Abstract
This report evaluates the role of interaction between glucocorticoid-induced tumor necrosis factor receptor (GITR) and GITR ligand (GITR-L) in the immunoinflammatory response to infection with herpes simplex virus (HSV). Both GITR and GITR-L were transiently upregulated after ocular HSV infection, on antigen-specific T cells and antigen-presenting cells, respectively, in the draining lymph node (DLN). In addition, virus-specific T-cell responses in the DLN and spleen were enhanced by anti-GITR antibody treatment, an outcome expected to result in more severe inflammatory lesions. Intriguingly, the treatment resulted in significantly diminished T-cell-mediated ocular lesions. The explanation for these findings was that anti-GITR antibody treatment caused a reduced production of ocular MMP-9, a molecule involved in ocular angiogenesis, an essential step in the pathogenesis of herpetic keratitis. Our results are the first observations to determine in vivo kinetics of GITR and GITR-L expression after virus infection, and they emphasize the role of GITR-GITR-L interaction to regulate virus-induced immunoinflammatory lesions.
The glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR) is a member of the TNF growth factor receptor family that includes CD40, CD27, 4-1BB, and OX40 (12). Among unstimulated lymphocytes, GITR is expressed predominantly on CD4+ CD25+ natural regulatory T cells (Treg) (8, 14). However, both activated CD4+ and CD8+ effector T cells also express GITR, which acts as a costimulator to enhance their effector function (3, 13). Initial studies indicated that GITR stimulation on Treg abrogated their suppressive activity (14), but this interpretation was questioned by the recent observation that GITR engagement on CD4+ CD25− T cells raised the threshold for immunosuppression by Treg (17). It has also become apparent that the ligand for mouse GITR (GITR-L) is constitutively expressed on B cells, macrophages, and dendritic cells and that in vitro Toll-like receptor (TLR) 4 or 9 stimulation transiently enhanced GITR-L expression, followed by their decline (17, 20). Recent in vivo studies using an agonistic antibody to engage GITR (DTA-1) have indicated that more robust protective immunity was generated against a persistent retrovirus infection and to poorly immunogenic tumors (2, 22). In addition, anti-GITR monoclonal antibody (MAb) treatment induced more severe experimental autoimmune encephalomyelitis (4). These reports emphasized the importance of GITR stimulation on T cells, but little is known about the consequences of in vivo manipulation of GITR stimulation for viral immunopathogenesis.
In this report, we analyze the effects of GITR manipulation in vivo on the expression of virus-induced immunoinflammatory lesions. The model used was corneal blindness caused by ocular infection with herpes simplex virus (HSV), an immunopathological lesion orchestrated mainly by effector CD4+ T cells (11). Previously, we showed that CD4+ CD25+ regulatory T cells modulate the severity of these keratitis lesions (18). We anticipated that treatment with agonistic anti-GITR MAb would cause more severe keratitis either because of interference with Treg suppressive activity or due to the costimulatory effect of GITR that could enhance antiviral T-cell effector function. Instead, the opposite result was obtained. Although anti-GITR MAb treatment enhanced HSV-specific T-cell immunity, virus-induced lesion severity was reduced. The diminished keratitis was attributed to the effects of the treatment on the reduced influx of CD4+ T cells into the infected corneas and decreased levels of ocular matrix metalloproteinase-9 (MMP-9), a molecule involved in ocular angiogenesis, an important step in the influx of inflammatory cells and pathogenesis of herpetic ocular lesions (7). Our results are discussed in terms of modulating GITR-GITR-L interactions where induced angiogenesis is detrimental to the host.
MATERIALS AND METHODS
Mice and virus.
Female 6- to 8-week-old Thy1.2+ C57BL/6 (B6) and congenic Thy1.1+ B6.PL (H-2b) mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and Jackson Laboratory (Bar Harbor, ME). gBT-I.1 mice were obtained from Francis Carbone, University of Melbourne, Australia. OT-II mice were bred and maintained in the Microbiology Department's animal facility. All investigations followed guidelines of the Committee on the Care of Laboratory Animals Resources, Commission on Life Science, National Research Council. HSV type 1 (HSV-1) and HSV-1 OVA (kind gifts from Chris Norbury, Penn State University) were grown in Vero cells obtained from the American Type Culture Collection (Manassas, VA). The viruses were concentrated, titrated, and stored in aliquots at −80°C until use.
Antibodies and reagents.
DTA-1 (anti-GITR MAb) was kindly provided by Shimon Sakaguchi (Kyoto University, Japan). Antibodies purchased from BD PharMingen (San Diego, CA) were enzyme-linked immunosorbent assay (ELISA) capture and biotinylated interleukin-2 (IL-2), IL-4, gamma interferon (IFN-γ), and IL-10; fluorescein isothiocyanate-conjugated anti-CD8 MAb; anti-rat immunoglobulin (Ig) G1; and phycoerythrin (PE)-conjugated anti-Thy1.2 MAb. Recombinant MMP-9, anti-MMP-9 capture biotinylated MAb, and fluorescein isothiocyanate-labeled anti-GITR MAb were obtained from R&D Systems, while PE-labeled anti-granzyme B antibody was obtained from Caltag Laboratories. Anti-GITR ligand (YGL383) MAb was produced by Herman Waldmann (Oxford University). HSV gB498-505 peptide (SSIEFARL) and chicken ovalbumin (OVA323-339) peptide were synthesized and supplied by Research Genetics, Huntsville, Ala.
Adoptive transfer and estimation of GITR and GITR-L expression.
A total of 2 × 106 magnetic cell sorting (MACS)-purified CD8+ T cells from gB transgenic mice (gBT) or CD4+ CD25− T cells from OT-II mice were adoptively transferred into the B6 mice. The recipient mice were Thy1.1+ in the case of OT-II transfer, and 24 h after adoptive transfer the mice were ocularly infected either with wild-type HSV-1 or HSV-1 encoding OVA protein. The draining cervical lymph nodes and spleens were collected after regular intervals of time, and GITR expression on antigen-specific CD8+ or CD4+ CD25− T cells was measured by flow cytometry.
Anti-GITR MAb treatment, HSV-1 infection, and clinical scoring.
B6 mice were given 1 mg of anti-GITR MAb intravenously 1 day before ocular HSV-1 infection (1 × 105 PFU/eye). Some mice were killed on day 7 and day 14 postinfection, and the immune responses in draining lymph nodes (DLN) and spleens were measured. Mice were examined at different times after infection for the development of corneal angiogenesis and clinical lesions by slit lamp biomicroscopy (Kowa Co., Nagoya, Japan) as described previously (24). Briefly, the clinical lesion score of herpetic stromal keratitis was determined as follows: 0, normal cornea; 1, mild haze; 2, moderate haze, iris visible; 3, severe haze, iris not visible; 4, severe haze and corneal ulcer; 5, corneal rupture. In reference to the angiogenic scoring system, the method relied on quantifying the degree of neovessel formation based on the centripetal growth of the longest vessels in each quadrant of the eye, and the longest neovessel in each quadrant was identified and graded between 0 (no neovessel) and 4 (neovessel in the corneal center). The scores for the four quadrants of the eye were then summed to derive a neovessel index (range, 0 to 16) for each eye at a given time point.
Viral titration.
Viral titers were calculated as described earlier (16). Briefly, eye swabs were taken from the infected corneas (three mice/group) using sterile cotton swabs soaked in Dulbecco's modified Eagle's medium only, and samples were plated on Vero cells for 48 h. Finally, medium was aspirated, Dulbecco's modified Eagle's medium containing 1% low-melting-point agarose and 0.2% neutral red was added to each well, and viral plaques in each well were quantitated.
Flow cytometric analysis of inflammatory cells in the inflamed corneas.
Single-cell preparations of virus-infected corneal samples were prepared at different time points postinfection using a method described earlier (11). Briefly, corneal samples were digested with Liberase (60 U/ml) in complete RPMI medium at 37°C for 1 h. After incubation, the corneas were disrupted with a syringe plunger and cells were counted with trypan blue. A total of 2 × 105 cells/well were used for flow cytometric analysis. Briefly, cells from corneal samples were incubated with anti-CD16/CD32 MAb prior to the addition of fluorochrome-labeled antibodies. After 30 min of incubation, cells were washed three times in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline, 2% fetal bovine serum, 0.1% sodium azide) and samples were acquired on a FACScan (BD Biosciences). The data were analyzed using Cell Quest 3.1 software (BD Biosciences).
In vivo CTL assay and intracellular granzyme B staining.
Cytotoxicity of CD8+ T cells in rat Ig- and anti-GITR MAb-treated animals was ascertained by in vivo cyotoxic-T-lymphocyte (CTL) assay as reported earlier (19). At 12 days postinfection, peptide-pulsed target splenocytes (Thy1.1) were adoptively transferred into the control and test groups of animals. Briefly, splenocytes from naive B6 mice (Thy1.1) were used as target cells and equally split into three populations. One population was pulsed with 2.5 μg of gB498-505 for 45 min at 37°C and then labeled with a high concentration (2.5 μM) of carboxyfluorescein diacetate succinimidyl ester (CFSE). The second population was pulsed with 0.25 μg of gB peptide and labeled with 0.25 μM of CFSE. The third population was pulsed with 2.5 μg of SIINFEKL (irrelevant) peptide and labeled with 0.025 μM of CFSE. An equal number of cells from each population (107) was mixed together and adoptively transferred intravenously into naive and HSV-1-infected rat Ig- and anti-GITR MAb-treated B6 mice. Mice were killed 1 h after adoptive transfer, erythrocytes were lysed, and cell suspensions were analyzed by FACS Vantage system. Each population was distinguished by its respective fluorescence intensity. Assuming that the number of peptide-pulsed cells injected was equivalent in all three populations, the percent killing of each population of SSIEFARL-specific target cells in infected animals was determined as follows: ratio = (percentage of CFSE low/percentage of CFSE high), and percent specific lysis = [1 − (ratio uninfected/ratio infected) × 100]. Intracellular levels of granzyme B in CD8+ T cells were determined by flow cytometry. Briefly, splenocytes obtained from both groups of animals were cell surface stained with anti-CD8-percp MAb, followed by their permeabilization to stain granzyme B with PE-labeled anti-granzyme B MAb.
Cytokine and MMP-9 ELISA.
The MACS-purified CD4+ T cells from the DLN and spleen were stimulated in vitro for 48 h with UV-inactivated, HSV-pulsed, T cell-depleted splenocytes. The culture supernatants were screened for the presence of IL-2 and IFN-γ cytokines by ELISA as described previously (18). For MMP-9 ELISA, four HSV-infected corneas from each group were isolated on different days postinfection and sonicated in radioimmunoprecipitation assay buffer (10 mM phosphate buffer, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate). Levels of MMP-9 in the corneal extract were estimated and quantitated with recombinant MMP-9 by ELISA.
Gelatin zymography.
Zymographic assays for gelatinase were performed as described previously (7). In brief, four corneas were excised and pooled from each group and sonicated in radioimmunoprecipitation assay buffer. Protein extracts (20 μg) of corneas from mice were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on gelatin-containing acrylamide gels (Bio-Rad) under nonreducing conditions, and the gel was incubated in 1× zymogram renaturing buffer (Bio-Rad) for 30 min at room temperature. This was followed by incubation with 1× zymogram developing buffer at 37°C for at least 4 h. Finally, the gel was stained with Coomassie blue R-250 (Sigma-Aldrich) for 30 min and then saturated with destaining solution (methanol-acetic acid-water, 50:10:40) until the area of protease activity appeared as a clear band.
Statistical analysis.
All analyses for statistically significant differences were performed with Student's paired t test. P values of <0.01 were considered significant. Results are expressed as means ± standard deviations.
RESULTS
HSV-1 infection transiently enhanced GITR-L expression on APCs, which was followed by their decline to below prestimulation levels.
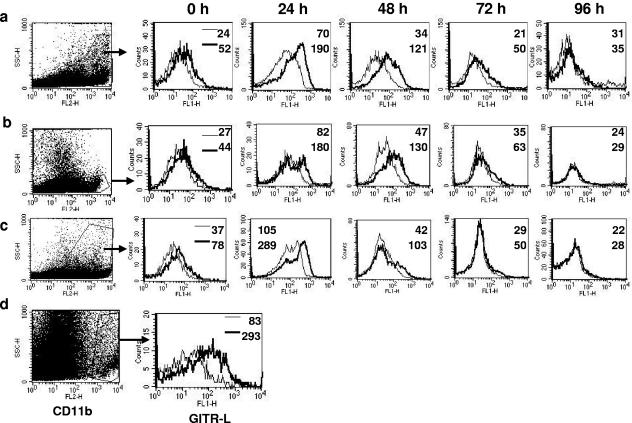
In vitro, antigen-presenting cells (APCs) were reported to downregulate GITR-L expression after TLR stimulation (17, 20). However, the in vivo kinetics of GITR-L expression after virus infection are not known. We monitored the GITR-L expression on three different types of APCs after ocular HSV-1 infection. At 24 h postinfection of B6 animals, enhanced GITR-L expression was detectable on CD11b+ (mean fluorescence intensity [MFI] = 52 to 190), CD11c+ (MFI = 44 to 180), and B220+ (MFI = 78 to 289) cells isolated from the draining cervical lymph nodes (Fig. 1). However, by 96 h postinfection, GITR-L expression declined to below prestimulation levels (CD11b MFI = 35, CD11c MFI = 29, and B220 MFI = 28). GITR-L expression was also monitored in the virus-infected corneal tissue. At 48 h postinfection, GITR-L expression was evident mainly on CD11b+ cells in the infected corneas (Fig. 1d). Very few CD11c+ cells were also GITR-L+ (data not shown). Thus, HSV-1 infection transiently upregulated the GITR-L expression on APCs.
FIG. 1.
Transient upregulation of GITR-L on antigen-presenting cells after ocular HSV infection. (a to c) Isotype control rat Ig or anti-GITR-L MAb was used to stain for the expression of GITR-L on CD11b+ (a), CD11c+ (b), and B220+ (c) cells at regular intervals of time after HSV infection. Histograms are gated on either CD11b+, CD11c+, or B220+ cells present in the DLN populations. (d) Expression of GITR-L on CD11b+ cells present in the HSV-infected cornea on day 2 postinfection. The numbers in each plot indicate the MFI of isotype (thin line) and anti-GITR-L MAb (thick line). Results are representative of three independent experiments.
HSV-1 infection transiently upregulated GITR expression on antigen-specific CD4+ and CD8+ T cells.
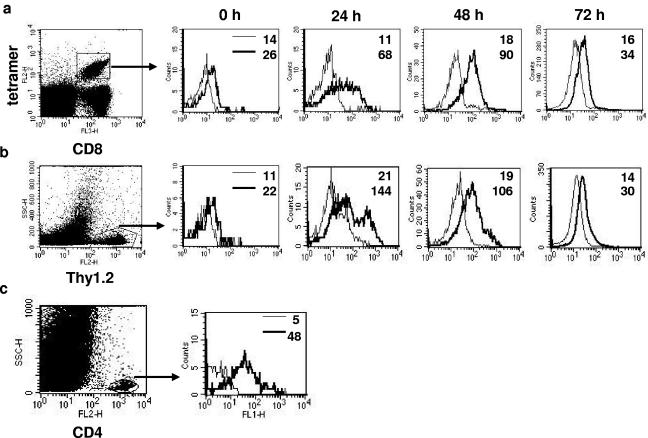
GITR expression has been reported on both CD25+ regulatory T cells and effector T cells (8). We ascertained the in vivo kinetics of GITR expression on antigen-specific effector T cells after virus infection. SSIEFARL-specific CD8+ T cells from gBT-I.1 mice or CD25− CD4+ T cells from OT-II (Thy1.2) mice were adoptively transferred into B6 recipients (Thy1.1), and GITR expression was determined on donor cells isolated from DLN at various time points after ocular HSV-1 or HSV-1 OVA infection. As is evident in Fig. 2, GITR expression levels increased around threefold (MFI = 26 to 68) on donor SSIEFARL tetramer+ CD8 and up to sixfold (MFI = 22 to 144) on donor OT-II CD4+ T cells by 24 h postinfection but declined to prestimulation levels by 72 h. Such changes on donor cells were not evident in splenic populations (data not shown). In separate experiments, GITR expression was also determined in the HSV infected corneas and was detectable on day 8 postinfection on CD4+ T cells (Fig. 2c) (MFI = 48).
FIG. 2.
Enhanced expression of GITR on antigen-specific T cells after HSV infection. (a) Flow cytometric analysis of GITR expression after ocular HSV infection on SSIEFARL-specific CD8+ T cells isolated from draining cervical lymph nodes. Histograms are gated on tetramer+ cells. (b) GITR expression on adoptively transferred OVA-specific CD4+ CD25− T cells isolated from DLN after ocular HSV-OVA infection. Histograms are gated on Thy1.2+ cells. (c) GITR expression on gated CD4+ T cells in the HSV-infected cornea on day 8 postinfection. The numbers in each plot indicate the mean fluorescence intensity of isotype (thin line) and anti-GITR antibody (thick line). Results are representative of three independent experiments.
Persistent GITR stimulation enhanced HSV-specific CD8+ and CD4+ T-cell effector function but decreased HSV-induced corneal lesion severity.
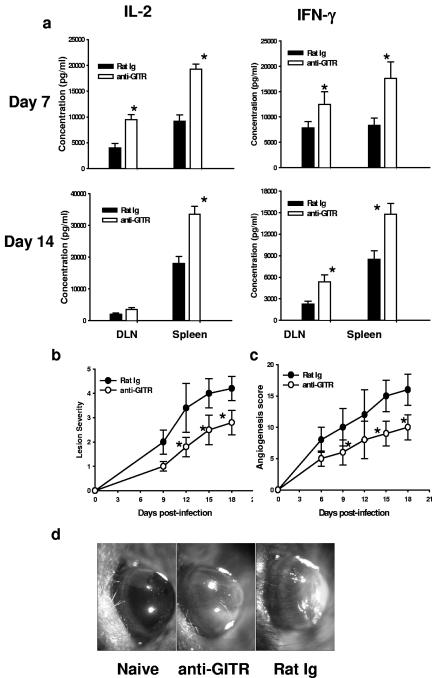
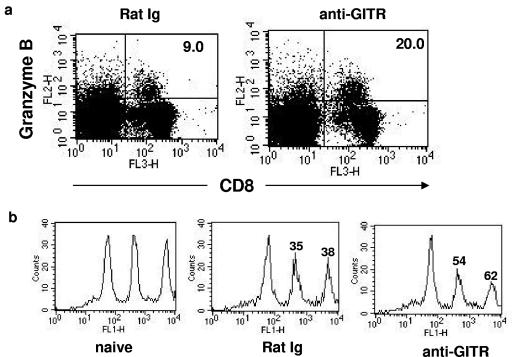
The immunological and pathological effects of prolonged GITR stimulation after ocular HSV infection were determined. Mice were given agonistic anti-GITR MAb 1 day before ocular HSV infection. Control animals were given rat Ig. Subsequently, HSV-specific CD4+ T-cell immunity was determined on day 7 and day 14 postinfection. As evident in Fig. 3a, CD4+ T cells isolated from DLN and spleens from the anti-GITR MAb-treated group on day 7 and day 14 postinfection produced large amounts of IL-2 and IFN-γ upon in vitro HSV restimulation. Treated mice were also analyzed for the severity of ocular lesions and the extent of angiogenesis caused by ocular HSV infection. As is evident in Fig. 3, anti-GITR MAb treated animals had significantly less angiogenesis (Fig. 3c) and milder lesions (Fig. 3b) (P < 0.01) than rat Ig-treated control animals. No significant difference in viral load was seen in the virus-infected eyes from both groups as measured in eye swabs at different time points postinfection (data not shown). Furthermore, the effect of anti-GITR MAb treatment on cytotoxicity of HSV-specific CD8+ T cells was determined during the contraction phase (day 12 postinfection) of the immune response. As is evident in Fig. 4a, GITR stimulation also enhanced the level of granzyme B in virus-specific CD8+ T cells isolated from the spleen on day 12 postinfection, and this was associated with enhanced cytotoxic function of these cells as measured by the in vivo CTL assay (Fig. 4b).
FIG. 3.
Anti-GITR MAb treatment enhanced HSV-specific CD4+ T-cell immunity but significantly reduced virus-induced angiogenesis and stromal keratitis. (a) Purified CD4+ T cells from HSV-infected groups of mice were restimulated in vitro with UV-inactivated, HSV-pulsed, T-cell-depleted splenocytes, and cytokine secretions in the culture supernatant were estimated 48 h later by ELISA. (b) Stromal lesion severity in rat Ig- and anti-GITR MAb-treated groups was measured at different time intervals. (c) Corneal angiogenesis in different groups was measured as described in Materials and Methods in a blinded study. (d) Corneal angiogenesis in the eyes, with maximum lesion severity from two different groups shown. Each group of mice consisted of six animals, and the results shown are representative of one of three independent experiments. *, P < 0.01 compared with rat Ig-treated HSV-infected mice. Error bars indicate standard deviations.
FIG. 4.
Anti-GITR MAb treatment enhanced granzyme B level and in vivo CD8+ T-cell cytotoxicity. (a) The percentages of granzyme B+ CD8+ T cells in the spleens of rat Ig- and anti-GITR MAb-treated mice on day 12 postocular infection were determined by flow cytometry. (b) The FACS plot indicates the in vivo cytotoxicity of SSIEFARL-specific CD8+ T cells after HSV infection in rat Ig- and anti-GITR MAb-treated mice on day 12 postinfection. The number on each peak indicates the percent antigen-specific lysis of target cells.
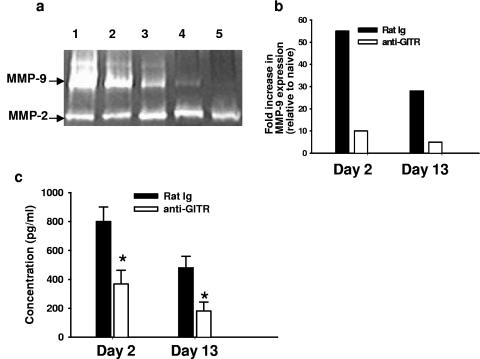
Anti-GITR MAb administration significantly reduced ocular MMP-9 levels.
In vivo GITR-GITR-L interaction might provide bidirectional signaling between T cells and APCs. However, the ability of any of the members of the TNF (ligand) family to transduce signaling is highly controversial. Earlier, it was reported that in vitro engagement of GITR-L on macrophages by the soluble GITR molecule resulted in enhanced production of MMP-9 (15), a matrix-degrading enzyme involved in tumor- and virus-induced angiogenesis (7). Angiogenesis is a necessary early step in the pathogenesis of keratitis lesions (24). Since angiogenesis was significantly reduced in the anti-GITR MAb-treated group, ocular MMP-9 levels were compared between control (rat Ig-treated) and test groups by ELISA and gelatin zymography assays. HSV-infected corneas were excised from the test and control groups at early (day 2) and late (day 13) times after infection and sonicated as described in Materials and Methods. As is evident in Fig. 5, corneal MMP-9 levels on day 2 and day 13 postinfection were significantly reduced in the anti-GITR MAb-treated groups (P < 0.01) compared with the rat Ig-treated control group. Taken together, we interpret these observations to mean that in vivo persistent GITR stimulation not only enhances virus-specific T-cell immunity but can also reduce angiogenesis in virus-induced immunoinflammatory lesions.
FIG. 5.
Anti-GITR MAb treatment reduced ocular MMP-9 production. (a) Corneal extracts obtained from rat Ig- and anti-GITR MAb-treated groups at early and late time points after infection were run on polyacrylamide gels as described in Materials and Methods. Lanes 1 and 2 show corneal extracts obtained from rat Ig-treated groups, while lanes 3 and 4 show corneal extracts from anti-GITR-treated groups on days 2 and 13 postinfection. Lane 5 shows corneal extracts from naive mice. (b) Densitometer scanning of the intensity of MMP-9 bands obtained from gelatin zymography. (c) MMP-9 levels in corneal extracts obtained on days 2 and 13 postinfection from rat Ig- and anti-GITR MAb-treated groups were estimated by ELISA. Results are representative of three independent experiments. *, P < 0.01 compared with rat Ig-treated HSV-infected mice. Error bars indicate standard deviations.
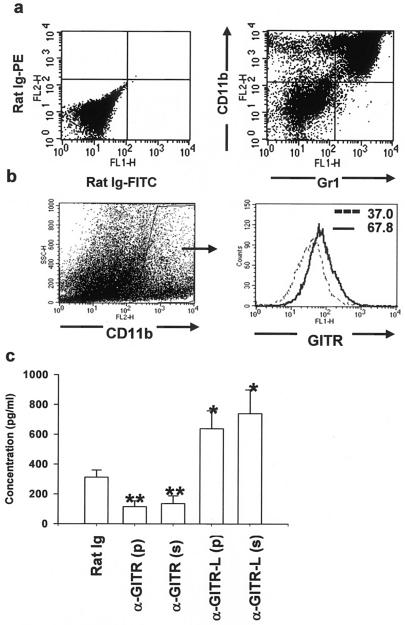
Plate-bound and soluble anti-GITR MAb induced MMP-9 production from ocularly isolated CD11b+ cells.
One aspect of this study was to determine the cell type that was influenced by anti-GITR MAb treatment and regulated ocular MMP-9 production. It was reported earlier that Gr1+ cells are the major inflammatory cell type (21) and a key player in MMP-9 production in virus-infected cornea (7). We observed that 48 h after ocular infection, >98% of Gr1+ cells in virus-infected corneas expressed the CD11b molecule (Fig. 6a). Moreover, most of the CD11b+ cells were GR1+ in HSV-infected corneas, as is evident in Fig. 6a. Thus, CD11b+ cells are the major inflammatory cell type and express the GITR molecule (Fig. 6b) in HSV-infected corneas. To ascertain the role of GITR-mediated signaling in downregulating the MMP-9 production from CD11b+ cells, these cells were enriched from the HSV-infected corneas by use of a MACS column purification system and were cultured with plate-bound (100 μg/ml) or soluble anti-GITR or GITR-L MAb for 48 h. In the control wells, soluble or plate-bound rat Ig was used. As is evident in Fig. 6b, blocking the GITR-GITR-L interaction on CD11b+ cells with either plate-bound or soluble anti-GITR-L MAb significantly induced (P < 0.001) MMP-9 secretion in the culture supernatant as measured by ELISA. In contrast, both plate-bound and soluble agonistic anti-GITR MAb treatment resulted in lesser secretion of MMP-9. We interpret these observations to mean that signaling through GITR expressed on CD11b+ cells is responsible for inhibiting the MMP-9 induction and that blocking of GITR-GITR-L interaction on CD11b+ cells leads to the production of matrix metalloproteinase-9.
FIG. 6.
CD11b+ cells isolated from virus-infected corneas expressed GITR, and blocking of GITR-GITR-L interaction induced MMP-9 secretion. (a) Corneal extracts obtained from HSV-infected groups 48 h postinfection were stained for CD11b+ cells and Gr1+ cells. (b) GITR expression on gated total CD11b+ cells was monitored. (c) MACS-purified CD11b+ cells from 16 corneas at 48 h postinfection were cultured with either plate-bound (p) or soluble (s) rat Ig or anti-GITR or anti-GITR-L MAb. Supernatant obtained from different wells 48 h postincubation was quantitated for MMP-9 production by ELISA. Results are representative of two independent experiments. *, P < 0.001, and **, P < 0.05 compared with rat-Ig treated cells. Error bars indicate standard deviations.
Anti-GITR MAb treatment significantly reduced the infiltration of CD4+ T cells in virus-infected corneas.
Herpetic stromal keratitis is considered to be orchestrated by activated CD4+ T cells in the cornea (11). Additionally, neovascularization of the virus-infected cornea represents an important step in the infiltration of inflammatory cells that regulate lesion pathogenesis (23). As mentioned above, anti-GITR antibody treatment diminished the ocular angiogenesis and lesion severity after HSV infection. Thus, the influx of CD4+ T cells in the virus-infected corneas was compared between anti-GITR and control rat Ig-treated animals. Infected corneas from each group of animals were Liberase digested as described in Materials and Methods, and the influx of CD4+ T cells was determined by flow cytometry after different times postinfection. As shown in Table 1, anti-GITR antibody treatment significantly reduced (P < 0.01) the infiltration of CD4+ T cells in ocular lesions compared with that in rat Ig-treated control animals. These results indicated that reduced ocular angiogenesis in anti-GITR antibody-treated animals leads to lesser influx of CD4+ T cells into the inflamed cornea and decreased lesion severity.
TABLE 1.
Effect of anti-GITR MAb treatment on infiltration of CD4+ T cells in HSV-infected corneas
| Day postinfection | Ocular CD4+ T cells recovered from rat Ig- and anti-GITR MAb-treated animalsa
|
|||
|---|---|---|---|---|
| % CD4+ T cells (mean ± SD)
|
No. of CD4+ T cells/eye
|
|||
| Rat Ig | Anti-GITR | Rat Ig | Anti-GITR | |
| 10 | 1.3 ± 0.3 | 0.6 ± 0.2* | 1.6 × 103 | 0.7 × 103* |
| 15 | 2.3 ± 0.5 | 1.1 ± 0.4* | 2.8 × 103 | 1.1 × 103* |
| 21 | 0.73 ± 0.2 | 0.5 ± 0.1 | 0.6 × 103 | 0.4 × 103 |
Four random corneal samples from each group of infected animals were liberase digested and stained for CD4+ T cells. A total of 105 ocular cells were acquired, and a gate was set up on CD4+ T cells. The percentage of the gated population is shown. The number of CD4+ T cells per eye was calculated from total viable ocular cells obtained after collagenase digestion from both groups. *, P < 0.01 compared with the rat Ig-treated control group.
DISCUSSION
This report evaluates the role of GITR-GITR-ligand interaction in the immunoinflammatory response to the herpes simplex virus infection. Both GITR and GITR-L were transiently upregulated after HSV infection on antigen-specific T cells and APCs, respectively, in the DLN. Intriguingly, agonistic anti-GITR MAb treatment significantly diminished T-cell-mediated stromal keratitis lesions subsequent to HSV ocular infection. In contrast, virus-specific T-cell responses in the DLN and spleen were enhanced compared with those in control animals. The explanation for these findings was that anti-GITR MAb treatment caused a reduced production of ocular MMP-9, a molecule involved in ocular angiogenesis, an essential step in the pathogenesis of herpetic keratitis and lesser influx of CD4+ T cells into the cornea. Our results are the first observations to determine in vivo kinetics of GITR and GITR-L expression after virus infection, and they emphasize the role of GITR-GITR-L interaction in regulating virus-induced immunoinflammatory lesions.
The TNF receptor molecule GITR is readily expressed on CD25+ natural regulatory T cells (14), but it is also rapidly upregulated on antigen-stimulated T cells (8). In vitro studies on the effect of GITR stimulation were initially interpreted as a blocking effect on the action of Treg (14). However, more recently GITR engagement was indicated to result in effector T cells becoming more resistant to suppression by Treg (17). Our results showed that GITR was rapidly upregulated on effector T cells after HSV infection when measured in DLN. However, this upregulation was transient, and it likely functions early in the immune response. The GITR-L expression was also upregulated transiently on APC in DLN but then declined to below prestimulation levels after HSV infection. We believe that transient upregulation of GITR-L expression on APCs after HSV infection of B6 animals is likely to be the indirect effect of either virus-induced inflammatory signals or perhaps viral proteins with TLR-inducing activity (5). Thus, although HSV has been shown to infect APCs in vitro (9), no report has documented that this is a significant event in vivo (1). We anticipate that the decline in GITR-L expression after 72 h is not because of the direct killing of APCs by HSV infection but more likely represents a change in the activation status of antigen-presenting cells resulting from the downregulation of inflammatory signals. A similar sequence of events was described in vitro for GITR-L expression on APCs after TLR 9 stimulation (17). Upregulation of GITR and GITR-L on T cells and APCs, respectively, during the initiation of an immune response may make them resistant to immunosuppression by CD25+ regulatory T cells. However, by day 4, when GITR-L expression has declined, effector T cells could be more susceptible to immunoregulation.
GITR-GITR-L interaction represents an attractive target for immunomodulation. Continuous stimulation of GITR with agonistic antibody results in enhanced T-cell responses as reported for tumor, autoimmune, and allograft models (4, 10, 22). For such systems it was not clarified if the increased responses were the consequence of effects on Treg or were solely directed at effector cell function. Since HSV infection resulted in changes in levels of both GITR and GITR-L expression, we expected that modulating GITR-GITR-L interaction would affect the outcome of viral immunity and immunopathology. Our in vivo studies revealed that stimulating GITR with agonistic MAb resulted in diminished angiogenesis, lesser influx of CD4+ T cells, and decreased ocular lesion severity even though GITR stimulation enhanced T-cell immunity. This observation was unexpected, since lesion severity is mediated by CD4+ T cells and enhancing their activity, while using anti-GITR MAb, was expected to generate more severe disease. An explanation for the observed phenotype was indicated by an earlier observation that the in vitro engagement of GITR-L on macrophages by the soluble GITR molecule resulted in enhanced secretion of the matrix-degrading enzyme MMP-9 (6). As shown previously, MMP-9, which is not normally present in cornea, is rapidly induced upon ocular HSV infection (7). Moreover, this molecule functions in angiogenesis, an essential step in the pathogenesis of keratitis. Interestingly, our results showed that in vivo anti-GITR MAb treatment led to diminished corneal MMP-9 expression, lesser angiogenesis, and reduced influx of CD4+ T cells into the cornea. Conceivably, this occurred as a result of continuous signaling through GITR on CD11b+ cells, as the latter expressed GITR and anti-GITR is an agonistic antibody. Our in vitro studies revealed that both GITR and GITR-L are expressed on ocular CD11b+ cells and that blocking of GITR signaling in CD11b+ cells while using anti-GITR-L MAb induced MMP-9.
The implications of our results are that GITR-GITR-L interaction not only represents an attractive target to overcome Treg-mediated immunosuppression but may also target induced angiogenesis under certain conditions. Hence, stimulating GITR on T cells and antigen-presenting cells at the same time not only will enhance T-cell immunity but could also reduce angiogenesis. Our results might have broader implication in tumor immunotherapy, where enhanced T-cell immunity and reduced angiogenesis are much needed to block tumor growth.
Acknowledgments
This work was supported by National Institutes of Health and National Eye Institute research grants AI-14981 and EYO5093.
REFERENCES
- 1.Carbone, F. R., and W. R. Heath. 2003. The role of dendritic cell subsets in immunity to viruses. Curr. Opin. Immunol. 15:416-420. [DOI] [PubMed] [Google Scholar]
- 2.Dittmer, U., H. He, R. J. Messer, S. Schimmer, A. R. Olbrich, C. Ohlen, P. D. Greenberg, I. M. Stromnes, M. Iwashiro, S. Sakaguchi, L. H. Evans, K. E. Peterson, G. Yang, and K. J. Hasenkrug. 2004. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity 20:293-303. [DOI] [PubMed] [Google Scholar]
- 3.Kanamaru, F., P. Youngnak, M. Hashiguchi, T. Nishioka, T. Takahashi, S. Sakaguchi, I. Ishikawa, and M. Azuma. 2004. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 172:7306-7314. [DOI] [PubMed] [Google Scholar]
- 4.Kohm, A. P., J. S. Williams, and S. D. Miller. 2004. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J. Immunol. 172:4686-4690. [DOI] [PubMed] [Google Scholar]
- 5.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, H. S., S. Y. Park, H. W. Lee, and H. S. Choi. 2004. Secretions of MMP-9 by soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) mediated by protein kinase C (PKC)delta and phospholipase D (PLD) in murine macrophage. J. Cell Biochem. 92:481-490. [DOI] [PubMed] [Google Scholar]
- 7.Lee, S., M. Zheng, B. Kim, and B. T. Rouse. 2002. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Investig. 110:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh, R. S., M. J. Whitters, C. A. Piccirillo, D. A. Young, E. M. Shevach, M. Collins, and M. C. Byrne. 2002. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311-323. [DOI] [PubMed] [Google Scholar]
- 9.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muriglan, S. J., T. Ramirez-Montagut, O. Alpdogan, T. W. Van Huystee, J. M. Eng, V. M. Hubbard, A. A. Kochman, K. H. Tjoe, C. Riccardi, P. P. Pandolfi, S. Sakaguchi, A. N. Houghton, and M. R. Van Den Brink. 2004. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J. Exp. Med. 200:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemialtowski, M. G., and B. T. Rouse. 1992. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J. Immunol. 149:3035-3039. [PubMed] [Google Scholar]
- 12.Nocentini, G., L. Giunchi, S. Ronchetti, L. T. Krausz, A. Bartoli, R. Moraca, G. Migliorati, and C. Riccardi. 1997. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:6216-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronchetti, S., O. Zollo, S. Bruscoli, M. Agostini, R. Bianchini, G. Nocentini, E. Ayroldi, and C. Riccardi. 2004. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 34:613-622. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135-142. [DOI] [PubMed] [Google Scholar]
- 15.Shin, H. H., S. G. Kim, M. H. Lee, J. H. Suh, B. S. Kwon, and H. S. Choi. 2003. Soluble glucocorticoid-induced TNF receptor (sGITR) induces inflammation in mice. Exp. Mol. Med. 35:358-364. [DOI] [PubMed] [Google Scholar]
- 16.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens, G. L., R. S. McHugh, M. J. Whitters, D. A. Young, D. Luxenberg, B. M. Carreno, M. Collins, and E. M. Shevach. 2004. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 173:5008-5020. [DOI] [PubMed] [Google Scholar]
- 18.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123-4132. [DOI] [PubMed] [Google Scholar]
- 19.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tone, M., Y. Tone, E. Adams, S. F. Yates, M. R. Frewin, S. P. Cobbold, and H. Waldmann. 2003. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci. USA 100:15059-15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumpey, T. M., S. H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turk, M. J., J. A. Guevara-Patino, G. A. Rizzuto, M. E. Engelhorn, and A. N. Houghton. 2004. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 200:771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng, M., D. M. Klinman, M. Gierynska, and B. T. Rouse. 2002. DNA containing CpG motifs induces angiogenesis. Proc. Natl. Acad. Sci. USA 99:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng, M., M. A. Schwarz, S. Lee, U. Kumaraguru, and B. T. Rouse. 2001. Control of stromal keratitis by inhibition of neovascularization. Am. J. Pathol. 159:1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]