Abstract
The smallpox vaccine is the prototypic vaccine, yet the viral targets critical for vaccine-mediated protection remain unclear in humans. We have produced protein microarrays of a near-complete vaccinia proteome and used them to determine the major antigen specificities of the human humoral immune response to the smallpox vaccine (Dryvax). H3L, an intracellular mature virion envelope protein, was consistently recognized by high-titer antibodies in the majority of human donors, particularly after secondary immunization. We then focused on examining H3L as a valuable human antibody target. Purified human anti-H3L antibodies exhibited substantial vaccinia virus-neutralizing activity in vitro (50% plaque reduction neutralization test [PRNT50] = 44 μg/ml). Mice also make an immunodominant antibody response to H3L after vaccination with vaccinia virus, as determined by vaccinia virus protein microarray. Mice were immunized with recombinant H3L protein to examine H3L-specific antibody responses in greater detail. H3L-immunized mice developed high-titer vaccinia virus-neutralizing antibodies (mean PRNT50 = 1:3,760). Importantly, H3L-immunized mice were subsequently protected against lethal intranasal challenges with 1 or 5 50% lethal doses (LD50) of pathogenic vaccinia virus strain WR, demonstrating the in vivo value of an anti-H3L response. To formally demonstrate that neutralizing anti-H3L antibodies are protective in vivo, we performed anti-H3L serum passive-transfer experiments. Mice receiving H3L-neutralizing antiserum were protected from a lethal challenge with 3 LD50 of vaccinia virus strain WR (5/10 versus 0/10; P < 0.02). Together, these data show that H3L is a major target of the human anti-poxvirus antibody response and is likely to be a key contributor to protection against poxvirus infection and disease.
Vaccines are one of the most cost-effective medical treatments in modern civilization (51). A smallpox vaccine was the first human vaccine, and vaccinia virus (VV) is considered the most successful human vaccine, having brought about the worldwide eradication of smallpox disease (20). Nevertheless, the mechanisms of adaptive immune protection elicited by the smallpox vaccine in humans generally remain unclear. There is currently greatly renewed interest in smallpox immunity due to the possible threat of bioterrorism (29). Given this concern, there has been much discussion about both the mechanisms of protection afforded by the smallpox vaccine and the possible development of safer alternatives to Dryvax, the current U.S. licensed human smallpox vaccine.
Our goal is to identify key antigenic targets of VV that are recognized by vaccinated humans and that are critical for protection against disease. These efforts are important for developing a clear understanding of the mechanisms of protection afforded by this prototypic vaccine. In addition, knowledge of key antigenic targets will be instructive for ongoing efforts to design alternative smallpox vaccines, as development and assessment of novel smallpox vaccines will be dependent on a detailed understanding of correlates of immunity.
Vaccines elicit three major types of immune responses that are each considered important in protective long-term immunity: antibodies, memory T cells, and memory B cells (11, 49, 56). Humans with either cellular or humoral immune deficiencies exhibit heightened susceptibility to poxvirus infection (38, 41). Antibodies are the body's first line of defense against infection, and circulating antibodies are the primary indicator of immunity for most human vaccines (11, 49). Antibodies can be protective against smallpox (variola virus) infection of humans (20, 39), presumably both by neutralizing the initial virus inoculum and by limiting the spread of virus particles within the host after infection is initiated. It is now clear from many studies that memory T cells (CD8, CD4, or a combination) are valuable for protection against a variety of infectious diseases (63), including poxviruses (4, 58, 60, 68). The smallpox vaccine is known to elicit T-cell responses in humans (12, 16, 22, 27), and VV-specific memory T cells are likely to be important components of the vaccine-mediated protection against smallpox virus (38, 41, 56). Memory B cells are also likely contributors to human immunity to smallpox, both by their ability to rapidly respond to infection with an anamnestic antibody response and by their potential ability to replenish long-lived plasma cells to maintain long-term serum antibody levels (5, 12). Given the renewed interest in smallpox, recent research efforts by a number of groups have focused on identifying the smallpox vaccine targets recognized by the different arms of the adaptive immune system, in both mice and humans (3, 21, 45, 58-60), to obtain information regarding potential correlates of immunity.
A variety of immunogenic VV antigens eliciting antibody responses have been identified in the literature, but the vast majority of that work was done in animal models. We have focused our efforts on understanding the human humoral immune response to VV. These efforts have centered on identifying the antigen specificities of the human anti-VV antibody response and determining which antibody targets are likely to be functionally valuable in protection. To do this, we utilized a novel proteomics approach to first globally identify the VV antigens recognized by sera from human Dryvax vaccinees. We found that H3L was a dominant antigen in the human antibody response. That observation led us to focus on understanding the biological relevance of H3L as a major target of human anti-VV antibody responses. We demonstrated that affinity-purified human anti-H3L exhibited VV-neutralizing activity in vitro. We then showed that mice immunized with recombinant H3L protein generated high titers of neutralizing antibodies. Importantly, H3L-immunized mice were subsequently protected against lethal intranasal challenges with pathogenic VV. We then formally demonstrated that neutralizing anti-H3L antibodies are protective in vivo by serum passive-transfer experiments. These data show that H3L is a major target of the human anti-poxvirus antibody response and is likely to be a key contributor to protection against poxvirus infection and disease.
MATERIALS AND METHODS
Sera.
For human antibodies we used vaccinia immune globulin (VIG; Cangene Corp., Winnipeg, Canada) and sera from a panel of 15 volunteers who donated blood before and 30 days after vaccination with Dryvax. Sera were stored as aliquots at −70°C. The individual donors were either vaccinia virus naïve at the time of vaccination (n = 4) or had been vaccinated against smallpox in childhood and were receiving a booster vaccination (n = 11). Mouse anti-vaccinia virus serum was obtained by retro-orbital bleed from BALB/c mice at various time points postinfection with 5 × 105 PFU VVWR or 1 × 105 PFU VVNYBOH administered intraperitoneally (i.p.). Mouse anti-H3L sera were generated by immunizing mice with purified recombinant H3L protein emulsified in Freund's complete adjuvant and boosting 3 weeks later with antigen in incomplete Freund's adjuvant (200 μg H3L/dose). Rabbit anti-H3L sera for passive-transfer experiments were generated by immunizing two rabbits with recombinant H3L protein emulsified in Freund's complete adjuvant and boosting seven times over 12 weeks with antigen in incomplete Freund's adjuvant (200 μg H3L/dose).
Viruses.
VVWR stocks were grown on HeLa cells in T175 flasks, infecting at a multiplicity of infection of 0.5. Cells were harvested at 60 h, and virus was isolated by rapidly freeze-thawing the cell pellet three times in a volume of 2.3 ml RPMI plus 1% fetal calf serum (FCS). Cell debris was removed by centrifugation. Clarified supernatant was frozen at −80°C as virus stock. VVWR stocks were titered on Vero cells (∼2 × 108 PFU/ml). VVNYBOH stocks were generated as low-passage stocks from commercial Dryvax, using the same conditions described for VVWR above.
Production and probing of vaccinia proteome microarray.
The high-throughput cloning and expression platform is described in detail elsewhere (15). Briefly, custom PCR primers comprising 20 bp of gene-specific sequence with 33 bp of “adapter” sequences were used in PCRs with vaccinia virus WR strain genomic DNA as a template. The adapter sequences, which become incorporated into the termini flanking the amplified gene, were homologous to the cloning site of the T7 expression vector pNHisCHA (Gene Therapy Systems, San Diego, CA) and allowed the PCR products to be cloned by homologous recombination in competent DH5α cells. The adapters also incorporated a 5′ polyhistidine epitope, an ATG translation start codon, and a 3′ hemagglutinin epitope and T7 terminator. Sequence-confirmed plasmids were expressed in 5-h in vitro transcription-translation reactions (RTS 100 kits from Roche) according to the manufacturer's instructions. Protein expression was monitored either by dot blot or microarray using both monoclonal anti-polyhistidine (clone His-1 from Sigma) and monoclonal anti-hemagglutinin (clone 3F10 from Roche) antibodies, followed by appropriate secondary antibodies. Microarrays were printed onto nitrocellulose-coated glass slides (FAST from Schleicher & Schuell Bioscience) using an Omni Grid 100 microarray printer (Gene Machines). Prior to array staining, the sera were diluted to 1/50 in Protein Array Blocking Buffer (Schleicher & Schuell Bioscience) containing Escherichia coli lysate at a final concentration of 10% and incubated at 18°C for 1 h with constant mixing. The arrays were rehydrated in blocking buffer for 30 min and probed with the pretreated sera for 1 to 2 h at 18°C with constant agitation. The slides were then washed five times in Tris buffer containing 0.05% Tween 20, and bound antibodies were detected by incubation in Cy3-conjugated goat anti-human immunoglobulin A (IgA) plus IgG plus IgM (heavy and light chains) secondary antibody (Jackson ImmunoResearch) diluted 1/2,500 in blocking buffer. After being washed five times, the slides were air dried under brief centrifugation and stored at 18°C in a desiccator. The arrays were examined in a GSI Lumonics ScanArray 4000 confocal glass slide scanner, and intensities were quantified using QuantArray software. All signal intensities were corrected for background, and vaccinia virus-specific signals were plotted as signal intensities.
Whole vaccinia virus ELISA.
VV antigen preparation for antibody enzyme-linked immunosorbent assay (ELISA) use was done by UV inactivating stock VVNYBOH with trioxsalen-psoralen (4′ aminomethyl-trioxsalen HCl; Calbiochem) (11, 12). VVNYBOH (108 PFU/ml) in 0.1% bovine serum albumin and 10 μg/ml trioxsalen was incubated for 10 min at room temperature and then UV inactivated with 2.25 J/cm2 (Stratalinker 1800; Stratagene, CA). This resulted in a >108-fold reduction in PFU. The UV-inactivated virus was then used at a 1:25 dilution in phosphate-buffered saline (PBS) with bovine serum albumin supplemented to a final concentration of 0.1%. Direct ELISA was done using Nunc Polysorp flat-bottomed 96-well plates coated overnight with 100 μl/well VV antigen. The plates were washed, and serum samples were added to the plates and serially diluted (twofold dilutions) in PBS plus 0.05%Tween 20 plus 10% FCS. Caltag horseradish peroxidase-conjugated goat anti-mouse IgGγ diluted 1:1,000 in PBS plus 0.05% Tween 20 plus 10% FCS was used for detection. The plates were developed using o-phenylenediamine, and the optical density at 490 nm (OD490) was read on a SpectraMax 250 (Molecular Devices). Anti-VV serum IgG antibody titers were determined as endpoint titers 0.1 OD unit more than background (no serum well).
Purification of H3L.
One-liter cultures of E. coli (strain BL21) transformed with pNHisCHA/H3L (VVWR strain H3L) were grown in the presence of kanamycin to an OD600 of 0.4 to 0.6 and induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) overnight. The cell pellets were resuspended in 5 ml lysis buffer (Bugbuster reagent from Novagen) per gram of cells. Lysates were clarified at 30,500 × g and frozen at −80°C. Cell extracts were loaded onto 1 ml or 5 ml nickel-coated columns (Amersham), washed in buffer A (500 mM NaCl, 20 mM NaH2PO4, 20 mM imidazole, 10% glycerol), and eluted in a stepwise gradient (200 to 500 mM) of imidazole. H3L elutes at 325 mM imidazole. The eluted protein was then dialyzed in PBS and adjusted to 1 to 1.5 mg/ml.
Affinity purification of H3L antibodies from VIG.
Recombinant H3L obtained from 1-liter cultures of IPTG-induced E. coli was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted onto nitrocellulose membranes. The H3L band was cut from the nitrocellulose and cut into fragments, blocked for 4 h in 5% fat-free milk powder in PBS, washed, and then incubated overnight with constant agitation at 4°C in 1 ml vaccinia immune globulin (VIG) diluted 1/10 in blocking buffer. After extensive washing (10 times in Tris-buffered saline plus 0.05% Tween 20), the bound antibody was eluted for 1 min in 0.1 M glycine-0.15 M NaCl, pH 2.4, and collected into 1 M Tris-HCl, pH 8.2. The eluate was then concentrated and transferred into serum-free Iscove’s modified Dulbecco’s medium using Centricon columns. Prior to neutralization assay, the purified antibody was quantified by Bradford assay, and the purity of the antibody was confirmed using Western blots of whole vaccinia virus particles transferred to nitrocellulose. Pure antibody gave a single band on Western blotting corresponding to H3L.
Plaque reduction neutralization tests (PRNT50).
CV-1 cells or VeroE6 cells were seeded into 12-well Costar plates (Corning Inc., Corning, NY) and used within 2 days of reaching confluence. Test sera were heat inactivated (56°C; 30 min), and twofold serial dilutions were performed in serum-free Iscove’s modified Dulbecco’s medium. The diluted sera were then incubated in an equal volume of sonicated vaccinia virus WR (103 PFU/ml) overnight at 37°C according to the method of Newman et al. (47). The CV-1 cells were rinsed in serum-free medium, the medium was aspirated, and 100 μl of virus-serum mixture was added to each well in duplicate and left to adsorb for 60 min at 37°C with periodic swirling. One milliliter of complete medium was then added, and the plates were incubated for 2 days for the plaques to develop. The medium was then aspirated, the cells were fixed and stained in one step with 0.1% crystal violet in 20% ethanol, and the plaques were quantified over white-light transillumination. The data were plotted, and a curve of best fit was applied by eye. The neutralization titer (NT50) was defined as the dilution of the serum that reduced the average number of plaques by 50% compared to the mean number of plaques of controls with no serum, according to the following formula (9): NT50 = DL + [(P50 − PL) (DH − DL)/(PH − PL)], where DL is the reciprocal of the lower dilution bracketing the 50% endpoint, DH is the reciprocal of the higher dilution bracketing the 50% endpoint, P50 is the number of plaques at the 50% endpoint, PL is the number of plaques at the lower dilution bracketing the 50% endpoint, and PH is the number of plaques at the higher dilution bracketing the 50% endpoint.
Vaccinia intranasal infections and protection studies.
Age-matched female BALB/c mice were used in all experiments. To infect the mice, VVWR was placed on the nares of the mouse, using a Pipetman, in a volume of 10 μl, which was then inhaled by the mouse. The mice were weighed daily from day 0 to day 21 to assess disease progression and the protective efficacies of vaccinations. After intranasal infection with VVWR, mice develop a systemic infection and exhibit severe weight loss. Unprotected mice begin to exhibit some weight loss by day 4 and have >30% weight loss by day 8, with nadir or death between days 8 and 12. A dose of 105 PFU VVWR was the standard lethal dose given to 10-week-old BALB/c females. In a series of pilot experiments, virus doses were titrated to establish LD50s for BALB/c females at different ages (data not shown), so that an appropriate LD50 dose was chosen for each vaccination experiment based on the age of the mice used. The mice used were all 6 to 13 weeks old at the time of challenge, depending on the experiment. We have validated that we obtain consistent results with this VVWR intranasal-challenge system (see Fig. 5; additional data not shown). Slight variations of this protection model are used by a number of laboratories, and it is a well-accepted murine model of protection against vaccinia virus (1, 4, 50, 52, 69). For H3L protection studies, female BALB/c mice (6 weeks old) were immunized with 50 μg purified H3L protein emulsified in Ribi adjuvant (first series of experiments) or complete Freund's adjuvant (Difco Laboratories, Detroit, MI) (second series of experiments) and then boosted 3 weeks later with H3L protein in Ribi (first series of experiments) or incomplete Freund's adjuvant (second series of experiments). Control mice received adjuvant alone. In passive-transfer protection experiments, rabbit polyclonal anti-H3L antiserum or control preimmune rabbit serum was injected intraperitoneally (200 μl) into groups of 10 BALB/c mice 1 day prior to intranasal challenge with VVWR as described above.
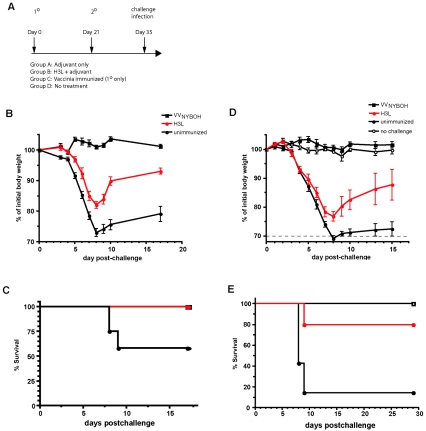
FIG. 5.
Protection of mice immunized with recombinant H3L protein against an intranasal challenge with VVWR. (A) Immunization and challenge protocol schematic. Mice were immunized twice with H3L protein in adjuvant, with the immunizations spaced 3 weeks apart. Control groups of mice received injections of adjuvant alone (first experiment only), or no injections, or a single immunization with 105 PFU VVNYBOH. 1°, primary; 2°, secondary. (B) Body weight was tracked after intranasal challenge with 1 LD50 of VVWR. H3L-immunized mice exhibited significantly less weight loss (P < 0.01) than group A and D control mice (unimmunized). No difference was observed between groups A and D (not shown). Groups A and D combined as “unimmunized,” n = 12; VVNYBOH, n = 4; H3L, n = 4. (C) Survival curve after intranasal challenge with 1 LD50 of VVWR. Approximately 50% of the unimmunized mice died from the intranasal VVWR infection, while all H3L-immunized mice survived (P < 0.02). (D) Body weight was tracked after intranasal challenge with 5 LD50 of VVWR. Weight loss was less severe in the H3L-immunized cohort (P < 0.01). (E) Survival curve after intranasal challenge with 5 LD50 of VVWR. H3L-immunized mice exhibited substantial protection from death compared to unimmunized mice (P < 0.02; 4/5 H3L, 1/7 unimmunized).
Statistical analysis.
Tests were performed using Prism 4.0 (GraphPad, San Diego, CA). Statistics were done using a two-tailed, unpaired t test with 95% confidence bounds unless otherwise indicated. Data from multiple experiments to be concurrently analyzed were first normalized prior to statistical testing. Data involving three or more groups were analyzed by one-way analysis of variance with a Bonferroni multiple-comparisons posttest. Error bars are ± 1 standard error of the mean unless otherwise indicated. Kaplan-Meier survival analysis was used for survival curves. Weight loss data were analyzed as “area under the curve” comparisons, with each mouse treated as an individual data point, and the significance of differences between experimental conditions was determined by one-way analysis of variance.
RESULTS
Proteome microarray screening identifies H3L as an immunodominant human antibody target.
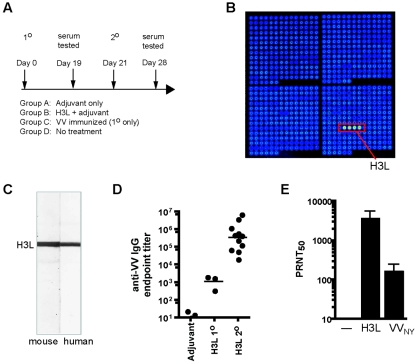
We developed VV proteome microarrays to enable us to elaborate a more comprehensive understanding of the human humoral immune response to the smallpox vaccine (15). We validated the microarray approach using VIG (15), and then we used the VV microarrays printed with 185 different VV proteins to screen serum samples from a panel of human volunteers both before and after VV immunization (Fig. 1). Intriguingly, H3L was a dominant component of the anti-VV human antibody response (Fig. 1). H3L is a VV surface protein expressed on intracellular mature virions (IMV) and participates in attachment of VV to target cells (13, 14, 44). Typically, the human anti-VV antibody response after secondary immunization was focused on a small subset of antigens, with H3L, A10L, and D13L targeted most heavily (Fig. 1A). H3L was of particular interest, as it is the only surface envelope protein among the three dominant antigens. Figure 1B shows H3L staining from representative human donors undergoing primary and secondary responses to vaccinia virus. A primary response is characterized by moderate titers of anti-H3L antibodies, although individuals vaccinated many decades previously often have detectable antibodies to H3L still remaining from the primary infection. A strong anamnestic response to H3L is observed after booster or secondary immunization (Fig. 1A to D). In addition, anti-H3L antibodies are heavily represented in VIG (Fig. 1B) (15). These observations led us to focus our immediate efforts on understanding H3L as a major target of the anti-VV human antibody response. A full survey of the proteomic data from vaccinated humans will be reported elsewhere (D. H. Davies, S. Crotty, and P. L. Felgner, unpublished data); here, we will focus on the relevance of H3L.
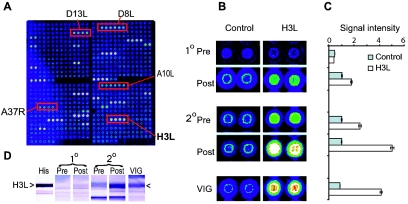
FIG. 1.
Protein microarray analysis of human antibodies generated during vaccinia infection reveals dominance of H3L. (A) VV proteome microarray showing representative antibody profile of a donor undergoing a secondary vaccinia virus infection. This donor was immunized 40 years previously and boosted with Dryvax; serum for probing the array was obtained 30 days after the boost. Annotated spots are vaccinia virus-specific antibody reactivities, whereas nonannotated signals are irrelevant non-VV “background” antibody reactivities that are also seen in Dryvax-naïve human sera (15). (B) Detail of proteome microarrays showing spots for H3L and control protein (nonexpressing plasmid) probed with sera from human donors before (Pre) and after (Post) Dryvax immunization. The data shown are representative of primary (1°) and secondary (2°) infections by a vaccinia virus-naive individual and a different previously vaccinated donor, respectively. In both cases, sera were taken 30 days after a Dryvax immunization. The previously vaccinated donor shows a typical anamnestic response to H3L. VIG is shown for comparison. Neutralization titers (PRNT50) were as follows: naive donor, pre = <1/10, post = 1/2,000; previously vaccinated, pre = 1/40, post = 1/3,000; VIG > 1/15,000. (C) Quantification of anti-H3L and control signal intensities from the arrays shown in panel B. (D) Immunoblot of H3L expressed in vitro, probed with the same sera used in panel B. The location of vaccinia virus H3L among the E. coli bands was determined by anti-histidine tag antibody (His). In arrays and immunoblots, sera were used at 1/50 dilution, whereas VIG was used at 1/500.
Human antibodies against H3L neutralize vaccinia virus.
H3L was a known target of human antibody responses from Western blot studies published by us and others (12, 17). Our new proteomics studies have put this into the context of the broader anti-VV humoral immune response (Fig. 1), showing that H3L is an immunodominant target of human anti-VV antibody responses. However, it is important to determine whether the human anti-H3L immunoglobulin (Ig) is valuable for protection against poxvirus infections. Does human anti-H3L possess poxvirus-neutralizing activity? To address this question, we affinity purified human anti-H3L Ig and tested it in vitro.
VIG recognizes 14 specific antigens on the VV proteome microarray (15), including H3L (Fig. 1B and C), and VIG is available in high concentration. Therefore, we used VIG as a source of human anti-H3L antibodies. Anti-H3L was affinity purified from VIG by panning over nitrocellulose-bound H3L protein and then eluting the anti-H3L Ig by low-pH wash (see Materials and Methods). Each batch of affinity-purified anti-H3L was of high purity, as verified by immunoblotting against whole vaccinia particles (Fig. 2A). The purified immunoglobulin concentration was determined by Bradford assay prior to use in experiments. Affinity-purified human anti-H3L was then tested for activity by VV PRNT50 (Fig. 2B). Human anti-H3L exhibited substantial neutralization activity against VV in vitro, with a top PRNT50 of 44 μg/ml (Fig. 2B). Statistical analysis of three independent virus neutralization experiments revealed a high level of statistical significance (P < 0.0001).
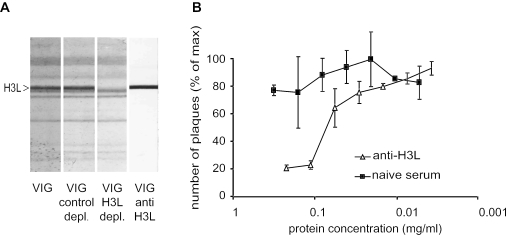
FIG. 2.
Human anti-H3L antibodies neutralize vaccinia virus in vitro. (A) Representative immunoblot of whole vaccinia virus particles used for screening affinity-purified anti-H3L antibodies. VIG control depl., VIG control depleted against E. coli proteins; VIG H3L depl., VIG depleted against H3L protein; VIG anti H3L, human anti-H3L antibodies affinity purified from VIG. The right middle lane unequivocally determines the location of the H3L band. (B) Plaque neutralization assays of monospecific human anti-H3L antibodies affinity purified from VIG. The PRNT50 of purified human anti-H3L was 44 μg/ml. One of three independent experiments is shown. Neutralization of VV by anti-H3L was statistically significant (P < 0.0001).
In reciprocal experiments, we depleted anti-H3L antibodies from human VIG or VV-immunized human serum (Fig. 2A), and we were able to demonstrate a reduction of neutralizing-antibody titers (data not shown). However, those data exhibited substantial variability, indicating the presence of additional neutralizing-antibody specificities contributing to total VV-neutralizing activity.
Mice immunized against H3L develop neutralizing antibodies.
Having established that human anti-H3L antibodies are a major component of the humoral immune response to the smallpox vaccine, and having established that human anti-H3L antibodies are VV neutralizing in vitro, we wanted to determine whether anti-H3L antibody responses are protective in vivo. Various experimental systems have shown that there is not a direct link between the in vitro virus neutralization activity of an antibody and the in vivo protection against disease afforded by antibodies (2, 18, 43, 61, 66). Therefore, it was necessary to test whether anti-H3L antibody responses are protective against poxvirus infection in vivo as well as in vitro. In addition, there has been substantial discussion in the literature about the putative protective value of anti-IMV versus anti-extracellular enveloped virus (EEV) antibody responses in vivo (see Discussion), and therefore, again, in vivo tests of the protective capacity of anti-H3L immunity were necessary.
We planned to address the value of an anti-H3L antibody response by using a murine VV challenge model system. However, first we needed to establish the validity of this model by determining the similarity of the murine and human antibody responses to VV and H3L after immunization. Mice were therefore immunized with VV, and their sera were analyzed by VV proteome array staining (Fig. 3). Some 21 different antigens were recognized by murine sera after VV immunization, including a strong antibody response to H3L (Fig. 3A). Quantification of signal intensities of the immunoreactive antigens for six different BALB/c mice is shown in Fig. 3B. Strikingly, the anti-H3L response was the strongest detected against a VV surface antigen, which was similar to what we observed in vaccinated humans (Fig. 1) (15). This conserved immunodominance of the anti-H3L antibody response in humans and mice indicated that H3L is a highly immunogenic protein.
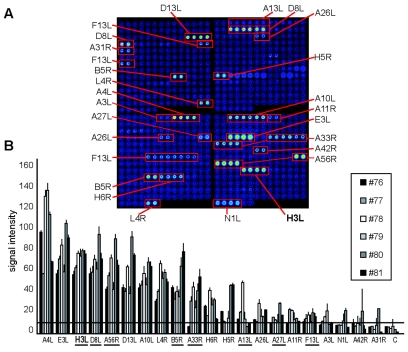
FIG. 3.
Mice immunized with vaccinia virus develop an immunodominant anti-H3L response. (A) Representative scan of a vaccinia virus proteome microarray probed with sera from an individual BALB/c mouse (no. 80) immunized i.p. 21 days previously with VVWR. The identities of these antigens have been described previously (15). Arrays probed with preimmune sera showed no reactivity in this array (15). (B) Quantification of arrays stained with sera from six individual mice immunized 21 days previously with VVWR illustrating the consistency of the responses. Underlined proteins are known envelope proteins. The horizontal line is a stringent cutoff, defined as the mean control signals plus three times the standard deviation. H3L is the strongest VV envelope protein recognized.
Mice were then immunized with purified recombinant H3L protein in adjuvant (Fig. 4A). Sera from the immunized mice specifically recognized H3L in the VV proteome arrays (Fig. 4B). In addition, the antiserum recognized authentic H3L from vaccinia virions in both immunoblots (Fig. 4C) and ELISAs (Fig. 4D) and was demonstrated to be IgG by ELISA. Most importantly, we needed to establish whether murine anti-H3L antibodies neutralize VV, as human anti-H3L antibodies do. Sera from H3L-immunized mice were tested by standard PRNT50 assay. Strong VV-neutralizing activity was detected in the murine anti-H3L serum (Fig. 4E). H3L protein-immunized mice actually possessed substantially higher neutralizing-antibody levels than VVNYBOH-immunized mice, demonstrating the potency of H3L as an antigenic target (Fig. 4E). In summary, there were strong similarities between the immunodominances and neutralizing activities of anti-H3L Ig in both VV-immunized humans and mice.
FIG. 4.
Mice immunized with H3L protein develop potent anti-VV neutralizing antibodies. (A) Immunization schematic. Mice were immunized twice with H3L protein in adjuvant, with the immunizations spaced 3 weeks apart. Control groups of mice received injections of adjuvant alone, or no injections, or a single i.p. immunization with 105 PFU VVNYBOH. The mice were monitored for anti-H3L responses at multiple time points postimmunization. 1°, primary; 2°, secondary. (B) Representative array scan of serum from a single mouse immunized with recombinant H3L adjuvanted in Ribi. (C) Representative immunoblot of serum from a single mouse immunized against recombinant H3L protein (left lane) probed against whole vaccinia virus particles (Fig. 2A). The right lane shows affinity-purified human anti-H3L to localize the H3L band. (D) Anti-VV IgG in H3L-immunized mice was quantified by ELISA. Sera from H3L-immunized mice were tested against VV-infected cell lysate. Anti-VV IgG levels were significantly above baseline after primary (P < 0.01; endpoint titer = 1,366) and secondary (P < 0.001; endpoint titer = 1.27 × 106) immunizations with H3L. (E) Anti-VV neutralizing-antibody titers (PRNT50) were measured in H3L-immunized mice and mice immunized with VVNYBOH. Mean neutralizing antibody titers in H3L-immunized mice were 3,760. Mean neutralizing antibody titers in 105 PFU VVNYBOH-immunized mice were 172.
Mice immunized with H3L are protected against intranasal vaccinia virus challenge.
On the basis of the neutralizing properties of anti-H3L antibodies, H3L was evaluated for its capacity to protect mice from a lethal intranasal challenge with VVWR. After intranasal infection with VVWR, unimmunized mice develop pneumonia and exhibit rapid weight loss and death within 7 to 9 days (data not shown; 1, 4, 21, 50, 52, 69). Immunization of mice i.p. with VVNYBOH or VVWR engenders full protective immunity against a subsequent intranasal challenge of virus (Fig. 5) (4, 21, 68).
Cohorts of mice were immunized twice with purified H3L protein in adjuvant (Fig. 5A). Anti-H3L responses were tracked by PRNT50, array-based ELISA, and traditional ELISA. High anti-H3L titers were present in all H3L-immunized mice prior to challenge (Fig. 4), with a mean PRNT50 of 1:3,760. Mice were then challenged with one 50% lethal dose (LD50) of VVWR (Fig. 5B and C). Control groups of mice immunized with the human smallpox vaccine (VVNYBOH) or nothing (adjuvant alone or no injection) were also challenged. Approximately 50% of the unimmunized mice died from the intranasal VVWR infection, whereas all H3L-immunized mice survived (Fig. 5C) (P < 0.02). H3L mice also exhibited significantly less weight loss than control mice (Fig. 5B) (P < 0.01). A parallel group of mice was challenged with a very high dose of VVWR (50 LD50), and the H3L-immunized mice were not protected against that high challenge dose (data not shown) (VVNYBOH-immunized mice were still protected against 50 LD50).
In a second experiment, H3L-immunized mice were challenged with 5 LD50 of VVWR, and those H3L-immunized mice exhibited substantial protection from death compared to unimmunized mice (survival, 4/5 H3L immunized and 1/7 unimmunized; P < 0.02) (Fig. 5E). In addition, morbidity and weight loss were much less severe in the H3L-immunized cohort (P < 0.01) (Fig. 5D). Therefore, immunization against H3L using adjuvanted protein can provide substantial protection against pathogenic respiratory poxvirus infection.
Passive transfer of H3L-neutralizing antibodies protects against intranasal VV challenge.
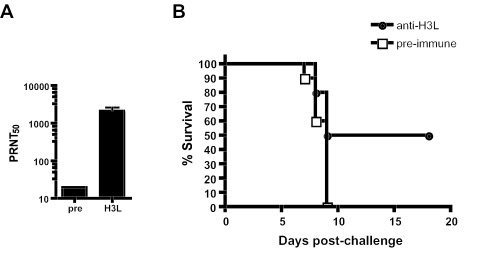
To formally demonstrate that neutralizing anti-H3L antibodies are protective, we performed passive-transfer experiments. A sufficient quantity of anti-H3L immunoglobulin for use in passive transfers was produced by immunizing rabbits with H3L protein and subsequently collecting the anti-H3L rabbit serum. The rabbit anti-H3L serum was H3L specific by proteome array (not shown) and was shown to be neutralizing by PRNT50 (Fig. 6A). The polyclonal anti-H3L antiserum was then injected intraperitoneally (200 μl) into BALB/c mice 1 day prior to intranasal challenge with 3 LD50 of VVWR; 100% of the mice that received irrelevant serum (preimmune) died, while passive immunotherapy with anti-H3L antibodies protected 50% of the mice (survival, 0/10 versus 5/10; P < 0.02) (Fig. 6B). In a repeat passive-transfer experiment, significant protection against morbidity and mortality was observed against a 1-LD50 challenge (P < 0.03), but not a 3-LD50 challenge. The partial protective efficacy in these experiments is likely related to the limited neutralization capacity of the volume of passively transferred anti-H3L serum once it was diluted throughout the mouse, as it would be a lower titer than in a mouse directly immunized with H3L protein (Fig. 4). In summary, anti-H3L neutralizing antibodies provide substantial protection against a lethal infection with a pathogenic poxvirus.
FIG. 6.
Passive immunization with anti-H3L neutralizing antibodies provides partial protection from a lethal VVWR challenge. (A) VV neutralization activity of rabbit anti-H3L serum. (B) Survival curve after 3-LD50 VVWR intranasal challenge. Ten mice per group. H3L-immunized mice were protected (P < 0.02).
DISCUSSION
Vaccinia virus provides excellent prophylactic immunity to variola virus, the causative agent of smallpox. However, there is considerable interest in developing safer alternative vaccines due to the risk of complications associated with vaccinia virus in immunocompromised individuals. Determining the antigen specificities and protective value of vaccine-induced antibody and T-cell responses is an important issue for understanding vaccinia virus-induced immunity and for the design of safer vaccines. In order to gain a clearer understanding of the humoral response to vaccinia virus, we have examined sera from a panel of Dryvax-immunized human volunteers for potential target antigens, using a novel proteome microarray approach (15). This global approach to antigen discovery has revealed several candidate antigens for consideration for inclusion in a prophylactic subunit vaccine. H3L protein was among the VV targets most frequently recognized by sera from vaccinees. Additionally, the response to H3L was strongly anamnestic in individuals undergoing a recall response to VV (Fig. 1). Those observations led us to focus on understanding the immunological relevance of H3L as a major target of human anti-VV antibody responses. In the work reported here, we establish H3L as an immunodominant target and take the key steps of demonstrating functional protective roles for human anti-H3L antibodies in vitro and protective roles for anti-H3L antibodies in vivo in the mouse model system.
Vaccinia virions are complex macromolecular structures consisting of some 100 different proteins, of which at least 18 are known envelope proteins (57). Given the antigenic complexity of the VV envelope, antibodies to multiple envelope polypeptides should have the potential to contribute to the overall protection afforded to an immunized person. This view is supported by our human antibody depletion experiments and by protein immunization experiments by multiple investigators in animal models (discussed below). However, very limited work has been done to directly identify VV envelope targets of human neutralizing antibodies. Only B5R has clearly been shown to be targeted by human neutralizing antibodies (targeting EEV particles) (3), and now our work adds anti-H3L antibodies to the list as a substantial component of the neutralizing human anti-IMV immune response.
Protective antibodies confer protection through the recognition of structures on the surfaces of virus particles. Four main assembly states of mature VV particles are identified, which differ in their envelope protein compositions (46, 57). The most abundant particle is the IMV, which accumulates in infected cells and which is released as the cells die (46). IMVs are robust infectious virus particles and may represent the principal type involved in transmission between hosts. An alternate maturation pathway is taken by a proportion of IMVs. They become wrapped in a double membrane from the trans-Golgi to form the intracellular enveloped virus, which then becomes translocated along microtubules to the cell surface, where the outermost intracellular enveloped virus membrane fuses with the plasma membrane (55). These virions may remain on the cell surface as cell-associated enveloped virus (CEV) or be released from the cell surface as EEV (7). Both extracellular forms are more fragile and less abundant than the IMV and are considered to be primarily involved in transmission within the same host rather than between hosts (48). Studies have demonstrated that antibodies against either IMV or EEV particles can be protective (discussed below). In the context of a vaccine to prevent host-to-host transmission, antibodies against IMV surface antigen might be expected to play a major role in protection. As such, our data suggest that anti-H3L antibodies may be a major contributor in humans for protection against variola virus infection. In general, anti-IMV antibodies may protect primarily by neutralizing the virus inoculum, and anti-EEV/CEV antibodies may protect primarily by limiting viral spread after infection, though there remains debate as to the relative in vivo contributions of antibodies against IMV versus EEV/CEV forms of the virion.
IMV antigens so far identified as being targets of VV-neutralizing antibodies in any species include 5 of the 11 known IMV membrane proteins. These are L1R (35, 36, 65), D8L (34), A27L (17, 40, 50, 53, 54), A17L (62), and H3L (10, 44). Vaccinia virus-neutralizing antibodies also recognize envelope proteins found on other assembly states, including the important EEV envelope protein B5R (24, 42). Human VIG is effective at limiting smallpox infection in humans (39) and contains antibodies against both IMV and EEV targets (15). In animal challenge studies, various levels of protection against lethal infection have been achieved by immunization with envelope proteins. In mice, protection using protein-based vaccines has been achieved with A27L (17, 40); L1R, B5R, or A33R alone (21, 24); or cocktails of A33R plus B5R (24), A33R plus L1R (21), or A33R plus L1R plus B5R (21). In general, the best protection was achieved with combinations of recombinant proteins, particularly at higher doses of virus challenge. Mice are also partially protected by DNA vaccine encoding L1R alone, which is markedly improved by the coinjection of A33R DNA (30) or by a combination of four genes, A27L plus A33R plus L1R plus B5R (31). Consistent with the protective value of antibodies against those VV targets, protection of monkeys from lethal challenge with monkeypox virus was achieved by a combination DNA vaccine including A27L plus A33R plus L1R plus B5R, and measurable protection was also achieved with L1R alone (32). Separately, passive-transfer studies have been performed to show the protective value of anti-B5R (24) and anti-A27L antibodies (50) in animal models. Together, those studies demonstrated that effective protection against infection can be achieved by antibody. However, again, there have been few data that speak to the relevance of these and others targets in VV-immunized humans.
Understanding the relevant protein targets on a pathogen is always important for understanding mechanisms and roles of humoral immunity, and VV is no exception. Hyperimmune human serum (VIG) is the licensed therapy for smallpox virus infection or disseminated vaccinia virus infection (23, 29). Which envelope proteins are the major protective/neutralizing targets of the human anti-VV antibody response? This is a complex issue, made more complex by the existence of both IMV and EEV virion forms. H3L is clearly a major target of the human neutralizing-antibody response, and it is an IMV surface protein. In the experiments presented here, anti-H3L antibody responses were able to provide only partial protection in vivo from lethal challenges with VV. Though there is clear evidence in the literature that antibody responses against either IMV or EEV proteins can be protective in mice (24, 50), it makes intuitive sense that antibody responses against both IMV and EEV would be better, to block all means of virus spread. The partial protective efficacy of anti-H3L antibodies observed here may be because responses against both IMV and EEV surface proteins are necessary for complete protection. There is some experimental evidence in mice supporting this concept (21).
The 35-kDa polypeptide (p35) encoded by the H3L open reading frame is an integral membrane protein that is accessible to detergent extraction (13, 44). p35 is found predominantly in the envelope of IMVs (13, 14, 44). Soluble H3L protein binds to cell-surface heparan sulfate and blocks the adsorption of IMVs, indicating that p35 is a VV receptor involved in target cell binding. VV H3L deletion mutants exhibited a small-plaque phenotype and decreased viral yields, and electron microscopic examination revealed defective virion morphogenesis (13, 14, 44). Multiple vaccinia virus antigens of 30 to 35 kDa were recognized by Western blotting in experiments using antisera from rabbits, mice, or humans immunized with VV (17, 54, 64). Antigenic p35 was first identified as the product of the H3L gene by Zinoviev et al. and Chertov et al. (10, 70), and rabbit antiserum to H3L was subsequently shown to be neutralizing by Lin and colleagues (44). (A separate group possibly studied the same protein but misidentified it as the VVIHD H6R gene product [25, 26]). Similarly, antibodies to the orthologs of H3L are also seen after infection with other orthopoxviruses, including orf virus, capripox virus, and fowlpox virus (8, 28, 33). The H3L gene is strongly conserved among the orthopoxviruses. For example, the H3L proteins from VVWR and variola virus share 96% amino acid identity, which may contribute to the observed cross-protection against variola virus afforded by vaccinia virus immunization.
As discussed earlier, T cells are also of great importance for control and clearance of poxvirus infections (4, 6, 37, 67, 68). We have not addressed issues of T-cell immunity in this study, as we have focused instead on humoral immune responses. Our experiments show that H3L is an immunodominant target of neutralizing antibodies after immunization of humans with VV and after immunization of mice with H3L protein. While memory T cells are highly capable of protection against poxvirus infections—and the presence of T cells is required for clearance of poxvirus infections—antibodies are sufficient to protect against a challenge without the involvement of vaccine-induced T cells (4, 19). Nevertheless, in the context of future vaccine design, memory T cells are quite valuable, and any subunit smallpox vaccine will likely need to stimulate both T- and B-cell responses to reach a high level of protective efficacy similar to that of vaccinia virus immunization.
In summary, we have studied the human antibody profile in response to vaccinia virus using proteome microarray screening and have focused on H3L, as antibodies to this protein are a dominant component of the response to vaccinia virus surface antigens. We demonstrated that human anti-H3L antibodies are capable of neutralizing VV in vitro. Furthermore, immunization of mice against H3L induces strong neutralizing antibodies, and H3L-immunized animals or animals that receive passive transfer of anti-H3L antibodies are protected against a lethal challenge with pathogenic vaccinia virus. This work identifies H3L as a good candidate for inclusion in a subunit vaccine against smallpox.
Acknowledgments
We thank Kandarp Shah and Chris Ma for help with H3L purification, Stacy Hernandez for help with PRNT assays, Rafi Ahmed for seed VVWR stocks, Denis Heck for microarray printing, and Paul Gershon and Ian Gibbons for stimulating discussion.
The program was supported by NIH/NIAID grants U01AI056464, AI058365, and 1U01AI061363-01 and a Cancer Research Institute Investigator Award to S.C.
This is LIAI manuscript no. 719.
REFERENCES
- 1.Alcami, A., and G. L. Smith. 1992. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71:153-167. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 6.Blanden, R. V. 1970. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J. Exp. Med. 132:1035-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulanger, D., P. Green, B. Jones, G. Henriquet, L. G. Hunt, S. M. Laidlaw, P. Monaghan, and M. A. Skinner. 2002. Identification and characterization of three immunodominant structural proteins of fowlpox virus. J. Virol. 76:9844-9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burleson, F. G., T. M. Chambers, and D. L. Weidbrauk. 1992. Virology: a laboratory manual. Academic Press, New York, N.Y.
- 10.Chertov, O., I. N. Telezhinskaya, E. V. Zaitseva, T. B. Golubeva, V. V. Zinov'ev, L. G. Ovechkina, L. B. Mazkova, and E. G. Malygin. 1991. Amino acid sequence determination of vaccinia virus immunodominant protein p35 and identification of the gene. Biomed. Sci. 2:151-154. [PubMed] [Google Scholar]
- 11.Crotty, S., and R. Ahmed. 2004. Immunological memory in humans. Semin. Immunol. 16:197-203. [DOI] [PubMed] [Google Scholar]
- 12.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969-4973. [DOI] [PubMed] [Google Scholar]
- 13.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 74:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J. Virol. 74:7518-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demkowicz, W. E., Jr., R. A. Littaua, J. Wang, and F. A. Ennis. 1996. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietzschold, B., M. Kao, Y. M. Zheng, Z. Y. Chen, G. Maul, Z. F. Fu, C. E. Rupprecht, and H. Koprowski. 1992. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc. Natl. Acad. Sci. USA 89:7252-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edghill-Smith, Y., M. Bray, C. A. Whitehouse, D. Miller, E. Mucker, J. Manischewitz, L. R. King, M. Robert-Guroff, A. Hryniewicz, D. Venzon, C. Meseda, J. Weir, A. Nalca, V. Livingston, J. Wells, M. G. Lewis, J. Huggins, S. H. Zwiers, H. Golding, and G. Franchini. 2005. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 191:372-381. [DOI] [PubMed] [Google Scholar]
- 20.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 21.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey, S. E., F. K. Newman, J. Cruz, W. B. Shelton, J. M. Tennant, T. Polach, A. L. Rothman, J. S. Kennedy, M. Wolff, R. B. Belshe, and F. A. Ennis. 2002. Dose-related effects of smallpox vaccine. N. Engl. J. Med. 346:1275-1280. [DOI] [PubMed] [Google Scholar]
- 23.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 24.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 25.Gordon, J., T. Kovala, and S. Dales. 1988. Molecular characterization of a prominent antigen of the vaccinia virus envelope. Virology 167:361-369. [PubMed] [Google Scholar]
- 26.Gordon, J., A. Mohandas, S. Wilton, and S. Dales. 1991. A prominent antigenic surface polypeptide involved in the biogenesis and function of the vaccinia virus envelope. Virology 181:671-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 28.Heine, H. G., M. P. Stevens, A. J. Foord, and D. B. Boyle. 1999. A capripoxvirus detection PCR and antibody ELISA based on the major antigen P32, the homolog of the vaccinia virus H3L gene. J. Immunol. Methods 227:187-196. [DOI] [PubMed] [Google Scholar]
- 29.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, K. Tonat, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 30.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 31.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Housawi, F. M., G. M. Roberts, J. A. Gilray, I. Pow, H. W. Reid, P. F. Nettleton, K. J. Sumption, M. H. Hibma, and A. A. Mercer. 1998. The reactivity of monoclonal antibodies against orf virus with other parapoxviruses and the identification of a 39 kDa immunodominant protein. Arch. Virol. 143:2289-2303. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao, J. C., C. S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichihashi, Y., and M. Oie. 1996. Neutralizing epitope on penetration protein of vaccinia virus. Virology 220:491-494. [DOI] [PubMed] [Google Scholar]
- 36.Ichihashi, Y., T. Takahashi, and M. Oie. 1994. Identification of a vaccinia virus penetration protein. Virology 202:834-843. [DOI] [PubMed] [Google Scholar]
- 37.Karupiah, G., R. M. Buller, N. Van Rooijen, C. J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempe, C. H. 1960. Studies of smallpox and complications of smallpox vaccination. Pediatrics 26:176-189. [PubMed] [Google Scholar]
- 39.Kempe, C. H., C. Bowles, G. Meiklejohn, T. O. Berge, L. St Vincent, B. V. Babu, S. Govindarajan, N. R. Ratnakannan, A. W. Downie, and V. R. Murthy. 1961. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull. World Health Organ. 25:41-48. [PMC free article] [PubMed] [Google Scholar]
- 40.Lai, C. F., S. C. Gong, and M. Esteban. 1991. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J. Virol. 65:5631-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane, J. M., F. L. Ruben, J. M. Neff, and J. D. Millar. 1969. Complications of smallpox vaccination, 1968. N. Engl. J. Med. 281:1201-1208. [DOI] [PubMed] [Google Scholar]
- 42.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280:132-142. [DOI] [PubMed] [Google Scholar]
- 43.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856-860. [DOI] [PubMed] [Google Scholar]
- 44.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew, A., M. Terajima, K. West, S. Green, A. L. Rothman, F. A. Ennis, and J. S. Kennedy. 2005. Identification of murine poxvirus-specific CD8+ CTL epitopes with distinct functional profiles. J. Immunol. 174:2212-2219. [DOI] [PubMed] [Google Scholar]
- 46.Moss, B. 2001. Poxviridae: the viruses and their replication. In D. M. Knipe and P. Howley (ed.), Fundamental virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 47.Newman, F. K., S. E. Frey, T. P. Blevins, M. Mandava, A. Bonifacio, Jr., L. Yan, and R. B. Belshe. 2003. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 41:3154-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin, S. A. 2003. Vaccines, vaccination, and vaccinology. J Infect. Dis. 187:1349-1359. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83:1059-1067. [DOI] [PubMed] [Google Scholar]
- 51.Rappuoli, R., H. I. Miller, and S. Falkow. 2002. Medicine. The intangible value of vaccination. Science 297:937-939. [DOI] [PubMed] [Google Scholar]
- 52.Reading, P. C., and G. L. Smith. 2003. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J. Gen. Virol. 84:1973-1983. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez, J. F., and M. Esteban. 1987. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J. Virol. 61:3550-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez, J. F., R. Janeczko, and M. Esteban. 1985. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J. Virol. 56:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roos, N., M. Cyrklaff, S. Cudmore, R. Blasco, J. Krijnse-Locker, and G. Griffiths. 1996. A novel immunogold cryoelectron microscopic approach to investigate the structure of the intracellular and extracellular forms of vaccinia virus. EMBO J. 15:2343-2355. [PMC free article] [PubMed] [Google Scholar]
- 56.Slifka, M. K. 2004. Immunological memory to viral infection. Curr. Opin. Immunol. 16:443-450. [DOI] [PubMed] [Google Scholar]
- 57.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 58.Snyder, J. T., I. M. Belyakov, A. Dzutsev, F. Lemonnier, and J. A. Berzofsky. 2004. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 78:7052-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyler, K. L., M. A. Mann, B. N. Fields, and H. W. T. Virgin. 1993. Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 67:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallengren, K., C. Risco, J. Krijnse-Locker, M. Esteban, and D. Rodriguez. 2001. The A17L gene product of vaccinia virus is exposed on the surface of IMV. Virology 290:143-152. [DOI] [PubMed] [Google Scholar]
- 63.Welsh, R. M., L. K. Selin, and E. Szomolanyi-Tsuda. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22:711-743. [DOI] [PubMed] [Google Scholar]
- 64.Wilton, S., J. Gordon, and S. Dales. 1986. Identification of antigenic determinants by polyclonal and hybridoma antibodies induced during the course of infection by vaccinia virus. Virology 148:84-96. [DOI] [PubMed] [Google Scholar]
- 65.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53-63. [DOI] [PubMed] [Google Scholar]
- 66.Wright, K. E., and M. J. Buchmeier. 1991. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J. Virol. 65:3001-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu, R., A. J. Johnson, D. Liggitt, and M. J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 172:6265-6271. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, W. H., D. Wilcock, and G. L. Smith. 2000. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J. Virol. 74:11654-11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinoviev, V. V., N. A. Tchikaev, O. Chertov, and E. G. Malygin. 1994. Identification of the gene encoding vaccinia virus immunodominant protein p35. Gene 147:209-214. [DOI] [PubMed] [Google Scholar]