Abstract
Background
Transcutaneous peripheral nerve electrical stimulation using high-frequency pulse clusters has been shown to relieve muscle fatigue, though its efficacy remains limited. Furthermore, this approach tends to exacerbate pain during stimulation, which constrains its clinical applications. This paper proposed a novel stimulation waveform to reduce muscle fatigue and the discomfort associated with high-frequency electrical stimulation, and compares it with previously reported high-frequency pulsed cluster stimulation.
Methods
We evaluated our waveform experimentally and through model simulations. During the experiment, two distinct high-frequency narrow pulse clusters were applied to the proximal segment of the median/ulnar nerve bundles: asymmetric random (aSymR) and previously reported symmetric (Sym) stimulation, both with a carrier frequency of 10 kHz. The two stimulation modes aimed to elicit the same contraction level and were maintained for 5 min to induce muscle fatigue. Finger force, high-density electromyographic (EMG) signals of the flexor muscles and the pain score were recorded. In addition, we developed a finite element model of the upper arm and a motor fiber model to simulate motor axon activation of the peripheral nerve induced by the two electrical stimulation modes.
Results
Compared with the Sym stimulation, the aSymR stimulation resulted in less pain and a significant reduction of muscle fatigue rate, which was characterized by slower force decay rate, less absolute force decay, greater plateau force, and ultimately greater force output. In addition, the simulation results showed that the delay for different fibers to reach the threshold was increased by the aSymR mode. Consistent with this, the experiment study showed that the EMG amplitude under the aSymR stimulation condition was smaller before fatigue onset, indicating the less synchronized activation of different muscle fibers.
Conclusions
Compared with the Sym stimulation, the aSymR stimulation can significantly relieve muscle fatigue possibly by reducing the synchronous activation across different fibers. This proposed aSymR stimulation mode not only reduces fatigue but also relieves pain, potentially contributing to the wide application of electrical stimulation in motor function rehabilitation for people with stroke.
Trial registration
Ethics committee of the Medical College of Xi’an Jiaotong University, 2021 − 1550. Registered 4 November 2021.
Keywords: Transcutaneous nerve electrical stimulation, Kilohertz stimulation, Muscle fatigue, Asynchronous axon activation
Background
Upper extremity motor dysfunction is one of the most prevalent chronic disabilities that remain after stroke, profoundly affecting their quality of life [1]. Neuromuscular electrical stimulation (NMES) is a technique that induces functional muscle contraction by transcutaneously stimulating superficial muscles or motor nerves [2], which has also been shown to promote post-stroke motor function recovery [3–5]. Consequently, NMES has been widely adopted in the rehabilitation of motor function in stroke survivors [6, 7]. In conventional NMES techniques, stimulation electrodes are typically positioned near the motor point of the muscle belly to stimulate the distal branches of motor neuron axons [8]. The current pulse width used in these techniques often exceeds several hundred microseconds, which is intended to simultaneously depolarize a substantial number of motor neuron axons causing them all to generate action potentials simultaneously [9]. This leads to synchronous, non-physiological activation of the motor units (MUs) [10] and muscle fibers [11, 12], rendering the stimulated muscle highly susceptible to fatigue and causing a rapid decline in evoked force.
Researchers have explored various strategies to mitigate this rapid muscle fatigue induced by electrical stimulation. For instance, fatigue can be delayed by modifying stimulation parameters such as current amplitude, frequency, and pulse width [13] or by employing spatially and/or temporally distributed stimulation across multiple electrodes in the muscle belly [14]. Shin et al. [15] demonstrated that transcutaneous electrical stimulation of proximal peripheral nerve bundles could slow muscle fatigue by activating different muscle fibers. However, the activation of motor units remained highly synchronous, which still resulted in a significant decline in muscle strength over time. Zheng et al. [16] then proposed using a cluster of narrow pulses with a kilohertz carrier frequency with the peripheral nerve stimulation, wherein a cluster of narrow pulses with a pulse width of less than 100 µs replaced the conventional rectangular pulse. Each narrow pulse provides only subthreshold stimulation to the axon [17], requiring multiple pulses to cumulatively reach the activation threshold [18], thereby reducing synchronized axonal activation. While this high-frequency stimulation alleviated muscle fatigue, it still differs somewhat from natural physiological activation and was reported to substantially increase pain levels [19], thus limiting its potential for clinical application. This study, therefore, aims to expand upon this previously developed high-frequency stimulation technique which will be referred to as the Symmetric (Sym) mode stimulation for the overall symmetric shape of anodic and cathodic pulses.
This study extended and improved upon the previously reported Sym stimulation by proposing an asymmetric random (aSymR) electrical stimulation waveform, with the intention to further reduce fatigue and pain caused by stimulation. The anti-fatigue performance of the proposed waveform was evaluated through experimental investigations and model simulations. Experimentally, we compared muscle activity (i.e., contraction force and EMG) and pain levels induced by the aSymR mode electrical stimulation with those induced by the previously developed Sym mode stimulation in healthy subjects. In the model simulation, a simplified concentric cylindrical model was employed to simulate the potential distribution within the human upper arm during electrical stimulation. These extracellular potentials were then applied as stimulus inputs to a multicompartmental double-cable model of mammalian myelinated nerve fibers, enabling the obtaining of membrane potentials for motor fibers of varying locations and diameters. We hypothesize that our proposed aSymR stimulation reduces muscle fatigue by decreasing the synchronization of axonal activation while also alleviating discomfort, which has the potential to promote the application of electrical stimulation technology in the clinical rehabilitation of stroke survivors.
Methods
To compare the effects of the proposed aSmyR stimulation waveform with the previously reported Sym stimulation mode in mitigating muscle fatigue and alleviating pain, we applied two stimulation modes to healthy subjects to induce muscle fatigue. Muscle fatigue level was assessed by recording and analyzing finger contraction force and EMG activity, while pain level was evaluated through pain score analysis. Additionally, we developed a computational model of the upper arm and motor fibers to simulate nerve activation under two stimulation modes, providing further analysis into the underlying mechanisms of fatigue resistance.
Experiment study
Subjects
Sixteen healthy subjects (eleven males and five females; age: 22–37 years old) with an average age of 24.5 (± 3.95) years were recruited for the study. Inclusion criteria were as follows: (1) healthy adults aged 18–70 years, (2) no self-reported history of neurological/neuromuscular disorders, (3) no cognitive impairment, (4) intact skin and sensory function in bilateral upper extremities, (5) no implanted medical devices or metal prosthetics, and (6) signed informed consent. Exclusion criteria were: (1) diagnosed with any clinically significant or unstable medical disorder, (2) pregnancy/lactation status (confirmed via verbal questioning), (3) suffering from psychiatric disorders such as schizophrenia, bipolar disorder, obsessive-compulsive disorder, personality disorders and major depression, (4) skin damage, infection, hyperalgesia and intolerance at the stimulation areas, (5) wearing of electronic devices such as cardiac pacemakers, and (6) lack of informed consent. All participants signed an informed consent approved by the Ethics Committee of the Medical College of Xi’an Jiaotong University.
Apparatus and data recording
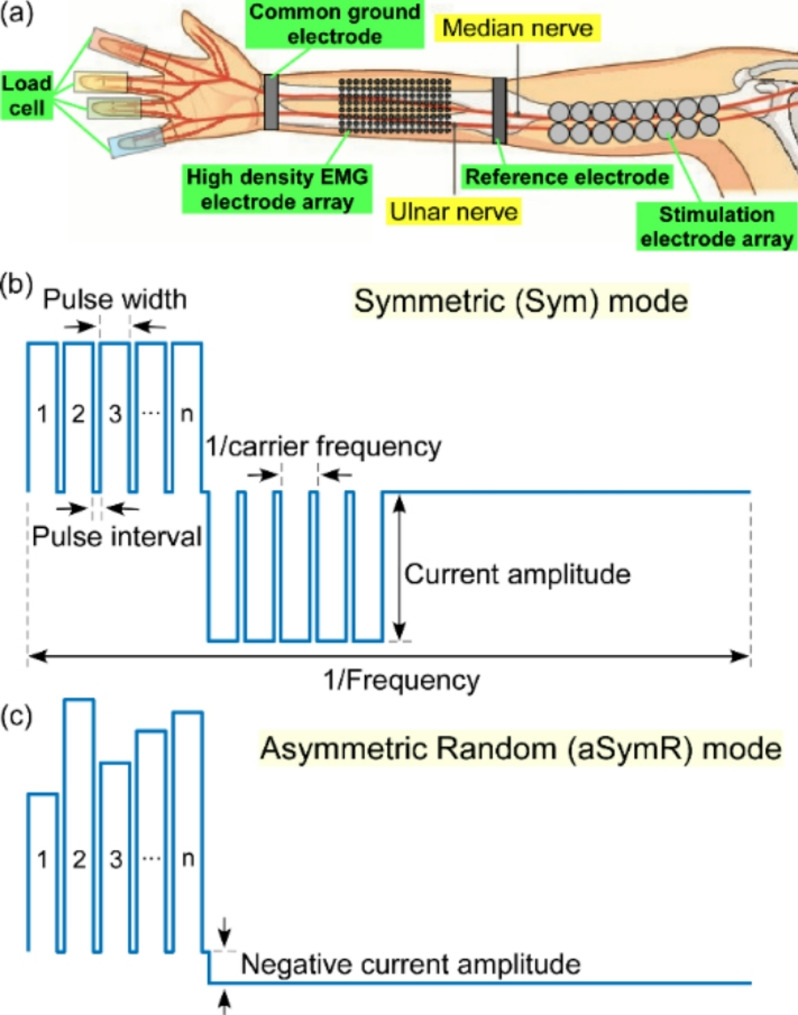
During the experiment, subjects were seated in a chair with their right forearm in a neutral position and the elbow supported by an adjustable frame. The wrist position was stabilized using two rigid resin boards to prevent force contamination arising from wrist movement. Flexion forces of the four fingers—Digit 2 (Index Finger), Digit 3 (Middle Finger), Digit 4 (Ring Finger) and Digit 5 (Little Finger) [20]—were measured using four miniature load cells (DJSX-50 Pressure Sensor, Shanghai Di Jia, China). Four fingers were positioned in a comfortable abducted posture, and Velcro straps were employed to ensure secure contact between the fingers and the load cells for accurate force measurement (Fig. 1(a)). Force data were sampled at 1000 Hz and continuously monitored by the experimenter via a screen. The thumb force was not measured mainly because: (1) The thumb flexors (flexor pollicis longus/brevis) constitute distinct neuromuscular compartments that are mainly innervated by radial nerve and anterior interosseous nerve. However, the median and ulnar nerves were stimulated in this study. (2) The load cells were positioned on one flat plane, whereas the thumb’s oppositional movement trajectory and force transmission pathways are different from the other four fingers.
Fig. 1.
(a) The experimental setup including the stimulation of the ulnar/median nerve, the EMG signals from the finger flexors and the flexion forces of individual fingers. (b) The symmetric high-frequency carrier pulse cluster electrical stimulation train in the Sym mode. (c) The asymmetric random high-frequency carrier pulse cluster electrical stimulation train in the aSymR mode
Two 8 × 8 high-density EMG electrode arrays with an inter-electrode distance of 10 mm and a single-electrode diameter of 3 mm (OT Bioelettronica, Torino, Italy) were placed over the flexor digitorum superficialis (FDS) and the flexor digitorum profundus (FDP) muscles (innervated by the median and ulnar nerves) in the forearm to obtain 128 channels of surface EMG (Fig. 1(a)). The EMG grid covering the entire muscle area enabled the quantification of the spatial distribution of muscle activation. To decrease the contact resistance, the skin was cleaned using an abrasive gel scrub and alcohol pads prior to electrode placement. The placement of the electrode arrays was initially guided by palpating the forearm flexor muscles while the subjects were instructed to voluntarily flex their fingers. Monopolar EMG signals were amplified using an EMG-USB2 + system (OT Bioelettronica, Torino, Italy) with a sample rate of 2048 Hz and a gain of 150. The amplifier’s bandwidth was set to 10–500 Hz. The reference electrode was positioned at the elbow and the common ground electrode was attached at the wrist to reduce stimulation artifacts.
Electrical stimuli were delivered via sixteen gel-based stimulation electrodes (approximately 1 cm in diameter) arranged in a 2 × 8 array, and placed beneath the short head of the biceps brachii along the median and ulnar nerves on the medial side of the upper arm (Fig. 1(a)). This position was selected due to the superficial location of the median and ulnar nerves [19]. The electrodes were connected to the columns of a matrix switch (DAQ970A + DAQM904A, Data Acquisition System + 4 × 8 Two-wire Matrix Switch, Keysight Technologies, USA) with the rows of the matrix switch linked to the anode and the cathode of one channel from the programmable multi-channel stimulator (STG4008, Multichannel Systems, Reutlingen, Germany). A custom-made MATLAB user interface was used to generate stimulation trains with varying parameters and to deliver electrical stimuli to any pair of the 16 electrodes, which enabled the selection of electrode pairs capable of eliciting the desired muscle contraction.
Stimulation paradigm
The asymmetric random (aSymR) stimulation waveform was designed with two main goals: to further reduce muscle fatigue and limit pain during stimulation. Towards the first goal, given that more rapid muscle fatigue during stimulation is associated with the synchronization of axonal activation [21], we introduced random fluctuations in the amplitude of the narrow pulses of the Sym mode stimulation. This approach holds promise for increasing the variability in the number of pulses required for different axons to reach their activation threshold, thereby introducing greater time delays among axonal activations. This strategy was anticipated to further mitigate muscle fatigue during stimulation. Towards the second goal, we incorporated an asymmetric design for the negative pulse in the high-frequency electrical stimulation waveform. Conventional electrical stimulation waveforms are typically symmetrical biphasic pulse waveforms [22], characterized by positive and negative phases with identical intensity and pulse width. This symmetry ensures that the net electrical charges delivered to the anode and cathode cancel out, thereby preventing localized skin burns [18]. However, while the cathodic pulse is known to effectively induce muscle contractions (effective pulse) [23], the anodic pulse primarily balances the electrical charge without contributing to muscle activation (ineffective pulse) [24]. Instead, the anodic pulse may activate pain-conducting fibers when their intensity surpasses the sensory threshold, causing user discomfort. To address this issue, researchers have proposed asymmetric electrical stimulation waveforms, which reduce the current intensity of the ineffective pulse while increasing its pulse width. This adjustment preserves charge balance and muscle force generation by the effective pulse while likely alleviating stimulation-induced discomfort.
The stimulation paradigm was designed to compare contraction force, EMG activity and pain level between asymmetric random (aSymR) high-frequency pulse cluster electrical stimulation and the previously reported symmetric (Sym) high-frequency pulse cluster electrical stimulation [16]. The sample stimulation trains for both modes are illustrated in Figs. 1(b) and (c), respectively. In both modes, clusters of pulses were delivered at an overall stimulation frequency of 40 Hz, which approximates the discharge rates of the motoneurons during voluntary activation [19]. Each pulse cluster then contained a set of high-frequency carrier pulses, each containing n positive-phase narrow pulses. The inter-pulse interval was fixed at 20 µs and the pulse width was set to 80 µs, resulting in a carrier frequency of 10 kHz. These parameters have previously been shown to be able to induce subthreshold depolarization [16]. The stimulation frequency and carrier frequency were consistent across both stimulation methods and all subjects. In the Sym stimulation mode, the amplitude of the high-frequency carrier pulses remains constant and both the positive and negative pulses maintain identical shapes (Fig. 1(b)). In the aSymR stimulation mode, the positive current amplitude of each high-frequency pulse is randomly varied around a set value, while the negative phase is a single low-amplitude pulse that has a duration that extends for the remainder of the 40 Hz stimulation cycle to minimize pain sensations (Fig. 1(c)). Since the duration of this negative pulse is determinable, to ensure charge balance, the negative-phase current amplitude is determined by the ratio of the total charge of the positive-phase pulses to the duration of the negative-phase pulse.
Force-matched stimulation and fatiguing
The maximum voluntary contraction (MVC) of individual fingers was first measured by instructing subjects to sequentially flex one finger at a time maximally against the load cell. For each subject, a pair of electrodes from the 2 × 8 array was selected using the Sym stimulation to identify the stimulation site that induced moderate muscle contractions with at most medium levels of pain. The stimulation current intensity and the number of narrow pulses were then adjusted until at least 30% of MVC was achieved in at least one finger. In the aSymR mode, the number of narrow pulses was adjusted until the elicited force matched that in the Sym mode. In the subsequent experiment, this current amplitude was maintained constant across both modes, such that the mean amplitude of the positive pulses in the aSymR mode was equivalent to the current amplitude in the Sym mode.
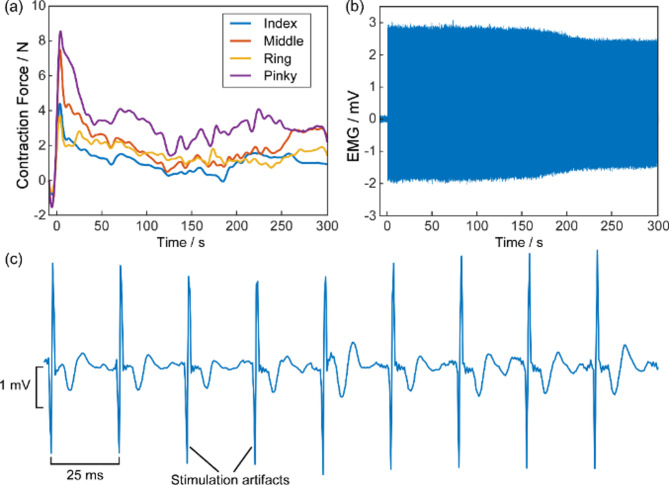
Each subject completed two experimental trials: the Sym stimulation trial and the aSymR stimulation trial. Each trial was stimulated continuously for 5 min to induce muscle fatigue. The order of the two stimulation trials was randomized across subjects to mitigate any potential order effects on the results. A 10-minute rest period was provided between the two trials to facilitate full recovery from muscle fatigue [25]. Pain levels were assessed using the Numerical Rating Scale (NRS) [26]. At the end of the experiment, subjects reported pain scores for each of the two stimulation modes. Although there was age variation among the subjects, resulting in variations in muscle strength and pain tolerance, each subject was required to complete both experimental trials and thus served as their own control in the paired comparisons between the two stimulation modes. This within-subject comparative design inherently controls for age-related variability. Fig. 2 illustrates the time courses of finger flexion forces and EMG signals from a representative Sym stimulation trial of a subject.
Fig. 2.
The finger flexion forces recordings (a) and the EMG recordings from the finger flexors (b), respectively from a representative Sym trial. The first stimulation started at 0. (c) Enlarged EMG segments of the original EMG are shown in (b)
Modeling study
The development of computational models helps to comprehensively analyze and understand the effects of these randomized pulse waveforms on neural activation. Neuronal activation in response to electrical stimulation is generally modeled by calculating the electric potentials generated by the applied stimulus and determining their effects on neuronal firing [27]. Finite element (FE) modeling has been employed to incorporate the distinct electrical properties of tissue layers surrounding the electrodes and nerves [28]. A simplified FE model of the human arm, composed of concentric cylinders representing bone, muscle, fat and skin, has been used to predict neural responses to transcutaneous electrical stimulation [29]. The double-cable mammalian motor axon model (McIntyre-Richardson-Grill neuron model, MRG) predicts axonal activation by simulating voltages along the axon [30], making it compatible with FE models of the arm. The MRG model has been applied in various contexts, including studies of peripheral nerve activation [31, 32], high-frequency stimulation [33, 34] and pain predictions [35]. In this section, we combined the MRG model with the arm model to predict neural responses during our proposed random pulse cluster electrical stimulation.
Upper arm model
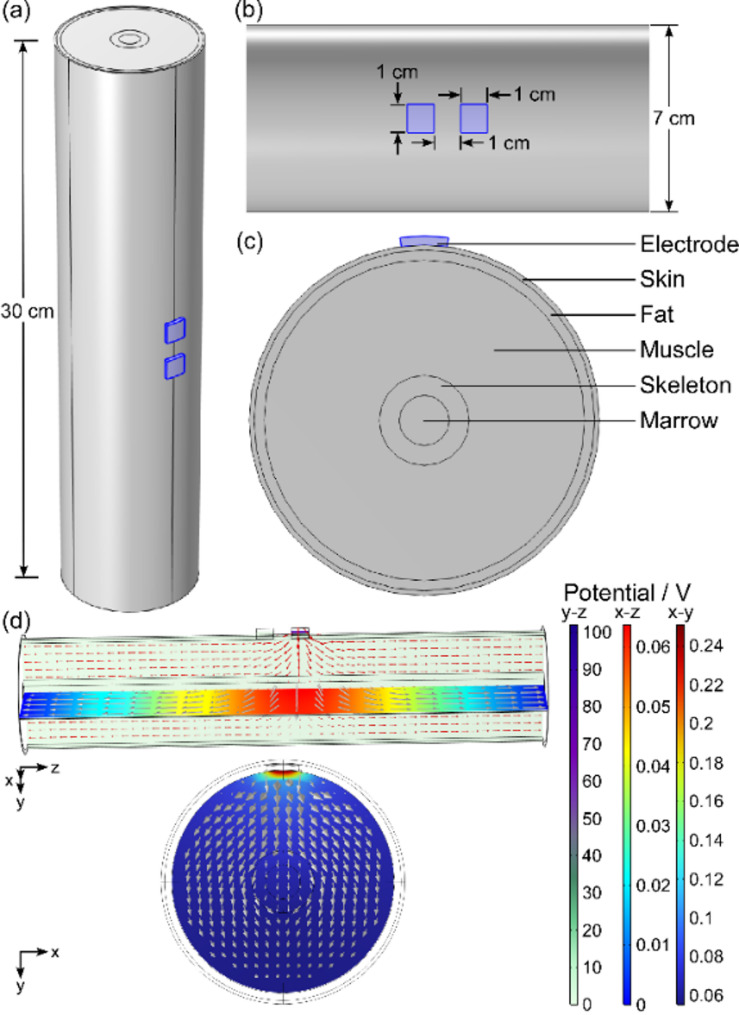
The electric field distribution within human upper arm tissue was analyzed using a finite element method implemented in COMSOL software. As shown in Fig. 3, a simplified hierarchical concentric cylindrical model was constructed based on human anatomical data of the human upper arm with a diameter of 7 cm and a length of 30 cm. The model consists of concentric layers representing the skin, fat, muscle, skeleton and marrow arranged from the outermost to the innermost layers. This concentric cylindrical model provides a reasonable approximation of the upper arm for studying non-microscopic surface effects. Two surface electrodes (1 × 1 cm) were positioned at the center of the skin’s surface, with a conductive gel used at the electrode-tissue interface. Each tissue layer in the model was assumed to be a homogeneous dielectric, characterized by its electrical conductivity (σ) and relative permittivity (ε) [36]. The dimensions and electrical properties of the model are summarized in Table 1 [37].
Fig. 3.
(a) Overall view of the upper arm concentric cylindrical model with a length of 30 cm. (b) Local view of the concentric cylindrical model with a diameter of 7 cm and two electrodes with a side length of 1 cm affixed to the middle of the surface. (c) Cross-sectional view of the concentric cylindrical model and the individual tissue components. (d) Potential (color change) and current density (arrows) distributions of the upper arm model with different planes in aSymR stimulation mode. Three color bars represent the potential in the y-z, x-z and x-y planes, respectively, and the unit is V
Table 1.
Dimensions and electrical properties of the different tissues in the human upper arm model
| Organization name | Thicknesses / mm | Conductivity / (S/m) | Relative permittivity |
|---|---|---|---|
| Skin | 1 | 0.0025 | 8000 |
| Fat | 2 | 0.01 | 20,000 |
| Muscle | 23 | 1 | 100,000 |
| Skeleton | 4 | 0.05 | 5000 |
| Marrow | 5 | 0.1 | 10,000 |
| Electrode | 2 | 0.0006 | 63 |
Two modes of current stimulation were implemented by designating one electrode as the terminal (anode) and the other as the ground (cathode). The boundary conditions of the ends of the model were set to ground [38]. One cycle of a current pulse cluster was applied to the anode, with the mean current amplitude set to 3 mA and the pulse number fixed at 8 for both stimulation modes. A customizable mesh refinement process was employed to generate a final mesh consisting of 918,808 domain cells, 322,758 boundary cells and 4474 edge cells. Simulations were performed using the Electric Currents (EC) transient solver in COMSOL.
Axon model
To reduce computational cost while maintaining acceptable accuracy, the following assumptions were made in the axon modeling process: (1) Only the motor axon model (MRG model [39]) was implemented in the NEURON [40] programming environment. (2) Fibers were assumed to be straight with no bends or undulations along their length. (3) All fibers were considered parallel to one another. (4) The nerve cross-section was assumed to remain constant along its length. (5) The axoplasm is assumed to be a homogeneous isotropic conductive medium. (6) The resting membrane potential is assumed to be constant.
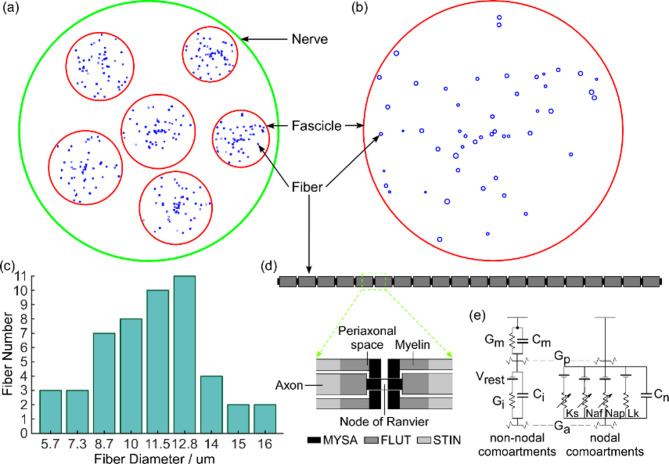
The number of fascicles within the median nerve according to histological data ranges from 3 to 37, varying with location along the arm and individual differences [41]. Based on previous literature [42, 43], the nerve model was designed to contain six randomly distributed fascicles with varying diameters, where the nerve diameter was set to 4000 μm and the fascicle diameters ranged between 800 and 1200 μm (Fig. 4(a)). Approximately 15–33% of the 250 fibers within each fascicle were classified as motor fibers [28], and 50 motor fibers were selected in the model for computational convenience. These 50 fibers with different diameters were randomly distributed within each fascicle (Fig. 4(b)). Nine fiber diameters were generated, ranging from 5.7 to 16.0 μm [30], and the fiber diameter distribution was assigned according to the literature [28] (Fig. 4(c)).
Fig. 4.
(a) Schematic cross-section of the nerve. The nerve (green circle) contains six fascicles (red circle) of different positions and diameters, with 50 fibers (blue circle) of different diameters randomly distributed in each fascicle. (b) Enlarged schematic of one of the fascicles. (c) Distribution of fiber diameters in each fascicle. (d) Multi-compartment double cable model of a mammalian fiber. (e) Electrical representation of the MRG double cable axon model
The MRG model is a multi-compartment double-cable representation of mammalian nerve fibers comprising 21 nodes of Ranvier interspersed with 20 internodes [30]. Each internode is subdivided into ten segments: two paranodal myelin attachment segments (MYSA), two paranodal main segments (FLUT), and six internodal segments (STIN) (Fig. 4(d)). The nodal membrane dynamics incorporate fast sodium (Naf), persistent sodium (Nap), slow potassium (Ks) and linear leakage (Lk) conductance arranged in parallel with the nodal capacitance (Cn). The internodal segments are modeled using a double-cable structure of linear conductance that explicitly represents the myelin sheath (characterized by Gm in parallel with Cm) and the internodal axolemma (characterized by Gi in parallel with Ci) [30] (Fig. 4(e)). Specific model parameters are provided in reference [30], with the temperature set at 37 °C and the resting potential of the motor axon model established at − 80 mV.
Data processing
Finger force
The raw force data were initially smoothed using the LOESS method. The initial peak forces of each finger were then compared between the two stimulation modes. Subsequently, the force data were segmented into 30 segments with a 10-second averaging window. Within each window, the force data were first averaged for individual fingers and then summed across all four fingers to represent the total force level. To further assess and compare the decline in contraction forces between the two stimulation modes, the force data were fitted to an exponential function y = Fp+ Fdeτt, where Fp represents the plateau force level at steady state, Fd is the absolute force decay (i.e., the difference between the initial force and the plateau force), and τ < 0 denotes the rate of force decline. Since the plateau force levels could differ between the two modes, a direct comparison of the τ-values would not accurately capture the force decay. Instead, the time required for the force to decline below 60% of the initial contraction force (termed the 60%-peak period) was estimated using the smoothed force data. A longer 60%-peak period indicates a slower force decay rate. The 60%-peak period was chosen over the commonly used 50% decay time because, in some trials, the elicited forces remained above 50% of peak force throughout the stimulation period. In addition, the force-time integral over the entire 5-minute stimulation period was calculated to quantify overall fatigue also using the smoothed force data.
EMG activity
The EMG signals were pre-processed using a 10 Hz high-pass filter and a 50 Hz notch filter to remove noise and baseline drift. To examine the temporal variations in EMG activity, the same 10-second averaging window used for the force signals was applied. Within each 10-second window, EMG signals were extracted starting 5 ms before the stimulation onset (defined as the first rise of the positive-phase pulse cluster in both stimulation modes) and ending 30 ms after the onset. These extracted segments were then averaged to obtain the average EMG signal for individual channels. Fig. 5 illustrates the EMG segments and corresponding average EMG signals within the second window of a representative channel for a subject under the Sym and aSymR stimulation modes, respectively. After identifying stimulation artifacts, the average EMG signal between the two stimulation artifacts was extracted to obtain the pure EMG signal for further analysis. We calculated the peak-to-peak magnitudes of the average EMG, defined as the difference between the maximum and minimum signal values. The peak-to-peak EMG magnitudes across all 128 channels were then averaged to represent the overall EMG activity level.
Fig. 5.
The EMG signals and average EMG within the 2nd window of a channel for a representative subject under the Sym stimulation mode (a) and the aSymR stimulation mode (b). The peak-to-peak magnitudes were calculated from the average EMG for each channel
Simulation data
The center of the nerve cross-section was placed 4 mm away from and parallel to the electrodes, as superficial peripheral nerves are typically located 1 mm beneath the fat layer [44]. It was assumed that the electrode was aligned with the midpoint of the fiber, such that the tenth Ranvier node coincided with the anode as the stimulation node. The sixth Ranvier node was selected to extract membrane potential to ensure appropriate propagation of the action potential along the fiber. The fiber model was initialized to a steady-state condition using a 10 ms delay before applying the stimulation waveform [45]. In summary, the electrical potentials at each fiber location beneath the anode, computed in COMSOL, were exported to MATLAB and then used as the stimulus input for fiber models of varying diameters in NEURON separately to obtain membrane potentials for fibers of different diameters. Finally, the activation time for each motor fiber was determined as the duration between the stimulation onset and the peak membrane potential.
Statistical analysis
In this study, measurements related to the contraction force levels, stimulation pulse number, NRS pain scores and fiber activation time were first calculated for each subject and then compared between the two stimulation modes using a two-tailed paired t-test. The peak-to-peak EMG magnitudes between the two stimulation modes were compared using the Paired Samples Wilcoxon Signed-Rank Test. The normality assumption was evaluated using the Kolmogorov-Smirnov test. The significant level of α = 0.05 was applied for all statistical tests.
Results
Contraction force
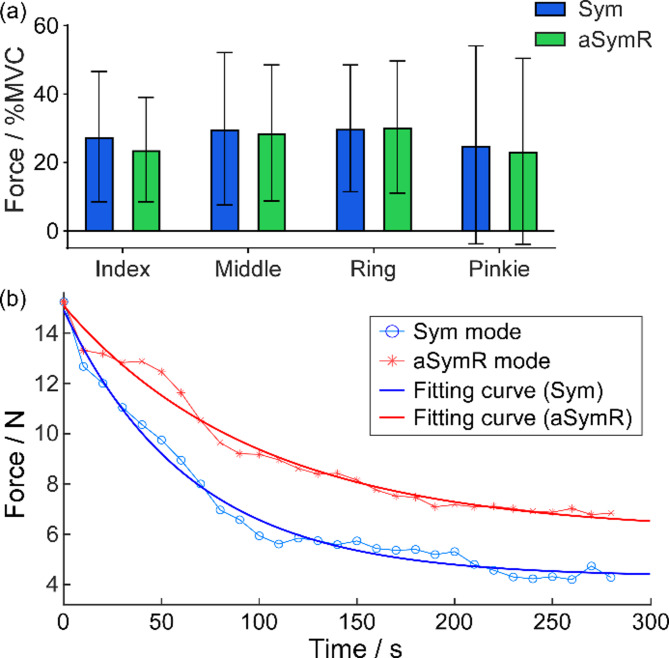
Fig. 6(a) illustrates the initial peak forces of individual fingers under the two stimulation modes, expressed as a percentage of the MVC of each finger. Statistical analysis revealed no significant differences in the initial forces for any finger between the two stimulation modes (Index: t(15) = 1.565, p = 0.138498; Middle: t(15) = 0.5172, p = 0.612565; Ring: t(15) = 0.1143, p = 0.910553; Pinkie: t(15) = 1.146, p = 0.269767). The average force level of the finger exhibiting the maximum elicited force was 30.00% MVC under the Sym mode and 30.33% MVC under the aSymR mode.
Fig. 6.
(a) The initial peak force of individual fingers for all subjects. Error bars represent standard deviations. (b) The change of the elicited finger force over 300 s continuous stimulation under the aSymR and Sym mode respectively across all subjects. The thick curves represent the regression fits
Since the individual finger forces were matched between the two stimulation modes, the force was averaged across four fingers for the subsequent analysis. Fig. 6(b) illustrates the average finger force over time for all subjects in both stimulation modes. The thick curves represent the regression fits. The regression analysis indicated that the forces decreased exponentially, exhibiting a rapid initial decline followed by stabilization in both stimulation modes. However, the force decay was slower under the aSymR mode compared to the Sym mode, resulting in a higher final force plateau after prolonged stimulation under the aSymR mode.
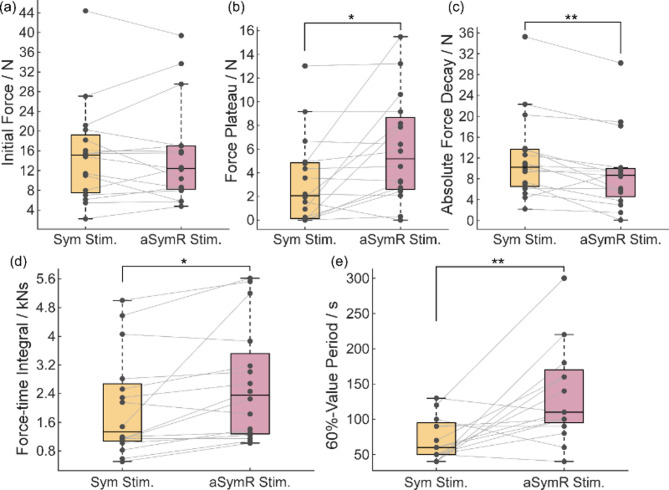
The statistical analyses of the regression results for contraction force across all subjects are shown in Figs. 7(a)~(c). The results show that the average initial peak force (Fp + Fd) across fingers was well-matched between the two stimulation modes (t(15) = 0.02411, p = 0.9811; Fig. 7(a)), consistent with the individual finger forces observed at the initial stimulation (Fig. 6(a)). The force plateau (Fp) under the aSymR stimulation was significantly higher than that under the Sym Stimulation (t(15) = 2.72, p = 0.0158; Fig. 7(b)), while the absolute force decay (Fd) under the aSymR stimulation was significantly lower compared to the Sym Stimulation (t(15) = 3.288, p = 0.005; Fig. 7(c)). These findings indicate that the reduction in conduction force after sustained aSymR stimulation is less pronounced, and the elicited force under the aSymR mode was prolonged compared to the Sym mode. Moreover, the force-time integral (Fig. 7(d)) under the aSymR mode was significantly greater than that under the Sym mode (t(15) = 2.777, p = 0.0141), suggesting that aSymR stimulation produces a higher overall force output even when the initial force levels are matched. Additionally, the 60%-peak value period (Fig. 7(e)) under the aSymR mode was significantly longer than that under the Sym mode (t(15) = 3.667, p = 0.0023), demonstrating that aSymR stimulation induces more sustained force and a slower rate of force decay over time.
Fig. 7.
The initial force (a), the force plateau (b), the absolute force decay (c), the force-time integral (d) and the 60%-value period (e) of all the subjects. *p < 0.05, **p < 0.01
EMG activity
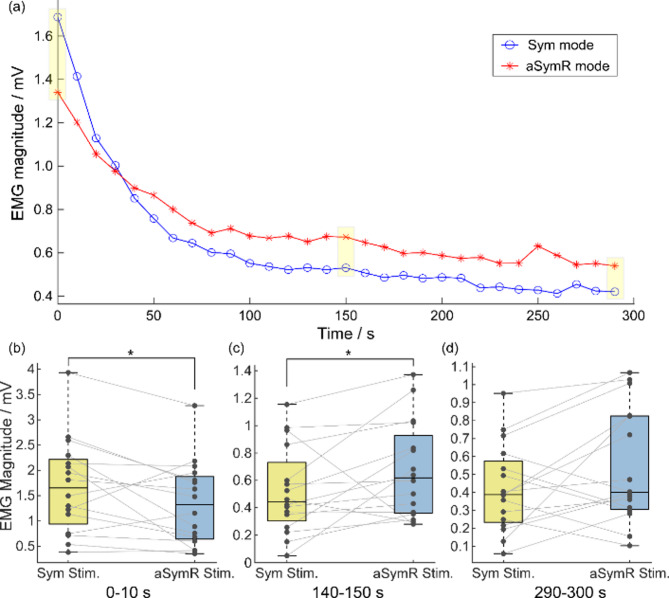
Fig. 8(a) shows the temporal changes in average peak-to-peak EMG magnitude across all subjects. As electrical stimulation progressed, EMG magnitude decreased in both stimulation modes, indicating the onset of muscle fatigue. During the early phase of stimulation, the EMG magnitude under the aSymR mode was lower than that under the Sym mode, potentially due to the aSymR stimulation reducing the synchronization of the motor unit discharges, resulting in a smaller average EMG magnitude [19]. During the middle and late phases of stimulation, the EMG magnitude in the aSymR mode exceeded that of the Sym mode, suggesting greater sustained muscle excitability under continuous aSymR stimulation. The EMG magnitude was further analyzed statistically at three specific time intervals: the beginning (0–10 s), middle (140–150 s) and end (290–300 s) of the stimulation trial as shown in Figs. 8(b)~(c). The results indicate that at the beginning of the stimulation (0–10 s), the EMG magnitude was significantly lower in the aSymR mode compared to the Sym mode (w = -78, p = 0.0443; Fig. 8(b)). This reduction can be attributed to asynchronous discharges in the aSymR mode, which tend to cancel each other out, thereby decreasing the overall EMG amplitude. This phenomenon may serve as a potential mechanism for reducing muscle fatigue in aSymR stimulation compared to Sym stimulation. As shown in Fig. 8(c), the EMG magnitude at the middle period (140–150 s) was significantly higher in the aSymR stimulation than in the Sym stimulation (w = 88, p = 0.0214). Similarly, at the end of the stimulation period (290–300 s), the EMG magnitude remained higher in the aSymR mode than in the Sym mode, although this difference did not reach statistical significance (w = 60, p = 0.1297; Fig. 8(d)).
Fig. 8.
(a) The change of the average peak-to-peak EMG magnitude over 300 s continuous stimulation under the aSymR and Sym mode respectively for all subjects. (b) The EMG magnitude of all the subjects during the 0–10 s (b), 140–150 s (c) and 290–300 s (d), respectively. *p < 0.05
Pain sensation
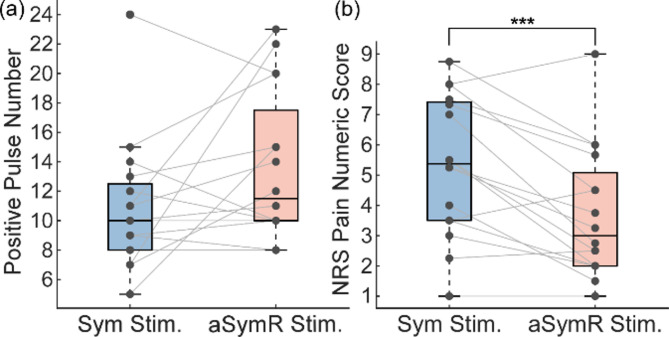
Considering that the pain levels may be related to the amount of applied charge, we first evaluated the number of narrow pulses delivered in the aSymR and Sym modes. The results showed that while the number of positive pulses in the aSymR mode was higher than in the Sym mode, the difference was not statistically significant (t(15) = 1.951, p = 0.07; Fig. 9(a)). We then compared the pain levels of all subjects under the two stimulation modes using the NRS pain numeric score, which assesses pain on a scale from 0 (no pain) to 10 (most intense pain). As shown in Fig. 9(b), the pain level under the aSymR mode was significantly lower than under the Sym mode (t(15) = 4.13, p = 0.0009). These findings demonstrated that the aSymR mode elicited significantly less pain and substantially reduced discomfort compared to the Sym mode.
Fig. 9.
(a) Number of positive narrow pulses of stimulation current under the Sym stimulation and the aSymR stimulation for all subjects. (b) The mean pain scores across all subjects for different stimulation modes. ***p < 0.001
Simulation results
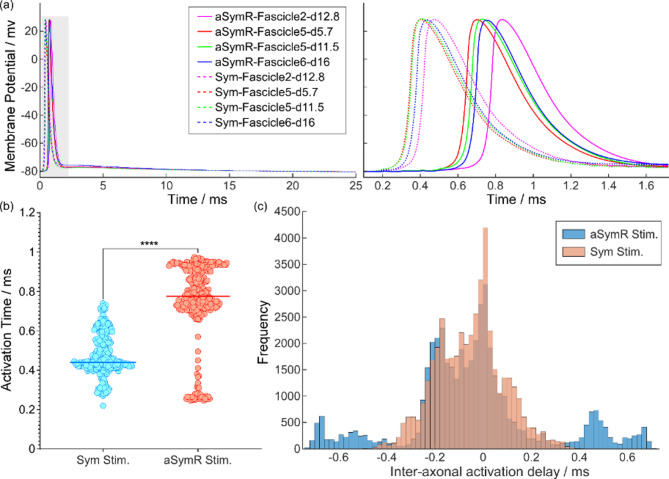
The membrane potentials of all 300 motor fibers were computed by applying the electrical potentials at different positions to their corresponding fibers. Fig. 10(a) shows the membrane potentials of several representative fibers and the zoomed-in views of specific segments (grey area). The results indicate that under identical stimulation conditions, axonal discharge was influenced by both position and diameter. Moreover, under different stimulation conditions, axonal activation occurred significantly later under the aSymR mode than under the Sym mode. In addition, the delay in activation across different axons was more pronounced under the aSymR mode compared to the Sym mode.
Fig. 10.
(a) The membrane potentials of four representative fibers and their partial zoomed-in views (grey area) under two stimulation modes. (b) The mean activation time of all fibers for different stimulation modes. (c) The histogram of the inter-axonal activation time delay for both modes. ****p < 0.0001
Fig. 10(b) further provides a statistical analysis of the activation time across all fibers, revealing that activation times under the aSymR mode were significantly delayed compared to those under the Sym mode (t(299) = 37.78, p < 0.0001). To further quantify the inter-fiber activation time differences, the time delays to activation were calculated for all fiber combinations (300 × 299/2 = 44850) and analyzed using a histogram. As illustrated in Fig. 10(c), the inter-axonal activation time delays for both stimulation modes exhibited an approximately normal distribution. However, under the aSymR mode, the frequency distribution was broader and flatter compared to the Sym mode, indicating that the aSymR mode expands the activation delay between axons. This effect is likely attributable to the random fluctuation in pulse amplitude under the aSymR mode, which amplifies the variation in the number of narrow pulses required for different axons to reach the threshold, thereby extending the activation time delays among axons.
Discussion
In this study, we compared the anti-fatigue properties of the Sym stimulation and the aSymR stimulation conditions using experiments and model simulations. The aSymR stimulation was hypothesized to mitigate muscle fatigue by decentralizing motor unit activation and to reduce discomfort compared to previously explored Sym stimulation [16]. To evaluate these effects, we compared contraction force, EMG activity of finger flexor and pain scores under both stimulation conditions at equivalent levels of muscle contraction. The findings revealed that the aSymR stimulation significantly prolonged evoked muscle force, attenuated the rate of muscle fatigue, preserved greater muscle activatability following sustained stimulation, and elicited lower pain levels compared to the Sym stimulation. Additionally, the activation time of motor fibers was significantly delayed under the aSymR stimulation, with increased variability in activation time across fibers. This indicates a more asynchronous activation pattern of muscle fibers during the aSymR stimulation, potentially explaining the enhanced fatigue suppression observed. Overall, the results demonstrate that the aSymR stimulation effectively relieves muscle fatigue and alleviates pain and discomfort during electrical stimulation, which has the potential to enhance the efficacy of NMES in motor function rehabilitation for people with neurological injuries such as stroke.
Evoked contraction forces
A significant limitation of electrical stimulation is the rapid onset of muscle fatigue, often characterized by a reduction in force output during sustained or repeated contractions [46]. In our experimental findings, both stimulation modes elicited an exponential decline in contractile force, but the decline was notably slower in the aSymR mode. Given that initial force output is a critical determinant of muscle fatigue, we ensured that the initial forces across the four fingers were closely matched in both modes to standardize the starting conditions. From this similar starting point, the force-time integral values were greater under the aSymR mode compared to the Sym mode, indicating enhanced force generation in the aSymR condition. Furthermore, the aSymR mode demonstrated a larger force plateau, smaller absolute force decline, and a significantly longer duration at 60%-peak value period compared to the Sym mode. These results provide clear evidence that the aSymR stimulation mode mitigates muscle fatigue and sustains evoked force more effectively than the Sym stimulation mode. Notably, people with neurological disorders typically experience greater muscle fatigability than individuals with healthy musculature, further exacerbating the challenges associated with muscle fatigue during electrical stimulation [47]. To counteract muscle fatigue, increasing current intensity is often necessary to maintain muscle contraction. However, this approach can heighten user discomfort, and prolonged exposure to high-intensity electrical stimulation may even lead to electrical tissue damage. Our proposed stimulation mode not only significantly reduces muscle fatigue and maintains muscle contraction despite fatigue but also alleviates pain, thereby enhancing the feasibility of prolonged and more effective electrical stimulation in stroke rehabilitation. Additionally, it demonstrates the potential to better accommodate the demands of high-intensity training in motor function rehabilitation for stroke survivors.
Dispersed axon and muscle fiber activation
Asynchronous activation of muscle fibers has been shown to delay the onset of muscle fatigue [48]. Meanwhile, this mechanism facilitates improved control of muscle force, as asynchronous force twitches reduce variability in the generated force [45]. Various stimulation strategies have been developed to achieve dispersed activation. For instance, the placement of multiple electrodes can spatially and sequentially distribute stimulation of different regions of muscle [49]. Moreover, our previous study employed high-frequency subthreshold current stimulation of the proximal end of peripheral nerve bundles to induce dispersed activation of motor unit axons, resulting in temporally segregated muscle fiber activation. This approach offers several advantages: First, the proximal nerve bundle contains axons that innervate muscle fibers across different depths and muscles [50]. Consequently, even with a single electrode pair, muscles in varied locations can be activated. Synergistic activation of multiple muscles promotes more natural grip patterns [51] and prolongs elicited force output. Second, a previous study has demonstrated that peripheral nerve bundle stimulation enhances cortical activation levels and functional connectivity compared to conventional muscle motor point stimulation [5], which may contribute to improve outcomes in NMES-based rehabilitation interventions for stroke survivors. Lastly, as the proximal peripheral nerve bundles are located superficially, significantly lower current amplitudes (2 ~ 6 mA) are sufficient to achieve the desired muscle activation compared to the typical values (10 ~ 20 mA) required by conventional surface NMES methods [52]. People with stroke may require greater stimulus intensity to elicit muscle contractions due to impaired neural pathways. This reduction in current amplitude not only minimizes user discomfort but also supports the development of wearable battery-powered stimulation devices.
In addition to the stimulation location, the waveform of the stimulation pulse directly influences the activation mechanisms of nerve fibers. Our previous studies have demonstrated the beneficial effects of high-frequency subthreshold stimulation pulses in alleviating muscle fatigue (i.e. the Sym mode). Under high-frequency pulse cluster stimulation, each narrow pulse induces only subthreshold depolarization, a phenomenon corroborated by our simulation results. The asynchronous discharge pattern observed under high-frequency stimulation arises from the amount differences in the subthreshold depolarizing current required by different axons for the sum of time. The degree of asynchronous fiber activation differs between the two stimulation modes, with the aSymR mode eliciting more pronounced asynchronous responses from fibers of varying positions and diameters compared to the Sym mode. In the Sym mode, the amplitude of each narrow pulse remains constant, causing the cumulative depolarizing current to increase steadily, with fewer depolarizing pulses required to reach the activation threshold of the fibers. While in the aSymR mode, the pulse amplitudes fluctuate randomly, introducing variability in the cumulative subthreshold depolarizing currents. Consequently, a greater number of subthreshold pulses is needed to reach the activation threshold, postponing the average activation time of the fibers. Additionally, the randomized pulse amplitudes increase the difference in the number of narrow pulses required to activate different fibers, further extending the activation time delay across fibers.
The more asynchronous activation of fibers can be further demonstrated by the experimental results. During the early phase of stimulation, the EMG amplitude in the aSymR mode was lower than in the Sym mode, possibly because motor unit activation was temporally distributed in the aSymR mode, resulting in reduced amplitude of the averaged EMG. In both stimulation modes, the amplitude of the EMG activity decreased progressively with continued stimulation, indicating the onset of muscle fatigue. This phenomenon is likely attributable to the blockage of action potential propagation caused by the sustained depletion of neurotransmitters [53, 54], leading to reduced muscle activity. However, during the middle and late phases of stimulation, the EMG amplitude in the aSymR mode exceeded that in the Sym mode. This may be because the EMG activity in the Sym stimulation mode becomes weakened due to muscle fatigue, suggesting that the aSymR stimulation is more effective in activating muscles under fatigued conditions. These results demonstrated that our proposed novel asymmetric random high-frequency stimulation waveform facilitates asynchronous axonal activation, thereby mitigating muscle fatigue, which holds promise for enhancing the clinical applications of electrical stimulation technologies.
Pain sensation
The sensory threshold has been reported to be inversely proportional to stimulation frequency [55], such that high-frequency electrical stimulation lowers the sensory threshold and thus increases user discomfort, as confirmed in previous studies [19, 56]. Since only the cathodic phase of a biphasic pulse effectively induces muscle contraction, this study designed charge-balanced but asymmetrical high-frequency carrier pulse cluster stimulation waveforms to ensure that equivalent positive and negative charges were delivered, mitigating the risk of electrochemical skin damage during prolonged stimulation. On the other hand, the width of the negative-phase pulse was extended to reduce its amplitude, with the negative-phase pulse amplitude used in experiments maintained at approximately 0.1 mA—below the sensation threshold and potentially alleviating pain. Another contributing factor may involve that different types of nerve fibers in signaling are activated, as nociceptive sensory signals engage both myelinated A-delta fibers and unmyelinated C-fibers. Due to their smaller diameters, C-fibers require stronger stimuli to reach activation thresholds [57, 58]. Overall, the experimental results demonstrated that user discomfort during stimulation was minimized, aligning with our expectations and supporting the potential for higher intensity electrical stimulation in rehabilitation exercises for stroke survivors.
Limitations and future work
In the present study, the current amplitude was determined based on achieving a moderate level of muscle contraction—30% MVC. This strength was chosen to avoid excessive contraction, which could induce pain from the undue pressure exerted by the hand against the experimental apparatus, potentially interfering with the subject’s judgment of pain level at the site of localized electrical stimulation. Although lower finger strengths are predominantly used in daily activities [59], a wide range of contraction levels may be used in clinical applications. Therefore, future investigations will consider fatigue resistance across varying contraction intensities. Additionally, while the NRS pain scale employed in this study has been validated in both theory and practice, some pain descriptions were difficult for some subjects to understand. Moreover, pain sensitivity can vary with factors such as age and gender, with older participants tending to exhibit slightly higher sensitivity [60]. Finally, to reduce computational complexity, we used simplified models to predict nerve axon fiber discharge patterns under Sym and aSymR Stimulation modes. While simplified models successfully simulate membrane potentials, more accurate results are anticipated with the use of anatomically realistic models.
Conclusions
We presented a novel asymmetric random high-frequency carrier pulse cluster electrical stimulation waveform, which demonstrated significant advantages over previously reported symmetric high-frequency stimulation. Specifically, the aSymR stimulation waveform effectively prolonged contraction force during sustained stimulation, reduced the rate of force decay, mitigated muscle fatigue and substantially reduced pain levels. Furthermore, our simulation results revealed that aSymR stimulation significantly delayed the activation time of motor fibers and induced asynchronous activation across fibers. These findings suggest that aSymR stimulation may better meet the needs of high-intensity training in motor function rehabilitation for stroke survivors, thus promoting the wide application of electrical stimulation technology in clinical rehabilitation.
Acknowledgements
Not applicable.
Abbreviations
- NMES
Neuromuscular electrical stimulation
- aSymR
Asymmetric random high-frequency pulse cluster electrical stimulation
- Sym
Symmetric high-frequency pulse cluster electrical stimulation
- FDS
Flexor digitorum superficialis
- FDP
Flexor digitorum profundus
- EMG
Electromyographic
- Mus
Motor units
- FE
Finite element
- MRG
McIntyre-Richardson-Grill neuron model
- MVC
Maximum voluntary contraction
- EC
Electric Currents
Author contributions
R. Y. and Y. Z. conceptualized the study. R. Y., Y. Z., H. S., S. F. and Z. D. did the methodology. R. Y. and Y. Z. performed the experiment and data analysis. R. Y., Y. Z. and H. S. wrote the main manuscript text. G. X, S. F. and Z. D. wrote part of the discussion. All authors reviewed the manuscript.
Funding
This work was supported by the STI 2030—Major Projects under Grant No. 2022ZD0209800, the Qin Chuang Yuan Talent Project under Grant No. QCYRCXM-2022-34, the Key Research and Development Program of Shaanxi Province under Grant 2022ZDLSF04-10, and the National Natural Science Foundation of China Research Fund for International Scholars under Grant No. W2433195.
Data availability
The data that support the findings of this study are available upon reasonable request from the authors.
Declarations
Ethics approval and consent to participate
All participants involved in the study signed informed consent approved by the Ethics Committee of the Department of Medicine at Xi’an Jiaotong University (Approval #: 2021–1550).
Consent for publication
All authors have read and approved the final manuscript. We confirm that this work is original and has not been published elsewhere.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Zheng, Email: yzheng@mail.xjtu.edu.cn.
Henry Shin, Email: henryhongsukshin@uhrs.edu.cn.
References
- 1.Loh M-S, Kuan Y-C, Wu C-W, et al. Upper extremity contralaterally controlled functional electrical stimulation versus neuromuscular electrical stimulation in Post-Stroke individuals: A Meta-Analysis of randomized controlled Trials. Neurorehabilit Neural Repair. 2022;36(7):472–82. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa K, Bergquist AJ, Yamashita T, et al. Motor point stimulation primarily activates motor nerve. Neurosci Lett. 2020;736:135246. [DOI] [PubMed] [Google Scholar]
- 3.Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve. 2007;35(5):562–90. [DOI] [PubMed] [Google Scholar]
- 4.Quandt F, Hummel FC. The influence of functional electrical stimulation on hand motor recovery in stroke patients: a review. Experimental & Translational Stroke Medicine. 2014;6:9. [DOI] [PMC free article] [PubMed]
- 5.Yuan R, Peng Y, Ji R, et al. Comparison of the activation level in the sensorimotor cortex between motor point and proximal nerve bundle electrical stimulation. J Neural Eng. 2024;21(2):026029. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Khan A, Song R, et al. Rewiring the lesioned brain: electrical stimulation for Post-Stroke motor Restoration. J Stroke. 2020;22(1):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Tanino G, Miyasaka H. Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation. Medical Devices (Auckland, N.Z.), 2017, 10: 207–13. [DOI] [PMC free article] [PubMed]
- 8.Gobbo M, Maffiuletti N, Orizio C, et al. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J Neuroeng Rehabil. 2014;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barss T, Ainsley E, Claveria-Gonzalez F et al. Utilizing physiological principles of motor unit recruitment to reduce fatigability of Electrically-Evoked contractions: A narrative Review. Arch Phys Med Rehabil, 2017, 99. [DOI] [PubMed]
- 10.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol. 2011;111(10):2399–407. [DOI] [PubMed] [Google Scholar]
- 11.Gregory C, Bickel C. Recruitment patterns in human skeletal muscle during electrical Stimulation. Phys Ther. 2005;85:358–64. [PubMed] [Google Scholar]
- 12.Vargas L, Baratta J, Hu X. Distribution of M-Wave and H-Reflex in Hand Muscles Evoked via Transcutaneous Nerve Stimulation: A Preliminary Report[C]. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 2021: 5897-5900Mexico: IEEE, 2021: 5897–5900[2023-04-06]. [DOI] [PubMed]
- 13.Vromans M, Faghri PD. Functional electrical stimulation-induced muscular fatigue: effect of fiber composition and stimulation frequency on rate of fatigue development. J Electromyogr Kinesiology: Official J Int Soc Electrophysiological Kinesiol. 2018;38:67–72. [DOI] [PubMed] [Google Scholar]
- 14.Buckmire A, Lockwood D, Doane C et al. Distributed stimulation increases force elicited with functional electrical stimulation. J Neural Eng, 2017, 15. [DOI] [PMC free article] [PubMed]
- 15.Shin H, Chen R, Hu X. Delayed fatigue in finger flexion forces through transcutaneous nerve stimulation. J Neural Eng. 2018;15(6):066005. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Hu X. Reduced muscle fatigue using kilohertz-frequency subthreshold stimulation of the proximal nerve. J Neural Eng. 2018;15(6):066010. [DOI] [PubMed] [Google Scholar]
- 17.Boulet J, White M, Bruce IC. Temporal considerations for stimulating spiral ganglion neurons with cochlear Implants. J Assoc Res Otolaryngol. 2016;17(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neudorfer C, Chow TC, Boutet A, et al. Kilohertz-frequency stimulation of the nervous system: A review of underlying mechanisms. Brain Stimul. 2021;14(3):513–30. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Hu X. Improved muscle activation using proximal nerve stimulation with subthreshold current pulses at kilohertz-frequency. J Neural Eng. 2018;15(4):046001. [DOI] [PubMed] [Google Scholar]
- 20.Biesecker LG, Aase JM, Clericuzio C, et al. Elements of morphology: standard terminology for the hands and feet. Am J Med Genet Part A. 2009;149A(1):93–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Shin H, Hu X. Muscle fatigue Post-stroke elicited from Kilohertz-Frequency subthreshold nerve Stimulation. Front Neurol. 2018;9:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasluosta C, Kiele P, Stieglitz T. Paradigms for restoration of somatosensory feedback via stimulation of the peripheral nervous system. Clin Neurophysiol. 2018;129(4):851–62. [DOI] [PubMed] [Google Scholar]
- 23.Formento E, D’Anna E, Gribi S, et al. A biomimetic electrical stimulation strategy to induce asynchronous stochastic neural activity. J Neural Eng. 2020;17(4):046019. [DOI] [PubMed] [Google Scholar]
- 24.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141(2):171–98. [DOI] [PubMed] [Google Scholar]
- 25.Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-Frequency and fatigue properties of motor units in muscles that control digits of the human Hand. J Neurophysiol. 1999;81(4):1718–29. [DOI] [PubMed] [Google Scholar]
- 26.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS pain), Numeric Rating scale for pain (NRS pain), McGill pain Questionnaire (MPQ), Short-Form McGill pain Questionnaire (SF-MPQ), Chronic pain Grade scale (CPGS), Short Form-36 Bodily pain scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis pain (ICOAP). Volume 63. Arthritis Care & Research; 2011. pp. S240–252. Suppl 11. [DOI] [PubMed]
- 27.Goffredo M, Schmid M, Conforto S, et al. A two-step model to optimise transcutaneous electrical stimulation of the human upper arm. COMPEL: Int J Comput Math Electr Electron Eng. 2014;33(4):1329–45. [Google Scholar]
- 28.Gaines JL, Finn KE, Slopsema JP, et al. A model of motor and sensory axon activation in the median nerve using surface electrical stimulation. J Comput Neurosci. 2018;45(1):29–43. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn A, Keller T, Lawrence M, et al. A model for transcutaneous current stimulation: simulations and experiments. Medical & Biological Engineering & Computing; 2009;47(3):279–89. [DOI] [PubMed]
- 30.McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery Cycle. J Neurophysiol. 2002;87(2):995–1006. [DOI] [PubMed] [Google Scholar]
- 31.Peterson EJ, Izad O, Tyler DJ. Predicting myelinated axon activation using Spatial characteristics of the extracellular field. J Neural Eng. 2011;8(4):046030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wongsarnpigoon A, Woock JP, Grill WM. Efficiency analysis of waveform shape for electrical excitation of nerve Fibers. IEEE Trans Neural Syst Rehabil Eng. 2010;18(3):319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina LE, Grill WM. Nerve excitation using an amplitude-modulated signal with kilohertz-frequency carrier and non-zero offset. J Neuroeng Rehabil. 2016;13(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelot NA, Behrend CE, Grill WM. Modeling the response of small myelinated axons in a compound nerve to Kilohertz frequency signals. J Neural Eng. 2017;14(4):046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillen A, Truong DQ, Cakmak YO et al. Understanding the effect of pulse width on activation depth in TENS: A computational study[Z]. medRxiv, 2024: 2024.04.10.24305618(2024-04-15)[2025-01-09]. [DOI] [PMC free article] [PubMed]
- 36.Filipovic ND, Peulic AS, Zdravkovic ND, et al. Transient finite element modeling of functional electrical stimulation. Gen Physiol Biophys. 2011;30(1):59–65. [DOI] [PubMed] [Google Scholar]
- 37.Filipović N, Nedeljković M, Peulić A. Finite element modeling of a transient functional electrical stimulation. J Serbian Soc Comput Mech. 2007;1(1):154–63. [Google Scholar]
- 38.Grinberg Y, Schiefer MA, Tyler DJ, et al. Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Trans Neural Syst Rehabilitation Engineering: Publication IEEE Eng Med Biology Soc. 2008;16(6):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntyre CC, Grill WM. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal Output. J Neurophysiol. 2002;88(4):1592–604. [DOI] [PubMed] [Google Scholar]
- 40.Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9(6):1179–209. [DOI] [PubMed] [Google Scholar]
- 41.Anonymous. Nerves and Nerve Injuries[M]. [2024-12-17].
- 42.Choi AQ, Cavanaugh JK, Durand DM. Selectivity of multiple-contact nerve cuff electrodes: a simulation analysis. IEEE Trans Biomed Eng. 2001;48(2):165–72. [DOI] [PubMed] [Google Scholar]
- 43.Capllonch-Juan M, Sepulveda F. Modelling the effects of ephaptic coupling on selectivity and response patterns during artificial stimulation of peripheral nerves. PLoS Comput Biol. 2020;16(6):e1007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn A, Keller T, Micera S, et al. Array electrode design for transcutaneous electrical stimulation: A simulation study. Med Eng Phys. 2009;31(8):945–51. [DOI] [PubMed] [Google Scholar]
- 45.Vargas L, Musselman ED, Grill WM et al. Asynchronous axonal firing patterns evoked via continuous subthreshold Kilohertz stimulation. J Neural Eng, 2023, 20(2). [DOI] [PMC free article] [PubMed]
- 46.Binder-Macleod SA, Snyder-Mackler L. Muscle fatigue: clinical implications for fatigue assessment and neuromuscular electrical stimulation. Phys Ther. 1993;73(12):902–10. [DOI] [PubMed] [Google Scholar]
- 47.Gerrits HL, Hopman MTE, Offringa C, et al. Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflug Arch: Eur J Physiol. 2003;445(6):734–40. [DOI] [PubMed] [Google Scholar]
- 48.Sayenko DG, Nguyen R, Popovic MR, et al. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources. Eur J Appl Physiol. 2014;114(4):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckmire AJ, Lockwood DR, Doane CJ, et al. Distributed stimulation increases force elicited with functional electrical stimulation. J Neural Eng. 2018;15(2):026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, Hawari MA, Hu X. Activation of superficial and deep finger flexors through transcutaneous nerve Stimulation. IEEE J Biomedical Health Inf. 2021;25(7):2575–82. [DOI] [PubMed] [Google Scholar]
- 51.Arnet U, Muzykewicz DA, Fridén J, et al. Intrinsic hand muscle function, part 1: creating a functional Grasp. J Hand Surg. 2013;38(11):2093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howlett OA, Lannin NA, Ada L, et al. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2015;96(5):934–43. [DOI] [PubMed] [Google Scholar]
- 53.Keyser RE, Peripheral Fatigue. High-Energy phosphates and hydrogen Ions. PM&R; 2010;2(5):347–58. [DOI] [PubMed]
- 54.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geng B, Yoshida K, Jensen W. Impacts of selected stimulation patterns on the perception threshold in electrocutaneous stimulation. J Neuroeng Rehabil, 2011, 8(1). [DOI] [PMC free article] [PubMed]
- 56.Laufer Y, Elboim M. Effect of burst frequency and duration of kilohertz-frequency alternating currents and of low-frequency pulsed currents on strength of contraction, muscle fatigue, and perceived discomfort. Phys Ther. 2008;88(10):1167–76. [DOI] [PubMed] [Google Scholar]
- 57.Li CL, Bak A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol. 1976;50(1):67–79. [DOI] [PubMed] [Google Scholar]
- 58.Howson DC. Peripheral neural excitability. Implications for transcutaneous electrical nerve stimulation. Phys Ther. 1978;58(12):1467–73. [DOI] [PubMed] [Google Scholar]
- 59.Redmond B, Aina R, Gorti T et al. Haptic characteristics of some activities of daily living. 2010 IEEE Haptics Symposium. 2010:71–76[2025-01-10].
- 60.Cvetkoska A, Maček-Lebar A, Trdina P, et al. Muscle contractions and pain sensation accompanying high-frequency electroporation pulses. Sci Rep. 2022;12(1):8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the authors.