Abstract
Topical antimicrobicides hold great promise in reducing human immunodeficiency virus (HIV) transmission. Amphibian skin provides a rich source of broad-spectrum antimicrobial peptides including some that have antiviral activity. We tested 14 peptides derived from diverse amphibian species for the capacity to inhibit HIV infection. Three peptides (caerin 1.1, caerin 1.9, and maculatin 1.1) completely inhibited HIV infection of T cells within minutes of exposure to virus at concentrations that were not toxic to target cells. These peptides also suppressed infection by murine leukemia virus but not by reovirus, a structurally unrelated nonenveloped virus. Preincubation with peptides prevented viral fusion to target cells and disrupted the HIV envelope. Remarkably, these amphibian peptides also were highly effective in inhibiting the transfer of HIV by dendritic cells (DCs) to T cells, even when DCs were transiently exposed to peptides 8 h after virus capture. These data suggest that amphibian-derived peptides can access DC-sequestered HIV and destroy the virus before it can be transferred to T cells. Thus, amphibian-derived antimicrobial peptides show promise as topical inhibitors of mucosal HIV transmission and provide novel tools to understand the complex biology of HIV capture by DCs.
Antimicrobial peptides (AMPs) are key effectors of the innate immune response of animals (31). The skin of anuran amphibians (frogs and toads) is a rich source of these peptides, accounting for >20% of the 880 known AMPs from all species (44, 68). (An online catalog of all reported molecules can be found at http://www.bbcm.units.it/∼tossi/pag2.htm.) Amphibian AMPs (referred to throughout as A-AMPs) are typically 11 to 46 amino acid residues in length and are produced and stored in specialized granular glands within the skin. The majority of A-AMPs consist of two distinct classes: linear α-helical peptides or peptides with cysteines forming a C-terminal disulfide-bonded loop (44).
A common structural feature of A-AMPs is the clustering of hydrophobic and cationic residues on opposite faces of the peptide α-helix, rendering these molecules amphipathic (39, 44). These net positively charged regions bind to the negatively charged head groups of bacterial membranes (35). Interestingly, A-AMPs possess activity against a variety of microbes, including bacteria (44), fungi (20, 46-49), viruses (5, 16, 17, 67), and malaria parasites (24).
Antimicrobial agents have been proposed as a form of prophylaxis against human immunodeficiency virus (HIV) transmission. Cyanovirin-N, an 11-kDa protein derived from cyanobacteria, binds the HIV envelope glycoprotein gp120 and inhibits the establishment of chronic infection (10, 58, 59). Retrocyclin, a θ-defensin, binds to cell surface glycoproteins and blocks HIV entry (18, 38, 63). Other examples of antimicrobial agents, such as bovine indolicidin, the human cathelicidin LL37, rhesus macaque myeloid α-defensin-4, and procine protegrin-1, inhibit infection at an early step in the replication program (45, 56).
Sexual transmission of HIV remains the most common mode of transmission worldwide. Exposure of HIV to mucosal regions likely results in its capture by dendritic cells (DCs) whose normal function is to internalize invading pathogens and process them for presentation by major histocompatibility complex molecules (37, 43). HIV exploits the DC's ability to capture pathogens to facilitate its delivery to T cells (26, 33, 53, 54, 62). Understanding how HIV interacts with DCs and how it exploits DCs to infect T cells could be important in determining the mechanism by which HIV gains a foothold in the host. This knowledge in turn could aid in developing new approaches to prevent HIV infection.
In this report, we identified several A-AMPs that prevent HIV infection by disrupting the integrity of the virion membrane at concentrations that are not toxic to target cells. Remarkably, these peptides also inhibit the transfer of HIV from DCs to T cells, even when DCs are transiently exposed to peptides hours after capture of virus. These findings suggest that A-AMPs could be utilized as an approach for HIV prophylaxis and importantly reveal that HIV is not fully protected after DC capture.
MATERIALS AND METHODS
Amphibian peptides.
The sequence of the peptides tested and species of origin can be found in Table 1. Caerin 1.1, caerin 1.9, caerin 4.1, dahlein 5.6, maculatin 1.1, and uperin 3.6 were synthesized by Chiron Mimotopes, Clayton, Victoria, Australia, using l-amino acids and standard 9-fluorenylmethoxycarbonyl chemistry. Dermaseptin and magainin II were purchased from Sigma (St. Louis, MO). Esculentin-2P and ranatuerin-2P were synthesized by Sigma Genosys (Houston, TX). Esculentin-1ARb, melittin-related peptide (MRP), and palustrin-3ARa were isolated from skin secretions of Rana areolata and Rana tagoi by J. M. Conlon (1, 19). Ranatuerin-6 was synthesized using standard 9-fluorenylmethoxycarbonyl chemistry.
TABLE 1.
Amphibian antimicrobial peptides
| Peptide | Species of origin | Sequence |
|---|---|---|
| Caerin 1.1 | Litoria caerulea | GLLSVLGSVAKHVLPHVVPVIAEHL-NH2 |
| Caerin 1.9 | Litoria chloris | GLFGVLGSIAKHVLPHVVPVIAEKL-NH2 |
| Caerin 4.1 | Litoria caerulea | GLWQKIKSAAGDLASGIVEGIKS-NH2 |
| Dahlein 5.6 | Litoria dahlii | GLLASLGKVFGGYLAEKLKPK |
| Dermaseptin | Phyllomedusa sauvagii | ALWKTMLKKLGTMALHAGKAALGAAADTISQGTQ |
| Esculentin-1ARb | Rana areolata | GLFPKFNKKKVKTGIFDIIKTVGKEAGMDVLRTGIDVIGCKIKGEC |
| Esculentin-2P | Rana pipiens | GFSSIFRGVAKFASKGLGKDLARLGVNLVACKISKQC |
| Maculatin 1.1 | Litoria genimaculata | GLFGVLAKVAAHVVPAIAEHF-NH2 |
| Magainin II | Xenopus laevis | GIGKFLHSAKKFGKAFVGEIMNS |
| MRP | Rana tagoi | AIGSILGALAKGLPTLISWIKNR-NH2 |
| Palustrin-3AR | Rana areolata | GIFPKIIGKGIVNGIKSLAKGVGMKVFKAGLNNIGNTGCNNRDEC |
| Ranatuerin-6 | Rana catesbeiana | FISAIASMLGKFL-NH2 |
| Ranatuerin-2P | Rana pipiens | GLMDTVKNVAKNLAGHMLDKLKCKITGC |
| RCCP | Rana catesbeiana | Natural mixture of peptides |
| Uperin 3.6 | Uperoleia mjobergii | GVIDAAKKWNVLKNLF-NH2 |
Cell culture and primary cell isolation.
Peripheral blood mononuclear cells were isolated from adult blood using a Ficoll-Hypaque gradient. Monocytes and CD4+ T cells were isolated from peripheral blood mononuclear cells as previously described (36, 40). DCs were generated by culturing CD14+ monocytes in RPMI complete medium (10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin) supplemented with interleukin-4 (50 μg/ml; R&D Systems) and granulocyte-macrophage colony-stimulating factor (50 μg/ml; R&D Systems) for 5 days and subsequently matured by addition of lipopolysaccharide (100 ng/ml; Sigma) for 1 to 2 days. Mature DC production was assessed by staining cells with antibodies to CD14, CD83, CD86, and HLA-DR (all from BD Biosciences). Hut 78 T cells expressing CCR5 (Hut/CCR5) were prepared and maintained as previously described (40, 65). 293T, Mus dunni, and HeLa cells were maintained in Dulbecco's modified Eagle medium. Murine L cells used to titer reovirus were maintained in Joklik minimal essential medium supplemented to contain 5% fetal bovine serum and 100 mg/ml of penicillin G, 100 μg/ml of streptomycin/ml, and 250 ng/ml of amphotericin B.
Virus production.
Vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped replication-incompetent HIV particles (HDV-VSV-G), or replication-competent virus expressing either the HIV envelope BaL that uses CCR5 as a coreceptor (HIV-R5) or LAI that uses CXCR4 as coreceptor (HIV-X4) were generated as previously described (60). All these viruses also contain enhanced green fluorescent protein (EGFP) (Clontech) in place of the nef gene. Reovirus strain type 1 Lang (T1L) is a Dermody laboratory stock. Reovirus titers were determined by plaque assay with L-cell monolayers (21). Infectious subvirion particles (ISVPs) were prepared by treatment of purified T1L virions with Nα-p-tosyl-l-lysine chloromethyl ketone-treated bovine chymotrypsin (Sigma) as previously described (2). Murine leukemia virus (MLV) pseudotyped with VSV-G was produced by transfecting 293T cells with pCIG-B (7), pBABE-EGFP, and pHCMV-G.
HIV infection and cell viability assay.
Virus was cultured with Hut or primary CD4+ T cells activated as previously described (41) in the presence of antimicrobial peptides at various time points and concentrations. Infection of T cells was analyzed through GFP expression after 3 days with a FACSCalibur four-color cytometer (BD Biosciences) and CELLQuest software (BD Biosciences). Aliquots of cells were removed at different time points post-peptide treatment and incubated with propidium iodide (PI; 25 μg/ml, Sigma). Cells were analyzed by flow cytometry for PI exclusion as an indicator of viability. Infection and PI data were normalized against phosphate-buffered saline (PBS) treatment controls set at 100% infectivity or viability, respectively.
T-cell proliferation assay.
The effect of A-AMPs on T-cell activation and proliferation was measured by labeling the T cells with carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). Purified cells were washed and resuspended in PBS. CFSE was added at a final concentration of 1.65 μM and incubated at 37°C for 3 min. Labeling was quenched by addition of 50% fetal calf serum in PBS, followed by three washes with RPMI-supplemented medium. All CFSE labeling and cell culture were performed in the dark. Cells were activated by plate bound anti-CD3 and anti-CD28 as previously described (41). The proliferation index was calculated as the sum of the total cells in all generations divided by the estimated number of original parent cells (determined by dividing the final number of cells in each division by the squared generation number).
Reovirus and MLV infection assays.
Caerin 1.9 was added to samples of T1L virions or ISVPs at a final concentration of 2.5 μM, 5 μM, or 7.5 μM and incubated at 37°C for 45 min. Virus was incubated with a related but inactive peptide, caerin 4.1, at a final concentration of 5 μM or buffer only (PBS) as controls. Virus was adsorbed to confluent HeLa cell monolayers at a multiplicity of infection (MOI) of 2 PFU per cell at 4°C for 1 h. Following two washes with PBS, fresh medium was added, and cells were incubated at 37°C for 24 h. Cells and medium were subjected to three cycles of freezing and thawing, and virus titer was determined by plaque assay (21). Assays were performed in triplicate, and the data presented are representative of two experiments. Caerin 1.9 and caerin 4.1 were added to MLV (30,000 infectious units [IFU]) and incubated at 37°C for 45 min. Virus was added to 3 × 104 Mus dunni cells and cultured for 3 days before cells were fixed and analyzed for infection by GFP expression by flow cytometry.
DC-mediated infection assays.
Monocyte-derived DCs were pulsed with replication-competent HIV-R5 at an MOI of 2. Virus-cell mixtures were centrifuged at 2,000 rpm for 1 h and cultured for an additional 2 h to allow DCs to efficiently capture virus. DCs were washed three times with complete RPMI medium to remove non-cell-associated virus. Peptide was added to cells at a final concentration of 1 to 32 μM and incubated for 45 min. DCs were washed three times with complete RPMI medium and incubated with 3 × 104 Hut/CCR5 cells for 3 days. Cells were harvested, fixed with 1% paraformaldehyde, and analyzed for expression of GFP by flow cytometry.
Virus-cell fusion assay.
HIV fusion assays were performed essentially as previously described (13). Briefly, viruses carrying a β-lactamase reporter protein fused to the amino terminus of the virion protein Vpr (BlaM-Vpr), were treated with caerin 1.9 (1 to 10 μM) for 1 h. These viruses plus peptide mixtures were added to Hut/CCR5 cells at 37°C for 2 h to allow virus-cell fusion. CCF2/AM (20 μM; Aurora Biosciences Corp.) was added, and the cultures were incubated for 14 h at room temperature. Cells were pelleted and resuspended in PBS, and the fluorescence was measured at 447 and 520 nm with a microplate fluorometer after excitation at 409 nm. Uncleaved CCF2 fluoresces green, due to fluorescence resonance energy transfer between the coumarin and fluorescein groups; however, cleavage by BlaM results in the dissociation of these fluorophores, and the emission spectrum shifts to blue. Thus, the ratio of blue to green cellular fluorescence is proportional to the overall extent of virus-cell fusion. Fluorescence ratios were calculated after subtraction of the average background fluorescence of control cultures containing no virus (blue values) and wells containing PBS (green values).
HIV p24 release assay.
HIV-VSV-G was incubated with either peptides or PBS at a concentration range of 1 to 32 μM for 30 min in complete RPMI medium. The medium was then assayed for the presence of viral core protein p24 by enzyme-linked immunosorbent assay (ELISA) as previously described (40). Plates were analyzed by microplate reader (Molecular Devices) at 405 nm absorbance. Total p24 was calculated using linear regression analysis from standards included on each plate.
RESULTS
Amphibian-derived AMPs inhibit HIV infection of T cells.
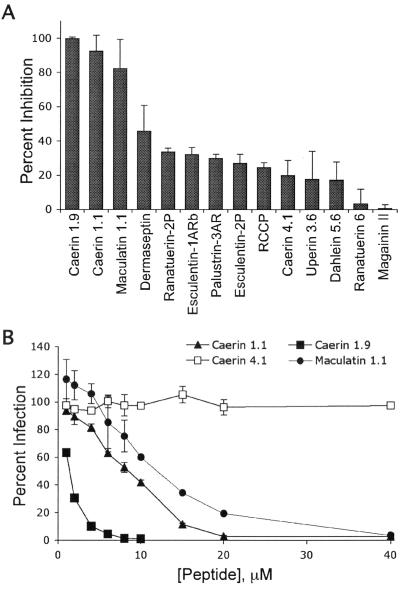
Because AMPs have activity against some viruses, including frog virus 3, channel catfish virus, and herpes simplex virus 1 infection (5, 17, 67), we investigated whether A-AMPs can inhibit infection by HIV. AMPs from diverse amphibian species (Table 1) were tested for their ability to block HIV infection of T cells (Fig. 1A). Prior to fixation, a portion of the cells were removed and stained with PI to determine cell viability. We identified three peptides (caerin 1.1, caerin 1.9, and maculatin 1.1) that inhibited HIV infection without adversely affecting T-cell viability. Other peptides, including esculentin-1ARb, esculentin-2P, magainin II, MRP, palustrin-3AR, and ranatuerin-2P, were not effective in inhibiting HIV infection of T cells at nontoxic concentrations (Fig. 1A). Dermaseptin partially inhibited HIV infection; however, higher doses were toxic to cells (Fig. 1A and data not shown). To determine the MIC, we incubated HIV and T cells with increasing doses of the effective peptides for 3 days. All three peptides were effective over a relatively narrow concentration range, with caerin 1.9 having the lowest IC50 (calculated as the concentration of peptide necessary to inhibit 50% of PBS-treated HIV infection of T cells) of 1.2 μM, caerin 1.1 having an IC50 of 7.8 μM, and maculatin 1.1 having an IC50 of 11.3 μM (Fig. 1B). Because of the higher concentration of maculatin 1.1 required for inhibition, we focused on caerin 1.1 and caerin 1.9 for the remainder of the study. A related noninhibitory peptide, caerin 4.1, was used as a control in all experiments.
FIG. 1.
Amphibian peptides inhibit HIV infection of T cells. (A) Fourteen purified peptides and one natural peptide mixture (RCCP) derived from the skin of anuran amphibians were incubated at 10 μg/ml (RCCP), 1 μM (ranatuerin-2P, esculentin-1ARb, palustrin-2AR, and esculentin-2P), 5 μM (caerin 1.9 and magainin II) or 10 μM (all others) with 3 × 104 T cells and HIV-R5 at an MOI of 1 per cell at 37°C for 3 days. Cells were harvested and analyzed for GFP expression. The data are presented as the mean of three replicate samples from one representative experiment of two independent experiments. Error bars indicate standard deviations. Data are presented as percent inhibition normalized to infection in the presence of PBS. (B) T cells (3 × 104) were incubated with HIV-R5 at an MOI of 1 and increasing concentrations of caerin 1.1, caerin 1.9, maculatin 1.1, caerin 4.1, or PBS at 37°C for 3 days. Cells were harvested and analyzed for GFP expression. The data are presented as the mean of three replicate samples from one representative experiment of six independent experiments. Error bars indicate standard deviations. IC50s were calculated by regression analysis.
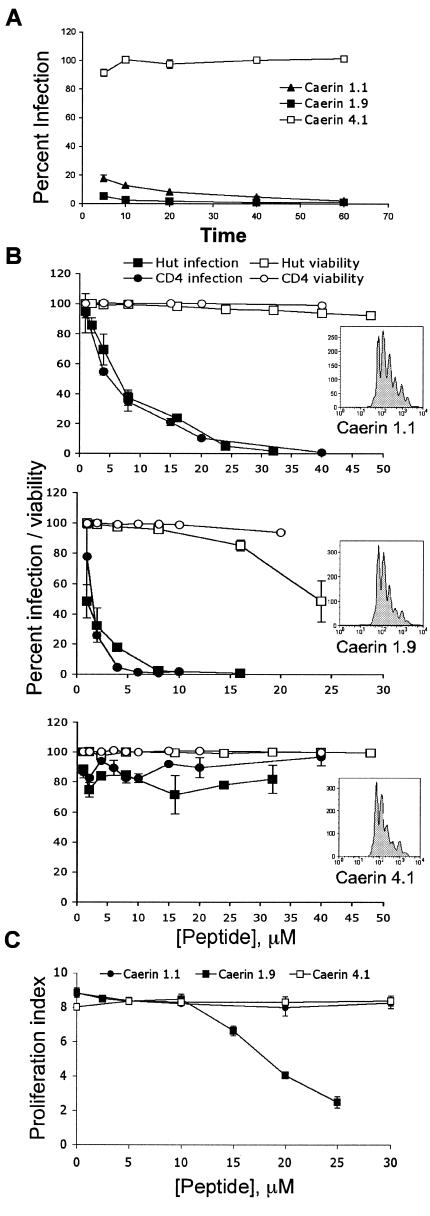
It has been proposed that amphibians secrete these peptides to combat topical microbial pathogens (68). Because endopeptidases rapidly degrade them, the peptides are at a high concentration for only a short time (52). Thus, they must exert their cytopathic effects faster than the generational doubling time of the pathogen. We therefore tested the kinetics of A-AMP-mediated inhibition of HIV infection. Both caerin 1.9 and caerin 1.1 inhibited infection by >99% and 90%, respectively, after only 5 to 10 min of exposure to HIV (Fig. 2A). Furthermore, these short incubation periods also increased the tolerance of T cells to much higher concentrations of peptide while retaining the effective IC50 of the peptides to block HIV (Fig. 2B). In addition, to exclude any adverse effects of the peptides on T-cell activation and proliferation, we measured cell division upon activation using CFSE labeling (Fig. 2B, inserts). These experiments demonstrate that even at twofold-higher concentrations that completely inhibit HIV infection, there was no effect on T-cell proliferation (Fig. 2C). The peptides were equally effective at blocking HIV infection of either Hut/CCR5 or primary CD4+ T cells (Fig. 2B). These results show that A-AMPs potently and rapidly inhibit HIV infection at significantly lower concentrations than those required to affect the viability of the T cells.
FIG.2.
Peptide inhibition occurs rapidly with no effect on cell viability. (A) Caerin 1.1 (20 μM), caerin 1.9 (5 μM), caerin 4.1 (20 μM), or PBS was incubated with 3 × 104 IFU of HIV-R5. At the times shown, virus-peptide solutions were diluted to a concentration that resulted in minimal activity based the results shown in Fig. 1B (fourfold) in complete RPMI medium with 3 × 104 T cells and incubated at 37°C for 3 days. Cells were harvested, fixed, and analyzed for GFP expression by flow cytometry. Data were normalized to infection following PBS treatment and are presented as the mean of three replicate samples from one representative experiment of three independent experiments, with error bars indicating standard deviation. (B) Peptides were incubated for 5 min at increasing concentrations (1 to 48 μM) with 5 × 104 activated primary CD4+ T cells (circles) or Hut/CCR5 cells (squares) infected with HIV-R5 at an MOI of 1, diluted fourfold with complete RPMI medium, and incubated at 37°C for 3 days. Cells were harvested and analyzed for GFP expression (closed circles and squares). Data are normalized to infection following PBS treatment and are presented as the mean of three replicate samples from one representative experiment of three independent experiments with error bars indicating standard deviation. At 2 h postinfection, 2 × 104 T cells were removed from the culture, stained with PI, and analyzed for viability by flow cytometry (open circles and squares). (C) Peptides were incubated for 5 min at increasing concentrations (1 to 50 μM) with 5 × 104 CFSE-labeled primary CD4+ T cells. Cells were then diluted fourfold with complete RPMI medium and activated by T-cell receptor engagement as previously described (41). The activated cells were cultured for 4 days, and CFSE fluorescence intensity was measured by flow cytometry. Representative histograms (panel B, insets) from each peptide treatment are shown (40 μM caerin 1.1, 10 μM caerin 1.9, and 40 μM caerin 4.1), which were identical to those of the cells not treated with peptides.
Amphibian AMPs can inhibit other enveloped viruses.
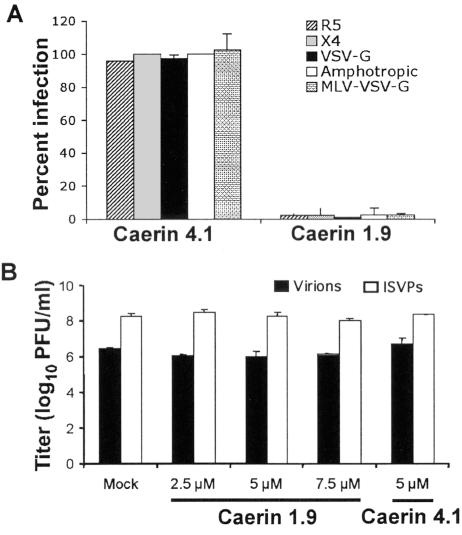
To determine whether the effects of A-AMPs are limited to a particular HIV strain or envelope coreceptor usage, we preincubated caerin 1.9 with HIV-LAI (CXCR4-tropic virus), HIV pseudotyped with VSV-G (coreceptor independent), HIV pseudotyped with the MLV amphotropic envelope glycoproteins, or MLV pseudotyped with VSV-G. T cells (or Mus dunni cells for the MLV infections) were then added and analyzed for infection at day 3. Caerin 1.9 potently inhibited infection of cells by HIV with different envelopes, as well as VSV-G pseudotyped MLV (Fig. 3A). These results suggest that A-AMPs inhibit both HIV and MLV infection independently of envelope glycoproteins.
FIG. 3.
Peptide activity against HIV with different envelopes and diverse viral species. (A) Caerin 1.9 (5 μM) and caerin 4.1 (5 μM) were incubated with 3 × 104 T cells and HIV-R5, HIV-X4, HDV-VSV-G, or HDV pseudotyped with the MLV amphotropic envelope at an MOI of 1 at 37°C for 3 days. Caerin 1.9 (10 μM) and caerin 4.1 (10 μM) were added to MLV (30,000 IFU) and incubated at 37°C for 45 min. Virus was then added to 3 × 104 Mus dunni cells and cultured at 37°C for 3 days. Following incubation, cells were harvested and analyzed for GFP expression by flow cytometry. Data are normalized to infection following PBS treatment and are presented as the mean of three replicate samples from one representative experiment of three independent experiments, with error bars indicating standard deviation. (B) Reovirus T1L virions or ISVPs were incubated with caerin 1.9, caerin 4.1, or PBS (mock) at the concentrations shown at 37°C for 45 min. Virus was adsorbed to confluent HeLa cell monolayers at an MOI of 2 PFU per cell at 4°C for 1 h. Following two washes with PBS, fresh medium was added, and cells were incubated at 37°C for 24 h. Cells and medium were subjected to three cycles of freezing and thawing, and virus titer was determined by plaque assay. The results are presented as the means of two independent experiments. Error bars represent standard deviations.
We next asked whether the A-AMPs could inhibit infection of a nonenveloped virus. For this experiments we used mammalian reoviruses, which are nonenveloped viruses that contain a segmented, double-stranded RNA genome. After engagement of cell-surface receptors including junctional adhesion molecule-A (4) and sialic acid (14, 23, 28, 42), reovirus virions enter cells by receptor-mediated endocytosis and are uncoated to produce ISVPs (9, 50, 55). ISVPs penetrate endosomal membranes, thereby delivering transcriptionally active core particles into the cytoplasm (8, 30, 34, 57). In sharp contrast to the results gathered using retroviruses HIV and MLV, the peptides did not inhibit reovirus infection (Fig. 3B).
Amphibian AMPs directly inactivate HIV by disrupting viral envelope integrity.
To define the mechanism by which A-AMPs inhibit HIV, we determined whether caerin 1.9 directly affects the integrity of the virus or interacts with the target cell to prevent infection. Caerin 1.9 was added to T cells at various intervals extending to 48 h and then washed away prior to virus exposure. If the peptide interfered with cellular components required for infection, fewer GFP-positive cells would be expected following this pretreatment. Instead, there was no difference in infection of the T cells regardless of the caerin 1.9 pretreatment interval (data not shown).
To determine the stage of HIV life cycle that is inhibited by these A-AMPs, we first analyzed the effect of delayed addition of peptide to T cells after infection with HIV. The reverse transcription of HIV after infection of cells was assessed by the presence of strong-stop, minus-strand viral DNA (R/U5 DNA) by quantitative real-time PCR. Addition of caerin 1.9 to cells after initiation of reverse transcription (4 to 8 h after infection) did not have any inhibitory effect (data not shown). These data suggest that caerin 1.9 inhibits HIV infection prior to the initiation of reverse transcription.
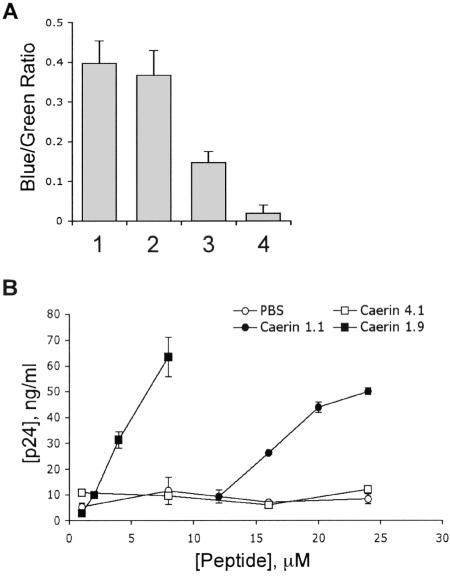
We therefore asked whether these A-AMPs prevent the entry of HIV by blocking its fusion to target cells. For this experiment, we employed a recently developed reporter assay to quantify HIV particle entry (13). In this assay, pretreatment of HIV with caerin 1.9 inhibited viral fusion to target cells (Fig. 4A), suggesting that these A-AMPs block infection prior to viral entry of the target cells.
FIG. 4.
Caerin 1.1 and caerin 1.9 disrupt the HIV envelope. (A) HIV-R5 carrying a β-lactamase reporter protein was incubated with 5 μM caerin 1.9 (lane 3) or PBS (lane 1) for 1 h prior to inoculation of Hut/CCR5 cells (105) by centrifugation at 600 × g for 1.5 h at 35°C. These cells or uninoculated cells (lane 4) were incubated at 37°C for 2 h to allow virus-cell fusion. CCF2/AM (20 μM) was added, the cultures were incubated at room temperature for 14 h, and fluorescence was measured at 447 and 520 nm by using a microplate fluorometer after excitation at 409 nm. Fluorescence ratios were calculated after subtraction of the average background fluorescence and are presented as relative fusion. Caerin 1.9 (5 μM) was added during the CCF2/AM incubation to control for peptide effects on dye loading (lane 2). Duplicate determinations were performed for each virus dilution. (B) HIV-VSV-G was incubated with peptides or PBS at the concentrations shown for 30 min in complete RPMI. Medium was then diluted 1:1,000 in assay buffer without detergent and analyzed for the presence of viral core protein p24 by ELISA. Total p24 was calculated from dilutions of replicate samples using linear regression analysis from p24 standards included on each plate. Data are presented as the mean of two replicate samples and are representative of two experiments. Error bars indicate standard deviation.
The inhibition of HIV entry by caerin 1.9 may have been caused by peptide attack of the virion membrane prior to cell binding and fusion. To determine whether A-AMPs directly inactivate virions by disrupting the envelope, virus was preincubated with increasing concentrations of A-AMPs or controls as described above and the release of HIV core protein p24 was measured by an ELISA. In this assay, p24 would only be detected if the viral envelope was disrupted by A-AMPs. Indeed, treatment of virions with A-AMPs released p24 into culture supernatant (Fig. 4B), indicating disruption of the viral membrane.
Amphibian AMPs inhibit DC-mediated trans infection of T cells.
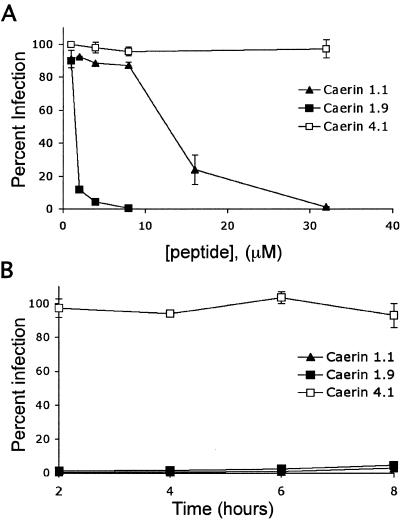
DCs resident at mucosal sites are thought to be among the first cells that interact with HIV upon exposure to the virus and possibly pass the virus on to T cells (26). An ideal topical antimicrobial would interfere with this “capture” process and inactivate HIV particles that have already been captured by DCs before they are transmitted to T cells. We therefore tested whether A-AMPs inhibit DC-mediated infection of T cells after the virus has been captured by DCs. For this experiment, DCs were first incubated with HIV, washed to remove cell-free virus, and subsequently incubated with different concentrations of peptide for 45 min. DCs were again washed to remove the peptide and incubated with T cells for 3 days to allow trans infection. Treatment of HIV-adsorbed DCs with A-AMPs inhibited the subsequent infection of T cells with similar IC50 values (caerin 1.1 to 12.6 μM and caerin 1.9 to 1.6 μM) (Fig. 5A) found for inhibition of direct T-cell infection (Fig. 1B). The A-AMPs also inhibited the trans infection of T cells with X4-tropic viruses captured by DCs (data not shown).
FIG. 5.
Amphibian antimicrobial peptides inhibit DC-mediated trans infection of T cells. (A) DCs (4 × 104) were pulsed with HIV-R5 at an MOI of 2. Virus-cell mixtures were centrifuged for 1 h at 2,000 rpm and incubated at 37°C for 2 h to allow DCs to efficiently capture the virus. DCs were washed three times with complete RPMI medium to remove unbound virus. Peptide was then added to the DCs at the concentrations shown and incubated at 37°C for 45 min. DCs were washed and incubated with 3 × 104 T cells at 37°C for 3 days. Cells were harvested, fixed with 1% paraformaldehyde, and analyzed for GFP expression. Data are normalized to infection following PBS treatment and are presented as the mean of three replicate samples from one representative experiment of three independent experiments, with error bars indicating standard deviation. (B) DCs (4 × 104) were incubated with HIV-R5 at an MOI of 1. At the times shown postinoculation, 30 μM caerin 1.1, 4 μM caerin 1.9, and 30 μM caerin 4.1 were added to cultures and incubated at 37°C for 45 min. DCs were washed three times in complete RPMI medium, incubated with 2 × 104 T cells at 37°C for 3 days, and analyzed for GFP expression. Data are normalized as above and are representative of four independent experiments.
We then asked how long after capture of HIV by DCs A-AMPs were able to inhibit virus transmission to T cells. To answer this question, DCs were first incubated with HIV and washed to remove unbound virus. The A-AMPs were then added after different time intervals, washed to remove the peptide, and cultured with T cells for 3 days. Exposure of DCs to A-AMPs as late as 8 h post-HIV capture resulted in >95% inhibition of trans infection (Fig. 5B). In these experiments, the viability of the DCs was not affected, as assessed by PI staining, even at threefold-higher peptide concentrations (data not shown).
The inhibition of DC-mediated transfer of virus by A-AMPs was remarkable, since free peptide was washed away before addition of the target T cells. These findings suggested that the peptides somehow access HIV captured by DCs either on the cell surface or intracellularly. It is also conceivable that some peptide sticks to the DC surface and interacts with the virus as it is being passed on to T cells. To test this possibility, we first incubated the DCs with high doses of peptides (8 μM caerin 1.9, 16 μM caerin 1.1, 16 μM caerin 4.1, or PBS) for 30 min, subsequently washed the cells several times to remove free peptide, and added virus to DCs. After 2 h of incubation, the DC-plus-virus cultures were extensively washed, and target T cells were added. We did not observe any inhibition of the infection of T cells (data not shown). We concluded that pretreatment of the DCs with A-AMPs, prior to addition of the virus, does not alter the capacity of DCs to capture HIV virions and trans infect T cells.
DISCUSSION
Our findings demonstrate that A-AMPs are highly effective in inhibiting HIV infection by disrupting the viral membrane and preventing the entry of the virus into target cells. Remarkably, the A-AMPs also were capable of inhibiting the transfer of HIV by DCs to T cells even when DCs were transiently exposed to peptides 8 h after virus capture. These data suggest that A-AMPs can access DC-sequestered HIV and destroy the virus before it can be transferred to T cells. This finding has broader implications for understanding how HIV is captured and transmitted to T cells.
DCs are thought to “capture” HIV virions at the mucosal surfaces and transfer the captured virus to its primary targets, CD4+ T cells (26). This capture process is generally thought to occur via a protein highly expressed on the surface of mucosal DCs, DC-SIGN (DC-specific ICAM grabbing nonintegrin) (22, 26, 27). After binding to DC-SIGN or as-yet-unidentified molecules on DCs, HIV is internalized into late-endosomal-stage compartments near the surface, where the virus remains in an infectious state for up to 5 days (26, 32). However, it is not clear whether these internalized virions are indeed infectious or whether the few viral particles that remain on the cell surface are preferentially transmitted to T cells. Our finding that the virus captured by DCs is effectively neutralized by A-AMPs suggests that either the virions are destroyed at the DC cell surface or the peptides gain access to the same compartment within DCs in which internalized virus resides.
The prevailing mechanistic model of A-AMP activity, called the “carpet” mechanism, is that positively charged regions of the α-helical peptides bind to negatively charged lipids in the membrane (35, 51, 66). The peptides integrate into the outer membrane, causing an increase in surface area that strains the bilayer. This alteration leads to transient pore formation, transport of outer lipids to the inner leaflet, and finally collapse of the membrane into small AMP-coated vesicles. An alternative model of activity called the “barrel-stave” mechanism proposes that α-helical peptides bundle on the surface of the membrane and assume an orientation to allow the hydrophobic surfaces to interact with the lipid core, while the hydrophilic surfaces orient inward (51). This arrangement creates an aqueous channel. Death of the microbe would then result from either loss of polarization (64), leaking of cellular contents (66), disturbance of membrane function from lipid redistribution (35), or activation of hydrolases that destroy the cell wall (6). In both of these models, A-AMPs are likely to be bound, either to the outer leaflet of the membrane or to internal regions of the bilayer. Based on these models, a likely mechanistic action of peptides would be to disrupt virions while they are exposed at the cell surface of the DCs. However, if most infectious HIV is internalized by DCs, then the destruction of only cell surface-bound virus would be hard to reconcile with this mode of action. Indeed, it has been shown that transient trypsin treatment of DCs that have captured HIV does not prevent transmission of virus to T cells, suggesting that DCs protect the virus from cleavage at the cell surface (32). Furthermore, the DC-captured HIV is also protected from neutralizing anti-HIV antibodies (25).
A particularly appealing scenario would be that the virus cycles from a vesicular compartment to the surface of the DC, where it is transiently exposed to peptides. This mechanism would explain how A-AMPs could inhibit trans infection even after 8 h of capture, since the internalized virus would be exposed to peptide during the cycling period. This model could also provide a mechanism for viral transmission from the surface of the DC to CD4+ T cells at the immunological synapse (36). In support of this model, we observed that A-AMP activity in the DC capture assays reached maximal inhibition after 30 min (compared to 10 min for virus alone) (Fig. 2A and data not shown). This slower rate of neutralization could be due to time required for viral particles to cycle to the cell surface and thus be exposed to destruction by the peptides. A radical alternative explanation for the destruction of DC-captured HIV by A-AMPs could be that only the virions that remain on the cell surface of the DCs are infectious and therefore their disruption by A-AMPs is sufficient to neutralize trans infection by DCs to T cells.
Although the above possibilities are consistent with the mode of action of the peptides, they do not rule out the inhibition of trans infection of HIV by A-AMPs by accessing the internal DC compartments to seek and destroy viral particles. Because the interaction of A-AMPs with biomembranes is dependent on many variables, such as lipid constituency, charge, and the presence of proteins that might affect stability (3, 15, 66, 68), it is conceivable that they may uniquely be internalized by DCs to gain access to the same compartment as HIV. An exciting prospect will be the utilization of A-AMPs as tools to dissect these unresolved questions about the mechanism of HIV capture and presentation by DCs.
Our findings demonstrate that the A-AMPs inhibit HIV infection by disrupting the virion envelope. Importantly, this disruption occurs at much lower peptide concentrations than that which is toxic to cells. This observation could be explained by the small surface area of the virion relative to that of the cell. If we assume that the peptides function by carpeting the outer leaflet of the membrane, we would expect an increase in surface area that strains the lipid bilayer (35, 51). The extreme rate of curvature in the viral membrane compared to the cell membrane could cause the viral bilayer to succumb to stresses induced by peptide binding on the outer leaflet at lower concentrations. Another plausible explanation is that differences in the lipid content of the HIV envelope could increase the binding rate or affinity of the amphibian peptides. Although HIV acquires its envelope by budding through cellular membranes, the lipid constituency of the viral envelope differs from that of a normal human cell. HIV envelopes enrich in Brij 98-insoluble lipid rafts, which contain elevated levels of cholesterol, sphingomyelin, and glycophospholipids (11, 12). The presence of cholesterol and sphingomyelin contributes to tighter packing of the membrane lipids, resulting in a stabilized bilayer. Transient disruptions of the glycerophospholipid regions of the envelope by peptides that adhere to the viral surface could be preferentially stabilized by the high cholesterol content of the envelope, similar to fusion pore stabilization.
The rapid kinetics and the large dose range of peptides that inhibit virus infectivity without producing cell toxicity, coupled with the capacity to inhibit DC-captured HIV, render these peptides ideal candidates as topical microbicides for preventing HIV transmission. It is conceivable that certain A-AMPs might elicit a specific immune response, which would limit the long-term use of A-AMPs. However, peptides in the absence of an adjuvant are typically not immunogenic. For example, studies with the HIV fusion inhibitor peptide T20 (enfuvirtide) suggest that even upon repeated systemic application this peptide did not elicit functionally relevant antibody response (29). Subtle modifications in the amino acid sequence of A-AMPs may improve their inhibitory characteristics, decreasing IC50s while further increasing 50% lethal doses. A veritable treasure trove of unexplored A-AMPs (61) might hold even greater promise as antiviral compounds against a wide array of important human pathogens. The remarkable capacity of A-AMPs to gain access to HIV sequestered within DCs also suggests that these peptides serve as highly effective probes and may help to gain insight into the complex and intricate biology of DC-mediated HIV capture and trans infection of T cells.
Acknowledgments
This work was supported by Public Health Service awards R01 AI049131 (D.U.), R01 AI47506 (C.A.), and R01 AI32539 (T.S.D.); the Elizabeth B. Lamb Center for Pediatric Research (B.E.Y. and T.S.D.); and National Science Foundation awards DEB-0213851 and IBN-0131184 (L.A.R.-S).
REFERENCES
- 1.Ali, M. F., K. R. Lips, F. C. Knoop, B. Fritzsch, C. Miller, and J. M. Conlon. 2002. Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Biochim. Biophys. Acta 1601:55-63. [DOI] [PubMed] [Google Scholar]
- 2.Baer, G. S., and T. S. Dermody. 1997. Mutations in reovirus outer-capsid protein σ3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 71:4921-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balla, M. S., J. H. Bowie, and F. Separovic. 2004. Solid-state NMR study of antimicrobial peptides from Australian frogs in phospholipid membranes. Eur. Biophys. J. 33:109-116. [DOI] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 5.Belaid, A., M. Aouni, R. Khelifa, A. Trabelsi, M. Jemmali, and K. Hani. 2002. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66:229-234. [DOI] [PubMed] [Google Scholar]
- 6.Bierbaum, G., and H. G. Sahl. 1985. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch. Microbiol. 141:249-254. [DOI] [PubMed] [Google Scholar]
- 7.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsa, J., B. D. Morash, M. D. Sargent, T. P. Copps, P. A. Lievaart, and J. G. Szekely. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45:161-170. [DOI] [PubMed] [Google Scholar]
- 9.Borsa, J., M. D. Sargent, P. A. Lievaart, and T. P. Copps. 1981. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology 111:191-200. [DOI] [PubMed] [Google Scholar]
- 10.Boyd, M., K. Gustafson, J. McMahon, R. Shoemaker, B. O'Keefe, T. Mori, R. Gulakowski, L. Wu, M. Rivera, C. Laurencot, M. Currens, J. Cardellina II, R. Buckheit, Jr., P. Nara, L. Pannell, R. Sowder II, and L. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, S., K. Gaus, R. Bittman, W. Jessup, S. Crowe, and J. Mak. 2004. The raft-promoting property of virion-associated cholesterol, but not the presence of virion-associated Brij 98 rafts, is a determinant of human immunodeficiency virus type 1 infectivity. J. Virol. 78:10556-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 14.Chappell, J. D., J. L. Duong, B. W. Wright, and T. S. Dermody. 2000. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74:8472-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia, C. S., J. Torres, M. A. Cooper, I. T. Arkin, and J. H. Bowie. 2002. The orientation of the antibiotic peptide maculatin 1.1 in DMPG and DMPC lipid bilayers. Support for a pore-forming mechanism. FEBS Lett. 512:47-51. [DOI] [PubMed] [Google Scholar]
- 16.Chinchar, V. G., L. Bryan, U. Silphadaung, E. Noga, D. Wade, and L. Rollins-Smith. 2004. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 323:268-275. [DOI] [PubMed] [Google Scholar]
- 17.Chinchar, V. G., J. Wang, G. Murti, C. Carey, and L. Rollins-Smith. 2001. Inactivation of frog virus 3 and channel catfish virus by esculentin-2P and ranatuerin-2P, two antimicrobial peptides isolated from frog skin. Virology 288:351-357. [DOI] [PubMed] [Google Scholar]
- 18.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conlon, J. M., A. Sonnevend, M. Patel, V. Camasamudram, N. Nowotny, E. Zilahi, S. Iwamuro, P. F. Nielsen, and T. Pal. 2003. A melittin-related peptide from the skin of the Japanese frog, Rana tagoi, with antimicrobial and cytolytic properties. Biochem. Biophys. Res. Commun. 306:496-500. [DOI] [PubMed] [Google Scholar]
- 20.Conlon, J. M., A. Sonnevend, M. Patel, C. Davidson, P. F. Nielsen, T. Pal, and L. A. Rollins-Smith. 2003. Isolation of peptides of the brevinin-1 family with potent candidacidal activity from the skin secretions of the frog Rana boylii. J. Pept. Res. 62:207-213. [DOI] [PubMed] [Google Scholar]
- 21.Coombs, K. M., S. C. Mak, and L. D. Petrycky-Cox. 1994. Studies of the major reovirus core protein sigma 2: reversion of the assembly-defective mutant tsC447 is an intragenic process and involves back mutation of Asp-383 to Asn. J. Virol. 68:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dermody, T. S., M. L. Nibert, R. Bassel-Duby, and B. N. Fields. 1990. A σ1 region important for hemagglutination by serotype 3 reovirus strains. J. Virol. 64:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efron, L., A. Dagan, L. Gaidukov, H. Ginsburg, and A. Mor. 2002. Direct interaction of dermaseptin S4 aminoheptanoyl derivative with intraerythrocytic malaria parasite leading to increased specific antiparasitic activity in culture. J. Biol. Chem. 277:24067-24072. [DOI] [PubMed] [Google Scholar]
- 25.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 27.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 28.Gentsch, J. R., and A. F. Pacitti. 1985. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J. Virol. 56:356-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg, M., N. Cammack, M. Salgo, and L. Smiley. 2004. HIV fusion and its inhibition in antiretroviral therapy. Rev. Med. Virol. 14:321-337. [DOI] [PubMed] [Google Scholar]
- 30.Hooper, J. W., and B. N. Fields. 1996. Role of the μ1 protein in reovirus stability and capacity to cause chromium release from host cells. J. Virol. 70:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimbrell, D. A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256-267. [DOI] [PubMed] [Google Scholar]
- 32.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 33.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucia-Jandris, P., J. W. Hooper, and B. N. Fields. 1993. Reovirus M2 gene is associated with chromium release from mouse L cells. J. Virol. 67:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzaki, K. 1999. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1462:1-10. [DOI] [PubMed] [Google Scholar]
- 36.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 37.McGreal, E. P., L. Martinez-Pomares, and S. Gordon. 2004. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol. Immunol. 41:1109-1121. [DOI] [PubMed] [Google Scholar]
- 38.Munk, C., G. Wei, O. O. Yang, A. J. Waring, W. Wang, T. Hong, R. I. Lehrer, N. R. Landau, and A. M. Cole. 2003. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retrovir. 19:875-881. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277-304. [DOI] [PubMed] [Google Scholar]
- 40.Oswald-Richter, K., S. M. Grill, M. Leelawong, and D. Unutmaz. 2004. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur. J. Immunol. 34:1705-1714. [DOI] [PubMed] [Google Scholar]
- 41.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacitti, A., and J. R. Gentsch. 1987. Inhibition of reovirus type 3 binding to host cells by sialylated glycoproteins is mediated through the viral attachment protein. J. Virol. 61:1407-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reis e Sousa, C. 2004. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin. Immunol. 16:27-34. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldi, A. C. 2002. Antimicrobial peptides from amphibian skin: an expanding scenario. Curr. Opin. Chem. Biol. 6:799-804. [DOI] [PubMed] [Google Scholar]
- 45.Robinson, W. E., Jr., B. McDougall, D. Tran, and M. E. Selsted. 1998. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 63:94-100. [DOI] [PubMed] [Google Scholar]
- 46.Rollins-Smith, L. A., C. Carey, J. M. Conlon, L. K. Reinert, J. K. Doersam, T. Bergman, J. Silberring, H. Lankinen, and D. Wade. 2003. Activities of temporin family peptides against the chytrid fungus (Batrachochytrium dendrobatidis) associated with global amphibian declines. Antimicrob Agents Chemother. 47:1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollins-Smith, L. A., C. Carey, J. Longcore, J. K. Doersam, A. Boutte, J. E. Bruzgal, and J. M. Conlon. 2002. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev. Comp. Immunol. 26:471-479. [DOI] [PubMed] [Google Scholar]
- 48.Rollins-Smith, L. A., J. K. Doersam, J. E. Longcore, S. K. Taylor, J. C. Shamblin, C. Carey, and M. A. Zasloff. 2002. Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev. Comp. Immunol. 26:63-72. [DOI] [PubMed] [Google Scholar]
- 49.Rollins-Smith, L. A., L. K. Reinert, V. Miera, and J. M. Conlon. 2002. Antimicrobial peptide defenses of the Tarahumara frog, Rana tarahumarae. Biochem. Biophys. Res. Commun. 297:361-367. [DOI] [PubMed] [Google Scholar]
- 50.Rubin, D. H., D. B. Weiner, C. Dworkin, M. I. Greene, G. G. Maul, and W. V. Williams. 1992. Receptor utilization by reovirus type 3: distinct binding sites on thymoma and fibroblast cell lines result in differential compartmentalization of virions. Microb. Pathog. 12:351-365. [DOI] [PubMed] [Google Scholar]
- 51.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 52.Steinborner, S. T., R. J. Waugh, J. H. Bowie, J. C. Wallace, M. J. Tyler, and S. L. Ramsay. 1997. New caerin antibacterial peptides from the skin glands of the Australian tree frog Litoria xanthomera. J. Pept. Sci. 3:181-185. [DOI] [PubMed] [Google Scholar]
- 53.Steinman, R. M. 2000. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100:491-494. [DOI] [PubMed] [Google Scholar]
- 54.Steinman, R. M., A. Granelli-Piperno, M. Pope, C. Trumpfheller, R. Ignatius, G. Arrode, P. Racz, and K. Tenner-Racz. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 276:1-30. [DOI] [PubMed] [Google Scholar]
- 55.Sturzenbecker, L. J., M. L. Nibert, D. B. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanabe, H., A. J. Ouellette, M. J. Cocco, and W. E. Robinson, Jr. 2004. Differential effects on human immunodeficiency virus type 1 replication by alpha-defensins with comparable bactericidal activities. J. Virol. 78:11622-11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosteson, M. T., M. L. Nibert, and B. N. Fields. 1993. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc. Natl. Acad. Sci. USA 90:10549-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai, C. C., P. Emau, Y. Jiang, M. B. Agy, R. J. Shattock, A. Schmidt, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2004. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 20:11-18. [DOI] [PubMed] [Google Scholar]
- 59.Tsai, C. C., P. Emau, Y. Jiang, B. Tian, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 19:535-541. [DOI] [PubMed] [Google Scholar]
- 60.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanhoye, D., F. Bruston, P. Nicolas, and M. Amiche. 2003. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 270:2068-2081. [DOI] [PubMed] [Google Scholar]
- 62.van Kooyk, Y., A. Engering, A. N. Lekkerkerker, I. S. Ludwig, and T. B. Geijtenbeek. 2004. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr. Opin. Immunol. 16:488-493. [DOI] [PubMed] [Google Scholar]
- 63.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 170:4708-4716. [DOI] [PubMed] [Google Scholar]
- 64.Westerhoff, H. V., D. Juretic, R. W. Hendler, and M. Zasloff. 1989. Magainins and the disruption of membrane-linked free-energy transduction. Proc. Natl. Acad. Sci. USA 86:6597-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, L., T. M. Weiss, R. I. Lehrer, and H. W. Huang. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 79:2002-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasin, B., M. Pang, J. S. Turner, Y. Cho, N. N. Dinh, A. J. Waring, R. I. Lehrer, and E. A. Wagar. 2000. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 19:187-194. [DOI] [PubMed] [Google Scholar]
- 68.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]