Abstract
The killer cell lectin-like receptor G1 (KLRG1) is a natural killer cell receptor expressed by T cells that exhibit impaired proliferative capacity. Here, we determined the KLRG1 expression by virus-specific T cells. We found that repetitive and persistent antigen stimulation leads to an increase in KLRG1 expression of virus-specific CD8+ T cells in mice and that virus-specific CD8+ T cells are mostly KLRG1+ in chronic human viral infections (human immunodeficiency virus, cytomegalovirus, and Epstein-Barr virus) but not in resolved infection (influenza virus). Thus, by using KLRG1 as a T-cell marker, our results suggest that the differentiation status and function of virus-specific CD8+ T cells are directly influenced by persistent antigen stimulation.
Inhibitory NK cell receptors are expressed in variable frequency by memory-phenotype T cells (11, 16, 18, 30). These receptors include the killer cell immunoglobulin-like receptors (KIRs) and the leukocyte immunoglobulin-like receptors (LIRs) in humans and the Ly49 molecules in mice. In humans and mice, CD94/NKG2A and the killer cell lectin-like receptor G1 (KLRG1) represent additional inhibitory NK cell receptors expressed by T cells (33). Classical and nonclassical major histocompatibility complex (MHC) molecules serve as ligands for KIRs, LIRs, CD94/NKG2A, and Ly49 molecules, while the ligand of KLRG1 is unknown.
In humans and mice, KLRG1 is expressed by CD4 and CD8 T cells that exhibit a memory cell phenotype and by a large proportion of NK cells but not by monocytes, granulocytes, or mast cells (6, 7, 14, 24, 31, 32). Human KLRG1 is also found in a substantial subset of γ/δ T cells (12) and, surprisingly, in a large proportion of naive-phenotype CD4 and CD8 T cells in umbilical cord blood (15). After birth, naive-phenotype KLRG1+ T cells disappear rapidly from peripheral blood. Experiments with P14 T-cell receptor (TCR) transgenic mice specific for lymphocytic choriomeningitis virus (LCMV) further showed that P14 memory cells expressing KLRG1 were impaired in their proliferation potential but not in their capacity to perform immediate effector cell functions (31). Similar to the data in mice, human T cells expressing KLRG1 also exhibit a poor proliferation potential, while their ability to secrete gamma interferon is preserved (32).
Stimulation of T cells in vitro fails to induce KLRG1 expression, while infection of mice with viruses dramatically and transiently increases the frequency of KLRG1-expressing CD8 T cells (6, 7, 17, 24, 32). However, information about the expression of KLRG1 by virus-specific CD8+ T cells during repetitive or persistent antigen stimulation in mice and humans is limited (22). To address this important question, we examined KLRG1 expression by antigen-experienced CD8 T cells in different viral infections using antibodies specific for human and mouse KLRG1 and MHC class I tetramers loaded with viral epitopes to identify virus-specific CD8 T cells in both species.
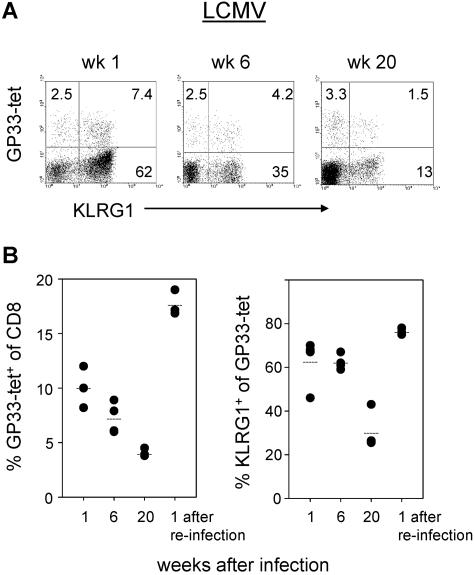
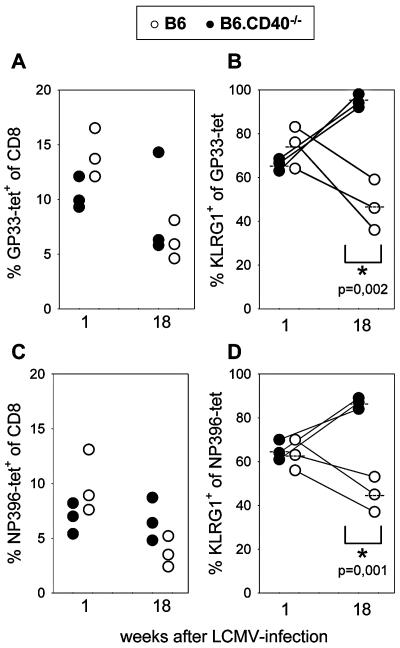
To determine the kinetics of KLRG1 expression by antigen-specific CD8 T cells during repetitive antigen stimulation, B6 mice were first infected intravenously with 200 PFU and rechallenged intraperitoneally with 2 × 106 LCMV (LCM-WE virus strain). Virus-specific CD8 T cells were identified by Db tetramers containing the GP33 peptide that belongs to one of the immunodominant epitopes in H-2b mice, and KLRG1 expression was determined by specific antibody staining. Peripheral blood lymphocytes (PBLs) from mice were stained as described previously (31). At the acute phase of the infection, about 10% of CD8+ T cells in the peripheral blood were stained with tetramers, and as previously reported, the majority of these cells expressed KLRG1 (62%). Importantly, CD8+ T cells not specific for this epitope also upregulated KLRG1. It is likely that these cells were also responding specifically to the virus, as it has been shown that at the height of the immune response more than 50% of peripheral T cells are LCMV specific (20). Six weeks after infection, the percentages of GP33 tetramer+ cells were slightly reduced with almost stable KLRG1 expression levels. After 28 weeks, GP33 tetramer+ cells were reduced to about 5% of CD8+ cells and KLRG1 expression was also lower than that at week 6 (Fig. 1). Importantly, reinfection of these LCMV-immune mice with a high dose (2 × 106 PFU) of LCMV induced a secondary response of GP33-specific T cells that was accompanied by clonal expansion and a strong increase (30% versus 76%) in the proportion of KLRG1+ cells (Fig. 1B, bottom right). These results suggest that repetitive antigen stimulation enhances KLRG1 expression by antigen-reactive CD8 T cells. To provide further evidence for this concept, KLRG1 expression by LCMV-specific CD8 T cells was examined in CD40-deficient mice. Previous studies of these mice have demonstrated that CD40 deficiency does not affect the primary LCMV-specific CD8 T-cell response. However, in the absence of neutralizing antibodies that cannot be generated by CD40-deficient mice, increased antigen load has been observed and long-term control of LCMV fails to occur (5, 8, 9, 27). One week after LCMV infection, similar numbers of LCMV-specific T cells, determined by GP33 and NP396 Db-tetramer staining, were found in wild-type and B6.CD40−/− mice. Expression of KLRG1 by GP33 and NP396 tetramer+ cells was also comparable in the two mouse strains 1 week after infection (Fig. 2). However, 18 weeks after infection, a significant difference in KLRG1 expression became evident: KLRG1 was present in 45 to 47% of GP33- or NP396-specific T cells in B6 mice, while 87 to 95% of these cells expressed KLRG1 in CD40-deficient mice. Although LCMV was not detectable in the spleen of CD40-deficient mice by conventional virus plaque assay (sensitivity, ∼103 PFU per g of spleen), intraperitoneal injection of cell-free supernatant from spleen homogenate (10 mg) from LCMV-infected B6.CD40−/− mice but not from similarly infected B6 mice induced an LCMV infection in naive mice (data not shown). Thus, these results indicate that stimulation by persisting viral antigen leads to an enhanced expression of KLRG1 by virus-specific CD8 T cells in mice. Noteworthily, Vivier and Anfossi have recently proposed a model of stochastic induction of NK cell receptors in T cells after antigen stimulation (30). Our data with KLRG1 can be explained by this model since it implies that the likelihood of a T cell to express KLRG1 increases with the number of TCR-triggering events. But additional factors such as duration and strength of antigen stimulation and cytokine environment could also modify induction of KLRG1. In this context it is noteworthy that, in contrast to KLRG1, Ly49 inhibitory receptors are not induced in murine CD8 T cells upon various settings of antigen immunization or microbial challenge (2, 17).
FIG. 1.
Expression of KLRG1 by LCMV-specific CD8 T cells. B6 mice obtained from Harlan Winkelmann (Borchen, Germany) were infected with LCMV (A and B). At the indicated times after infection, PBLs were stained with Db/LCMV GP33-41 tetramers and with monoclonal antibodies specific for CD8 and KLRG1. To stain murine lymphocytes, fluorescein isothiocyanate- or allophycocyanin-labeled anti-CD8 monoclonal antibodies were used that were purchased from BD PharMingen (San Diego, CA). Murine KLRG1 was detected by 2F1 monoclonal antibody (10) purified from hybridoma supernatants by affinity chromatography over protein G-agarose and conjugated with Alexa 488 (Molecular Probes, Eugene, OR) according to the protocol of the manufacturer. The tetramers were prepared by us as described previously (1). The dot plots shown in panel A were gated on CD8 T cells. In panel B, percent tetramer+ cells of CD8 T cells and percent KLRG1+ cells of tetramer+ cells from individual mice are shown.
FIG. 2.
Increased KLRG1 expression by LCMV-specific memory CD8 T cells from CD40-deficient mice. B6 (open circles) and B6.CD40−/− (closed circles) mice (obtained from the Jackson Laboratory, Bar Harbor, ME) were infected with LCMV, and at the indicated times after infection, PBLs were stained with Db/LCMV GP33-41 or Db/LCMV NP396-404 tetramers and with monoclonal antibodies specific for CD8 and KLRG1. In panels A and C, percent GP33-41 tetramer+ (A) and NP396-404 tetramer+ (C) cells of CD8 T cells from individual mice are shown. In panels B and D, percent KLRG1+ cells of GP33-41 (B) and of NP396-404 (D) tetramer+ cells from the same mice are depicted. Data are derived from three individual mice (indicated by lines) per group that were analyzed at weeks 1 and 18 after LCMV infection, and relevant P values are indicated.
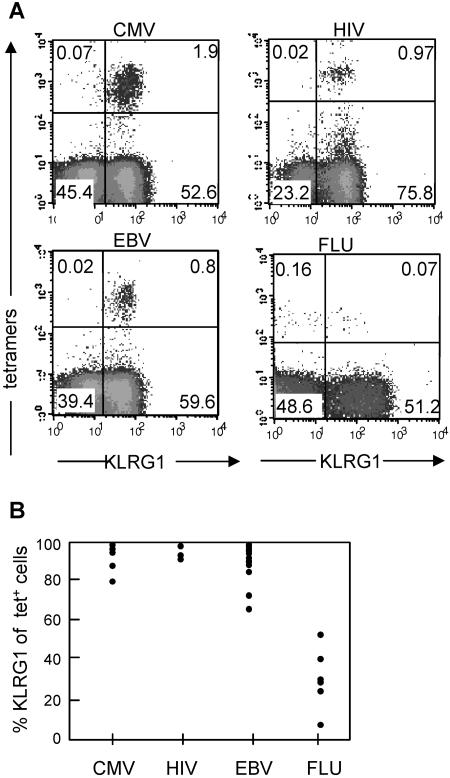
Next, we determined whether increased KLRG1 expression can also be observed in chronic viral infections in humans. For these experiments, KLRG1 expression by virus-specific CD8 T cells was determined using MHC class I tetramers loaded with viral epitopes to identify T cells specific for cytomegalovirus (CMV), human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), and influenza virus (FLU) (Table 1). The analyses were performed with PBLs from several donors in the chronic (CMV, HIV, and EBV) or resolved (FLU) phase of infection using different epitopes from the same virus (Table 1). It revealed a remarkably clear-cut picture: the vast majority of CMV- (93% ± 8%), HIV- (94% ± 3%) and EBV-specific (90% ± 10%) CD8 T cells expressed KLRG1, while the frequency of KLRG1+ cells within FLU-specific (34% ± 15%) T cells was considerably lower (Fig. 3). These results support the hypothesis that ongoing antigen triggering in chronic human viral infection increases the abundant expression of KLRG1 by CMV-, HIV-, and EBV-specific CD8 T cells. In contrast, FLU represents a resolved infection with efficient elimination and antibody-mediated neutralization of the virus. Therefore, expression of KLRG1 by FLU-specific CD8 T cells was probably found to be considerably lower. By using KLRG1 as a marker, our results suggest that persistent viral antigen stimulation leads to T cells with impaired proliferative capacity and that this is a general feature of virus-specific CD8+ T cells in chronic infections (23, 26).
TABLE 1.
MHC class I-restricted viral epitopes used in this study
| Virus | MHC type | Protein | Epitope | Sequence |
|---|---|---|---|---|
| LCMV | Db | Glycoprotein | 33-41 | KAVYNFATM |
| LCMV | Db | Nucleoprotein | 396-404 | FQPQNGQFI |
| CMV | A*0201 | Matrix pp65 | 495-503 | NLVPMVATV |
| CMV | B7 | Matrix pp65 | 417-426 | TPRVTGGGAM |
| HIV | B8 | Nef | 89-96 | FLKEKGGL |
| EBV | B8 | BZLF1 | 190-197 | RAKFKQLL |
| EBV | B8 | EBNA3A | 325-333 | FLRGRAYGL |
| FLU | A*0201 | Matrix | 58-66 | GILGFVFTL |
FIG. 3.
Expression of KLRG1 by virus-specific CD8 T cells in humans. (A) PBLs from donors that were known to possess traceable numbers of virus-specific T cells were stained by the indicated tetramers (A2/pp65 for CMV, B8/Nef for HIV, B8/BZLF1 for EBV, and A2/matrix for FLU) and with monoclonal antibodies specific for CD8 and KLRG1. Antibody and tetramer staining was performed as previously described using either heparinized blood, buffy coats, or peripheral blood mononuclear cells isolated from blood by using Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density centrifugation. Antibodies were obtained from BD PharMingen. Human KLRG1 was detected by Alexa 488-labeled 13A2 monoclonal antibody or by biotinylated or phycoerythrin-labeled 13F12F2 monoclonal antibody (15, 32). Shown are representative dot plots gated on CD8 T cells with percentages of cells present in the different quadrants. (B) Percent KLRG1+ cells of CMV-, HIV-, EBV-, and FLU-specific CD8 T cells identified with the tetramers. Each circle within one particular virus group represents the value from one donor. All donors were analyzed in the chronic (CMV, HIV, and EBV) or postacute (FLU) phase of the infection. KLRG1 expression levels in CMV- and EBV-specific T cells were independent of the HIV status, and FLU-specific T cells were analyzed from HIV-seronegative donors. Peripheral blood mononuclear cells from healthy adult donors (>20 years) were obtained from the Blood Transfusion Center, University Hospital Freiburg, Freiburg, Germany. Further samples were obtained from healthy laboratory workers and from patients chronically infected with HIV, EBV, or CMV, attending clinics in Oxford, United Kingdom. The medical history of each subject was recorded, and blood samples were drawn for serological, virological, and immunological analyses. The study protocol was approved by the local ethics committee.
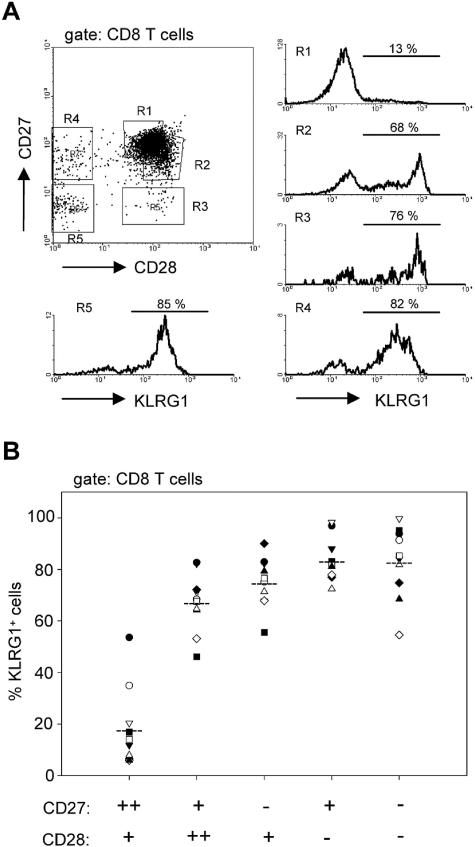
Finally, we determined at what step in T-cell differentiation KLRG1 is upregulated in human cells. Human CD8 T cells have been classified into different subsets according to the expression of the costimulatory receptors CD27 and CD28 (3, 4, 13, 28). Figure 4 shows that the frequency of KLRG1+ cells increased along the widely accepted differentiation pattern from CD27++ CD28+ to CD27− and/or CD28− cell subsets. Remarkably, the most prominent increase in the number of KLRG1+ cells (18% versus 67%) occurred between the CD27++ CD28+ and CD27+ CD28++ cell population. The transition between these two “early” cell subsets is characterized by a slight downregulation of CD27 and a small CD28 upregulation. Hence, these data suggest that KLRG1 expression in humans is induced at a relatively early phase in the differentiation pathway of CD8 T cells. Thus, CMV-, HIV-, and EBV-specific CD8+ T cells are all KLRG1 positive although they differ significantly in their CD27 or CD28 expression (3).
FIG. 4.
Expression of KLRG1 in CD27/CD28 subsets of human CD8 T cells. PBLs from healthy donors were stained with monoclonal antibodies specific for CD8, CD27, CD28, and KLRG1. (A) Histograms on the right display KLRG1 expression gated on the regions R1 to R4 that are indicated in the CD27/CD28 dot plot on the left. A representative staining from one donor is shown. (B) Percent KLRG1+ cells in the indicated CD27/CD28 subsets of CD8 T cells. Symbols represent values from individual donors.
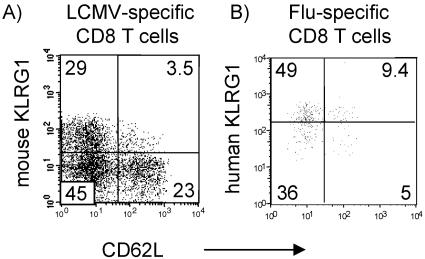
In this respect, KLRG1 differs from other NK receptors expressed in human T cells such as KIRs or LIR1 that are present predominantly in CD8 T cells that have lost CD27 and/or CD28 expression (34). Since CD27/CD28 cannot be used as memory cell markers in mice, it is difficult to directly compare KLRG1 expression in memory T-cell subsets in humans and mice. Nonetheless, our data might indicate that the differentiation state at which KLRG1 is induced differs in humans and mice. In mice, KLRG1 is induced probably rather late during T-cell differentiation since P14 T cells have to divide at least 10 times before they express KLRG1 after antigen stimulation in vivo (31). At this state in differentiation, all other known memory cell markers in mice such as CD44, CD49D, CD62L, CD122, and Ly6C are already up- or downregulated (19, 21, 35). Memory T cells have been divided into CCR7+ CD62L+ central and CCR7− CD62L− effector memory cells (25). CD62L/KLRG1 coexpression analysis of LCMV-specific CD8 cells in mice and FLU-specific CD8 cells in humans revealed that the majority of KLRG1+ cells were CD62L negative in both species (Fig. 5). These results are in agreement with our previous results showing that KLRG1 is expressed preferentially by CCR7− effector memory compared to CCR7+ central memory T cells (32).
FIG. 5.
Coexpression of KLRG1 and CD62L by virus-specific CD8 T cells in humans and mice. (A) P14 TCR-transgenic memory T cells were generated in an adoptive transfer system as described previously (29). Three weeks after LCMV infection, splenic P14 memory cells, identified by the Thy.1 marker, were examined for mouse KLRG1 versus CD62L expression. (B) KLRG1 versus CD62L expression by human FLU tetramer+ CD8 T cells from a healthy donor.
In conclusion, our data show that expression of KLRG1 by virus-specific CD8 T cells in humans and mice is induced by repetitive antigen stimulation and that it differs in chronic versus resolved human viral infection. They also show that KLRG1 is a convenient marker for early T-cell differentiation in humans. Thus, our results indicate that the differentiation status and function (e.g., proliferative capacity) of virus-specific CD8+ T cells are directly influenced by persistent viral antigen stimulation.
Acknowledgments
We thank Stephen Batsford for comments on the manuscript; Marlies Rawiel, Nadine Kersting, and Natalja Nazarova for excellent technical assistance; and Theresa Treuer, Sonja Wagenknecht, Rainer Bronner, and Thomas Imhof for animal husbandry.
This work was supported by the Deutsche Forschungsgemeinschaft DFG (SFB620, Teilprojekt B2 and C6 and Emmy Noether-Programm TH 719/2-1).
REFERENCES
- 1.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Anfossi, N., S. H. Robbins, S. Ugolini, P. Georgel, K. Hoebe, C. Bouneaud, C. Ronet, A. Kaser, C. B. DiCioccio, E. Tomasello, R. S. Blumberg, B. Beutler, S. L. Reiner, L. Alexopoulou, O. Lantz, D. H. Raulet, L. Brossay, and E. Vivier. 2004. Expansion and function of CD8+ T cells expressing Ly49 inhibitory receptors specific for MHC class I molecules. J. Immunol. 173:3773-3782. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 4.Azuma, M., J. H. Phillips, and L. L. Lanier. 1993. CD28− T lymphocytes. Antigenic and functional properties. J. Immunol. 150:1147-1159. [PubMed] [Google Scholar]
- 5.Bachmann, M. F., L. Hunziker, R. M. Zinkernagel, T. Storni, and M. Kopf. 2004. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur. J. Immunol. 34:317-326. [DOI] [PubMed] [Google Scholar]
- 6.Beyersdorf, N. B., X. Ding, K. Karp, and T. Hanke. 2001. Expression of inhibitory “killer cell lectin-like receptor G1” identifies unique subpopulations of effector and memory CD8 T cells. Eur. J. Immunol. 31:3443-3452. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, C., M. Kaufmann, and H. Pircher. 1998. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. J. Immunol. 161:6451-6454. [PubMed] [Google Scholar]
- 8.Borrow, P., A. Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone, and R. A. Flavell. 1996. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 183:2129-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow, P., D. F. Tough, D. Eto, A. Tishon, I. S. Grewal, J. Sprent, R. A. Flavell, and M. B. Oldstone. 1998. CD40 ligand-mediated interactions are involved in the generation of memory CD8+ cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 72:7440-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corral, L., T. Hanke, R. E. Vance, D. Cado, and D. H. Raulet. 2000. NK cell expression of the killer cell lectin-like receptor G1 (KLRG1), the mouse homolog of MAFA, is modulated by MHC class I molecules. Eur. J. Immunol. 30:920-930. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea, A., and L. L. Lanier. 1998. Killer cell inhibitory receptor expression by T cells. Curr. Top. Microbiol. Immunol. 230:25-39. [DOI] [PubMed] [Google Scholar]
- 12.Eberl, M., R. Engel, S. Aberle, P. Fisch, H. Jomaa, and H. Pircher. 2005. Human Vgamma9/Vdelta2 effector memory T cells express the killer cell lectin-like receptor G1 (KLRG1). J. Leukoc. Biol. 77:67-70. [DOI] [PubMed] [Google Scholar]
- 13.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke, T., L. Corral, R. E. Vance, and D. H. Raulet. 1998. 2F1 antigen, the mouse homolog of the rat “mast cell function-associated antigen,” is a lectin-like type II transmembrane receptor expressed by natural killer cells. Eur. J. Immunol. 28:4409-4417. [DOI] [PubMed] [Google Scholar]
- 15.Marcolino, I., G. K. Przybylski, M. Koschella, C. A. Schmidt, D. Voehringer, M. Schlesier, and H. Pircher. 2004. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur. J. Immunol. 34:2672-2680. [DOI] [PubMed] [Google Scholar]
- 16.McMahon, C. W., and D. H. Raulet. 2001. Expression and function of NK cell receptors in CD8+ T cells. Curr. Opin. Immunol. 13:465-470. [DOI] [PubMed] [Google Scholar]
- 17.McMahon, C. W., A. J. Zajac, A. M. Jamieson, L. Corral, G. E. Hammer, R. Ahmed, and D. H. Raulet. 2002. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8+ T cells. J. Immunol. 169:1444-1452. [DOI] [PubMed] [Google Scholar]
- 18.Moretta, L., C. Romagnani, G. Pietra, A. Moretta, and M. C. Mingari. 2003. NK-CTLs, a novel HLA-E-restricted T-cell subset. Trends Immunol. 24:136-143. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna, K., and R. Ahmed. 2000. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165:1733-1737. [DOI] [PubMed] [Google Scholar]
- 20.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 21.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161:5338-5346. [PubMed] [Google Scholar]
- 22.Ouyang, Q., W. M. Wagner, D. Voehringer, A. Wikby, T. Klatt, S. Walter, C. A. Muller, H. Pircher, and G. Pawelec. 2003. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp. Gerontol. 38:911-920. [DOI] [PubMed] [Google Scholar]
- 23.Papagno, L., C. A. Spina, A. Marchant, M. Salio, N. Rufer, S. Little, T. Dong, G. Chesney, A. Waters, P. Easterbrook, P. R. Dunbar, D. Shepherd, V. Cerundolo, V. Emery, P. Griffiths, C. Conlon, A. J. McMichael, D. D. Richman, S. L. Rowland-Jones, and V. Appay. 2004. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins, S. H., S. C. Terrizzi, B. C. Sydora, T. Mikayama, and L. Brossay. 2003. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J. Immunol. 170:5876-5885. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 26.Tesselaar, K., R. Arens, G. M. van Schijndel, P. A. Baars, M. A. van der Valk, J. Borst, M. H. van Oers, and R. A. van Lier. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49-54. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen, A. R., A. Nansen, J. P. Christensen, S. O. Andreasen, and O. Marker. 1998. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J. Immunol. 161:4583-4590. [PubMed] [Google Scholar]
- 28.Tomiyama, H., H. Takata, T. Matsuda, and M. Takiguchi. 2004. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur. J. Immunol. 34:999-1010. [DOI] [PubMed] [Google Scholar]
- 29.Unsoeld, H., and H. Pircher. 2005. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J. Virol. 79:4510-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivier, E., and N. Anfossi. 2004. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat. Rev. Immunol. 4:190-198. [DOI] [PubMed] [Google Scholar]
- 31.Voehringer, D., C. Blaser, P. Brawand, D. H. Raulet, T. Hanke, and H. Pircher. 2001. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167:4838-4843. [DOI] [PubMed] [Google Scholar]
- 32.Voehringer, D., M. Koschella, and H. Pircher. 2002. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100:3698-3702. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama, W. M., and B. F. Plougastel. 2003. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 3:304-316. [DOI] [PubMed] [Google Scholar]
- 34.Young, N. T., M. Uhrberg, J. H. Phillips, L. L. Lanier, and P. Parham. 2001. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J. Immunol. 166:3933-3941. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman, C., K. Brduscha-Riem, C. Blaser, R. M. Zinkernagel, and H. Pircher. 1996. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 183:1367-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]