Abstract
Research begins with a problem that must be translated into an answerable research question. The research question is a structured interrogative statement based on an unsolved problem, which the researcher tries to answer through the study. The art of articulating a good research question is a crucial part of the research process as it sets the stage for the rest. A critical challenge in biomedical research lies in the frequent shortcomings of research question formulation and presentation. This paper aims to provide a practical guide to assist researchers in formulating answerable and worth-answering research questions in biomedical research. The PICOT/PECOT strategy (addresses population, intervention/exposure, comparator, outcome, and time) is essential to develop an excellent relational quantitative research question. The formulated research question should pass the FINER (feasible, interesting, novel, ethical, and relevant) criteria, determining the worth-answering aspect of the research question. Research questions are presented as questions, hypotheses, and propositions.
Keywords: Biomedical research, Research problem, Research question
Introduction
Research always begins with a problem.1 The problem must be narrowed down to a research question that can be answered.2 This question is a structured interrogative statement3 and a more precise and detailed expression of the statement of the problem.4 The research question is what the authors want to know5 and try to answer it by conducting the study.6-8 The main objective of research is to provide precise and unbiased answers to important questions,5 and new knowledge comes from answerable questions.9 Thus, good research depends on good questions.10 John Ruskin said, “That you should be able to ask a question clearly, is two-thirds of the way to getting it answered.” This quote emphasizes the importance of having a clear research question.
Having a clear research question is crucial because it helps in promoting clarity of thought,11 retrieving relevant information,6,12,13 determining research methodology,3,11,14,15 setting the stage for design, measurement model, and analysis,16-18 and affecting the length, cost, and external validity (generalizability) of the study.19 Additionally, having a clear research question allows one to generate hypotheses and present clear objectives,6 ultimately determining the study’s course.17 If the research question is poorly formulated, it can result in an unfocused project.20 This can lead to subsequent efforts in research methodology, data collection and analyses, and writing going to waste.11 Moreover, researchers may adopt an erroneous design14 or conduct an unethical study.17
Despite the pivotal role of a well-articulated research question as the cornerstone of the research process21,22 and a crucial step in ensuring research rigor,5 research questions are often not articulated and presented effectively in biomedical research. For instance, approximately 65% of research questions posed in papers in the rehabilitation literature did not clearly indicate what the researcher wanted to know.5 Similarly, only 44% of papers published in quality-of-life journals had adequately framed questions.16 A systematic review of papers published in anesthesia journals revealed that 96% of papers failed to adopt appropriate criteria to frame a good research question; the lack of a stated time frame and the failure to identify a comparator were the primary reasons for the research questions not meeting appropriate criteria.14 This paper aims to fill this gap by providing a practical guide to assist researchers in formulating answerable research questions in biomedical research.
Translation of the research problem to the research question
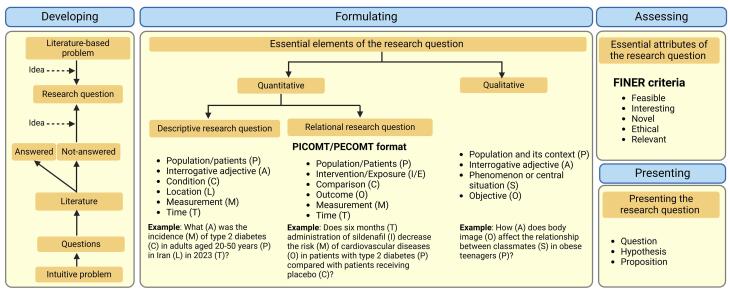
The process of developing a research question usually begins by having potential ideas for addressing a particular problem.6 Research problems are primarily raised by a critical review of existing literature (i.e., literature-driven approach); however, insightful observations or clinical experiences can also spark novel research inquiries (i.e., intuition-driven approach).1 The idea, a feat of association, is a new combination of old elements that aims to bring together different aspects of nature using a process called cross-fertilization or bisociation.23 To transform an idea into a research question, discuss it with your team, develop it further, write down the question, and refine it in conceptual and practical (methodological) ways.24 This way generally proceeds from a broad problem area to developing a specific question amenable to empirical inquiry,10 a process called narrowing the research question.25 One of the key steps in the process of developing a research question is conducting a thorough literature review,25,26 which will provide you with a solid foundation for your research.27 Prior knowledge of a given topic determines the type of question asked; background questions (fact-finding questions) are general questions already answered in the literature and asked by those relatively inexperienced in a field.7,12 Foreground questions, which need a much greater level of prior knowledge, are asked by those who have more experience in a field; these questions arise when one is already aware of alternative choices of action but needs to decide which one to adopt (e.g., two drugs for treatment a disease).7,12 The success of a research project depends on the ability of researchers to translate a problem into research questions5 (Figure 1).
Figure 1.
Developing, formulating, assessing, and presenting the research question. An intuitive research problem may raise several questions, which may have been answered previously (background questions) or still need to be answered (foreground questions) and are transformed into the research question. Quantitative (descriptive and relational) and qualitative research questions are formulated to include essential elements and then subjected to the FINER criteria to determine questions worth answering. The final research question can be presented as a question, hypothesis, or proposition. Created with Biorender.com
Elements of a research question
The first step in evidence-based practice is transforming practice-based information needs into structured questions, a process known as formulating a research question that requires knowing what we need to know.12 Depending on the research objective (specific outcome expected by the researcher), questions can take on a quantitative or qualitative nature.28 With a quantitative research question, researchers want to describe, explain, and predict the phenomenon under the study29 and seek the magnitude of difference in an outcome between groups or the strength of the association between variables.30,31 Quantitative research questions may be descriptive or relational/analytical/inferential.6,28 Description questions, also known as classification questions,6 include an interrogative adjective (e.g., what), measurement (e.g., prevalence, incidence), a condition (e.g., diabetes), population (e.g., Iranian adults), location/place (Tehran), and time (e.g., 2024)28 (Figure 1).
PICOT/PECOT framework describes essential elements of a research question in relational questions posed in analytical observational and interventional studies.8 The first framework for formulating a clear foreground question was suggested by Richardson et al. as PICO or PECO to address Patients/Population, Intervention/Exposure, Comparison, and Outcome.32 PICO has then undergone some variations; for example, in 2005, Fineout-Overholt and Johnston proposed adding timeframe (PICOT)33 as an assessment of outcome is done over a specified time that is chosen based on evidence (e.g., clinical consideration).14 It has also been proposed that measurement (M) should be added to this strategy (PICOMT) to include what researchers want to determine (e.g., the effect of an intervention, the risk of an outcome, or characteristics of a diagnostic factor).28 To see other versions of the original PICO, see Davies.13 According to the clinical context (etiology/causality, diagnosis, intervention, and prognosis), different versions of the PICO/PECO format can be applied.28 Nevertheless, for formulating a good and explicit research question in interventional studies, PICOT criteria is a good option,16 particularly for the study that wants to answer a question about evidence to guide practice, such as effectiveness (effect in actual conditions) studies,5 i.e., RCTs.34 In analytical observational studies, PECOT is used to replace “I” with “E” (exposure)16 (Figure 1).
With a qualitative research question, researchers want to describe and interpret a phenomenon that is not appropriate to quantify.28,35 In qualitative research questions that do not conform to the PICO strategy, researchers propose a central question started with an interrogative adjective (which, how, what, rather than why) that has three components: population and its context, the phenomenon or situation, and what they what to find about the phenomenon (the objective of the study).28
Figure 1 presents examples of quantitative (descriptive and relational) and qualitative research questions for type 2 diabetes and obesity as research problems. If we consider alcohol use disorder as a research problem, a quantitative descriptive question would be: What [A] are the most prevalent socioeconomic factors associated with alcohol use disorder [C] using a self-report questionnaire [M] among the adult population [P] in East Asian countries [L] during last decade [T]? A relational quantitative research question is: Does a 6-month [T] cognitive behavioral therapy [I] decrease frequency [M] of alcohol drinking [O] in alcohol-dependent adolescents [P] compared to opioid antagonists[C]? Finally, a qualitative research question is: How [A] do alcohol-dependent adults [P] describe the role of their social networks (family, friends, communities)[O] on experience of sustained abstinence of alcohol [S]? Interested readers are referred to excellent classification and examples of research questions provided by Canon and Buitrago-Gomez.28
Population
Apopulation is a group of persons for whom knowledge is required,5 e.g., middle-aged adults between 45 and 65 with stage 1 hypertension.36 Defining the population determines who is excluded from or included in the study and, thus, to whom the outcome can be applied.19 By determining exclusion and inclusion criteria, the research question affects the external validity (generalizability) of the study.19 Identifying the patient population is a difficult aspect of articulating the research question; if a restricted group is selected, potential sources of bias and variability are limited but at the expense of external validity.11,22 If a broader population is selected, the results are more generalizable, but it increases bias and decreases internal validity (integrity).11,22 Thus, defining the study population affects the study’s internal and external validity.19 Internal validity is the degree to which the study draws correct conclusions from the findings, and external validity is the degree to which the study’s conclusions are applied outside the study.37 Since the purpose of research is to draw inferences from the findings of the study about the nature of the universe around it,37 it is desirable to pose a more generalizable question, but as a general rule, “do not sacrifice internal validity for generalizability” and keep in mind that an internally invalid study should not be done.9 The sample should be representative of that population to allow the generalization of the study findings to the target population.38
Intervention/ exposure
Intervention is a particular predictor variable that investigators manipulate.37 In the clinical setting, intervention may be a pharmacological treatment11; however, all interventions are not pharmacologic and can be preventive, educational, or nutritional.28 Intervention should be defined in detail to allow others to reproduce the study.19 In analytical observational studies, exposure is considered instead of intervention.11
Comparator
In the clinical setting, thecomparator may be the accepted standard therapy,11 a placebo (placebo-controlled study), an alternative intervention (head-to-head study), or an alternative patient population (e.g., the same intervention in young and old patients).19 In epidemiological studies, non-exposed subjects are used as the comparator.11
Outcome
Thestudy’s outcome should be clearly defined because it affects statistical analysis and sample size.11,25 In the case of clinical trials, it is recommended that researchers select one primary outcome and avoid multiple outcomes or composite outcomes, which is a combination of multiple primary outcomes (e.g., respiratory adverse events).25 Secondary outcomes can be preserved to document potential side effects and the safety of the intervention.25 A good primary outcome should be objective, valid, reliable, easily quantifiable, specific, sensitive, and straightforward.14 An optimal research question should be clear about the relation between exposure and outcome.16
Worth-answering research question: FINER criteria
Not all research questions raised and formulated deserve answers.6,25 If the question posed in a scientific study is feasible, interesting, novel, ethical, and relevant, criteria collectively known as FINER, it is worth answering.6,22 Investigators must review the literature to determine whether the research question fulfills these criteria.6,19,27 FINER criteria define the essential attributes of a research question8 (Figure 1).
Feasibility
The research question should be limited to one that can be solved with the resources at hand,34 that is, researchers being practically able to implement the planned study.8 Feasibility means that the research question is within the researcher’s ability6,8,39 considering technical expertise,6,8 budget,6,8,25 time,6,8 and the possibility of recruiting participants.8,25,36 Some strategies for achieving success with the feasibility of a study include conducting a pilot study,8,14 consulting with experts,14 collaborating or conducting multicenter studies,8,14 and choosing common outcomes.14
Interesting
The research question should primarily interest investigators,8,25 stakeholders, and the scientific community.14,25 Nonetheless, being interesting is subjective and depends on novelty.6,25
Novel
The research question should be novel and address a knowledge gap.8,19 To be novel, the study should provide findings that advance or improve the field.25,36,40 If the research question were not novel, it would not interest peers.6 When previous studies are replicated to resolve a controversy, some improvements (e.g., increasing sample size or the follow-up period) are needed.36
Ethical
Ethical aspects of the study should be considered at the early stages of the research question formulation.11 To do this, authors must be familiar with research ethics guidelines, including the Nuremberg Code41 and the Declaration of Helsinki,42 which are cornerstones for conducting ethical biomedical research,41 and also get Research Ethics approval before starting research.14 Research should be ethical, considering the protection of human and animal subjects in data collection, data storage, and reporting of the results.36 In human studies, the cardinal ethical principle governing research is human dignity, and research should be based on respect for autonomy (e.g., informed consent, privacy), beneficence (maximizing benefits), non-maleficence (minimizing harm), and justice.25,43
Relevant
The research question should be relevant to scientific knowledge, decision-making, practice, or future directions to guide further research.8,39 The Research question is the main part of a research project that reflects research relevancy.39,44 The research question is relevant if answering the question has the potential to improve the decision-making of practitioners or solve a problem in a given field.44 Relevance addresses whether the question is significant/important enough to be worth asking and whether the study findings can affect what others in the field think or do.39 Importance in the clinical setting means that the answer to the question improves patient care.11 Relevancy also refers to practical implications, methodological advances, and theory building.26 Practical relevance means how new knowledge obtained by the study contributes to the practice in that field.45 A relevant question addresses a critical issue and may change practice or influence policy.36
Presenting a research question
Research questions are presented as questions, hypotheses, and propositions.46 All research has a question, whereas a hypothesis is required for studies addressing relational questions.6 Thus, all experimental and analytical observational studies address at least one hypothesis.10,47 Purely descriptive studies such as case reports and case series or even cross-sectional studies (e.g., the prevalence of diabetes in a population) do not involve tests of statistical significance and do not require a hypothesis.21,37,48 Systematic reviews and meta-analyses also do not have hypotheses.4 A systematic review starts with a question to be answered by review.36 In two types of genetic association studies, i.e., candidate gene association and genome-wide association, the former is hypothesis-driven, but the latter has no previous hypothesis.49 In quantitative research, hypotheses are stated at the beginning of the study and then tested by hypothesis testing. In contrast, qualitative studies generate new hypotheses for future testing because they observe relationships in the natural setting rather than hypothesize them.48
A single research question can suggest several hypotheses.48 Hypotheses are the classic, scientific way of formulating a research question,46 enabling researchers to make evidence-based predictions based on previous knowledge.48 A hypothesis, as a version of the research question, also provides a basis for testing the statistical significance of the findings,19,48 which is the heart of the scientific method and indicates relationships between variables, which are directional or non-direction and causal or correlational.46 The difference between the hypothesis and the research question is that the latter presents an idea, but the former aims to answer the research question.15
Like hypotheses, propositions are another version of the research question. They are declarative and testable factual statements about what research expects to find.46 However, propositions are useful formats of research questions when an uncharted territory is investigated in which evidence does not provide a well-established set of theories for proposing a hypothesis; thus, propositions are mainly based on inspiration rather than established knowledge.46
Conclusion
The research question is a structured and worth-answering interrogative statementbased on an unsolved problem, which the researcher tries to answer through the study. The art of articulating a good research question is the most critical part of the research process. Developing a research question starts with ideas for solving a problem and refining it through a comprehensive literature review. Quantitative (descriptive and relational) and qualitative research questions are formulated depending on the study objective. Population, intervention/exposure, comparator, outcome, and time (PICOT/PECOT strategy) help formulate a relational quantitative research question. The formulated research question is then subjected to the FINER (feasible, interesting, novel, ethical, and relevant) criteria to determine questions worth answering.
Research questions are presented as questions, hypotheses, and propositions. In experimental and analytical observational studies, the research question is more appropriately stated as a hypothesis, a declarative sentence that predicts the expected answer to the research question. Hypotheses are the classic, scientific way of formulating a research question. A hypothesis also provides a basis for testing the statistical significance of the findings, which is the heart of the scientific method. In summary, the research question should be clear, well-structured, novel, relevant, and ethical to be worth conducting a research project.
Citation: Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Biomedical research: formulating a well-built and worth-answering research question. Addict Health. 2025;17:1564. doi:10.34172/ahj.1564
Funding Statement
None.
Footnotes
Competing Interests
None declared.
Ethical Approval
Not applicable.
References
- 1. Bahadoran Z, Mirmiran P, Ghasemi A. Biomedical research: The research problem matters. Addict Health 2025; Forthcoming.
- 2.Bahcekapili E, Bahcekapili T, Fis Erümit S, Göktas Y, Sözbilir M. The factors affecting definition of research problems in educational technology researches. Educ Sci Theory Pract. 2013;13(4):2330–5. [Google Scholar]
- 3.Onwuegbuzie AJ, Leech NL. Linking research questions to mixed methods data analysis procedures. Qual Rep. 2006;11(3):474–98. [Google Scholar]
- 4.McGaghie WC, Bordage G, Shea JA. Problem statement, conceptual framework, and research question. Acad Med. 2001;76(9):923–4. [Google Scholar]
- 5.Mayo NE, Asano M, Barbic SP. When is a research question not a research question? J Rehabil Med. 2013;45(6):513–8. doi: 10.2340/16501977-1150. [DOI] [PubMed] [Google Scholar]
- 6.Tully MP. Research: articulating questions, generating hypotheses, and choosing study designs. Can J Hosp Pharm. 2014;67(1):31–4. doi: 10.4212/cjhp.v67i1.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31(1):47–50. doi: 10.4103/0253-7184.69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanan S, Parameswaran N. FINER criteria–what does it mean? CosmoDerma. 2022;2:115. doi: 10.25259/csdm_123_2022. [DOI] [Google Scholar]
- 9.Brian Haynes R. Forming research questions. J Clin Epidemiol. 2006;59(9):881–6. doi: 10.1016/j.jclinepi.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polit DF, Beck CT. Nursing Research: Principles and Methods. Lippincott Williams & Wilkins; 2004.
- 11.Stone P. Deciding upon and refining a research question. Palliat Med. 2002;16(3):265–7. doi: 10.1191/0269216302pm562xx. [DOI] [PubMed] [Google Scholar]
- 12.Booth A. Clear and present questions: formulating questions for evidence-based practice. Libr Hi Tech. 2006;24(3):355–68. doi: 10.1108/07378830610692127. [DOI] [Google Scholar]
- 13.Davies KS. Formulating the evidence-based practice question: a review of the frameworks for LIS professionals Evid. Based Libr Inf Pract. 2011;6(2):75–80. doi: 10.18438/b8ws5n. [DOI] [Google Scholar]
- 14.Thabane L, Thomas T, Ye C, Paul J. Posing the research question: not so simple. Can J Anaesth. 2009;56(1):71–9. doi: 10.1007/s12630-008-9007-4. [DOI] [PubMed] [Google Scholar]
- 15.Parathasarathy S, Samantaray A, Jain D. A well-formulated research question: the foundation stone of good research. Indian J Anaesth. 2023;67(4):326–7. doi: 10.4103/ija.ija_226_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayo NE, Ow N, Asano M, Askari S, Barclay R, Figueiredo S, et al. Reducing research wastage by starting off on the right foot: optimally framing the research question. Qual Life Res. 2022;31(10):2889–99. doi: 10.1007/s11136-022-03117-y. [DOI] [PubMed] [Google Scholar]
- 17.Barroga E, Matanguihan GJ. A practical guide to writing quantitative and qualitative research questions and hypotheses in scholarly articles. J Korean Med Sci. 2022;37(16):e121. doi: 10.3346/jkms.2022.37.e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahan BC, Morris TP, Cro S. We must let the research question drive study methods. BMJ. 2024;384:q173. doi: 10.1136/bmj.q173. [DOI] [PubMed] [Google Scholar]
- 19. Batty LM, Lording T, Ek ET. How to get started: from idea to research question. In: Musahl V, Karlsson J, Hirschmann MT, Ayeni OR, Marx RG, Koh JL, et al, eds. Basic Methods Handbook for Clinical Orthopaedic Research: A Practical Guide and Case Based Research Approach. Berlin, Heidelberg: Springer; 2019. p. 57-63. 10.1007/978-3-662-58254-1_7. [DOI]
- 20.Doody O, Bailey ME. Setting a research question, aim and objective. Nurse Res. 2016;23(4):19–23. doi: 10.7748/nr.23.4.19.s5. [DOI] [PubMed] [Google Scholar]
- 21.Lipowski EE. Developing great research questions. Am J Health Syst Pharm. 2008;65(17):1667–70. doi: 10.2146/ajhp070276. [DOI] [PubMed] [Google Scholar]
- 22.Farrugia P, Petrisor BA, Farrokhyar F, Bhandari M. Practical tips for surgical research: Research questions, hypotheses and objectives. Can J Surg. 2010;53(4):278–81. [PMC free article] [PubMed] [Google Scholar]
- 23. Foster J. How to Get Ideas. Berrett-Koehler Publishers; 2007.
- 24.Clouse RE. Proposing a good research question: a simple formula for success. Gastrointest Endosc. 2005;61(2):279–80. doi: 10.1016/s0016-5107(04)02579-9. [DOI] [PubMed] [Google Scholar]
- 25.Fandino W. Formulating a good research question: pearls and pitfalls. Indian J Anaesth. 2019;63(8):611–6. doi: 10.4103/ija.IJA_198_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bordage G, Dawson B. Experimental study design and grant writing in eight steps and 28 questions. Med Educ. 2003;37(4):376–85. doi: 10.1046/j.1365-2923.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 27.Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Scientific publishing in biomedicine: information literacy. Int J Endocrinol Metab. 2022;20(3):e128701. doi: 10.5812/ijem-128701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cañón M, Buitrago-Gómez Q. The research question in clinical practice: a guideline for its formulation. Rev Colomb Psiquiatr (Engl Ed) 2018;47(3):193–200. doi: 10.1016/j.rcp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Watson R. Quantitative research. Nurs Stand. 2015;29(31):44–8. doi: 10.7748/ns.29.31.44.e8681. [DOI] [PubMed] [Google Scholar]
- 30.Peterson SJ, Foley S. Clinician’s guide to understanding effect size, alpha level, power, and sample size. Nutr Clin Pract. 2021;36(3):598–605. doi: 10.1002/ncp.10674. [DOI] [PubMed] [Google Scholar]
- 31. Browner WS. Publishing and Presenting Clinical Research. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
- 32.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–3. [PubMed] [Google Scholar]
- 33.Fineout-Overholt E, Johnston L. Teaching EBP: asking searchable, answerable clinical questions. Worldviews Evid Based Nurs. 2005;2(3):157–60. doi: 10.1111/j.1741-6787.2005.00032.x. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbroucke JP, Pearce N. From ideas to studies: how to get ideas and sharpen them into research questions. Clin Epidemiol. 2018;10:253–64. doi: 10.2147/clep.S142940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorne S. Data analysis in qualitative research. Evid Based Nurs. 2000;3(3):68–70. doi: 10.1136/ebn.3.3.68. [DOI] [Google Scholar]
- 36.Capili B. How does research start? Am J Nurs. 2020;120(10):41–4. doi: 10.1097/01.NAJ.0000718644.96765.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hulley SB, Newman TB, Cummings SR. Getting started: the anatomy and physiology of clinical research. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB, eds. Designing Clinical Research. 4th ed. China: Lippincott Williams & Wilkins; 2013. p. 2-13.
- 38.Röhrig B, du Prel JB, Blettner M. Study design in medical research: part 2 of a series on the evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(11):184–9. doi: 10.3238/arztebl.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pangaro L, McGaghie WC. Relevance. Acad Med. 2001;76(9):927–9. [PubMed] [Google Scholar]
- 40.Kram JJ, Sullivan Vedder L, Fay B, Simpson D. A clear, succinct research question portends the rest of the story. J Patient Cent Res Rev. 2023;10(4):198–200. doi: 10.17294/2330-0698.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuster E. Fifty years later: the significance of the Nuremberg code. N Engl J Med. 1997;337(20):1436–40. doi: 10.1056/nejm199711133372006. [DOI] [PubMed] [Google Scholar]
- 42.Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall R, McKnight D, Cox R, Coonan T. Guidelines on the ethics of clinical research in anesthesia. Can J Anaesth. 2011;58(12):1115–24. doi: 10.1007/s12630-011-9596-1. [DOI] [PubMed] [Google Scholar]
- 44.Toffel MW. Enhancing the practical relevance of research. Prod Oper Manag. 2016;25(9):1493–505. doi: 10.1111/poms.12558. [DOI] [Google Scholar]
- 45.Ringsted C, Hodges B, Scherpbier A. ‘The research compass’: an introduction to research in medical education: AMEE Guide no 56. Med Teach. 2011;33(9):695–709. doi: 10.3109/0142159x.2011.595436. [DOI] [PubMed] [Google Scholar]
- 46. Denscombe M. Research Proposals: A Practical Guide: A Practical Guide. 2nd ed. McGraw-Hill Education (UK); 2020.
- 47.Fisher WE. Abstract writing. J Surg Res. 2005;128(2):162–4. doi: 10.1016/j.jss.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 48. Fraenkel JR, Wallen NE, Hyun HH. How to Design and Evaluate Research in Education. 11th ed. New York: McGraw-Hill; 2023.
- 49.Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, et al. How to use an article about genetic association: A: Background concepts. JAMA. 2009;301(1):74–81. doi: 10.1001/jama.2008.901. [DOI] [PubMed] [Google Scholar]