Abstract
The spread of highly pathogenic avian influenza H5N1 viruses across Asia in 2003 and 2004 devastated domestic poultry populations and resulted in the largest and most lethal H5N1 virus outbreak in humans to date. To better understand the potential of H5N1 viruses isolated during this epizootic event to cause disease in mammals, we used the mouse and ferret models to evaluate the relative virulence of selected 2003 and 2004 H5N1 viruses representing multiple genetic and geographical groups and compared them to earlier H5N1 strains isolated from humans. Four of five human isolates tested were highly lethal for both mice and ferrets and exhibited a substantially greater level of virulence in ferrets than other H5N1 viruses isolated from humans since 1997. One human isolate and all four avian isolates tested were found to be of low virulence in either animal. The highly virulent viruses replicated to high titers in the mouse and ferret respiratory tracts and spread to multiple organs, including the brain. Rapid disease progression and high lethality rates in ferrets distinguished the highly virulent 2004 H5N1 viruses from the 1997 H5N1 viruses. A pair of viruses isolated from the same patient differed by eight amino acids, including a Lys/Glu disparity at 627 of PB2, previously identified as an H5N1 virulence factor in mice. The virus possessing Glu at 627 of PB2 exhibited only a modest decrease in virulence in mice and was highly virulent in ferrets, indicating that for this virus pair, the K627E PB2 difference did not have a prevailing effect on virulence in mice or ferrets. Our results demonstrate the general equivalence of mouse and ferret models for assessment of the virulence of 2003 and 2004 H5N1 viruses. However, the apparent enhancement of virulence of these viruses in humans in 2004 was better reflected in the ferret.
From December 2003 to April 2005, highly pathogenic avian influenza (HPAI) H5N1 viruses caused outbreaks of disease in domestic poultry in nine Asian countries (World Organization for Animal Health [OIE] [http://www.oie.int]). This unprecedented spread of HPAI virus was associated with a total of 79 human infections and 49 deaths in Vietnam, Thailand, and Cambodia (World Health Organization [WHO; http://www.who.int/en/]). With continued H5N1 virus circulation in poultry and further human cases likely, the potential for the emergence of an H5 avian-human reassortant with pandemic potential is a clear and present threat to public health worldwide.
HPAI viruses were first recognized to cause human respiratory infection and death in 1997, when, during outbreaks of disease in domestic poultry in Hong Kong, avian-to-human transmission of a purely avian H5N1 virus resulted in 18 human cases, of which 6 were fatal (5, 6, 35). The outbreak ended with the culling of all poultry in Hong Kong's poultry farms and markets. Although routine surveillance in Hong Kong repeatedly detected H5N1 virus in poultry between 1999 and 2003 (9, 13-15, 33), no further human cases were reported until February 2003, when H5N1 viruses were isolated from two family members with respiratory illness, one of whom died (26).
In December 2003, South Korean authorities first reported outbreaks of HPAI H5N1 virus in poultry (OIE [http://www.oie.int]; 21). By February 2004, seven additional Asian countries announced poultry outbreaks due to HPAI H5N1 virus (OIE [http://www.oie.int]). The outbreaks in Vietnam and Thailand were widespread, with approximately 90% and 60% of provinces affected, respectively. In contrast, outbreaks in Japan, Cambodia, Laos, Indonesia, and China were reported to be more regional. In August 2004, Malaysian authorities reported outbreaks in a single district (OIE [http://www.oie.int]).
Although the true numbers of human infections during the H5N1 outbreaks remain unknown, the 62% mortality rate among humans with documented H5N1 disease in 2004 and 2005 was markedly higher (WHO [http://www.who.int/en/]) than the 33% fatality rate among documented human H5N1 cases in 1997. Patients evaluated in Vietnam and Thailand generally presented with fever, respiratory symptoms, diarrhea, lymphopenia, and thrombocytopenia (2, 36), and although rare, presentation with fever and gastrointestinal symptoms but no respiratory symptoms was also reported (1, 7). Pneumonia with severe impairment of respiratory gas exchange was common, and despite assisted ventilation, most of these patients progressed to respiratory failure and death.
Because there is only limited information on the biologic and molecular properties that may confer virulence on HPAI H5N1 virus in humans, studies in mammalian models are necessary. Nonhuman primates, ferrets, and mice have been used as mammalian models to study influenza virus pathogenesis. Experimental infection of cynomolgous macaques with an H5N1 virus from the index case of the 1997 outbreak reproduced the acute respiratory distress syndrome and multiple-organ dysfunction observed in humans (19, 30, 31, 39). However, additional studies with other avian H5N1 strains have not been reported, likely due in part to the practical, ethical, and economic limitations of this mammalian model. On the other hand, the ferret, a naturally susceptible host to influenza A viruses, has been effectively used to evaluate H5N1 virus virulence, as well as the safety and efficacy of H5N1 vaccine candidates (12, 22, 38, 41). We previously established criteria for the assessment of H5N1 virus virulence in ferrets and demonstrated an equivalence in virulence for two 1997 H5N1 strains studied in this model (41). In contrast, the same two 1997 H5N1 strains caused two distinct phenotypes of disease in BALB/c mice: a highly pathogenic phenotype with systemic replication, lymphopenia, and death, and a low-pathogenic phenotype with efficient respiratory viral replication but no systemic lethal infection (11, 16, 17, 23, 37).
Here, we evaluate the relative virulence of selected H5N1 viruses isolated from humans or avian species during the 2003 and 2004 outbreak in Asia in both the mouse and ferret models and compare them to the earlier 1997 H5N1 strains isolated from humans. Our results demonstrate that, in general, the levels of virulence of H5N1 viruses in these two models are comparable. Furthermore, the ferret model demonstrates an increase in virulence of the 2004 human H5N1 isolates compared with the 1997 human isolates and with the 2003 and 2004 avian isolates studied.
MATERIALS AND METHODS
Viruses.
Highly pathogenic avian influenza A (H5N1) viruses isolated from birds and humans in various countries in Asia were used in this study (Table 1). Virus stocks for each of these viruses were propagated in the allantoic cavities of 10-day-old embryonated hen's eggs following incubation at 37°C for 24 to 29 h. Allantoic fluid from multiple eggs was pooled, clarified by centrifugation, aliquoted, and stored at −70°C. The 50% tissue culture infectious dose (TCID50) and 50% egg infectious dose (EID50) titers were determined by serial titration of viruses in Madin-Darby canine kidney (MDCK) cells and eggs, respectively, and were calculated by the method of Reed and Muench (27). VN1203 and VN1204 viruses were plaque purified in MDCK cells, and stocks were produced in 10-day-old embryonated hen's eggs, as described above. All research with HPAI viruses was conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agent Program (29). Animal research was conducted under the guidance of the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility.
TABLE 1.
H5N1 avian influenza viruses
| Virus | Name in this study | Country of origin | Patient age (yr) | Patient gender | Patient status |
|---|---|---|---|---|---|
| A/Vietnam/1203/2004a | VN1203 | Vietnam | 10 | Male | Fatal |
| A/Vietnam/1204/2004a | VN1204 | Vietnam | 10 | Male | Fatal |
| A/Thailand/16/2004 | Thai16 | Thailand | 7 | Male | Fatal |
| A/Thailand/SP/83/2004 | SP83 | Thailand | 58 | Female | Fatal |
| A/Thailand/Kan/353/2004 | Kan353 | Thailand | 6 | Male | Fatal |
| A/Hong Kong/483/1997 | HK483 | Hong Kong | 13 | Male | Fatal |
| A/Hong Kong/486/1997 | HK486 | Hong Kong | 5 | Female | Recovered |
| A/Chicken/Vietnam/NCVD/8/2003 | CkNCVD8 | Vietnam | NAb | NA | NA |
| A/Chicken/Vietnam/NCVD/31/2004 | CkNCVD31 | Vietnam | NA | NA | NA |
| A/Chicken/Indonesia/7/2003 | CkIndon | Indonesia | NA | NA | NA |
| A/Chicken/Korea/ES/2003 | CkKorea | South Korea | NA | NA | NA |
VN1203 and VN1204 were isolated from the same individual.
NA, not applicable.
Sequence and phylogenetic analyses.
Viral RNA extracted with an RNAeasy kit (QIAGEN, Hilden, Germany) was used in a One-Step reverse transcription-PCR (QIAGEN, Hilden, Germany). PCR products were purified using a QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and sequenced directly using the ABI BigDye terminator cycle-sequencing kit with products resolved on an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequences of primers are available upon request. DNA sequence analysis was performed using version 10 of the Genetics Computer Group sequence analysis package (8). Sequences were aligned with ClustalW software (4), and trees were generated by neighbor-joining analysis with the Tamura-Nei gamma model, implemented in the MEGA3 program (Life Sciences, Tempe, AZ). Identical relationships were found with the PAUP program (version 4.0 beta; Florida State University). The Influenza Sequence Database accession numbers for the HA sequences used in phylogeny inference are as follows (25): ISDN40864, A/Chicken/Cambodia/1/2004; ISDN40925, A/Chicken/Laos/44/2004; ISDN40922, A/Chicken/Laos/7191/2004; ISDN38693, A/Chicken/Vietnam/NCVD31/2004; ISDN49016, A/Chicken/Yamaguchi/7/2004; ISDN48989, A/Duck/GuangXi/13/2004; ISDN48957, A/Duck/HuNan/15/2004; ISDN40341, A/Thailand/16/2004; ISDN40917, A/Thailand/SP83/2004; ISDN40918, A/Thailand/Kan353/2004; ISDN38687, A/Vietnam/1203/2004; ISDN38688, A/Vietnam/1204/2004; ISDN40921, A/Chicken/Korea/ES/2003; ISDN40326, A/Chicken/Vietnam/NCVD8/2003; ISDN38262, A/Hong Kong/213/2003; AY575874, A/Duck/HongKong/821/02; ISDN38689, A/Duck/Vietnam/NCVD1/2002; AF468837, A/Duck/Anyang/AVL-1/2001; ISDN38260, A/Goose/Vietnam/113/2001; AF036356, A/HongKong/156/97; AF046097, A/HongKong/483/97; AF102671, A/HongKong/486/97; AF144305, A/Goose/Guangdong/1/96; and X07869, A/Chicken/Scotland/59. The Influenza Sequence Database accession numbers for the sequences used in comparisons (see Tables 4 and 5) are as follows: ISDN40379, ISDN40842, ISDN40934, and ISDN40016 for A/Vietnam/1203/04; ISDN40380, ISDN40843, ISDN121932, and ISDN40017 for A/Vietnam/1204/04; ISDN40383, ISDN40859, ISDN40940, ISDN40341, ISDN40086, ISDN48790, and ISDN40040 for A/Thailand/16/04; ISDN49457, ISDN40931, ISDN121933, ISDN40917, ISDN41067, ISDN 48792, and ISDN41028 for A/Thailand/SP/83/04.
TABLE 4.
Amino acid differences between H5N1 influenza viruses VN1203 and VN1204
| Virus | Amino acid
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PB2
|
PB1
|
PA
|
NS1a
|
|||||
| 456 | 627 | 207 | 436 | 85 | 142 | 615 | 87 | |
| VN1203 | N | K | R | H | T | E | K | S |
| VN1204 | D | E | K | Y | M | K | E | P |
NS1 numbering includes a 5-amino-acid deletion.
NA numbering includes a 20-amino-acid deletion.
NS1 numbering includes a 5-amino-acid deletion.
TABLE 5.
Amino acid differences between H5N1 influenza viruses Thai16 and SP83
| Virus | Amino acid
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2
|
PB1
|
PA
|
HA1a
|
HA2a
|
NP
|
NAb
|
NS1c
|
||||||
| 192 | 627 | 177 | 627 | 127 (131) | 457 (456) | 454 | 44 | 100 | 200 | 381 | 438 | 128 | |
| Thai16 | E | K | D | R | V | K | E | H | H | N | T | A | V |
| SP83 | D | E | E | G | A | R | D | Q | Y | S | I | T | I |
H3 numbering is in parentheses.
NA numbering includes a 20-amino-acid deletion.
NS1 numbering includes a 5-amino-acid deletion.
Mouse experiments.
Female BALB/c mice, 6 to 8 weeks old (Charles River Laboratories, Wilmington, MA), were lightly anesthetized with CO2 before infection. Fifty microliters of infectious virus diluted in phosphate-buffered saline (PBS) was inoculated intranasally (i.n.). Fifty percent mouse infectious dose (MID50) and fifty percent lethal dose (LD50) titers were determined by inoculating groups of eight mice i.n. with serial 10-fold dilutions of virus. Three days later, three mice from each group were euthanatized; lungs were collected, immediately frozen on dry ice, and stored at −70°C until they were processed. The frozen tissues were later thawed, homogenized in 1 ml of cold PBS, and clarified by centrifugation (2,200 × g) at 4°C. Virus titers present in clarified homogenates were determined in eggs. The five remaining mice in each group were monitored daily for clinical signs for 14 days postinfection (p.i.). Any mouse that lost more than 25% of its body weight was euthanatized. MID50 and LD50 titers were calculated using the method of Reed and Muench (27) and were expressed as the EID50 value corresponding to 1 MID50 or LD50. Replication of the H5N1 viruses in lung, spleen, thymus, heart, and brain tissues of mice (three per group) were determined 3 and 6 days p.i. Clarified homogenates of these tissues were titrated for virus infectivity in eggs from an initial dilution of 1:10 in PBS. The statistical significance of the virus titer data was determined by using analysis of variance.
Blood samples were collected from infected mice on days 0, 3, 5, 7, and 9 p.i. Absolute leukocyte counts were determined with a hemocytometer on heparinized blood diluted 1:10 with Turks solution (2% acetic acid, 0.01% methylene blue). Cell numbers were determined in triplicate from two individual mice. For differential counts, peripheral blood was obtained from two or three mice on the days indicated. Blood smears were prepared in duplicate (two slides per mouse) at each bleeding and were stained with Hema 3 stain (Fisher Diagnostics, Middleton, VA). Monocytes, polymorphonuclear neutrophils, and lymphocyte numbers were determined. At least 100 cells were counted for each slide at a magnification of ×1,000.
Ferret experiments.
Four to six male Fitch ferrets, 8 to 12 months of age (Triple F Farms, Sayre, PA), serologically negative by hemagglutination inhibition for currently circulating influenza viruses, were used to assess the virulence of each virus included in this study. The ferrets were housed in cages within a Duo-Flo Bioclean mobile clean room (Lab Products, Seaford, DE) throughout each experiment. At least 2 days prior to infection, baseline serum, temperature, and weight measurements were obtained. After the ferrets were anesthetized with an intramuscular injection of a ketamine hydrochloride (24 mg/kg)-xylazine (2 mg/kg)-atropine (0.05 mg/kg) cocktail, they were inoculated i.n. with 107 EID50 of virus, unless otherwise indicated, in 1 ml of PBS. The ferrets were monitored for changes in body temperature and weight and the presence of the following clinical signs: sneezing, lethargy, anorexia, nasal or ocular discharge, dyspnea, diarrhea, and neurological dysfunction. Body temperatures were measured using an implantable subcutaneous temperature transponder (BioMedic Data Systems, Inc., Seaford, DE). Lethargy was measured based on a scoring system of 0 to 3, and the scores were used to calculate a relative inactivity index (RII) as previously described (28, 41). Any ferret that lost more than 25% of its body weight or exhibited neurological dysfunction was euthanatized and submitted to postmortem examination. Statistical significance of lethality was determined using Fisher's exact probability test. The ferrets were bled via the anterior vena cava on days 0, 1, 3, 5, 7, and 14 p.i. for collection of peripheral blood and subsequent differential blood counts. Blood smears were prepared and processed as described above.
Virus shedding was measured in nasal washes collected on days 1, 3, 5, and 7 p.i. from anesthetized ferrets as previously described (41). The nasal washes were immediately frozen on dry ice and stored at −70°C until they were processed. Prior to euthanasia, the ferrets were heavily sedated and exsanguinated, and they were euthanatized via intracardiac injection of Euthanasia V solution (1 ml/kg). A postmortem examination was conducted immediately, and the following tissues were aseptically collected in this order: spleen, kidneys, intestines, liver, heart, lungs-trachea, brain, olfactory bulb, and nasal turbinates. Tissue specimens collected for virus titration were immediately frozen on dry ice and stored at −70°C until they were processed. The remainder of the lungs and brains were fixed in 10% neutral-buffered formalin, including infusion of the lungs, for subsequent histological analysis. Frozen tissue specimens were thawed, weighed, rinsed, and then homogenized in cold PBS using disposable sterile tissue grinders (Kendall, Mansfield, MA). Tissue homogenates were clarified by centrifugation (2,200 × g) at 4°C. Virus titers in clarified homogenates, peripheral blood, and nasal washes were determined in eggs. Nasal wash, nasal turbinate, and peripheral blood virus titers were expressed as EID50/ml, while virus titers in all other tissues were expressed as EID50/g. The limit of virus detection was 101.5 EID50/ml.
Histological analyses.
Necropsies were performed on all infected ferrets that died suddenly or were euthanatized on days 3, 5, 6, 7, 8, 9, or 14 p.i. Tissues were fixed in formalin, and representative samples from each lung lobe (in one cassette) and a coronal section, including the parietal and temporal brain cortex (in one cassette), were paraffin-embedded and stained with hematoxylin and eosin (H&E) or immunohistochemistry (IHC). IHC analysis was performed using a monoclonal antibody to influenza A nucleoprotein on tissues harvested prior to 14 days p.i. Detection of the attached primary antibody was carried out with the LSAB2 Universal alkaline phosphatase system (Dako Corp., Carpinteria CA) and naphthol fast red as a chromogen (40). Only cells with red-staining nuclei were considered positive with the IHC assay. Statistical significance was determined using Fisher's exact probability test.
RESULTS
Characteristics of HPAI H5N1 viruses used in this study.
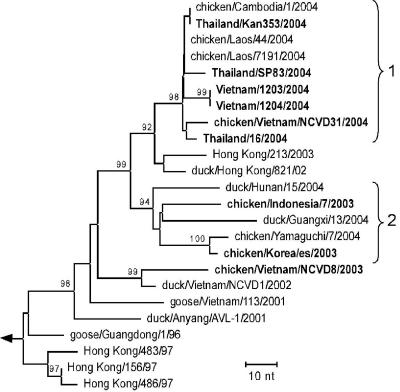
A panel of representative viruses isolated from humans or chickens during the 2003 and 2004 H5N1 virus outbreak were evaluated for their relative virulence in BALB/c mice and ferrets and compared to the well-characterized H5N1 viruses isolated from humans in Hong Kong in 1997 (Table 1) (23, 41). Viruses were selected to be representative of the geographic origins and the various genetic lineages of H5N1 viruses that were available at the time of the study. All H5N1 viruses tested had high infectivity titers in the allantoic cavities of 10-day-old embryonated eggs or MDCK cells with titers ranging from 8.5 to 9.8 log10 EID50/ml and 7.0 to 8.0 log10 TCID50/ml, respectively (Table 2). Two isolates, VN1203 and VN1204, were cultivated from different clinical specimens (pharyngeal swab and tracheal aspirate, respectively) from the same individual, a 10-year-old male from Vietnam. All 2004 H5N1 viruses isolated from humans were from fatal cases. As shown in Fig. 1, the phylogenetic analysis of the HA1 genes of the nine representative viruses demonstrates that, except for one, all of the viruses included in this study fall into two distinct genetic clades. All human viruses from Thailand and Vietnam, as well as the chicken virus CkNCVD31, belong to clade 1. The avian isolates CkIndon and CkKorea fall into clade 2, whereas CkNCVD8 is the most divergent and falls outside of these two clades.
TABLE 2.
Virulence of H5N1 viruses in BALB/c mice
| Virus | EID50/mla (log10) | TCID50/mlb (log10) | PB2 at 627c | Wt lossd (%) | Lung titerse (log10) | MID50f (log10) | LD50f (log10) | Virulence in miceg |
|---|---|---|---|---|---|---|---|---|
| HK483 | 8.5 | 7.7 | K | 16.6 | 7.5 ± 0.3 | ND | 1.6 | High |
| Thai16 | 8.8 | 7.0 | K | 16.1 | 7.7 ± 0.5 | 1.3 | 1.7 | High |
| Kan353 | 9.5 | ND | K | ND | ND | ND | ND | ND |
| VN1203 | 9.5 | 7.5 | K | 18.8 | 6.3 ± 0.2 | 1.8 | 2.2 | High |
| VN1204 | 9.8 | 7.3 | E | 17.6 | 6.9 ± 0.4 | 2.3 | 3.8 | High |
| SP83 | 9.5 | 8.0 | E | 12.8 | 6.6 ± 0.7 | 1.8 | 5.5 | Low |
| CkKorea | 9.3 | 7.5 | E | 13.3 | 6.9 ± 0.3 | 2.3 | >7.0 | Low |
| CkIndon | 9.3 | 7.3 | E | 3.7 | 4.3 ± 0.5 | 5.3 | >7.0 | Low |
| CkNCVD8 | 9.5 | 7.3 | E | 11.4 | 3.0 ± 1.6 | 5.8 | 5.8 | Low |
| CkNCVD31 | 8.5 | 7.0 | E | 6.9 | 3.8 ± 0.4 | 3.5 | >7.0 | Low |
Titer of virus stocks prepared in 10-day-old embryonated eggs.
Titer of virus stocks prepared on MDCK cells; ND, not determined.
Amino acid at position 627 of PB2; K, lysine; E, glutamic acid.
Maximum percent weight loss (mean of five mice per group) following infection with 106 EID50 of virus.
Average lung titers of three mice on day 3 p.i., expressed as EID50/ml ± SD.
MID50 and LD50 are expressed as the EID50 required to give one MID50 or one LD50.
Highly virulent viruses had LD50 doses 50- to 1.9 × 105-fold less than the low-virulence viruses.
FIG. 1.
Phylogenetic relationships of the H5 hemagglutinin genes. The tree includes avian influenza isolates collected during the 2003 and 2004 outbreak in Asia and selected ancestors dating back to 1996 (see Materials and Methods for database accession numbers). The viruses evaluated in this study are in boldface type. HA clades are indicated by curved brackets. Phylogenetic trees were inferred from nucleotide sequences by the neighbor-joining method with A/Chicken/Scotland/56 genes as the outgroup (not shown; the branch position is indicated by the arrow). Bootstrap analysis values of ≥90% are shown above the branches. The scale bar indicates the number of nucleotide (nt) changes per unit length of the horizontal branches.
Pathogenicity of H5N1 viruses in mice.
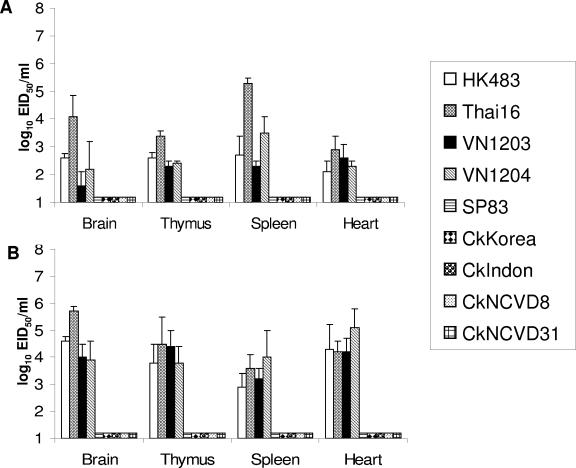
The MID50 and LD50 titers of eight 2003 and 2004 H5N1 viruses were determined in BALB/c mice and compared to a group of animals infected with the highly pathogenic HK483 virus, previously shown to be lethal in mice (11, 23). As shown in Table 2, all eight 2003 and 2004 H5N1 isolates replicated in mouse lungs without prior host adaptation. However, three of the four avian viruses tested achieved significantly (P < 0.01) lower lung virus titers and had higher MID50 titers than did all human isolates and the CkKorea isolate. The 2003 and 2004 H5N1 viruses also varied in their abilities to cause severe disease and death in this species. Mice infected with doses of ≥104 EID50 of the human H5N1 isolates (Thai16, VN1203, VN1204, and HK483) began to lose weight within 2 days; showed signs of illness, such as ruffled fur and listlessness, during the first week of infection; and succumbed to infection by day 9 p.i. The Thai16 and VN1203 viruses, like HK483 virus, had an LD50 of ≤103.0 EID50, whereas VN1204 virus had an LD50 of 103.8 EID50, requiring 40 times more virus than VN1203 virus to kill mice. The biological disparity between the VN1203 and VN1204 isolates, despite being cultured from the same individual, might have been due to amino acid differences at key residues or to the presence of a mixture of viruses in one or both preparations. To test the latter possibility, we plaque purified VN1203 and VN1204 viruses on MDCK cells, and seed viruses derived from single plaques were amplified in eggs and used to determine the LD50. The plaque-purified VN1203 and VN1204 viruses had LD50 titers of 101.5 and 103.8, respectively, confirming the phenotypes of the original virus stocks and indicating that mixed virus populations did not account for the observed differences in virulence. We next examined the ability of the human H5N1 viruses to replicate in organs outside of the respiratory tract, including the brain. Thai16, VN1203, and VN1204 viruses could be detected in brain, thymus, spleen, and heart tissues of mice on days 3 and 6 p.i., recapitulating the outcome of infection with the HK483 H5N1 virus (Fig. 2). Among the human H5N1 isolates, Thai16 virus was recovered from the brain at the highest levels, with titers increasing 20- to 400-fold from days 3 to 6 p.i. In general, the titers of the human isolates on day 6 were higher than those on day 3 for each of the tissues examined. Evaluation of peripheral blood leukocyte counts on days 0, 1, 3, 5, and 7 p.i. showed that the highly virulent Thai16, VN1203, and VN1204 viruses induced leukopenia in mice as early as 3 days p.i., which was sustained until the deaths of these mice. Differential blood counts revealed that lymphocyte numbers in Thai16-, VN1203-, and VN1204-infected mice dropped up to 89% by day 5 p.i. in comparison to mock-infected mice (data not shown).
FIG. 2.
Comparison of mean titers of influenza A H5N1 virus recovered from mouse tissues. Mice were inoculated i.n. with 106 EID50 of each virus, and tissues were collected on days 3 (A) and 6 (B) p.i. Tissue homogenates were prepared and titrated in eggs. Virus endpoint titers are expressed as mean log10 EID50 per milliliter plus standard deviation. The limit of virus detection was 101.5 EID50/ml.
The lethalities of the four avian H5N1 isolates and the human SP83 virus were substantially lower than those of other H5N1 viruses (Table 2). All mice infected with CkKorea, CkIndon, or CkNCVD31 virus survived infection (LD50 > 107.0), whereas CkNCVD8 and SP83 viruses caused lethal disease only at the highest virus doses (LD50 ≥ 105.5). Although SP83 and CkKorea viruses replicated in mouse lungs to titers similar to those of the lethal human H5N1 isolates, infectious virus was not recovered from organs outside of the respiratory tract on days 3 and 6 p.i. in mice infected with these two viruses or the other three avian strains. CkIndon and CkNCVD31 viruses induced only minimal clinical illness and weight loss, and virus was restricted to the respiratory tract at titers below 104.5 EID50/ml on days 3 and 6 p.i. (Fig. 2). Evaluation of peripheral blood leukocyte counts on days 0, 1, 3, 5, and 7 p.i. indicated that mice infected with the low-virulence SP83 virus induced a transient drop (6 to 25%) in leukocyte numbers on days 3 and 5 p.i. with recovery to normal levels by day 7 p.i. (data not shown). These data indicate that the majority of 2004 human H5N1 strains were highly pathogenic for mice, causing systemic infection, whereas the avian isolates and the human SP83 virus replicated only in the respiratory tract and were considerably less lethal.
Clinical response of ferrets to infection with H5N1 viruses.
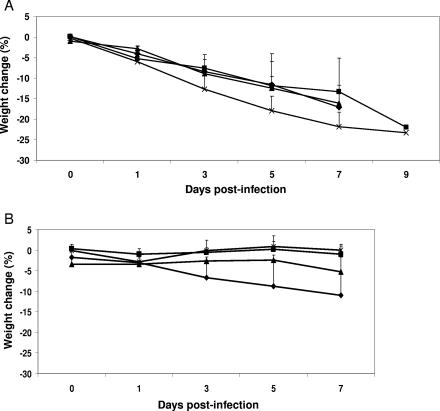
The severity of clinical disease caused by H5N1 viruses isolated during the 2003 and 2004 outbreak was evaluated in four to six naïve ferrets inoculated i.n. with 107 EID50 of each virus. Two or three ferrets were monitored up to 14 days p.i. for clinical signs of disease, while two or three ferrets were euthanatized on day 3 p.i. to assess pathological and virologic parameters. All viruses caused fever, with the peak mean change in body temperature ranging from 1.2 to 2.9°C over baseline (range, 37.1 to 38.7°C). Four viruses isolated from humans, VN1203, VN1204, Thai16, and Kan353, caused severe lethargy in all infected ferrets that was accompanied by anorexia, rhinorrhea, dyspnea, diarrhea, and a mean maximal weight loss of 16 to 23% (Fig. 3 and Table 3). Furthermore, at least two-thirds of the animals died by day 9 p.i., indicating that these viruses are highly virulent for ferrets. Three additional ferrets were inoculated with either 105 or 105.5 EID50 of the VN1203 isolate; none of these ferrets survived beyond day 7 p.i. (data not shown), confirming the virulence of this virus, even at lower infectious doses. Lymphocytes were depleted in the peripheral blood of ferrets infected with the highly virulent viruses by 76 to 79% compared to preinfection levels, with the greatest depletion occurring 5 days p.i. (data not shown). Transient lymphopenia was observed in the two ferrets that survived infection with these virulent viruses. In contrast to the severe and usually fatal disease induced by the majority of human H5N1 isolates, SP83 virus caused only modest weight loss and relatively mild illness (RII = 1.6), and all ferrets recovered fully. Likewise, ferrets infected with any of the three avian H5N1 isolates (CkKorea, CkIndon, or CkNCVD31) exhibited only minor weight loss, minimal clinical signs, and modest and transient lymphopenia and survived the 14-day experimental period. Therefore, the 2003 and 2004 H5N1 viruses evaluated here can be clustered into two distinct groups according to their virulence phenotypes in ferrets (Table 3). For comparison, a parallel experiment was performed using a 1997 human H5N1 isolate, HK486. Ferrets inoculated with HK486 virus developed signs of disease, including severe lethargy (RII = 2.1), dyspnea, and modest weight loss, but all survived infection (Table 3). Based on our observations and those previously reported (41), the data suggest that the highly virulent 2004 H5N1 viruses caused more severe disease in ferrets than the 1997 H5N1 HK486 virus.
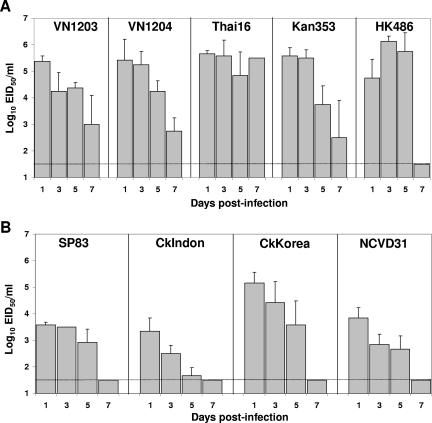
FIG. 3.
Weight change of H5N1-infected ferrets. All ferrets were inoculated i.n. with 107 EID50 of H5N1 virus. The percent weight change was determined by comparing the weight of each animal at each time point to its preinfection weight. The mean percentage weight change is shown ± standard deviation. (A) Ferrets were inoculated with either VN1203 (⧫), VN1204 (▪), Thai16 (▴), or Kan353 (×) and were weighed on days −1, 0, 1, 3, 5, 7, and 9 p.i. Only a single ferret remained on day 7 p.i. for Thai16 and on day 9 p.i. for VN1204 and Kan353. (B) Ferrets were inoculated with either SP83 (⧫), CkIndon (▪), CkKorea (▴), or CkNCVD31 (×) and were weighed on days −1, 0, 1, 3, 5, and 7 p.i.
TABLE 3.
Summary of results in ferrets inoculated with H5N1 influenza viruses
| Virus | Number of animals
|
PB2 627 amino acid | Virulence in ferrets | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical symptoms up to day 14 p.i.
|
Virus titer on day 3 p.i. (log10 mean EID50/ml)
|
||||||||||
| Wt lossa (%) | Lethargy (RIIb)c | Respiratoryc | Neurologicalc | Day of death (MDT) | Lungsc | Spleenc | Olfactory bulbc | Brainc | |||
| Thai16 | 16 | 3/3 (2.4) | 3/3 | 0/3 | 6, 7, 7d (6.6) | 3/3 (5.3) | 2/3 (2.0) | 3/3 (3.5) | 3/3 (2.9) | K | High |
| VN1203 | 17 | 2/2 (2.4) | 2/2 | 0/2 | 8, 8 (8.0) | 2/2 (4.5) | 2/2 (3.4) | 2/2 (5.0) | 2/2 (3.6) | K | High |
| VN1204 | 22 | 2/3 (1.8) | 2/3 | 1/3 | 8, 9,e survf (10.3) | 3/3 (3.8) | 0/3 (≤1.5) | 2/3 (3.1) | 1/3 (1.7) | E | High |
| Kan353 | 23 | 3/3 (2.2) | 2/3 | 1/3 | 5, 8,g surv (9.0) | 3/3 (6.2) | 2/3 (3.4) | 3/3 (5.0) | 3/3 (3.5) | K | High |
| HK486 | 10 | 2/2 (2.1) | 2/2 | 0/2 | Survived | 2/3 (4.7) | 1/3 (2.3) | 3/3 (4.2)h | 3/3 (4.2)h | E | High |
| SP83 | 11 | 2/3 (1.6) | 0/3 | 0/3 | Survived | 3/3 (3.7) | 0/3 (≤1.5) | 3/3 (3.9) | 1/3 (1.7) | E | Low |
| CkNCVD31 | 3 | 3/3 (1.3) | 0/3 | 0/3 | Survived | 2/3 (2.5) | 0/3 (≤1.5) | 1/3 (2.1) | 0/3 (≤1.5) | E | Low |
| CkKorea | 5 | 0/3 (1.0) | 0/3 | 0/3 | Survived | 3/3 (3.1) | 0/3 (≤1.5) | 3/3 (4.0) | 2/3 (2.5) | E | Low |
| CkIndon | 1 | 1/3 (1.1) | 0/3 | 0/3 | Survived | 1/3 (1.9) | 0/3 (≤1.5) | 0/3 (≤1.5) | 0/3 (≤1.5) | E | Low |
The percent mean maximum weight loss is shown.
Relative inactivity index.
No. of animals/total.
Euthanatized due to severity of clinical symptoms.
Euthanatized due to torticollis.
Survived the 14-day experimental period.
Euthanatized due to hind-limb paresis.
Olfactory bulb and brain tissues were processed together for this virus group only.
Comparison of lethality of H5N1 viruses for ferrets.
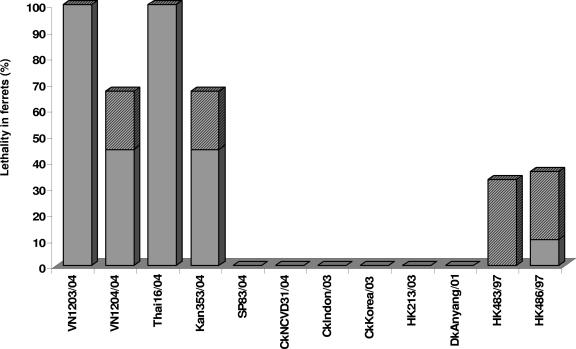
We next compared the virulence of the recent H5N1 viruses with that of other H5N1 viruses isolated since 1997 and previously characterized in the ferret model in this laboratory. The relative lethality observed in ferrets reported here or elsewhere (24, 41), shown in Fig. 4, demonstrates that of 34 ferrets infected with H5N1 viruses isolated in 1997, 35% did not recover from infection. In general, these ferrets developed neurological symptoms 7 to 13 days p.i., necessitating euthanasia (41). In contrast, most ferrets infected with a comparable dose of the 2004 highly virulent H5N1 viruses (VN1203, VN1204, Thai16, or Kan353; n = 13) died acutely (mean death time [MDT] = 8.5) without signs of neurological dysfunction. All other H5N1 viruses characterized to date, including the other 2003 and 2004 isolates evaluated in the current study, a 2001 avian isolate (A/Duck/Anyang/AVL-1/2001 [24]), and a 2003 human isolate (A/Hong Kong/213/03), did not induce severe or fatal disease in ferrets. Thus, the 2004 highly virulent H5N1 viruses isolated from humans identified in this study were significantly more lethal for ferrets than the H5N1 viruses isolated from 2001 to 2004, exhibiting low virulence (P < 0.001), or the 1997 H5N1 viruses, HK483 and HK486 (P < 0.01).
FIG. 4.
Lethality and neurological dysfunction exhibited by ferrets infected with H5N1 viruses. Infected ferrets were monitored for a 14-day experimental period for clinical signs of illness. Any ferret that exhibited neurological signs or lost more than 25% of its body weight was euthanatized. Each bar indicates the percentage of ferrets that died or were euthanatized before the end of the experimental period, while the dark shaded portion of the bar indicates the portion of those ferrets exhibiting neurological dysfunction. Data from ferrets infected with A/Duck/Anyang/AVL-1/01 (24) or A/Hong Kong/483/97 virus (41) were published elsewhere. The percentage for A/Hong Kong/486/1997 virus was determined based on data obtained here and from Zitzow et al. (41).
Replication of H5N1 viruses in ferrets.
To assess the efficiency and kinetics of virus replication in the upper respiratory tract of ferrets infected with the 2003 and 2004 H5N1 isolates, we analyzed nasal wash specimens collected on alternate days for 7 days p.i. (Fig. 5). All viruses that caused severe disease or death in ferrets (VN1203, VN1204, Thai16, Kan353, and HK486) achieved peak mean titers of ≥105.4 EID50/ml and had sustained titers of ≥104.0 EID50/ml for 3 to 5 days p.i. Ferrets infected with the highly virulent 2004 H5N1 human isolates failed to clear infectious virus from the upper respiratory tract by day 7 p.i. The mean peak titer for Thai16-infected animals remained high through day 7 p.i., when two ferrets died or were euthanatized in extremis. In contrast, low-virulence viruses, SP83, CkIndon, and CkNCVD31, reached peak mean titers of <104.0 EID50/ml in nasal washes over the 7-day period, while CkKorea virus reached a peak titer of 105.2 EID50/ml on day 1 p.i. All mean titers for the low-virulence viruses fell below 104.0 EID50/ml by day 5 p.i., and all animals cleared the infection by day 7 p.i. Thus, all four of the highly virulent 2004 H5N1 viruses replicated to higher levels and were shed for a longer period of time than the viruses exhibiting low virulence in ferrets.
FIG. 5.
Virus replication in the upper respiratory tract of H5N1-infected ferrets. Virus titers were measured in nasal washes collected on the days indicated from ferrets inoculated i.n. with 107 EID50 of either an H5N1 virus characterized as highly virulent (A) or an H5N1 virus found to be of low virulence (B). Mean titers are shown and are expressed as the log10 mean (plus standard deviation) EID50/ml, with the limit of detection at 101.5 EID50/ml.
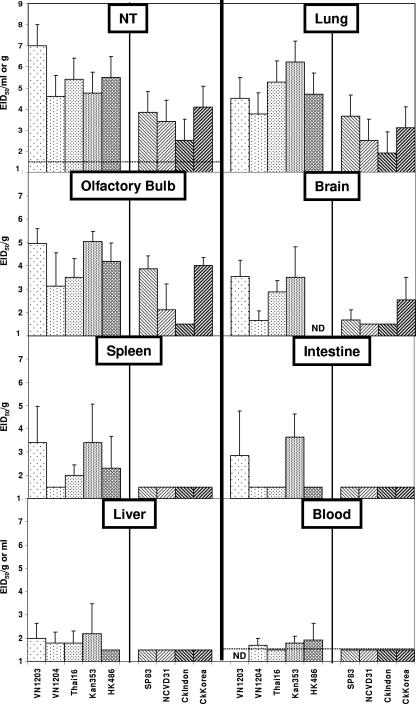
To investigate the ability of the 2003 and 2004 H5N1 viruses to cause systemic infection in ferrets, we determined the viral titers in major organs of two or three animals euthanatized on day 3 p.i. (Fig. 6 and Table 3). All viruses tested were detected in the upper and lower respiratory tracts of ferrets. Highly virulent viruses had mean titers in the nasal turbinates of ≥104.6 EID50/ml, whereas low-virulence virus titers were ≤104.1 EID50/ml. Likewise, the mean lung viral titers for the highly virulent viruses, ranging between 103.8 and 106.2 EID50/g, tended to be higher than those for ferrets inoculated with the low-virulence viruses, which had mean lung titers of ≤103.7 EID50/g. All viruses except one of the low-virulence isolates, CkIndon, were detected in the olfactory bulb of the brain. All of the highly virulent viruses and two of the low-virulence isolates, SP83 and CkKorea, were also detected in the brain posterior to the olfactory bulb. Titers in this organ for the highly virulent viruses were generally higher than those of the low-virulence viruses. In a previous study, human H3N2 virus was found in the brains of ferrets that did not exhibit any severe clinical signs of disease (41). Therefore, in this animal model, isolation of virus from the brain is not necessarily an indicator of the level of virulence of a particular influenza A virus. Some highly virulent viruses were also detected in the spleen, intestine, liver, or peripheral blood of ferrets, whereas none of the low-virulence viruses were detected in these organs. Taken together, these data indicate that, compared with low-virulence strains, highly virulent viruses, in general, replicated to higher titers and for a longer duration in the respiratory tract and spread to multiple organs.
FIG. 6.
Systemic replication of H5N1 viruses in ferrets. Virus titers in tissues collected 3 days p.i. from ferrets infected with 107 EID50 of the viruses indicated were measured in eggs. Mean viral titers are shown and are expressed as EID50/g plus standard deviation for all tissues except the nasal turbinates, which are expressed as EID50/ml. The limit of detection was 101.5 EID50/ml of tissue homogenate. ND, not determined.
Gross and histological pathology observed in H5N1-infected ferrets.
Ferrets infected with the highly virulent 2004 H5N1 viruses exhibited severe macroscopic pathology 5 to 9 days p.i., including focal areas of pulmonary discoloration in 100% of animals examined and hemorrhages that ranged from 0.5 to 2 cm in diameter in adipose tissue surrounding the liver, kidneys, and bladder in 63% of animals. In contrast, no ferrets infected with the 2003 and 2004 H5N1 low-virulence viruses exhibited equivalent macroscopic pathological changes at 14 days p.i. While ferrets infected with HK486 virus exhibited some pulmonary discoloration, there was no evidence of hemorrhages, confirming previous findings (41).
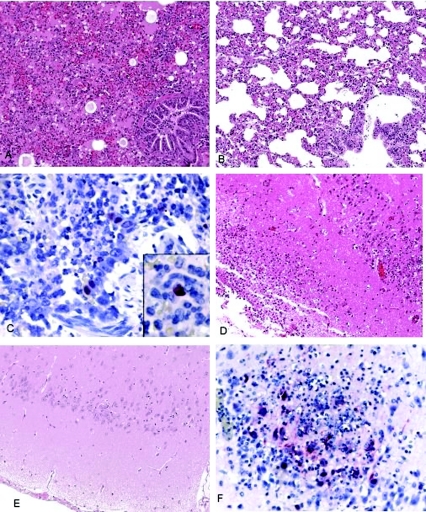
Histopathologic evaluation of lung tissue samples of ferrets infected with any of the 2003 and 2004 H5N1 viruses demonstrated diffuse inflammation of interalveolar septa accompanied by intra-alveolar edema regardless of the time postinfection. The inflammatory infiltrate present in the interalveolar septa was predominantly composed of mononuclear cells. However, the severity of inflammation observed in the lungs of ferrets infected with the 2004 highly virulent isolates (Fig. 7A) expanded to larger areas of the lung than the inflammation observed with the viruses of low virulence (Fig. 7B), which tended to be more localized. Influenza virus antigens were detected by IHC in about 67% of lung specimens collected from ferrets 3 days p.i. with either the high- or low-virulence viruses. Detection of viral antigens in tissues was limited to day 3 p.i., except for lungs harvested at 5 and 7 days p.i. from two ferrets infected with the highly virulent virus Kan353 or Thai16, respectively (data not shown). Viral antigens were detected in the bronchial cells present in the alveoli (inset in Fig. 7C) or bronchiles (Fig. 7C), although the latter was primarily observed in ferrets infected with the highly virulent viruses.
FIG. 7.
Representative images of histopathologic changes and immunostaining of tissues from ferrets infected with H5N1 viruses of high or low virulence. (A) H&E staining of the lungs of a VN1204-infected ferret 3 days p.i. showing diffuse interstitial inflammation accompanied by intra-alveolar edema. (B) H&E staining of the lungs of an SP83-infected ferret 3 days p.i. showing more modest inflammation and more gas-filled lumina than in those infected with a highly virulent virus. (C) Immunostaining of influenza virus antigen in the lungs of a VN1204-infected ferret 3 days p.i. was observed in bronchiolar epithelial cells and interstitial cells (inset). (D) H&E staining of the parietal cortex of the brain from a VN1204-infected ferret 9 days p.i. showing inflammatory infiltrate in the meninges and brain parenchyma. (E) H&E staining of the parietal cortex of the brain tissue from a ferret infected with SP83 virus 14 days p.i. showing a lack of inflammatory infiltrate. (F) Immunostaining of brain tissue from a VN1204-infected ferret 9 days p.i. exhibiting influenza virus antigen in neurons in an area of brain inflammation. Original magnifications, ×50 (A, B, D, and E); ×157.5 (C); ×100 (F).
Brain specimens from ferrets infected with the highly virulent viruses exhibited inflammation (Fig. 7D) in 48% of the infected ferrets in tissues collected after 6 days p.i. Only 18% of ferrets infected with low-virulence viruses showed some inflammation in the brain; all others exhibited normal histology (Fig. 7E). Inflammation was observed significantly more often in brain tissue from ferrets infected with highly virulent viruses than in those infected with low-virulence viruses (P < 0.05) and was predominantly composed of mononuclear cells present in the meninges, choroid plexus, and brain parenchyma. Influenza virus antigens were detected in neurons (3 to 9 days p.i.) in the same areas of the brain where inflammation was observed (Fig. 7F) among 43% of ferrets infected with the highly virulent viruses. In contrast, no viral antigen was detected in the ferrets infected with low-virulence viruses (P < 0.05).
Molecular correlates of viral pathogenicity phenotypes in mice and ferrets.
The availability of two isolates from the same patient, VN1203 and VN1204, with different pathogenicity phenotypes provided a pair of viruses that might yield information regarding the molecular determinants of virulence. Alignments of the deduced amino acid sequences of the 10 viral proteins of VN1203 and VN1204 viruses showed that they had a total of eight amino acid differences distributed among PB2, PB1, PA, and NS1 (Table 4). VN1203 virus has a Lys at residue 627 of the PB2 gene, while VN1204 has a Glu residue. VN1203 virus was shown to be highly virulent in mice, with an LD50 of 102.2, whereas the LD50 for VN1204 virus was 103.8, approximately 40-fold less lethal for mice than VN1203 virus (Table 2). This was a modest effect compared to previous studies, in which the Lys-to-Glu 627 substitution in PB2 of the 1997 H5N1 virus HK483 resulted in a 3-log-unit decrease in lethality for mice (16). Our results indicate that the Lys/Glu disparity at position 627 in PB2 was insufficient to abolish virulence in mice for this virus pair and suggest that the ability of this residue to attenuate a given H5N1 virus is dependent on an additional amino acid sequence(s).
Thai16 and SP83 viruses, both isolated from humans in Thailand, are genetically similar but were strikingly different in their virulence for mice and ferrets (Tables 2 and 3). The deduced amino acid sequences of these viruses differ by 13 amino acids in seven genes, including 627 of PB2 (Table 5). Thai16 virus has a Lys at PB2 627, while SP83 has a Glu, which may contribute to the difference in virulence in mice. Because reduced virulence was not observed in ferrets infected with VN1204 virus, the PB2 627 Lys/Glu disparity alone is unlikely to account for the difference in virulence for ferrets between the Thai 16 and SP83 viruses. Therefore, other amino acid changes between these viruses likely confer the differences in the pathogenic phenotypes observed in both mice and ferrets.
DISCUSSION
HPAI H5N1 viruses continue to cause disease in poultry and humans in southeastern Asia. To better understand the potential of H5N1 viruses isolated during the 2003 and 2004 epizootic event to cause disease in mammalian species, we compared the virulence of viruses representing multiple genetic groups in two well-established animal models. With one exception, all 2004 H5N1 viruses isolated from humans were highly lethal for mice and ferrets, whereas isolates from chickens were not, including one avian isolate that belongs to the same genetic group as the human isolates. Our results demonstrate that these two models generally offer similar results in the evaluation of the virulence of H5N1 viruses in mammals; however, results in ferrets may better reflect the apparent increase in the mortality rate observed among humans with H5N1 disease in 2004 and 2005.
Of the 2003 and 2004 H5N1 isolates examined in the current study, the most virulent were the four human isolates, VN1203, VN1204, Thai16, and Kan 353. These viruses replicated to high titers in the mouse and ferret respiratory tract and were isolated from multiple organs, including the brain, of both species. In contrast, the four avian isolates, all from chickens, and one human isolate (SP83) infected only the respiratory tract in mice and showed limited dissemination from the respiratory tract in the ferret. Recently, Govorkova et al. (12) reported that 2004 H5N1 viruses isolated in Vietnam or Thailand from humans, duck, or quail, but not those isolated from chickens, induced severe disease in ferrets; however, the virulence of these H5N1 viruses in mice was not reported. In the present study, the highly virulent viruses could be isolated from the upper respiratory tract of ferrets for a period of at least 7 days p.i., whereas all 2003 and 2004 low-virulence strains were cleared by that time. Similar to the significant lymphopenia among 10 human patients with confirmed H5N1 virus infection (36), the number of circulating lymphocytes in mice and ferrets infected with these highly virulent 2004 H5N1 viruses was significantly reduced. In contrast, the low-virulence H5N1 viruses caused transient lymphopenia that rebounded by the end of the infectious period. Alterations in lymphocyte numbers may be due to the differential induction of apoptosis between highly virulent and low-virulence H5N1 viruses (37).
Compared with other H5N1 viruses isolated in Asia since 1997, including those isolated from humans, the 2004 human H5N1 isolates were clearly more virulent in the ferret model. Severe systemic pathology, rapid progression of disease, and lethality distinguish the highly virulent 2004 H5N1 viruses from the 1997 H5N1 viruses. The severe pulmonary damage observed in ferrets infected with the highly virulent 2004 human H5N1 viruses in this study may account for the low MDT values. Systemic replication is a feature of the disease caused by H5N1 viruses in both mice and ferrets, but not in the macaque model, in which multiple-organ dysfunction syndrome was attributed to diffuse alveolar damage rather than systemic virus replication following infection with a 1997 H5N1 strain (19). Evidence for extrapulmonary replication of H5N1 virus in humans, in general, has been lacking. However, recently the isolation of H5N1 virus from cerebrospinal fluid and feces from an atypical pediatric case was reported (7). The high lethality rate observed in ferrets in the current study is consistent with the high fatality rate observed in documented human infections in 2004 (2, 36) and provides further evidence for the suitability of this animal model for investigating H5N1 virus virulence.
Enhanced virulence, as well as an expanded host range, of recent H5N1 viruses has also been reported elsewhere. For example, Chen et al. (3) reported that 1999 to 2002 H5N1 strains isolated from apparently healthy ducks in mainland China had become increasingly pathogenic for mice, although their virulence in ferrets was not reported. H5N1 viruses isolated in 2002 from migratory birds in Hong Kong caused higher morbidity and mortality in ducks than did earlier H5N1 isolates from the same region (34). The recent detection of H5N1 virus in domestic and zoo felids that were fed infected bird carcasses further demonstrates the potential extended host range of recent H5N1 viruses (18, 20). Interestingly, the multiorgan hemorrhagic lesions and encephalitis detected in zoo felids is consistent with the disseminated disease observed in ferrets infected with human H5N1 strains reported here. The apparent increase in virulence and expanded host range underscore a heightened risk to human health posed by recent H5N1 viruses.
Amino acid sequence comparison of VN1203 and VN1204 revealed eight differences within coding regions, including a Lys and Glu at 627 in PB2, respectively, but only a modest degree of attenuation of the VN1204 virus was observed in mice. Previously, the PB2 627 Glu/Lys substitution was identified as a molecular determinant of virulence in a pair of 1997 H5N1 viruses in inbred mice (16), although certain 1997 H5N1 viruses which lacked this substitution were also highly lethal for mice (17). The precise contribution of the 627 Glu/Lys substitution in the lethal phenotype is not known, but it may influence the replication efficiency of the H5N1 virus in murine cells (32), resulting in a more widespread infection with prolonged neutrophil infiltration in the lungs of mice. Nevertheless, a Lys at 627 of PB2 was observed in a majority of the 2004 human H5N1 isolates, as well as in an HPAI H7N7 virus isolated in 2003 from a fatal human case in The Netherlands (10). Our results suggest that other, as yet undefined, amino acid differences within VN1204 may also contribute to virulence in mice and compensate for the lack of Lys at PB2 627. In contrast, both VN1203 and VN1204 clearly exhibited a highly virulent phenotype in ferrets. This is consistent with our previous studies that showed that a 1997 H5N1 virus with a Glu at 627 PB2 was virulent in ferrets (41). Based on these data, a Lys at 627 of PB2 is not required for a high level of virulence in ferrets. Additional studies are needed to fully understand the molecular correlates determining virulence of H5N1 viruses in the mouse and ferret model systems. To address this, we have identified two H5N1 viruses isolated from humans (Thai16 and SP83) that cause substantially different diseases in both mice and ferrets but differ by only 13 amino acids, including the Lys/Glu difference at residue 627 of PB2 (Tables 4 and 5). Thai16 and SP83 viruses are candidates for reverse-genetics-based studies to identify molecular correlates for virulence using mice and ferrets as animal model systems.
This study confirms the validity of using animal models, such as the mouse and ferret, to better understand the potential of HPAI viruses to cause severe disease in humans. Although the inbred mouse may be more sensitive for the detection of differences in virulence associated with single amino acid substitutions, the ferret more closely reflects the symptoms of disease observed in humans. These mammalian systems will be useful in identifying molecular correlates associated with virulence of HPAI viruses in mammals in order to predict the potential of newly emerging HPAI viruses to infect and cause severe disease in humans.
Acknowledgments
We thank Mark Simmerman and Scott Dowell, International Emerging Infections Program, CDC, Bangkok, Thailand; Long V. Nguyen, Department of Animal Health, Hanoi, Vietnam; and David E. Swayne, U.S. Department of Agriculture, Southeast Poultry Research Laboratory, Athens, Georgia, for facilitating access to viruses and the Influenza Branch genomic sequencing group for sequencing support. We also thank Timothy M. Uyeki and Randy A. Albrecht for helpful discussions and for critical reviews of the manuscript.
REFERENCES
- 1.Apisarnthanarak, A., R. Kitphati, K. Thongphubeth, P. Patoomanunt, P. Anthanont, W. Auwanit, P. Thawatsupha, M. Chittaganpitch, S. Saeng-Aroon, S. Waicharoen, P. Apisarnthanarak, G. A. Storch, L. M. Mundy, and V. J. Fraser. 2004. Atypical avian influenza (H5N1). Emerg. Infect. Dis. 10:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2004. Cases of influenza A (H5N1)—Thailand, 2004. Morb. Mortal. Wkly. Rep. 53:100-103. [PubMed] [Google Scholar]
- 3.Chen, H., G. Deng, Z. Li, G. Tian, Y. Li, P. Jiao, L. Zhang, Z. Liu, R. G. Webster, and K. Yu. 2004. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 101:10452-10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. de Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 6.de Jong, J. C., E. C. Claas, A. D. Osterhaus, R. G. Webster, and W. L. Lim. 1997. A pandemic warning? Nature 389:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong, M. D., V. C. Bach, T. Q. Phan, M. H. Vo, T. T. Tran, B. H. Nguyen, M. Beld, T. P. Le, H. K. Truong, V. V. Nguyen, T. H. Tran, Q. H. Do, and J. Farrar. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352:686-691. [DOI] [PubMed] [Google Scholar]
- 8.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis, T. M., R. B. Bousfield, L. A. Bissett, K. C. Dyrting, G. S. Luk, S. T. Tsim, K. Sturm-Ramirez, R. G. Webster, Y. Guan, and J. S. Malik Peiris. 2004. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 33:492-505. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, Y., J. S. Peiris, A. S. Lipatov, T. M. Ellis, K. C. Dyrting, S. Krauss, L. J. Zhang, R. G. Webster, and K. F. Shortridge. 2002. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 99:8950-8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan, Y., M. Peiris, K. F. Kong, K. C. Dyrting, T. M. Ellis, T. Sit, L. J. Zhang, and K. F. Shortridge. 2002. H5N1 influenza viruses isolated from geese in Southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology 292:16-23. [DOI] [PubMed] [Google Scholar]
- 15.Guan, Y., J. S. Peiris, L. L. Poon, K. C. Dyrting, T. M. Ellis, L. Sims, R. G. Webster, and K. F. Shortridge. 2003. Reassortants of H5N1 influenza viruses recently isolated from aquatic poultry in Hong Kong SAR. Avian Dis. 47:911-913. [DOI] [PubMed] [Google Scholar]
- 16.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 17.Katz, J. M., X. Lu, T. M. Tumpey, C. B. Smith, M. W. Shaw, and K. Subbarao. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J. Virol. 74:10807-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keawcharoen, J., K. Oraveerakul, T. Kuiken, R. A. Fouchier, A. Amonsin, S. Payungporn, S. Noppornpanth, S. Wattanodorn, A. Theambooniers, R. Tantilertcharoen, R. Pattanarangsan, N. Arya, P. Ratanakorn, D. M. Osterhaus, and Y. Poovorawan. 2004. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 10:2189-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiken, T., G. F. Rimmelzwaan, G. van Amerongen, and A. D. Osterhaus. 2003. Pathology of human influenza A (H5N1) virus infection in cynomolgus macaques (Macaca fascicularis). Vet. Pathol. 40:304-310. [DOI] [PubMed] [Google Scholar]
- 20.Kuiken, T., G. Rimmelzwaan, D. van Riel, G. van Amerongen, M. Baars, R. Fouchier, and A. Osterhaus. 2004. Avian H5N1 influenza in cats. Science 306:241. [DOI] [PubMed] [Google Scholar]
- 21.Lee, C. W., D. L. Suarez, T. M. Tumpey, H. W. Sung, Y. K. Kwon, Y. J. Lee, J. G. Choi, S. J. Joh, M. C. Kim, E. K. Lee, J. M. Park, X. Lu, J. M. Katz, E. Spackman, D. E. Swayne, and J. H. Kim. 2005. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J. Virol. 79:3692-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, S., C. Liu, A. Klimov, K. Subbarao, M. L. Perdue, D. Mo, Y. Ji, L. Woods, S. Hietala, and M. Bryant. 1999. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 179:1132-1138. [DOI] [PubMed] [Google Scholar]
- 23.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, X., D. Cho, H. Hall, T. Rowe, H. Sung, W. Kim, C. Kang, I. Mo, N. Cox, A. Klimov, and J. Katz. 2003. Pathogenicity and antigenicity of a new influenza A (H5N1) virus isolated from duck meat. J. Med. Virol. 69:553-559. [DOI] [PubMed] [Google Scholar]
- 25.Macken, C., H. Lu, J. Goodman, and L. Boykin. 2001. The value of a database in surveillance and vaccine selection, p. 103-106. In A. Osterhaus, N. Cox, and A. Hampson (ed.), Options for the control of influenza IV. Elsevier Science, Amsterdam, The Netherlands.
- 26.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 28.Reuman, P. D., S. Keely, and G. M. Schiff. 1989. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods 24:27-34. [DOI] [PubMed] [Google Scholar]
- 29.Richmond, J. Y., and R. W. M. McKinney. 1999. Laboratory biosafety level criteria, p. 17-52. In J. Y. Richmond and R. W. McKinney (ed.), Biosafety in microbiological and biomedical laboratories, 4th ed. Centers for Disease Control and Prevention, Atlanta, Ga.
- 30.Rimmelzwaan, G. F., T. Kuiken, G. van Amerongen, T. M. Bestebroer, R. A. Fouchier, and A. D. Osterhaus. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 75:6687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimmelzwaan, G. F., T. Kuiken, G. van Amerongen, T. M. Bestebroer, R. A. Fouchier, and A. D. Osterhaus. 2003. A primate model to study the pathogenesis of influenza A (H5N1) virus infection. Avian Dis. 47:931-933. [DOI] [PubMed] [Google Scholar]
- 32.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 33.Sims, L. D., T. M. Ellis, K. K. Liu, K. Dyrting, H. Wong, M. Peiris, Y. Guan, and K. F. Shortridge. 2003. Avian influenza in Hong Kong 1997-2002. Avian Dis. 47:832-838. [DOI] [PubMed] [Google Scholar]
- 34.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 36.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, D. J. Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 37.Tumpey, T. M., X. Lu, T. Morken, S. R. Zaki, and J. M. Katz. 2000. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 74:6105-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webby, R. J., D. R. Perez, J. S. Coleman, Y. Guan, J. H. Knight, E. A. Govorkova, L. R. McClain-Moss, J. S. Peiris, J. E. Rehg, E. I. Tuomanen, and R. G. Webster. 2004. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 363:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 40.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, and A. S. Khan. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]
- 41.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]