Abstract
Background
The single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) can be performed as a primary or (planned) secondary metabolic bariatric procedure. The aims of this study were to compare mid-term outcomes up to 5 years after primary vs secondary SADI-S and between different common channel (CC) lengths.
Methods
Multicenter retrospective cohort study including 103 patients who underwent SADI-S between 06–2015 and 02–2019. Outcomes on weight loss, nutrient status, health-related quality of life (HRQoL) and gastro-intestinal symptoms until 5 years postoperatively were evaluated and compared between primary (n = 19) vs secondary SADI-S (n = 84), and CC length ≤ 250 cm (n = 66,) vs > 250 cm (n = 33).
Results
Mean total weight loss (TWL) at 5 years of follow-up was higher for patients who underwent primary SADI-S compared to secondary SADI-S (34.8 (29.8–39.9)% vs 15.9 (13.0–18.9)%, p < 0.001) and for CC length ≤ 250 cm compared to > 250 cm (25.3 (21.8–28.9)% vs 21.3 (17.2–25.4)%, p = 0.12). Patients who underwent primary SADI-S also had significantly higher scores on the domains of the BODY-Q HRQoL questionnaire (p < 0.05 for all), with the exception of sexual well-being. Nutrient status and gastro-intestinal symptoms were comparable between the indication groups, but CC length ≤ 250 cm tended to result in more nutrient deficiencies and higher defecation frequency.
Conclusion
Both primary and secondary SADI-S result in durable weight loss outcomes up to 5 years postoperatively. It is imperative that CC length should be at least 250 cm to prevent malnutrition and gastro-intestinal complaints. Furthermore, focus on HRQoL is essential in future research into SADI-S.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11695-025-07888-4.
Keywords: Sleeve gastrectomy, Single-anastomosis duodeno-ileal bypass, SADI-S, Conversion surgery, Secondary surgery, Recurrent weight gain
Introduction
Sleeve gastrectomy is the most performed metabolic bariatric surgical procedure worldwide [1], and can be performed either as a stand-alone or as a first stage procedure in patients with a body mass index (BMI) ≥ 50 kg/m2 [2]. Multiple surgical techniques are available as conversion procedure or planned second step after SG, including single-anastomosis duodeno-ileal bypass (SADI), Roux-en-Y gastric bypass (RYGB), one anastomosis gastric bypass (OAGB) and duodenal switch (DS) [3–5].
In 2007, Sánchez-Pernaute et al. first introduced the SADI with SG (SADI-S) as a single step procedure [6], with total weight loss (TWL) ranging between 22.5–38.0% beyond five years postoperatively [7–9]. Later, the effectiveness of the SADI as a second step after SG was also shown with TWL ranging between 15.0–41.0% beyond five years postoperatively [7–9]. SADI-S is currently considered a safe and effective treatment for severe obesity and related complications by the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) with acceptable early complication rates (5.3%) and late complications mostly related to patients’ nutritional status (e.g. severe protein energy malnutrition, iron deficiency) [10].
Still, there is a wide range in postoperative outcomes following SADI-S, and potential factors contributing to this variation may include common channel (CC) length, primary versus secondary procedure, and indication for secondary SADI-S (e.g. planned second stage, recurrent weight gain, suboptimal initial response) [11]. Furthermore, only few studies consider the implications of SADI-S on patient-reported outcomes, such as health-related quality of life (HRQoL) and gastro-intestinal symptoms including gastroesophageal reflux disease (GERD) and constipation or diarrhea [12].
The primary aim of this study is to compare mid-term outcomes on weight loss, nutrient status, and patient-reported HRQoL and gastro-intestinal symptoms up to 5 years after primary versus secondary SADI-S. Secondary aims are to explore differences in weight loss outcomes between different indications for secondary SADI-S and between different CC lengths (≤ 250 cm or > 250 cm).
Methods
Study Design and Population
All adult patients who underwent primary or secondary SADI-S between June 2015 and February 2019 at one of four participating centers in the Netherlands (Rijnstate Hospital, Arnhem; Catharina Hospital, Eindhoven; St. Antonius Hospital, Nieuwegein; Dutch Obesity Clinic, The Hague) were included in this study. Exclusion criteria were known pregnancy during follow-up, malnutrition due to other causes (e.g. malignancies, alcoholism), previous metabolic bariatric surgery (other than laparoscopic adjustable banding or sleeve gastrectomy) and loss to follow-up directly after SADI-S.
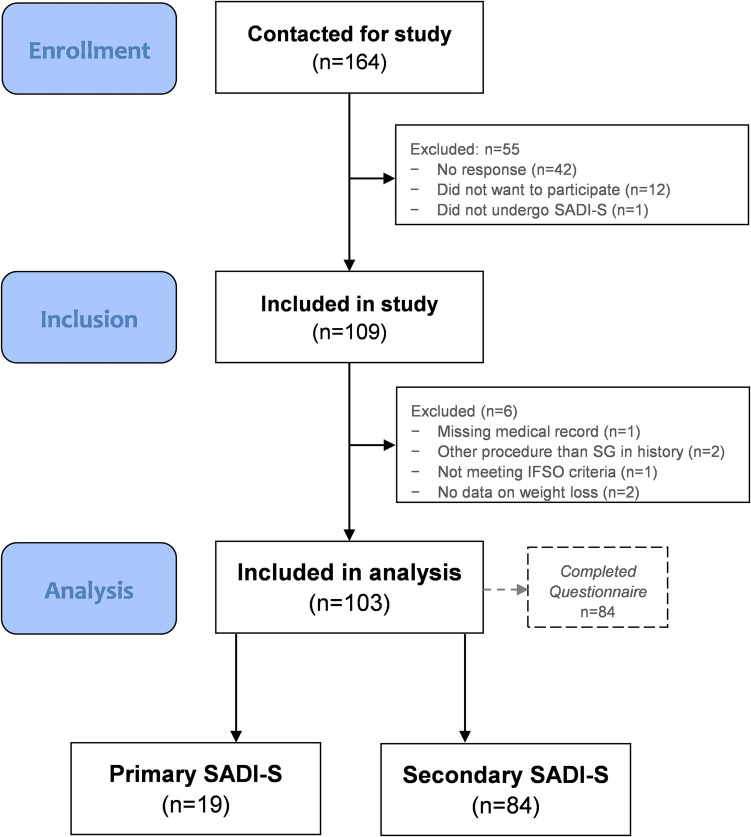
Patients were contacted by email and had the opportunity to digitally consent to either record-based research only (retrospective cohort) or to record-based research supplemented with additional questionnaires (cross-sectional). Of the 164 patients that were contacted for the study, 109 (66%) agreed to participate of whom six were excluded because of missing medical records (n = 1), history of other metabolic bariatric procedures (n = 2), not meeting IFSO criteria at the time of SADI-S (n = 1) or missing data on weight loss (n = 2). This resulted in a total study population of 103 participants (Fig. 1). Additional questionnaires on HRQoL and gastro-intestinal symptoms were completed by n = 84 at a median follow-up of 32 months after SADI-S.
Fig. 1.
Flowchart patient selection and inclusion population
The study protocol was reviewed and approved by the institutional review boards of the participating centers and all participants agreed to participate in this study.
All patients were divided into two groups: primary SADI-S or secondary SADI-S. Patients in the secondary SADI-S group were subsequently categorized into one of the following three indications for secondary SADI-S; 1: persistent ≥ class 2 obesity following SG (defined as patients that had a preoperative BMI ≥ 35 kg/m2 pre SADI-S and therefore still met IFSO-criteria for metabolic bariatric surgery (MBS) after SG), 2: suboptimal clinical response (defined as TWL < 20% at nadir after SG) or 3: recurrent weight gain (defined as a 30% increase of total weight lost in kilograms from nadir after SG). Additionally, the total cohort was divided into two groups based on their CC length after SADI-S: ≤ 250 cm or > 250 cm. In four cases, data on CC length was missing.
SADI-S Procedure and Follow-up
First, a SG is performed, either during the same procedure (primary SADI-S) or at a previous occasion (secondary SADI-S). In both primary and secondary SADI-S, SG was performed in a similar fashion. When performing the SG, the abdomen was insufflated with CO2 using a Verress needle up to a maximum pressure of 19 mmHg. The first trocar was placed using a Blunttip-trocar under sight of the Endo-eye laparoscope after which the other trocars and the liver retractor were placed. Access to the omental bursa was obtained via the large curvature of the stomach and the stomach and the omentum were separated. The fundus and the angle of His were dissected and subsequently the stomach was exposed 2–4 cm proximal of the pylorus. Next, a 40-French gastric tube was advanced up to the pylorus. A linear stapled sleeve gastrectomy (Echelon, Ethicon, Johnson&Johnson) was performed parallel to the gastric tube, sparing 4 cm of gastric antrum proximal to the pylorus.
To complete the SADI-S configuration, the stomach was held upwards to identify the pylorus and dissect the duodenum 3 cm distal to the pylorus. The duodenum was then transected with a linear stapler. From the ileocecal junction, the surgeon measured the ileum with 5-cm intervals up to a length of 200–300 cm, after which the point for anastomosis was marked. The designated CC limb was pulled cranially to be anastomosed with the proximal duodenal stump using a stapler and/or V-loc sutures according to the surgeon’s preference.
After discharge, patients received follow-up at the outpatient clinic for 5 years. Standard laboratory blood tests were performed at least twice during the first year and yearly afterwards. Evaluated laboratory parameters included hemoglobin, ferritin, folic acid, vitamins A, B1, B6, B12 and D, calcium, parathyroid hormone (PTH) and albumin. Nutrient supplementation was advised to all patients, consisting of a (weight loss surgery) multivitamin supplement with additional calcium/vitamin D supplementation daily.
Data Collection
Data on patient characteristics (gender, age, anthropometrics and presence of obesity-related complications), the SADI-S procedure (timing, duration, CC length, hospital stay, complications), weight loss and nutrient status were retrospectively extracted from medical records.
Complications were divided into short-term complications (≤ 30 days) and long-term complications (> 30 days), and were scored using the Clavien-Dindo classification [13]. Weight loss was defined as percentage TWL (weight loss at follow-up divided by preoperative weight). A nutrient deficiency was defined as a serum level below the local reference value at the time of blood collection.
HRQoL was measured using the BODY-Q questionnaire using the domains body image, physical function, psychological function, sexual well-being and social function. Scores in the BODY-Q questionnaires range from 0 to 100 with 0 being the worst score and 100 the best [14]. Gastro-intestinal symptoms that were evaluated included GERD, constipation and diarrhea. Complaints of GERD were assessed using the GERD-Health Related Quality of Life Questionnaire (GERD-HRQoL), which contains ten questions concerning reflux and dysphagia. A total score of 0 is equal to no complaints and a score of 50 to very severe complaints [15]. Information on defecation pattern was retrieved via the Fecal Score (FS). The FS is based on fecal frequency, fecal consistency and hinder in daily life. Fecal frequency and consistency were scored on a 5-point scale. Hinder in daily life was scored on a 6-point scale [16].
Data Analysis
Data are reported as mean ± standard deviation (normal distribution) or as median [Q1, Q3] (non-normal distribution) for continuous variables, and as frequency (percentage) for categorical variables, unless stated otherwise.
Differences in patient characteristics between different indications for SADI-S and CC-length were compared using independent samples t-tests, Mann–Whitney U tests and Chi-square tests for normal continuous data, non-normal continuous data and count data, respectively.
Differences in weight loss outcomes between the groups were analyzed using linear mixed-effects models. The crude model consisted of fixed effects for group (SADI-S indication or CC length), follow-up time (baseline, 6 mo, 1y, 2y, 3y, 4, 5y) and their interaction term, plus a random effect for participants. Time entered the model as a repeated measure using an autoregressive covariance structure. Log-likelihood ratio tests were performed to explore potential confounders. Final models included gender and CC length (≤ 250 cm/> 250 cm) as confounder for weight loss per SADI-S indication and gender and SADI-S indication (primary/secondary) as confounder for weight loss per CC length. Results are presented as estimated marginal means and 95% confidence intervals (95% CI). Means and standard deviations of the original data at the different follow-up times can be found in Tables S1a and S1b.
The prevalence of nutrient deficiencies during follow-up was compared between the groups using Chi-Square tests or Fisher’s Exact test (if > 20% of expected counts were less than 5). Differences in HRQoL and patient-reported symptoms were analyzed using independent samples t-tests, and Chi-Square tests, respectively.
All statistical analyses were performed using IBM SPSS Statistics 29 for Windows (IBM Corp., Armonk USA). A two-sided p-value below 0.05 was considered statistically significant.
Results
The majority of the total study population was female (81%) with a mean age of 43.3 ± 11.1 years (Table 1). A total of 19 patients (18%) underwent SADI-S as a primary procedure and 84 patients (82%) as a secondary procedure after SG: 49 (58%) because of persistent ≥ class 2 obesity following SG, 15 (18%) due to suboptimal clinical response and 20 (24%) because of recurrent weight gain.
Table 1.
General patient characteristics for the total cohort, and primary and secondary SADI-S
| Total cohort (n = 103) |
Primary SADI-S (n = 19) |
Secondary SADI-S (n = 84) |
p value | |
|---|---|---|---|---|
| Gender (female) | 83 (80.6) | 14 (73.3) | 69 (82.1) | 0.52 |
| Age (years) | 43.3 ± 11.1 | 45.9 ± 12.7 | 42.7 ± 10.7 | 0.26 |
| Interval between SG and SADI-S (months) | - | NA | 34.5 [23.0, 58.5] | NA |
| Duration of SADI-S procedure (min)a | 73.5 [60, 94] | 104 [90, 114] | 69 [59, 88] | < 0.001 |
| Length of common channel (cm)b | 250 [250, 300] | 300 [250, 300] | 250 [250, 250] | < 0.001 |
| Hospital stay (days) | 2.0 [1.0, 2.0] | 2.0 [2.0, 2.0] | 1.0 [1.0, 2.0] | 0.006 |
Data represented as mean ± standard deviation, median [Q1, Q3] or frequency (%)
SG sleeve gastrectomy, SADI-S single-anastomosis duodeno-ilial bypass with sleeve gastrectomy
a missing for n = 5
b missing for n = 4
The median interval between SG and SADI-S for patients undergoing SADI-S as a second step procedure was 34.5 [23.0, 58.5] months. Median duration of the procedure, length of CC and hospital stay were shorter in the secondary SADI-S group compared to the primary SADI-S group (p < 0.05; Table 1).
Completed follow-up rates were 91% at 1 year, 73% at 2 years, 69% at 3 years, 62% at 4 years, and 53% at 5 years. All other patients were lost to follow-up.
Complications
A total of six complications (5.8%) were registered in the first 30 days postoperatively (Table 2). Five patients had a Clavien-Dindo grade III complication: one due to jejnunal perforation, two due to anastomotic leakage, one due to anastomotic bleeding and one due to an intra-abdominal abscess. All < 30 day complications occurred in the secondary SADI-S group.
Table 2.
Short- and long-term complications after SADI-S
| Complications | n (%) |
|---|---|
| Short term (< 30 days) | 6 (5.8) |
| Clavien-Dindo I | 0 (0) |
| Clavien-Dindo II | 1 (0.9) |
| Deep venous thrombosis | 1 (0.9) |
| Clavien-Dindo III | 5 (4.8) |
| Jejunal perforation | 1 (0.9) |
| Anastomotic leakage | 2 (1.9) |
| Anastomotic bleeding | 1 (0.9) |
| Intra-abdominal abscess | 1 (0.9) |
| Clavien-Dindo IV | 0 (0) |
| Clavien-Dindo V | 0 (0) |
| Long term (> 30 days) | 7 (6.7) |
| Clavien-Dindo I | 1 (0.9) |
| Surgical site infection | 1 (0.9) |
| Clavien-Dindo II | 0 (0) |
| Clavien-Dindo III | 6 (5.8) |
| Revision cc length | 4 (3.8) |
| Conversion to RYGB | 1 (0.9) |
| Internal herniation | 1 (0.9) |
| Clavien-Dindo IV | 0 (0) |
| Clavien-Dindo V | 0 (0) |
Data are represented as valid frequency (%)
Seven long term complications (6.7%) were registered, six of these where Clavien-Dindo grade III complications. One patient underwent revisional surgery due to excessive weight loss, one due to invalidating diarrhea and two due to insufficient weight loss, one was converted to a RYGB because of a relative stenosis in the gastric pouch and one internal herniation was reported. One CD II complication occurred in the primary SADI-S group after > 30 days and all other complications occurred in the secondary SADI-S group. There were no complications with a Clavien-Dindo classification of IV or V.
Weight Loss
For patients who underwent SADI-S as a secondary procedure, mean BMI decreased from 55.6 ± 8.1 kg/m2 before SG to 40.1 ± 6.4 kg/m2 at nadir (median 12 [12, 24] months post-SG). Maximum TWL at nadir was 27.2 ± 8.5% and 81% had reached a TWL of ≥ 20% after SG.
Before SADI-S, weight and BMI were comparable between the primary and secondary SADI-S group (Table 3). During follow-up after SADI-S, TWL was significantly higher in the primary SADI-S group compared to the secondary SADI-S group at all time points (p < 0.001 for all), resulting in a nadir TWL of 37.2 ± 5.8% after primary SADI-S (at median 24 [12, 36] months) and 21.9 ± 10.6% after secondary SADI-S (at median 12 [7.5, 36] months). At 5 years of follow-up, TWL was 34.8 (29.8–39.9)% for primary SADI-S vs 15.9 (13.0–18.9)% for secondary SADI-S (p < 0.001).
Table 3.
Weight outcomes over time before and after primary and secondary SADI-S
| n | Total cohort | n | Primary SADI-S | n | Secondary SADI-S | p valuea | ||
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | < 0.001 | |||||||
| Before SADI-S | 99 | 128.9 (123.3–134.6) | 19 | 126.1 (116.7–135.5) | 80 | 131.8 (126.0–137.6) | 0.30 | |
| 6 months | 93 | 102.7 (97.1–108.3) | 19 | 93.5 (84.1–102.9) | 74 | 111.8 (106.0–117.6) | 0.001 | |
| 1 year | 90 | 96.3 (90.6–101.9) | 17 | 85.2 (75.7–94.6) | 73 | 107.4 (101.5–113.2) | < 0.001 | |
| 2 years | 72 | 95.0 (89.3–100.6) | 17 | 82.6 (73.1–92.0) | 55 | 107.4 (101.5–113.3) | < 0.001 | |
| 3 years | 68 | 96.8 (91.1–102.5) | 15 | 84.7 (75.1–94.2) | 53 | 108.9 (102.9–114.8) | < 0.001 | |
| 4 years | 61 | 98.2 (92.4–104.0) | 14 | 87.0 (77.4–96.7) | 47 | 109.3 (103.3–115.4) | < 0.001 | |
| 5 years | 53 | 98.3 (92.3–104.4) | 10 | 86.3 (76.1–96.4) | 43 | 110.4 (104.2–116.6) | < 0.001 | |
| BMI (kg/m2) | < 0.001 | |||||||
| Before SADI-S | 99 | 42.3 (40.4–44.1) | 19 | 41.0 (37.8–44.1) | 80 | 43.6 (41.6–45.5) | 0.16 | |
| 6 months | 93 | 33.2 (31.3–35.1) | 19 | 29.7 (26.6–32.9) | 74 | 36.7 (34.7–38.6) | < 0.001 | |
| 1 year | 90 | 31.0 (29.2–32.9) | 17 | 26.9 (23.7–30.0) | 73 | 35.2 (33.2–37.1) | < 0.001 | |
| 2 years | 72 | 30.6 (28.7–32.4) | 17 | 26.0 (22.8–29.1) | 55 | 35.1 (33.2–37.1) | < 0.001 | |
| 3 years | 68 | 31.2 (29.3–33.1) | 15 | 26.7 (23.5–29.9) | 53 | 35.7 (33.7–37.6) | < 0.001 | |
| 4 years | 61 | 31.7 (29.7–33.6) | 14 | 27.6 (24.4–30.8) | 47 | 35.7 (33.7–37.8) | < 0.001 | |
| 5 years | 53 | 31.7 (29.7–33.7) | 10 | 27.4 (24.0–30.7) | 43 | 36.1 (34.0–38.1) | < 0.001 | |
| TWL (%) | < 0.001 | |||||||
| 6 months | 93 | 21.9 (19.3–24.5) | 19 | 29.0 (24.7–33.4) | 74 | 14.8 (12.2–17.3) | < 0.001 | |
| 1 year | 90 | 26.9 (24.3–29.4) | 17 | 35.6 (31.3–40.0) | 73 | 18.1 (15.5–20.7) | < 0.001 | |
| 2 years | 72 | 28.0 (25.4–30.6) | 17 | 37.7 (33.4–42.1) | 55 | 18.3 (15.6–21.0) | < 0.001 | |
| 3 years | 68 | 26.6 (23.9–29.2) | 15 | 36.0 (31.5–40.4) | 53 | 17.2 (14.4–19.9) | < 0.001 | |
| 4 years | 61 | 25.4 (22.7–28.2) | 14 | 34.2 (29.7–38.8) | 47 | 16.6 (13.8–19.5) | < 0.001 | |
| 5 years | 53 | 25.4 (22.4–28.3) | 10 | 34.8 (29.8–39.9) | 43 | 15.9 (13.0–18.9) | < 0.001 |
Data represented as estimated marginal mean (95% CI)
SADI-S single-anastomosis duodeno-ilial bypass with sleeve gastrectomy, BMI body mass index, TWL total weight loss
aModel adjusted for gender (male/female) and CC length (≤ 250 cm/> 250 cm)
When subdividing the secondary SADI-S group into the three different indications, TWL in the recurrent weight gain group was significantly higher compared to the suboptimal clinical response group at 6 months (19.5 (15.2–23.8)% vs 11.0 (5.9–16.1)%, p = 0.049) and 1 year (24.0 (19.6–28.4)% vs 14.5 (9.4–19.6)%, p = 0.02) after SADI-S (Fig. 2). Additionally, TWL in this group was higher than in the persistent ≥ class 2 obesity group at 1 year (16.6 (13.4–20.0)%, p = 0.02). Thereafter, weight loss was similar between the three groups.
Fig. 2.
TWL (%) after SADI-S for the total study population and per SADI-S indication. Data are presented as estimated marginal means (95% CI)
When weight loss of the SG was taken into account in the secondary SADI group, total TWL at 5 years in this group (32.0 ± 14.9%) was comparable to the primary SADI-S group (34.4 ± 7.6%), although BMI at 5 years was still lowest in the primary SADI-S group (Fig. 3).
Fig. 3.
TWL (%) and BMI per SADI-S indication at 5 years postoperatively, including TWL after SG before the secondary SADI-S procedure
Nutrient Status
The prevalence of nutrient deficiencies for ferritin (12–20%), folic acid (19–27%), vitamin D (24–31%) and zinc (57–76%) as well as anemia (19–26%) and elevated PTH levels (61–77%) increased after SADI-S (Table 4). However, vitamin B12 deficiency decreased from 47% before SADI-S to 3–13% during follow-up. Deficiencies for vitamins A, B1, B6 and calcium, and low albumin levels were not common before and after SADI-S (Table 4).
Table 4.
Nutrient deficiencies over time before and after SADI-S
| Critical range | Before SADI-Sa | After SADI-S | |||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Anemia |
F: < 7.5 mmol/L M: < 8.5 mmol/L |
6/93 (6.5) | 22/85 (25.9) | 14/55 (25.5) | 7/37 (18.9) |
| Ferritin | < 20 µg/L | 8/76 (10.5) | 9/78 (11.5) | 10/49 (20.4) | 6/36 (16.7) |
| Iron | < 10 µmol/L | 6/36 (16.7) | 4/35 (11.4) | 7/23 (30.4) | 1/15 (6.7) |
| Folic acid | < 10 nmol/L | 12/76 (15.8) | 21/79 (26.6) | 10/48 (20.8) | 7/36 (19.4) |
| Vitamin A | < 0.7 µmol/L | 0/3 (0.0) | 0/36 (0.0) | 0/21 (0.0) | 0/8 (0.0) |
| Vitamin B1 | < 70 nmol/L | 0/36 (0.0) | 1/56 (1.8) | 1/31 (3.2) | 0/22 (0.0) |
| Vitamin B6 | < 35 nmol/L | 0/36 (0.0) | 0/58 (0.0) | 0/31 (0.0) | 0/23 (0.0) |
| Vitamin B12 | < 295 pmol/L | 36/76 (47.4) | 9/82 (11.0) | 6/47 (12.8) | 1/34 (2.9) |
| Vitamin D | < 50 mmol/L | 16/77 (20.8) | 26/84 (31.0) | 15/53 (28.3) | 9/37 (24.3) |
| Calcium | < 2.15 mmol/L | 0/59 (0.0) | 7/85 (8.2) | 7/53 (13.2) | 1/37 (2.7) |
| PTHb | > 7 pmol/L | 16/76 (21.1) | 49/81 (60.5) | 34/49 (69.4) | 27/35 (77.1) |
| Albumin | < 35 g/L | 0/49 (0.0) | 6/73 (8.2) | 3/52 (5.8) | 1/34 (2.9) |
| Zinc | < 10 µmol/L | 1/4 (25.0) | 25/33 (75.8) | 15/22 (68.2) | 4/7 (57.1) |
Data are represented as valid frequency (%)
F female, M male, PTH parathyroid hormone
a Only Hb was assessed before primary SADI-S
b elevated PTH levels
There was no significant difference in nutrient status between primary and secondary SADI-S (data not shown).
Health-Related Quality of Life and Gastro-Intestinal Symptoms
On a scale of 0–100, highest scores regarding HRQoL were found for the subscales ‘physical function’ (78.0 ± 20.4), ‘social function ‘ (68.9 ± 22.4) and ‘psychological function’ (62.9 ± 22.9) (Table 5).
Table 5.
Health-related quality of life (BODY-Q) scores after SADI-S
| Total cohort (n = 84) |
Primary SADI-S (n = 17) |
Secondary SADI-S (n = 67) |
p value |
Persistent ≥ class 2 obesity (n = 40) |
Suboptimal clinical response (n = 13) |
Recurrent weight gain (n = 14) |
|
|---|---|---|---|---|---|---|---|
| Body image | 44.1 ± 24.2 | 62.9 ± 24.6 | 39.3 ± 21.7 | < 0.001 | 40.6 ± 22.3 | 37.9 ± 20.7 | 36.9 ± 22.3 |
| Physical function | 78.0 ± 20.4 | 90.2 ± 14.3 | 75.0 ± 20.7 | 0.001 | 77.2 ± 20.0 | 70.9 ± 21.4 | 72.3 ± 22.6 |
| Psychological function | 62.9 ± 22.9 | 74.2 ± 21.8 | 60.0 ± 22.5 | 0.02 | 61.6 ± 23.4 | 57.4 ± 25.2 | 58.0 ± 17.9 |
| Sexual well-being | 48.9 ± 24.4 | 57.9 ± 20.8 | 46.6 ± 24.9 | 0.09 | 51.9 ± 23.0 | 38.4 ± 23.8 | 39.1 ± 28.7 |
| Social function | 68.9 ± 22.4 | 80.3 ± 21.8 | 66.0 ± 21.8 | 0.02 | 69.8 ± 22.7 | 63.5 ± 17.3 | 57.9 ± 21.6 |
Data represented as mean ± standard deviation
BODY-Q scores were analyzed at a median of 32 months post SADI-S
When comparing HRQoL, patients who underwent primary SADI-S had significant higher scores for ‘body image’, ‘physical function’, ‘psychological function’ and ‘social function’ compared to patients who underwent a secondary SADI-S (p < 0.05 for all; Table 5). For the sub-indications of secondary SADI- S, HRQoL was comparable between the groups for all domains of the BODY-Q questionnaires (p > 0.05 for all).
The severity of GERD symptoms after SADI-S was low with a median score of 2 [0, 10], and a maximum reported score of 34. Still, 43 patients (51%) reported using a proton pump inhibitor (PPI) at a median of 32 months postoperatively.
Most patients reported a defecation frequency of more than two times daily (48%) with a median frequency of 4 [3, 5] times daily after SADI-S. Consistency was mostly pulpy and soft (71%), and 26% of patients reported never experiencing hinder in daily life due to their defecation frequency or consistency, whereas 19% experienced this on a daily basis. There were no differences in fecal score between primary and secondary SADI-S (data not shown).
Common Channel Length
Total range for CC length was 120–300 cm, with CC length ≤ 250 cm in 66 patients (67%) and > 250 cm in 33 patients (33%). Both groups significantly differed with respect to age, preoperative weight and BMI and length of hospital stay (Table S2).
During follow-up after SADI-S, TWL tended to be higher in the CC ≤ 250 cm group compared to the > 250 cm group, resulting in 25.3 (21.8–28.9)% vs 21.3 (17.2–25.4)% TWL at 5 years of follow-up (p = 0.12; Table 6, Fig. 4).
Table 6.
Weight outcomes over time between SADI with CC ≤ 250 cm and > 250 cm
| n | Total cohort | n | CC ≤ 250 cm | n | CC > 250 cm | p valuea | ||
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 0.70 | |||||||
| Before SADI-S | 99 | 125.6 (120.0–131.1) | 66 | 131.8 (124.9–138.7) | 33 | 119.4 (111.9–126.9) | 0.01 | |
| 6 months | 93 | 103.2 (97.6–108.7) | 62 | 109.3 (102.4–116.2) | 31 | 97.0 (89.5–104.5) | 0.01 | |
| 1 year | 90 | 98.1 (92.6–103.7) | 61 | 103.8 (96.9–110.7) | 29 | 92.5 (84.9–100.0) | 0.02 | |
| 2 years | 72 | 97.5 (91.8–103.1) | 45 | 103.5 (96.5–110.5) | 27 | 91.4 (83.8–99.0) | 0.01 | |
| 3 years | 68 | 99.4 (93.8–105.1) | 43 | 104.3 (97.2–111.3) | 25 | 94.6 (86.9–102.3) | 0.05 | |
| 4 years | 61 | 100.6 (94.9–106.4) | 39 | 104.2 (97.0–111.4) | 22 | 97.1 (89.2–104.9) | 0.15 | |
| 5 years | 53 | 101.2 (95.3–107.1) | 34 | 105.7 (98.4–113.1) | 19 | 96.6 (88.5–104.7) | 0.07 | |
| BMI (kg/m2) | 0.64 | |||||||
| Before SADI-S | 99 | 41.1 (39.3–43.0) | 66 | 42.6 (40.3–44.9) | 33 | 39.7 (37.2–42.2) | 0.07 | |
| 6 months | 93 | 33.4 (31.5–35.2) | 62 | 34.9 (32.6–37.2) | 31 | 31.9 (29.3–34.4) | 0.05 | |
| 1 year | 90 | 31.7 (29.8–33.5) | 61 | 33.1 (30.8–35.4) | 29 | 30.3 (27.8–32.8) | 0.08 | |
| 2 years | 72 | 31.4 (29.5–33.3) | 45 | 32.9 (30.6–35.2) | 27 | 29.9 (27.4–32.4) | 0.06 | |
| 3 years | 68 | 32.1 (30.2–34.0) | 43 | 33.2 (30.8–35.5) | 25 | 31.0 (28.4–33.6) | 0.18 | |
| 4 years | 61 | 32.5 (30.6–34.4) | 39 | 33.1 (30.7–35.5) | 22 | 31.9 (29.3–34.5) | 0.47 | |
| 5 years | 53 | 32.6 (30.6–34.6) | 34 | 33.6 (31.1–36.0) | 19 | 31.7 (29.0–34.4) | 0.27 | |
| TWL (%) | 0.49 | |||||||
| 6 months | 93 | 21.7 (19.1–24.2) | 62 | 22.4 (19.3–25.6) | 31 | 20.9 (17.3–24.5) | 0.49 | |
| 1 year | 90 | 25.5 (22.9–28.1) | 61 | 26.4 (23.2–29.6) | 29 | 24.6 (21.0–28.3) | 0.44 | |
| 2 years | 72 | 26.2 (23.6–28.9) | 45 | 27.0 (23.7–30.2) | 27 | 25.5 (21.8–29.2) | 0.52 | |
| 3 years | 68 | 24.7 (22.0–27.3) | 43 | 26.4 (23.1–29.7) | 25 | 22.9 (19.2–26.7) | 0.14 | |
| 4 years | 61 | 23.6 (20.8–26.4) | 39 | 26.5 (23.0–29.9) | 22 | 20.7 (16.9–24.6) | 0.02 | |
| 5 years | 53 | 23.3 (20.4–26.2) | 34 | 25.3 (21.8–28.9) | 19 | 21.3 (17.2–25.4) | 0.12 |
Data represented as estimated marginal mean (95% CI)
SADI-S single-anastomosis duodeno-ilial bypass with sleeve gastrectomy, BMI body mass index, TWL total weight loss
aModel adjusted for gender and SADI indication (primary/secondary)
Fig. 4.
TWL (%) after SADI-S per CC length. Data are presented as estimated marginal means (95% CI)
There was no significant difference in the prevalence of nutrient deficiencies between the CC groups after SADI-S (Table S3). However, anemia and folic acid deficiencies at 1 year post-SADI tended to be more prevalent in the CC ≤ 250 cm group compared to the CC > 250 cm group (32% vs 15%, p = 0.11; 33% vs 17%, p = 0.17, respectively). Furthermore, deficiencies for iron (15–22% vs 0–8%, p > 0.05) and vitamin D (29–32% vs 13–17%, p > 0.05) tended to be more common in the CC ≤ 250 cm group at 3 and 5 years of follow-up. Severe protein energy malnutrition, presented as low albumin levels, were also only observed in patients with a CC ≤ 250 cm (4–12%).
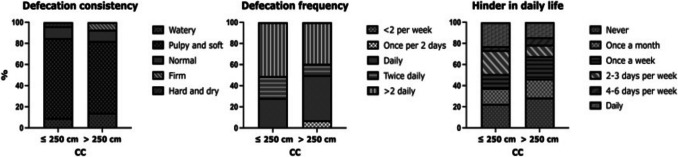
Patients with a CC length of ≤ 250 cm tended to defecate more often with 50% of patients reporting more than two times per day (median 4 times) compared to 39% in the CC > 250 cm group (median 3 times, p = 0.09) (Fig. 5). Fecal consistency and hinder in daily life were similar, and there were no differences in HRQoL between the CC groups (data not shown).
Fig. 5.
Fecal score after SADI-S according to CC length
Discussion
The aim of this study was to describe mid-term outcomes on weight loss, nutrient status and patient-reported HRQoL and gastro-intestinal symptoms up to 5 years after primary versus secondary SADI-S, and to explore differences in these outcomes between different indications for secondary SADI-S (persistent ≥ class 2 obesity, suboptimal clinical response, recurrent weight gain) and between different CC lengths (≤ 250 cm or > 250 cm).
A significantly higher mean TWL of 34.8 (29.8–39.9)% versus 15.9 (13.0–18.9)% at five years was found for primary SADI-S compared to secondary SADI-S. In a recent study by Osorio et al. [17], TWL was 27.3% for primary SADI-S and 24.4% for secondary SADI-S at 5 years postoperatively. The discrepancy between these findings could be attributed to the fact that %TWL in the study of Osorio et al. was measured 5 years from the primary procedure whilst %TWL in our cohort is reported at 5 years post-secondary SADI-S. Furthermore, our findings are also not in line with the systematic review by Esparham et al. [18], who reported a %TWL for primary SADI-S of 38.8% and 37.0% for secondary SADI-S at 5 years postoperatively. We do not have a valid explanation for the discrepancies between our results and the study by Esparham et al. Similar studies comparing primary versus secondary MBS procedures were performed for OAGB and RYGB [19, 20]. Both studies found a higher %TWL in primary procedures compared to secondary procedures, which is in line with our findings in SADI-S.
When the initial weight loss of the SG procedure is taken into account for patients who underwent SADI-S as a second step procedure, weight loss outcomes at 5 years were similar for primary and secondary SADI-S (34.4% vs 32.0% TWL). This indicates that both procedures are equally effective in terms of weight loss. Yet, BMI at 5 years was still lowest in the primary SADI-S group. This can be attributed to several factors, for example: a lower initial BMI, a relatively long median interval to the secondary procedure (34 months) leading to some amount of recurrent weight gain and thus a suboptimal result of the combined procedures. Also, part of the secondary procedures were performed due to a suboptimal clinical response following SG, which might indicate a lower response to metabolic surgery overall.
Interestingly, the recurrent weight loss group showed higher %TWL until 1 year postoperatively, compared to the suboptimal clinical response and persistent ≥ class 2 obesity group, after which it decreased to similar weight loss for all groups. These variations in weight loss patterns might be explained by individual differences in demographic, biological, psychological and behavioral determinants of weight loss [21].
Although not statistically different for all time points, we also found a trend towards higher weight loss for CC length ≤ 250 cm compared to > 250 cm. An explanation for this difference is that CC length ≤ 250 cm per definition result in more malabsorption. To our knowledge, there are no previous studies that compare differences in TWL following SADI-S according to CC lengths.
When assessing HRQoL, patients who underwent secondary SADI-S had significant lower scores for ‘body image’, ‘physical function’, ‘psychological function’ and ‘social function’ compared to patients who underwent a primary SADI-S, although overall HRQoL was acceptable. These BODY-Q scores are comparable to the scores that Makarawung et al. found in their multicenter, cross-sectional studies in patients that underwent RYGB or SG more than 3 years postoperatively [22]. Dalaei et al. investigated the normative scores in patients that did not undergo any type of MBS [23]. On the domains of psychological and physical function, scores found in our study were comparable to their findings: 62.9 vs 61.5 for psychological function and 78.0 vs 81.2 for physical function, respectively. Patients that underwent MBS did score differently on the domains of social wellbeing, sexual wellbeing and body image compared to the general population: 68.9 vs 59.5 for social wellbeing, 48.9 vs 67.0 for sexual wellbeing and 44.1 vs 53.5 for body image. Surprisingly, patients in our cohort that underwent SADI-S as a secondary procedure scored significantly lower on all BODY-Q domains, except for sexual wellbeing. We hypothesize that this difference can be attributed to the fact that patients who underwent SADI-S as a secondary procedure had their primary procedure (SG) earlier on in time. As shown by the study of Makarawung et al. [22], BODY-Q scores tend to decline over time in patients that underwent MBS. Admella et al.[12] analyzed patient-reported outcomes and quality of life after SADI-S using the SF- 36 physical and mental components. Their study showed improvement on both the physical and mental components at 3 years postoperatively compared to preoperatively. In our study, the BODY-Q scores were only measured at a median of 32 months post-SADI-S and therefore a comparison with preoperative data was not possible.
Our study showed a low prevalence of GERD and low scores in the GERD-HRQoL questionnaire which is comparable to the currently available literature [12]. However, almost half of the patients used PPIs at a median of 32 months postoperatively. It is unknown why PPI usage is this high and if this influenced the results in our study on the reported prevalence of GERD. However, GERD symptoms are known to increase after SG in roughly 20% of patients [24] and a study by Salminen et al. reported PPI use in 64% of patients at ten years follow up post- SG [25]. It can therefore be hypothesized that (chronic) PPI use is already high pre SADI-S and that patients have relatively low complaints of GERD due to using PPIs.
Most patients reported a defecation frequency of more than two times daily (48%), and consistency was mostly pulpy and soft (71%). Admella et al. [12] used the Bristol stool chart to evaluate the consistency of stools post-SADI-S. Although the fecal score and Bristol stool chart are not directly comparable, a Bristol score ≥ 5 could be compared to a defecation consistency that is described as watery in the fecal score questionnaire. Admella et al. found a higher percentage of patients having diarrhea at 2–3 years postoperatively compared to our results (25.4% vs 11.0%).
Patients with a CC length of ≤ 250 cm did not report different defecation patterns compared to patients with a CC > 250 cm, which may be attributed to the limited range of CC lengths in our study. However, they did tend to defecate more often with 50% of patients reporting more than two times per day (median 4 times) compared to 39% in the CC > 250 group (median 3 times).
Furthermore, anemia, iron deficiency and severe protein energy malnutrition tended to be more present in the CC ≤ 250 cm group. These findings also correspond to what is described in existing literature [26, 27]. Therefore, it is advised to use a CC length of at least 250 cm.
Complication rates in our study on both short- (≤ 30 days; 5.8%) and long-term (> 30 days; 6.7%) were acceptable and comparable to previously reported complication rates [28]. Interestingly, all < 30 day complications and six out of seven of the > 30 day complications occurred in the secondary SADI-S group. We hypothesize that this difference is due to the fact that revisional procedures are more difficult to perform. Franken et al. [29] also found a similar major complication rate of 6% (CD ≥ III), but a much higher minor complication rate of 36% (CD I-II) post SADI-S. This can be explained by the fact that nutrient deficiencies were scored as minor complications whereas we did not score deficiencies as minor complications.
Strengths and Limitations
This study has several strengths. First, to our knowledge this is the first study that describes weight loss patterns in SADI-S for different indications and CC lengths. Second, the extensive description of health-related quality of life using the BODY-Q, fecal score, and GERD-HRQoL questionnaires gives important insights of patient reported outcomes following SADI-S.
This study also has some limitations. First, as many retrospective studies on MBS procedures, we also experienced a relatively high loss to follow-up rate. Despite all effort, we were only able to achieve a complete follow-up rate of 53% after 5 years resulting in a relatively small, heterogeneous study population. Outside of research om MBS procedures, loss to follow-up unfortunately also is a widespread challenging problem in daily clinical practice. Second, the total cohort consisted of 103 patients with only 19 patients undergoing SADI-S as a primary procedure, all coming from the same center. This may hamper a solid comparison of primary versus conversion SADI-S. Third, due to the retrospective design, general characteristics were not equally distributed between the different indications for SADI-S. Fourth, in case of secondary SADI-S after SG, information about whether or not a re-sleeve was performed during the SADI-S procedure was not reported. Fifth, potential influences of multivitamin supplementation use on nutrient status was not taken into account. Therefore, our outcomes on nutrient status and subsequent deficiencies following SADI-S should be interpreted with some caution. Finally, the cut-off for CC length at 250 cm is artificial and results may have been influenced due to two patients in the ≤ 250 cm group with a very short CC (respectively, 120 and 200 cm).
Conclusion
Both primary and secondary SADI-S are safe procedures which result in good and durable weight loss outcomes up to 5 years postoperatively. It is imperative that common channel length should be at least 250 cm to prevent malnutrition and gastro-intestinal complaints. Furthermore, the implications of SADI-S procedures on health-related quality of life should not be overlooked, especially after secondary SADI-S. In future research, it is essential to broaden the focus beyond weight-related outcomes and to include patient-reported outcomes, allowing for a more comprehensive understanding of the impact on patients'daily lives.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Rhiannon Stacie for her support in data collection
Author Contribution
MH and LH contributed equally in writing the main manuscript, collected data from their affiliated center, analyzed the data, prepared the figures and tables and contributed equally to forming this manuscript VM, MVh, MAN collected data from their respective affiliated center and provided feedback on the draft manuscript of this study All authors reviewed the manuscript and provided feedback.
Funding
No funding was received for this study.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests
Footnotes
Key Points
• Both primary and secondary SADI-S are safe procedures which lead to good and durable weight loss outcomes up to 5 years postoperatively
• A CC length of ≥ 250 cm is advised to minimize risks of malnutrition and lower HrQoL
• HrQoL was lower for patients that underwent SADI-S as a secondary procedure
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mitchell J. R. Harker and Laura Heusschen contributed equally.
Contributor Information
Mitchell J. R. Harker, Email: mharker@rijnstate.nl
Eric J. Hazebroek, Email: ehazebroek@rijnstate.nl
References
- 1.IFSO. IFSO 8th Global Registry Report 2023. Available from: https://www.ifso.com/ifso-registry.php. Accessed 26-09-2023.
- 2.Sanchez-Pernaute A, Rubio MA, Cabrerizo L, Ramos-Levi A, Perez-Aguirre E, Torres A. Single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) for obese diabetic patients. Surg Obes Relat Dis. 2015;11(5):1092–8. 10.1016/j.soard.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Andalib A, Alamri H, Almuhanna Y, Bouchard P, Demyttenaere S, Court O. Short-term outcomes of revisional surgery after sleeve gastrectomy: a comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis). Surg Endosc. 2021;35(8):4644–52. 10.1007/s00464-020-07891-z. [DOI] [PubMed] [Google Scholar]
- 4.Martini F, Paolino L, Marzano E, D’Agostino J, Lazzati A, Schneck AS, et al. Single-Anastomosis Pylorus-Preserving Bariatric Procedures: Review of the Literature. Obes Surg. 2016;26(10):2503–15. 10.1007/s11695-016-2310-1. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Pernaute A, Herrera MA, Perez-Aguirre ME, Talavera P, Cabrerizo L, Matia P, et al. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) One to three-year follow-up. Obes Surg. 2010;20(12):1720–6. 10.1007/s11695-010-0247-3. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Pernaute A, Rubio Herrera MA, Perez-Aguirre E, Garcia Perez JC, Cabrerizo L, DiezValladares L, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2017;17(12):1614–8. 10.1007/s11695-007-9287-8. [DOI] [PubMed] [Google Scholar]
- 7.Dijkhorst PJ, Al Nawas M, Heusschen L, Hazebroek EJ, Swank DJ, Wiezer RMJ, et al. Single Anastomosis Duodenoileal Bypass or Roux-en-Y Gastric Bypass After Failed Sleeve Gastrectomy: Medium-Term Outcomes. Obes Surg. 2012;31(11):4708–16. 10.1007/s11695-021-05609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osorio J, Lazzara C, Admella V, Franci-Leon S, Pujol-Gebelli J. Revisional Laparoscopic SADI-S vs. Duodenal Switch Following Failed Primary Sleeve Gastrectomy: a Single-Center Comparison of 101 Consecutive Cases. Obes Surg. 2021;31(8):3667–74. 10.1007/s11695-021-05469-9 [DOI] [PubMed]
- 9.Sanchez-Pernaute A, Rubio MA, Perez N, Marcuello C, Torres A, Perez-Aguirre E. Single-anastomosis duodenoileal bypass as a revisional or second-step operation after sleeve gastrectomy. Surg Obes Relat Dis. 2020;16(10):1491–6. 10.1016/j.soard.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Ponce de Leon-Ballesteros G, Romero-Velez G, Higa K, Himpens J, M OK, Torres A, et al. Single Anastomosis Duodeno-Ileostomy with Sleeve Gastrectomy/Single Anastomosis Duodenal Switch (SADI-S/SADS) IFSO Position Statement-Update 2023. Obes Surg. 2024. 10.1007/s11695-024-07490-0 [DOI] [PubMed]
- 11.Ortiz-Zuniga AM, Costa Forner P, Cirera de Tudela A, Garcia Ruiz A, Comas Martinez M, Palmas F, et al. The Impact of the Length of the Common Intestinal Loop on Metabolic and Nutritional Outcomes of Patients with Severe Obesity Who Undergo of Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy: 5-Year Follow-Up. J Laparoendosc Adv Surg Tech A. 2022;32(9):955–61. 10.1089/lap.2021.0863. [DOI] [PubMed] [Google Scholar]
- 12.Admella V, Lazzara C, Sobrino L, Acrich E, Biondo S, Pujol-Gebelli J, et al. Patient-Reported Outcomes and Quality of Life After Single-Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S): a Cross-Sectional Study with 283 Patients from a Single Institution. Obes Surg. 2023;33(6):1754–63. 10.1007/s11695-023-06554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klassen AF, Cano SJ, Alderman A, Soldin M, Thoma A, Robson S, et al. The BODY-Q: A Patient-Reported Outcome Instrument for Weight Loss and Body Contouring Treatments. Plast Reconstr Surg Glob Open. 2016;4(4):e679. Epub 2016/05/21. 10.1097/GOX.0000000000000665. PubMed PMID: 27200241; PubMed Central PMCID: PMCPMC4859238 Surgery Foundation. The international field-test was funded by a grant from the Canadian Institutes for Health Research (CIHR). The BODY-Q is owned by McMaster University and Memorial Sloan-Kettering Cancer Center. Stefan Cano, Anne Klassen and Andrea Pusic are co-developers of the BODY-Q and, as such, could potentially receive a share of any license revenues as royalties based on their institutions inventor sharing policy. Stefan Cano is cofounder of Modus Outcomes, an outcomes research and consulting firm that provides services to pharmaceutical, medical device, and biotechnology companies. Dr. Andrea Pusic received support through the NIH/NCI Cancer Center Support Grant P30 CA008748. The Article Processing Charge was paid from the CIHR grant. [DOI] [PMC free article] [PubMed]
- 15.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130–4. 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 16.Schijns W, Aarts EO, Berends FJ, Janssen IM, Schweitzer DH. Loose and frequent stools and PTH levels are positively correlated post-gastric bypass surgery due to less efficient intestinal calcium absorption. Surg Obes Relat Dis. 2016;12(8):1548–53. 10.1016/j.soard.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Osorio J, Admella V, Merino D, Sobrino L, Tuero C, Vilarrasa N, et al. One-Stage Vs. Two-Step One Anastomosis Duodenal Switch (OADS/SADI-S): A Safety and Efficacy Single-Center Propensity-Score Matched Analysis. Obes Surg. 2024;34(7):2293–302. 10.1007/s11695-024-07280-8. [DOI] [PubMed]
- 18.Esparham A, Roohi S, Ahmadyar S, Dalili A, Moghadam HA, Torres AJ, et al. The Efficacy and Safety of Laparoscopic Single-Anastomosis Duodeno-ileostomy with Sleeve Gastrectomy (SADI-S) in Mid- and Long-Term Follow-Up: a Systematic Review. Obes Surg. 2023;33(12):4070–9. 10.1007/s11695-023-06846-2. [DOI] [PubMed] [Google Scholar]
- 19.Abdulrazzaq S, Elhag W, El Ansari W, Mohammad AS, Sargsyan D, Bashah M. Is Revisional Gastric Bypass as Effective as Primary Gastric Bypass for Weight Loss and Improvement of Comorbidities? Obes Surg. 2020;30(4):1219–29. 10.1007/s11695-019-04280-x. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Abeid A, Bendayan A, Yuval JB, Eldar SM, Lahat G, Lessing Y. Primary versus Revisional One Anastomosis Gastric Bypass: Outcomes of Patients with at Least 8-Year Follow-Up. Obes Facts. 2024;17(3):303–10. 10.1159/000538768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eykelenboom M, van Stralen MM, Poelman MP, Steenhuis IHM. Patterns of weight loss and their determinants in a sample of adults with overweight and obesity intending to lose weight. Nutr Diet. 2020;77(2):240–6. 10.1111/1747-0080.12489. [DOI] [PubMed] [Google Scholar]
- 22.Makarawung DJS, de Vries CEE, List EB, Monpellier VM, Mou D, Klassen AF, et al. Patient-Level Factors Associated with Health-Related Quality of Life and Satisfaction with Body After Bariatric Surgery: a Multicenter. Cross-Sectional Study Obes Surg. 2022;32(9):3079–87. 10.1007/s11695-022-06214-6. [DOI] [PubMed] [Google Scholar]
- 23.Dalaei F, de Vries CEE, Poulsen L, Kaur MN, Pfob A, Mou D, et al. General population normative scores for interpreting the BODY-Q. Clin Obes. 2022;12(4):e12528. 10.1111/cob.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does Sleeve Gastrectomy Expose the Distal Esophagus to Severe Reflux?: A Systematic Review and Meta-analysis. Ann Surg. 2020;271(2):257–65. 10.1097/SLA.0000000000003275. [DOI] [PubMed] [Google Scholar]
- 25.Salminen P, Gronroos S, Helmio M, Hurme S, Juuti A, Juusela R, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients With Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2022;157(8):656–66. Epub 2022/06/23. 10.1001/jamasurg.2022.2229. PubMed PMID: 35731535; PubMed Central PMCID: PMCPMC9218929 Research Foundation awarded to Turku University Hospital and the Mary and Georg C. Ehrnrooth Foundation during the conduct of the study. Dr Gronroos reported grants from the Orion Research Foundation, the Paulo Foundation, and the Gastroenterological Research Foundation during the conduct of the study. No other disclosures were reported.
- 26.Balibrea JM, Vilallonga R, Hidalgo M, Ciudin A, Gonzalez O, Caubet E, et al. Mid-Term Results and Responsiveness Predictors After Two-Step Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy. Obes Surg. 2017;27(5):1302–8. 10.1007/s11695-016-2471-y. [DOI] [PubMed] [Google Scholar]
- 27.Barajas-Gamboa JS, Moon S, Romero-Velez G, Strong AT, Allemang M, Navarrete S, et al. Primary single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) versus sleeve gastrectomy to SADI conversions: a comparison study of prevalence and safety. Surg Endosc. 2023;37(11):8682–9. 10.1007/s00464-023-10305-5. [DOI] [PubMed] [Google Scholar]
- 28.Verhoeff K, Mocanu V, Zalasky A, Dang J, Kung JY, Switzer NJ, et al. Evaluation of Metabolic Outcomes Following SADI-S: a Systematic Review and Meta-analysis. Obes Surg. 2022;32(4):1049–63. 10.1007/s11695-021-05824-w. [DOI] [PubMed] [Google Scholar]
- 29.Franken RJ, Sluiter NR, Franken J, de Vries R, Souverein D, Gerdes VEA, et al. Treatment Options for Weight Regain or Insufficient Weight Loss After Sleeve Gastrectomy: a Systematic Review and Meta-analysis. Obes Surg. 2022;32(6):2035–46. 10.1007/s11695-022-06020-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.