Abstract
Objective
Patients with type 2 diabetes mellitus (T2DM) have high risk of frailty. The clinical frailty scale (CFS) has been used to evaluate clinical frailty. To evaluate the association between CFS and visit-to-visit glycated hemoglobin (HbA1C) variability in T2DM patients.
Methods
Patients who were hospitalized with T2DM and received at least three HbA1C tests after discharging from endocrinology department and general practice department during 12-month follow-up were retrospectively enrolled. The patients were divided into the low-HbA1C variability group and the high-HbA1C variability group according to the results of the HbA1C variability score (HVS). The baseline clinical information, including CFS during hospitalization, was collected and compared between the two groups. We performed a propensity score match (PSM) to eliminate the influences of other confounding factors.
Results
A total of 370 patients were included in this study. Most baseline demographic, clinical parameters and metabolic parameters were comparable between the two groups except age, baseline HbA1C, albumin, and comorbidities including hypertension and dyslipidemia between the two groups before PSM. All of the relative parameters were comparable after a 1:1 PSM. Uni-variable and multi-variable logistic analysis revealed that higher CFS was associated with higher HbA1C variability and receiver operating characteristic curve showed that CFS had good predictive value in HVS.
Conclusion
Higher CFS was associated with higher visit-to-visit HbA1C variability.
Keywords: clinical frailty scale, HbA1C variability score, type 2 diabetes mellitus, propensity score match
Background
With the rapidly increasing prevalence of type 2 diabetes mellitus (T2DM) during the past few decades, the epidemic of this disorder has become a serious concern in modern society worldwide.1 International guidelines highlight the importance of proper management of blood glucose in patients with T2DM.2,3 Lately, the “low and stable” strategy in managing T2DM has been recognized.4 Visit-to-visit glycemic variability (including blood glucose and HbA1C variability) has been acknowledged as a risk factor for higher mortality and disability rates for T2DM patients.5–9 Hence, these patients are at high risk of unfavorable clinical outcomes and need more attention in clinical practice.
However, we did not get any reports regarding the risk factors for high visit-to-visit glycemic variability even though previous studies have issued that age, food intake, exercise, medication usage, treatment compliance, and comorbidities are all associated with glycemic variability.10–12
Frailty, which has a high prevalence in patients with T2DM, has been demonstrated to be associated with a worse prognosis of different medical conditions.13–17 For patients with T2DM, frailty was associated with worse clinical outcomes, especially for older people.18,19 The underlying mechanism of frailty in T2DM patients is complicated. Previous studies show that malnutrition and sarcopenia are potential mechanism of frailty in these patients.20,21 The compromised skeletal muscle function, vascular function are also related to frailty in T2DM patients.22 High glycemic variability has been demonstrated to be a risk factor for frailty. In a pilot study using continuous glucose monitoring, T2DM patients were divided into frailty patients and non-frailty patients, after multivariate adjustments, the post-lunch time above range was associated with higher risk of frailty.23 This study showed the potential relationship between glycemic variability and frailty.
In clinical practice, the evaluation of frailty often needs a comprehensive history and physical examination. Lately, the clinical frailty scale (CFS), which is a simple scoring system, is introduced and shows good clinical value in evaluating frailty.24,25
In this study, we aim to evaluate the association between CFS and visit-to-visit HbA1C variability in patients with T2DM.
Materials and Methods
This is a single-center retrospective study. T2DM patients hospitalized in endocrinology department and general practice department from January 1, 2018, to December 31, 2022, were enrolled. Patients with acute diabetic complications, patients younger than 18 years old; patients with other types of DM, patients with severe cerebrovascular diseases, cognitive impairment, acute coronary syndrome, infection, tumor, and severe hepatic and renal dysfunction were excluded. Patients with missing clinical data and patients who refused to or could not participate in our study (CFS≥8) were excluded. Patients who did not have at least three HbA1C test results during 12-month follow-up were also excluded.
The baseline demographic and clinical information including CFS were collected. The on-admission and follow-up laboratory test results were also collected. Blood samples were analyzed by Sysmex XE2100 automatic blood analyzer from Donga Company, Japan; biochemical function (hs-CRP, liver function, kidney function, lipid profile, blood glucose, etc).
The CFS is a 9-point scale, which has been detailed and illustrated elsewhere. All patients were evaluated by trained nurses on admission and the CFS results were extracted from electrical medical records.
HbA1c was measured in whole blood using turbidimetric inhibition immuno assay by Siemens DCA vantage analyzer. The HbA1c variability score (HVS) developed by Forbes A.et al in 2018, was used to evaluate the visit-to-visit variability of HbA1C in our study. The HVS is the frequency of 0.5% (5.5 mmol/mol) change in the first three HbA1c results during clinical follow-up when compared with baseline HbA1C (the value of HbA1C during hospitalization).26 Patients were divided into the high HbA1C variability group (HVS ≥ 2) and the low HbA1c group (HVS ≤ 1) according to the HVS results.
All of the enrolled patients and their family members provided informed consent. This study was approved by the ethics committee of Beijing Tongren Hospital, Capital Medical University and it fully met the requirements of the Helsinki Declaration for clinical research.
Statistical Analysis
Categorical variables were presented as numbers and frequencies (percentages) and were compared using the chi-square test or exact Fisher test, as appropriate. Continuous variables were expressed as mean SD ± mean or median (quartile 1, quartile 3) based on normality assumption and were compared using the independent sample t-test or Mann–Whitney test, as appropriate. A logistic regression model with propensity scores matching (PSM) of a 1:1 ratio was performed with variables associated with HVS to balance the patient characteristics between the two groups. The predictive value of CFS in HVE was evaluated by the receiver operating characteristic (ROC) curve. Data processing and analysis were performed using R version 4.4.0 (2024–04-24), along with Zstats 1.0 (www.zstats.net).
Results
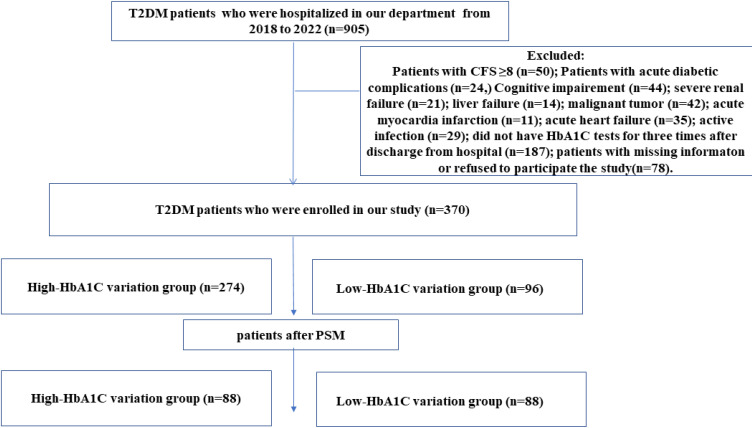
A total of 370 patients were enrolled, 274 patients were in the high-HbA1C variability group and 96 patients were in the low-HbA1C variability group (Figure 1).
Figure 1.
Study flow chart.
The comparison results of baseline demographic and clinical data before PSM were shown in Table 1 and Table 2: Most of the parameters between the two groups were comparable except that patients in the high-HbA1C variability group were older [66.00 (52.00, 77.00) vs 61.50 (47.00, 72.25), years, p = 0.032], the medical history of T2DM were longer [10.00 (7.25, 13.00) vs 8.00 (6.00, 12.00), years, p < 0.001], the CFS were higher [5.00 (3.25, 6.00) vs 3.00 (2.00, 4.00), p < 0.001], the proportion of hypertension was higher (74.82% vs 62.50%, p = 0.021),)and the proportion of dyslipidemia was higher (89.05% vs 77.08%, p = 0.004), and the level of serum albumin was lower [41.00 (38.00, 43.30) vs 42.20 (39.77, 44.50), g/L, p = 0.014], the level of baseline HbA1C were higher [8.50 (7.90, 9.10) vs 8.10 (7.80, 8.50), %, p < 0.001].
Table 1.
Comparison Results of Demographic and Clinical Parameters Between the Two Groups Before PSM
| Variable | Total (n = 370) | Low- HbA1C Variability Group (n = 96) |
High- HbA1C Variability Group (n = 274) |
Statistic | P | SMD |
|---|---|---|---|---|---|---|
| Age, years,M (Q₁, Q₃) | 65.50 (51.00, 75.75) | 61.50 (47.00, 72.25) | 66.00 (52.00, 77.00) | Z=−2.150 | 0.032 | 0.263 |
| Gender, n (%) | χ²=0.027 | 0.903 | ||||
| Female | 140 (37.84) | 37 (38.54) | 103 (37.59) | 0.027 | ||
| Male | 230 (61.89) | 59 (61.46) | 171 (62.40) | |||
| Medical history length of T2DM, years, M (Q₁, Q₃) | 10.00 (7.00, 13.00) | 8.00 (6.00, 12.00) | 10.00 (7.25, 13.00) | Z=−3.352 | <0.001 | 0.351 |
| Insulin usage, n (%) | χ²=0.100 | 0.752 | ||||
| No | 136 (36.76) | 34 (35.42) | 102 (37.23) | 0.037 | ||
| Yes | 234 (63.24) | 62 (64.58) | 172 (62.77) | −0.037 | ||
| CVD, n (%) | χ²=0.875 | 0.350 | ||||
| Yes | 288 (77.84) | 78 (81.25) | 210 (76.64) | −0.109 | ||
| No | 82 (22.16) | 18 (18.75) | 64 (23.36) | 0.109 | ||
| CAD, n (%) | χ²=0.035 | 0.852 | ||||
| Yes | 261 (70.54) | 67 (69.79) | 194 (70.80) | 0.022 | ||
| No | 109 (29.46) | 29 (30.21) | 80 (29.20) | −0.022 | ||
| Hypertension, n (%) | χ²=5.307 | 0.021 | ||||
| Yes | 105 (28.38) | 36 (37.50) | 69 (25.18) | −0.284 | ||
| No | 265 (71.62) | 60 (62.50) | 205 (74.82) | 0.284 | ||
| CKD, n (%) | χ²=0.009 | 0.923 | ||||
| Yes | 195 (52.7) | 51 (53.12) | 144 (52.55) | −0.011 | ||
| No | 175 (47.3) | 45 (46.88) | 130 (47.45) | 0.011 | ||
| Dyslipidemia, n (%) | χ²=8.430 | 0.004 | ||||
| Yes | 52 (14.05) | 22 (22.92) | 30 (10.95) | −0.383 | ||
| No | 318 (85.95) | 74 (77.08) | 244 (89.05) | 0.383 | ||
| Smoking, n (%) | χ²=1.649 | 0.199 | ||||
| Yes | 195 (52.7) | 56 (58.33) | 139 (50.73) | −0.152 | ||
| No | 175 (47.3) | 40 (41.67) | 135 (49.27) | 0.152 | ||
| Alcoholic, n (%) | χ²=0.613 | 0.434 | ||||
| Yes | 310 (83.78) | 78 (81.25) | 232 (84.67) | 0.095 | ||
| No | 60 (16.22) | 18 (18.75) | 42 (15.33) | −0.095 | ||
| BMI, M (Q₁, Q₃) | 23.00 (21.00, 25.50) | 22.70 (20.90, 24.90) | 23.20 (21.10, 25.70) | Z=−1.010 | 0.313 | 0.101 |
| Anti-platelet therapy, n (%) | χ²=0.333 | 0.564 | ||||
| Yes | 55 (14.86) | 16 (16.67) | 39 (14.23) | −0.070 | ||
| No | 315 (85.14) | 80 (83.33) | 235 (85.77) | 0.070 | ||
| ACEi or ARB, n (%) | χ²=0.000 | 0.987 | ||||
| Yes | 166 (44.86) | 43 (44.79) | 123 (44.89) | 0.002 | ||
| No | 204 (55.14) | 53 (55.21) | 151 (55.11) | −0.002 | ||
| βBlocker, n (%) | χ²=0.229 | 0.632 | ||||
| Yes | 212 (57.3) | 57 (59.38) | 155 (56.57) | −0.057 | ||
| No | 158 (42.7) | 39 (40.62) | 119 (43.43) | 0.057 | ||
| CCB, n (%) | χ²=0.408 | 0.523 | ||||
| Yes | 275 (74.32) | 69 (71.88) | 206 (75.18) | 0.077 | ||
| No | 95 (25.68) | 27 (28.12) | 68 (24.82) | −0.077 | ||
| Diuretic, n (%) | χ²=0.007 | 0.934 | ||||
| Yes | 294 (79.46) | 76 (79.17) | 218 (79.56) | 0.010 | ||
| No | 76 (20.54) | 20 (20.83) | 56 (20.44) | −0.010 | ||
| Statins, n (%) | χ²=0.180 | 0.672 | ||||
| Yes | 51 (13.78) | 12 (12.50) | 39 (14.23) | 0.050 | ||
| No | 319 (86.22) | 84 (87.50) | 235 (85.77) | −0.050 |
Notes: *bold font indicates statistically significance.
Abbreviations: PSM, propensity score-matched method; eGFR, estimated glomerular filtration rate; CAD, coronary artery disease; CVD, cerebrovascular disease; CKD, chronic kidney disease; HbA1C, Hemoglobin A1C; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker. CCB, calcium channel blocker.
Table 2.
Comparison Results of Laboratory Parameters Between the Two Groups Before PSM
| Variable | Total (n = 370) | Low- HbA1C Variability Group (n = 96) |
High- HbA1C Variability Group (n = 274) |
Statistic | P | SMD |
|---|---|---|---|---|---|---|
| eGFR, mL/min,Mean ± SD | 73.71 ± 14.68 | 74.64 ± 15.38 | 73.39 ± 14.44 | t=0.722 | 0.471 | −0.087 |
| Albumin, g/L, M (Q₁, Q₃) | 41.20 (38.05, 43.70) | 42.20 (39.77, 44.50) | 41.00 (38.00, 43.30) | Z=−2.456 | 0.014 | −0.297 |
| Uric acid, mmol/L, M (Q₁, Q₃) | 402.00 (318.00, 455.75) | 377.00 (310.00, 439.75) | 406.00 (323.75, 462.00) | Z=−1.946 | 0.052 | 0.192 |
| Fast glucose, mmol/L, M (Q₁, Q₃) | 9.42 (8.50, 11.87) | 9.30 (8.20, 11.85) | 9.50 (8.60, 11.87) | Z=−0.587 | 0.557 | −0.026 |
| Baseline HbA1C,%, M (Q₁, Q₃) | 8.40 (7.90, 9.10) | 8.10 (7.80, 8.50) | 8.50 (7.90, 9.10) | Z=−3.524 | <0.001 | 0.402 |
| hs-CRP, g/L, M (Q₁, Q₃) | 7.50 (5.32, 9.40) | 7.70 (5.80, 9.75) | 7.50 (5.30, 9.40) | Z=−0.883 | 0.377 | −0.090 |
| RBC, 1012/L, M (Q₁, Q₃) | 4.50 (3.80, 5.20) | 4.45 (3.77, 5.10) | 4.50 (3.80, 5.20) | Z=−0.409 | 0.683 | 0.036 |
| Hemoglobin, g/L, M (Q₁, Q₃) | 126.00 (115.25, 138.00) | 127.00 (118.00, 138.75) | 126.00 (115.00, 137.75) | Z=−1.209 | 0.227 | −0.139 |
| Platelets,109/L, M (Q₁, Q₃) | 203.00 (151.50, 241.00) | 204.00 (167.25, 240.25) | 201.00 (150.25, 241.00) | Z=−0.424 | 0.672 | −0.065 |
| TC, mmol/L, M (Q₁, Q₃) | 4.69 (4.31, 5.30) | 4.85 (4.44, 5.46) | 4.67 (4.27, 5.28) | Z=−1.579 | 0.114 | −0.134 |
| TG, mmol/L, M (Q₁, Q₃) | 2.23 (1.76, 2.60) | 2.17 (1.85, 2.54) | 2.25 (1.70, 2.64) | Z=−0.431 | 0.667 | 0.079 |
| HDL-C, mmol/L, M (Q₁, Q₃) | 0.97 (0.87, 1.29) | 0.98 (0.88, 1.31) | 0.96 (0.86, 1.27) | Z=−0.568 | 0.570 | −0.053 |
| LDL-C, mmol/L, M (Q₁, Q₃) | 3.01 (2.53, 3.42) | 3.12 (2.64, 3.40) | 2.96 (2.51, 3.45) | Z=−0.705 | 0.481 | 0.008 |
Notes: *bold font indicates statistically significance.
Abbreviations: PSM, propensity score-matched method; eGFR, estimated glomerular filtration rate; HbA1C, Hemoglobin A1C; hs-CRP, hypersensitive C-reactive protein; TC, total cholesterol; TG, total triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
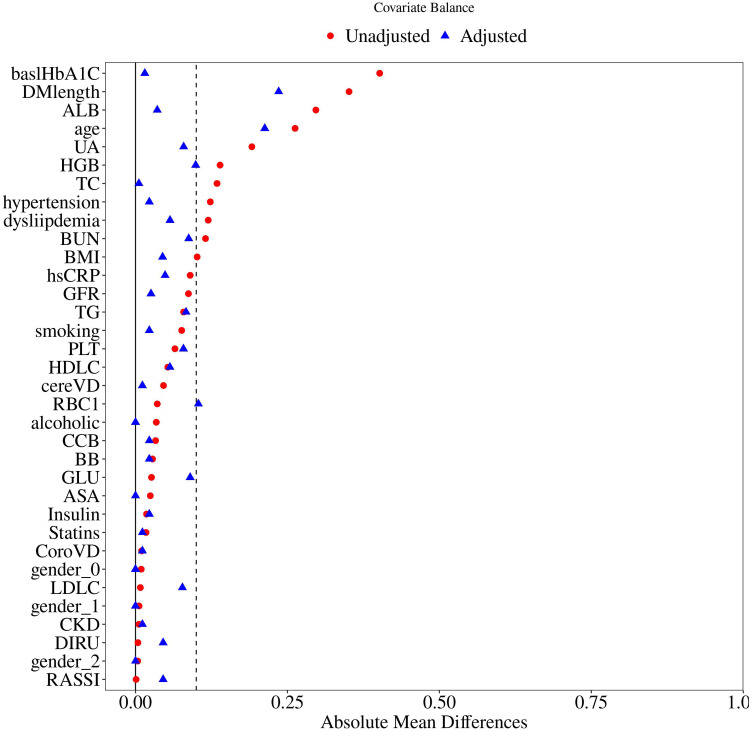
We further performed PSM to reduce the potential influences of other covariates. The detailed PSM process is shown in Figure 2. In conclusion, after a 1:1 ratio PSM, there was no significant difference between the two groups regarding relative parameters after PSM (Tables 3 and 4).
Figure 2.
The process of propensity score-matched analysis.
Table 3.
Comparison Results of Demographic and Clinical Parameters Between the Two Groups After PSM#
| Variable | Total (n = 176) | Low- HbA1C Variability Group (n = 88) | High- HbA1C Variability Group (n = 88) | Statistic | P | SMD |
|---|---|---|---|---|---|---|
| Age, M (Q₁, Q₃) | 65.00 (48.00, 75.00) | 62.50 (47.75, 74.00) | 66.00 (51.25, 77.00) | Z=−1.379 | 0.168 | 0.209 |
| Gender, n (%) | χ²=0.000 | 1.000 | ||||
| No | 66 (37.5) | 33 (37.50) | 33 (37.50) | 0.000 | ||
| Yes | 110 (62.5) | 55 (62.50) | 55 (62.50) | 0.000 | ||
| Medical history length of T2DM, years, M (Q₁, Q₃) | 9.00 (7.00, 13.00) | 9.00 (6.00, 12.00) | 10.00 (7.00, 13.00) | Z=−1.758 | 0.079 | 0.240 |
| Insulin usage, n (%) | χ²=0.096 | 0.757 | ||||
| No | 68 (38.64) | 33 (37.50) | 35 (39.77) | 0.046 | ||
| Yes | 108 (61.36) | 55 (62.50) | 53 (60.23) | −0.046 | ||
| CAD, n (%) | χ²=0.036 | 0.850 | ||||
| No | 141 (80.11) | 70 (79.55) | 71 (80.68) | 0.029 | ||
| Yes | 35 (19.89) | 18 (20.45) | 17 (19.32) | −0.029 | ||
| CVD, n (%) | χ²=0.026 | 0.871 | ||||
| No | 121 (68.75) | 60 (68.18) | 61 (69.32) | 0.025 | ||
| Yes | 55 (31.25) | 28 (31.82) | 27 (30.68) | −0.025 | ||
| Hypertension, n (%) | χ²=0.101 | 0.750 | ||||
| No | 60 (34.09) | 31 (35.23) | 29 (32.95) | −0.048 | ||
| Yes | 116 (65.91) | 57 (64.77) | 59 (67.05) | 0.048 | ||
| CKD, n (%) | χ²=0.023 | 0.880 | ||||
| No | 89 (50.57) | 45 (51.14) | 44 (50.00) | −0.023 | ||
| Yes | 87 (49.43) | 43 (48.86) | 44 (50.00) | 0.023 | ||
| Dyslipidemia, n (%) | χ²=0.979 | 0.322 | ||||
| No | 31 (17.61) | 18 (20.45) | 13 (14.77) | −0.160 | ||
| Yes | 145 (82.39) | 70 (79.55) | 75 (85.23) | 0.160 | ||
| Smoking, n (%) | χ²=0.094 | 0.759 | ||||
| No | 104 (59.09) | 51 (57.95) | 53 (60.23) | 0.046 | ||
| Yes | 72 (40.91) | 37 (42.05) | 35 (39.77) | −0.046 | ||
| Alcoholic, n (%) | χ²=0.000 | 1.000 | ||||
| No | 146 (82.95) | 73 (82.95) | 73 (82.95) | 0.000 | ||
| Yes | 30 (17.05) | 15 (17.05) | 15 (17.05) | 0.000 | ||
| BMI, M (Q₁, Q₃) | 22.71 (20.80, 25.50) | 22.70 (20.88, 24.95) | 22.90 (20.75, 25.83) | Z=−0.317 | 0.752 | 0.043 |
| Antiplatelet therapy, n (%) | χ²=0.000 | 1.000 | ||||
| No | 26 (14.77) | 13 (14.77) | 13 (14.77) | 0.000 | ||
| Yes | 150 (85.23) | 75 (85.23) | 75 (85.23) | 0.000 | ||
| ACEi or ARB, n (%) | χ²=0.371 | 0.543 | ||||
| No | 76 (43.18) | 40 (45.45) | 36 (40.91) | −0.092 | ||
| Yes | 100 (56.82) | 48 (54.55) | 52 (59.09) | 0.092 | ||
| βBlocker, n (%) | χ²=0.093 | 0.761 | ||||
| No | 100 (56.82) | 51 (57.95) | 49 (55.68) | −0.046 | ||
| Yes | 76 (43.18) | 37 (42.05) | 39 (44.32) | 0.046 | ||
| CCB, n (%) | χ²=0.115 | 0.735 | ||||
| No | 128 (72.73) | 63 (71.59) | 65 (73.86) | 0.052 | ||
| Yes | 48 (27.27) | 25 (28.41) | 23 (26.14) | −0.052 | ||
| Diuretic, n (%) | χ²=0.485 | 0.486 | ||||
| No | 132 (75) | 68 (77.27) | 64 (72.73) | −0.102 | ||
| Yes | 44 (25) | 20 (22.73) | 24 (27.27) | 0.102 | ||
| Statins, n (%) | χ²=0.050 | 0.823 | ||||
| No | 23 (13.07) | 11 (12.50) | 12 (13.64) | 0.033 | ||
| Yes | 153 (86.93) | 77 (87.50) | 76 (86.36) | −0.033 |
Notes: #The following variables were used in the propensity score (PSM) model: age, sex, medical history of diabetes, hypertension, dyslipidemia, BMI, previous history of coronary artery disease, cerebrovascular disease, smoking, hypertension, CKD, previous use of antiplatelet therapy, ACEI or ARB, β-blockers, statins. *Red font indicates statistically significance.
Abbreviations: PSM, propensity score-matched method; eGFR, estimated glomerular filtration rate; CAD, coronary artery disease; CVD, cerebrovascular disease; CKD, chronic kidney disease; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker. CCB, calcium channel blocker.
Table 4.
Comparison Results of Laboratory Parameters Between the Two Groups After PSM#
| Variable | Total (n = 176) | Low- HbA1C Variability Group (n = 88) | High- HbA1C Variability Group (n = 88) | Statistic | P | SMD |
|---|---|---|---|---|---|---|
| eGFR, mL/min,Mean ± SD | 73.48 ± 14.79 | 73.66 ± 15.32 | 73.29 ± 14.33 | t=0.165 | 0.870 | −0.026 |
| Albumin, g/L, M (Q₁, Q₃) | 42.00 (38.95, 44.35) | 42.00 (38.95, 44.52) | 42.05 (39.03, 44.23) | Z=−0.287 | 0.774 | −0.035 |
| Uric acid, mmol/L, M (Q₁, Q₃) | 380.50 (310.75, 445.50) | 377.00 (310.75, 439.75) | 392.50 (315.25, 461.00) | Z=−0.555 | 0.579 | 0.080 |
| Fast glucose, mmol/L, M (Q₁, Q₃) | 9.55 (8.60, 12.33) | 9.35 (8.43, 12.35) | 9.60 (8.70, 12.25) | Z=−1.033 | 0.302 | 0.083 |
| Baseline HbA1C,%, M (Q₁, Q₃) | 8.20 (7.80, 8.60) | 8.20 (7.88, 8.50) | 8.20 (7.70, 8.72) | Z=−0.391 | 0.695 | −0.018 |
| hs-CRP, g/L,M (Q₁, Q₃) | 7.65 (5.80, 9.90) | 7.70 (5.97, 9.75) | 7.45 (5.75, 9.90) | Z=−0.577 | 0.564 | −0.047 |
| RBC, 1012/L,M (Q₁, Q₃) | 4.50 (3.80, 5.20) | 4.50 (3.77, 5.10) | 4.50 (3.90, 5.20) | Z=−0.758 | 0.448 | 0.106 |
| Hemoglobin, g/L, M (Q₁, Q₃) | 127.00 (117.00, 140.25) | 128.00 (119.00, 141.00) | 126.50 (116.00, 139.25) | Z=−0.724 | 0.469 | −0.095 |
| Platelets,109/L, M (Q₁, Q₃) | 205.00 (168.75, 241.75) | 202.00 (148.50, 237.00) | 213.00 (177.00, 244.25) | Z=−0.886 | 0.375 | 0.083 |
| TC, mmol/L, M (Q₁, Q₃) | 4.78 (4.31, 5.42) | 4.85 (4.40, 5.42) | 4.72 (4.26, 5.42) | Z=−0.450 | 0.653 | −0.005 |
| TG, mmol/L, M (Q₁, Q₃) | 2.17 (1.83, 2.52) | 2.17 (1.87, 2.57) | 2.16 (1.69, 2.48) | Z=−0.913 | 0.361 | −0.085 |
| HDLC, mmol/L, M (Q₁, Q₃) | 0.98 (0.88, 1.33) | 0.98 (0.88, 1.35) | 0.98 (0.88, 1.31) | Z=−0.155 | 0.877 | 0.050 |
| LDLC, mmol/L, M (Q₁, Q₃) | 3.12 (2.57, 3.49) | 3.13 (2.64, 3.42) | 3.02 (2.56, 3.54) | Z=−0.012 | 0.991 | 0.071 |
Notes: #The following variables were used in the propensity score (PSM) model: age, sex, medical history of diabetes, hypertension, dyslipidemia, BMI, previous history of coronary artery disease, cerebrovascular disease, smoking, hypertension, CKD, previous use of antiplatelet therapy, ACEI or ARB, β-blockers, statins. *Red font indicates statistically significance.
Abbreviations: PSM, propensity score-matched method; eGFR, estimated glomerular filtration rate; HbA1C, Hemoglobin A1C; hs-CRP, hypersensitive C-reactive protein; TC, total cholesterol; TG, total triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Both the uni-variable and multi-variable logistic analysis between the two groups after PSM were performed and the results were shown in Table 5: The CFS was the independent risk factor for high HVS (OR = 2.05 95% CI (1.62 ~ 2.61), p < 0.001).
Table 5.
Results of Uni-Variable and Multi-Variable Logistic Analysis in Predicting HVS After PSM
| Variable | Uni-Variable Analysis | Multi-Variable Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | t | P | OR (95% CI) | β | S.E | t | P | OR (95% CI) | |
| Hypertension | ||||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Yes | 0.10 | 0.32 | 0.32 | 0.750 | 1.11 (0.59 ~ 2.06) | 0.23 | 0.37 | 0.63 | 0.529 | 1.26 (0.61 ~ 2.59) |
| Dyslipidemia | ||||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Yes | 0.39 | 0.40 | 0.99 | 0.324 | 1.48 (0.68 ~ 3.25) | 0.85 | 0.47 | 1.79 | 0.073 | 2.33 (0.92 ~ 5.90) |
| Baseline HbA1C | −0.03 | 0.20 | −0.13 | 0.898 | 0.97 (0.66 ~ 1.44) | −0.24 | 0.24 | −1.00 | 0.315 | 0.79 (0.49 ~ 1.26) |
| Albumin | −0.01 | 0.04 | −0.23 | 0.818 | 0.99 (0.92 ~ 1.07) | −0.01 | 0.04 | −0.12 | 0.904 | 0.99 (0.91 ~ 1.08) |
| CFS | 0.68 | 0.12 | 5.82 | <0.001 | 1.97 (1.57 ~ 2.47) | 0.72 | 0.12 | 5.89 | <0.001 | 2.05 (1.62 ~ 2.61) |
| Age | 0.01 | 0.01 | 1.39 | 0.165 | 1.01 (0.99 ~ 1.04) | 0.00 | 0.01 | 0.22 | 0.826 | 1.00 (0.97 ~ 1.03) |
| Medical history length of T2DM | 0.05 | 0.03 | 1.64 | 0.101 | 1.05 (0.99 ~ 1.11) | 0.03 | 0.04 | 0.80 | 0.423 | 1.03 (0.95 ~ 1.12) |
Notes: *bold indicates statistically significance.
Abbreviations: HVS, HbA1C variability score; PSM, propensity score-matched method; OR: Odds Ratio; CI, Confidence Interval; CFS, clinical frailty scale.
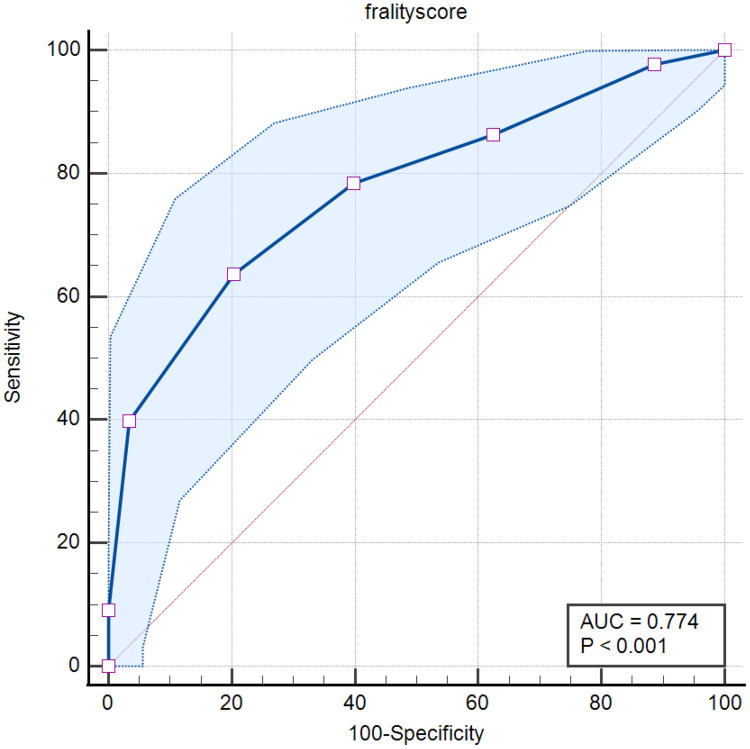
The predictive value of CFS in HVS was further evaluated by using the ROC curve and the result showed that CFS had good predictive value in predicting HVS [AUC = 0.774, 95% CI: (0.705 to 0.833), p < 0.001], the cut-off value of HVS was 4.0 with sensitivity = 63.64% and specificity = 79.55% (Figure 3).
Figure 3.
Results of the receiver operating characteristic curve.
Discussion
The association between high visit-to-visit HbA1C variability in T2DM patients and adverse clinical outcomes has been well-recognized. However, studies that explore the risk factors for the visit-to-visit HbA1C variability are scarce. So far as we know, our study is the first study evaluating the association between CFS and visit-to-visit HbA1C variability in T2DM patients. Our study revealed that higher CFS was associated with higher visit-to-visit HbA1C variability in patients with T2DM.
T2DM, one of the most common metabolic disorders, contributes to the mortality and disability in millions of people worldwide by causing macro-vascular (ischemia heart disease stroke, etc) and micro-vascular (including blindness, lower limb amputation and renal failure, etc) complications.27,28 The underlying mechanism of the complications caused by T2DM is complex, insulin resistance, inflammation, oxidized stress, mitochondrial dysfunction, and endothelial dysfunction are all believed to be associated with the development of T2DM-related complications.1,2,28–30
Higher visit-to-visit glycemia variability was demonstrated to be associated with these pathology changes of T2DM. Xu Jet al included 1856 community residents without T2DM and all of the participants received tests for insulin resistance evaluation by using the homeostatic model assessment. A total of 153 (8.2%) participants developed T2DM during follow-up and high visit-to-visit fasting plasma glucose variability was associated with a 1.48 increased risk of diabetes after adjusting other covariates. Further mediation analyses suggested that changes in insulin resistance might mediate 17.3% of the association between increased visit-to-visit fasting plasma glucose variability and elevated diabetes risk. This study demonstrated high visit-to-visit fasting plasma glucose variability was associated with increased insulin resistance.31 The association between visit-to-visit glycemia variability and oxidative stress was also confirmed in a study by Saito Y et al which explored the association between visit-to-visit HbA1C variability and cancer development in T2DM patients.32 Fang Q et al who demonstrated that long-term visit-to-visit HbA1c variability was independently associated with aortic stiffness progression in T2DM patients.5
Frailty is a common condition in patients with chronic diseases, especially in aged people.13 T2DM patients are at high risk of frailty because insulin resistance produces a negative impact on skeletal muscle function and on vascular function.22 Since higher visit-to-visit glycemia variability can aggravate insulin resistance, these patients are at higher risk of frailty. High visit-to-visit blood pressure variability was demonstrated to be associated with an increased incident rate of frailty in older adults from a large cohort study.33
The clinical frailty scale (CFS) is a promising frailty screening tool that has been proven to be associated with clinical outcomes in different diseases as we mentioned above. In the prospective, multi-center, longitudinal study, CFS was confirmed as a good independent negative prognostic factor of long-term mortality in the patients who were hospitalized in the intensive care unit.34 In another study by Bradley NA et al, CFS showed good prognostic value in patients with chronic limb-threatening ischemia.35 Han SJ et al evaluated the association between CFS and clinical outcomes and CFS was demonstrated to be useful in screening high-risk patients.36 In a meta-analysis that enrolled 17 studies (n = 45,022), CFS was confirmed as an accurate and reliable tool for predicting short-term mortality in emergency patients.25 The usefulness of CFS in T2DM patients was also evaluated in a study that enrolled 400 patients (35.3% of them were T2DM patients), the study showed that frailty was more common in patients with diabetes and patients with diabetes, the CFS was higher, and with worse clinical outcomes.37
Our study, so far as we know, for the first time revealed that higher CFS was associated with higher visit-to-visit glycemia variability in T2DM patients. This result highlights the importance of stable glycemic control could be beneficial to reduce the potential risk of frailty.
Our study has some limitations. First, this is a retrospective study, even if we performed PSM to limit the influence of other parameters, potential biases still might exist, so prospective studies are needed to confirm our findings. Second, our study is a single-center study with relatively limited participants, so multi-center studies with more enrolled patients are required. Third, we applied CFS to evaluate the frailty status and the predictive value of other frailty scoring systems still needs to be verified. Fourth, the visit-to-visit glycemic variability in our study was evaluated by HVS, which was relatively simple and easy compared with other variability parameters including coefficient of variation, standard deviation, corrected variability independent of the mean (cVIM), etc. cVIM), etc. So the predictive value of these parameters still needs to be verified.
In conclusion, our study reveals that high CFS is associated with high visit-to-visit HbA1C variability and CFS has good clinical value in predicting HVS. Future studies are still needed to confirm our findings.
Data Sharing Statement
The authors will supply the relevant data in response to reasonable requests.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Beijing Tongren Hospital, Capital Medical University (TR-LCHL-23-105), which met the requirements of the Helsinki Declaration for clinical research. Informed consent was obtained from the patients or their family members who participated in our study.
Consent for Publication
All authors agreed to publish this study in our journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am. 2021;50(3):337–355. doi: 10.1016/j.ecl.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Chan JCN, Yang A, Chu N, et al. Current type 2 diabetes guidelines: individualized treatment and how to make the most of metformin. Diabetes Obes Metab. 2024;26(Suppl 3):55–74. doi: 10.1111/dom.15700 [DOI] [PubMed] [Google Scholar]
- 3.Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. doi: 10.1002/dmrr.3158 [DOI] [PubMed] [Google Scholar]
- 4.Li F, Zhang L, Shen Y, et al. Higher glucose fluctuation is associated with a higher risk of cardiovascular disease: insights from pooled results among patients with diabetes. J Diabetes. 2023;15(5):368–381. doi: 10.1111/1753-0407.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Q, Shi J, Zhang J, et al. Visit-to-visit HbA1c variability is associated with aortic stiffness progression in participants with type 2 diabetes. Cardiovasc Diabetol. 2023;22(1):167. doi: 10.1186/s12933-023-01884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echouffo-Tcheugui JB, Zhao S, Brock G, et al. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: the allhat study. Diabetes Care. 2019;42(3):486–493. doi: 10.2337/dc18-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Dong S, Fu EL, et al. Long-term visit-to-visit variability in hemoglobin A1c and kidney-related outcomes in persons with diabetes. Am J Kidney Dis. 2023;82(3):267–278. doi: 10.1053/j.ajkd.2023.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu JC, Yang YY, Chuang SL, et al. Long-term visit-to-visit glycemic variability as a predictor of major adverse limb and cardiovascular events in patients with diabetes. J Am Heart Assoc. 2023;12(3):e025438. doi: 10.1161/JAHA.122.025438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wu S, Li M, et al. Long-term glycemic variability is associated with arterial stiffness in Chinese adults. Front Endocrinol 2021;12:711540. doi: 10.3389/fendo.2021.711540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Ao L, Hu X, et al. Influence of blood glucose fluctuation, C-peptide level and conventional risk factors on carotid artery intima-media thickness in Chinese Han patients with type 2 diabetes mellitus. Eur J Med Res. 2019;24(1):13. doi: 10.1186/s40001-019-0370-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502–510. doi: 10.2337/dc15-2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monnier L, Bonnet F, Colette C, Renard E, Owens D. Key indices of glycaemic variability for application in diabetes clinical practice. Diabetes Metab. 2023;49(6):101488. doi: 10.1016/j.diabet.2023.101488 [DOI] [PubMed] [Google Scholar]
- 13.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 14.Verduri A, Carter B, Laraman J, et al. Frailty and its influence on mortality and morbidity in COPD: a systematic review and meta-analysis. Intern Emerg Med. 2023;18(8):2423–2434. doi: 10.1007/s11739-023-03405-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetrano DL, Palmer KM, Galluzzo L, et al. Hypertension and frailty: a systematic review and meta-analysis. BMJ Open. 2018;8(12):e024406. doi: 10.1136/bmjopen-2018-024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin U, Yoon M, Ha J, et al. Association between frailty and physical performance in older patients with heart failure. Clin Cardiol. 2023;46(12):1530–1537. doi: 10.1002/clc.24142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quach J, Kehler DS, Giacomantonio N, et al. Association of admission frailty and frailty changes during cardiac rehabilitation with 5-year outcomes. Eur J Prev Cardiol. 2023;30(9):807–819. doi: 10.1093/eurjpc/zwad048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Xiong T, Tan X, Chen L. Frailty and risk of microvascular complications in patients with type 2 diabetes: a population-based cohort study. BMC Med. 2022;20(1):473. doi: 10.1186/s12916-022-02675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He D, Li J, Li Y, et al. Frailty is associated with the progression of prediabetes to diabetes and elevated risks of cardiovascular disease and all-cause mortality in individuals with prediabetes and diabetes: evidence from two prospective cohorts. Diabet Res Clin Pract. 2022;194:110145. doi: 10.1016/j.diabres.2022.110145 [DOI] [PubMed] [Google Scholar]
- 20.Tamura Y, Omura T, Toyoshima K, et al. Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients. 2020;12(11):3367. doi: 10.3390/nu12113367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, et al. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health. 2022;19(14):8677. doi: 10.3390/ijerph19148677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assar ME, Laosa O, Rodríguez Mañas L. Diabetes and frailty. Curr Opin Clin Nutr Metab Care. 2019;22(1):52–57. doi: 10.1097/MCO.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 23.Chung SM, Lee YH, Kim CO, et al. Daytime glycemic variability and frailty in older patients with diabetes: a pilot study using continuous glucose monitoring. J Korean Med Sci. 2021;36(27):e190. doi: 10.3346/jkms.2021.36.e190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walston J, Buta B, Xue Q-L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34(1):25–38. doi: 10.1016/j.cger.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falk Erhag H, Guðnadóttir G, Alfredsson J, et al. The association between the clinical frailty scale and adverse health outcomes in older adults in acute clinical settings - a systematic review of the literature. Clin Interv Aging. 2023;18:249–261. doi: 10.2147/CIA.S388160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476–486. doi: 10.1016/S2213-8587(18)30048-2 [DOI] [PubMed] [Google Scholar]
- 27.Mizukami H, Kudoh K. Diversity of pathophysiology in type 2 diabetes shown by islet pathology. J Diabetes Investig. 2022;13(1):6–13. doi: 10.1111/jdi.13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Militaru M, Lighezan DF, Tudoran C, et al. Connections between cognitive impairment and atrial fibrillation in patients with diabetes mellitus type 2. Biomedicines. 2024;12(3):672. doi: 10.3390/biomedicines12030672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. doi: 10.3390/ijms21176275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunton S. Pathophysiology of type 2 diabetes: the evolution of our understanding. J Fam Pract. 2016;65(4 Suppl):supp_az_0416. [PubMed] [Google Scholar]
- 31.Xu J, Li L, Huang S, et al. Impact of visit-to-visit fasting plasma glucose variability on the development of diabetes: the mediation by insulin resistance. J Diabetes. 2022;14(3):205–215. doi: 10.1111/1753-0407.13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Noto H, Takahashi O, Kobayashi D. Visit-to-visit hemoglobin a1c variability is associated with later cancer development in patients with diabetes mellitus. Cancer J. 2019;25(4):237–240. doi: 10.1097/PPO.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 33.Rouch L, De Souto Barreto P, Hanon O, et al. Visit-to-visit blood pressure variability and incident frailty in older adults. J Gerontol a Biol Sci Med Sci. 2021;76(8):1369–1375. doi: 10.1093/gerona/glab112 [DOI] [PubMed] [Google Scholar]
- 34.Remelli F, Scaramuzzo G, Capuzzo M, et al. Frailty trajectories in ICU survivors: a comparison between the clinical frailty scale and the Tilburg frailty indicator and association with 1-year mortality. J Crit Care. 2023;78:154398. doi: 10.1016/j.jcrc.2023.154398 [DOI] [PubMed] [Google Scholar]
- 35.Bradley NA, Walter A, Roxburgh CSD, McMillan DC, Guthrie GJK. The relationship between clinical frailty score, CT-derived body composition, systemic inflammation, and survival in patients with chronic limb-threatening ischemia. Ann Vasc Surg. 2024;104:18–26. doi: 10.1016/j.avsg.2023.06.012 [DOI] [PubMed] [Google Scholar]
- 36.Han SJ, Jung HW, Lee JH, et al. Clinical frailty scale, K-FRAIL questionnaire, and clinical outcomes in an acute hospitalist unit in Korea. Korean J Intern Med. 2021;36(5):1233–1241. doi: 10.3904/kjim.2020.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKenzie HT, Tugwell B, Rockwood K, Theou O. Frailty and diabetes in older hospitalized adults: the case for routine frailty assessment. Can J Diabetes. 2020;44(3):241–245. doi: 10.1016/j.jcjd.2019.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will supply the relevant data in response to reasonable requests.