function of chemokine receptor usage. The emergence of viruses using CXCR4 is generally associated with a more-rapid decrease in the number of CD4+ T cells and with faster disease progression than those observed in patients with a persistent R5 virus population (12, 44). This observation supports the hypothesis that X4 variants are responsible for the deterioration of the immune function. Alternatively, the emergence of X4 variants can be interpreted as the consequence, and not the cause, of the loss of immune competence. Selection against X4 variants, exerted by the immune system, could explain their delayed and infrequent emergence despite the fact that only a few amino acid changes in the envelope are sufficient to switch to CXCR4 usage (11, 15, 18). Neutralizing antibodies could participate to limit the spread of X4 viruses. Accordingly, T-cell-line-adapted X4 viruses have been shown to be more sensitive to neutralizing antibodies than R5 viruses (7, 48). This difference in sensitivity, however, was not confirmed when primary X4 and R5 viruses were compared (9, 24, 30, 50, 56), suggesting that increased sensitivity of T-cell-line-adapted strains was the result of virus growth in culture (7, 48). We took advantage of the specificity of our clones, which represent closely related coexisting primary viruses, to address this point. The neutralization curves of R5 and X4 (or R5X4) viruses were indistinguishable and intersected each other. Two exceptions were observed; these would, however, support opposing interpretations: R5 clones from patient T28 appeared more resistant than X4 clones when tested with pool P1, while R5 clones from patient T5 appeared more sensitive than CXCR4-using clones when tested with late serum T5. Altogether, these results argue against an intrinsic difference in neutralization sensitivity associated with virus tropism.
Finally, it was demonstrated that not only the overall number but also the position of glycosylation sites is a determinant of virus sensitivity to neutralization (4, 10, 39, 48, 54). Viral clones isolated from patient T5 showed a lower number of potential N-glycosylation sites (between 25 and 28 sites) compared to the clones from patient T28 (30 to 31 sites). Viral clones from patient T5 were more sensitive to neutralization than those of patient T28 with all sera tested here. The differences in neutralization sensitivity among different clones from each patient, despite the conservation of the final number of glycosylation sites, is in agreement with the recent glycan shield model (54), according to which differences in the position of glycosylation sites (and not necessarily in the overall number) can result in major differences in sensitivity to neutralization, a strategy used by HIV to escape antibody-mediated neutralization.
Acknowledgments
This work was supported in part by the French National Agency for AIDS Research (ANRS) and Sidaction. K.S. was the recipient of a fellowship from ANRS.
REFERENCES
- 1.Aasa-Chapman, M. M., A. Hayman, P. Newton, D. Cornforth, I. Williams, P. Borrow, P. Balfe, and A. McKnight. 2004. Development of the antibody response in acute HIV-1 infection. AIDS 18:371-381. [DOI] [PubMed] [Google Scholar]
- 2.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Back, N. K., L. Smit, J. J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199:431-438. [DOI] [PubMed] [Google Scholar]
- 5.Barin, F., S. Brunet, D. Brand, C. Moog, R. Peyre, F. Damond, P. Charneau, and F. Barre-Sinoussi. 2004. Interclade neutralization and enhancement of human immunodeficiency virus type 1 identified by an assay using HeLa cells expressing both CD4 receptor and CXCR4/CCR5 coreceptors. J. Infect. Dis. 189:322-327. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R. 1997. A vaccine for HIV type 1: the antibody perspective. Proc. Natl. Acad. Sci. USA 94:10018-10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 9.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchi, F., A. L. DeVico, A. Garzino-Demo, A. Cara, R. C. Gallo, and P. Lusso. 1996. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat. Med. 2:1244-1247. [DOI] [PubMed] [Google Scholar]
- 12.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delwart, E. L., H. W. Sheppard, B. D. Walker, J. Goudsmit, and J. I. Mullins. 1994. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 68:6672-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derdeyn, C. A., J. M. Decker, F. Bibollet-Ruche, J. L. Mokili, M. Muldoon, S. A. Denham, M. L. Heil, F. Kasolo, R. Musonda, B. H. Hahn, G. M. Shaw, B. T. Korber, S. Allen, and E. Hunter. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019-2022. [DOI] [PubMed] [Google Scholar]
- 15.Fouchier, R. A., M. Groenink, N. A. Kootstra, M. Tersmette, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1992. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 66:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geffin, R., C. Hutto, C. Andrew, and G. B. Scott. 2003. A longitudinal assessment of autologous neutralizing antibodies in children perinatally infected with human immunodeficiency virus type 1. Virology 310:207-215. [DOI] [PubMed] [Google Scholar]
- 17.Haigwood, N. L., and L. Stamatatos. 2003. Role of neutralizing antibodies in HIV infection. AIDS B(Suppl.):S67-S71. [DOI] [PubMed]
- 18.Hwang, S. S., T. J. Boyle, H. K. Lyerly, and B. R. Cullen. 1992. Identification of envelope V3 loop as the major determinant of CD4 neutralization sensitivity of HIV-1. Science 257:535-537. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, M. A., and A. B. van 't Wout. 2003. Predicting HIV-1 coreceptor usage with sequence analysis. AIDS Rev. 5:104-112. [PubMed] [Google Scholar]
- 20.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 23.Labrosse, B., A. Brelot, N. Heveker, N. Sol, D. Schols, E. De Clercq, and M. Alizon. 1998. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J. Virol. 72:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCasse, R. A., K. E. Follis, T. Moudgil, M. Trahey, J. M. Binley, V. Planelles, S. Zolla-Pazner, and J. H. Nunberg. 1998. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J. Virol. 72:2491-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lathey, J. L., R. D. Pratt, and S. A. Spector. 1997. Appearance of autologous neutralizing antibody correlates with reduction in virus load and phenotype switch during primary infection with human immunodeficiency virus type 1. J. Infect. Dis. 175:231-232. [DOI] [PubMed] [Google Scholar]
- 26.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowitz, M., M. Louie, A. Hurley, E. Sun, M. Di Mascio, A. S. Perelson, and D. D. Ho. 2003. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J. Virol. 77:5037-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 30.Montefiori, D. C., R. G. Collman, T. R. Fouts, J. Y. Zhou, M. Bilska, J. A. Hoxie, J. P. Moore, and D. P. Bolognesi. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori, D. C., T. S. Hill, H. T. Vo, B. D. Walker, and E. S. Rosenberg. 2001. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J. Virol. 75:10200-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moog, C., H. J. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantaleo, G., S. Menzo, M. Vaccarezza, C. Graziosi, O. J. Cohen, J. F. Demarest, D. Montefiori, J. M. Orenstein, C. Fox, L. K. Schrager, et al. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 332:209-216. [DOI] [PubMed] [Google Scholar]
- 34.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed] [Google Scholar]
- 36.Pellegrin, I., E. Legrand, D. Neau, P. Bonot, B. Masquelier, J. L. Pellegrin, J. M. Ragnaud, N. Bernard, and H. J. Fleury. 1996. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J. Acquir. Immune Defic. Syndr. Hum. Retrovir. 11:438-447. [DOI] [PubMed] [Google Scholar]
- 37.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924-932. [DOI] [PubMed] [Google Scholar]
- 38.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 39.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 40.Resch, W., N. Hoffman, and R. Swanstrom. 2001. Improved success of phenotype prediction of the human immunodeficiency virus type 1 from envelope variable loop 3 sequence using neural networks. Virology 288:51-62. [DOI] [PubMed] [Google Scholar]
- 41.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 43.Sanders-Buell, E., M. O. Salminen, and F. E. McCutchan. 1995. Sequencing primers for HIV-1, p. III 15-III 21. In G. Myers, B. Hahn, K. T. Jeang, J. W. Mellors, F. McCutchan, L. E. Henderson, and G. N. Pavlakis (ed.), Human retroviruses and AIDS 1995. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N. Mex.
- 44.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, H. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X. L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73:10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 47.Skrabal, K., V. Trouplin, B. Labrosse, V. Obry, F. Damond, A. J. Hance, F. Clavel, and F. Mammano. 2003. Impact of antiretroviral treatment on the tropism of HIV-1 plasma virus populations. AIDS 17:809-814. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trkola, A., T. Ketas, V. N. Kewalramani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trouplin, V., F. Salvatori, F. Cappello, V. Obry, A. Brelot, N. Heveker, M. Alizon, G. Scarlatti, F. Clavel, and F. Mammano. 2001. Determination of coreceptor usage of human immunodeficiency virus type 1 from patient plasma samples by using a recombinant phenotypic assay. J. Virol. 75:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 53.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 55.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 56.York, J., K. E. Follis, M. Trahey, P. N. Nyambi, S. Zolla-Pazner, and J. H. Nunberg. 2001. Antibody binding and neutralization of primary and T-cell line-adapted isolates of human immunodeficiency virus type 1. J. Virol. 75:2741-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]