Abstract
Herpes simplex virus (HSV) spreads rapidly and efficiently within epithelial and neuronal tissues. The HSV glycoprotein heterodimer gE/gI plays a critical role in promoting cell-to-cell spread but does not obviously function during entry of extracellular virus into cells. Thus, gE/gI is an important molecular handle on the poorly understood process of cell-to-cell spread. There was previous evidence that the large extracellular (ET) domains of gE/gI might be important in cell-to-cell spread. First, gE/gI extensively accumulates at cell junctions, consistent with being tethered there. Second, expression of gE/gI in trans interfered with HSV spread between epithelial cells. To directly test whether the gE ET domain was necessary for gE/gI to promote virus spread, a panel of gE mutants with small insertions in the ET domain was constructed. Cell-to-cell spread was reduced when insertions were made within either of two regions, residues 256 to 291 or 348 to 380. There was a strong correlation between loss of cell-to-cell spread function and binding of immunoglobulin. gE ET domain mutants 277, 291, and 348 bound gI, produced mature forms of gE that reached the cell surface, and were incorporated into virions yet produced plaques similar to gE null mutants. Moreover, all three mutants were highly restricted in spread within the corneal epithelium, in the case of mutant 277 to only 4 to 6% of the number of cells compared with wild-type HSV. Therefore, the ET domain of gE is indispensable for efficient cell-to-cell spread. These observations are consistent with our working hypothesis that gE/gI can bind extracellular ligands, so-called gE/gI receptors that are concentrated at epithelial cell junctions. This fits with similarities in structure and function of gE/gI and gD, which is a receptor binding protein.
Herpes simplex virus (HSV) gE/gI is a heterodimer of two glycoproteins, gE and gI, encoded by the US7 and US8 genes, which functions to promote cell-to-cell spread (29). In vivo, HSV resides primarily in epithelial and neuronal tissues and spreads rapidly and efficiently, often in a directed fashion, between cells. Swift spread in tissues, racing against the effects of host immunity, appears to involve highly evolved viral machineries that promote sorting of virions to sites of cell-to-cell contact and which speed movement from one cell to an adjacent cell. One aspect of HSV cell-to-cell spread in epithelial and neuronal tissues involves a process by which newly assembled virus particles are directed to specialized cell surface domains, e.g., epithelial cell junctions or synapses, and can then rapidly enter an adjacent connected cell (29, 32). In this process, enveloped virions released into the narrow spaces between adjacent epithelial cells apparently enter the neighboring cell by a process similar to entry of extracellular or cell-free virus particles (29). This process is distinct from cell fusion, where an infected cell expressing late viral proteins fuses with an uninfected cell, combining two nuclei and mixing cytoplasm from both infected and uninfected cells. Moreover, as there is no evidence for spread of nonenveloped capsids from the cytoplasm of an infected cell directly into that of uninfected cell, the virus envelope appears to be essential.
Both cell-to-cell spread and entry of extracellular virus particles share a number of properties. Both processes require gD, encoded by the US6 gene adjacent to the gI gene, which acts as a receptor-binding protein (7, 30, 34) to bind receptors such as nectin-1, an adhesion molecule found in cell junctions (22, 33, 49). HSV glycoproteins gB and gH/gL are also required for both entry and cell-to-cell spread, apparently to promote fusion between virion and cellular membranes (6, 21, 47).
Unlike gB, gD, and gH/gL, HSV gE/gI is not required for the entry of extracellular virus. However, when gE or gI is mutated, HSV displays markedly reduced cell-to-cell spread in cultured epithelial cells, as well as in epithelial and neuronal tissues (14-16). Based on earlier studies in highly transformed cultured cells such as HeLa, Hep-2, or Vero cells, which form few cell junctions or where discrete cell surface domains are not formed, it was suggested that gE/gI was dispensable both for entry and cell-to-cell spread (14, 15, 35, 56, 58). However, this picture is inaccurate, as these cells poorly mimic those that the virus infects in vivo with respect to gE/gI. Spread of gE-null mutants in cultured keratinocytes or retinal epithelial cells is reduced to only 15% of the number infected by wild-type HSV (58; T. Wisner and D. C. Johnson, unpublished data). However, the effects of gE/gI are more profound in epithelial tissues. In recent studies of virus spread in the cornea, gE- and gI-null mutants spread to only 4 to 6% of the number of corneal epithelial cells as did rescued viruses (45), and gE/gI double mutants were further restricted such that only 2% as many corneal cells were infected (T. Wisner, K. Polcicova, and D. C. Johnson, unpublished data). Spread of gE- and gI-null mutants within the neuron-rich retina was also severely curtailed, as was spread from the retina to retinorecipient regions of the brain (15). Based on these observations in tissues, gE/gI can be described as indispensable for efficient cell-to-cell spread.
Other alphaherpesviruses, the pig pseudorabies virus (PRV), and human varicella zoster virus (VZV) express gE and gI homologues that have revealed properties similar to those of HSV gE/gI and have highlighted other important principles. Elegant studies of PRV gE and gI mutants demonstrated a role for gE/gI and the related membrane protein US9 in directed spread within neuronal networks and the movement of virus along neuronal axons and across synaptic junctions (8-10, 19, 54). As with HSV, PRV gE and gI mutants exhibit major defects in epithelial spread (51, 52). However, PRV does not require gD to spread, either in cultured epithelial cells or in tissues in vivo, and apparently relies on gE/gI for this spread instead of gD (41, 44, 46). VZV has no gD homologue. VZV mutants lacking gE are not viable (40; J. Cohen and N. Nguyen, unpublished data), although gI-null mutants have been constructed (11, 37). Unlike HSV and PRV, it is thus likely that VZV relies upon gE/gI for both entry and cell-to-cell spread. VZV gE is larger than HSV and PRV gE molecules and thus may possess sequences that act in place of gD.
The question of how gE/gI promotes cell-to-cell spread has been difficult to unravel, even compared with the complex problem of how HSV enters cells (49). Our studies of HSV gE/gI and those of PRV gE/gI have indicated that the cytoplasmic (CT) domains of gE and gI act to sort the complex to the trans-Golgi network (TGN)/endosomes and are essential for gE/gI to promote cell-to-cell spread (32, 39, 51, 52, 58). In polarized epithelial cells, HSV gE/gI extensively accumulates in the TGN/endosomes (39, 58), the site of virus envelopment (24). The gE CT domain is both necessary and sufficient for TGN localization (39, 58). In addition, HSV enveloped particles, once formed in the TGN/endosomes, traffic specifically to cell junctions, and this requires the gE CT domain (32). Further, gE/gI, together with gD, is essential for secondary envelopment of HSV, a process whereby tegument-coated nucleocapsids acquire an envelope by budding into TGN/endosomes (20). Based on these three pieces of information, we proposed that gE/gI promotes virus envelopment into subdomains of the TGN that are known to be important in the sorting of cellular proteins to basolateral or lateral cell surfaces (29, 32). Later in the infection, HSV causes redistribution of the TGN/endosomes to cell junctions, and gI plays an essential role in this redistribution (39, 59). Therefore, one way gE/gI promotes cell-to-cell spread is by directing assembly of virions into intracellular compartments so that virus progeny is ultimately delivered to cell junctions.
Beyond these intracellular events involving sorting of nascent virions, there is evidence that the extracellular (ET) domains of gE/gI can also play important roles in cell-to-cell spread. First, gE/gI moves from the TGN/endosomes at intermediate to late times of infection and accumulates at cell junctions, while gD and other HSV glycoproteins are located much more extensively on apical cell surfaces (16, 39, 58). Similarly, gE lacking the CT domain (and TGN sorting signals) and coexpressed with gI using adenovirus vectors largely localized at cell junctions (12). There could be a number of reasons why a viral glycoprotein accumulates specifically at lateral cell surfaces. However, the most likely possibility is that there is selective retention or tethering of gE/gI by cellular components of cell junctions. Second, a mutant form of gE without the CT domain and coexpressed with gI in epithelial cells interfered with spread of HSV between cells (12). Thus, we proposed that the ET domains of gE/gI could promote cell-to-cell spread by binding ligands or receptors at cell junctions to promote entry into adjacent cells (12, 29, 39, 58). It was noted that gI, gE, and gD are encoded by adjacent genes, show structural similarity in their ET domains, including the placement of cysteine residues, and may have evolved from a common ancestor (38). Observations that gD is a receptor binding protein are consistent with the notion that gE/gI may also bind cell surface ligands that promote cell-to-cell spread (29).
Here, we tested the hypothesis that the ET domain of gE is important for cell-to-cell spread by constructing and characterizing a panel of gE mutants with small insertions in the ET domain. Several of these mutants formed complexes with gI and were processed into mature forms that were incorporated into virions yet behaved similarly to gE-null mutants, failing to spread in cultured epithelial cells and in the cornea. Together, the results support the notion that gE/gI can promote HSV spread between epithelial cells by binding extracellular ligands, components of cell junctions.
MATERIALS AND METHODS
Cells and viruses.
HaCaT cells (human keratinocytes) were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and ARPE-19 cells were grown in DMEM-F12 (50:50) containing 10% FBS. R-970 cells and Vero cells were grown in DMEM with 8% FBS. HSV-1 wild-type strain F, F-BAC (an HSV-1 derived from the bacterial artificial chromosome [BAC] copy of HSV-1 strain F) (25, 26), and HSV gE mutants derived from bacterial artificial chromosome (BAC) were propagated, and their titers were determined on Vero cells.
Construction of HSV gE mutants and transfer into HSV-BAC.
Previously, HSV-1 strain F gE sequences (762 bp upstream of the gE start codon extending to 692 bp downstream of the stop codon) were subcloned into plasmid pUC19, producing plasmid pUC-US7/8 (58). pUC-US7/8 was modified by constructing an additional PacI site immediately to the left of the EcoRI site and an AscI site to the right of the HindIII site (both flanking US7 and US8 sequences), producing plasmid pUC-US7/8PA. In order to insert small oligonucleotides throughout gE coding sequences, pUC-US7/8PA was digested with restriction enzyme HaeIII, HhaI, EagI, BstEII, MluI, StyI, or TfiI. Each of these enzymes cuts pUC-US7/8PA multiple times and was used at a limiting enzyme concentration and then linearized-plasmid purified on agarose gels and ligated with 12- or 15-base oligonucleotides that contained an EcoRV site and which inserted the codons described in Fig. 1. Each plasmid was exhaustively digested with EcoRV and religated to remove tandem inserts and then characterized by restriction analyses and sequenced carefully in both directions to confirm that other mutations were not produced. An additional mutant, BACgE-448, was derived from a plasmid used to construct F-gEΔCT (58) and contains a stop codon so that the majority of the CT domain of gE was removed, leaving three residues intact. Mutant gE sequences with insertions at various sites were excised from pUC-US7/8, using PacI and AscI, and transferred into a BAC shuttle plasmid, pSTPA, which was derived from pST76K_SR (5) by altering the KpnI and SacI sites to PacI and AscI sites, respectively. PST76K-SR contains a temperature-sensitive origin of replication, the SacB gene (encoding sucrose sensitivity), a kanamycin resistance gene, and a RecA gene. Other gE mutants derived from the HSV-1 NS strain were described previously by Basu et al. (3) and were kindly provided by Harvey Friedman (University of Pennsylvania School of Medicine). These gE mutants had been inserted into plasmid pCMV3 and were digested with KpnI and BglI to transfer the gE coding sequences into pST76K_SR that had been digested with KpnI and BamHI. To recombine gE sequences from pSTPA or pSTK6K_SR into HSV-BAC, a protocol modified from that described by Horsburgh et al. (25, 26) was used. Briefly, shuttle plasmids derived from pST76K_SR were electroporated into bacteria containing HSV-BAC and which were plated twice on chloramphenicol (20 μg/ml)-kanamycin (30 μg/ml) plates at 43°C before being grown at 30°C. Bacteria were then grown on plates containing choramphenicol and sucrose (5% wt/vol). Sucrose-resistant colonies were then replica plated on kanamycin and chloramphenicol plates to confirm loss of the shuttle plasmid. Mutations were then confirmed in the BAC by using PCR and restriction analyses. HSV-BAC DNA was prepared by growing bacteria in media containing chloramphenicol, followed by alkaline sodium dodecyl sulfate lysis and isopropanol precipitation of BAC DNA or chromatography on QIAGEN columns (Valencia, CA). Vero cells in Opti-MEM lacking serum were transfected with HSV-BAC DNA using Lipofectamine (Invitrogen), and the cytopathic effects of viruses were observed after 4 to 5 days. Rescued versions of gE mutants 277 and 348-4 were produced by preparing viral DNA and cotransfecting Vero cells with the DNA and with plasmid pUC-US7/8 that contains wild-type gE sequences. Large plaques were screened by restriction analyses for the presence of EcoRV sites.
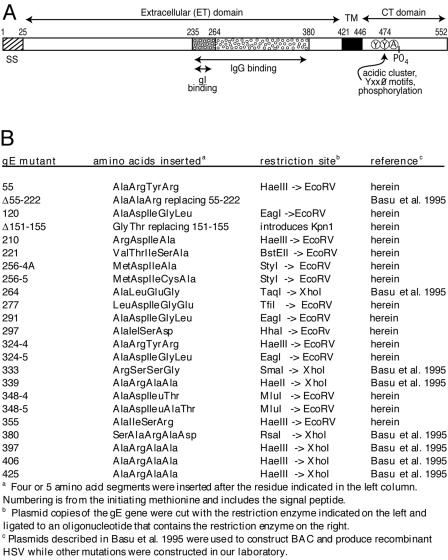
FIG. 1.
Cartoon of HSV-1 gE and description of gE ET domain mutants. (A) HSV gE is a type 1 membrane glycoprotein with a 25-amino-acid signal sequence (SS), a 396-residue extracellular (ET) domain, a 25-amino-acid transmembrane (TM) domain, and a 106-residue cytoplasmic (CT) domain that contains acidic cluster residues, YXXØ motifs, and phosphorylated residues. The ET domain contains a region from positions 235 to 380 that is necessary for binding IgG as well as a region (positions 235 to 264) that is important for binding gI (3). Note that the numbering of gE residues here refers to the HSV-1 17 strain, whereas HSV-1 F strain gE has two additional residues (58). (B) Mutations in the ET domain were constructed by inserting the indicated amino acid sequences (four or five amino acids) directly following the residue indicated. In mutant Δ55-222 a large fragment was replaced by three amino acids, and in Δ151-155, five residues were removed and two were inserted. herein, plasmids containing gE mutations constructed in this study; Basu et al. 1995, plasmids constructed by Basu et al. (3).
Cell-to-cell spread in cultured epithelial cells and single-step growth.
The spread of HSV in cultured HaCaT and ARPE-19 cells was examined as described previously (58). Briefly, monolayers of cells were infected using low multiplicities, and cells were incubated in media containing 1% FBS and 0.2% human gamma globulin for 48 h and then fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 10 to 20 min. The cells were washed, permeabilized with 0.2% Tween 20, and stained with rabbit anti-HSV-1 serum (Dako, Copenhagen, Denmark) followed by donkey anti-rabbit immunoglobulin G (IgG) antibodies conjugated with horseradish peroxidase (Amersham), and plaques were visualized by the addition of a peroxidase substrate, 3,3′-diaminobenzidine hydrochloride (Sigma). For single-step growth analyses, HaCaT cells were infected with HSV at 10 PFU/cell, the inoculum was removed, the cells were washed, and after various times the cells were scraped into the media. Cells and culture fluid were frozen at −70°C and sonicated extensively, and plaque titrations were performed using Vero cells.
HSV spread in the cornea.
BALB/c mice were subjected to corneal scarification as described previously (14, 45) using 3 × 105 to 5 × 105 PFU in 1.5 μl. After 2 days, the mice were sacrificed, their eyes were removed, and the corneas were dissected and permeabilized and then stained with anti-HSV antibodies and Alexa 594-conjugated secondary antibodies, as described previously (45). The immunostained corneas were flat mounted, examined using a fluorescent microscope equipped with a wide-field image restoration system, and photographed using a Nikon TE200 inverted fluorescent microscope with an Applied Precision Deltavision wide-field image restoration system.
Staining of cell surface gE and gD and fluorescent-activated cell sorter (FACS) analysis.
HaCaT cells were infected with wild-type HSV-1 or gE mutants (using 10 PFU/cell) for 10 h and then removed from dishes by incubation with PBS containing 0.53 mM EDTA and 0.25% (wt/vol) trypsin. The cells were washed twice with FACS buffer (PBS containing 2% horse serum and 0.1 mM NaN3) and stained with either anti-gD monoclonal antibody (MAb) DL6 or anti-gE MAb 3114 for 45 to 60 min on ice. The cells were washed in FACS buffer, stained with secondary Alexa 488 goat anti-mouse IgG for 45 to 60 min on ice (Molecular Probes, Eugene, OR), washed, fixed with 1% paraformaldehyde for 30 min on ice, and then analyzed with a Becton Dickinson FACSCalibur flow cytometer.
Radiolabeling of cells, virus purification, and immunoprecipitation of gE.
R-970 cells were infected with HSV at a count of 10 PFU/cell, virus was removed after 2 h, and cells were incubated with DMEM lacking methionine and cysteine and containing [35S]methionine-cysteine (50 to 75 μCi/ml) from 6 to 9 h postinfection. Radiolabeled cells were scraped and lysed in 1% NP-40-0.5% deoxycholate (DOC) buffer containing 2 mg/ml bovine serum albumin and 1.0 mM phenylmethylsulfonyl fluoride (PMSF), frozen at −70°C, and centrifuged at 25,000 rpm in a Beckman 70Ti rotor, and then gE/gI or gD was immunoprecipitated with MAb 3114 (anti-gE), 3063 (anti-gE), or DL6 (anti-gD) as described previously (58). Precipitated glycoproteins were subjected to electrophoresis as described previously (58). Radiolabeled HSV particles were prepared by infecting and labeling the cells from 6 to 19 h postinfection in DMEM that contained 5% of the normal levels of methionine and cysteine and had [35S]methionine-cysteine (150 uCi/ml) added and harvesting cell culture supernatants. Supernatants were centrifuged at 1,000 × g for 10 min to remove cells and debris and then centrifuged on top of step gradients composed of 20% sucrose on top of 50% sucrose in PBS at 20,000 rpm in a Beckman SW32 rotor for 2 h. Virus bands at the 20%/50% sucrose interface were aspirated and diluted two to three times with PBS, and virus was pelleted at 25,000 rpm in a Beckman SW41 rotor. Viruses were disrupted in NP-40-DOC buffer containing 2 mg/ml bovine serum albumin and 1.0 mM PMSF, frozen at −70°C, and then centrifuged to remove insoluble material and immunoprecipitated as described above.
Binding of IgG-coated sheep RBC to HSV-infected cells.
Labeling of sheep red blood cells (RBC; Lampire Biological Labs) with 51Cr and coating with anti-sheep RBC antibodies were done as described previously (23) with some exceptions. RBC were washed and labeled with 51Cr (50 to 100 μCi/ml) in PBS containing 10 mM EDTA and 0.2% fish gelatin (Sigma) and then washed in this buffer and coated with rabbit anti-sheep RBC IgG (Rockland). HaCaT cells infected with HSV were incubated with saturating quantities of IgG-coated RBC for 2 h at 37°C in cell culture media containing 1% FBS, incubated for 15 min on ice, and washed four times with PBS containing 1% FBS. Cells were lysed using NP-40-DOC buffer and label was counted using a Beckman scintillation counter.
RESULTS
Construction of HSV-1 gE ET domain mutants.
To characterize the role of the ET domain of gE in HSV cell-to-cell spread, a panel of insertion mutants was constructed (Fig. 1). Mutations were specifically designed with the intention of maintaining the gross folding of gE, so that the glycoprotein trafficked to cell surfaces and into virion envelopes and bound gI. Most gE ET mutations were constructed in this laboratory, while other mutants were previously constructed as plasmids by Basu et al. in order to measure IgG binding after transfection into cells (3). The gE ET domain mutations were transferred into a BAC copy of the HSV strain F genome and Vero cells transfected with BACs to produce infectious HSV (25, 26). Although BAC mutagenesis rarely produces secondary mutations (1, 55), we also constructed rescued versions of two interesting mutants, 277 and 348-4, denoted 277R and 348-4R, by standard marker transfer.
Cell-to-cell spread of gE ET domain mutants in cultured epithelial cells.
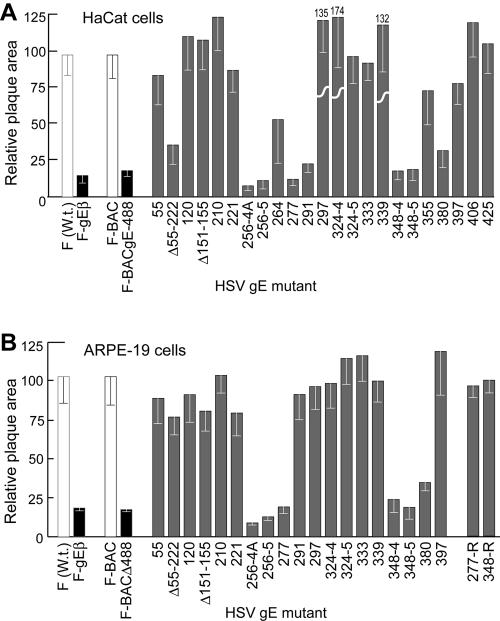
Previously, we showed that gE-null mutants and mutants lacking just the gE CT domain produced plaques that included approximately 15 to 20% as many infected epithelial cells as with wild-type HSV-1 (12, 58). Again, in the present experiments F-gEβ, a mutant lacking all gE sequences, formed plaques encompassing 15 to 18% as many cells as with wild-type HSV-1 strain F on both HaCaT human keratinocytes and ARPE-19 human retinal epithelial cells (Fig. 2). F-BAC, an HSV-1 strain derived from the BAC copy (without mutating gE), produced plaques that were slightly smaller than those produced by wild-type HSV-1 strain F, encompassing ∼90% as many cells as with F. This was apparently due to the fact that bacterial sequences in F-BAC were inserted into thymidine kinase sequences, as thymidine kinase-HSV mutants produced slightly smaller plaques on these epithelial cells (data not shown). For this reason, we compared plaques formed by gE ET domain mutants in HaCaT and ARPE-19 cells with those formed by F-BAC. A mutant derived from F-BAC and lacking all but three residues of the large CT domain of gE (F-BACΔ488) produced plaques that were 16% of the area of those produced by F-BAC on HaCaT and ARPE-19 cells (Fig. 2). Thus, F-BACΔ488 was phenotypically similar to a gE-null mutant, as was described for F-gEΔCT, a gE mutant lacking the CT domain and derived from HSV-1 (strain F) (58).
FIG. 2.
Cell-to-cell spread of HSV gE ET domain mutants in HaCaT and ARPE-19 cells. Human HaCaT keratinocytes or retinal epithelial ARPE-19 cells were infected with wild-type HSV-1 strain F or F-gEβ (derived from F), F-BAC, or F-BACgE448, which lacks all but 3 residues of the gE CT domain (derived from F-BAC), or gE ET mutants at low multiplicities. After 2 days, the cells were stained with anti-HSV polyclonal antibodies, peroxidase-conjugated secondary antibodies, and substrate to reveal HSV-infected cells. Ten plaques were photographed, and NIH Image software was used to calculate plaque areas. The area of wild-type F [F (W.t.)] plaques was arbitrarily set at a value of 100 for comparison with F-gEβ (derived from F), and the area of F-BAC plaques was arbitrarily set at 100 for comparison with gE ET domain mutants and F-BACΔ488 (all derived from F-BAC). Mutant 324-4 produced plaques that were 174% of the area of F-BAC plaques. 277-R and 348-4R were rescued versions of gE ET mutants 277 and 348-4, respectively.
A number of mutations in the gE ET domain, including 4- or 5-amino-acid inserts after residue 256, a 4-residue insert after residue 277, and 4- or 5-residue inserts after residue 348, reduced cell-to-cell spread to that seen with null mutants on both ARPE-19 and HaCaT cells (Fig. 2). Rescued versions of mutants 277 and 348-4, 277R and 348-4R, respectively, produced plaques of wild-type size on ARPE-19 cells (Fig. 2B). Of special interest was mutant 291, which produced small plaques in HaCaT cells but plaques that were similar to those produced by F-BAC on ARPE-19 cells. Such a cell-type-specific defect in entry or spread has not been reported, to our knowledge.
Three other mutants, Δ55-222, 264, and 380, produced plaques of intermediate sizes, 30 to 75% of the area of wild-type-produced plaques, compared with F-BAC (Fig. 2). Again, the effects of these mutations tended to be more pronounced on HaCaT cells than on ARPE-19 cells. Previously, Friedman and colleagues ascribed a “severe” phenotype to a mutation (SerAlaArgAlaAsp inserted after residue 380) rescued in HSV-1 strain NS (48). In our studies, an identical residue 380 mutation introduced into HSV-1 strain F did not result in a reduction in cell-to-cell spread as severe as that of null mutants or other gE ET domain mutants (277 or 348-4). Mutant 324-4 consistently produced plaques that were larger than those of F-BAC on HaCaT cells but for unknown reasons, not on ARPE-19 cells. This may not relate to specific effects of the residues inserted at position 324, because mutant 324-5, which contains the same 4-residue insert and an additional fifth residue, did not show this phenotype. In summary, mutations in two regions of the gE ET domain, residues 256 to 291 and 348 to 380, most severely affect HSV cell-to-cell spread.
Expression of gE ET domain mutant proteins, maturation, and complex formation with gI.
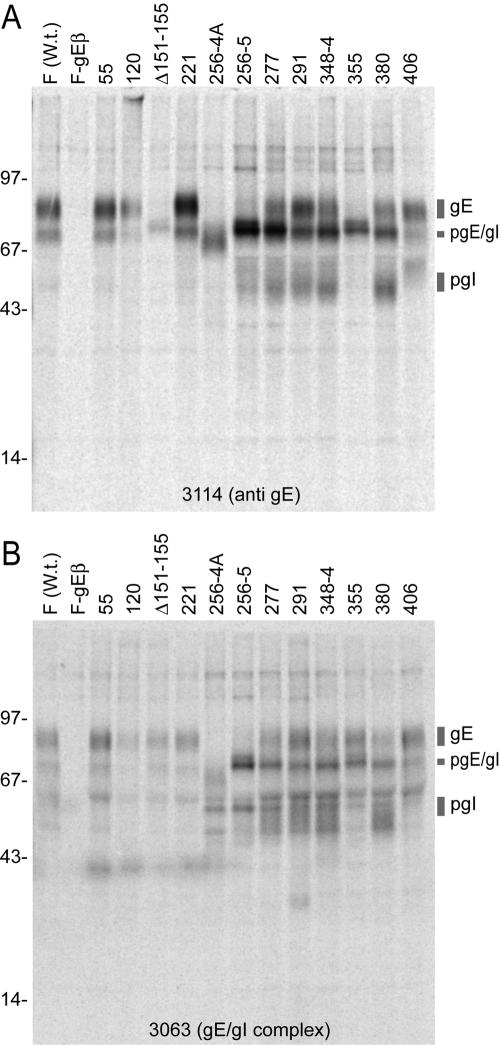
We were especially interested in forms of gE that did not mediate cell-to-cell spread yet were not altered globally, i.e., so that gE complexed with gI and moved beyond the endoplasmic reticulum to the Golgi apparatus, into virions, and to the cell surface. Thus, we characterized expression of several of the gE mutants, focusing on interesting mutants that did not mediate cell-to-cell spread. HSV-infected cells were radiolabeled with [35S]methionine-cysteine, and gE/gI was immunoprecipitated. For technical reasons, R-970 cells were used in these experiments rather than HaCaT or ARPE-19 cells, because gE and gI are extremely fuzzy or diffuse when characterized using HaCaT and ARPE-19 cells, due to extensive posttranslational modification (13, 31, 58). MAb 3114 recognizes gE with or without gI bound, while a second MAb, 3063, recognizes gE, but only when complexed with gI (16, 58). Mature gE that migrates more slowly in sodium dodecyl sulfate-polyacrylamide gels was immunoprecipitated from cells infected with mutants 55, 120, 221, 277, 291, 348-4, 380, and 406 using both MAb 3114 and MAb 3063 (Fig. 3). There were also variable amounts of pgE, the precursor form of gE, as well as gI and pgI, the immature form of gI. Note that the gross levels of gE and gI were somewhat different due to the staging of infections. It was also obvious that more immature gE accumulated with certain mutants, especially mutants 277, 348-4, and 380, when precipitated with MAb 3114, although this was less the case with these mutants when precipitated with MAb 3063. Mutants 256-4 and 256-5 displayed primarily immature gE with both anti-gE MAbs (Fig. 3), suggesting that very little of these glycoproteins reached the Golgi apparatus. It appears likely that the epitopes for MAb 3114 in mature gE depend upon residues 151 to 155 and 355, because MAb 3114 did not recognize mutants Δ151-155 and 335 well, yet MAb 3063 precipitated mature gE from both. These studies produced the conclusion that gE mutants 277, 291, 348-4, and 380 produced mature gE.
FIG. 3.
Immunoprecipitation of radiolabeled gE/gI from cells infected with HSV gE ET domain mutants. R-970 cells were infected with wild-type HSV-1 (F), F-gEβ, and various gE mutants. The cells were labeled with [35S]methionine-cysteine from 6 to 9 h after infection. gE/gI was immunoprecipitated from cell extracts using MAb 3114 or 3063, and proteins were subjected to electrophoresis on polyacrylamide gels. The positions of gE, immature gE (pgE), g, and immature gI (pgI) as well as molecular mass markers of 97, 67, 43, and 14 kDa are shown. W.t., wild type.
Cell surface expression of gE ET domain mutants and incorporation into virions.
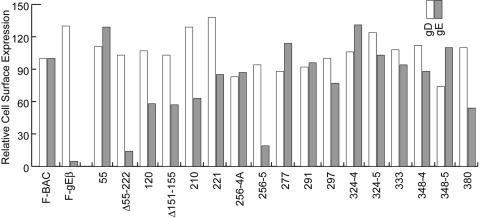
To further characterize mutant gE molecules for transport to the cell surface, infected HaCaT cells were stained with MAb 3114 and secondary fluorescent antibodies and analyzed by FACS. Cells infected in parallel were also stained with anti-gD MAb DL6. gD is a viral glycoprotein that should not be affected by mutations in gE and was used as a control for infection of cells. Again, we focused primarily on mutants that did not mediate cell-to-cell spread. A gE-null mutant, F-gEβ, served as a negative control. Mutants Δ55-222 and 256-5 were not present on cell surfaces (Fig. 4), and this likely explains their inability to mediate cell-to-cell spread (Fig. 2) and, for 256-5, is likely related to the inability to reach the Golgi apparatus, reflected in the accumulation of immature gE (Fig. 3). It is not clear why 256-4A reached the cell surface in these experiments, because immature gE was primarily detected in cells infected with this mutant (Fig. 3), although this may relate to loss of epitopes for MAbs 3114 and 3063. Mutant 380 displayed ∼50%-reduced cell surface expression, which may explain the reduced cell-to-cell spread (Fig. 2) (48). Note that there were other mutants (mutants 120 and 210) with reduced surface expression that spread well. Importantly, mutants 277, 291, 348-4, and 348-5 exhibited normal levels of gE on the cell surface (Fig. 4). Similar results were obtained in two other experiments. In summary, gE mutants 277, 291, 348-4, and 348-5 cannot mediate cell-to-cell spread yet fold relatively normally, bind gI, and reach the cell surface.
FIG. 4.
Cell surface expression of gE and gD. HaCaT cells were infected with F-BAC, FgEβ, or gE mutants for 10 h, and then the cells were removed from plastic dishes using EDTA and trypsin and stained with either anti-gD MAb DL6 or anti-gE MAb 3114 and fluorescent secondary antibodies. The cells were then characterized by FACS. The mean fluorescence for F-BAC with background fluorescence subtracted was arbitrarily set at a value of 100, and the mean fluorescence of cells infected with each mutant was compared to this value.
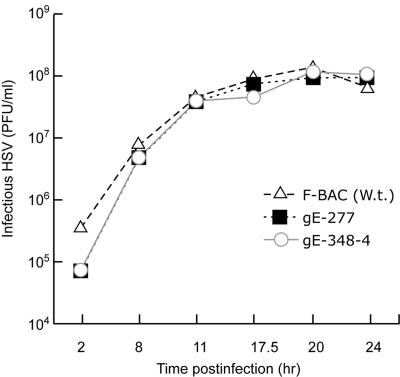
Replication of mutants 277 and 348-4 and incorporation into virions.
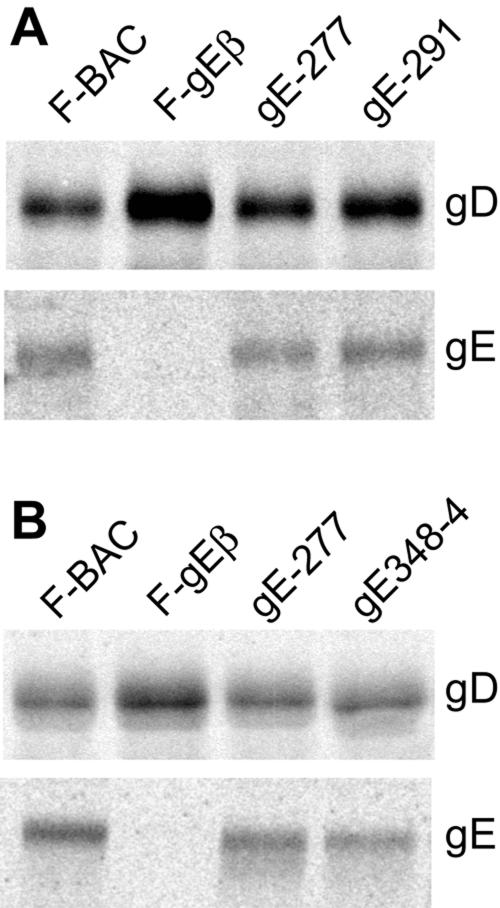
It was possible that gE mutants that were unable to spread also exhibited defects in some aspect of virus replication. While this was unlikely, as gE-null mutants replicate relatively normally, we characterized two of the most interesting mutants, 277 and 348-4, for single-step growth on HaCaT cells. Mutants 277 and 348-4 both produced similar quantities of infectious virus in HaCaT cells, with the same kinetics as wild-type F-BAC (Fig. 5). To determine whether gE mutants 277, 291, and 348-4 were incorporated into virions, infected cells were radiolabeled with [35S]methionine, culture supernatants were harvested before any obvious cell lysis, cellular debris was removed by low-speed centrifugation, and extracellular virus particles were partially purified using sucrose step gradients. The virions were pelleted and disrupted in detergents, and gE or gD was immunoprecipitated and then subjected to electrophoresis. F-gEβ, a gE-null mutant, displayed gD but no gE. There were mature gE and gD in mutants 277, 291, and 348-4 approximating the amounts seen in wild-type F-BAC (Fig. 6).
FIG. 5.
Replication gE mutants 277 and 348-4. HaCaT cells were infected with F-BAC and gE mutants 277 and 348-4 using 10 PFU/cell. At various times, cells were scraped from plastic dishes into cell culture media, frozen, and sonicated, and HSV titers were determined in plaque assays. The experiments were performed in duplicate, and differences in titers at each time point were small, so that error bars overlapped the symbols and were too small to be discerned. W.t., wild type.
FIG. 6.
Incorporation of gE mutants 277, 291, and 348-4 into extracellular virions. R-970 cells were infected with F-BAC, gE-null mutant F-gEβ, or gE ET domain mutant 277, 291, or 348-4 at 10 PFU/cell. The cells were labeled with [35S]methionine from 6 to 18 h postinfection, cell culture supernatants were harvested and centrifuged at low speed, and then virions were partially purified on 20%/50% sucrose step gradients. Virions were pelleted and disrupted in NP-40-DOC buffer and centrifuged at high speed to remove insoluble debris, gE/gI was immunoprecipitated using a mixture of MAb 3114 and 3063 or gD immunoprecipitated using MAb DL6, and the proteins were subjected to electrophoresis using polyacrylamide gels. (A) Mutants 277 and 291; (B) a different experiment including mutants 277 and 348-4.
Spread of HSV gE ET domain mutants in the cornea.
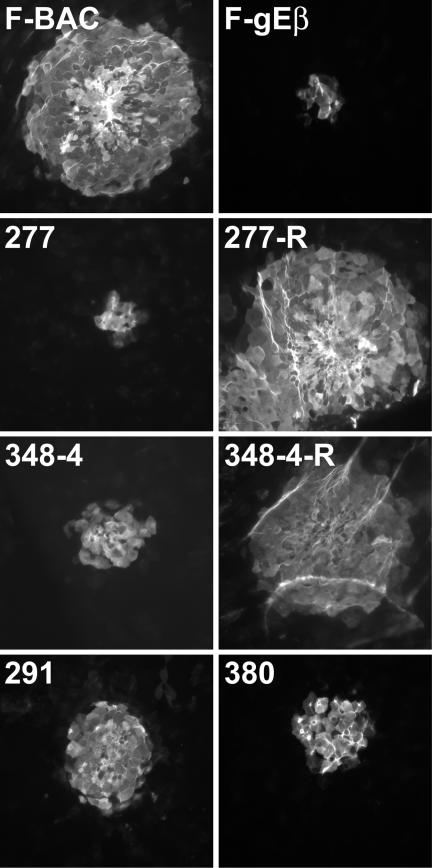
Previously, we showed that HSV-1 gE-null mutants that spread poorly in cultured epithelial cells also spread poorly in the corneal epithelium (14). Here, we tested spread of HSV gE ET domain mutants 277, 291, 348-4, and 380 in the cornea by infecting mice following scarification and then removing their corneas after 2 days and staining HSV lesions by using anti-HSV antibodies and secondary fluorescent antibodies. The immunostained corneas were flat mounted and examined using a fluorescent microscope equipped with a wide-field image restoration system, allowing characterization of very thin sections and counting of the infected cells in various layers of the corneal epithelium. The cell counts were also compared to those in the areas of virus lesions measured using imaging software. Wild-type HSV-1 F-BAC produced lesions that each consisted of 232 to 315 epithelial cells (Fig. 7). By contrast, gE-null mutant F-gEβ and mutant 277 infected 10 to 16 cells, so that ∼4 to 6% as many cells were infected as with F-BAC. Mutants 348-4 and 380 infected more cells, 26 to 42 cells per lesion, while mutant 291 infected 67 to 88 cells per lesion. Rescued viruses derived from mutants 277 and 348-4 produced lesions similar to those produced by F-BAC (Fig. 7). Therefore, mutants 277, 291, 348-4, and 380 were defective in cell-to-cell spread in the corneal epithelium, with mutant 277 showing a more profound phenotype similar to that of a gE-null mutant.
FIG. 7.
Spread of gE mutants 277, 291, 348-4, and 380 in corneal epithelium. BALB/c mice were infected in the cornea with F-BAC; FgEβ; gE mutant 277, 291, 348-4, or 380; or the rescued virus 277R or 348-4R for 2 days. The mice were sacrificed, and their corneas were removed, fixed, permeabilized, and stained with anti-HSV antibodies and fluorescent secondary antibodies. Wide-field fluorescent microscopy was performed on flat-mounted corneas in order to observe thin sections of the corneas amounting to single cells.
Immunoglobulin Fc receptor activity of gE ET domain mutants.
HSV-1 gE/gI is also an IgG Fc receptor (28). Studies by Friedman and colleagues mapped regions of gE involved in IgG binding by using a panel of gE insertion mutants as well as gE/gD fusion proteins expressed by transfection (2, 17). These studies concluded that a central region of the gE ET domain, between residues 235 and 380 (Fig. 1), was both necessary and sufficient for binding IgG. Two gE mutants, 339 and 380, were rescued in HSV with similar results (48, 57). Especially interesting was the observation that mutant 339 could mediate cell-to-cell spread but largely or entirely lacked Fc receptor activity. This led the authors to conclude that gE domains involved in Fc receptor activity differ from those that mediate cell-to-cell spread (57). It is not clear whether other mutants that lacked Fc receptor activity also lost the capacity to mediate cell-to-cell spread, because these gE mutants were not rescued in HSV and tested for cell-to-cell spread. This point is of interest because it is not clear whether the cell-to-cell spread and IgG binding functions overlap.
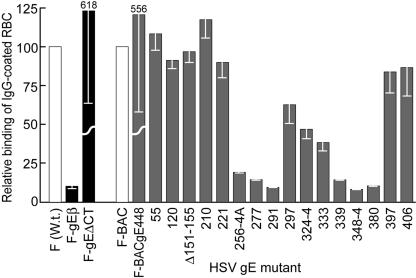
We tested the IgG Fc receptor activity of our gE mutants using rabbit IgG-coated sheep RBC labeled with 51Cr as in previous experiments (23). The polymeric nature of IgG-coated RBC allows better detection of the HSV Fc receptors, which bind monomeric IgG relatively poorly (4, 23). HaCaT cells infected with gE-null mutant F-gEβ bound ∼10% as many IgG-coated RBC as did cells infected with wild-type F (Fig. 8). Mutants 55, 120, Δ151-155, 210, 397, and 406 all bound amounts of IgG similar to that bound by F-BAC. By contrast, mutants 256-4A, 277, 291, 339, 348-4, and 380 showed dramatically reduced binding of Ig-coated RBC. Cells infected with mutants 297, 324-4, and 333 (all with mutations more centrally located within the previously mapped IgG-binding region) showed intermediate results, binding about 30 to 60% as many IgG-coated RBC as did wild-type HSV (Fig. 8). Therefore, as in the previous studies (57), gE mutant 339 lacked IgG binding activity yet could mediate cell-to-cell spread. However, all the other gE mutants that failed to spread, 277, 291 (on HaCaT cells), 348-4, and 380, also lost IgG binding activity. There were no mutations that abolished cell-to-cell spread without reducing IgG binding. These results are consistent with the notion that the regions of gE/gI that promote cell-to-cell spread extensively overlap domains involved in binding IgG and differ somewhat from previous conclusions on this point.
FIG. 8.
IgG binding of gE ET domain mutants. HaCaT cells were infected with wild-type HSV-1 (F); F-gEβ; F-gEΔCT; or F-BAC, F-BACgE488, or gE ET domain mutants at 10 PFU/cell for 18 h. The cells were incubated with 51Cr-labeled, IgG-coated sheep RBC for 2 h at 37°C and then washed, and the radioactivity in cell extracts was counted. The binding of wild-type HSV-1 (F) was arbitrarily set to a value of 100, and the binding levels of F-gEβ and F-ΔCT were compared to that value, while the binding of F-BAC was also arbitrarily set to 100, and the binding levels of F-gEBAC488 and all gE ET domain mutants were compared to that value. W.t., wild type.
Of interest was the IgG binding of gE molecules lacking the CT domain. F-gEΔCT (derived from wild-type HSV-1 strain F) and F-BACgE-448 (derived from F-BAC) both lack all but three residues of the large gE CT domain. HaCaT cells that were grown to confluent monolayers and infected with F-gEΔCT or F-BACgE-448 bound ∼sixfold more IgG than did wild-type HSV-1 or F-BAC (Fig. 8). Previous observations demonstrated that gE lacking the cytosolic domain and complexed with gI was much more extensively expressed on apical surfaces of epithelial cells than was wild-type gE/gI (12, 58). The gE CT domain both directs gE/gI to the TGN early, so that the glycoprotein complex is internal, and later promotes targeting to cell junctions and away from apical surfaces (12, 58). Clearly, without the CT domain, much more gE/gI escapes from cytoplasmic vesicles to apical surfaces where it can bind IgG.
DISCUSSION
The proposition that initiated these studies was that the ET domains of gE/gI function in an important manner in HSV cell-to-cell spread. Previously, there was extensive evidence that the CT domains of HSV and PRV gE/gI mediate important trafficking decisions and are essential for the glycoprotein to mediate cell-to-cell spread (12, 39, 50, 52, 53, 58). HSV gE CT mutants behave similarly to gE-null mutants in cultured epithelial cells and in the cornea (58; T. Wisner and D. C. Johnson, unpublished). However, the large ET domains of gE and gI have not been well characterized in cell-to-cell spread. To identify mutants that were not altered in global folding, complex formation with gI, traffic to the cell surface, and incorporation into virions, we constructed a panel of mutants and recombined them into HSV. Three mutants, 277, 291, and 348-4, met these criteria and yet did not spread well. Somewhat more immature gE accumulated within infected cells with certain of these mutants, e.g., 277 and 380; however, importantly, gE molecules from mutants 277, 291, and 348-4 reached the cell surface, and normal amounts of mature gE were found in extracellular virions. Mutants 277, 291, and 348-4 spread poorly in cultured HaCaT keratinocytes, infecting approximately as many cells as did gE-null mutants. Especially striking was the observation that mutant 277 spread to only 4 to 6% as many epithelial cells in the cornea as did F-BAC, suggesting that mutant 277 behaved as a gE-null mutant in terms of cell-to-cell spread function. Mutants 291, 348-4, and 380 were also substantially reduced in spread within the cornea, although not as severely as were mutant 277 and gE-null mutants. Assays of the cornea illustrate the speed of HSV spread in epithelial tissues, spreading to over 300 cells in 48 h, as well as the importance of gE/gI in this process.
Mutant 291 spread poorly on HaCaT cells and in the cornea yet, surprisingly, spread normally in ARPE-19 cells. A second mutant, 324-4, produced plaques that were consistently larger than those of wild-type HSV-1 on HaCaT cells but not on ARPE-19 cells. It was extraordinary that these mutants behaved so differently on human keratinocytes than on human retinal epithelial cells. To our knowledge, mutations that substantially reduce spread on some cells, but with no effect on spread on other cells, have not been reported. HaCaT and ARPE-19 cells may differ in either the quantity or quality of gE/gI ligands.
Our observations with gE mutants 277, 291, and 348-4 further support the conclusion that the ET domain of gE is essential in order for gE/gI to function in cell-to-cell spread. By virtue of their orientation on the surface of virions that are present in the narrow spaces between epithelial cells, the ET domains of gE/gI have the potential to interact with cell surface components of cell junctions. This hypothesis is further supported by previous observations that (i) gE/gI concentrates at cell junctions (12, 16, 39, 58, 59), consistent with tethering there, and (ii) gE/gI expressed in trans can interfere with cell-to-cell spread (12). Based on these data, we propose the working hypothesis that gE/gI binds gE/gI receptors (29, 32). Years ago, we proposed the notion of gD receptors, based on interference with entry by UV-inactivated HSV particles that either contained or did not contain gD as well as interference with a soluble form of gD (27, 30). Campadelli-Fiume et al. similarly found that HSV could not enter cells stably expressing gD, a third form of interference (7). The suggestion that gE/gI binds receptors is consistent with the notion that HSV-1 gD, gE, and gI appear to be a family of glycoproteins with globally similar structures, derived from a single progenitor (38), and sharing important roles in secondary envelopment (20). However, it is important to point out that establishing the existence of gE/gI receptors will depend upon their identification and characterization.
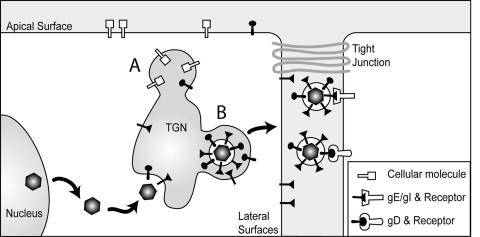
The cartoon shown in Fig. 9 describes two distinct mechanisms by which gE/gI can promote spread between epithelial cells. First, the CT domains of gE and gI promote accumulation in, and envelopment into, subcompartments of the TGN, where sorting to lateral cell surfaces occurs (20, 29, 32, 39). Other TGN subcompartments sort cellular proteins to apical surfaces. This selective sorting increases the delivery of nascent virions to cell junctions and, presumably, enhances virus spread (29, 32). Second, virions enter the narrow spaces between epithelial cells and engage receptors that promote entry by fusion between the virion envelope and the plasma membrane of the adjacent cell. Nectin-1 is one such receptor that gD binds and is concentrated at cell junctions (33). Interactions between gD and nectins (or other gD receptors) are essential for HSV cell-to-cell spread. By contrast, PRV gD is not required for cell-to-cell spread; apparently gE/gI can accomplish this without gD (41, 44, 46). Our results suggest that there are other extracellular ligands that HSV can interact with during the process of crossing cell junctions: gE/gI receptors that can promote or enhance HSV cell-to-cell spread. It is not yet clear whether gE/gI receptors act in a fashion similar to gD receptors to trigger the fusion of virions found at cell junctions with cell membranes. However, observations that gD is not required for PRV cell-to-cell spread support the argument that gD and gE/gI can perform similar functions in this process. The notion of redundancy and overlapping functions for gE/gI and gD is further illustrated by the process of secondary envelopment, which requires gE/gI or gD serving overlapping yet essential functions (20).
FIG. 9.
Model for how gE/gI functions in cell-to-cell spread. gE/gI and gD promote secondary envelopment of HSV into TGN/endosomal vesicles. gE/gI is sorted to specific TGN subcompartments that ultimately lead to sorting to lateral cell junctions (denoted B). Other TGN subcompartments contain proteins that are sorted to apical surfaces (denoted A). Virions that reach cell junctions move into the narrow spaces between cells and interact with gD (nectin-1) and gE/gI receptors that promote HSV entry into adjacent cells.
Since gE/gI does not obviously promote or enhance entry of cell-free virus, gE/gI receptors may be more exclusively localized to cell junctions. By contrast, gD receptors, such as nectin-1, must be on apical cell surfaces in order to mediate HSV entry into epithelial cells. Clearly, gE/gI and putative receptors are not absolutely essential for cell-to-cell spread. However, the relative importance of gE/gI and its receptors has been grossly underestimated based on studies of cultured cells that lack epithelial cell junctions. gE-null mutants or gE-277 spread to only ∼4 to 6% as many corneal epithelial cells as does wild-type HSV-1 (45), and double mutants lacking both gE and gI spread to only 2% as many cells as were infected by wild-type HSV-1 (Wisner and Johnson, unpublished). Therefore, we consider gE/gI and putative gE/gI receptors indispensable for efficient HSV spread in epithelial tissues.
An alternative possibility suggests that gE/gI binds to other HSV membrane proteins in the lumen of cytoplasmic vesicles and directs these proteins into the virion and ultimately to cell junctions. This would explain the essential character of the ET domain of gE in its capacity to mediate cell-to-cell spread. However, we have not observed interactions between gE/gI and other HSV membrane proteins, and virions lacking gE do not possess obvious deficiencies in other membrane proteins. Moreover, it is more difficult to understand how this idea explains the interference observed with gE/gI (lacking the gE CT domain), which primarily localizes to cell junctions and not to the TGN (12).
HSV gE/gI binds IgG (4, 23, 28, 43), and there is evidence in animal models that this can protect the virus from IgG and complement or antibody-dependent cellular cytotoxicity (18, 36, 42, 48). In previous studies involving transfection of plasmids into cells, regions of gE involved in binding IgG were mapped to a domain including residues 235 to 380 (2, 17). Our studies with gE mutants expressed in the context of HSV infection confirmed these conclusions. There was also evidence that the N- and C-terminal extremities of this region were more important. Mutations in the central region of this domain, including amino acids 297 to 333, less profoundly reduced IgG binding and also had less dramatic effects on cell-to-cell spread. In general, we concluded that mutations that affected IgG binding also affected cell-to-cell spread. This was somewhat different than conclusions drawn by Friedman and colleagues, who suggested that IgG binding and cell-to-cell spread were mediated through distinct domains (57). Their conclusion was largely based on studies of mutant 339. We similarly found that the same position 339 mutation could mediate cell-to-cell spread but was defective for IgG binding. However, all other mutations in gE that reduced spread also reduced IgG binding, and vice versa, and there was a quantitative correlation. Therefore, it appears that, for the most part, domains of gE that are important for IgG binding are also important for cell-to-cell spread. We speculate that gE/gI receptors might contain IgG folds or be IgG supergene family members. However, proving the existence of gE/gI receptors will require their identification and characterization in cell-to-cell spread. The mutants described here should be important tools in our efforts in this regard.
Acknowledgments
We thank Aurelie Snyder of the Microbiology Core Facility for valuable expertise with confocal microscopy and deconvolution software, Tiffani Howard for graphics, and Jeff Vandhey for help submitting this paper online. We are also grateful to Harvey Friedman, who provided plasmids encoding mutant gE molecules, as well as to Frank Tufaro and Brian Horsburgh for providing reagents and advice allowing us to establish the BAC system.
We acknowledge support from the National Eye Institute (EY11245) and the National Institute for Allergy and Infections Diseases (AI 73996).
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111-121. [DOI] [PubMed] [Google Scholar]
- 2.Basu, S., G. Dubin, M. Basu, V. Nguyen, and H. M. Friedman. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154:260-267. [PubMed] [Google Scholar]
- 3.Basu, S., G. Dubin, T. Nagashunmugam, M. Basu, L. T. Goldstein, L. Wang, B. Weeks, and H. M. Friedman. 1997. Mapping regions of herpes simplex virus type 1 glycoprotein I required for formation of the viral Fc receptor for monomeric IgG. J. Immunol. 158:209-215. [PubMed] [Google Scholar]
- 4.Bell, S., M. Cranage, L. Borysiewicz, and T. Minson. 1990. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J. Virol. 64:2181-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, W. Z., S. Person, S. C. Warner, J. H. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957-969. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, J. I., and H. Nguyen. 1997. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 71:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, W. J., and D. C. Johnson. 2003. Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread. J. Virol. 77:2686-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, A. M., R. G. Hope, and H. S. Marsden. 1987. Generation and properties of the glycoprotein E-related 32K/34K/35K and 55K/57K polypeptides encoded by herpes simplex virus type 1. J. Gen. Virol. 68:2093-2104. [DOI] [PubMed] [Google Scholar]
- 14.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingwell, K. S., and D. C. Johnson. 1998. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubin, G., S. Basu, D. L. Mallory, M. Basu, R. Tal-Singer, and H. M. Friedman. 1994. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J. Virol. 68:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enquist, L. W., M. J. Tomishima, S. Gross, and G. A. Smith. 2002. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 86:5-16. [DOI] [PubMed] [Google Scholar]
- 20.Farnsworth, A., K. Goldsmith, and D. C. Johnson. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 23.Hanke, T., F. L. Graham, V. Lulitanond, and D. C. Johnson. 1990. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology 177:437-444. [DOI] [PubMed] [Google Scholar]
- 24.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horsburgh, B. C., M. M. Hubinette, D. Qiang, M. L. MacDonald, and F. Tufaro. 1999. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 6:922-930. [DOI] [PubMed] [Google Scholar]
- 26.Horsburgh, B. C., M. M. Hubinette, and F. Tufaro. 1999. Genetic manipulation of herpes simplex virus using bacterial artificial chromosomes. Methods Enzymol. 306:337-352. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, D. C., and P. G. Spear. 1983. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell 32:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krummenacher, C., I. Baribaud, R. J. Eisenberg, and G. H. Cohen. 2003. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J. Virol. 77:8985-8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longnecker, R., and B. Roizman. 1987. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science 236:573-576. [DOI] [PubMed] [Google Scholar]
- 36.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 76:9232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 39.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 41.Mulder, W., J. Pol, T. Kimman, G. Kok, J. Priem, and B. Peeters. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J. Virol. 70:2191-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Para, M. F., R. B. Baucke, and P. G. Spear. 1982. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced Fc-binding receptors. J. Virol. 41:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peeters, B., J. Pol, A. Gielkens, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polcicova, K., P. S. Biswas, K. Banerjee, T. Wisner, B. T. Rouse, and D. C. Johnson. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 46.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycopreoteins gII and gp50 are essential for virus penetration. J. Virol. 64:5346-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saldanha, C. E., J. Lubinski, C. Martin, T. Nagashunmugam, L. Wang, H. van Der Keyl, R. Tal-Singer, and H. M. Friedman. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 74:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tirabassi, R. S., and L. W. Enquist. 1998. Role of the envelope protein gE in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirabassi, R. S., and L. W. Enquist. 2000. Role of the pseudorabies virus gI cytoplasmic tail in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J. Virol. 74:3505-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tirabassi, R. S., R. A. Townley, M. G. Eldridge, and L. W. Enquist. 1997. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J. Virol. 71:6455-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 55.Wagner, M., Z. Ruzsics, and U. H. Koszinowski. 2002. Herpesvirus genetics has come of age. Trends Microbiol. 10:318-324. [DOI] [PubMed] [Google Scholar]
- 56.Weber, P. C., M. Levine, and J. C. Glorioso. 1987. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science 236:576-579. [DOI] [PubMed] [Google Scholar]
- 57.Weeks, B. S., P. Sundaresan, T. Nagashunmugam, E. Kang, and H. M. Friedman. 1997. The herpes simplex virus-1 glycoprotein E (gE) mediates IgG binding and cell-to-cell spread through distinct gE domains. Biochem. Biophys. Res. Commun. 235:31-35. [DOI] [PubMed] [Google Scholar]
- 58.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisner, T. W., and D. C. Johnson. 2004. Redistribution of cellular and herpes simplex virus proteins from the trans-Golgi network to cell junctions without enveloped capsids. J. Virol. 78:11519-11535. [DOI] [PMC free article] [PubMed] [Google Scholar]