Abstract
Background
BTZ-043 is a promising novel drug candidate for antituberculosis treatment. This study aimed to apply a previously developed mouse-to-human translational modeling platform for antituberculosis drugs to predict phase 2A outcomes for BTZ-043 in humans and evaluate the impact of observed drug-drug interactions on the contribution of BTZ-043 to combotherapy in a mouse model.
Methods
The study utilized data from mouse experiments for BTZ-043 monotherapy and combotherapy with bedaquiline, pretomanid, and linezolid, and clinical information for BTZ-043 monotherapy. The translational models were applied to predict the colony-forming units as a measure of efficacy in humans treated with BTZ-043 monotherapy and evaluate the effect of BTZ-043 on the pharmacokinetics-pharmacodynamics of combotherapy bedaquiline, pretomanid, and linezolid.
Results
The mouse-pharmacokinetic and mouse-pharmacodynamic data for BTZ-043 monotherapy were best described by 2-compartmental and direct Emax models, respectively. The model-based prediction of efficacy in humans was comparable to the observed phase 2A efficacy. Single-compartmental models, developed separately, best described the mouse-pharmacokinetic data for bedaquiline, pretomanid, and linezolid in combotherapy. Coadministration with BTZ-043 was associated with at least a 2-fold reduction in bedaquiline, pretomanid, and linezolid exposures in mice, and model-based simulations suggested that the observed decreases in exposure to these drugs would have resulted in even lower efficacy than what was observed when bedaquiline, pretomanid, and linezolid are coadministered with BTZ-043.
Conclusions
The translational modeling platform adequately predicted the efficacy of BTZ-043 monotherapy. In the absence of drug-drug interactions, coadministration of BTZ-043 with bedaquiline, pretomanid, and linezolid in combotherapy is predicted to improve treatment efficacy.
Clinical Trials Registration. NCT0404400.
Keywords: translational PKPD, phase 2A prediction, early bacterial activity, drug interactions, tuberculosis
The previously developed translational modeling platform for antituberculosis drugs adequately predicted phase 2A efficacy outcomes for BTZ-043. In the absence of drug-drug interactions, BTZ-043 increases efficacy outcomes when coadministered with a combination regimen of bedaquiline, pretomanid, and linezolid.
Tuberculosis is caused by Mycobacterium tuberculosis and has remained among the leading global public health challenges for more than 40 years [1, 2]. An estimated global total of 10.6 million new tuberculosis cases and 1.3 million tuberculosis-associated deaths were reported in 2022 [2]. While there has been great progress toward the treatment of both drug-susceptible and drug-resistant forms of tuberculosis, the treatment duration and side effects remain challenging while treating patients with drug-resistant tuberculosis [3, 4]. Recent clinical trials have demonstrated that bedaquiline, pretomanid, and linezolid as a combotherapy (BPaL) regimen is effective for the treatment of drug-resistant tuberculosis within 6 months [5, 6]. Further studies have shown that combining or modifying currently established antituberculosis drug regimens, such as the BPaL regimen, with other promising compounds that have novel targets in diverse bacterial functions can enhance antibacterial activity [7].
Benzothiazinones are among the promising novel drug candidates being explored in optimizing antituberculosis drug regimens. Benzothiazinones have a high potency against both drug-susceptible and drug-resistant strains of M. tuberculosis as they inhibit decaprenylphosphoryl-D-ribose oxidase (DprE1), which is essential for cell wall biosynthesis in M. tuberculosis, by covalently binding to this enzyme [8]. DprE1 is targeted by 4 drug candidates currently being evaluated in clinical trials, including benzothiazinones PBTZ169 and BTZ-043, azaindole TBA-7371, and the carbostyril drug, quabodepistat [8–11]. It has been shown that benzothiazinones have high treatment efficacy compared to other DprE1 inhibitors in mouse models, especially during the second month [12, 13]. BTZ-043 has a minimum inhibitory concentration (MIC) of <0.01 mg/L [8], and is currently under evaluation in multiple combinations in phase 2 clinical trials.
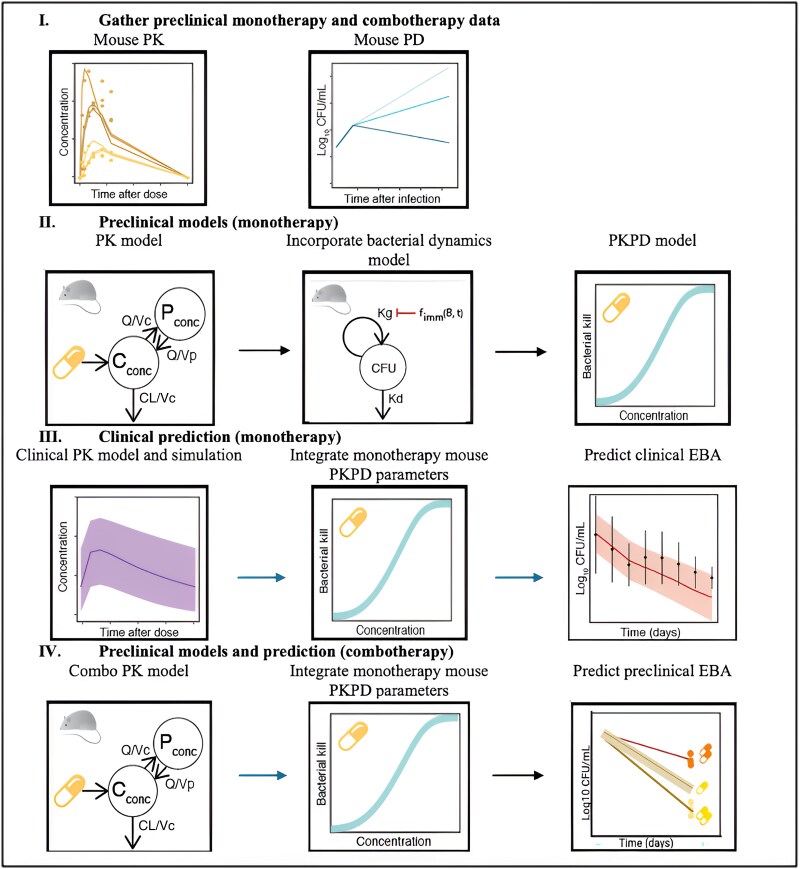
The Food and Drug Administration guidance on the development of drugs to treat pulmonary tuberculosis recognizes animal models in bridging between preclinical and clinical drug development, and investigation of the antimycobacterial effect of the drug [14]. Previously, a robust, mechanistic, mouse-to-human pharmacokinetic-pharmacodynamic (PKPD) model-based translational platform was developed and validated [15]. The translational modeling platform created a link from preclinical to clinical drug development and has been used to predict the early bactericidal activity (EBA) of several drugs in phase 2A clinical trials [15]. The workflow in Figure 1 shows how the translational modeling platform was applied to predict phase 2A EBA in terms of colony-forming units (CFU) for BTZ-043. Furthermore, we aimed to evaluate the PKPD properties of each drug in the BPaL regimen when coadministered with BTZ-043 (BPaLT).
Figure 1.
Concept framework and workflow. The figure shows the modeling workflow that was used on BTZ-043 preclinical and clinical modeling, which consisted of 4 major parts: (I) compiling monotherapy and combotherapy data from preclinical studies; (II) developing a mouse-PKPD model to relate concentration to the decline in CFU as the response of interest for efficacy; (III) simulating clinical PK profiles based on a published clinical model for BTZ-043 monotherapy and integrating with mouse-PD established in this work to predict clinical EBA; and finally (IV) developing combotherapy mouse-PK models and integrating with previously published monotherapy mouse-PKPD models for companion drugs in the BPaL regimen to predict preclinical EBA at adjusted doses of companion drugs in the BPaL regimen and make comparison with observed data. Abbreviations: BPaL, bedaquiline, pretomanid, linezolid; Cconc, central compartment drug concentration; CFU, colony-forming unit; EBA, early bactericidal activity; fimm, immunological function; Kd, bacterial death rate; Kg, bacterial growth rate; Pconc, peripheral compartment drug concentration; PD, pharmacodynamic; PK, pharmacokinetic; Q, intercompartmental clearance; Vc, central compartment volume of distribution; Vp, peripheral compartment volume of distribution.
METHODS
Sources of Data
Monotherapy Mouse-PK and Mouse-PD Data
Mouse-PK data were obtained from plasma samples of healthy BALB/c mice dosed orally by gavage for 5 days with BTZ-043 at doses ranging from 2.5 to 250 mg/kg/day to reach a steady state (Aurigon Toxicological Research Center, Ltd; ATRC 832.220.5501) [16], and from M. tuberculosis H37Rv-infected mice after 4 weeks of treatment (Johns Hopkins University Center for Tuberculosis Research; JHU2d2). On day 5 and day 26, respectively, plasma samples were collected (n = 3 to 5) predose (representing 24-hour value) and up to 8 hours postdosing for bioanalytical analysis. Mouse-PD data were generated from murine experiments conducted at JHU (Tyagi 170922) and at the Research Center Borstel, Germany (RCB; KW115) using female BALB/c mice [16]. Mice were aerosol-infected with M. tuberculosis H37Rv and the number of implanted bacteria in the lungs was determined the following day. After 3 weeks, mice (n = 3 to 5) were sacrificed to determine pulmonary bacterial burden at the start of treatment. The remaining animals were dosed orally by gavage with BTZ-043 doses ranging from 2.5 to 1000 mg/kg [16]. In the Tyagi 170922 experiment, drugs were administered 5 days per week, either once or twice daily. For all twice-daily dosing, the time between administrations was 7 to 8 hours. After 6 weeks of treatment, mice (n = 3 to 4) per treatment group were sacrificed to assess efficacy. In the KW115 experiment, drugs were administered 5 days per week in once-daily doses, and the mice (n = 5 to 6) were sacrificed after 4, 6, and 8 weeks of treatment to assess efficacy. Further description of the experimental designs and processing of biological samples is available in the earlier publication [16].
Combotherapy Mouse-PK and Mouse-PD Data
Data were obtained from experiments in infected female BALB/c mice conducted at JHU (JHU2d and JHU2d2). The mice were aerosol-infected with M. tuberculosis H37Rv. Treatment started 2 weeks after infection and the mice were randomized to receive either the BPaL or BPaLT regimen. The mice were administered bedaquiline, pretomanid, and linezolid at doses of 25, 100, and 100 mg/kg once daily, respectively, while the dosage for BTZ-043 was 100 mg/kg twice daily, administered 8 hours apart. Separate bedaquiline and pretomanid formulations were combined on the day of dosing and administered in 1 gavage 2 hours after the first daily dose of BTZ-043. Separate linezolid and BTZ-043 formulations were mixed and administered in 1 gavage with the second daily dose of BTZ-043. All drugs were administered 5 days per week for up to 16 weeks. Mouse-PK data were obtained from plasma samples of mice (n = 3) allocated to each regimen from predose (representing 24-hour value) to 6 hours postdose on the last day of dosing of the fourth week of treatment. Mouse-PD data were obtained from untreated mice sacrificed on the first day after infection (n = 6) and on the day the treatment was initiated (n = 9), and used to determine the lung CFU measurements implanted and present at the start of treatment, respectively. To assess efficacy, treated mice (n = 5) were sacrificed after 4 and 8 weeks of treatment.
Animal ethics approval ATRC 832 and KW115 experiments were approved by the animal research ethics committee of the federal state of Schleswig-Holstein prior to permission by the Ministry of Energy, Agriculture, the Environment, Nature, and Digitalization (Kiel, Germany; permits 3-1/15, 69-6/16, and 84-9/20). For experiments Tyagi 170922, JHU2d, and JHU2d2, all procedures involving the care and use of animals in the study were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee (protocol #MO15M479).
Clinical Data
A phase 1B/2A clinical trial was conducted in South Africa by the University of Munich (NCT0404400) [17]. Participants aged 18 to 64 years (n = 78), with newly diagnosed, uncomplicated, smear-positive, drug-susceptible pulmonary tuberculosis were enrolled in the study. Participants received BTZ-043 doses ranging from 250 to 1750 mg. Plasma PK samples were collected and a BTZ-043 population PK model was developed and validated [18]. Sputum samples were collected from participants at baseline just before treatment initiation and after up to 14 days of treatment with BTZ-043 monotherapy to determine the efficacy. Participants provided written informed consent 154 and the study was approved by ethics committees of the sites in South Africa and the sponsor in 155 Germany and by the South African Health Products Regulatory Agency (SAHPRA; Ref 20190606).
Model Development and Simulation
Mouse-PK and PKPD Model and Drug-Drug Interaction Evaluation
Compartmental PK models were developed to describe mouse-PK data for BTZ-043 monotherapy and combotherapy coadministering BPaL with and without BTZ-043 using single-compartment (Supplementary Eq. 1 and Eq. 2) or multicompartment models (Supplementary Eq. 3 and Eq. 5) with first-order (Supplementary Eq. 6) or nonlinear (Michaelis-Menten) absorption kinetics when appropriate (Supplementary Eq. 7). To assess the PK drug interactions of BTZ-043 with BPaL, coadministration with BTZ-043 was included in the mouse-PK model as a binary covariate. A compartmental PKPD model was developed to describe the exposure-response relationship of BTZ-043 as monotherapy from mouse-PD data. The mouse-PKPD model integrates the mouse-PK model to describe drug exposure, a bacterial dynamics model to account for the mouse immune system without (Supplementary Eq. 9) and during treatment (Supplementary Eq. 10), and described the effect using the Emax model (Supplementary Eq. 11), with delay (Supplementary Eq. 12). The bacterial dynamics model describes bacterial growth before the initiation of treatment based on a previously published baseline model [19]. Additive, proportional, or combination residual error models were tested for the best model fit. Model simulations were performed to predict PK exposure in mice at adjusted doses of bedaquiline, pretomanid, and linezolid in the BPaL regimen. The mouse-PK and PKPD model equations Supplementary Eq. 1 to Eq. 12 are available in the Supplementary Material.
Clinical EBA Prediction
To predict the phase 1B/2A clinical EBA by simulations, the estimated BTZ-043 monotherapy mouse-PD model parameters were integrated with clinical-PK model parameters [18]. Mouse-PD parameters determined by 5 days per week dosing can be translated to daily dosing in humans because the PD parameters are driven by the exposure (PK) and not by the dosing (5 or 7 days per week). Clinical predictions of EBA were based on simulating the exposure-response relationship in 1000 virtual patients at the dosing levels used in the phase 1B/2A clinical trial, ranging from 250 to 1750 mg once daily [17]. The baseline CFU values used in the simulations were derived as the mean baseline CFU from the phase 1B/2A clinical trial [17]. The protein binding ratio between humans and mice () of 0.98 from our internal laboratory findings was used to convert unbound plasma drug concentrations for BTZ-043 from human to mouse to translate the mouse-PKPD relationships. The EBA predictions were simulated at ±25% of 0.98 to evaluate the effects varying on efficacy. The model-based predictions of EBA were compared graphically and numerically with observed EBA from the phase 2A clinical trial to explore the model's predictive performance.
Effect of BTZ-043 on BPaL Efficacy
The previously established mouse-PD monotherapy model parameters for each drug comprising the BPaL regimen [15] were integrated with their respective mouse-PK model parameters in combotherapy to simulate predictions of the change in CFU count over time on treatment based on 1000 virtual mice. To estimate the effect of coadministering BTZ-043 with BPaL, the doses of bedaquiline, pretomanid, and linezolid were altered from the doses in the original experiments, 1 at a time during the first, second, and third scenarios of the simulations, respectively, while holding the doses of companion drugs constant to those used in the original experiment during each scenario. The dose alteration for the drugs comprising the BPaL regimen during simulations was based on the BTZ-043-induced alteration of the exposure to drugs comprising the BPaL regimen. The predicted CFU decline in each scenario was combined and compared to the observed CFU change for the BPaLT regimen.
Software and Statistical Considerations
All model development and validation were performed using NONMEM (version 7.5.1) [20], Perl speaks NONMEM (PsN, 5.4.0) [21], and R (version 4.4.1) [22] statistical program packages were utilized for model diagnostics and data visualization. The mouse-PK and PKPD model parameters were estimated using the first-order conditional estimation with interaction method. The best models were selected based on numerical statistics utilizing the change in objective function value (OFV) at a 5% significance level, graphically using the goodness of fit plots and simulation-based diagnostics using the visual predictive checks.
RESULTS
Mouse-PK and PKPD for BTZ-043 Monotherapy
The raw mouse-PK data are presented in Supplementary Figure 2. The final mouse-PK model parameter estimates for BTZ-043 monotherapy are presented in Table 1. A 2-compartmental model with nonlinear absorption kinetics fit by the Michaelis-Menten equation, linear clearance, and the combined proportional and additive error model best described the mouse-PK data for BTZ-043 in mice. The raw mouse-PD data presented in Supplementary Figure 3 showed a dose-response for BTZ-043 in mice. The final mouse-PKPD model parameter estimates for BTZ-043 monotherapy are presented in Table 1. The baseline empirical adjusted estimates of the bacterial growth parameters were 1.27 day−1 and 1.20 day−1 for the Tyagi 170922 and KW115 experiments, respectively. A direct Emax model and additive error model best described the mouse-PD data. The Emax parameter was estimated to be 0.181 day−1, and the EC50 parameter was estimated to be 0.005 mgL−1. The visual predictive checks for the final mouse-PK and mouse-PKPD models showed good fits, as presented in Supplementary Figure 2 and Supplementary Figure 3, respectively.
Table 1.
Mouse-PK and Mouse-PKPD Model Parameters of BTZ-043 Monotherapy, Baseline Bacterial Dynamics Model Parameters, and the Clinical PK Model Parameters of BTZ-043 Monotherapy
| Parameter, Unit | Estimates (RSE %) |
|---|---|
| Monotherapy mouse-PK model | |
| CL/F, Lh−1 | 0.119 (9) |
| Km, mgL−1 | 0.948 (15) |
| Vc/F, L | 0.041 (21) |
| Vmax, mgL−1h−1 | 1.9 (12) |
| Vp/F, L | 0.203 (15) |
| Q/F, Lh−1 | 0.042 (23) |
| IIV F, CV% | 17.4 (26) |
| Additive error, mgL−1 | 0.105 (57) |
| Proportional error, CV% | 14.1 (19) |
| Monotherapy mouse-PKPD model | |
| Emax, d−1 | 0.181 (3) |
| EC50, mgL−1 | 0.005135 (22) |
| Additive error, log CFU lung−1 | 1.416 (5) |
| Baseline bacterial dynamics model [19] | |
| Kg for study 1, d−1 | 1.27a |
| Kg for study 2, d−1 | 1.20a |
| Kd, d−1 | 0.41 |
| KB, % | 20.3 |
| B50, log10 CFU | 7.86 |
| γB | 3.11 |
| KT, % | 70.2 |
| γT | 5 |
| T50, d | 17.4 |
| Monotherapy clinical PK model [18] | |
| CL/F, Lh−1 | 404 |
| Ka, h−1 | 1.69 |
| Vc/F, L | 764 |
| F | 1b |
| MTT | 0.34 |
| ntr | 1 |
| Q/F | 68 |
| Vp/F | 382 |
| IIVCL/F, CV% | 27 |
| IIVV/F, CV% | 43 |
| IIVKa, CV% | 128 |
| IIVF, CV% | 27 |
| EBA prediction | |
| Observed CFU drop day−1, % | 12.7 |
| Predicted CFU drop day−1, % | 11.3 |
Abbreviations: B50, CFU counts to reach half of KB; CL/F, apparent clearance; EC50, concentration to achieve 50% of maximum effect; Emax, maximum effect; F, bioavailability; IIV, interindividual variability; Ka, absorption rate; KB, maximal inhibitory CFU-dependent adaptive immune effect; Kd, bacterial death rate; Kg, bacterial growth rate; Km, Michaelis-Menten constant; KT, maximal inhibitory time-dependent adaptive immune effect; MTT, mean transit time; ntr, number of transit compartments; Q/F, apparent intercompartmental clearance; PKPD, pharmacokinetic-pharmacodynamic; T50, time to reach half of the maximal time covariate; Vc/F, apparent central volume of distribution; Vmax, maximum absorption rate; Vp/F, apparent peripheral volume of distribution; γB, steepness of the CFU-dependent adaptive immune effect curve; γT, steepness of the time-dependent adaptive immune effect curve; CV, coefficient of variation = ; RSE, relative standard error.
aReestimated in the baseline model based on data from KW115 and Tyagi 170922 experiments.
bFixed parameter value.
Clinical EBA Predictions
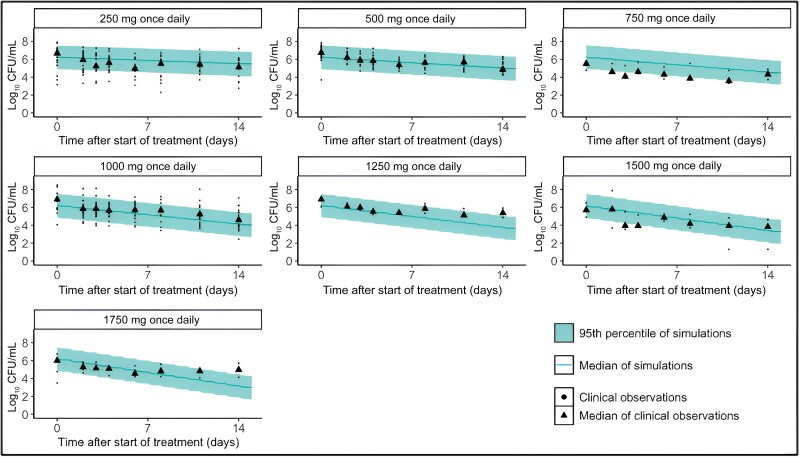
The BTZ-043 model-based phase 2A prediction of CFU adequately predicted the phase 1B/2A observed CFU for BTZ-043 monotherapy as explored graphically in Figure 2. The median of the CFU from model-based predictions was comparable to the median of the observed CFU, and the 95th percentile of prediction adequately overlapped the observed CFU values. The model predictions of CFU were approximately within the range of observed CFU for all doses. The overall model-based predicted CFU decline per day was 11.3%, comparable to the overall observed CFU decline per day of 12.7%, as presented in Table 1. Supplementary Figures 4 and 5 present sensitivity analyses of the impact of changes in the degree of protein binding of ±25% of 0.98, assumed value of the . The CFU predictions in Supplementary Figures 4 and 5 are all comparable to those in Figure 3, hence the change in the will unlikely influence the efficacy of BTZ-043 monotherapy.
Figure 2.
Early bactericidal activity (EBA) prediction. Clinical EBA predictions overlaying median clinical observations over 14 days. The baseline colony-forming units (CFU) of predictions was determined as the median of clinical observations from the same clinical trial.
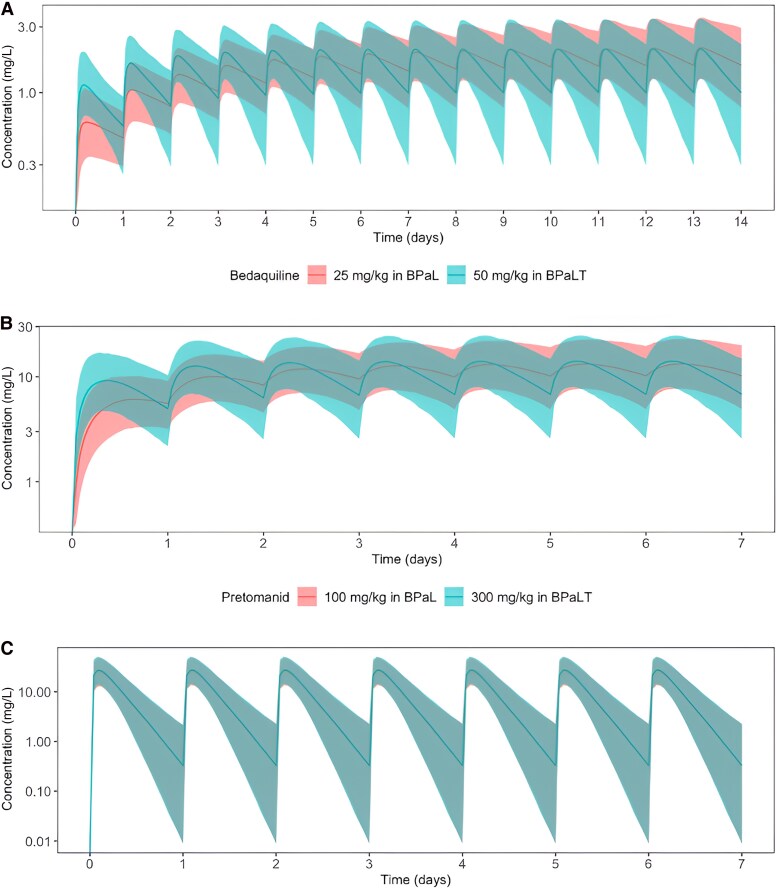
Figure 3.
Effect of adjusting the dose of BPaL drugs when they are coadministered with BTZ-043 in mice. The figure shows the median (solid lines) and 95th percentile (shaded area) of the model-based predictions of steady-state plasma concentrations for bedaquiline (A), pretomanid (B), and linezolid (C) at original experimental doses for these drugs in the BPaL regimen (red) and when dosing levels for bedaquiline only (A), pretomanid only (B), and linezolid only (C) have been adjusted in the BPaLT regimen (green). Abbreviations: BPaL, bedaquiline, pretomanid, linezolid; BPaLT, BPaL plus BTZ-043.
PK Interaction of BTZ-043 With BPaL Regimen
The raw mouse-PK data and the final mouse-PK model parameter estimates for bedaquiline, pretomanid, and linezolid in BPaL combotherapy are presented in Supplementary Figure 1A and Table 2, respectively. A single-compartmental model with linear absorption and clearance best described each drug's mouse-PK data. Coadministration of BPaL with BTZ-043 was identified as an influential covariate associated with a 2.48-fold increase in bedaquiline clearance, a 3.34-fold increase in pretomanid clearance, and a 0.34-fold decrease in linezolid bioavailability parameters based on changes in the OFV at a 5% significance level. Consequently, coadministration with BTZ-043 significantly reduced bedaquiline, pretomanid, and linezolid exposure, as measured by the area under curve (AUC) parameter, by 56%, 69%, and 66%, respectively. Simulations performed to estimate the increases in drug doses needed to compensate for this effect of BTZ-043 coadministration predicted that bedaquiline at 2-fold, pretomanid at 3-fold, and linezolid at 3-fold increases in dose when administered in the BPaLT regimen, would have comparable exposure to when they are administered in the BPaL regimen, as presented in Figure 3.
Table 2.
Mouse-PK of BPaL Drugs in Combination With BTZ-043 and the Respective Monotherapy Mouse-PKPD Model Parameters
| Parameter, Unit | Estimates (RSE%) |
|---|---|
| BPaL mouse-PK model | |
| Bedaquiline | |
| CL/F, Lh−1 | 0.01 (10) |
| Vc/F, L | 0.73 (17) |
| Ka, h−1 | 0.77 (35) |
| F | 1a |
| CLT/F, % | 248 (29) |
| AUCT, % | 56 - |
| Proportional error, CV % | 47 (13) |
| Pretomanid | |
| CL/F, Lh−1 | 0.01 (14) |
| Vc/F, L | 0.11 (32) |
| Ka, h−1 | 0.06 (30) |
| F | 1a |
| CLT/F, % | 334 (19) |
| AUCT, % | 69 - |
| Proportional error, CV % | 58 (15) |
| Linezolid | |
| CL/F, Lh−1 | 0.009 (11) |
| Vc/F, L | 0.013 (30) |
| Ka, h−1 | 0.22 (7) |
| F | 1a |
| FT, % | 34 (14) |
| AUCT, % | 66 - |
| Proportional error, CV % | 47 (20) |
| Monotherapy mouse-PKPD model [15] | |
| Bedaquiline | |
| Emax, d−1 | 0.52 |
| EC50, mgL−1 | 0.23 |
| Pretomanid | |
| Emax, d−1 | 0.43 |
| EC50, mgL−1 | 3.46 |
| γ, d−1 | 0.38 |
| Linezolid | |
| Emax, d−1 | 1.00 |
| EC50, mgL−1 | 2.77 |
| γ, d−1 | 0.21 |
| Kdelay, d−1 | 6.75 |
Abbreviations: AUCT, effect of BTZ-043 on the area under the concentration-time curve; BPaL, bedaquiline, pretomanid, linezolid; CL/F, apparent clearance; CLT/F, effect of BTZ-043 on clearance; EC50, concentration to achieve 50% of maximum effect; Emax, maximum effect; F, bioavailability; FT, effect of BTZ-043 on bioavailability; Ka, absorption rate; Kdelay, delay effect; PKPD, pharmacokinetic-pharmacodynamic; Vc/F, apparent central volume of distribution; γ, sigmoid; ; CV, coefficient of variation = ; ; RSE, relative standard error.
aFixed parameter value.
PD Interaction of BTZ-043 With BPaL Regimen
Coadministration of BTZ-043 was observed to lower the efficacy of the BPaL regimen in mice, as presented in Table 3 (first 2 rows) and Supplementary Figure 1B. After 28 days of treatment, the observed CFU in mice receiving the BPaLT regimen was 75% higher than the observed CFU in mice receiving the BPaL regimen. As presented in Figure 4 and Table 3, the simulated CFU with a 56% decrease in bedaquiline dose from 25 to 11 mg/kg for the BPaL regimen was 36% higher than the observed CFU, the simulated CFU with a 66% decrease in pretomanid dose from 100 to 31 mg/kg for the BPaL regimen was 26% higher than the observed CFU, the simulated CFU with a 66% decrease in linezolid dose from 100 to 34 mg/kg for the BPaL regimen was 17% higher than the observed CFU, and the simulated CFU when the doses of bedaquiline, pretomanid, and linezolid were lowered by 56%, 69%, and 66%, respectively, for the BPaL regimen was 80% higher than the observed CFU. Hence, the increase in CFU prediction for the BPaL regimen after the respective BTZ-043-induced reduction in exposure for each component drug approximated the increase in CFU observed when BTZ-043 was coadministered with BPaL (deviation, 5%).
Table 3.
Pharmacokinetic Drug-Drug Interaction, Here Reflected by Reduced BPaL Dosing, Approximate BPaLT Effect of BTZ-043 on CFU Decline
| Regimen | Day 28 Log10 CFU/Lung | Log10 CFU Increase Versus BPaL Observed (%) |
|---|---|---|
| BPaL, observed | 2.72 | … |
| BPaLT, observed | 4.77 | 2.05 (75) |
| BPaL, simulated 56% lower B dose | 3.70 | 0.98 (36) |
| BPaL, simulated 69% lower Pa dose | 3.44 | 0.72 (26) |
| BPaL, simulated 66% lower L dose | 3.19 | 0.47 (17) |
| BPaL, Additive effect no simulation | 4.89 | 2.17 (80) |
Observed mean log10 CFU/lung decline at day 28 for BPaL and BPaLT regimens in the experiment at the dosing levels of 50 mg/kg for bedaquiline once daily, 100 mg/kg for pretomanid once daily, 100 mg/kg for linezolid once daily, and BTZ-043 100 mg/kg twice daily, and then at simulated mean log10 CFU/lung decline at day 28 for BPaL at reduced doses for component drugs in the BPaL regimen.
Abbreviations: BPaL, bedaquiline, pretomanid, linezolid; BPaLT, bedaquiline, pretomanid, linezolid, BTZ-043; CFU, colony-forming unit.
Figure 4.
Effect of BTZ-043 on BPaL efficacy in mice. The figure shows the observed CFU (circles) and the median of observations (dashed lines) for the BPaL (red) and BPaLT (green) regimens in mice. Additionally, the graph shows the observed median (solid lines) and 95th percentile (shaded area) of the model-based prediction of CFU for BPaL at adjusted dose levels for bedaquiline only (A), pretomanid only (B), and linezolid only (C), in the BPaL regimen. Abbreviations: BPaL, bedaquiline, pretomanid, linezolid; BPaLT, BPaL plus BTZ-043; CFU, colony-forming unit.
DISCUSSION
The study further validates a previously established mouse-to-human translational modeling platform [15]. Using available preclinical and clinical-PK information for BTZ-043, the phase 1B/2A observed sputum CFU counts for BTZ monotherapy were adequately predicted using the translational model-based approach presented in Figure 2. This finding supports prior suggestions that the translational modeling platform can predict phase 2A clinical trial outcomes adequately [15]. Consequently, the cost associated with conducting a clinical trial can be reduced through efficient trial design or, in some cases, avoided completely if predictions show no efficacy during the clinical development of antituberculosis compounds. Both the observed and model-based predictions of CFU after treatment initiation show a highly significant reduction of the bacterial burden. The same result was observed in previous in vitro and in vivo studies of BTZ-043 or PBTZ169, which showed that benzothiazinones hold great promise as new antimycobacterial drugs [11, 13, 23].
With preclinical data to inform PK and PD interactions between drugs, the translational modeling platform can also be used to predict the change in CFU in mouse lungs or human sputum in combination therapy with novel drug regimens [24]. Here, we present the results of an experiment in an established murine model of tuberculosis in which the addition of BTZ-043 to the BPaL regimen had the surprising effect of increasing the lung CFU counts compared to treatment with BPaL alone. An embedded plasma PK analysis revealed significant reductions in exposure for each drug comprising the synergistic BPaL combination upon coadministration with BTZ-043, affirming the value of assessing for PK-related drug-drug interactions in preclinical trials [25, 26]. BTZ-043 lowered exposures for all the drugs in the BPaL regimen by at least 2-fold, in mice. To date, there are no other publications that have reported on either how coadministration with BTZ-043 affects the exposure of companion antituberculosis drugs, or its mechanism of action to explain the results found during modeling. However, preclinical drug-drug interaction studies in cytochrome and transporter models have not shown any indication that BTZ-043 will impact the exposure of other tuberculosis drugs in humans. In addition, interim analyses of phase 2B clinical trials have not shown any significant changes in exposure for BTZ-043 or other tuberculosis drugs during coadministration.
To explore whether the observed PK-related drug-drug interaction was sufficient to explain the observed antagonistic effect of adding BTZ-043 on the efficacy of the BPaL regimen in mice, simulations were performed using the combotherapy mouse-PKPD models to estimate the impact of lowering exposure to component drugs in the BPaL regimen by simulating BPaL efficacy with dose level reductions equivalent to the BTZ-043–induced percentage decline in the AUCs for each respective drug. We found that the simulated BPaL efficacy at reduced doses of the component drugs was approximately 5% lower than the observed efficacy for BPaLT. Thus, the model-based prediction of a decline in CFU predicts that BTZ-043 provides additional efficacy to BPaL without drug-drug interactions. Additional combotherapy studies in the murine models using dose adjustment or timing for BTZ-043 to compensate for the observed drug-drug interactions and a range of BTZ-043 doses are warranted to confirm these predictions and better estimate the potential value of combining BTZ-043 with BPaL and other novel regimens sharing similar component drugs, especially because previous in vitro studies have shown that BTZ-043 improves efficacy when combined with a range of drugs like pyrazinamide, rifampin, isoniazid, ethambutol, bedaquiline, pretomanid, moxifloxacin, meropenem, and SQ-109. Also, other in vitro studies have suggested that PBTZ169, another benzothiazinone, increases efficacy when combined with bedaquiline, pyrazinamide, or a combination of bedaquiline and pyrazinamide [11]. The direction of interactions for BTZ-043 when coadministered with other drugs on both PK and PD likely differs depending on whether it is a 2, 3, or 4 drugs combotherapy.
A potential limitation of this study is that model development relied on sometimes sparse PK data points available throughout the analysis. Indeed, the modeling dataset contained fewer observations but for a larger dose range. Therefore, we were able to statistically significantly quantify (change in OFV = 52; 1 degree of freedom) for example the Michaelis-Menten absorption kinetics, which improved the mouse-PK model fit for BTZ-043 data by accounting for the notable dose-dependent differences in the pattern of the PK profiles (Supplementary Figure 2). Some previous studies have also shown that drug absorption may be dose-dependent, which may be well explained by a saturation absorption process mathematically obeying Michaelis-Menten–type kinetics [27]. Also, while tuberculosis has cavitating lesions, this study utilized data from experiments using BALB/c mice only and lacks investigation related to caseating lung lesions. Recent studies have shown that BTZ-043 accumulates in caseating tuberculosis lesions and efficiently acts against M. tuberculosis in C3HeB/FeJ mice and mice overexpressing interleukin [13, 16], hence the inclusion of data obtained from such mice that are prone to developing these types of lesions [28, 29] could bring more insight into the translational modeling. Currently, efforts are underway to integrate experimental data on different mouse models reflecting various clinical risk phenotypes, to quantify and predict drug (regimen) efficacy in easy- and harder-to-treat patients. In addition, an investigation of metabolite information for companion drugs in the BPaL regimen when coadministered with BTZ-043 could be potentially helpful in establishing and quantifying the source of BTZ-043 interactions. Finally, any potential effects of coadministration of BTZ-043 on the unbound fraction of BPaL component drugs were not considered in the simulations of combination therapy in mice. The potential for such an interaction could be measured in vitro and included in future simulations, although the technical challenge of accurately measuring changes in the unbound fraction of very highly bound drugs like bedaquiline should be acknowledged.
In conclusion, this study explored the various aspects of BTZ-043 using for the first time preclinical to clinical translational PKPD modeling approaches. The model-based translational modeling platform to predict phase 2A clinical trials in human treatment outcomes of antituberculosis drugs was applied to preclinical data and clinical PK information to adequately predict the clinical EBA among tuberculosis patients treated with BTZ-043 monotherapy. BTZ-043 lowers the pharmacokinetic exposure of BPaL drugs in mice and, as a result, has an antagonistic interaction with BPaL in this model. Model-based simulations predict that, in the absence of drug-drug interaction in humans, the efficacy of the BPaL regimen in treating drug-resistant tuberculosis can be increased when coadministered with BTZ-043 as BPaLT.
Supplementary Material
Contributor Information
Bernard Ngara, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, California, USA.
Lorenzo Flori, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, California, USA; Department of Pharmacy, University of Pisa, Pisa, Italy.
Rob Christiaan van Wijk, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, California, USA.
Jacqueline P Ernest, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, California, USA.
Sandeep Tyagi, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Heena Soni, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Christoph Hölscher, Division of Infection Immunology, Research Center Borstel-Leibniz Lung Center, Borstel, Germany; Thematic Translational Unit Tuberculosis, German Center for Infection Research, Partner Site Hamburg-Lübeck-Borstel-Riems, Hamburg, Germany.
Kerstin Walter, Division of Infection Immunology, Research Center Borstel-Leibniz Lung Center, Borstel, Germany; Thematic Translational Unit Tuberculosis, German Center for Infection Research, Partner Site Hamburg-Lübeck-Borstel-Riems, Hamburg, Germany.
Julia Dreisbach, Institute of Infectious Diseases and Tropical Medicine, Ludwig Maximilian University Hospital, Ludwig Maximilian University Munich, Munich, Germany; German Center for Infection Research, partner site Munich, Munich, Germany.
Michael Hoelscher, Institute of Infectious Diseases and Tropical Medicine, Ludwig Maximilian University Hospital, Ludwig Maximilian University Munich, Munich, Germany; German Center for Infection Research, partner site Munich, Munich, Germany; Immunology, Infection and Pandemic Research, Fraunhofer Institute for Translational Medicine and Pharmacology, Munich, Germany; Unit Global Health, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg, Germany.
Eric L Nuermberger, Center for Tuberculosis Research, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Radojka M Savic, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, California, USA; Center for Tuberculosis, University of California, San Francisco, California, USA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. The authors thank Anu Patel who played a peer-mentoring role to the first author; Ann-Kathrin Lemm, Alexandra Hölscher, and Johanna Volz for expert technical assistance in the KW115 experiment; and Hapila GmbH, Gera produces BTZ-043 and provides all analytical standards for KUM and HKI.
Author contributions. B. N. wrote the manuscript and generated the figures and tables. L. F., R. C. W., J. P. E., C. H., K. W., J. D., M. H., E. L. N., and R. M. S. reviewed, commented, and edited the overall manuscript. B. N., L. F., R. C. W., J. P. E., J. D., M. H., E. L. N., and R. M. S. provided substantial scientific context for the study. B. N., L. F., and R. C. W. performed data management and the development of models. J. P. E. performed the code review. S. T., H. S., C. H., K. W., and E. L. N. provided preclinical data. J. D. and M. H. provided clinical data. R. M. S. supervised the whole study.
Financial support. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant number UM1 AI179699 from the Preclinical Design and Clinical Translation of TB Regimens Consortium, support for modeling and simulation work); TB Alliance (support for mouse model experiments conducted at Johns Hopkins University); German Center for Infection Research (support for mouse model experiments at Aurigon Toxicological Research Center and Research Center Borstel). The phase 1B/2A clinical study is part of the European and Developing Countries Clinical Trials Partnership 2 program (grant number TRIA2015-1102) with support from the German Ministry for Education and Research (grant number 01KA1701). Further support was from the German Center for Infection Research InfectControl (grant numbers 03ZZ0803A, 03ZZ0835A, and 03ZZ0826A); the Bavarian Ministry for Science and the Arts; the Swiss State Secretariat for Education, Research, and Innovation, and the Nederlandse Organisatie voor Wetenschappelijk Onderzoek.
References
- 1. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med 2020; 8:19. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global tuberculosis report 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023. Accessed 26 March 2024.
- 3. Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Medicine 2012; 9:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB Treatment–2017, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conradie F, Diacon AH, Ngubane N, et al. Bedaquiline, pretomanid and linezolid for treatment of extensively drug resistant, intolerant or non-responsive multidrug resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022; 387:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black TA, Buchwald UK. The pipeline of new molecules and regimens against drug-resistant tuberculosis. J Clin Tuberc Other Mycobact Dis 2021; 25:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makarov V, Manina G, Mikusova K, et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 2009; 324:801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterji M, Shandil R, Manjunatha MR, et al. 1,4-Azaindole, a potential drug candidate for treatment of tuberculosis. Antimicrob Agents Chemother 2014; 58:5325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariguchi N, Chen X, Hayashi Y, et al. OPC-167832, a novel carbostyril derivative with potent antituberculosis activity as a DprE1 inhibitor. Antimicrob Agents Chemother 2020; 64:e02020-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makarov V, Lechartier B, Zhang M, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med 2014; 6:372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robertson GT, Ramey ME, Massoudi LM, et al. Comparative analysis of pharmacodynamics in the C3HeB/FeJ mouse tuberculosis model for DprE1 inhibitors TBA-7371, PBTZ169, and OPC-167832. Antimicrob Agents Chemother 2021; 65:e0058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramey ME, Kaya F, Bauman AA, et al. Drug distribution and efficacy of the DprE1 inhibitor BTZ-043 in the C3HeB/FeJ mouse tuberculosis model. Antimicrob Agents Chemother 2023; 67:e0059723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pulmonary tuberculosis: developing drugs for treatment guidance for industry, 2022.
- 15. Ernest JP, Goh JJN, Strydom N, et al. Translational predictions of phase 2a first-in-patient efficacy studies for antituberculosis drugs. Eur Respir J 2023; 62:2300165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Römpp A, Treu A, Kokesch-Himmelreich J, et al. The clinical-stage drug BTZ-043 accumulates in murine tuberculosis lesions and efficiently acts against Mycobacterium tuberculosis. Nat Commun. 2025; 16:826. [DOI] [PMC free article] [PubMed]
- 17. Heinrich N, De Jager V, Dreisbach J, et al. BTZ-043 shows good safety and strong bactericidal activity in a combined phase1b/2a study in tuberculosis patients. SSRN, doi: doi 10.2139/ssrn.4601314, 16 October 2023, preprint: not peer reviewed. [DOI]
- 18. Koele S. BTZ-043-PK-PD/BTZ-043 PKPD supplementary files 17052024.docx. https://github.com/SimonKoele/BTZ-043-PK-PD/blame/main/BTZ-043 PKPD supplementary files 17052024.docx. Accessed 19 July 2024.
- 19. Zhang N, Strydom N, Tyagi S, et al. Mechanistic modeling of Mycobacterium tuberculosis infection in murine models for drug and vaccine efficacy studies. Antimicrob Agents Chemother 2020; 64:e01727–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ICON, plc . ICON, plc. NONMEM | Nonlinear mixed effects modelling | ICON plc [Internet]. [cited 11 September 2024]. Available from: https://www.iconplc.com/solutions/technologies/nonmem.
- 21. Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a perl module for NONMEM related programming. Comput Methods Programs Biomed 2004; 75:85–94. [DOI] [PubMed] [Google Scholar]
- 22. R Foundation . R: The R project for statistical computing. https://www.r-project.org/. Accessed 1 October 2024.
- 23. Eckhardt E, Li Y, Mamerow S, et al. Pharmacokinetics and efficacy of the benzothiazinone BTZ-043 against tuberculous mycobacteria inside granulomas in the guinea pig model. Antimicrob Agents Chemother 2023; 67:e01438-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strydom N, Ernest JP, Imperial M, et al. Dose optimization of TBI-223 for enhanced therapeutic benefit compared to linezolid in antituberculosis regimen. Nat Commun 2024; 15:7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuhlmann J. Drug interaction studies during drug development: which, when, how? Int J Clin Pharmacol Ther 1994; 32:305–11. [PubMed] [Google Scholar]
- 26. Srinivas NR. Drug-drug interaction studies in preclinical species: should metabolite(s) kinetics be studied? Xenobiotica 2009; 39:193–6. [DOI] [PubMed] [Google Scholar]
- 27. Wood JH, Thakker KM. Michaelis-Menten absorption kinetics in drugs: examples and implications. Eur J Clin Pharmacol 1982; 23:183–8. [DOI] [PubMed] [Google Scholar]
- 28. Driver ER, Ryan GJ, Hoff DR, et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 2012; 56:3181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irwin SM, Driver E, Lyon E, et al. Presence of multiple lesion types with vastly different microenvironments in C3HeB/FeJ mice following aerosol infection with Mycobacterium tuberculosis. Dis Model Mech 2015; 8:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.