Abstract
Background
A significant proportion of patients starting dialysis do so with a temporary or tunnelled haemodialysis catheter. Insertion of these catheters can be achieved either by using the anatomical landmarks for the veins into which they are inserted or using ultrasound guidance. It has been suggested that the use of ultrasound guidance reduces the immediate complications of haemodialysis catheter insertions such as pneumothorax or arterial puncture.
Objectives
The aim of the review was to compare the use of real‐time 2‐dimensional (2‐D) Doppler ultrasound venous imaging in the insertion of percutaneous central venous catheters for dialysis versus the traditional "blind" landmark method.
Search methods
We searched the Cochrane Renal Group's Specialised Register, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL). Reference lists of identified studies and relevant narrative reviews were also screened. Search date: January 2011.
Selection criteria
All randomised controlled trials (RCTs) and quasi‐RCTs evaluating ultrasound guidance in the percutaneous insertion of central venous catheters for dialysis (both cuffed and uncuffed) against the traditional blind landmark method.
Data collection and analysis
Two authors assessed risk of bias and extracted data. Statistical analyses were performed using the random effects model and the results expressed as risk ratios (RR) for dichotomous outcomes or mean difference (MD) for continuous data with 95% confidence intervals (CI).
Main results
We identified seven studies enrolling 767 patients and with 830 catheter insertions. Three of seven studies described the method of random sequence generation, none described allocation concealment, and blinding of participants and personnel was not possible. Real‐time ultrasound guidance was found to significantly reduce the risk of catheter placement failure on the first attempt (5 studies, 595 catheters): RR 0.40, 95% CI 0.30 to 0.52), significantly reduce the risk of arterial puncture (6 studies, 535 catheters: RR 0.13, 95% CI 0.04 to 0.37) and haematomas (4 studies, 323 catheters: RR 0.22, 95% CI 0.06 to 0.81) when compared to the landmark method. The time taken for successful cannulation was significantly lower with the use of real‐time ultrasound guidance (1 study, 73 catheters: MD ‐1.40 min, 95% CI ‐2.17 to ‐0.63) and there were less attempts/catheter insertion (1 study, 110 catheters: ‐0.35, 95% CI ‐0.54 to ‐0.16).
Authors' conclusions
Use of real‐time 2‐D Doppler ultrasound guidance has significant benefits with respect to the number of catheters successfully inserted on the first attempt, reduction in the risk of arterial puncture and haematomas and the time taken for successful vein puncture.
Keywords: Humans; Anatomic Landmarks; Catheterization, Central Venous; Catheterization, Central Venous/methods; Hematoma; Hematoma/prevention & control; Randomized Controlled Trials as Topic; Renal Dialysis; Renal Dialysis/instrumentation; Ultrasonography, Interventional; Ultrasonography, Interventional/methods; Wounds, Stab; Wounds, Stab/prevention & control
Plain language summary
Ultrasound use for the placement of haemodialysis catheters
Insertion of haemodialysis catheters can be achieved either by using the anatomical landmarks for the veins into which they are inserted or using ultrasound guidance. It has been suggested that the use of ultrasound guidance reduces the immediate complications of haemodialysis catheter insertions such as pneumothorax or arterial puncture. We identified seven studies, enrolling 767 patients that compared haemodialysis catheter insertion using the traditional 'blind' landmark method and insertion using ultrasound imaging. The use of ultrasound imaging was found to be associated with significantly less risk of arterial puncture and haematomas and less time to insert the catheter as well as a higher success rate for inserting the catheter on the first attempt.
Background
With the prevalence of end‐stage kidney disease (ESKD) growing at a rate of between 7% and 9% per year, it is projected that there will be greater than 350,000 such patients worldwide by the year 2010. Patients preferring hospital‐based haemodialysis varied from 70% to 85% in UK and USA respectively (Renal Association (UK) 2008; USRDS 2008).
Vascular access for haemodialysis is best achieved via an Arterio‐Venous Fistula (AVF) and is considered to be the 'gold standard'. However an increasing number of patients are starting dialysis with either temporary catheters or tunnelled cuffed catheters (DOPPS 2008). Typically, insertion of percutaneous haemodialysis catheters is indicated for:
those requiring vascular access while waiting for creation, or maturation of, an AVF;
contraindication for the creation of an AVF (e.g. terminal heart failure);
absence of suitable sites for AVF formation;
life expectancy of less than 12 months;
those expecting a kidney transplant in the immediate future.
Insertion of these percutaneous catheters is an invasive procedure with a small but definite morbidity and mortality. Reasons for this include anatomical variation of the vascular structures, thick neck in obese individuals, and inexperience of the operator.
Venous cannulation is performed using three different techniques.
Surgical cut down technique: usually done by a skilled operator and is the recommended technique because of lower risk of peri‐insertion complications.
Percutaneous Seldinger technique using traditional anatomical landmarks ("blind"): preferred by most non‐surgical staff.
Percutaneous Seldinger technique under real time ultrasound guidance.
Central venous cannulation can be achieved using various puncture sites, but the right internal jugular vein (RIJV) is recommended for this purpose. RIJV joins the subclavian vein beneath the sternal border of the clavicle to form the brachiocephalic vein. This is then joined by the left brachiocephalic to form the superior vena cava (SVC) which drains into the right atrium forming a straight venous drainage system ideal for catheterisation. The left internal jugular vein (LIJV) on the other hand has a sharp turn at the left jugular and subclavian junctions making catheterisation more challenging and potentially hazardous.
Ultrasound guidance has become increasingly popular in an endeavour to minimise complications of central venous catheter insertion. It provides real‐time grey scale imaging of the anatomy. Portable ultrasound machines are convenient and can be used at the bedside, radiology suites, theatres and high care or intensive care settings.
Occasionally the carotid artery can be anterior and even lateral to the vein (Gordon 1998). The recommended order for insertion of tunnelled lines is the RIJV, the LIJV followed by the femoral veins.
Use of landmark techniques for puncture of the jugular veins may result in significant immediate complications. Puncture of the carotid artery, haematoma, pneumothorax and haemothorax are the commonly reported complications.
Data from insertion of central venous catheters in the non‐dialysis population using the blind landmark technique showed cannulation failure rates between 7% and 19.4% and the peri‐operative complications between 0.2% and 35.4% (Rosen 1992). Use of ultrasound guidance has been shown to reduce catheterisation related morbidity (successful cannulation 100%, first pass 91% versus 38%, and carotid artery puncture 1.7% versus 38%) when compared with the blind technique (Denys 1990). The National Institute for Clinical Excellence (NICE 2002) and the Renal Association (UK) (Renal Association (UK) 2007) both recommend the use of 2‐dimensional (2‐D) ultrasound imaging for central venous catheterisation. This review assessed the potential advantages of the use of ultrasound imaging for the insertion of haemodialysis catheters when compared to blind insertion.
Objectives
The aim of the review was to compare the use of 2‐D ultrasound venous imaging in the insertion of percutaneous central venous catheters for dialysis versus the traditional "blind" landmark method.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) evaluating ultrasound guidance in the percutaneous insertion of central venous catheters for dialysis (both cuffed and uncuffed) against the traditional "blind" landmark method.
Types of participants
Inclusion criteria
We included all studies that involved adults or children requiring haemodialysis via a percutaneous catheter, and published in any language.
Exclusion criteria
Patients requiring central venous access for non‐dialysis indications (e.g. central venous pressure monitoring, drug administration, total parenteral nutrition, and management of peri‐operative fluids in intensive care and operating theatres) were excluded. We also excluded studies using audio doppler ultrasound techniques.
Types of interventions
Use of real‐time 2‐D Doppler ultrasound guidance (referred to as real‐time ultrasound guidance or simply ultrasound guidance in the subsequent sections and figures in the review) for the placement of percutaneous haemodialysis catheters.
Types of outcome measures
Failure to place catheters: overall and on first attempt
Attempts/cannulation: number of catheters inserted in the first attempt, number of attempts/catheter insertion
Time/cannulation: minutes/catheter insertion
Carotid artery puncture, central vein perforation, neck haematoma: number of catheter insertions associated with these complications
Radiological and clinical evidence of pneumo‐ or haemothorax: number of catheter insertions associated with pneumo‐ haemothorax
Number of patients with brachial plexus injury
Search methods for identification of studies
Cochrane Renal Group's specialised register
MEDLINE (1950 to July 2010)
EMBASE (1980 to July 2010)
Cochrane Central Register of Controlled Trials ‐ CENTRAL (in The Cochrane Library ‐ up to the most recent issue)
Authors of studies identified as potentially eligible for inclusion were contacted both to clarify missing data or methodological details and to ask for additional published or unpublished studies.
Studies presented in conference proceedings were included. No additional search strategy to identify these was used.
Reference lists of previous reviews (including systematic reviews) and previous studies were included
Papers in languages other than English were planned to be included and translation facilities within the Cochrane Collaboration were used when needed.
See Appendix 1 for search terms.
A final search was performed by the Cochrane Renal Group in January 2011 using the modified search strategies in Appendix 2
Data collection and analysis
Selection of studies
All titles and abstracts were independently assessed by two authors. Full papers were obtained for those studies that might fulfil the inclusion criteria. Two authors independently assessed these studies to determine if they fulfilled the inclusion criteria. Disagreement were resolved by discussion or if necessary the decision of a third author.
Data extraction and management
Two authors extracted data independently. Data extraction was done using the Cochrane Renal Group prescribed data extraction form. Discrepancies were resolved by discussion with a third author.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (seeAppendix 3).
Was there adequate sequence generation?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the study?
Were incomplete outcome data adequately addressed?
Are reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous data (number of catheters successfully inserted, number of catheters inserted in first attempt, number of patients with complications) the risk ratio (RR) was used with 95% confidence intervals (CI). For continuous data (attempts/catheter insertion, amount of time/catheter insertion), the mean difference (MD) with 95% CI was used.
Dealing with missing data
Letters were to be sent to authors to clarify missing or unclear data however no addition data were obtained.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
if sufficient RCTs were found we planned to analysis for publication bias will be made using the funnel plot method (Higgins 2011). There were insufficient studies to identified to do this.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model will also be analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis were to be undertaken according to type of catheter inserted (tunnelled and temporary haemodialysis catheters) and type of patients (adult and children). There were insufficient studies identified to do this.
Results
Description of studies
Results of the search
The combined search identified 2616 articles, of which 2598 articles were excluded initially. Full‐text assessment of 18 potentially eligible reports identified seven eligible studies (Bansal 2005; Ibrik 2000; Korogolu 2006; Kumwenda 2003; Nadig 1998; Prabhu 2010; Zafar‐Khan 1995) with a total of 787 patients and 830 catheter insertions (see Figure 1).
1.
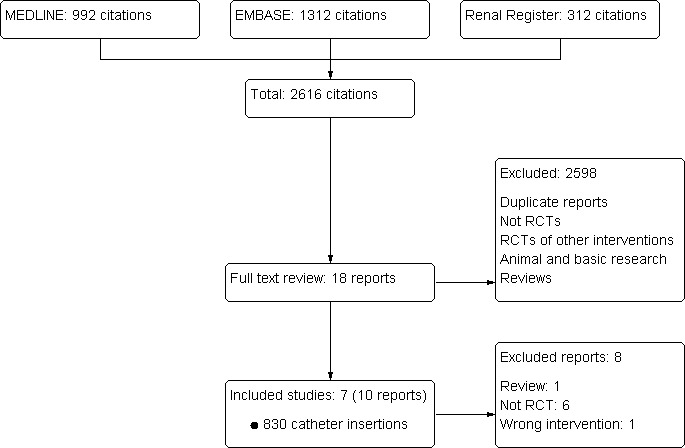
Study flow diagram illustrating the process from literature searching to study selection
Included studies
In all seven studies the experimental intervention consisted of inserting the haemodialysis catheter using real‐time ultrasound guidance. Only one study (Ibrik 2000) included all three major central veins (i.e. femoral, subclavian and internal jugular) and in Prabhu 2010 all catheters were placed in the femoral vein. The internal jugular vein was chosen for catheter insertion in the five remaining studies. Ibrik 2000 and Korogolu 2006 included both tunnelled and temporary haemodialysis catheter insertions. Kumwenda 2003 included only tunnelled haemodialysis catheter placements. In Nadig 1998 the control intervention was insertion of catheter after making a skin mark corresponding to the internal jugular vein using ultrasound scanning to identify the course of the vein. In all the other studies the catheter was inserted “blindly” using the known anatomical landmarks corresponding to the internal jugular vein.
Excluded studies
Major reasons for exclusion were: duplicate references (1); non‐RCTs (2); RCTs of other interventions not stated in the inclusion criteria (3); and animal and basic research studies (4).
Risk of bias in included studies
We have presented the risk of bias assessment in the Characteristics of included studies table, including commentary about each of the domains. An overall assessment of the risk of bias of included studies is displayed in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomised sequence generation was adequate in three studies (Korogolu 2006; Nadig 1998; Prabhu 2010) and unclear in all other studies. None of the studies described how the randomisation was assigned.
Blinding
Due to the nature of the investigation we did not expect blinding of participants and investigators. None of the studies reported blinding of outcome assessors.
Incomplete outcome data
Of the outcomes of interest in this review one study reported six outcomes that could be meta‐analysed (Nadig 1998), two reported five outcomes (Bansal 2005; Prabhu 2010), three reported four outcomes (Ibrik 2000; Korogolu 2006; Kumwenda 2003) and one study reported one outcome (Zafar‐Khan 1995).
Selective reporting
None of the included patients in any of the studies were lost to follow‐up.
Other potential sources of bias
All the studies analysed the results on an intention‐to‐treat basis.
Effects of interventions
For the purpose of this review, real‐time ultrasound guided catheter insertion is considered the treatment intervention and other forms of localising the central vein for catheter insertion are considered the control intervention.
Catheter placement failure (overall)
Real‐time ultrasound guidance was found to significantly reduce the overall risk of catheter placement failure compared to the landmark method (Analysis 1.1 (7 studies, 830 catheters): RR 0.11, 95% CI 0.03 to 0.35). There was no significant heterogeneity between the studies (Chi² = 1.31, P = 0.86, I² = 0%).
1.1. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 1 Failed catheter placement (overall).
Catheter placement failure (first attempt)
Real‐time ultrasound guidance was found to significantly reduce the risk of catheter placement failure on the first attempt compared to the landmark method (Analysis 1.2 (5 studies, 595 catheters): RR 0.40, 95% CI 0.30 to 0.52). There was no significant heterogeneity between the studies (Chi² = 4.26, P = 0.37, I² = 6%).
1.2. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 2 Failed catheter placement (first attempt).
Attempts/catheter insertion
Only Prabhu 2010 reported this outcome in a format that could be meta‐analysed. Ultrasound guidance was associated with significantly less attempts/catheter placement (Analysis 1.3 (1 study, 110 catheters): MD ‐0.35, 95% CI ‐0.54 to ‐0.16). In Nadig 1998 there were 100 attempts for 37 catheter insertions (2.7 attempts/catheter inserted) in the control group compared to 40 attempts for 36 catheter insertion (1.11 attempts/catheter inserted) in the real‐time ultrasound guidance group. In Korogolu 2006 there were 99 attempts for the insertion of 40 catheters (2.47 attempts/catheter inserted) in the blind insertion group compared to 44 attempts for the insertion of 40 catheters in the real‐time ultrasound guidance group (1.1 attempts/catheter inserted). In Zafar‐Khan 1995 there were 3.5 attempts/successful catheter insertion in the blind method group compared to 1.5 attempts/successful catheter insertion in the real‐time ultrasound group.
1.3. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 3 Attempts/catheter insertion.
Time taken for venous cannulation (from skin anaesthesia to successful puncture of the vein)
Nadig 1998 reported significantly less time required for successful vein puncture from the time the skin was anaesthetised with real‐time ultrasound guidance (Analysis 1.4 (1 study, 73 catheters): MD –1.40 min, 95% CI ‐2.17 to –0.63).
1.4. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 4 Time for cannulation (anaesthesia to vein puncture).
Complications
Arterial puncture
Real‐time ultrasound guidance significantly reduced the risk of carotid artery puncture (Analysis 1.5 (6 studies, 785 catheters): RR 0.22, 95% CI 0.06 to 0.81). Heterogeneity measures were moderately significant (Chi² = 7.99, P = 0.09, I² = 50%). Kumwenda 2003 was responsible for the heterogeneity, which was the only study where catheters inserted using real‐time ultrasound were associated with higher number of arterial punctures compared to the control group (4/125 versus 3/125). When this study was removed from the analysis the results favour placing catheters using real time ultrasound guidance (5 studies, 535 catheters, RR 0.13, 95% CI 0.04 to 0.37) and heterogeneity was no longer significant (Chi² = 1.24, P = 0.74, I² = 0%).
1.5. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 5 Arterial puncture.
Haematoma
Real‐time ultrasound guidance was associated with a significantly reduced risk of haematoma (Analysis 1.6 (4 studies, 323 catheters): RR 0.27, 95% CI 0.08 to 0.88). Heterogeneity was not significant (Chi² = 1.12, P = 0.57, I² = 0%).
1.6. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 6 Haematoma.
Pneumo‐ or haemothorax
There was no difference between either patient groups for the risk for pneumo‐ or haemothorax (Analysis 1.7 (5 studies, 675 catheters): RR 0.23, 95% CI 0.04 to 1.38). Heterogeneity was not significant (Chi² = 0.09, P = 0.96, I² = 0%)
1.7. Analysis.

Comparison 1 Ultrasound guided versus "blind" haemodialysis catheter insertion, Outcome 7 Pneumothorax or haemothorax.
None of the studies assessed the number of catheter insertions associated with central vein perforation or brachial plexus injury. We did not have a sufficient number of studies to perform a funnel plot analysis for publication bias or subgroup analyses.
Discussion
Real‐time ultrasound guidance has been found to have a number of clinically important benefits by significantly:
reducing the risk of failed catheter placement overall and also on first‐attempt;
reducing the time needed for successful vein puncture;
reducing the risk of arterial punctures and haematoma formation.
Several studies have previously shown the advantages of ultrasound guidance in central venous cannula placements, i.e. non‐haemodialysis central venous catheters (Denys 1990; Hind 2003). The literature search done to inform the NICE report on the use of ultrasound for central venous catheter placements (2002) identified 20 RCTs (6 using audio doppler, 13 using 2‐D Doppler and one using both) comparing ultrasound versus traditional landmark methods for central venous catheter insertion (NICE 2002). This review found that the use of ultrasound techniques significantly reduced the risk of failed catheter placements ( 9 studies, RR 0.16, 95% CI 0.09 to 0.90), risk of failure of catheter insertion at first attempt (4 studies, RR 0.59, 95% CI 0.39 to 0.88) and risk of any complications (7 studies, RR 0.36, 95% CI 0.17 to 0.76). These findings are similar to the results from this systematic review.
Although the evidence for the use of ultrasound guidance for central venous catheter insertion has been evaluated thoroughly in the NICE 2002 report no such review of evidence had been conducted so far for the use of ultrasound in the setting of haemodialysis catheter insertion. The strength of this analysis is that this is a comprehensive systematic review of RCTs assessing the efficacy of the use of real‐time ultrasound guidance for the insertion of haemodialysis catheters. We had rigid inclusion criteria of including RCTs alone and have used a very comprehensive search strategy of all major medical electronic databases and other sources.
The major limitation of this review has been the small number of studies identified and the fact that in seven studies there was only a total of 830 catheter insertions. Despite this, the meta‐analysis of these studies has managed to highlight several advantages for real‐time ultrasound guidance as described in the beginning of this section. Three of the studies (Ibrik 2000; Kumwenda 2003; Zafar‐Khan 1995) were reported only in an abstract form. It was therefore difficult to obtain details on patient characteristics from these studies.
Both the National Institute for Health and Clinical Excellence (NICE 2002) and the Renal Association (UK) (Renal Association (UK) 2007) recommend the use of ultrasound guidance for central venous catheterisation. The NKF‐KDOQI guidelines recommends the use of real‐time Doppler guidance for the placement of all tunnelled catheters and for the placement of internal jugular vein nontunnelled catheters (NKF‐KDOQI 2000). The findings from our systematic review add further support to these recommendations
Authors' conclusions
Implications for practice.
The results from this review support the use of real‐time doppler ultrasound guidance for the insertion of haemodialysis central venous catheters.
Implications for research.
The use of doppler ultrasound guidance in the setting of femoral vein dialysis catheter insertion needs to be explored further as only one study included in the review looked the use of ultrasound guidance for femoral vein catheter insertions.
What's new
| Date | Event | Description |
|---|---|---|
| 22 November 2011 | Amended | correction of spelling |
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Author's electronic search strategies
| Database | Search terms |
| CENTRAL via Ovid |
|
| MEDLINE via Ovid |
|
| EMBASE via Ovid |
|
Appendix 2. Cochrane Renal Group's modified electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE via Ovid |
|
| EMBASE via Ovid |
|
Appendix 3. Risk of bias assessment tool
| Potential sources of bias | Assessment criteria |
| Was there adequate sequence generation? | Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Was allocation adequately concealed? | Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk of bias: No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. |
| High risk of bias: No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Were incomplete outcome data adequately addressed? | Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Are reports of the study free of suggestion of selective outcome reporting? | Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Was the study apparently free of other problems that could put it at a risk of bias? | Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. |
Data and analyses
Comparison 1. Ultrasound guided versus "blind" haemodialysis catheter insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failed catheter placement (overall) | 7 | 830 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.03, 0.35] |
| 2 Failed catheter placement (first attempt) | 5 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.52] |
| 3 Attempts/catheter insertion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Time for cannulation (anaesthesia to vein puncture) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Arterial puncture | 6 | 785 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.81] |
| 6 Haematoma | 4 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.88] |
| 7 Pneumothorax or haemothorax | 5 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bansal 2005.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Low risk | All patients accounted for |
| Other bias | Low risk | ITT analysis performed |
Ibrik 2000.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, all patients accounted for |
| Other bias | Low risk | ITT analysis performed |
Korogolu 2006.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Low risk | All patients accounted for |
| Other bias | Low risk | ITT analysis performed |
Kumwenda 2003.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, all patients accounted for |
| Other bias | Unclear risk | ITT analysis performed |
Nadig 1998.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using lots |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Low risk | All patients accounted for |
| Other bias | Low risk | ITT analysis performed |
Prabhu 2010.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'computer‐generated randomisation chart' |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/7 outcomes relevant to this review were reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Low risk | All patients accounted for |
| Other bias | Low risk | ITT analysis performed |
Zafar‐Khan 1995.
| Methods |
|
|
| Participants | Inclusion criteria
Exclusion criteria: NS |
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or investigators however this was not expected |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only, 1/7 outcomes reported and could be meta‐analysed |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, all patients accounted for |
| Other bias | Low risk | ITT analysis performed |
HD ‐ haemodialysis; ITT ‐ intention‐to‐treat analysis; NS ‐ not stated; USS ‐ ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altman 1994 | Intervention not relevant to review |
| Conz 1997 | Non‐randomised study |
| Docktor 1999 | Non‐randomised study |
| Ewing 2002 | Non‐randomised study |
| Farrell 1997 | Non‐randomised study |
| Kumwenda 1997 | Non‐randomised study |
| Kwon 1997 | Non‐randomised study |
| Muhm 2002 | Review article |
Contributions of authors
KSR: Screening of titles and abstracts for inclusion, risk of assessment, data extraction, data entry, data analysis, writing of review
RS: Risk of bias assessment, data extraction, data entry, data analysis, writing of review
EK: Screening of titles and abstracts for inclusion
EV: Arbitration with regards to study and data inclusion, data analysis, writing of review
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Bansal 2005 {published data only}
- Bansal R, Agarwal SK, Tiwari SC, Dash SC. A prospective randomized study to compare ultrasound‐guided with non‐ultrasound guided double lumen internal jugular catheter insertion as a temporary hemodialysis access. Renal Failure 2005;27(5):561‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ibrik 2000 {published data only}
- Ibrik O, Samon Guasch R, Roca Tey R, Viladoms Guerra J. Ultrasound‐guided cannulation versus the landmark‐guided technique for hemodialysis vascular access [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association. European Kidney Research Organisation; 2000 Sept 17‐20; Nice, France. 2000:270.
- Ibrik O, Samon Guasch R, Roca Tey R, Viladoms Guerra J. Venous cannulation of large veins for hemodialysis vascular access study of 212 catheters [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association. European Kidney Research Organisation; 2000 Sept 17‐20; Nice, France. 2000:270.
Korogolu 2006 {published data only}
- Koroglu M, Demir M, Koroglu BK, Sezer MT, Akhan O, Yildiz H, et al. Percutaneous placement of central venous catheters: comparing the anatomical landmark method with the radiologically guided technique for central venous catheterization through the internal jugular vein in emergent hemodialysis patients. Acta Radiologica 2006;47(1):43‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumwenda 2003 {published data only}
- Kumwenda M. A randomised study to compare the success rate and complications of the landmark and ultrasound guide techniques of the insertion of tunnelled haemodialysis catheters in patients with end‐stage renal failure [abstract]. Journal of American Society of Nephrology 2003;14(Nov):767A. [Google Scholar]
Nadig 1998 {published data only}
- Nadig C, Leidig M, Schmiedeke T, Hoffken B. The use of ultrasound for the placement of dialysis catheters. Nephrology Dialysis Transplantation 1998;13(4):978‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nadig C, Leidig M, Schmiedeke T, Hoffken B. The use of ultrasound for the placement of dialysis catheters [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):168A. [DOI] [PubMed] [Google Scholar]
Prabhu 2010 {published data only}
- Prabhu MV, Juneja D, Gopal PB, Sathyanarayanan M, Subhramanyam S, Gandhe S, et al. Ultrasound‐guided femoral dialysis access placement: a single‐center randomized trial. Clinical Journal of the American Society of Nephrology ‐ CJASN 2010;5(2):235‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu MV, Juneja D, Gopal PB, Subhramanyam S, Sathyanarayanan M, Gandhe S, et al. Role of ultrasonography in insertion of femoral dialysis catheters: a single‐centre prospective randomized trial [abstract no: SA538]. World Congress of Nephrology; 2009 May 22‐26; Milan Italy. 2009.
Zafar‐Khan 1995 {published data only}
- Zafar‐Khan F, Largoza MV, Hannani A, Lee J, Ahmed Z. Use of ultrasound in the placement of hemodialysis catheters: a comparison [abstract]. Journal of the American Society of Nephrology 1995;6(3):506. [Google Scholar]
References to studies excluded from this review
Altman 1994 {published data only}
- Altman SD, Epstein ML, Beasley RE, Shane RW, Jamnadas P, Work J. Ultrasound guided angioplasty of hemodialysis access stenosis [abstract]. Journal of the American Society of Nephrology 1994;5(3):406. [Google Scholar]
Conz 1997 {published data only}
- Conz PA, Dissegna D, Rodighiero MP, La Greca G. Cannulation of the internal jugular vein: Comparison of the classic Seldinger technique and an ultrasound guided method. Journal of Nephrology 1997;10(6):311‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Docktor 1999 {published data only}
- Docktor BL, Sadler DJ, Gray RR, Saliken JC, So CB. Radiologic placement of tunneled central catheters: Rates of success and of immediate complications in a large series. American Journal of Roentgenology 1999;173(2):457‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ewing 2002 {published data only}
- Ewing F, Patel D, Petherick A, Winney R, McBride K. Radiological placement of the AshSplit haemodialysis catheter: A prospective analysis of outcome and complications. Nephrology Dialysis Transplantation 2002;17(4):614‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farrell 1997 {published data only}
- Farrell J, Gellens M. Ultrasound‐guided cannulation versus the landmark‐guided technique for acute haemodialysis access. Nephrology Dialysis Transplantation 1997;12(6):1234‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumwenda 1997 {published data only}
- Kumwenda M. Two different techniques and outcomes for insertion of long‐term tunnelled haemodialysis catheters. Nephrology Dialysis Transplantation 1997;12(5):1013‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwon 1997 {published data only}
- Kwon TH, Kim YL, Cho DK. Ultrasound‐guided cannulation of the femoral vein for acute haemodialysis access. Nephrology Dialysis Transplantation 1997;12(5):1009‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muhm 2002 {published data only}
- Muhm M. Ultrasound guided central venous access. BMJ 2002;325(7377):1373‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Denys 1990
- Denys BS, Uretsky BB, Reddy FS. Ultrasound assisted cannulation of the internal jugular veins. 63rd Scientific Session of American Heart Association; 1990 November; Dallas (USA). 1990.
DOPPS 2008
- Dialysis Outcome and Practice Outcome Study. www.dopps.org (accessed 22 August 2011).
Gordon 1998
- Gordon AC, Saliken JC, Johns D, Owen R, Gray RR. US‐guided puncture of the internal jugular vein: complications and anatomic considerations. Journal of Vascular & Interventional Radiology 1998;9(2):854‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from www.cochrane‐handbook.org.
Hind 2003
- Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, Thomas S. Ultrasonic locating devices for central venous cannulation: meta‐analysis. BMJ 2003;327(7411):361‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2002
- The National Institute for Clinical Excellence (UK). Central venous catheters ‐ ultrasound locating devices: Guidance. http://www.nice.org.uk/nicemedia/pdf/Ultrasound_49_GUIDANCE.pdf (accessed 22 August 2011).
NKF‐KDOQI 2000
- NKF‐KDOQI. NKF‐KDOQI Vascular Access Guidelines. http://www.kidney.org/professionals/kdoqi/guidelines_updates/doqiupva_i.html#doqiupva5 (accessed 22 August 2011).
Renal Association (UK) 2007
- Mactier R. Renal Association‐ Clinical Practice Guidelines ‐ Haemodialysis. http://www.renal.org/guidelines/module3a.html#VascularAccess (accessed 22 August 2011).
Renal Association (UK) 2008
- Renal Association. www.renal.org (accessed 22 August 2011).
Rosen 1992
- Rosen M, Latto P, Ng S. Percutaneous central venous catheterisation. 2nd Edition. London: WB Saunders, 1992. [Google Scholar]
USRDS 2008
- United States Renal Data System (USRDS). www.usrds.org (accessed 22 August 2011).
References to other published versions of this review
Vaux 2009
- Vaux EC, Shail R, Rabindranath KS. Ultrasound use for the placement of haemodialysis catheters. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005279.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


