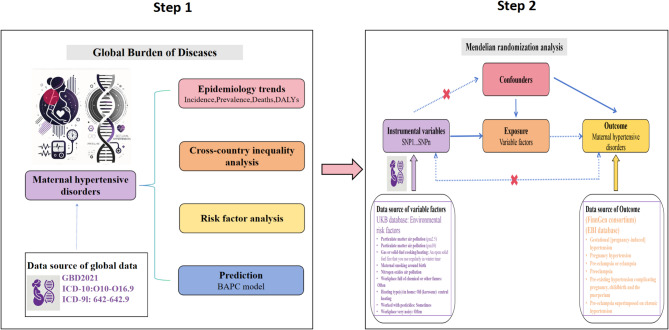
Abstract
Background
Maternal Hypertensive Disorders (MHD), encompassing gestational hypertension, chronic hypertension, preeclampsia, and eclampsia, which was a significant contributor to maternal morbidity and mortality, particularly in regions with lower socioeconomic status.
Methods
Using data from the 2021 Global Burden of Disease (GBD) study, we analyzed the burden of MHD globally. We used the slope index and concentration index to measure cross-country inequality in MHD burden, and employed a Bayesian age-period-cohort (BAPC) model to project the burden from 2022 to 2045. Additionally, we conducted a two-sample Mendelian randomization (MR) analysis based on genome-wide association study (GWAS) to investigate potential causal relationships between occupational exposures and MHD.
Results
Overall, the global incidence, prevalence, mortality, and DALYs for MHD have declined. However, incidence and prevalence rose in Central Asia, Eastern Europe, and Western Europe, while the Caribbean increasing in mortality and DALYs. Iron deficiency emerged as the leading risk factor. Significant SDI-related inequalities in MHD burden were observed, especially in lower SDI countries. Projections suggest ongoing reductions in MHD burden through 2045. MR results revealed a significant causal link between frequent exposure to chemical or other fumes in the workplace and MHD, while no clear causal relationships were identified for particulate matter or other assessed exposures.
Conclusions
Although the global MHD burden is decreasing, marked regional disparities persist. Efforts focusing on addressing iron deficiency, improving nutritional support, and mitigating occupational exposures may further reduce the burden. Strengthening maternal healthcare services—especially in low-SDI will be crucial for achieving sustainable declines in MHD worldwide.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-025-07766-y.
Graphical Abstract
Keywords: Maternal hypertensive disorders (MHD), Global burden disease (GBD), Cross-country inequality, Occupational exposures, Mendelian randomization (MR)
Introduction
Maternal Hypertensive Disorders (MHD) are a major global public health concern. They include gestational hypertension, chronic hypertension, preeclampsia, eclampsia, and preeclampsia superimposed on chronic hypertension, collectively affecting approximately 5–15% of pregnant women worldwide [1, 2]. Research has shown that women who have experienced MHD—particularly those with eclampsia and severe preeclampsia—are linked to higher rates of all-cause mortality, perinatal mortality, cardiovascular disease mortality, digestive system disease mortality, as well as mortality from endocrine, nutritional, and metabolic diseases among their offspring from birth through adolescence [3–7]. Furthermore, the ongoing global burden of maternal hypertensive disorders (MHD), particularly in low- and middle-income countries, is associated with an increasing trend in long-term maternal morbidity and mortality [8].
Maternal hypertensive disorders (MHD) are caused by the interaction of multiple factors including genetic susceptibility, environmental and occupational exposures, pre-existing clinical conditions (chronic hypertension, diabetes), and sociodemographic factors (maternal age, parity, and access to health care) [3–11], underscoring the importance of further epidemiological research and policy support to improve maternal health and reduce global health inequalities. Air pollution is a major occupational health issue affecting populations in low-, middle-, and high-income countries. Although previous epidemiological and large-scale cohort studies indicate that occupational pollutants are risk factors for MHD, contributing to increased incidence and mortality [12–15], emerging evidence on environmental and occupational determinants of hypertension outcomes in pregnancy, including exposure to air pollutants, noise, and chemical fumes, remains underexplored in causal epidemiology. To address this gap, we conducted a two-sample Mendelian randomization study, using genome-wide genetic variations such as single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to estimate the causal relationship between occupational exposures and MHD.
Using the 2021 Global Burden of Disease (GBD) database, we evaluated the trends and burden of MHD from 1990 to 2021, examined health inequalities among countries in different regions, analyzed its attributable risk factors, and projected the disease burden from 2022 to 2045. We further explored the potential causal relationship between air pollutants and gestational hypertension through Mendelian randomization, thereby enhancing our understanding of air pollution as a risk factor. Our study provides the most up-to-date epidemiological data on MHD worldwide, offering profound insights into the promotion of maternal and infant health and the achievement of global health goals. This analysis not only informs healthcare professionals and policymakers but also assists governments worldwide in formulating maternal health policies.
Methods
Study data
The GBD analysis process of this study is shown in Step 1 in the Central Illustration. The burden data originated from the GBD 2021. The GBD 2021 database, which serves as a public resource, is supplied by the Institute for Health Metrics and Evaluation of the University of Washington [16]. The GBD 2021 study conducts a thorough assessment of health consequences related to 371 diseases and injuries [17]. We produced estimates for 204 countries and territories that were grouped into 21 regions and seven super-regions (Table 1). The seven super-regions are central Europe, eastern Europe, and central Asia; high income; Latin America and the Caribbean; north Africa and the Middle East; south Asia; southeast Asia, east Asia, and Oceania; and sub-Saharan Africa. In GBD 2021 we continue to analyse at subnational levels countries that were added in previous cycles including Brazil, China, Ethiopia, India, Indonesia, Iran, Italy, Japan, Kenya, Mexico, New Zealand, Nigeria, Norway, Pakistan, Russia, the Philippines, Poland, South Africa, Sweden, the UK, and the USA. The GBD 2021 study uses the Bayesian meta-regression tool DisMod-MR 2.1 as its core model to estimate the burden of disease across countries, years, age groups, and sexes. The model integrates data from a wide range of sources (including surveys, registries, hospital records, and demographic data) while adjusting for known biases (e.g., misclassification or underreporting). It assumes internal consistency among key epidemiological indicators (incidence, prevalence, remission, and mortality) and applies differential equations to ensure consistency. When original data are sparse or missing, covariate-based predictions are used to take advantage of data from similar countries, regions, or years. Its study design and methodology have been extensively detailed in existing GBD literature (https://ghdx.healthdata.org/gbd-results‐tool).
Table 1.
The case number and ASR of Incidence of MHD in 1990 and 2021 for female by SDI quintiles and by GBD regions, with EAPC from 1990 to 2021
| Incidence | 1990 | 2021 | |||
|---|---|---|---|---|---|
| Number (95% UIs) | ASIR(95% UIs) | Number (95% UIs) | ASIR(95% UIs) | EAPC (95% CI) 1990–2021 | |
| Global | 15,662,895(12,935,145 − 19,254,513) | 919.34(759.23-1,130.15) | 18,050,085(15,356,124 − 21,519,204) | 723.53(615.54-862.59) |
-0.51 (-0.56 to -0.45) |
| High SDI | 1,233,522(966,651-1,612,143) | 440.55(345.24-575.78) | 1,235,244(1,023,404-1,513,215) | 399.58(331.05–489.50) |
-0.49 (-0.67 to -0.31) |
| High-middle SDI | 1,488,518(1149,315-1,967,034) | 427.48(330.07-564.91) | 1,249,379(1,008,834-1,562,214) | 319.54(258.02-399.55) |
0.05 (-0.31 to 0.41) |
| Middle SDI | 4,056,969(3,303,930-5,098,008) | 716.47(583.48-900.31) | 3,701,256(3,110,329-4,468,478) | 468.48(393.68-565.59) |
-0.72 (-0.88 to -0.57) |
| Low-middle SDI | 4,734,331(3,973,547-5,762,420) | 1,320.24(1,108.08-1,606.94) | 5,103,004(4,291,790-6,055,102) | 794.26(668-942.45) |
-1.83 (-1.99 to -1.68) |
| Low SDI | 4,139,304(3,487,342-4,867,143) | 2,776.52(2,339.20-3,264.73) | 6,749,882(5,727,623-7,955,523) | 1,874.36(1,590.49-2,209.15) |
-1.28 (-1.4 to -1.16) |
| Andean Latin America | 60,880(54,015–69,612) | 492.72(437.16-563.39) | 102,409(95,875 − 110,222) | 468.01(438.15-503.72) |
-0.16 (-0.36 to 0.04) |
| Australasia | 35,035(29,359 − 42,314) | 532.62(446.32-643.28) | 33,846(26,068 − 42,709) | 369.09(284.26-465.73) |
-1.03 (-1.23 to -0.84) |
| Caribbean | 93,720(74,276 − 120,931) | 796.5(631.26-1027.76) | 83,402(67,141 − 104,886) | 545.37(439.04-685.86) |
-0.95 (-1.02 to -0.87) |
| Central Asia | 70,849(56,869 − 89,435) | 323.69(259.82-408.61) | 91,670(73,030–116,744) | 298.04(237.44-379.56) |
0.4 (0.11 to 0.7) |
| Central Europe | 112,704(83,118–154,106) | 285.66(210.67-390.59) | 80,855(66,374 − 100,780) | 247.17(202.90-308.08) |
-0.29 (-0.64 to 0.06) |
| Central Latin America | 684,950(574,021–824,555) | 1,258.84(1,054.97-1,515.42) | 621,918(545,662–717,391) | 722.16(633.61-833.02) |
-0.99 (-1.33 to -0.65) |
| Central Sub-Saharan Africa | 563,799(466,899 − 670,890) | 3,413.33(2,826.687-4,061.68) | 878,783(721,967-1,077,617) | 2,022.87(1,661.90-2,480.57) |
-1.51 (-1.63 to -1.39) |
| East Asia | 1,278,231(942,274-1,787,350) | 313.09(230.80-437.80) | 710,146(548,939 − 920,215) | 163.57(126.45-211.98) |
-0.57 (-1.7 to 0.58) |
| Eastern Europe | 429,926(320,324–586,671) | 597.86(445.45-815.83) | 372,397(286,377–479,586) | 608.35(467.82-783.45) |
1.12 (0.65 to 1.59) |
| Eastern Sub-Saharan Africa | 1,976,139(1,638,189-2,341,423) | 3,414.77(2,830.79-4,045.98) | 3,087,073(2,599,544-3,620,626) | 2,204.79(1,856.59-2,585.85) |
-1.39 (-1.52 to -1.26) |
| High-income Asia Pacific | 179,021(141,199–232,968) | 312.17(246.21-406.23) | 115,278(98,177 − 135,473) | 234.77(199.94-275.89) |
-1.52 (-1.99 to -1.05) |
| High-income North America | 581,683(445,278–766,637) | 643.34(492.47-847.89) | 626,403(539,595–736,474) | 584.1(503.15-686.72) |
-0.48 (-0.71 to -0.25) |
| North Africa and Middle East | 1,091,557(866,925-1,390,333) | 1,055.518(838.301-1,344.43) | 1,205,613(946,504-1,540,737) | 597.84(469.35-764.02) |
-1.49 (-1.66 to -1.33) |
| Oceania | 17,955(14,074 − 23,015) | 887.73(695.81-1,137.90) | 33,574(26,578 − 42,939) | 758.02(600.05-969.45) |
-0.72 (-0.76 to -0.67) |
| South Asia | 4,018,273(3,329,687-4,920,134) | 1,207.95(1,000.95-1,479.06) | 3,478,494(2,875,246-4,288,315) | 559.77(462.69-690.09) |
-2.8 (-3.17 to -2.44) |
| Southeast Asia | 1,195,303(945,536-1,507,583) | 770.38(609.40-971.64) | 1,143,820(917,858-1,420,431) | 493.36(395.89-612.66) |
-1.33 (-1.39 to -1.26) |
| Southern Latin America | 107,883(82,156 − 140,036) | 678.62(516.79-880.87) | 134,877(113,450 − 162,874) | 616.64(518.68-744.64) |
-0.1 (-0.2 to 0.01) |
| Southern Sub-Saharan Africa | 342,754(283,702 − 409,228) | 1,979.57(1,638.52-2,363.49) | 366,803(307,516 − 431,853) | 1,339.08(1,122.64-1,576.55) |
-1.09 (-1.14 to -1.03) |
| Tropical Latin America | 413,752(331,184–535,494) | 804.11(643.65-1,040.71) | 375,061(321,710 − 447,913) | 497.09(426.38-593.64) |
-1.22 (-1.48 to -0.96) |
| Western Europe | 372,227(285,328–488,578) | 312.83(239.80-410.62) | 387,665(309,193–488,869) | 321.12(256.12-404.95) |
0.41 (0.27 to 0.54) |
| Western Sub-Saharan Africa | 2,036,247(1,723,735-2,377,706) | 3,508.93(2,970.40-4,097.34) | 4,119,997(3,534,414-4,761,707) | 2,593.41(2,224.81-2,997.35) |
-0.84 (-1.03 to -0.66) |
Abbreviations: ASIR, age-standardized incidence rate; MHD, Maternal Hypertensive Disorders; SDI, sociodemographic index; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study; EAPC, estimated annual percentage change; UIs, uncertainty intervals; CI, confidence interval
Indicator Definitions
According to the International Classification of Diseases, Ninth Revision (ICD-9: 642–642.9) and Tenth Revision (ICD-10: O10–O16.9), Maternal Hypertensive Disorders (MHD) are defined as follows: (1)Pre-existing hypertension complicating pregnancy, childbirth and the puerperium.(2)Pre-existing hypertension with pre-eclampsia.(3)Gestational [pregnancy-induced] edema and proteinuria without hypertension.(4)Gestational [pregnancy-induced] hypertension without significant proteinuria.(5)Pre-eclampsia.(6)Eclampsia. (7)Unspecified maternal hypertension. Mapping was performed using the GBD etiology hierarchy to ensure consistency across data sources and years. GBD defines each disease using a standardized algorithm applied to administrative data, surveys, and vital registries.
Data acquisition and preliminary analysis
The 95% uncertainty intervals (UI) for incidence, prevalence, mortality, and disability-adjusted life years (DALYs) are derived directly from the GBD 2021 estimates based on 1,000 posterior draws generated by a Bayesian meta-regression model (DisMod-MR 2.1). New cases and incident cases were distinguished from prevalent cases based on the temporal patterns of diagnostic coding and record structure. However, we acknowledge that some degree of misclassification (possibly including recurrent cases) is possible due to the limitations of routinely collected data. These draws reflect uncertainty from multiple sources, including variability in the input data, sampling error, and model assumptions. For each health indicator, the 2.5 and 97.5 percentiles of the 1,000 draws were used to construct the 95% UI, ensuring that the probabilities reflect the range of estimates. The estimated annual percentage change (EAPC) was computed by employing a least squares linear regression model, and the results of the regression were organized and analyzed using the R package. For Estimated Annual Percentage Change (EAPC) calculations, we fit a linear regression model to the natural logarithm of the age-standardized rates over time. The 95% CIs of the EAPCs were derived from the standard error of the regression coefficient, assuming a normal distribution [18]. It is computed using the formula: ln(y) = α + βx + ε, where (y) stands for the age-standardized incidence, (α) is the intercept, (x) represents the year, (β) is the slope, and (ε) is the normally distributed error term. The EAPC formula is (exp^β − 1)×100%. An upward trend in age-standardized incidence is shown when the lower bound of the EAPC 95% confidence interval (CI) is greater than 0; a downward trend is indicated when the upper bound of the EAPC 95% CI is less than 0; otherwise, the incidence is considered relatively stable. For Mendelian randomization (MR) estimates, 95% CIs were calculated using the delta method or standard error propagation methods, depending on the MR method applied (e.g., IVW, MR Egger) [19]. In order to analyze the global distribution and regional differences in MHD, the generated global maps were conducted regional comparative analyses. Data were aggregated according to the geographic regions defined by the GBD study to visualize the geographic distribution of the disease burden. Based on population to explore the distribution of MHD across age groups. Additionally, the relationship between the SDI and MHD burden were investigated. The SDI classification (low, low-middle, middle, high-middle, and high) was used to compare disease burden across different levels of socioeconomic development [20].
Cross-country inequality analysis
Health inequality monitoring can lay a foundation for evidence-based health planning and further refine relevant policies, programs, and practices in order to reduce differences in health distribution. In the present study, two standard metrics for absolute and relative gradient inequality, specifically the slope index of inequality and the concentration index, were employed to measure the inequality in the distribution of the Maternal Hypertensive Disorders (MHD) burden across countries. The slope index of inequality was determined by regressing national DALYs rates of all age populations on a sociodemographic development-related relative position scale. The concentration index was calculated by numerically integrating the area under the Lorenz concentration curve, which was fit using the cumulative fraction of DALYs and the cumulative relative distribution of the population ranked by SDI [18].
Prediction analysis
Using a Bayesian age-period-cohort (BAPC) analysis based on the 1990–2021 data from the Global Burden of Disease (GBD) database for global AF/AFL incidence, prevalence, mortality, and DALYs, we projected the future burden of MHD for the period 2022–2045. Population estimates were obtained from the 2019 revision of the United Nations World Population Prospects, disaggregated by year (up to 2100), age, and sex (https://population.un.org/wpp/downloads). The BAPC model builds on the age-period-cohort (APC) framework—a model commonly used to analyze trends in the incidence and mortality of chronic diseases—and employs the integrated nested Laplace approximation (INLA) algorithm for parameter estimation [21]. The classic APC model describes changes in incidence or mortality as functions of age, period, and cohort, thus enabling predictions based on these trends [22].
MR analysis
In this study, the Mendelian randomization (MR) flowchart is presented in Step 2 of the Central Illustration. This Mendelian randomization analysis was conducted and reported in accordance with the STROBE-MR (Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization) guidelines [23], and the STORBE-MR checklist is provided in the Supplementary Table 22. We applied an MR approach combined with genome-wide association study (GWAS) data to investigate the potential causal relationship between occupational exposures and Maternal Hypertensive Disorders (MHD). Specifically, particulate matter was regarded as the exposure variable (Supplementary Table 1), while MHD was considered the outcome variable (Supplementary Table 2). Exposure data on occupational pollutants and MHD case data were obtained from the UK Biobank and FinnGen databases, respectively, covering participants in both cohorts. We identified independent SNPs associated with particulate matter based on two criteria. First, we selected SNPs at a genome-wide significance threshold of p < 5 × 10^(-6). Second, we used pairwise linkage disequilibrium (LD) analyses to assess SNP independence, selecting SNPs that were at least 10,000 kb apart and excluding those with LD (R^2 < 0.01). The instrumental variables used in this study are listed in Supplementary Table 3. To bolster the reliability of our analysis, we applied five different methods: MR-Egger, weighted median, inverse variance weighting (IVW), simple mode, and weighted mode. Among these, IVW was our primary approach because—when no horizontal pleiotropy exists among the instrumental variables—it provides the most robust evidence [24]. However, if horizontal pleiotropy is present, other causal pathways beyond the exposure of interest may confound the results. Thus, we employed the other four complementary methods to ensure that our findings remained robust to potential confounding factors. We then compared the conclusions from all five methods to evaluate consistency and reliability. To further validate the robustness of our results, we performed sensitivity analyses including the MR-Egger intercept test and MR pleiotropy residual analysis [25]. Additionally, we conducted leave-one-out analyses and created scatter plots to visually identify and examine potential outliers.
Statistical analysis
The projected absolute figures for each metric spanning from 2022 to 2045 were computed by multiplying the yearly age-sex-country-specific projection ratios with the population projections of the corresponding subgroups within the same year. These estimates were then combined to showcase the outcomes at the global scale, as well as at the World Bank income group levels and on a country-by-country basis. To calculate the age-adjusted rates for incidence, prevalence, mortality, and DALYs, the direct standardization technique was employed, with the World Health Organization standard population serving as the benchmark. Eventually, the average annual percentage alteration and associated 95% confidence interval for each measure during the period from 2022 to 2045 were determined to gauge the shift in the global age-adjusted rates. All of the analytical procedures were carried out using the R statistical software (version 4.3.2). MR analysis was performed using the R packages “TwoSampleMR” and “data.table”.
Result
Analysis of the trend of global disease burden of MHD
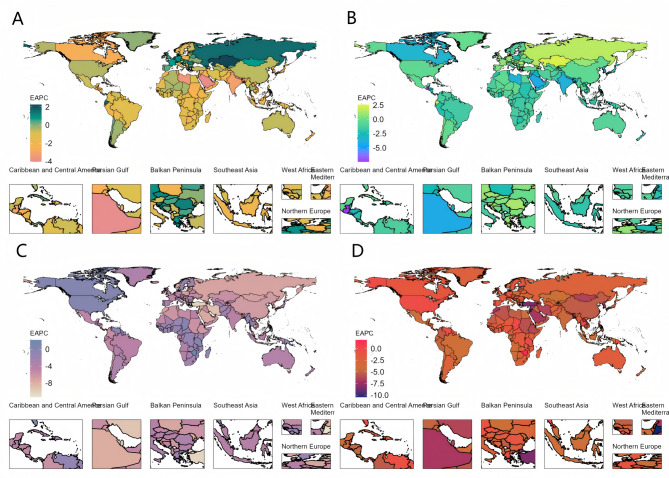
Analyses of the Global Burden of Disease (GBD) database indicate a clear trend in the worldwide epidemiology of Maternal Hypertensive Disorders (MHD). While the total number of incident cases increased from 15,662,895 (95% UI: 12,935,146–19,254,514) in 1990 to 18,050,085 (95% UI: 15,356,124–21,519,204) in 2021, the incidence rate per 100,000 population decreased from 919.34% (95% UI: 759.23%–1,130.15%) in 1990 to 723.53% (95% UI: 615.54–862.59%) in 2021, marking an overall decline of 21.3%. The estimated annual percentage change (EAPC) was − 0.51% (95% CI: -0.56 to -0.45) (Table 1; Fig. 1A, Supplementary Fig. 1A-C). However, in Central Asia, Eastern Europe, and Western Europe, incidence rates moved in the opposite direction of the global trend, with EAPCs of 0.40% (95% CI: 0.11 to 0.70), 1.12% (95% CI: 0.65 to 1.59), and 0.41% (95% CI: 0.27 to 0.54), respectively (Table 1; Fig. 1A).
Fig. 1.
The trend in ASR of EAPC from 1990 to 2021. A The trend in ASR of incidence (EAPC) from 1990 to 2021; B The trend in ASR of prevalence (EAPC) from 1990 to 2021; C The trend in ASR of deaths (EAPC) from 1990 to 2021; D The trend in ASR of DALYs (EAPC) from 1990 to 2021, of MHD worldwide. Abbreviations: ASR, age-standardized rate; EAPC, estimated annual percentage change; DALYs, disability-adjusted life-years; MHD, Maternal Hypertensive Disorders
Consistent with incidence, the overall prevalence of MHD declined over the past 30 years. Although the total number of prevalent cases rose from 3,076,691 (95% UI: 1,997,627–4,481,074) in 1990 to 3,520,625 (95% UI: 2,279,278–5,084,397) in 2021, the prevalence rate decreased from 180.59% (95% UI: 117.25–263.02) per 100,000 in 1990 to 141.12% (95% UI: 91.36–203.81) in 2021, for an overall reduction of 21.90%. The EAPC was − 0.54% (95% CI: -0.60 to -0.47) (Table 2; Fig. 1B, Supplementary Fig. 1D-F). Nonetheless, in Central Asia, Eastern Europe, and Western Europe, prevalence showed an upward trend contrary to the global pattern, with EAPC of 0.49% (95% CI: 0.17 to 0.81), 1.13% (95% CI: 0.66 to 1.60), and 0.38% (95% CI: 0.22 to 0.54), respectively (Table 2; Fig. 1B).
Table 2.
The case number and ASR of prevalence of MHD in 1990 and 2021 for female by SDI quintiles and by GBD regions, with EAPC from 1990 to 2021
| Prevalence | 1990 | 2021 | |||
|---|---|---|---|---|---|
| Number (95% UIs) | ASPR(95% UIs) | Number (95% UIs) | ASPR(95% UIs) | EAPC (95% CI) 1990–2021 | |
| Global |
3,076,691 (1,997,627-4,481,074) |
180.59 (117.25-263.02) |
3,520,625 (2,279,278-5,084,397) |
141.12 (91.36-203.81) |
-0.54 (-0.6 to -0.47) |
| High SDI |
261,686 (167,014–391,787) |
93.46 (59.65-139.93) |
259,630 (168,010–377,648) |
83.99 (54.35–22.16) |
-0.51 (-0.7 to -0.32) |
| High-middle SDI | 328,87 (206,50–499,19 | 94.34(59.41–3.54) |
281,971 (176,919 − 418,676) |
72.12 (45.25-107.08) |
0.15 (-0.21 to 0.51) |
| Middle SDI |
817,326 (526,616-1,201,473) |
144.34 (93.00-212.18) |
734,683 (467,492 1,071,245) |
92.99 (59.17-135.59) |
-0.79 (-0.94 to -0.63) |
| Low-middle SDI |
861,716 (555,045 − 1,267,717) |
240.30 (154.78-353.52) |
939,109 (610,340-1,360,617 |
146.17 (94.00-211.77) |
-1.89 (-2.1 to -1.68) |
| Low SDI |
805,351 (529,167-1,171,500) |
540.21 (354.95-785.81) |
1,302,927 (860,960-1,902,981) |
361.81 (239.08-528.43) |
-1.3 (-1.44 to -1.15) |
| Andean Latin America |
10,374 (6,930 − 14,915) |
83.96 (56.09-120.71) |
18,220 (12,690 − 25,001) |
83.26 (57.99-114.26) |
-0.16 (-0.49 to 0.17) |
| Australasia |
8,037 (5,349 − 11,686) |
122.19 (81.32-177.66) |
7,409 (4,517 − 10,925) |
80.79 (49.26-119.13) |
-1.19 (-1.42 to -0.97) |
| Caribbean |
18,586 (11,972 − 29,352) |
157.96 (101.74-249.46) |
15,638 (9,868 − 23,418) |
102.26 (64.53-153.13) |
-1.15 (-1.23 to -1.06) |
| Central Asia |
14,389 (8,962 − 22,087) |
65.74 (40.95-100.91) |
19,104 (11,959 − 28,730) |
62.11 (38.88–93.41) |
0.49 (0.17 to 0.81) |
| Central Europe |
25,131 (15,405 − 39,897) |
63.7 (39.04-101.12) |
17,869 (11,497 − 26,001) |
54.62 (35.15–79.48) |
-0.34 (-0.7 to 0.02) |
| Central Latin America |
115,079 (74,433 − 168,867) |
211.5 (136.8-310.35) |
102,308 (68,856 − 145,556) |
118.8 (79.95-169.02) |
-0.82 (-1.23 to -0.4) |
| Central Sub-Saharan Africa |
114,184 (75,279 − 167,133) |
691.29 (455.75-1,011.85) |
176,527 (114,494 − 257,551) |
406.35 (263.55-592.86) |
-1.55 (-1.72 to -1.38) |
| East Asia |
276,325 (166,315–440,395) |
67.68 (40.74-107.87) |
156,013 (94,867 − 238,503) |
35.94 (21.85–54.94) |
-0.46 (-1.74 to 0.84) |
| Eastern Europe |
102,064 (62,996 − 156,566) |
141.93 (87.60-217.72) |
88,184 (54,981 − 134,308) |
144.06 (89.82-219.41) |
1.13 (0.66 to 1.6) |
| Eastern Sub-Saharan Africa |
388,816 (255,009-569813) |
671.88 (440.66-984.64) |
602,266 (396,782–876,263) |
430.14 (283.38-625.83) |
-1.41 (-1.57 to -1.25) |
| High-income Asia Pacific |
31,578 (19,282 − 48,836) |
55.06 (33.62–85.15) |
18,741 (12,085 − 27,290) |
38.17 (24.61–55.58) |
-1.93 (-2.51 to -1.34) |
| High-income North America |
124,115 (77,999 − 192,941) |
137.27 (86.27-213.39) |
131,064 (85,938 − 185,150) |
122.21 (80.13-172.65) |
-0.49 (-0.73 to -0.26) |
| North Africa and Middle East |
239,480 (151,545 − 352,545) |
231.57 (146.54-340.91) |
270,126 (166,026-405191) |
133.95 (82.33-200.93) |
-1.4 (-1.57 to -1.23) |
| Oceania |
3732 (2,366-5,547) |
184.502 (116.969-274.256) |
6,866 (4,293 − 10,429) |
155.02 (96.92-235.46) |
-0.78 (-0.82 to -0.74) |
| South Asia |
690,077 (431,663-1,057,306) |
207.45 (129.77-317.84) |
597,394 (374,209–901,826) |
96.13 (60.22-145.12) |
-3.06 (-3.58 to -2.55) |
| Southeast Asia |
250,255 (156,168–370,483) |
161.29 (100.65-238.78) |
245,050 (153,080–364,422) |
105.7 (66.03-157.18) |
-1.26 (-1.34 to -1.19) |
| Southern Latin America |
24,774 (15,451 − 37,691) |
155.84 (97.19-237.09) |
31,021 (20,449 − 45,051) |
141.83 (93.49-205.97) |
-0.09 (-0.2 to 0.02) |
| Southern Sub-Saharan Africa |
72,228 (47,321 − 104,626) |
417.15 (273.3-604.27) |
74,400 (48,546 − 106,736) |
271.61 (177.22-389.66) |
-1.19 (-1.25 to -1.13) |
| Tropical Latin America |
75,485 (47,196 − 119,826) |
146.70 (91.72-232.88) |
65,638 (43,014–94,496) |
86.99 (57.01-125.24) |
-1.5 (-1.89 to -1.12) |
| Western Europe |
82,654 (51,188 − 127,530) |
69.46 (43.02-107.18) |
85,626 (53,336 − 127,824) |
70.93 (44.18-105.88) |
0.38 (0.22 to 0.54) |
| Western Sub-Saharan Africa |
409,329 (272,978 − 588,062) |
705.37 (470.41-1,013.37) |
791,163 (528,610-1,132,040) |
498.01 (332.74-712.58) |
-0.89 (-1.12 to -0.65) |
Abbreviations: ASPR, age-standardized prevalence rate; MHD, Maternal Hypertensive Disorders; SDI, sociodemographic index; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study; EAPC, estimated annual percentage change; UIs, uncertainty intervals; CI, confidence interval
Following the same pattern as incidence and prevalence, overall mortality and DALYs for MHD declined in the past 30 years. The number of deaths decreased by 29.20%, from 53,894 (95% UI: 47,676–59,819) in 1990 to 38,147 (95% UI: 31,879–46,096) in 2021. Meanwhile, DALYs dropped by 29.00%, from 3,479,885 (95% UI: 3,085,882–3,873,201) in 1990 to 2,469,636 (95% UI: 2,083,398–2,958,212) in 2021. The mortality rate per 100,000 population declined from 3.16% (95% UI: 2.80–3.51) in 1990 to 1.53% (95% UI: 1.28–1.85) in 2021, representing an overall reduction of 56.70%. The EAPC for mortality was − 2.16% (95% CI: -2.21 to -2.10) (Table 3; Fig. 1C, Supplementary Fig. 1G-I). The DALY rate per 100,000 population decreased by 51.50%, from 204.25% (95% UI: 181.13–227.34) in 1990 to 98.99% (95% UI: 83.51–118.58) in 2021, yielding an EAPC of -2.10% (95% CI: -2.15 to -2.04) (Table 4; Fig. 1D, Supplementary Fig. 1J-L). However, in the Caribbean region, mortality and DALYs showed an upward trend contrary to the global pattern, with EAPC of 0.80% (95% CI: 0.58 to 1.02) and 0.75% (95% CI: 0.53 to 0.96), respectively (Tables 3 and 4). For detailed country-specific trends in the MHD burden, please refer to Supplementary Tables 4–7.
Table 3.
The case number and ASR of deaths of MHD in 1990 and 2021 for female by SDI quintiles and by GBD regions, with EAPC from 1990 to 2021
| Deaths | 1990 | 2021 | |||
|---|---|---|---|---|---|
| Number (95% UIs) | ASDR(95% UIs) | Number (95% UIs) | ASDR(95% UIs) | EAPC (95% CI) 1990–2021 | |
| Global |
53,895 (47,676 − 59,819) |
3.16 (2.8–3.51) |
38,147 (31,879 − 46,096) |
1.53 (1.28–1.85) |
-2.16 (-2.21 to -2.1) |
| High SDI |
338 (285–397) |
0.12 (0.10–0.14) |
135 (114–163) |
0.04 (0.04–0.05) |
-2.71 (-2.87 to -2.55) |
| High-middle SDI | 2,022 (1,625-2,412) |
0.58 (0.47–0.69) |
361 (300–448) |
0.09 (0.08–0.12) |
-5.33 (-5.45 to -5.21) |
| Middle SDI |
10,549 (9,346 − 11,815) |
1.86 (1.65–2.09) |
4,961 (4,235-5,981) |
0.63 (0. 54-0.76) |
-3.22 (-3.29 to -3.15) |
| Low-middle SDI |
23,080 (19,957 − 25,795) |
6.44 (5.57–7.19) |
14,069 (11,322 − 17,616) |
2.19 (1.76–2.74) |
-3.46 (-3.55 to -3.37) |
| Low SDI |
17,872 (15,191 − 20,320) |
11.99 (10.19–13.63) |
18,588 (15,234 − 22,700) |
5.16 (4.23–6.30) |
-2.6 (-2.7 to -2.5) |
| Andean Latin America | 643 (548–749) |
5.20 (4.44–6.06) |
337 (248–444) |
1.54 (1.14–2.03) |
-4.29 (-4.53 to -4.04) |
| Australasia |
4 (3–5) |
0.07 (0.05–0.08) |
1 (1–2) |
0.02 (0.01–0.02) |
-3.88 (-4.51 to -3.25) |
| Caribbean |
383 (307–483) |
3.25 (2.61–4.10) |
511 (326–750) |
3.34 (2.13–4.91) |
0.8 (0.58 to 1.02) |
| Central Asia |
252 (224–283) |
1.15 (1.02–1.3) |
80 (66–97) |
0.26 (0.22–0.31) |
-4.53 (-4.84 to -4.22) |
| Central Europe |
52 (45–60) |
0.13 (0.11–0.15) |
7 (6–8) |
0.02 (0.02–0.03) |
-4.88 (-5.3 to -4.44) |
| Central Latin America |
1,128 (1,030 − 1,233) |
2.07 (1.89–2.27) |
495 (402–605) |
0.57 (0.47–0.7) |
-3.92 (-4.15 to -3.69) |
| Central Sub-Saharan Africa |
2,464 (1,735-3,273) |
14.92 (10.50-19.82) |
2,679 (1,860-3,719) |
6.17 (4.28–8.56) |
-2.08 (-2.4 to -1.77) |
| East Asia |
1,822 (1,245-2,462) |
0.45 (0.31–0.60) |
194 (140–268) |
0.05 (0.03–0.06) |
-6.52 (-6.78 to -6.26) |
| Eastern Europe |
185 (159–211) |
0.26 (0.22–0.29) |
17 (14–21) |
0.03 (0.02–0.03) |
-6.67 (-7.02 to -6.31) |
| Eastern Sub-Saharan Africa |
7,510 (6,429-8,642) |
12.98 (11.11–14.93) |
6,404 (5,098 − 7,877) |
4.57 (3.64–5.63) |
-3.24 (-3.38 to -3.09) |
| High-income Asia Pacific |
53 (43–62) |
0.092 (0.075–0.108) |
7 (6–8) |
0.01 (0.01–0.02) |
-5.65 (-5.99 to -5.31) |
| High-income North America |
88 (72–108) |
0.1 (0.08–0.12) |
82 (66–102) |
0.08 (0.06–0.1) |
0.12 (-0.16 to 0.4) |
| North Africa and Middle East |
4,350 (3,443-5,186) |
4.21 (3.33–5.01) |
1,654 (1,188-2,209) |
0.82 (0.59–1.1) |
-5.12 (-5.3 to -4.93) |
| Oceania |
43.604 (24.091–64.759) |
2.16 (1.19–3.20) |
79 (54–110) |
1.78 (1.21–2.49) |
-0.57 (-0.77 to -0.38) |
| South Asia |
20,401 (17,206 − 23,202) |
6.13 (5.17–6.97) |
11,967 (8,863 − 15,766) |
1.93 (1.43–2.54) |
-3.92 (-4.11 to -3.74) |
| Southeast Asia |
6,405 (5,359-7,510) |
4.13 (3.45–4.84) |
3,097 (2,487-3,898) |
1.34 (1.07–1.68) |
-3.44 (-3.52 to -3.36) |
| Southern Latin America |
114 (97–135) |
0.72 (0.61–0.85) |
37 (29–46) |
0.17 (0.13–0.21) |
-3.65 (-3.96 to -3.34) |
| Southern Sub-Saharan Africa |
623 (473–820) |
4 (3–4) |
475 (363–614) |
1.73 (1.33–2.24) |
-0.98 (-2.08 to 0.13) |
| Tropical Latin America |
1,225 (1,093 − 1,373) |
2.38 (2.12–2.67) |
345 (299–393) |
0.46 (0.4–0.52) |
-4.44 (-4.88 to -4) |
| Western Europe |
87 (77.- 98) |
0.07 (0.07–0.08) |
20 (17–22) |
0.02 (0.01–0.02) |
-3.99 (-4.28 to -3.69) |
| Western Sub-Saharan Africa |
6,064 (4,654-7,378) |
10.45 (8.02–12.71) |
9,660 (7,354 − 12,669) |
6.08 (4.63–7.97) |
-1.51 (-1.6 to -1.43) |
Abbreviations: ASDR, age-standardized deaths rate; MHD, Maternal Hypertensive Disorders; SDI, sociodemographic index; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study; EAPC, estimated annual percentage change; UIs, uncertainty intervals; CI, confidence interval
Table 4.
The case number and ASR of dalys of MHD in 1990 and 2021 for female by SDI quintiles and by GBD regions, with EAPC from 1990 to 2021
| DALYs | 1990 | 2021 | |||
|---|---|---|---|---|---|
| Number (95% UIs) | ASDAR(95% UIs) | Number (95% UIs) | ASDAR(95% UIs) | EAPC (95% CI) 1990–2021 | |
| Global |
3,479,885 (3,085,882-3,873,201) |
204.25 (181.13-227.34) |
2,469,636 (2,083,398-2,958,212) |
98.99 (83.51-118.58) |
-2.10 (-2.15 to -2.04) |
| High SDI |
33,250 (26,096 − 42,851) |
11.88 (9.32–15.30) |
20,491 (14,260 − 29,256) |
6.63 (4.61–9.46) |
-1.70 (-1.81 to -1.6) |
| High-middle SDI |
139,303 (112,428 − 168,990) |
40.01 (32.29–48.53) |
3488,4 (27,185 − 44,882) |
8.92 (6.95–11.48) |
-4.19 (-4.27 to -4.10) |
| Middle SDI |
690,767 (607,609–780,248) |
121.99 (107.30-137.79) |
329,586 (282,911 − 391,530) |
41.72 (35.81–49.56) |
-3.05 (-3.12 to -2.99) |
| Low-middle SDI |
1,480,228 (1,288,978-1,643,500) |
412.78 (359.45-458.32) |
888,004 (717,495-1,101,565) |
138.21 (111.68-171.45) |
-3.49 (-3.58 to -3.39) |
| Low SDI |
1,134,178 (960,803-1,289,414) |
760.77 (644.48–864.90) |
1,194,634 (983,739-1,454,751) |
331.74 (273.17-403.97) |
-2.57 (-2.67 to -2.47) |
| Andean Latin America |
39,982 (34,224 − 46,689) |
323.59 (276.98-377.87) |
20,884 (15,654 − 27,162) |
95.44 (71.54-124.13) |
-4.19 (-4.41 to -3.96) |
| Australasia | 655(471–921) |
9.96 (7.17–14.01) |
444.13 (270.34-699.83) |
4.84 (2.95–7.63) |
-2.08 (-2.32 to -1.85) |
| Caribbean |
24,004 (19,494 − 29,614) |
204.00 (165.67-251.68) |
30,763 (19,909 − 44,682) |
201.16 (130.19-292.18) |
0.75 (0.53 to 0.96) |
| Central Asia |
16,051 (14,253 − 18,012) |
73.33 (65.12–82.30) |
5,756 (4,747-6,995) |
18.72 (15.43–22.74) |
-4.09 (-4.41 to -3.78) |
| Central Europe |
4,418 (3,638-5,460) |
11.20 (9.22–13.84) |
1,277 (872-1,850) |
3.91 (2.66–5.66) |
-2.81 (-3.24 to -2.37) |
| Central Latin America |
76,045 (68,349 − 83,482) |
139.76 (125.62-153.43) |
35,211 (29,062 − 42,472) |
40.89 (33.75–49.32) |
-3.61 (-3.82 to -3.39) |
| Central Sub-Saharan Africa |
153,719 (108,575 − 202,463) |
930.64 (657.33-1225.74) |
169,383 (120,040–232,144) |
389.90 (276.32-534.37) |
-2.06 (-2.37 to -1.76) |
| East Asia |
125,963 (90,448 − 167,330) |
30.85 (22.16–40.99) |
18,997 (13,979 − 25,375) |
4.38 (3.22–5.85) |
-5.36 (-5.61 to -5.11) |
| Eastern Europe |
16,522 (13,599 − 20,732) |
22.98 (18.91–28.83) |
5,234 (3,197-8,326) |
8.55 (5.22–13.60) |
-2.62 (-2.97 to -2.26) |
| Eastern Sub-Saharan Africa |
470,623 (406,953 − 536,555) |
813.24 (703.22-927.17) |
411,016 (334,512 − 500,683) |
293.55 (238.91-357.59) |
-3.17 (-3.31 to -3.02) |
| High-income Asia Pacific |
4,774 (3,779-6,014) |
8.33 (6.59–10.49) |
1,393 (951-2,012) |
2.84 (1.94–4.10) |
-3.66 (-4.1 to -3.23) |
| High-income North America |
11,437 (8,401 − 15,916) |
12.65 (9.29–17.60) |
11,120 (7,956 − 14,920) |
10.37 (7.42–13.91) |
-0.32 (-0.51 to -0.13) |
| North Africa and Middle East |
272,820 (219,474 − 324,251) |
263.81 (212.23-313.54) |
112,009 (84,543 − 145,990) |
55.54 (41.92–72.39) |
-4.78 (-4.97 to -4.59) |
| Oceania |
2,858 (1,662-4,244) |
141.32 (82.17-209.85) |
5,144 (3,595-7,086) |
116.13 (81.17-159.99) |
-0.55 (-0.73 to -0.36) |
| South Asia |
1,315,573 (1,116,161-1,497,912) |
395.48 (335.53-450.29) |
746,671 (559,140–976,528) |
120.16 (89.98-157.15) |
-4.00 (-4.19 to -3.81) |
| Southeast Asia |
404,655 (338,524 − 473,087) |
260.80 (218.18-304.91) |
194,162 (158,026–242,903) |
83.75 (68.16-104.77) |
-3.37 (-3.44 to -3.29) |
| Southern Latin America |
8,168 (6,944-9,670) |
51.38 (43.68–60.83) |
3,729 (2,812-4,885) |
17.05 (12.86–22.33) |
-2.79 (-3.02 to -2.56) |
| Southern Sub-Saharan Africa |
41,239 (32,037–53,875) |
238.18 (185.03-311.16) |
31,625 (24,643 − 39,964) |
115.45 (89.96–145.90) |
-1.05 (-2.04 to -0.05) |
| Tropical Latin America |
77,629 (69,713 − 86,526) |
150.87 (135.49-168.16) |
24,210 (20,615 − 27,912) |
32.09 (27.32–36.99) |
-4.06 (-4.48 to -3.64) |
| Western Europe |
9,219 (7,253 − 12,289) |
7.75 (6.10-10.33) |
5,300 (3,362-8,270) |
4.39 (2.79–6.85) |
-1.36 (-1.51 to -1.2) |
| Western Sub-Saharan Africa |
403,528 (314,574 − 491,338) |
695.37 (542.08-846.69) |
635,307 (496,632–820,756) |
399.91 (312.62-516.64) |
-1.57 (-1.65 to -1.49) |
Abbreviations: ASDAR, age-standardized DALYs rate; DALYs, Disability-Adjusted Life Years; MHD, Maternal Hypertensive Disorders; SDI, sociodemographic index; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study; EAPC, estimated annual percentage change; UIs, uncertainty intervals; CI, confidence interval
MHD burden by age group: Temporal and regional trends
From a temporal perspective, the global burden of Maternal Hypertensive Disorders (MHD) gradually declined between 1990 and 2021 (Fig. 2). However, in examining different SDI categories, heat maps reveal that among individuals aged 10–54, Low SDI regions bear the heaviest burden (Fig. 2), exhibiting the highest incidence, prevalence, mortality, and DALY rates (Supplementary Tables 8–11). From an age-structure standpoint, the global burden of MHD is primarily concentrated in the 20–24 and 25–29 age groups. In High and High-Middle SDI regions, MHD incidence and prevalence peak in the 25–29 and 30–34 age groups. In contrast, in Middle and Low-Middle SDI regions, incidence, prevalence, mortality, and DALY rates are highest among those aged 20–24 and 25–29. Notably, in Low SDI regions—especially for individuals aged 25–29—incidence, prevalence, mortality, and DALY rates are the highest (Fig. 2). Detailed country-specific results on MHD burden by age group, time period, and region can be found in Supplementary Tables 12–15.
Fig. 2.
Heat map of burden trends in different age groups and regions. A The incidence rate of nine age groups (with 5-year intervals within 10–54 years) from 1990 to 2021, both globally and in five territories (ranging from low to high SDI); B The prevalence rate of nine age groups (with 5-year intervals within 10–54 years) from 1990 to 2021, both globally and in five territories (ranging from low to high SDI); C The deaths rate of nine age groups (with 5-year intervals within 10–54 years) from 1990 to 2021, both globally and in five territories (ranging from low to high SDI); D The DALYs rate of nine age groups (with 5-year intervals within 10–54 years) from 1990 to 2021, both globally and in five territories (ranging from low to high SDI). Abbreviations: DALYs, disability-adjusted life-years; SDI, sociodemographic index
Cross-country inequality analysis
Significant absolute and relative inequalities associated with SDI were observed in the burden of Maternal Hypertensive Disorders (MHD), and these inequalities decreased markedly over time (Fig. 3). Interestingly, the number of deaths was disproportionately concentrated in countries with lower levels of social and demographic development. According to the slope index of inequality, in 1990, the difference in deaths per 100,000 population between countries with the highest and lowest SDI was − 11.90 (95% CI: -12.80, -11.00); by 2021, it had decreased to -4.0 (95% CI: -4.40, -3.50) (Supplementary Table 16). Furthermore, the concentration index—measuring relative gradient inequality—was − 0.57 (95% CI: -0.64, -0.50) in 1990 and − 0.60 (95% CI: -0.68, -0.52) in 2021, indicating an unequal distribution of MHD burden among countries with varying SDI levels.
Fig. 3.
SDI-related health inequality regression (A) and concentration (B) curves for the Deaths of MHD worldwide, 1990 and 2021. Abbreviations: SDI, socio demographic index; MHD, Maternal Hypertensive Disorders
Risk factor analysis
Among all potential risk factors identified in GBD 2021, global age-standardized Maternal Hypertensive Disorders (MHD) mortality and DALYs were primarily attributed to iron deficiency. The age-standardized mortality rate was 0.27% (95% CI: 0.13–0.39), and the age-standardized DALY rate was 0.46% (95% CI: 0.22–0.66) (Supplementary Table 17). Between 1990 and 2021, both the age-standardized mortality and DALY rates associated with iron deficiency showed a downward trend (Fig. 4A–B). The burden of iron deficiency was particularly severe in Low SDI regions, as well as in Central, Eastern, and Western Sub-Saharan Africa.
Fig. 4.
Trends in the rate of risk factors attributable to MHD in 27 regions around the world from 1990 to 2019 to 2021. A Trends in the rate of risk factors to age-standardized DALYs of MHD in 27 regions around the world from 1990 to 2019 to 2021. B Trends in the rate of risk factors to age-standardized death of MHD in 27 regions around the world from 1990 to 2019 to 2021. Abbreviations: DALYs, disability-adjusted life-years; MHD, Maternal Hypertensive Disorders
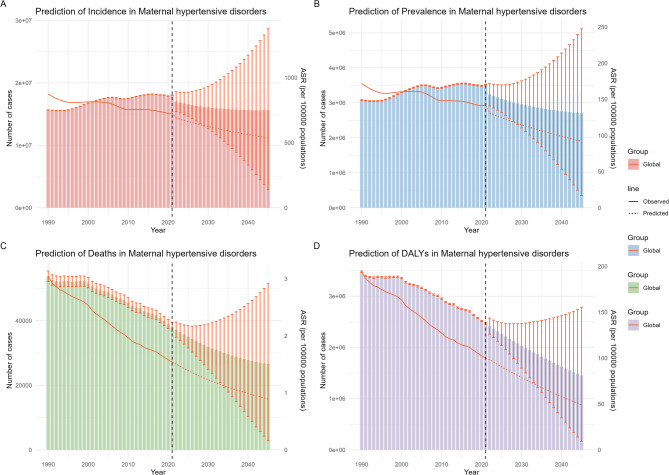
Prediction model analysis
Figure 5 presents the predicted number of Maternal Hypertensive Disorders (MHD) cases in 2045, along with the age-standardized rates (ASR) for incidence, prevalence, mortality, and DALYs. Globally, the number of incidence, prevalence, mortality, and DALY cases is expected to decrease by 2045, and each corresponding ASR is projected to decline annually. Detailed global values for the number of cases and ASRs are provided in Supplementary Table 18. Supplementary Fig. 2 illustrates the projected number of cases by age group in 2045. The number of incidence and prevalence cases is forecast to decrease, primarily concentrated among individuals aged 20–24, 25–29, and 30–34. Likewise, mortality and DALYs are also expected to show a marked decline, concentrated mainly in the 20–24 and 25–29 age groups. For specific values, please refer to Supplementary Table 19.
Fig. 5.
Projected the ASIR、ASPR、ASDR、ASDAR and the burden casess of MHD in global from 2022 to 2045
A The number of incidence casess and ASIR of MHD in global from 2022 to 2045. B The number of prevalence casess and ASPR of MHD in global from 2022 to 2045. C The number of deaths cases and ASDR of MHD in global from 2022 to 2045. D The number of DALYs cases and ASDAR of MHD in global from 2022 to 2045. Abbreviations: ASIR, age-standardized incidence rate; ASPR, age-standardized rate; ASDR, age-standardized deaths rate; ASDAR, age-standardized DALYs rate; DALYs, disability-adjusted life-years; MHD, Maternal Hypertensive Disorders
Two-Sample MR analysis
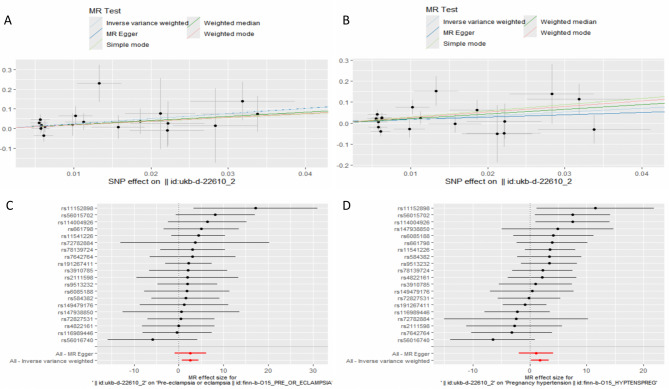
We included nine occupational exposure factors to explore their causal relationships with six MHD outcomes. Notably, gestational hypertension served as the outcome for six different cohorts. After selecting the instrumental variables (IVs), we identified 2,317 independent SNPs associated with these occupational exposures (Supplementary Table 3). We then performed a comprehensive two-sample MR analysis to investigate the causal relationship between occupational pollution and maternal hypertensive disorders. Specifically, we used a range of MR methods—IVW, MR-Egger, weighted median, simple mode, and weighted mode—to evaluate the effects of particulate matter air pollution (PM2.5, PM10), gas or solid-fuel cooking/heating (an open solid fuel fire used regularly in winter), maternal smoking around birth, nitrogen oxides air pollution, working in an environment often filled with chemical or other fumes, heating type(s) in the home (oil/kerosene central heating), occasional pesticide exposure at work, and frequent exposure to very noisy workplaces. Our findings indicated a significant causal relationship between Workplace full of chemical or other fumes: Often and both preeclampsia/eclampsia as well as gestational hypertension (Table 5). The forest and scatter plots visually depict the notable associations between Workplace full of chemical or other fumes: Often and preeclampsia/eclampsia as well as gestational hypertension (Fig. 6). Using the IVW method, we observed an odds ratio (OR) of 13.08 (95% CI, 2.04–83.65) for preeclampsia/eclampsia, and an OR of 5.88 (95% CI, 1.23–28.06) for gestational hypertension. Conversely, our results showed no substantial causal relationship between gestational hypertensive disorders and PM2.5, PM10, gas or solid-fuel cooking/heating, maternal smoking around birth, nitrogen oxides air pollution, oil (kerosene) central heating in the home, occasional pesticide exposure at work, or frequent exposure to very noisy workplaces. Although IVW is an efficient estimator of causal effects when its assumptions hold, the results may be unreliable in the presence of significant horizontal pleiotropy, heterogeneity in the IV effect, weak IVs or outliers, or complex nonlinear relationships between exposure and outcome. To address these concerns, we conducted MR-Egger intercept tests for pleiotropy, and the results for both outcomes (preeclampsia/eclampsia and gestational hypertension) showed Egger P-values > 0.05, indicating no significant horizontal pleiotropy (Supplementary Table 20). Heterogeneity tests using both MR-Egger and IVW (Q statistics) also yielded P-values > 0.05, suggesting no notable heterogeneity(Supplementary Table 21). The overall symmetry of funnel plots confirmed this finding, suggesting minimal heterogeneity among the included SNPs (Supplementary Fig. 3A–B). Additionally, a leave-one-out analysis underscored the stability of the MR effect estimates (Supplementary Fig. 3C–D). Taken together, these results suggest that the estimated causal effects from the selected instrumental variables are credible, thereby reinforcing the robustness and interpretability of our findings.
Table 5.
Mendelian randomization analysis identified several significant causal occupational exposure factors and maternal hypertensive disorders
| Outcomes | Exposure | Method | Nsnp | SE | Pval | OR | 95%CI |
|---|---|---|---|---|---|---|---|
| Pre-eclampsia or eclampsia | Workplace full of chemical or other fumes: Often | IVW | 20 | 0.95 | 0.007 | 13.08 | (2.04–83.65) |
| IVW | 20 | 1.80 | 0.165 | 13.5 | (0.40-457.9) | ||
| MR Egger | 20 | 1.30 | 0.105 | 8.21 | (0.64-104.42) | ||
| Weighted median | 20 | 2.01 | 0.368 | 6.39 | (0.12-330.39) | ||
| Simple mode | 20 | 1.70 | 0.273 | 6.77 | (0.24-188.26) | ||
| Pregnancy hypertension | Workplace full of chemical or other fumes: Often | IVW | 20 | 0.80 | 0.026 | 5.88 | (1.23–28.06) |
| MR Egger | 20 | 1.54 | 0.489 | 2.98 | (0.14–61.30) | ||
| Weighted median | 20 | 1.03 | 0.032 | 9.03 | (1.21–67.32) | ||
| Simple mode | 20 | 1.93 | 0.144 | 18.96 | (0.43-831.88) | ||
| Weighted mode | 20 | 1.70 | 0.135 | 14.25 | (0.51-401.56) |
Fig. 6.
Scatter plot and forest plots showed the genetic association between occupational exposure factors and MHD. A, C Workplace full of chemical or other fumes: Often on Pre-eclampsia or eclampsia. B, D Workplace full of chemical or other fumes: Often on Pregnancy hypertension. Abbreviations: MHD, Maternal Hypertensive Disorders
Discussion
Based on the 2021 Global Burden of Disease (GBD) data, this study systematically analyzed the global epidemiological trends of maternal hypertensive disorders (MHD) from 1990 to 2021 and projected their future burden. To our knowledge, this is the first comprehensive epidemiological study that considers global burden trends, cross-national inequalities, risk factor assessments, predictive analysis, and, importantly, the causal relationship between occupational exposures and MHD. Our findings indicate that, worldwide, the incidence, prevalence, mortality, and disability-adjusted life years (DALYs) of MHD have generally declined. However, in certain countries and regions, these indicators deviate from the global trend, underscoring the need for targeted public health strategies in these priority areas.
From the perspective of the Social Demographic Index (SDI) distribution, the MHD burden is mainly concentrated in regions with lower SDI (Low SDI). High SDI regions show peak incidence and burden at 30–34 years old, whereas Low SDI regions reach similar levels earlier, at 25–29 years. Cross-national inequality analyses reveal that, between 1990 and 2021, the slope index of inequality decreased significantly, suggesting that the uneven burden of MHD across different countries has improved. Iron deficiency remains the leading risk factor for MHD, posing the greatest challenge in Low SDI areas. Bayesian age-period-cohort (BAPC) model projections indicate that, by 2045, incidence, prevalence, mortality, and DALY cases of MHD will continue to decline, with decreasing age-standardized rates (ASRs). Furthermore, two-sample Mendelian randomization analysis shows a significant causal association between frequent exposure to chemicals or fumes in the workplace Workplace full of chemical or other fumes: Often and MHD, offering new evidence and perspectives on the role of occupational exposures as a primary risk factor for MHD.
Similar to previous GBD studies, analyses from 1990 to 2019 also suggested marked decreases in the age-standardized death rate (ASDR) and age-standardized DALY rate for MHD [26]. The cross-national inequality analysis demonstrates that the slope index of inequality has significantly declined from 1990 to 2021, reflecting an overall improvement in the uneven distribution of MHD burden among countries. This achievement can be attributed to the development and prioritization of the World Health Organization’s maternal health programs over the past three decades, as well as the continual progress of large-scale clinical research supported by various governments [27– 31]. These efforts have contributed to more effective primary prevention and improvement in mortality and DALY rates. Nevertheless, some regions—namely Central Asia, Eastern Europe, and Western Europe—have seen rising incidence and prevalence of MHD, while the Caribbean region has experienced increasing mortality and DALYs. Overall, the surge in incidence and prevalence of MHD in Central Asia, Eastern Europe, and Western Europe, as well as the rise in mortality and DALYs in the Caribbean, can be traced to multiple interacting factors. These include an aging population and increased pregnancies at advanced maternal age, lifestyle changes and rising obesity, a growing burden of chronic diseases, improved diagnostic and monitoring capacities, disparities in healthcare resources and medical conditions, and shifts in regional demographics [32, 33]. In response to the increase in prevalence in some regions (Central Asia, Eastern Europe, and Western Europe), we emphasize the need for enhanced early screening and prenatal risk stratification, which may help identify and manage maternal hypertensive disorders (MHD) before complications arise. The increase in prevalence may also reflect improved detection rather than an increase in burden, which requires nuanced interpretation and response. For regions where mortality and disability-adjusted life-year (DALY) rates are increasing but remain high (the Caribbean), we recommend targeted interventions to improve access to emergency obstetric care, ensure the availability of antihypertensive medicines during pregnancy, and train health workers in the early recognition and management of pre-eclampsia and eclampsia. Previous studies indicate that, in Latin America and the Caribbean, hypertensive disorders account for about 26% of maternal deaths, with 10% of these deaths linked to eclampsia [34]. This figure is noticeably higher than the 9% reported in Africa and Asia. Although maternal mortality in high-income countries is substantially lower than in developing regions, 16% of maternal deaths are still attributable to hypertensive disorders [35]. Moreover, high SDI regions generally exhibit higher economic and cultural levels, leading to more frequent pregnancies at advanced maternal age [36], whereas this trend is less pronounced in low SDI regions. These observations help explain why, in our results, incidence and prevalence rates in high SDI areas shift from the 25–29 age group to 30–34, whereas they remain predominantly within the 25–29 range in low SDI regions.
Quantifying cross-national inequalities in the burden of MHD across different SDI gradients could illuminate distribution patterns and highlight areas for improvement in prevention and control. It is widely accepted that high-SDI countries have better access to healthcare systems, which makes them more efficient—factors that may reduce the burden of disease. The concentration of MHD burden in low-SDI countries stems from two key issues. First, the risks associated with iron deficiency are most severe in these regions; second, low-SDI countries face enormous challenges due to population growth and insufficient health resources. To this end, we recommend expanding access to iron supplements and dietary fortification programmes for women of childbearing age. Integrate nutritional assessment and counselling into routine antenatal care in response to the World Health Organization’s global initiative to halve the prevalence of anaemia by 2025. Establish national health surveillance systems that can break down data by SDI, geography and age group to monitor trends are recommended. International support and collaboration are encouraged, particularly through WHO and UN initiatives to allocate resources and technical assistance to high-burden, low-SDI areas. In addition, we note that while the incidence and prevalence rates in other SDI categories (e.g., low-SDI and high-SDI regions) have declined significantly, the lack of significant changes in the incidence and prevalence rates in high-middle SDI regions and Andean Latin America may reflect multiple factors, such as: Stable access to and coverage of health care: Health system improvements in these regions may have reached a plateau, resulting in neither a significant increase nor a significant decrease in the detection or reporting of incidence. For example, in the Andean Latin America region, while national policies (maternal health programs) may have stabilized results, persistent local disparities—particularly in rural or indigenous communities—may mask local increases in morbidity or mortality. Sociodemographic transitions: Changes in maternal age, urbanization, and occupational patterns may offset each other in terms of risk factor burden. For example, in high-middle SDI regions, moderate improvements in health care (access to prenatal care) may be offset by rising risk factors such as delayed childbearing, obesity, or occupational exposures. Data quality limitations: Fluctuations in surveillance or registry completeness may dampen trends in some subregions. Accordingly, we recommend: Continue to invest in maternal health surveillance systems to improve trend sensitivity. In the Andean Latin America region, expand community prenatal services in underserved areas to address geographic inequalities, and strengthen nutrition programs, prioritizing iron supplementation and dietary support, given that iron deficiency is a major risk factor for MHD worldwide. Policy priorities should be to develop prevention strategies targeting occupational and environmental risk profiles, especially in urbanized high-middle SDI regions. Targeted occupational hazards can be reduced, and workplace safety regulations can be enforced to reduce exposure to chemical fumes, which are modifiable risk factors highlighted by our MR results. and incorporating obesity and hypertension screening into routine prenatal care to mitigate compounding metabolic risks.
Conduct targeted research on region-specific risk factors that may not be captured by current GBD models. These strategies align with our research’s emphasis on tailored interventions—combining risk-targeted mitigation measures and strengthening systemic health care—to address stagnation in regions where trends are not evident.
Within the GBD 2021 database, iron deficiency stands out as the primary risk factor for MHD. As in the GBD 2019 findings, reductions in iron deficiency–attributable ASDR, age-standardized DALYs, and years of life lost (YLL) have been notable among maternal diseases, including hypertensive disorders in pregnancy [26]. Research by Woodman et al. showed that perinatal iron deficiency combined with a high-salt diet can result in sex-dependent, long-term mitochondrial dysfunction and oxidative stress in the kidneys, potentially elevating the risk of hypertension and cardiovascular diseases [37]. However, large-scale clinical studies remain necessary to determine effect size and intervention efficacy in the broader population. In 2023, the WHO called for accelerated action to reduce iron deficiency–related anemia in pregnant women and introduced its first comprehensive framework for reducing anemia, launched at the International Maternal and Child Health Conference. The WHO urges countries to act quickly, aiming to halve the prevalence of anemia among women of reproductive age by 2025. In line with this target, public health policies should incorporate screening and management of iron deficiency and high-salt diets into routine prenatal checkups and nutritional counseling. As GBD data updates continue and basic research further illuminates the mechanisms linking perinatal nutrition and disease, clinical and public health efforts must keep emphasizing iron deficiency prevention and dietary interventions to lower maternal morbidity and diminish chronic disease risk for future generations.
This study employed a Bayesian age-period-cohort (BAPC) model based on GBD data from 1990 to 2021 to project the global burden of MHD from 2022 to 2045. The results suggest that, over the next three decades, the age-standardized rates (ASR) of incidence, prevalence, mortality, and DALYs for MHD will likely decline annually, alongside overall reductions in these four indicators. With continued advancements in diagnostic capabilities and relevant interventions, the MHD burden is expected to decrease further. Several points, however, require consideration: Suitability of BAPC: BAPC incorporates age, period, and cohort effects, allowing a more nuanced analysis of disease dynamics over time. Compared to traditional age-period-cohort analyses, the Bayesian approach partially mitigates the identifiability problem and manages uncertainties more effectively over a long projection horizon. Nonetheless, BAPC remains sensitive to model assumptions and priors; inappropriate choices may introduce bias [38]. Exogenous Shocks: These projections rely on WHO population estimates to 2050. Major policy changes, improvements in healthcare services, or major global events (e.g., pandemics, wars, economic or occupational crises) could alter future trends. Population Demographics: While population aging drives higher incidence and mortality for some chronic diseases, shifts in fertility rates and disease profiles might reduce the overall share of MHD in future populations [39]. Dynamic Monitoring and Model Adjustment: In a rapidly changing global health landscape marked by emerging infectious diseases, occupational changes, and public health emergencies, regular data updates and model recalibrations are crucial for accurate projections. Overall, using the BAPC approach with GBD data, our study is the first to forecast a year-by-year global burden for MHD from 2022 to 2045. The “optimistic” trend of declining rates suggests that, if the current effective healthcare interventions and public health measures are sustained and expanded, the global burden of MHD could be further reduced. Nonetheless, caution is advised in interpreting and applying these projections, as they remain subject to model assumptions, data limitations, and unforeseen external factors.
In addition, this study used Mendelian randomization (MR) to explore potential causal relationships between occupational pollutants and MHD through genome-wide association study (GWAS) data, thereby complementing existing research on occupational exposures as a risk factor for hypertensive disorders in pregnancy. Our findings suggest no causal relationship between PM2.5 exposure and MHD, whereas “Workplace full of chemical or other fumes: Often” is significantly associated with MHD. This contradicts some earlier research, which consistently identified significant correlations between PM2.5 and MHD [40–44]. Traditional epidemiological studies typically rely on occupational or individual monitoring data, which may introduce measurement error and bias. Variations in exposure windows (e.g., early vs. mid pregnancy) may also affect findings. Compared to PM2.5, exposure to chemicals or fumes in the workplace is often more concentrated, more intense, and more consistently accumulated during working hours. Moreover, GWAS data for such workplace exposures often include clearer questionnaire- or phenotype-based information (e.g., job type, frequency of exposure). Consequently, in the setting of “intense and specific occupational exposure,” genetic instruments may more robustly capture the relationship between exposure and outcomes, making it easier to detect causal associations via MR. However, our results also show that the 95% confidence interval for the association between “Workplace full of chemical or other fumes: Often” and MHD is extremely wide, indicating a high degree of uncertainty. Although the finding is statistically significant, its clinical interpretation warrants caution. A lower bound of 2.04 suggests at least a weak positive correlation, whereas an upper bound of 83.65 reflects a high degree of imprecision—one of the main limitations of this study. Future research could consider additional statistical methods or stratified analyses to minimize confounding influences. Our results suggest a potential causal relationship but do not provide definitive evidence and should be interpreted in the context of genetic and environmental parameters of the study population.
Limitations
Our research faces several limitations. Data sparsity remains a challenge in many low-income or conflict-affected settings, where estimates rely more on models than on empirical evidence. Misclassification bias or differences in diagnostic criteria (e.g., preeclampsia vs. gestational hypertension) can affect comparability across settings. The modeling framework assumes stationary covariate effects over time, which may not fully capture changing health care access, diagnostic practices, or sociodemographic changes. Besides, the use of age-standardized rates may mask important subnational or age-specific patterns that are policy-relevant but obscured in aggregate estimates. GBD estimates rely heavily on aggregated data from hospital records, health surveys, and demographic data, which may vary in completeness, diagnostic accuracy, and temporal consistency across regions. This may lead to systematic underreporting, especially in resource-poor settings, thereby potentially underestimating the burden in low-SDI countries and exaggerating inequality indicators. We hereby explicitly state that caution is warranted when interpreting regional comparisons based on model estimates. Because GBD data are aggregated at the aggregate level and parity is not tracked separately, it is indeed possible that some women contribute to the incidence count more than once in different pregnancies, which may slightly increase the burden. In our Mendelian randomization section, we noted that instrumental variables derived from genome-wide association studies (GWAS) of European ancestry may not be fully generalizable to global populations. In addition, pleiotropy or weak instrument bias may shift causal estimates in either direction. Although sensitivity analyses support the validity of our instruments, we now explicitly state that MR results should be interpreted with caution, especially in multiethnic or underrepresented populations, and large-scale cohort studies are necessary to validate these findings for clinical and public health applications.
Conclusion
Drawing on the GBD 2021 database, this study systematically examined maternal hypertensive disorders (MHD) from multiple angles—global burden trends, cross-national inequalities, risk factor assessments, and future projections—covering the period from 1990 to 2021. Overall, MHD incidence, prevalence, mortality, and DALYs have declined worldwide, but certain regions (such as Central Asia, Eastern Europe, Western Europe, and the Caribbean) continue to show sustained increases. Our two-sample Mendelian randomization analysis further revealed a potential causal link between frequent chemical or fume exposures in the workplace and MHD, warranting further investigation into their role in maternal health outcomes. We urge global public health policymakers to reinforce iron deficiency prevention, promote nutritional and high-salt diet interventions during pregnancy, and systematically monitor workplace exposures. Strengthening these primary prevention and precision interventions can help build on existing achievements and reduce the global burden of hypertensive disorders in pregnancy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank the participants and researchers involved in the FinnGen study, the GWAS databases and and researchers used in the study.
Author contributions
Jun-peng Xiong and Shu-Wen Chen conceived the study concept and designed the study. Jun-peng Xiong and Shu-Wen Chen acquired and curated the data. Jun-peng Xiong, Han Wang, Xiao-nan Yang, Xin-yi Chen conducted the data analysis and analysis visualizations. Shu-Wen Chen contributed to data analysis. Jun-peng Xiong the initial draft of the article. Rong-hui Yu and Bin-quan You are the guarantor for the study. All of the authors contributed to the revisions of the article and had full access to the data.
Funding
This study was supported by the Anhui Province Clinical Medicine Research Transformation Project (202427b10020101), Luliang Municipal Science and Technology Bureau High-level Scientific and Technological Talents Special Project (2024RC23),Suzhou Clinical Key Disease Diagnosis and Treatment Technology Special Project (LCZX202237).
Data availability
All data used in this study can be performed using published GWAS summary statistics and GBD 2021 portal (http://ghdx.healthdata.org/gbd-2021). In the prediction analysis, population estimates were obtained from the 2019 revision of the United Nations World Population Prospects, disaggregated by year (up to 2100), age, and sex (https://population.un.org/wpp/downloads). Exposure data on occupational pollutants and MHD case data were obtained from the UK Biobank and FinnGen databases, respectively, GWAS summary statistics on MHD were derived from the FinnGen consortium and downloaded from (https://www.finngen.fi/en). GWAS summary statistics of instrumental variables were obtained from (https://gwas.mrcieu.ac.uk/), and source data of the GWAS are available in the supplementary tables of the article.
Declarations
Ethics approval and consent to participate
Ethical approval and consent are not required because this study used publicly available secondary data that were aggregated and nonidentified.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junpeng Xiong and Shuwen Chen contributed equally to this work and shared first authorship.
Contributor Information
Binquan You, Email: youbinquan@126.com.
Ronghui Yu, Email: yuronghui1971@163.com.
References
- 1.Hinkle SN, Schisterman EF, Liu D, et al. Pregnancy complications and Long-Term mortality in a diverse cohort. Circulation. 2023;147(13):1014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garovic VD, Dechend R, Easterling T et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association [published correction appears in Hypertension. 2022;79(3):e70. 10.1161/HYP.0000000000000212]. Hypertension. 2022;79(2):e21–e41. 10.1161/HYP.0000000000000208. [DOI] [PMC free article] [PubMed]
- 3.Huang C, Wei K, Lee PMY, Qin G, Yu Y, Li J. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: national population based cohort study [published correction appears in BMJ. 2022;379:o2726. 10.1136/bmj.o2726]. BMJ. 2022;379:e072157. Published 2022 Oct 19. 10.1136/bmj-2022-072157. [DOI] [PMC free article] [PubMed]
- 4.Crump C, Sundquist J, Sundquist K. Adverse pregnancy outcomes and Long-Term mortality in women. JAMA Intern Med. 2024;184(6):631–40. 10.1001/jamainternmed.2024.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leon LJ, McCarthy FP, Direk K, et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: A CALIBER study. Circulation. 2019;140(13):1050–60. 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 6.Barrett PM, McCarthy FP, Evans M, et al. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: A Swedish registry-based cohort study. PLoS Med. 2020;17(8):e1003255. 10.1371/journal.pmed.1003255. Published 2020 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L, Tang K, Magee LA, et al. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat Rev Endocrinol. 2022;18(12):760–75. 10.1038/s41574-022-00734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Cheng F, Fu Q, Cheng P, Zuo J, Wu Y. Time trends in maternal hypertensive disorder incidence in Brazil, Russian Federation, India, China, and South Africa (BRICS): an age-period-cohort analysis for the GBD 2021. BMC Pregnancy Childbirth. 2024;24(1):731. Published 2024 Nov 8. 10.1186/s12884-024-06931-z. [DOI] [PMC free article] [PubMed]
- 9.Kobashi G. Genetic and occupational factors associated with the development of hypertension in pregnancy. J Epidemiol. 2006;16(1):1–8. 10.2188/jea.16.1. [DOI] [PMC free article] [PubMed]
- 10.Niu Z, Habre R, Yang T, et al. Increased risk of gestational hypertension by periconceptional exposure to ambient air pollution and effect modification by prenatal depression. Hypertension. 2024;81(6):1285–95. 10.1161/HYPERTENSIONAHA.123.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honigberg MC, Truong B, Khan RR, et al. Polygenic prediction of preeclampsia and gestational hypertension. Nat Med. 2023;29(6):1540–9. 10.1038/s41591-023-02374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Wang P, Zhang L, et al. Ozone exposure during pregnancy and risk of gestational hypertension or preeclampsia in China. JAMA Netw Open. 2023;6(4):e236347. 10.1001/jamanetworkopen.2023.6347. Published 2023 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Melo NC, Sampaio E, Souza PC, Marques RC, Bernardi JVE, Bastos WR, Cunha MPL. Occupational exposure to metal(loid)s and hypertensive disorders of pregnancy: A systematic review. Environ Res. 2024;257:119391. 10.1016/j.envres.2024.119391. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen M, Nobile F, Stayner LT, de Hoogh K, Brandt J, Stafoggia M. Ambient air pollution and hypertensive disorders of pregnancy in Rome. Environ Res. 2024;251(Pt 1):118630. 10.1016/j.envres.2024.118630. [DOI] [PubMed] [Google Scholar]
- 15.Meeker JD, McArthur KL, Adibi JJ, et al. Urinary concentrations of phthalate metabolites in relation to preeclampsia and other hypertensive disorders of pregnancy in the occupational influences on child health outcomes (ECHO) program. Environ Int. 2024;187:108678. 10.1016/j.envint.2024.108678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Health Data Exchange. Institute for Health Metrics and Evaluation. 2024. Accessed June 26, 2024. https://ghdx.healthdata.org/gbd-2021
- 17.Ferrari AJ, Santomauro DF, Aali A, Abate YH, Abbafati C, Abbastabar H, Abd ElHafeez S, Abdelmasseh M, Abd-Elsalam S, Abdollahi A. Global incidence, prevalence, years lived with disability (YLDs), disability‐adjusted life‐years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. 2024;403:2133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao F, Xu Z, Li XX, et al. Trends and cross-country inequalities in the global burden of osteoarthritis, 1990–2019: A population-based study. Ageing Res Rev. 2024;99:102382. 10.1016/j.arr.2024.102382. [DOI] [PubMed] [Google Scholar]
- 19.Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78. 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD 2021 Diabetes Collaborators. Global, regional, and National burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021 [published correction appears in lancet. 2023;402(10408):1132. Doi: 10.1016/S0140-6736(23)02044-5]. Lancet. 2023;402(10397):203–34. 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed]
- 21.Blangiardo M, Cameletti M, Baio G, Rue H. Spatial and spatio-temporal models with R-INLA. Spat Spatiotemporal Epidemiol. 2013;4:33–49. 10.1016/j.sste.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Lai H, Shi X, et al. Global Temporal trends and projections of acute hepatitis E incidence among women of childbearing age: Age-period-cohort analysis 2021. J Infect. 2024;89(4):106250. 10.1016/j.jinf.2024.106250. [DOI] [PubMed] [Google Scholar]
- 23.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21. 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 24.Hosier H, Lipkind HS, Rasheed H, DeWan AT, Rogne T. Dyslipidemia and risk of preeclampsia: A multiancestry Mendelian randomization study. Hypertension. 2023;80(5):1067–76. 10.1161/HYPERTENSIONAHA.122.20426. [DOI] [PubMed] [Google Scholar]
- 25.Brumpton B, Sanderson E, Heilbron K, et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat Commun. 2020;11(1):3519. 10.1038/s41467-020-17117-4. Published 2020 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu N, Ye E, Ba Y et al. The global burden of maternal disorders attributable to iron deficiency related sub-disorders in 204 countries and territories: an analysis for the Global Burden of Disease study. Front Public Health. 2024;12:1406549. Published 2024 Sep 5. 10.3389/fpubh.2024.1406549 [DOI] [PMC free article] [PubMed]
- 27.Wu P, Green M, Myers JE. Hypertensive disorders of pregnancy. BMJ. 2023;381:e071653. 10.1136/bmj-2022-071653. Published 2023 Jun 30. [DOI] [PubMed] [Google Scholar]
- 28.Qiao J, Wang Y, Li X, et al. A lancet commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet. 2021;397(10293):2497–536. 10.1016/S0140-6736(20)32708-2. [DOI] [PubMed] [Google Scholar]
- 29.Souza JP, Day LT, Rezende-Gomes AC, et al. A global analysis of the determinants of maternal health and transitions in maternal mortality. Lancet Glob Health. 2024;12(2):e306–16. 10.1016/S2214-109X(23)00468-0. [DOI] [PubMed] [Google Scholar]
- 30.Conway F, Portela A, Filippi V, Chou D, Kovats S. Climate change, air pollution and maternal and newborn health: an overview of reviews of health outcomes. J Glob Health. 2024;14(04128). 10.7189/jogh.14.04128. Published 2024 May 24. [DOI] [PMC free article] [PubMed]
- 31.Lee S, Adam AJ. Designing a logic model for mobile maternal health e-Voucher programs in Low- and Middle-Income countries: an interpretive review. Int J Environ Res Public Health. 2021;19(1):295. 10.3390/ijerph19010295. Published 2021 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Requejo J, Diaz T, Park L et al. Assessing coverage of interventions for reproductive, maternal, newborn, child, and adolescent health and nutrition. BMJ. 2020;368:l6915. Published 2020 Jan 26. 10.1136/bmj.l6915 [DOI] [PMC free article] [PubMed]
- 33.Faye CM, Wehrmeister FC, Melesse DY, et al. Large and persistent subnational inequalities in reproductive, maternal, newborn and child health intervention coverage in sub-Saharan Africa. BMJ Glob Health. 2020;5(1):e002232. 10.1136/bmjgh-2019-002232. Published 2020 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99(7):547–53. 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 35.Gestational Hypertension and Preeclampsia. ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135(6):e237–60. 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 36.Fu R, Li Y, Li X, Jiang W. Hypertensive disorders in pregnancy: global burden from 1990 to 2019, current research hotspots and emerging trends. Curr Probl Cardiol. 2023;48(12):101982. 10.1016/j.cpcardiol.2023.101982. [DOI] [PubMed] [Google Scholar]
- 37.Woodman AG, Mah R, Keddie DL, et al. Perinatal iron deficiency and a high salt diet cause long-term kidney mitochondrial dysfunction and oxidative stress. Cardiovasc Res. 2020;116(1):183–92. 10.1093/cvr/cvz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volker J, Schmid. Leonhard held. BAMP– Bayesian age-period-cohort modeling and prediction. J Stat Softw. 2007;21. 10.18637/jss.v021.i08.
- 39.GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. 2024;403(10440):2133–61. 10.1016/S0140-6736(24)00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Sun M, Liang Q, et al. The relationship between long-term exposure to PM2.5 and hypertension in women: A meta-analysis. Ecotoxicol Environ Saf. 2021;208:111492. 10.1016/j.ecoenv.2020.111492. [DOI] [PubMed] [Google Scholar]
- 41.Cao L, Wang L, Wu L, et al. Particulate matter and hypertensive disorders in pregnancy: systematic review and meta-analysis. Public Health. 2021;200:22–32. 10.1016/j.puhe.2021.08.013. [DOI] [PubMed]
- 42.Sun Y, Bhuyan R, Jiao A, et al. Association between particulate air pollution and hypertensive disorders in pregnancy: A retrospective cohort study. PLoS Med. 2024;21(4):e1004395. 10.1371/journal.pmed.1004395. Published 2024 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun M, Yan W, Fang K, et al. The correlation between PM2.5 exposure and hypertensive disorders in pregnancy: A Meta-analysis. Sci Total Environ. 2020;703:134985. 10.1016/j.scitotenv.2019.134985. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Chen J, Wang K, et al. Exposure to ambient black carbon and particulate matter during pregnancy in associations with risk of pre-eclampsia: A meta-analysis based on population-based studies. Environ Pollut. 2024;343:123230. 10.1016/j.envpol.2023.123230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study can be performed using published GWAS summary statistics and GBD 2021 portal (http://ghdx.healthdata.org/gbd-2021). In the prediction analysis, population estimates were obtained from the 2019 revision of the United Nations World Population Prospects, disaggregated by year (up to 2100), age, and sex (https://population.un.org/wpp/downloads). Exposure data on occupational pollutants and MHD case data were obtained from the UK Biobank and FinnGen databases, respectively, GWAS summary statistics on MHD were derived from the FinnGen consortium and downloaded from (https://www.finngen.fi/en). GWAS summary statistics of instrumental variables were obtained from (https://gwas.mrcieu.ac.uk/), and source data of the GWAS are available in the supplementary tables of the article.