Abstract
Background
Adjunctive host-directed therapies that modulate host immune responses to reduce excessive inflammation and prevent tissue damage in tuberculosis are being investigated. Macrolides, including azithromycin, were shown to possess anti-inflammatory and immune-modulatory effects in addition to their antibacterial effects. In the current trial, we investigated whether azithromycin enhances resolution of systemic and pulmonary inflammation and decreases extracellular matrix-related tissue turnover in tuberculosis patients.
Methods
An open-label, randomized, controlled trial was performed. Adult patients with drug-susceptible, pulmonary tuberculosis aged above 18 years were randomly assigned to receive standard antituberculosis care or azithromycin 250 mg orally once daily in addition to standard care (SOC) for 28 days.
Results
Twenty-eight patients were included within 4 weeks after initiating antituberculosis treatment. Twelve patients in both arms completed the trial. Participants were mostly young, male, had a history of smoking, and had no comorbidities. No differences in baseline characteristics were observed between the study arms. In blood, azithromycin treatment significantly enhanced the reduction of the tuberculosis marker interferon-γ-induced protein-10 (SOC plus azithromycin, −38% vs SOC alone, −24% vs SOC, P < .05) and the collagen type IV degradation product C4M (−26% vs −11%, P < .05). In sputum, treatment with azithromycin significantly reduced neutrophils (−24% vs 0%, P < .001), neutrophil elastase (−88% vs 75%, P < .01), and transforming growth factor-β (−86% vs −68%, P < .05). No significant effects were observed on other parameters. Treatment with azithromycin appeared to be safe.
Conclusions
The addition of azithromycin to standard antituberculosis treatment appears to diminish excess neutrophilic inflammation in patients with pulmonary tuberculosis.
Clinical Trials Registration. NCT03160638.
Keywords: tuberculosis, azithromycin, host-directed therapy, inflammation, extracellular matrix
Addition of azithromycin to standard antituberculosis treatment appears to diminish excess sputum neutrophilic inflammation in patients with pulmonary tuberculosis. Azithromycin could be a potential host-directed therapy for prevention of post-tuberculosis sequelae.
Tuberculosis remains a global concern, causing an estimated 10.8 million new cases and 1.25 million deaths in 2023 [1]. Treatment is focused on eradication of Mycobacterium tuberculosis; however, even after successful treatment approximately half of patients are left with permanent physiological, radiological, and clinical abnormalities [2]. Residual pulmonary impairment after completion of therapy, known as post-tuberculosis lung disease, has a significant impact on quality of life with patients experiencing persistent cough and breathlessness; life expectancy is reduced [3, 4]. This impairment may be explained by lung remodeling, including cavitation, fibrosis, and bronchiectasis [2]. Damage caused by the complex interplay between M. tuberculosis and the host immune response is considered to be key in post-tuberculosis lung disease [5]. Treatment and pharmacological approaches to manage the host-pathogen interaction in post-tuberculosis lung disease are identified as important research areas [6].
After infection with M. tuberculosis, both pro- and anti-inflammatory processes may contribute to disease establishment and progression depending on temporal and microenvironmental circumstances. The immune system plays a crucial role in M. tuberculosis-dependent lung remodeling [5]. Host-directed therapies aim to reduce excessive inflammation and prevent subsequent pathological tissue damage [7]. Several immunomodulatory and inflammatory drugs, including doxycycline, metformin, and everolimus, have been studied as host-directed therapies [8–11]. In other lung diseases, azithromycin decreases production of proinflammatory cytokines, such as interleukin 6 (IL-6), IL-8, tumor necrosis factor-α (TNF-α), and matrix metalloproteases (MMPs), and increases neutrophil apoptosis resulting in reduced inflammation and enhanced tissue repair [12].
Lung inflammation in tuberculosis triggers degradation of the extracellular matrix (ECM) contributing to pulmonary cavitation and tissue injury. Expression of several MMPs is increased in sputum of tuberculosis patients [13]. In particular, MMP-8 appears to play a prominent role in the immunopathology of tuberculosis [14]. MMP-1 has been identified as the primary collagen degrading MMP, and is most likely to be responsible for the destruction of ECM collagens in the lung [15]. In line with these observations, turnover products of collagen III and elastin are increased in tuberculosis [16].
The immunomodulatory effects of azithromycin in tuberculosis are currently unknown. Therefore, we performed a phase 2 randomized, open-label pilot trial to investigate whether the addition of azithromycin to standard of care (SOC) treatment enhances resolution of systemic and pulmonary inflammation and decreases tissue turnover in persons with drug-susceptible pulmonary tuberculosis.
METHODS
Trial Design
This was a prospective, randomized open-label pilot trial of azithromycin as adjunct to first-line antituberculosis treatment in persons with pulmonary tuberculosis at the Tuberculosis Center Beatrixoord, University Medical Center Groningen (UMCG), the Netherlands. This trial was approved by the ethical review board of the UMCG (IRB 2017-404) and registered at ClinicalTrials.gov (NCT03160638). The trial was performed in accordance with the Helsinki Declaration. The trial protocol can be found in the Supplementary Material. Recruitment started on 1 February 2018 and was completed on 21 April 2022, with interruptions due to the coronavirus disease 2019 (COVID-19) pandemic.
Study Participants
Persons were eligible if they had a microbiologically confirmed diagnosis of drug-susceptible pulmonary tuberculosis (by culture and/or molecular test) and provided written informed consent. Persons with a previous diagnosis of tuberculosis, age <18 years, pregnant, or breast feeding, hypersensitivity to macrolides, treatment with macrolides or tetracyclines in previous month, treatment with analgesic/immunosuppressant drugs or digoxin, gastrointestinal complaints, other respiratory diseases, human immunodeficiency virus (HIV)/AIDS, impaired liver function, or corrected QT interval ≥500 ms were excluded.
Study Procedures
As this was a pilot trial, no power calculation was performed. Twenty-four participants were randomly assigned (1:1 ratio), using a randomization list with random permutated blocks of 4, to receive azithromycin 250 mg orally once daily for 28 days in addition to SOC or SOC alone. An azithromycin loading dose of 500 mg was administered on day 1. Participants were randomized within 4 weeks after the start of antituberculosis treatment.
Blood and sputum samples were collected before the start of azithromycin treatment and at day 28 (end of azithromycin treatment). Venous blood samples were collected before administration and 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after administration of antituberculosis medication on day 7, to determine the pharmacokinetic parameters of the antituberculosis drugs.
Sputum was collected using a stepwise approach. Initially, spontaneous sputum was collected according to standard hospital procedures. If this was ineffective, sputum production was facilitated by nebulization of hypertonic (4%) saline using a validated and standardized technique [17]. Two sequential sputum samples were collected. The first sample was treated with dithiothreitol to analyze cell differentials. The second sample was used for analysis of inflammatory cytokines and MMPs. To this aim, samples were diluted with an equal amount of phosphate-buffered saline, centrifuged, and sterile filtered through a 0.22-μm low protein binding Durapore polyvinylidene fluoride membrane (Merck Millipore) to remove M. tuberculosis.
Outcome Measures
Blood Inflammatory Parameters and ECM-Associated Proteins
Blood inflammatory parameters and ECM-associated proteins were analyzed as per protocol, with the exception of interferon-γ-induced protein-10 (IP-10), nordicC4M (degradation fragment of collagen type IV), and nordicPro-C4 (a fragment of the internal 7S domain of collagen type IV), which were added to the panel of analyzed parameters before start of the analyses. Leukocytes, cell differentiation, and C-reactive protein (CRP) were determined by routine clinical laboratory tests. Procalcitonin, cytokines (interferon-γ [IFN-γ], IL-2, IL-6, IL-8, IL-10, TNF-α, and IP-10) and MMPs (MMP-1 and MMP-8) were determined in serum using a multiplex assay (catalog No. LXSAHM-12, R&D systems), according to the manufacturer's instructions. Myeloperoxidase (MPO; catalog No. DY3174, R&D systems), neutrophil elastase (catalog No. DY9167-05, R&D systems), and transforming growth factor-β (TGF-β; catalog No. DY240-05, R&D systems) were analyzed in serum by enzyme-linked immunosorbent assay, according to the manufacturer's instructions.
ECM turnover-related neoepitopes of collagen were assessed in plasma samples at Nordic Bioscience (Herlev, Denmark), see Supplementary Material for the list, as described previously [18]. Elastin fragment generated by human neutrophil elastase (ELA-HNE), C3M, and Pro-C4 were measured by automated chemiluminescence immune analysis in a random-access IDS-i10 device (Immunodiagnostic Systems Holdings).
Sputum Inflammatory Parameters and ECM-Associated Proteins
Processed whole-sputum samples were stained with May-Grünwald Giemsa to obtain cell differentials by counting 600 viable, nonsquamous cells. Sputum results were rejected if the percentage of squamous cells was >80% or if the total number of nonsquamous cells was <600. Analysis for cytokines and MMPs was performed as described above.
Pharmacokinetics
Plasma drug levels of isoniazid, rifampicin, pyrazinamide, and ethambutol were analyzed according to validated methods by the Department of Clinical Pharmacy and Pharmacology, UMCG [19, 20]. Pharmacokinetic parameters for isoniazid, rifampicin, and pyrazinamide (area under the curve 0–24 hours [AUC0–24], volume of distribution, clearance, absorption rate constant, and lag time) were calculated using MwPharm++ version 2.4.0.328 (Mediware) [21–23]. AUC0–24 for ethambutol was calculated using the trapezoid rule.
Susceptibility Testing
Susceptibility testing was performed by the National Institute for Public Health and the Environment (Bilthoven, The Netherlands) using TREK Sensititre MYCOTB and Equin1F plates according to the manufacturer's instructions.
Statistical Analyses
Results of the trial are reported in accordance with the CONSORT guidelines. Statistical evaluations were performed using SPSS, version 28.0.1.0 (IBM). Parameters were summarized as frequency, percentage, and median (interquartile range). Comparisons between the arms were performed using a Mann–Whitney U test or Fisher exact test. Differences were considered statistically significant at P < .05.
RESULTS
Study Design and Participants
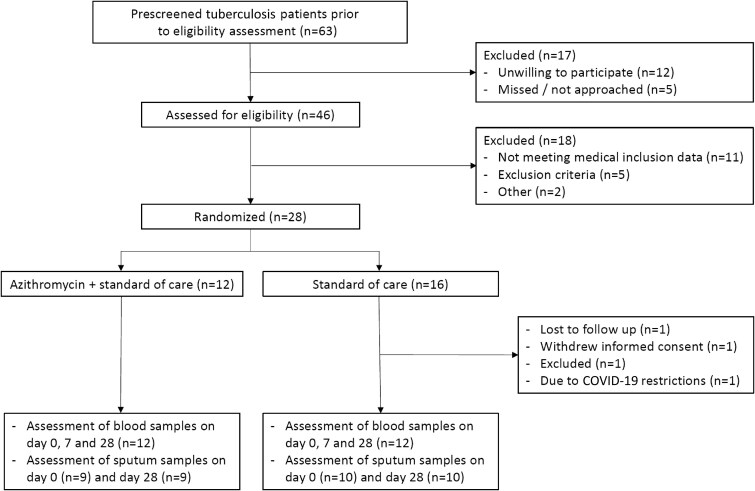
Between February 2018 and April 2022, 63 persons with pulmonary tuberculosis were prescreened of which 46 were assessed for eligibility (Figure 1). After admission, patients were directly started on first-line treatment. As soon as microbiologically confirmed diagnosis of drug-susceptible pulmonary tuberculosis was established, participants were enrolled. Patients were randomly assigned to the azithromycin group or SOC group 13 days (interquartile range [IQR], 9–19 days) after initiating first-line antituberculosis treatment (Table 1). As per protocol, participants that withdrew from the trial were replaced. In the end, 12 participants were allocated to the azithromycin group and 16 to the SOC group. Of the SOC group, 1 participant was withdrawn due to COVID-19 restrictions. This person participated for 21 days and was included in the safety analyses. The other participants that withdrew were included in the SOC group and withdrew for other reasons before any study-related actions were performed. Twelve persons in each group (24 in total) completed the trial and were included in the final analysis.
figure 1.
Flowchart of the selection and enrolment process. Safety analysis included all participants who underwent randomization and received azithromycin or started in the trial (standard of care arm). The end point assessment included all participants who underwent randomization and returned for follow-up on day 28. Blood inflammatory parameters and ECM-associated proteins are reported on day 0 and on day 28. Pharmacokinetic sampling was performed on day 7. Abbreviation: COVID-19, corona virus disease 2019.
Table 1.
Baseline Characteristics
| Characteristic | Total (n = 24) | Standard of Care + Azithromycin (n = 12) | Standard of Care Alone (n = 12) | P a |
|---|---|---|---|---|
| Age, years (IQR) | 33 (21–40) | 32 (21–40) | 33 (20–43) | .84 |
| Sex, male (%) | 23 (96) | 11 (92) | 12 (100) | 1.00b |
| Weight, kg (IQR) | 60.7 (56.6–68.9) | 60.5 (53.3–68.9) | 61.0 (56.8–69.9) | .63 |
| BMI, kg/m2 (IQR) | 20.0 (18.7–21.4) | 19.8 (18.5–21.9) | 20.0 (18.9–21.4) | .63 |
| Smoking, n (%) | 17 (71) | 9 (75) | 8 (67) | 1.00b |
| Alcohol abuse, n (%) | 6 (25) | 2 (17) | 4 (33) | .64b |
| Time between initiating first-line antituberculosis treatment and randomization, days (IQR) | 13 (9–19) | 13 (9–19) | 12 (7–20) | .71 |
| Tuberculosis diagnosis, n (%) | ||||
| Pulmonary | 24 (100) | 12 (100) | 12 (100) | 1.00b |
| Extrapulmonary | 0 (0) | 0 (0) | 0 (0) | |
| Ethnicity, n (%) | ||||
| Black | 13 (54) | 7 (58) | 6 (50) | 1.00b |
| White | 11 (46) | 5 (42) | 6 (50) | |
| Comorbidity, n (%) | ||||
| None reported | 18 (75) | 10 (83) | 8 (67) | .64b |
| Chronic pancreatitis | 2 (8) | 1 (8) | 1 (8) | |
| Other | 4 (17) | 1 (8) | 3 (25) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
aVariables were compared by Mann-Whitney U test unless stated otherwise.
bFisher exact test.
No differences in baseline characteristics were observed between the study groups (Table 1). The majority of the persons with pulmonary tuberculosis were young, male (96%), had a smoking history (71%), and no comorbidities (75%).
Blood Inflammatory Parameters
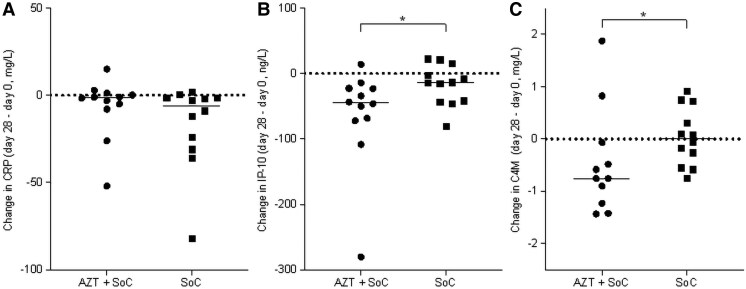
Baseline levels of inflammatory markers in blood were similar between the study arms at baseline (Supplementary Table 1). Most parameters of inflammation improved in both allocation arms, but without differences between the groups (Table 2 and Supplementary Figures 1–3). Specifically, no difference was observed between the groups for CRP (Figure 2A). Only the decrease in IP-10 was significantly larger in patients treated with azithromycin (P < .05; Figure 2B).
Table 2.
Change in Blood Leukocytes, Inflammatory Parameters, and Extracellular Matrix-Associated Proteins
| Parameter | Change Day 28 − Day 0 | P a | |
|---|---|---|---|
| Azithromycin + Standard of Care (n = 12) | Standard of Care (n = 12) | ||
| Hemocytometry | |||
| Leukocytes, × 109/L | −0.25 (−3.08 to 0.70) | −0.75 (−2.58 to 0.15) | .67 |
| Neutrophils, × 109/L | −0.15 (−2.39 to 0.86) | −1.07 (−2.31 to −0.41) | .35 |
| Lymphocytes, × 109/L | 0.00 (−0.26 to 0.51) | 0.12 (−0.13 to 0.33) | .71 |
| Monocytes, × 109/L | −0.02 (−0.20 to 0.06) | −0.11 (−0.18 to 0.07) | .67 |
| Eosinophils, × 109/L | −0.06 (−0.31 to 0.10) | 0.02 (−0.06 to 0.09) | .32 |
| Basophils, × 109/L | 0.00 (−0.03 to 0.01) | −0.01 (−0.01 to 0.01) | .93 |
| Inflammatory mediators | |||
| C-reactive protein, mg/L | −1.5 (−7.3 to 1.0) | −6.0 (−29.3 to −1.6) | .18 |
| Procalcitonin, ng/L | −16.6 (−31.9 to −7.1) | 5.1 (−22.1 to 33.2) | .14 |
| Interferon-γ, ng/L | −17.2 (93.8 to 25.7) | −17.5 (−195.1 to 0.0) | .29 |
| Interferon-γ-induced protein-10, ng/L | −45.1 (−71.3 to −22.8) | −14.1 (−43.4 to −10.3) | .03 |
| Interleukin-2, ng/L | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.0) | 1.00 |
| Interleukin-6, ng/L | −6.8 (−7.4 to −0.5) | −3.8 (−10.8 to 0.0) | .93 |
| Interleukin-8, ng/L | −6.8 (−21.2 to 2.4) | 7.8 (−12.5 to 16.2) | .29 |
| Interleukin-10, ng/L | 0.0 (0.0 to 0.0) | 0.0 (−2.9 to 0.0) | .84 |
| Myeloperoxidase, µg/L | −102.2 (−243.1 to 11.5) | −59.3 (−196.0 to −7.3) | .67 |
| Tumor necrosis factor-α. ng/L | 0.0 (−4.9 to 0.0) | 0.0 (−4.9 to 4.9) | .55 |
| Extracellular matrix-associated proteins | |||
| Matrix metalloprotease-1, µg/L | −1.6 (−3.1 to −0.4) | −1.1 (−2.7 to −0.1) | .51 |
| Matrix metalloprotease-8, µg/L | −9.0 (−15.0 to −1.1) | −5.0 (−17.6 to −1.1) | .80 |
| Neutrophil elastase, mg/L | −0.2 (−1.2 to 0.1) | −0.3 (−1.4 to 0.3) | .89 |
| Transforming growth factor-β, µg/L | −1.2 (4.8 to 1.4) | −0.1 (−3.9 to 1.9) | .55 |
| ELA-HNE, mg/L | 0.0 (−0.2 to 0.3) | 0.0 (−0.2 to 2.0) | .93 |
| C1 M, µg/L | 1.2 (−1.0 to 2.4) | −1.0 (−2.8 to 1.8) | .32 |
| C3 M, µg/L | −107.5 (−264.3 to 59.5) | −32.5 (−119.0 to 12.5) | .21 |
| C4M, mg/L | −0.8 (−1.3 to −0.4) | 0.0 (−0.3 to 0.4) | .04 |
| C6M, µg/L | 1.3 (−1.3 to 3.4) | −0.9 (−3.4 to 2.2) | .71 |
| Pro-C3, µg/L | 0.1 (−2.3 to 2.0) | 0.8 (−1.2 to 7.0) | .48 |
| Pro-C4, mg/L | −10.1 (−29.1 to −1.7) | −3.6 (−13.1 to 8.2) | .17 |
| Pro-C6, µg/L | 0.8 (−0.2 to 3.6) | 1.8 (0.0 to 9.4) | .14 |
Data are median (interquartile range).
Abbreviations: C1M, MMP-generated fragment of collagen type I; C3M, MMP-generated fragment of collagen type III; C4M, MMP-generated fragment of collagen type IV; C6M, MMP-generated fragment of collagen type VI; ELA-HNE, elastin fragment generated by human neutrophil elastase; MMP, matrix metalloproteases; Pro-C3, formation marker of collagen type III; Pro-C4, fragment of internal 7S domain of collagen type IV; Pro-C6, formation marker of collagen type VI.
aChange (day 28 − day 0) was compared by Mann-Whitney U test.
Figure 2.
Change in blood inflammatory parameters in tuberculosis patients treated with azithromycin or standard of care. Change in (A) C-reactive protein (CRP), (B) interferon-γ-induced protein-10 (IP-10), and (C) the collagen type IV degradation product C4M (C4M) blood concentrations in pulmonary tuberculosis patients in the azithromycin plus standard of care group (AZT + SOC) and the SOC arm. Change in parameters was calculated between end of treatment (day 28) and baseline (day 0). Each symbol represents a single participant. *P < .05.
Sputum Inflammatory Parameters
Nine persons in the azithromycin group and 10 persons in the SOC group were able to produce sputum (Supplementary Table 2). In the azithromycin group, sputum was induced in 3 persons at baseline. Of these, 1 could not produce sputum at the end of treatment. For the other 2 participants and 3 additional persons sputum had to be induced at the end of treatment. In the SOC group, sputum needed to be induced in only 1 person at baseline.
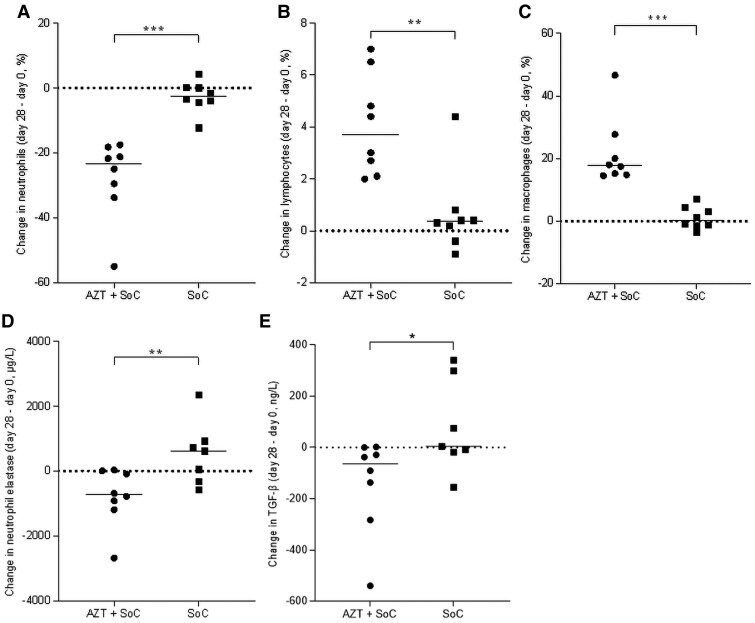
Baseline values of inflammatory cells, cytokines, and chemokines in sputum were comparable between the arms (Supplementary Table 3). There was a considerably larger reduction in sputum neutrophil percentages in the azithromycin group (P < .001; Figure 3); the increases in lymphocyte and macrophage percentages were considered reciprocal for the decrease in neutrophil percentage. Although not statistically significant, similar effects were observed for absolute cell numbers (Supplementary Figure 5). No significant between group differences in inflammatory mediators or markers were seen in sputum (Table 3 and Supplementary Figure 6).
Figure 3.
Change in sputum inflammatory parameters in tuberculosis patients treated with azithromycin or standard of care. Change in (A) neutrophil percentage, (B) lymphocyte percentage, (C) macrophage percentage, (D) neutrophil elastase, and (E) transforming growth factor-β (TGF-β) in sputum of pulmonary tuberculosis patients in the azithromycin plus standard of care group (AZT + SOC) and the SOC arm. Change in parameters was calculated between end of treatment (day 28) and baseline (day 0). Each symbol represents a single participant. *P < .05.
Table 3.
Change in Sputum Leukocytes, Inflammatory Parameters, and Extracellular Matrix-Associated Proteins
| Parameter | Change Day 28 − Day 0 | P a | |
|---|---|---|---|
| Azithromycin + Standard of Care (n = 12) | Standard of Care (n = 12) | ||
| Inflammatory cell differentials | |||
| Neutrophils, % | −23.4 (−32.7 to −19.0) | −2.6 (−4.5 to 0.2) | <.0001 |
| Lymphocytes, % | 3.7 (2.3 to 6.1) | 0.4 (−0.2 to 0.7) | .0002 |
| Macrophages, % | 17.8 (14.9 to 25.8) | 0.2 (−1.5 to 4.1) | <.0001 |
| Eosinophils, % | 1.2 (−0.5 to −2.0) | 0.1 (−0.5 to 0.50) | .38 |
| Basophils, % | 0.3 (0.0 to 0.7) | 0.4 (0.0 to 1.1) | .80 |
| Epithelial cells, % | 0.4 (0.0 to 1.2) | 0.0 (−0.3 to 0.3) | .24 |
| Inflammatory mediators | |||
| Procalcitonin, ng/L | −16.2 (−59.5 to 0.0) | −36.0 (−106.5 to −15.1) | .40 |
| Interferon-γ, ng/L | −17.0 (−331.9 to 792.6) | −34.4 (−692.0 to 424.3) | .87 |
| Interferon-γ-induced protein-10, ng/L | −143.4 (−503.7 to −10.8) | 319.7 (−2012.1 to 1027.8) | .23 |
| Interleukin 2, ng/L | −410.2 (−971.3 to 93.2) | −102.4 (−1468.2 to 421.2) | .96 |
| Interleukin 6, ng/L | −76.8 (−246.5 to 30.4) | 0.0 (−345.9 to 291.1) | .69 |
| Interleukin 8, µg/L | −5.8 (−19.7 to 1.6) | 0.3 (−31.8 to 6.1) | .78 |
| Interleukin 10, ng/L | −0.2 (−8.4 to 0.0) | 0.0 (−17.3 to 4.8) | .69 |
| Myeloperoxidase, mg/L | −0.3 (−0.8 to 0.1) | 0.0 (−1.2 to 2.2) | .46 |
| Tumor necrosis factor-α, ng/L | −9.6 (−125.2 to 11.4) | 10.8 (−266.7 to 220.4) | .78 |
| Extracellular matrix-associated proteins | |||
| Matrix metalloprotease-1, µg/L | −0.3 (−3.4 to 2.0) | 0.4 (−4.1 to 0.9) | 1.00 |
| Matrix metalloprotease-8, µg/L | −123.4 (−2577.1 to 56.2) | −165.3 (−3755.4 to 508.2) | .69 |
| Neutrophil elastase, µg/L | −731.0 (−1117.1 to −20.8) | 614.4 (−317.1 to 920.9) | .0009 |
| Transforming growth factor-β, ng/L | −64.1 (−346.7 to −7.2) | 4.2 (−18.7 to 298.6) | .04 |
Data are median (interquartile range).
aChange (day 28–day 0) was compared by Mann-Whitney U test.
ECM-Associated Proteins in Blood and Sputum
Baseline levels of ECM metabolites and proteases in blood and sputum were similar between the groups (Supplementary Tables 1 and 3). Changes in blood markers of ECM turnover showed no differences between the groups, except for the collagen type IV degradation product C4M (Figure 2C). Conversely, Pro-C4, the internal 7S domain a fragment of collagen IV, was reduced in the azithromycin group, but no statistically significant differences were observed between the groups. In sputum, the changes in the 2 MMPs we measured were not significantly different between groups. Sputum neutrophil elastase and TGF-β were reduced further in the azithromycin group compared to the SOC group (P < .05; Table 3, Figure 3, and Supplementary Figure 7).
Pharmacokinetics and Susceptibility Testing
No differences were observed between the SOC plus azithromycin and SOC only groups in dosing of rifampicin (600 mg once daily), isoniazid (300 mg once daily), pyrazinamide (27.1 [26.0–28.7] vs 28.1 [24.4–30.1] mg/kg once daily, respectively), or ethambutol (16.2 [15.5–17.1] vs 16.9 [15.9–21.8] mg/kg once daily, respectively, P > .29). One patient received a higher dose of rifampicin (1200 mg) and another patient received a higher dose of isoniazid (450 mg) based on results from therapeutic drug monitoring. Both patients were in the SOC group. No differences in pharmacokinetic parameters were observed between groups (Supplementary Table 4). A trend (P = .09) towards an increased lag time was observed for isoniazid in the azithromycin group.
All M. tuberculosis isolates of the participants were classified as susceptible to first-line antituberculosis drugs. No difference in minimum inhibitory concentration (MIC) values was observed between groups (Supplementary Table 5). All isolates were resistant (MIC >4 mg/L) to azithromycin.
Adverse Events and Concomitant Medication
None of the participants withdrew due to adverse events and no serious adverse events were observed (Supplementary Table 6). All adverse events reported were either grade 1 or grade 2. Most frequently observed adverse events were skin-related events and nausea, vomiting, and dyspepsia. No differences were observed between allocation groups. The total number of adverse events appeared to be lower in the azithromycin group.
In addition to first-line antituberculosis treatment, almost all participants (96%) used other forms of medication (Supplementary Table 7). No apparent differences between the groups were observed.
DISCUSSION
In this pilot trial we investigated whether azithromycin in addition to first-line antituberculosis treatment may reduce pulmonary inflammation in persons with pulmonary tuberculosis. In blood, azithromycin reduced IP-10 concentrations, and in sputum neutrophil percentages were reduced in patients treated with adjunctive azithromycin compared to SOC. Some markers of lung damage and remodeling, C4M in blood, and neutrophil elastase and TGF-β in sputum, were reduced further in the azithromycin group compared to the SOC group.
Alveolar macrophages and neutrophils are the main cells responsible for M. tuberculosis phagocytosis [24]. Phagocytosis of M. tuberculosis leads to the destruction of the pathogen. In tuberculosis disease this process is ineffective leading to ongoing inflammation and tissue damage [5]. In line with our observations, blood neutrophil numbers have previously been found to increase in persons with pulmonary tuberculosis and normalize during treatment [25]. Neutrophils are the predominant cell in sputum of persons with pulmonary tuberculosis as well [26, 27]. In line with an immunomodulatory role of macrolides in respiratory diseases [12], neutrophils in sputum were reduced by azithromycin treatment. These reductions were associated with reduced neutrophil elastase and TGF-β, shown to be involved in the immune response to M. tuberculosis [28, 29]. The significant increase in sputum macrophage and lymphocyte percentage we believe is reciprocal with the decrease in neutrophils.
In general, markers of inflammation decreased during antituberculosis treatment, as demonstrated by reductions in acute phase proteins (CRP, procalcitonin), cytokines, and chemokines (IFN-γ, IL-6, IP-10, and MPO). CRP, procalcitonin, IFN-γ, and IL-6 have been shown to reflect disease severity [30–32]. The observed reductions reflect effective antituberculosis treatment, but no difference was observed between study groups. IP-10, or C-X-C motif chemokine ligand 10 (CXCL10), is a proinflammatory chemokine which is released by antigen presenting and structural cells in response to IFN-γ [33]. In blood, IP-10 is increased in persons with tuberculosis upon diagnosis compared to persons without tuberculosis [34]. IP-10 was shown to decrease during successful treatment, whereas it increases during ineffective tuberculosis treatment, suggesting that IP-10 may be a marker of disease activity and treatment efficacy [35]. In our trial, IP-10 concentrations decreased as expected both in blood and sputum during adequate treatment of persons with pulmonary tuberculosis. Interestingly, this reduction in blood was enhanced by azithromycin treatment suggesting that azithromycin may enhance resolution of inflammation.
Changes in the lungs of persons with pulmonary tuberculosis, including airway narrowing, cavitation, and fibrosis, may lead to airflow obstruction and restrictive ventilatory defects [5]. Based on the reduction in inflammation, azithromycin could also reduce parameters of remodeling, including markers of ECM turn-over. TGF-β is a fibrogenic cytokine released by inflammatory and structural cells leading to fibrosis by increased ECM production [28, 36]. Expression of TGF-β was reduced in sputum of persons treated with azithromycin. Local inflammation characterized by neutrophils and their products, such as elastases, cause local damage [36]. Expression of several MMPs is increased in sputum of persons with tuberculosis and normalizes during treatment [13, 37]. Also in our trial, MMP-1 and MMP-8 decreased during treatment in blood and sputum, but no additional effects were observed with azithromycin. High concentrations of neutrophil elastase were found in both blood and sputum, which decreased in particular in persons treated with azithromycin, reflecting the decrease of neutrophils in sputum. This reduction in elastase may ultimately lead to a reduction in tissue injury. Collagen type IV is a major component of the basement membrane underlying the airway smooth muscle, endothelium, and epithelium, including in the alveoli [38]. In our trial, the degradation marker C4M, an MMP-mediated degradation product of collagen type IV found in basement membranes [39], representing epithelial damage, was decreased in plasma. Also, the collagen type IV formation marker Pro-C4 was decreased in both groups, suggesting damage and repair of alveolar basement membranes. Collectively, our findings suggest that reduced pulmonary inflammation in tuberculosis in response to azithromycin treatment may be associated with reduced tissue turnover.
No effects of azithromycin were observed on the pharmacokinetics of the first-line tuberculosis treatment. Exposure (AUC0–24) for the different antituberculosis drugs were as described previously [23, 40]. Moreover, treatment with azithromycin in combination with first-line tuberculosis treatment appeared to be safe.
Limitations of our trial include that the majority of participants were male, and we only provided azithromycin treatment to a small group of persons for a relatively short period of time (4 weeks). The high variability in the parameters studied, at baseline and the change during treatment, may have limited the power of this pilot trial to detect differences. Moreover, as we performed a pilot study, no corrections were performed for multiple testing. As microbiologically confirmed diagnosis of drug-susceptible pulmonary tuberculosis was an inclusion criterium, azithromycin treatment was started as soon as possible, but within 4 weeks, after initiation of first-line tuberculosis treatment. Some inflammatory mediators may already have decreased during this period. In future studies, treatment of azithromycin could be started together with first-line treatment to enhance immunomodulatory effects. Spontaneous sputum was not available for all participants. Especially in the azithromycin group, sputum had to be induced more frequently. Moreover, insufficient sputum was available to measure some parameters, such as neoepitopes and tissue inhibitors of metalloproteases (TIMPs). Future studies should be designed to address the effects of azithromycin treatment on structural and functional changes in persons with tuberculosis. Due to the short treatment period, the effects of azithromycin on radiological abnormalities or lung function were not determined. Eventually, future studies addressing the effects of long-term treatment with azithromycin in preventing or alleviating posttuberculosis lung disease should include these parameters.
In conclusion, the results from our pilot trial suggest that azithromycin treatment in addition to first-line tuberculosis treatment may reduce pulmonary inflammation and tissue turnover as shown by reduced IP-10 and C4M in blood and reduced neutrophils, neutrophil elastase, and TGF-β in sputum, and was well tolerated. Results justify further study on the role of azithromycin as a host-directed therapy for prevention of posttuberculosis lung disease.
Supplementary Material
Contributor Information
Bart G J Dekkers, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Huib A M Kerstjens, Department of Pulmonary Diseases and Tuberculosis, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Helene W Breisnes, Hepatic and Pulmonary Research, Nordic Bioscience, Herlev, Denmark; Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark.
Diana J Leeming, Hepatic and Pulmonary Research, Nordic Bioscience, Herlev, Denmark.
Richard M Anthony, National Tuberculosis Reference Laboratory, Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Henderik W Frijlink, Department of Pharmaceutical Technology and Biopharmacy, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, The Netherlands.
Tjip S van der Werf, Department of Pulmonary Diseases and Tuberculosis, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Jos G W Kosterink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Department of Pharmacotherapy, Epidemiology, and Economics, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, The Netherlands.
Jan-Willem C Alffenaar, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Sydney School of Pharmacy, Faculty of Medicine and Health, University of Sydney, Sydney, New South Wales, Australia.
Onno W Akkerman, Department of Pulmonary Diseases and Tuberculosis, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Tuberculosis Center Beatrixoord, University of Groningen, University Medical Center Groningen, Haren, The Netherlands.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to the people who participated in this trial. We thank Marcel van der Leij and Hessel Hovinga (Department of Laboratory Medicine) for expert technical assistance. We express gratitude to Marian de Jong (Department of Clinical Pharmacy and Pharmacology) for trial monitoring. We also thank the funding agency.
Author contributions . B. G. J. D., H. A. M. K., T. S. v. d. W., J. G. W. K., J. W. C. A., and O. W. contributed conception and design. B. G. J. D., H. W. B., and R. M. A. performed analysis. All authors contributed interpretation and edited the manuscript for important intellectual content.
Data sharing. Upon reasonable request, and subject to review, the authors will provide the data that support the findings of this study.
Financial support . This work was supported by the Stichting Beatrixoord Noord-Nederland (grant number 210.164 to B. G. J. D.).
References
- 1. World Health Organization . Global tuberculosis report. Geneva: World Health Organization, 2024. [Google Scholar]
- 2. Allwood BW, Byrne A, Meghji J, Rachow A, Van Der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021; 100:751–63. [DOI] [PubMed] [Google Scholar]
- 3. Pasipanodya JG, McNabb SJ, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health 2010; 10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Migliori GB, Marx FM, Ambrosino N, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021; 25:797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018; 27:170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pontali E, Akkerman OW, Zenner D, Migliori GB. Post-TB lung disease: keep going beyond TB!. Int J Tuberc Lung Dis 2024; 28:113–4. [DOI] [PubMed] [Google Scholar]
- 7. Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15:255–63. [DOI] [PubMed] [Google Scholar]
- 8. Ayodele S, Kumar P, van Eyk A, Choonara YE. Advances in immunomodulatory strategies for host-directed therapies in combating tuberculosis. Biomed and Pharmacother 2023; 162:114588. [DOI] [PubMed] [Google Scholar]
- 9. Miow QH, Vallejo AF, Wang Y, et al. Doxycycline host-directed therapy in human pulmonary tuberculosis. J Clin Invest 2021; 131:e141895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padmapriydarsini C, Mamulwar M, Mohan A, et al. Randomized trial of metformin with anti-tuberculosis drugs for early sputum conversion in adults with pulmonary tuberculosis. Clin Infect Dis 2022; 75:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallis RS, Ginindza S, Beattie T, et al. Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir Med 2021; 9:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cramer CL, Patterson A, Alchakaki A, Soubani AO. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med 2017; 129:493–9. [DOI] [PubMed] [Google Scholar]
- 13. Ugarte-Gil CA, Elkington P, Gilman RH, et al. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One 2013; 8:e61333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ong CWM, Elkington PT, Brilha S, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog 2015; 11:e1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salgame P, Yan B, Sloutsky A, Kobzik L, Kramnik I, Ramakrishnan L. MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest 2011; 121:1686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seddon J, Kasprowicz V, Walker NF, et al. Procollagen III N-terminal propeptide and desmosine are released by matrix destruction in pulmonary tuberculosis. J Infect Dis 2013; 208:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. in ‘t Veen JC, de Gouw HW, Smits HH, et al. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J 1996; 9:2441–7. [DOI] [PubMed] [Google Scholar]
- 18. Sand JMB, Leeming DJ, Byrjalsen I, et al. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD—results from the ECLIPSE study. Respir Res 2016; 17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Velde F, Alffenaar JWC, Wessels AMA, Greijdanus B, Uges DRA. Simultaneous determination of clarithromycin, rifampicin and their main metabolites in human plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877:1771–7. [DOI] [PubMed] [Google Scholar]
- 20. Sturkenboom MGG, van der Lijke H, Jongedijk EM, et al. Quantification of isoniazid, pyrazinamide and ethambutol in serum using liquid chromatography-tandem mass spectrometry. J Appl Bioanal 2015; 1:89–98. [Google Scholar]
- 21. Sturkenboom MG, Mulder LW, de Jager A, et al. Pharmacokinetic modeling and optimal sampling strategies for therapeutic drug monitoring of rifampin in patients with tuberculosis. Antimicrob Agents Chemother 2015; 59:4907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abolhassani-Chimeh R, Akkerman OW, Saktiawati AMI, et al. Population pharmacokinetic modelling and limited sampling strategies for therapeutic drug monitoring of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother 2022; 66:e0000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akkerman OW, Dijkwel RDC, Kerstjens HAM, et al. Isoniazid and rifampicin exposure during treatment in drug-susceptible TB. Int J Tuberc Lung Dis 2023; 27:772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu Rev Immunol 2013; 31:475–527. [DOI] [PubMed] [Google Scholar]
- 25. Moideen K, Kumar NP, Nair D, Banurekha VV, Bethunaickan R, Babu S. Heightened systemic levels of neutrophil and eosinophil granular proteins in pulmonary tuberculosis and reversal following treatment. Infect Immun 2018; 86:e00008-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eum S-Y, Kong J-H, Hong M-S, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010; 137:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nolan A, Condos R, Huie ML, et al. Elevated IP-10 and IL-6 from bronchoalveolar lavage cells are biomarkers of non-cavitary tuberculosis. Int J Tuberc Lung Dis 2013; 17:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warsinske HC, Pienaar E, Linderman JJ, Mattila JT, Kirschner DE. Deletion of TGF-β1 increases bacterial clearance by cytotoxic T cells in a tuberculosis granuloma model. Front Immunol 2017; 8:1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Meer AJ, Zeerleder S, Blok DC, et al. Neutrophil extracellular traps in patients with pulmonary tuberculosis. Respir Res 2017; 18:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miranda P, Gil-Santana L, Oliveira MG, et al. Sustained elevated levels of C-reactive protein and ferritin in pulmonary tuberculosis patients remaining culture positive upon treatment initiation. PLoS One 2017; 12:e0175278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leboueny M, Siawaya ACM, Bouanga LDJ, Ndjindji OM, Nzoghe AM, Siawaya JFD. Changes of C-reactive protein and procalcitonin after four weeks of treatment in patients with pulmonary TB. J Clin Tuberc Other Mycobact Dis 2023; 31:100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mattos AMM, de Almeida CS, Franken KLMC, et al. Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol 2010; 22:775–82. [DOI] [PubMed] [Google Scholar]
- 33. Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 2014; 43:1472–86. [DOI] [PubMed] [Google Scholar]
- 34. Chegou NN, Sutherland JS, Malherbe S, et al. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax 2016; 71:785–94. [DOI] [PubMed] [Google Scholar]
- 35. Azzurri A, Sow OY, Amedei A, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect 2005; 7:1–8. [DOI] [PubMed] [Google Scholar]
- 36. Singh S, Allwood BW, Chiyaka TL, et al. Immunologic and imaging signatures in post tuberculosis lung disease. Tuberculosis (Edinb) 2022; 136:102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muefong CN, Owolabi O, Donkor S, et al. Major neutrophil-derived soluble mediators associate with baseline lung pathology and post-treatment recovery in tuberculosis patients. Front Immunol 2021; 12:740933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dekkers BGJ, Saad SI, van Spelde LJ, Burgess JK. Basement membranes in obstructive pulmonary diseases. Matrix Biol Plus 2021; 12:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rønnow SR, Sand JMB, Langholm LL, et al. Type IV collagen turnover is predictive of mortality in COPD: a comparison to fibrinogen in a prospective analysis of the ECLIPSE cohort. Respir Res 2019; 20:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akkerman OW, Kerstjens HAM, Kingma M, Bolhuis MS, Sturkenboom MGG. Reply to ‘therapeutic drug monitoring for isoniazid and rifampicin exposure’. Int J Tuberc Lung Dis 2024; 28:169–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.