Abstract
Virion glycoproteins gB, gD, and gH/gL play essential roles for herpes simplex virus (HSV) entry. The function of gD is to interact with a cognate receptor, and soluble forms of gD block HSV entry by tying up cell surface receptors. Both gB and the nonessential gC interact with cell surface heparan sulfate proteoglycan (HSPG), promoting viral attachment. However, cells deficient in proteoglycan synthesis can still be infected by HSV. This suggests another function for gB. We found that a soluble truncated form of gB bound saturably to the surface of Vero, A431, HeLa, and BSC-1 cells, L-cells, and a mouse melanoma cell line expressing the gD receptor nectin-1. The HSPG analog heparin completely blocked attachment of the gC ectodomain to Vero cells. In contrast, heparin only partially blocked attachment of soluble gB, leaving 20% of the input gB still bound even at high concentrations of inhibitor. Moreover, heparin treatment removed soluble gC but not gB from the cell surface. These data suggest that a portion of gB binds to cells independently of HSPG. In addition, gB bound to two HSPG-deficient cell lines derived from L-cells. Gro2C cells are deficient in HSPG, and Sog9 cells are deficient in HSPG, as well as chondroitin sulfate proteoglycan (CSPG). To identify particular gB epitopes responsible for HSPG-independent binding, we used a panel of monoclonal antibodies (MAbs) to gB to block gB binding. Only those gB MAbs that neutralized virus blocked binding of soluble gB to the cells. HSV entry into Gro2C and Sog9 cells was reduced but still detectable relative to the parental L-cells, as previously reported. Importantly, entry into Gro2C cells was blocked by purified forms of either the gD or gB ectodomain. On a molar basis, the extent of inhibition by gB was similar to that seen with gD. Together, these results suggest that soluble gB binds specifically to the surface of different cell types independently of HSPG and CSPG and that by doing so, the protein inhibits entry. The results provide evidence for the existence of a cellular entry receptor for gB.
Herpes simplex virus (HSV) is responsible for mucocutaneous lesions, commonly known as cold sores, and genital lesions or herpes genitalis. After primary infection virus resides latent in sensory and autonomic neurons from where it reactivates periodically (66). Among the important steps in infection, attachment and entry have attracted attention as targets for therapeutic treatments and vaccines. Binding and entry are complex biochemical processes involving 5 of the 12 known surface glycoproteins of the virus (59).
Current models postulate an initial association of glycoprotein C (gC) and/or gB with cell surface heparan sulfate proteoglycan (HSPG) (56). Following binding, entry requires the interaction of gD with one of its receptors that include herpesvirus entry mediator (HVEM), nectin-1 and nectin-2, and specific sites in heparan sulfate generated by certain 3-O-sulfotransferases (18, 38, 55). The envelope-fusion mechanism is not solved yet, but this event requires gB, the heterodimer gH/gL, gD, and a gD entry receptor (7, 39, 48, 63).
HSV mutants lacking the gC gene exhibit reduced attachment, but the particles are still infectious (22). For that reason, gC is considered a nonessential glycoprotein for entry. Moreover, cells deficient in proteoglycan synthesis are still permissive to HSV (2). On the contrary, a gB null virus cannot penetrate target cells (9). A polylysine (pK) sequence located in the N terminus of gB is responsible for interaction of gB with HSPG. A virus mutant from which the pK coding sequence of gB is deleted is still infectious, although virus binding is reduced (32). Taken together, these data suggest that binding of HSV to HSPG through gC and/or gB is an important yet not essential event for entry. Since gB is essential for entry, this glycoprotein must carry out a function(s) other than binding to HSPG.
Various criteria were used to show that gD binds receptors, and these same criteria should apply to demonstrate the existence of a gB receptor(s). HSV entry should be blocked by (i) antibodies to gB, (ii) soluble forms of gB, (iii) antibodies to receptor, and (iv) soluble forms of receptor. A number of monoclonal antibodies (MAbs) for gB have neutralizing activity (24, 47, 54). However, a soluble form of HSV-2 gB failed to block entry of HSV-1 and HSV-2 (27, 67). In the present study, we found that a soluble truncated form of HSV-1 gB bound saturably to the surface of different cell types, including HSPG-deficient cells Gro2C and Sog9 (2). The key finding was that soluble gB, like gD, efficiently inhibited HSV-1 entry into Gro2C cells in a dose-dependent manner. The results provide evidence for the existence of an entry receptor specific for gB.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells, Vero or BSC-1, were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum (FCS). Human adenocarcinoma cervix cells (HeLa), human epidermoid carcinoma cells (A431), mouse fibroblast L-cells, and derived mutant Gro2C and Sog9 cells (2) were grown in DMEM with 10% FCS. B78-H1 mouse melanoma cells and derived C10 cells engineered to express the gD receptor nectin-1 (37) were grown in DMEM supplemented with 5% FCS and 500 μg/ml of G418. Stocks of HSV-1 KOS and HSV-1 KOS/tk12 that carries the lacZ gene under control of the ICP4 promoter (65) were purified on sucrose gradients as described previously (19), and titers were determined with Vero cells. HSV-1 KOS/tk12 and C10 cells were kindly provided by P. G. Spear. L-cells and Gro2C and Sog9 cells were a generous gift from F. Tufaro.
Antibodies and reagents.
Rabbit sera R47 and R69 were raised against HSV-1 gC and gB, respectively (16, 25). Rabbit serum R7 was raised against HSV-2 gD but cross-reacted with HSV-1 gD (25). MAbs DL16 and DL21 were selected among a panel of antibodies generated against cell extracts infected by HSV type 1 (HSV-1) and HSV-2. DL21 reacted with a conformational epitope on gB, and DL16 recognized a multimer-specific epitope on gB (D. Long, G. H. Cohen, and R. J. Eisenberg, unpublished results). MAb CK6 recognizes human nectin-1 (30). Hybridoma cell lines expressing MAbs to gB, numbered SS-10 to SS-118, were generated by fusing SP2/0 cells with spleen cells of mice immunized with full-length gB purified from HSV-1- and HSV-2-infected BHK cells. Hybridoma cells were injected into mice to raise ascites. Immunoglobulin G (IgG) was purified from sera and ascites fluid with HiTrap protein G 1-ml columns (Amersham Pharmacia Biotech) and dialyzed against phosphate-buffered saline (PBS). Anti-mouse and anti-rabbit secondary antibodies coupled to horseradish peroxidase (HRP) were purchased from Kirkegaard and Perry Laboratories.
Production and purification of HSV-1 glycoproteins.
gC(457t) or gD(306t) and gD(285t) contain the ectodomain of gC-1 truncated at residue 457 or gD-1 truncated at residue 306 and 285, respectively (50, 51). gB(730t) comprises the first 700 amino acids of the mature gB ectodomain expressed from a baculovirus expression system (4). The amino acids are numbered starting at the first methionine of gB. The truncated protein was purified from baculovirus-infected insect cells (sf9) using a DL16 immunosorbent column. Bound gB was eluted with 3 M KSCN, then dialyzed and concentrated essentially the same way as described for gD(306t) (58).
Binding of soluble glycoproteins to the cell surface by CELISA.
We used a modification of a published cellular enzyme-linked immunosorbent assay (CELISA) (15). Cells were seeded in 96-well plates and grown overnight to reach 4 × 104 cells per well. They were then incubated for 1 h at 4°C with serial dilutions of soluble glycoproteins diluted in DMEM containing 5% FCS and 30 mM HEPES (DFH). After three washes with ice-cold DFH, cells were fixed with 3% paraformaldehyde. Cells were then rinsed three times with PBS and incubated 1 h with 20 μg/ml of R7, R47, or R69 IgGs that had been diluted in PBS containing 5% nonfat dry milk. After three washes with cold PBS, cells were further incubated for 1 h with HRP-conjugated anti-rabbit antibodies. Following another three PBS washes, cells were rinsed with 20 mM citrate buffer, pH 4.5. 2,2′-Azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase substrate (Moss, Inc.) was added, the absorbance at 405 nm was measured at multiple time points with a micro titer plate reader, and the mean slopes were recorded.
Binding of soluble glycoproteins to the cell surface by Western blotting.
Cells were seeded overnight in 12-well plates. They were then incubated for 1 h at 4°C with gB(730t), gC(457t), or gD(285t) in DFH. After three washes with cold PBS, cells were extracted in MNE (25 mM MES [2-{N-morpholino}ethanesulfonic acid], pH 6.5, 150 mM NaCl, and 2 mM EDTA) containing 1% Brij 96 (Aldrich) and a cocktail of protease inhibitors (Roche). The nuclei were pelleted by centrifugation at 500 × g for 5 min, the supernatant was mixed with an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (Pierce) and proteins were resolved by SDS-PAGE (Novex) under denaturing conditions. Proteins were transferred to nitrocellulose membrane, reacted with specific antibodies, and then incubated with secondary antibodies coupled to HRP. Bound antibodies were revealed by enhanced chemiluminescence (ECL) (Amersham) and exposure to X-ray film.
Heparin inhibition studies.
Cells seeded in 96-well plates were incubated for 1 h at 4°C with solutions of 10 nM gB(730t) or 6 nM gC(457t) prepared in DFH. They were then incubated for 1 h at 4°C with serial dilutions of heparin (Sigma) prepared in DFH. After three washes in DFH, cells were fixed, and bound glycoproteins determined by CELISA. In other experiments, 10 nM gB(730t) or 6 nM gC(457t) was first incubated for 1 h at 4°C with serial dilutions of heparin made in DFH. Mixtures were then transferred onto cells and incubated for 1 h at 4°C. After three washes with DFH, cells were fixed with 3% paraformaldehyde, and bound glycoproteins were detected by CELISA.
Characterization of monoclonal antibodies to gB. (i) Western blot analysis.
MAbs to gB were screened by Western blotting against gB(730t) under native or denaturing conditions as previously described for gD (13). To denature gB(730t), the protein was boiled for 5 min in SDS-PAGE sample buffer containing 1% SDS and 100 mM dithiothreitol (Pierce). For native conditions, gB(730t) was mixed with sample buffer containing 0.1% SDS, but no reducing agent was added and the sample was not boiled. Proteins were resolved by SDS-PAGE. After transfer to nitrocellulose, individual strips were blocked and then incubated with 1 μg/ml of IgG. Bound MAbs were revealed with secondary antibodies coupled to HRP and ECL as described before.
(ii) ELISA.
In this assay, 50 μl of 200 nM gB(730t) was added to wells of 96-well plates and incubated overnight at 4°C. After being blocked with PBS containing 5% nonfat milk, antibodies (diluted in PBS containing 5% nonfat milk) were added. Bound MAbs were incubated with secondary antibodies coupled to HRP, rinsed with citrate buffer, and revealed by the addition of substrate ABTS as for CELISA.
(iii) Virus neutralization.
HSV-1 KOS/tk12 at a concentration of 4 × 106 PFU/ml was incubated with serial dilutions of MAbs to gB for 1 h at 4°C. The virus-MAb mixtures were then transferred onto a monolayer of C10 cells, L-cells, or Gro2C cells at a multiplicity of infection (MOI) of 10. The plates were incubated for another hour at 4°C to allow for virus attachment. The temperature was then shifted to 37°C to allow fusion to proceed, and β-galactosidase activity was determined 5 h postinfection (p.i.) as a measure of entry.
Blocking of gB binding to cells using MAbs to gB.
gB(730t) (1 μM) was incubated for 1 h at 4°C with various concentrations of MAb to gB. The protein-antibody mixture was added to a monolayer of C10 cells and incubated for 1 h at 4°C. After three washes with cold PBS, cells were extracted in MNE containing 1% Brij 96 and protease inhibitors. Association of gB with cells was detected by analyzing cell extracts by Western blots as described above. The protein-antibody mixture was also added to a monolayer of L-cells or Gro2C cells and incubated for 1 h at 4°C. After three washes with DFH, cells were fixed and the amount of gB(730t) remaining bound to cells was measured by CELISA as described above.
Glycoprotein binding and virus entry assay.
Cells in 96-well plates were incubated (or not) for 1 h at room temperature with serial dilutions of gB(730t), gD(306t), or gC(457t) in DFH. Medium containing glycoproteins was removed, and cells were either washed three times with DFH or not washed, then infected with HSV-1 KOS/tk12 at an MOI of 10, and incubated at 4°C for 1 h to allow virus attachment. The temperature was then shifted to 37°C to allow synchronous infection. Cells were lysed 5 h later by the addition of an equal volume of DMEM containing 1% NP-40. β-Galactosidase activity was determined by the addition of substrate (chlorophenol red-β-d-galactopyranoside; Roche) and measurement of absorbance at 570 nm in a microtiter plate reader.
RESULTS
Expression and purification of a soluble form of HSV-1 gB.
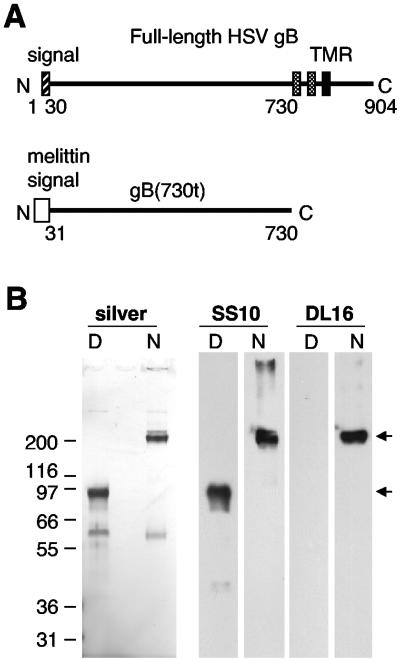
A soluble form of HSV-1 gB truncated at residue 730 [gB(730t)] was generated using the baculovirus system (Fig. 1A). Since the biologically active form of gB is proposed to be a multimer (8, 12, 23), gB(730t) was affinity purified on a immunosorbent column with MAb DL16, which only recognizes a multimeric form of virion gB. The protein was analyzed by SDS-PAGE under denaturing and nondenaturing conditions (Fig. 1B). Under denaturing conditions, the protein migrated as a major single band of 95 kDa. Under native conditions of electrophoresis, the major band was >200 kDa, indicative of a multimer of gB(730t). This suggested that all of the purified protein was oligomeric. Both multimeric and monomeric forms of gB(730t) reacted by Western blotting with MAb SS10. In contrast, MAb DL16 reacted selectively with multimeric gB(730t) under native conditions of electrophoresis. A second minor band of 60 kDa seen on the silver-stained gel was not bound by either MAb on Western blotting. These data indicate that baculovirus-expressed gB(730t) is similar in conformation to full-length virion gB. This protein was used in subsequent experiments.
FIG. 1.
Construction and expression of a soluble form of HSV-1 gB. (A) A schematic representation of full-length gB indicates the position of the 30-amino-acid signal sequence (hatched box) and the two short hydrophobic sequences (gray boxes) preceding the transmembrane region (TMR) (black box) (45). In the baculovirus-expressed gB construct [gB(730t)], the extracellular domain between the signal peptide and the first hydrophobic sequence was fused to the melittin signal sequence. (B) Purified gB(730t) was electrophoresed under denaturing (D) or nondenaturing “native” (N) conditions on SDS-PAGE gels, either silver stained or Western blotted, and probed with MAb SS10 or DL16. Migration positions of molecular mass markers are indicated (on the left, in kilodaltons) along with the expected position of the gB(730t) monomer and multimer (arrows).
The gB ectodomain binds saturably to the cell surface.
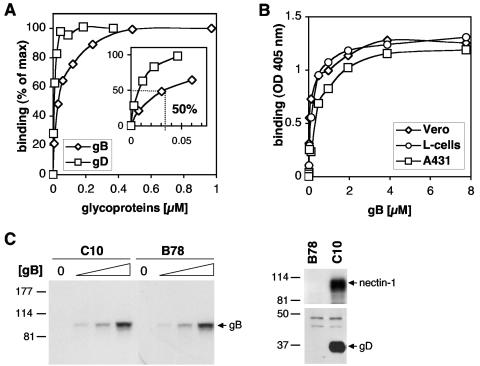
Although several studies implicate gB in attachment, direct experiments have not been described that characterize the interaction of HSV-1 gB with cell surface molecules (21, 32). To obtain this information, a panel of different cell types was incubated with gB(730t) or with gD(306t) (27, 43), and attachment to cells was evaluated by CELISA (Fig. 2). gB(730t) bound saturably to the surface of B78 cells expressing nectin-1, with 50% of saturation occurring with 35 nM glycoprotein (Fig. 2A). As expected (61), gD(306t) also bound saturably to the surface of these cells (Fig. 2A). gB(730t) bound saturably to the surface of Vero cells, L-cells, A431 cells, HeLa cells, BSC-1 cells, and B78 cells (Fig. 2B and data not shown), and the dose-response curve was similar to that for C10 cells. These results suggest that gB, like gD, may bind to a specific receptor. In a second experiment, C10 cells or the parental nonpermissive B78 cells were incubated with various concentrations of gB(730t), then washed, and lysed. gB(730t) association with cells was evaluated in cell extracts by SDS-PAGE and Western blotting (Fig. 2C). Importantly, the same amount of gB(730t) bound, regardless of whether nectin-1 was present. This last observation suggests that nectin-1 is not important for gB binding to the cell surface and is consistent with our previous results showing that gB does not interact directly with nectin-1 in vitro (31). By contrast, soluble gD bound to C10 cells but did not bind to the parental B78 cells.
FIG. 2.
A soluble form of gB binds saturably to the surface of different cell types. (A) C10 cells were seeded in 96-well plates and incubated at 4°C with increasing concentrations of gB(730t) or gD(306t). After 1 h, cells were washed, and CELISA was used to quantitate the amounts of the glycoproteins associated with the cell surface using R69 or R7 for gB and gD, respectively. A value of 100% is defined by the binding of one of the different glycoproteins at the maximal concentration [1 μM and 0.4 μM for gB(730t) and gD(306t), respectively] used in the experiment. Values were plotted after subtraction of background generated by individual polyclonal antibodies. The concentration of gB required to saturate 50% of the cell surface was estimated graphically (insert). Results are representative of at least three independent experiments run in duplicate. (B) Vero cells, L-cells, or A431 cells were seeded in 96-well plates and incubated at 4°C with increasing concentrations of gB(730t). After 1 h, cells were washed and amounts of glycoproteins associated with the cell surface were measured by CELISA with R69. (C) C10 or B78 cells were seeded in 12-well plates were incubated with 0, 0.1, 0.2, or 0.5 μM gB(730t) (reading from left to right) or 0.1 μM of gD(285t) for 1 h at 4°C. Cells were washed; proteins were extracted, resolved by SDS-PAGE, and transferred to membranes; and the amounts of gB or gD associated with the cell extract were measured by Western blotting with polyclonal antibody R69 or R7, respectively. Nectin-1 expression was revealed using MAb CK6. Migration positions of gB(730t), gD(285t), and nectin-1 are indicated.
Heparin removes all gC from the surface but does not remove all bound gB.
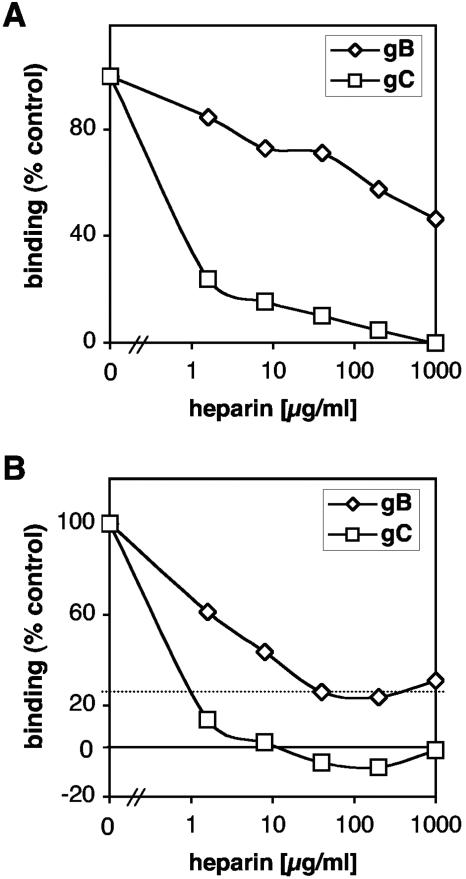
We asked whether heparin, a soluble analog of HSPG, could remove bound gB from the cell surface. Vero (Fig. 3A) or C10 (not shown) cells were first incubated for 1 h at 4°C with 10 nM gB(730t), a concentration that saturates slightly <50% of the cell surface (Fig. 2A, inset). Soluble gC(457t) was used as a control because it binds specifically to cell surface HSPG (61). Cells were then incubated with various concentrations of heparin. The amount of gB(730t) or gC(457t) remaining bound to cells was detected with polyclonal antibodies (Fig. 3A). As expected, heparin at a concentration of 1.5 μg/ml removed >70% of all bound gC(457t) from the cells. In fact, gC(457t) was completely removed from the surface with higher concentrations of heparin. In contrast, heparin had a more modest effect on gB(730t) binding, in that a concentration of 1 mg/ml was required to remove 50% of bound protein. These results suggest that a large proportion of gB(730t) remains attached to the cells through a non-HSPG cell surface molecule.
FIG. 3.
Heparin only partially removes gB or competes with attachment of gB to cells. (A) Vero cells seeded in 96-well plates were incubated with 10 nM gB(730t) or 6 nM gC(457t). After 1 h at 4°C, glycoprotein-containing medium was removed and replaced by fresh medium together with various concentrations of heparin. Cells were incubated for 1 h, washed, and fixed and the level of glycoprotein associated with the cell surface was measured by CELISA with polyclonal antibodies R47 and R69 to gC and gB, respectively. (B) gB(730t) or gC(457t) was incubated for 1 h in the presence of various concentrations of heparin. Glycoprotein-plus-heparin mixtures were added to Vero cells seeded in 96-well plates, and incubation continued for another hour. Cells were then washed and fixed, and glycoproteins associated with the cell surface were revealed by CELISA as described in the legend to panel A.
Heparin blocks attachment of gC to the surface of cells but only partially blocks attachment of gB.
To further examine the interaction of gB and gC with HSPG, we first incubated each soluble glycoprotein with heparin and then added the protein-heparin mixture to Vero cells (Fig. 3B) or C10 cells (not shown). Binding of gC(457t) was blocked in a dose-dependent manner, as previously reported (61), and complete inhibition required 8 μg of heparin/ml (Fig. 3B). Heparin also inhibited gB(730t) binding to cells in a dose-dependent manner, reaching a maximum of 80% inhibition at 40 μg/ml heparin (Fig. 3B). Higher concentrations of heparin (up to 1 mg/ml) did not increase the extent of inhibition. This confirmed the above data (Fig. 3A) that a fraction of gB binds to cells in an HSPG-independent manner.
The gB ectodomain can attach to cells that are deficient in proteoglycan synthesis.
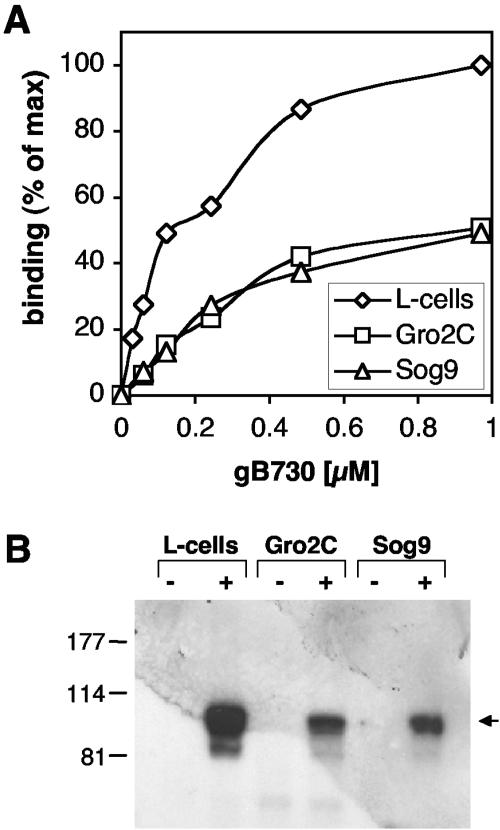
To address this possibility, we analyzed binding of gB(730t) to L-cells or to the L-cell-derived mutant cell lines Gro2C and Sog9, both deficient in proteoglycan synthesis (Fig. 4). Gro2C cells are deficient in HSPG expression and Sog9 cells are deficient in both HSPG and chondroitin sulfate proteoglycan (CSPG). gB(730t) was incubated with L-cells or mutant lines and attachment was measured by CELISA. As shown for Vero, A431, and C10 cells (Fig. 2), gB(730t) bound to the surface of parental L-cells (Fig. 2B and 4A). Interestingly, gB also bound to Gro2C and Sog9 cells (Fig. 4A). However, less gB bound to these cells than to L-cells. Association of gB with these cell types was also detected in cell extracts by SDS-PAGE and Western blotting (Fig. 4B). Although the amount detected was reduced compared to parental L-cells, similar amounts of gB were found in Gro2C and Sog9 cells. Binding of gD was similar in all three lines (reference 61 and data not shown). On the other hand, binding of gC(457t) was considerably reduced on Gro2C cells and almost null on Sog9 cells (reference 61 and data not shown). These results suggest that HSPG contribute to but are not essential for gB attachment to the surface. Since HSPG and CSPG are the only type of proteoglycans present on L-cells (2), the data provide evidence for the existence of another type of gB binding molecule.
FIG. 4.
Soluble gB binds saturably to the surface of cells deficient in HSPG. (A) L-cells, Gro2C cells, or Sog9 cells seeded in 96-well plates were incubated with increasing concentrations of gB(730t) for 1 h at 4°C. Cells were washed and fixed, and binding of gB was quantified by CELISA using anti-gB polyclonal antibody R69. A value of 100% is defined by the binding of gB(730t) to L-cells at the maximal concentration (1 μM) used in the experiment. The experiment shown is representative of three independent experiments. (B) L-cells, Gro2C cells, or Sog9 cells seeded in 12-well plates were incubated for 1 h at 4°C with 1 μM gB(730t). After being washed, total cell proteins were extracted, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with polyclonal antibody R69. Migration positions of molecular mass markers are indicated (on the left, in kilodaltons) along with the expected position of gB(730t) (arrow).
Monoclonal antibodies block binding of soluble gB to cells.
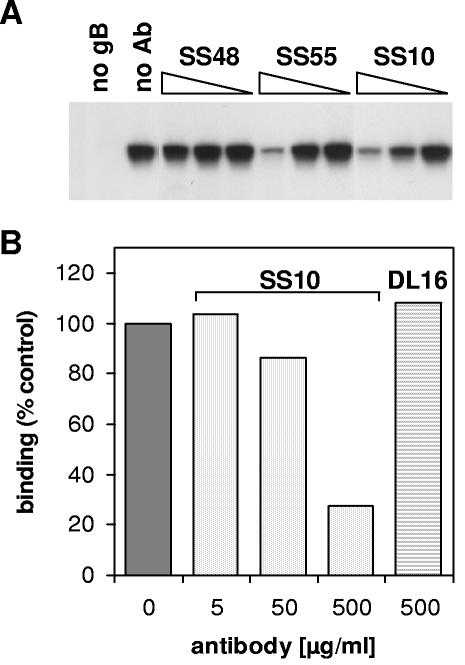
To show that gB binding to the cells was relevant, we used a panel of gB-specific MAbs (Fig. 5). gB(730t) was incubated with various concentrations of MAbs, and the mixture was added to C10 cells. Cells were washed to remove unbound glycoproteins. Cell extracts were prepared, and the level of gB binding was examined by SDS-PAGE and Western blotting. MAbs SS10, SS55, and SS118 blocked the association of gB(730t) with the cells, whereas MAbs SS19, SS48, DL16, and DL21 had no effect (Fig. 5A and Table 1). These results suggested that the association of gB with the cell surface is specific and involves particular regions or epitopes.
FIG. 5.
Monoclonal antibodies to gB block binding of soluble gB to the cells. (A) gB(730t) at a final concentration of 0.5 μM was incubated in the presence of MAb SS48, SS55, or SS10 at a final concentration of 300, 30, or 3 μg/ml (reading from left to right) or with no antibody (no Ab). The mixture was then added to C10 cells seeded into 12-well plates and incubated for 1 h. After being washed, total cell proteins were extracted, resolved by SDS-PAGE, transferred to membranes, and probed against gB with R69. (B) gB(730t) at a final concentration of 0.5 μM was incubated in the presence of MAb SS10 at a final concentration of 500, 50, or 5 μg/ml (reading from left to right) or DL16 at a final concentration of 500 μg/ml. The mixture was then added to Gro2C cells seeded into 12-well plates and incubated for 1 h. Cells were then washed and fixed, and the level of glycoprotein associated with the cell surface was measured by CELISA using R69.
TABLE 1.
Characterization of monoclonal antibodies to gB
| MAb | ELISAa | Western blotb
|
Neutralization in:
|
Blocks gB binding to:
|
||||
|---|---|---|---|---|---|---|---|---|
| Denatured | Native | Veroc | C10d | Gro2C | C10 | Gro2C | ||
| SS10 | ++ | + | +++ | + | + | + | + | + |
| SS55 | ++ | − | +++ | + | + | + | + | + |
| SS118 | ++ | − | +++ | + | + | ND | + | ND |
| SS19 | + | + | +++ | − | − | ND | − | ND |
| SS48 | − | − | + | − | − | ND | − | ND |
| DL16 | ++ | − | +++ | − | − | − | − | − |
| DL21 | ++ | − | +++ | − | − | ND | − | ND |
Serial dilutions of each antibody were reacted against gB(730t), and binding was determined by ELISA. Antibodies were grouped into good (++), moderate (+), or none (−) binder categories, as a function of their avidity for gB.
Antibodies at 1 μg/ml were reacted against Western blots of gB(730t) electrophoresed under native or denaturing conditions. Binding was strong (+++), moderate (+), or not detected according to the exposure time required to develop ECL reactions.
Measured as a 50% reduction in plaques on Vero cells.
Antibodies were considered positive for neutralization in C10 and Gro2C cells if <25 μg/ml inhibited entry by 50%, measuring β-galactosidase activity 5 hours p.i.
One possibility was that antibodies to gB reduced attachment of virus by preventing gB interaction with HSPG. To rule out this hypothesis, we used CELISA to test whether these antibodies blocked gB association with the surface of L-cells (not shown) or Gro2C cells (Fig. 5B and Table 1). Antibodies SS10 and SS55 blocked binding of gB(730t) to both cell types. This last result suggests that these antibodies primarily block interaction of gB with a non-HSPG receptor.
Monoclonal antibodies that block binding of soluble gB to cells inhibit entry.
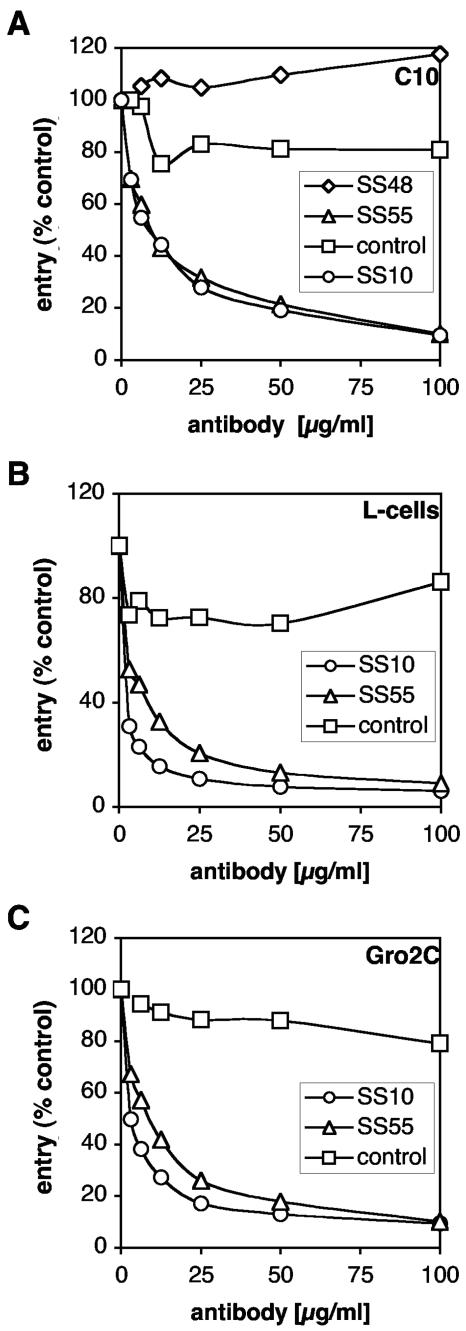
To assess the virus neutralizing activity of MAbs used in the blocking studies, HSV-1 KOS/tk12 was incubated with each of the MAbs and tested for virus entry (Fig. 6 and Table 1). Antibodies SS10, SS55, and SS118 blocked virus entry into C10 cells whereas SS19, SS48, and DL21 did not (Fig. 6A and Table 1). Similar results were obtained using a plaque reduction assay with Vero cells (Table 1). Thus, the ability of SS10, SS55, and SS118 to block gB binding to C10 cells and Vero cells correlates with neutralization of virus entry. Moreover, antibodies SS10 and SS55 neutralized entry into both L-cells and Gro2C cells (Fig. 6B and C; Table 1), ruling out the possibility that neutralizing antibodies blocked virus attachment to HSPG. Together; these observations indicate that interaction of gB with a non-HSPG structure on the cell surface is specific and is relevant in the context of virus entry.
FIG. 6.
Monoclonal antibodies to gB neutralize entry into L-cells and Gro2C. (A) HSV-1 KOS/tk12 was incubated for 1 h with increasing concentrations of MAb to gB (SS10, SS48, and SS55) or anti-myc antibody (control). The virus-antibody mixture was then used to infect C10 cells seeded in 96-well plates at an MOI of 10 PFU/cell. β-Galactosidase activity was assayed at 6 h p.i. as a measure of entry. (B and C) HSV-1 KOS/tk12 was incubated for 1 h with increasing concentrations of MAbs to gB (SS10 and SS55) or anti-myc antibody (control). Virus at a multiplicity of infection of 10 PFU/cell was then used to infect L-cells (B) and Gro2C cells (C) seeded in 96-well plates. β-Galactosidase activity was assayed at 6 h postinfection.
Soluble gB inhibits virus entry into cells deficient in HSPG synthesis.
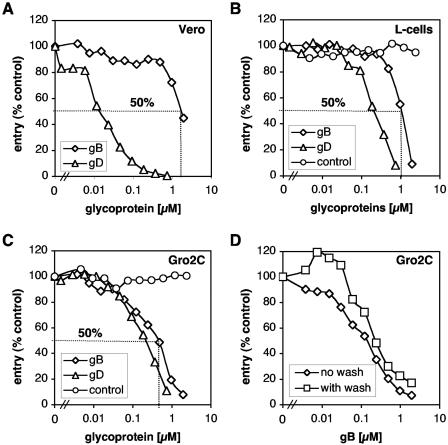
We next wanted to study the importance of gB for HSV entry, dependently or independently of expression of cell proteoglycans. Therefore, Vero cells were incubated with gB(730t) or gD(306t) and then infected with HSV-1 KOS/tk12 carrying the lacZ gene under the control of the HSV-1 ICP4 (for infected-cell polypeptide 4) promoter (Fig. 7A). Levels of β-galactosidase activity in cell extracts were determined at 6 h p.i. and used as a measure of virus entry. In agreement with our and other's reports, gD(306t) efficiently blocked virus entry into these cells in a dose-dependent fashion, with 50% inhibition requiring only 20 nM protein (27, 41). In contrast, gB(730t) did not block virus entry except at concentrations of ≥2 μM for 50% inhibition.
FIG. 7.
Soluble gB bound to cells deficient in HSPG inhibits HSV entry. Vero cells (A), L-cells (B), and Gro2C cells (C and D) seeded in 96-well plates were incubated with increasing concentrations of gB(730t) (A to D) or gD(306t) (A to C). After 1 h at 4°C, glycoprotein-containing medium was removed and cells were either directly infected or washed three times with DFH and then infected with HSV-1 KOS/tk12 at an MOI of 10 PFU per cell. Cells were transferred to 37°C, and β-galactosidase activity was assayed at 6 h postinfection. Concentrations of gB(730t) required to inhibit 50% of entry are estimated graphically for each cell line.
One possible explanation for the poor ability of gB to inhibit HSV entry into Vero cells was the presence of high levels of HSPG on the surface. To address this issue, L-cells and Gro2C cells were incubated with gB(730t) or gD(306t) and then infected with HSV-1 KOS/tk12. HSV can infect and replicate in Gro2C and Sog9 cells, although it is impaired in these cells compared to parental L-cells (2). Inhibition of replication occurred at the level of entry (data not shown). As with Vero cells, gD(306t) efficiently blocked virus entry into both L-cells and Gro2C cells in a dose-dependent fashion (Fig. 7B and C). However, in these cells 50% inhibition by gD(306t) required 200 nM protein, 10-fold more than for Vero cells. This result might simply reflect differences in expression levels of gD receptors in Vero and L-cells. Alternatively, gD might bind less efficiently to mouse nectin-1 and/or HVEM than to the primate homologs. Interestingly, in these cells, 1 μM gB(730t) inhibited entry by 50%, half the amount required for 50% blocking of entry into Vero cells. Of most importance was the observation that gB efficiently blocked entry into Gro2C cells and did so in a dose-dependent fashion, with 50% inhibition occurring with 250 nM protein. This level of inhibition was about four times more efficient than it was in the parental L-cells and eight times more efficient than for Vero cells. Moreover, gD(306t) and gB(730t) exhibited comparable blocking activity into Gro2C cells. On the other hand, gC(457t) was unable to block entry in any of these cells (reference 61 and data not shown). In some experiments, cells were incubated with gB(730t) and then washed to remove unbound glycoproteins before infection. gB(730t) efficiently blocked virus entry whether cells were washed or not (Fig. 7D). These experiments indicate that gB bound to the cell surface was responsible for the blocking of HSV entry. Together, these observations strongly suggest the existence of a gB receptor different than HSPG.
DISCUSSION
The major finding of this study is that soluble gB blocks virus entry into cells lacking HSPG. We hypothesize that gB binds a cell receptor as an essential step in the entry process. To our knowledge, this is the first evidence that a soluble form of gB can compete with a gB-specific receptor for HSV entry. Earlier studies demonstrated that a soluble form of HSV-2 gB did not inhibit entry of HSV-1 into human osteosarcoma cells, nor did it inhibit HSV-2 entry into Vero cells (27, 67). Although it is possible that gB2 functions differently than gB1, it is more likely that much of the gB2 was tied up with HSPG. Indeed in our studies with Vero cells, we only saw blocking at very high concentrations of gB(730t).
Also, the strategy used to purify soluble gB was different than the one used by others (44, 60). Most of the gB(730t) used in this study was multimeric and reacted with antibodies to conformational epitopes. Since the biological active form of gB is a multimer (8, 12, 23), gB(730t) represents a functionally active competitor.
Virion gB was initially reported to be a dimer (12, 52). Recent studies, however, suggest that gB(730t) actually forms an elongated trimeric molecule (K. Eldwein, personal communication). These findings are important, since fusion proteins from several enveloped viruses, including HIV gP41 and influenza hemagglutinin, are trimeric (10, 68).
gB interacts with a non-HSPG receptor on the cell surface.
Previous studies showed that gB binds with heparin and probably functions in attachment of HSV to HSPG on cell surfaces (22, 67). Here, we found that a soluble form of gB interacted with the surface of different cell types, including L-cells deficient in the synthesis of HSPG. gB bound similarly to cells deficient in HSPG or deficient in both HSPG and CSPG, even though virus entry was more severely impaired in cells lacking both types of proteoglycan. This suggests that CSPG is not directly involved in gB attachment but may play additional functions during entry, as suggested by others (3). Since HSPG and CSPG are the major and unique type of proteoglycan expressed by L-cells, a gB receptor might belong to a family other than proteoglycan (32). We are currently investigating the nature of such molecules.
All cells analyzed expressed the gD receptors nectin-1 and HVEM (29). We were unable to characterize gD receptors expressed in L-cells, since reagents specific for mouse nectin-1 and mouse HVEM are not yet available. Parental B78 cells are negative for both receptors (37). However, gB bound to B78 cells. We concluded that neither HVEM nor nectin-1 is important for gB binding.
Specificity of the interaction.
Certain monoclonal antibodies blocked the interaction of gB with C10 cells, L-cells, and Gro2C cells. Interestingly, those antibodies that blocked gB attachment also neutralized virus entry into these cells. These observations confirmed the specificity of gB binding to cells deficient in HSPG synthesis. They also provided evidence that particular domains of gB participate in the interaction. We are currently characterizing the domains recognized by the different monoclonal antibodies as a way of establishing functional domains that are important for attachment and entry.
Importance of HSPG on the cell surface for gB attachment.
Once gB was bound to the surface of Vero cells, it could not be detached with heparin. On the contrary, gC was detached with heparin. In addition, heparin competed for binding of both gB and gC with the cell, but unlike gC, 20% of gB was able to bind even at high concentrations of heparin. It is unlikely that the residual binding can be attributed to differences in affinity of the two glycoproteins for HSPG. According to published studies, gC-1 and gB-2 have similar affinity for heparin, but gB-2 forms a more stable complex with heparin than does gC-1 (50, 67). Moreover, purified soluble gB from bovine herpesvirus type 1 binds to live cells with a KD of 5 × 10−7 M, a value similar to that reported for the HSV-2 gB/heparin complex (34). This suggests that the affinity of gB for heparin is conserved in related alphaherpesviruses, which is not surprising, since gB is the most conserved glycoprotein in the family (46); we propose that the affinity of gB1 for heparin is similar to that reported for gB2 (20, 62). Another possibility is that the initial binding of gB to HSPG may enhance attachment of gB to a second type of receptor. Support for this idea is provided by the observation that gB from BHV-1 initially binds to HSPG and then binds with higher affinity to an unidentified non-HSPG receptor (34). This interaction involves the N-terminal subunit of BHV-1 gB, while the entire molecule is required for non-HSPG binding and for inhibition of plaque formation (33, 34). Similarly, varicella zoster virus gB interacts with HSPG, a process that takes part in the initial attachment of the virus to the cell. However, like HSV gB, varicella zoster virus gB can still attach to HSPG deficient cells (26). Also, cell surface HSPGs are not essential for infection by pseudorabies virus (28). It may be that the gB protein of all alphaherpesviruses shares the dual capacity to bind to HSPG and to another type of receptor. Moreover, the interaction of virion gC and gB with HSPG may also favor binding of gD to one of its entry receptors.
gB receptor for other herpesviruses.
Evidence for a gB receptor comes from other members of the herpesvirus family. In a recent study, it was shown that cytomegalovirus (CMV) gB binds to and activates the epidermal growth factor receptor (64). Integrin α3β1 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (1). Human herpes virus 7 utilizes CD4 as a receptor for infection, although gB does not bind to CD4 (36, 53). Cellular integrins also function as entry receptors for CMV and this interaction involves a highly conserved domain in all beta- but not alphaherpesviruses (17). The interaction of CMV gB with receptor induces cellular transcription, leading to a coordinated antiviral response (5, 6, 57). HSV also triggers early signaling events associated with entry such as tyrosine phosphorylation and calcium influx (11, 49). CMV and HSV gB are both essential for fusion, share 50% amino acid identity, bind to HSPG, and display a similar arrangement of disulfide bonds (14, 35, 46). Although the receptors might be different, gB from both viruses may share a similar role in triggering fusion and inducing receptor-mediated signaling in infected cells. We are currently working on this model.
Final considerations.
Future studies will focus on identifying the non-HSPG gB receptor and defining its role in entry. Recently, we showed that gB but not gC, gD, or gH interacts with lipid rafts during entry (4). One interesting possibility is that the non-HSPG receptor for gB resides in these microdomains. Since rafts are essential for entry, association of the gB receptor with these structures may be important. In addition, rafts may be enriched for a gB receptor and therefore represent a good starting place to look for such a receptor. Recently, it was observed that virus entry into some cell types occurs by endocytosis rather than by direct fusion (40, 42). Perhaps the putative gB receptor is required for endocytosis involving lipid rafts (4). We are currently investigating whether HSV enters L-cells via endocytosis and whether rafts are mobilized during entry into these cells. Characterization of a gB receptor thus represents a crucial step in our understanding of HSV direct fusion with the cells, pH-dependent endocytosis, or cell-induced signaling.
Acknowledgments
This work was supported by Public Service grants AI-056045 and AI-18289 from the National Institute of Allergy and Infectious Diseases to R.J.E. and G.H.C. and grant NS-36731 from the National Institute of Neurological Disorders and Stroke to R.J.E. and G.H.C.
We are grateful to P. G. Spear and F. Tufaro for reagents. We also thank all the members of the Cohen and Eisenberg laboratories for critical advice and helpful discussions.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.Banfield, B. W., Y. Leduc, L. Esford, K. Schubert, and F. Tufaro. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 69:3290-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield, B. W., Y. Leduc, L. Esford, R. J. Visalli, C. R. Brandt, and F. Tufaro. 1995. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 208:531-539. [DOI] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme, K. W., J. Singh, S. T. Perry, and T. Compton. 2004. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J. Virol. 78:1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 8.Cai, W. Z., S. Person, C. DebRoy, and B. H. Gu. 1988. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J. Mol. Biol. 201:575-588. [DOI] [PubMed] [Google Scholar]
- 9.Cai, W. Z., S. Person, S. C. Warner, J. H. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 11.Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 83:2247-2255. [DOI] [PubMed] [Google Scholar]
- 12.Claesson-Welsh, L., and P. G. Spear. 1986. Oligomerization of herpes simplex virus glycoprotein B. J. Virol. 60:803-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 15.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J. Virol. 76:10894-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 17.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 19.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 70:6067-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold, B. C., S. I. Gerber, T. Polonsky, B. J. Belval, P. N. Shaklee, and K. Holme. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 206:1108-1116. [DOI] [PubMed] [Google Scholar]
- 21.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 22.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Highlander, S. L., W. F. Goins, S. Person, T. C. Holland, M. Levine, and J. C. Glorioso. 1991. Oligomer formation of the gB glycoprotein of herpes simplex virus type 1. J. Virol. 65:4275-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland, T. C., S. D. Marlin, M. Levine, and J. Glorioso. 1983. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J. Virol. 45:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquet, A., M. Haumont, D. Chellun, M. Massaer, F. Tufaro, A. Bollen, and P. Jacobs. 1998. The varicella zoster virus glycoprotein B (gB) plays a role in virus binding to cell surface heparan sulfate proteoglycans. Virus Res. 53:197-207. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karger, A., A. Saalmuller, F. Tufaro, B. W. Banfield, and T. C. Mettenleiter. 1995. Cell surface proteoglycans are not essential for infection by pseudorabies virus. J. Virol. 69:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krummenacher, C., F. Baribaud, M. Ponce de Leon, I. Baribaud, J. C. Whitbeck, R. Xu, G. H. Cohen, and R. J. Eisenberg. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322:286-299. [DOI] [PubMed] [Google Scholar]
- 30.Krummenacher, C., I. Baribaud, J. F. Sanzo, G. H. Cohen, and R. J. Eisenberg. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J. Virol. 76:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laquerre, S., R. Argnani, D. B. Anderson, S. Zucchini, R. Manservigi, and J. C. Glorioso. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Y., X. Liang, S. van Drunen Littel-van den Hurk, S. Attah-Poku, and L. A. Babiuk. 1996. Glycoprotein Bb, the N-terminal subunit of bovine herpesvirus 1 gB, can bind to heparan sulfate on the surfaces of Madin-Darby bovine kidney cells. J. Virol. 70:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Y., S. van Drunen Littel-van den Hurk, L. A. Babiuk, and X. Liang. 1995. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J. Virol. 69:4758-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopper, M., and T. Compton. 2002. Disulfide bond configuration of human cytomegalovirus glycoprotein B. J. Virol. 76:6073-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lusso, P., P. Secchiero, R. W. Crowley, A. Garzino-Demo, Z. N. Berneman, and R. C. Gallo. 1994. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 91:3872-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 39.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 40.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicola, A. V., C. Peng, H. Lou, G. H. Cohen, and R. J. Eisenberg. 1997. Antigenic structure of soluble herpes simplex virus (HSV) glycoprotein D correlates with inhibition of HSV infection. J. Virol. 71:2940-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicola, A. V., S. H. Willis, N. N. Naidoo, R. J. Eisenberg, and G. H. Cohen. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J. Virol. 70:3815-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pachl, C., R. L. Burke, L. L. Stuve, L. Sanchez-Pescador, G. Van Nest, F. Masiarz, and D. Dina. 1987. Expression of cell-associated and secreted forms of herpes simplex virus type 1 glycoprotein gB in mammalian cells. J. Virol. 61:315-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellett, P. E., K. G. Kousoulas, L. Pereira, and B. Roizman. 1985. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J. Virol. 53:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira, L. 1994. Function of glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 3:9-28. [PubMed] [Google Scholar]
- 47.Pereira, L., D. Dondero, B. Norrild, and B. Roizman. 1981. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc. Natl. Acad. Sci. USA 78:5202-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 49.Qie, L., D. Marcellino, and B. C. Herold. 1999. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology 256:220-227. [DOI] [PubMed] [Google Scholar]
- 50.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 51.Rux, A. H., S. H. Willis, A. V. Nicola, W. Hou, C. Peng, H. Lou, G. H. Cohen, and R. J. Eisenberg. 1998. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator. J. Virol. 72:7091-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarmiento, M., and P. G. Spear. 1979. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J. Virol. 29:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Secchiero, P., D. Sun, A. L. De Vico, R. W. Crowley, M. S. Reitz, Jr., G. Zauli, P. Lusso, and R. C. Gallo. 1997. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 71:4571-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 56.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuve, L. L., S. Brown-Shimer, C. Pachl, R. Najarian, D. Dina, and R. L. Burke. 1987. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J. Virol. 61:326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tal-Singer, R., C. Peng, M. Ponce De Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trybala, E., J. A. Liljeqvist, B. Svennerholm, and T. Bergstrom. 2000. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J. Virol. 74:9106-9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 65.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 66.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 67.Williams, R. K., and S. E. Straus. 1997. Specificity and affinity of binding of herpes simplex virus type 2 glycoprotein B to glycosaminoglycans. J. Virol. 71:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]