Abstract
Background
Given the association between diabetic microvascular disease and bone metabolism, we aimed to investigate the correlation between the concentration of the serum bone turnover marker procollagen type I N-terminal propeptide (PINP) and diabetic retinopathy (DR) in patients with type 2 diabetes mellitus (T2DM) in this study.
Methods
This was a cross-sectional study. T2DM patients aged ≥18 years were consecutively recruited from the inpatient population of the Department of Endocrinology at Nanjing Drum Tower Hospital, between January 2016 and January 2018, and participants were divided into DR and non-DR groups. We compared clinical and laboratory data of patients in the two groups. Logistic regression analysis was employed to investigate the overall risk of DR at the PINP quartiles. Receiver operating characteristic (ROC) curves were conducted to estimate the predictive power of PINP for DR.
Results
A total of 509 patients with T2DM were included in this study (390 males and 194 females), including 148 patients with DR. Age and diabetes duration were independent risk factors for DR, PINP was also an independent protective factor (all P < 0.05). According to the interquartile range of PINP, all participants were divided into four groups. After adjustment for confounders, patients in Q2, Q3 and Q4 all had a decreased risk of DR compared with Q1 group (OR 0.501, 95% CI 0.280~0.894; OR 0.289, 95% CI 0.157~0.533; OR 0.077, 95% CI 0.036~0.165) respectively. Meanwhile, the AUC of DR diagnosed by the combined diagnostic model of PINP with age and duration of diabetes was 0.8271 (95% CI: 0.7911–0.863).
Conclusion
The PINP level is associated with diabetic retinopathy in patients with T2DM, and PINP was an independent protective factor for DR and may help to predict its progression.
Keywords: diabetic retinopathy, DR, type 2 diabetes mellitus, T2DM, type I N-terminal propeptide, PINP
Introduction
Diabetes Mellitus (DM), influenced by genetic and environmental factors, is one of the world’s most common chronic diseases. The International Diabetes Federation (IDF) estimated that the number of people with diabetes mellitus (DM) will reach 700 million globally in 2045, most that of type 2 diabetes mellitus (T2DM), with the largest absolute increase in middle-income countries, including China.1 Diabetic retinopathy (DR), as one of the most common microvascular complications of T2DM, remains a leading cause of preventable blindness in the adult working population and the global DR burden is expected to remain high through 2045.2,3 Approximately 19.5 million people have been diagnosed with DR,4 and the rise in DR has resulted in more blindness, higher healthcare costs, and a significant decline in patients’ quality of life. Therefore, it is crucial to explore the correlates of predictable DR to facilitate early screening and prevention of DR.
Accumulating studies have demonstrated that T2DM and its microvascular complications severely affect bone metabolism and damage the microstructure of the bone, thereby increasing fracture risk.5 As diabetes progresses, chronic hyperglycemia and acute glucose fluctuations lead to increased accumulation of reactive oxygen species (ROS) and oxidative stress, which in turn activate DNA and protein damage pathways that adversely affect bone health. Moreover, accumulation of advanced glycosylation end products (AGEs) in the bone matrix exacerbates oxidative stress in the bone microenvironment, leading to increased bone fragility and elevated fracture risk. In addition, microvascular damage in diabetic patients reduces blood flow to the bone, leading to hypoxia, which in turn affects cortical bone formation and resorption, indirectly leading to increased fracture risk.6
Currently, most studies have focused on the indirect effects of DR on bone. Considering DR and poor bone health shared the common pathologic mechanisms, we sought to explore the association between bone turnover marker and DR. The procollagen type I N-propeptide (PINP) from osteoblasts is a bone turnover marker that reflects collagen formation and osteoblast activation. Since PINP is not affected by exogenous factors such as hormones, it is a more specific and sensitive indicator of the stability of bone formation.7 The aim of this study was to investigate the relationship between PINP levels and DR in patients with T2DM to further understand the bone–vascular axis in glucose metabolism.
Materials and Methods
Study Design and Participants
This was a retrospective cross-sectional study including 509 patients with T2DM subjects who hospitalized at Nanjing Drum Tower Hospital between January 2016 and January 2018. The 2003 American Diabetes Association criteria were used to diagnose T2DM.8 All patients underwent examination or re-examination for DR, and the fundus photography was performed by trained specialists using non-mydriatic cameras (CR-2 AF, JAPAN). The International Clinical Diabetic Retinopathy Scale was used to group the presence or absence of retinopathy.9 The inclusion criteria were as follows: aged ≥ 18 years; completion of relevant clinical and laboratory data. The exclusion criteria were as follows: (1) acute metabolic complications such as diabetic ketoacidosis, hypoglycaemic hyperosmolar state and hypoglycaemic coma within the previous 3 months; (2) had a serious and unstable health condition (ie, severe kidney or liver dysfunction, severe cardiovascular or cerebrovascular diseases, or malignant tumors); and (4) had taken drugs affecting sex hormone levels within the previous 3 months. Finally, 509 patients were categorized into DR and non-DR groups based on whether or not have diabetic retinopathy.
Data Collection
Data collected from the electronic case system included age, gender, duration of diabetes, body mass index (BMI), and presence of diabetic retinopathy. All patients had venous blood collected on the day of admission on a fasting basis for more than 8 hours for clinical analysis. The blood biochemical indexes of patients included fasting plasma glucose (FPG), 2-hour postprandial glucose (2h-PG), glycosylated hemoglobin (HbA1c), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), creatinine (Cr), blood urinary nitrogen (BUN), uric acid (UA). In addition, procollagen type I N-propeptide (PINP) and 25-Hydroxyvitamin D (25 (OH) D) levels measured by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system according to the manufacturer’s instructions were also obtained.
Statistical Analysis
Statistical analysis was performed using SPSS 27.0 software. After normality testing, the mean ± standard deviation (SD) was utilized to describe the continuous parameters in a normal distribution, otherwise shown as median [interquartile range (IQR)]. Categorical parameters were described as percentages and numbers. For variables in a normal distribution, t-test was employed to compare the difference between two groups. Otherwise, the Mann–Whitney U-test was used for analysis. The logistic regression analysis was then utilized to assess the overall risk of DR according to PINP quartiles. The odds ratio (OR) and 95% confidence interval (CI) were calculated by two logistic regression models using categorical variables (quartiles). Receiver operating characteristic (ROC) curve was conducted to assess the predictive value of the PINP for identifying DR. When P < 0.05, it was considered statistically significant.
Results
A total of 509 patients with T2DM were included in this study (390 males and 194 females), including 148 patients with DR. The results showed that PINP values were lower in the DR group patients (P < 0.001) (Figure 1). Moreover, as shown in Table 1, compared with those without DR, participants with DR had higher levels of age, duration of T2DM, FPG and Cr (all P < 0.05). There were no statistically significant differences in other parameters (P > 0.05) (Table 1).
Figure 1.
Comparisons of PINP between non-DR and DR groups.
Note: ***p < 0.001.
Table 1.
Baseline Characteristics
| Variables | Non-DR (n=361) | DR (n=148) | P-value |
|---|---|---|---|
| Age (year) | 55.94±6.70 | 61.00±5.40 | <0.001 |
| Male (n, %) | 234, 73.6% | 84, 56.8% | 0.088 |
| SBP (mmHg) | 131.58±15.05 | 134.46±14.74 | 0.064 |
| DBP (mmHg) | 80.19±10.76 | 78.45±11.34 | 0.114 |
| BMI (kg/m2) | 24.81±3.06 | 24.90±2.74 | 0.575 |
| Duration (year) | 8.54±6.36 | 11.87±6.52 | <0.001 |
| FPG (mmol/L) | 8.34±2.86 | 8.69±2.58 | 0.023 |
| 2h-PG (pmol/L) | 14.85±4.81 | 15.55±3.81 | 0.052 |
| HbA1c (%) | 8.68±2.14 | 8.84±1.64 | 0.100 |
| AST (U/L) | 20.65±8.74 | 21.02±9.39 | 0.365 |
| ALT (U/I) | 25.97±16.15 | 24.28±13.38 | 0.920 |
| TG (mmol/l) | 1.62±0.98 | 1.69±1.24 | 0.953 |
| TC (mmol/l) | 4.33±1.07 | 4.36±1.12 | 0.696 |
| HDL-C (mmol/l) | 1.10±0.32 | 1.12±0.34 | 0.475 |
| LDL-C (mmol/l) | 2.51±0.88 | 2.56±0.92 | 0.478 |
| BUN (mmol/l) | 5.46±1.34 | 5.60±1.24 | 0.107 |
| Cr (umol/l) | 59.04±14.16 | 61.91±15.01 | 0.047 |
| UA (umol/l) | 323.63±87.56 | 322.70±84.12 | 0.780 |
| PINP (ng/mL) | 39.03±15.10 | 29.17±8.75 | <0.001 |
| 25(OH)D (ng/mL) | 18.07±6.91 | 17.29±8.00 | 0.127 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose level; 2hPG, 2-hour postprandial plasma glucose; HbA1c, glycated hemoglobin A; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; PINP, procollagen type I N-terminal propeptide; 25(OH)D, 25-Hydroxyvitamin D.
Next, according to the interquartile range of PINP, all participants were divided into four groups by PINP quartiles: Q1 (<25.59), Q2 (25.59, 33.60), Q3 (33.61, 43.05) and Q4 (>43.05). We found that the prevalence of DR increased with decreasing the PINP levels, 58 in Q1 group (39.2%), 44 in Q2 group (29.7%), 34 in Q3 group (23.0%) and 12 in Q4 group (8.1%) (Figure 2).
Figure 2.
Relationship between PINP quartile and the prevalence of DR.
Note: ***trend p < 0.001.
Then, a multivariate logistic regression analysis was performed on all variables independently associated with diabetic retinopathy to determine age, duration of diabetes, and PINP (Table 2). In this analysis, age, duration of diabetes, and PINP level were all continuous variables. Among these factors, age and diabetic duration were positively associated with the risk of DR, and PINP level was negatively associated with DR.
Table 2.
Results of the Multivariate Logistic Regression Analysis
| Variables | Beta Value | P-value | OR (95% CI) |
|---|---|---|---|
| Age (year) | 0.140 | <0.001 | 1.15 (1.104~1.197) |
| Duration (year) | 0.047 | 0.010 | 1.048 (1.011~1.086) |
| FPG (mmol/L) | 0.031 | 0.454 | 1.031 (0.952~1.117) |
| Cr (umol/l) | 0.008 | 0.328 | 1.008 (0.992~1.023) |
| PINP (ng/mL) | −0.089 | <0.001 | 0.915 (0.893~0.938) |
To further assess the relationship between the levels of PINP and the occurrence of DR, we performed the regression analysis in unadjusted model (model 1) compared with Q1, Q3 (Q3 versus Q1, OR, 0.430; 95% CI: 0.255~0.727) and Q4 (Q4 versus Q1, OR, 0.124; 95% CI: 0.062~0.247) kept an independent effect on DR presence (Table 3). After age, duration of diabetes, FPG and Cr adjustment (model 2), in comparison to Q1group, patients in Q2, Q3 and Q4 all had a significantly decreased risk of DR by 49.9% (OR, 0.501; 95% CI: 0.280~0.894), 71.1% (OR, 0.289; 95% CI: 0.157~0.533) and 92.3% (OR, 0.077; 95% CI: 0.036~0.165), respectively (Table 3).
Table 3.
Association of PINP as a Continuous Variable and Quartiles with DR
| PINP (Quartile) | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Q1 (<25.59) | 1.00 (Reference) | 1.00 (Reference) | ||
| Q2 (25.59, 33.60) | 0.631 (0.380~1.045) | 0.074 | 0.501 (0.280~0.894) | 0.019 |
| Q3 (33.61, 43.05) | 0.430 (0.255~0.727) | 0.002 | 0.289 (0.157~0.533) | <0.001 |
| Q4 (>43.05) | 0.124 (0.062~0.247) | <0.001 | 0.077 (0.036~0.165) | <0.001 |
Notes: Model 1: Unadjusted; Model 2: Adjusted for age, duration of diabetes, FPG, Cr.
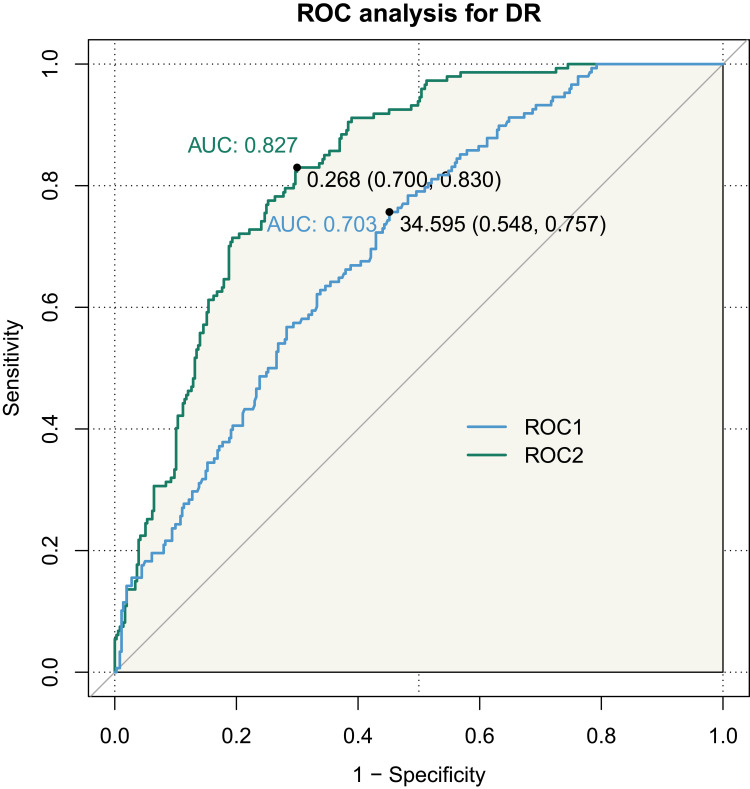
ROC curve analysis showed that the area under the curve (AUC) of DR diagnosed by PINP values was 0.703, 95% CI: 0.6562–0.75 in all T2DM patients (Figure 3, ROC 1). Meanwhile, the AUC of DR diagnosed by the combined diagnostic model of PINP with duration of diabetes and age was 0.8271, 95% CI: 0.7911–0.863 (Figure 3, ROC 2) (p < 0.05).
Figure 3.
ROC curve of PINP index predicting DR in patients with T2DM. (ROC1: PINP; ROC2: Combined with age, duration of diabetes).
Discussion
This study included 509 eligible T2DM participants and examined the relationship of PINP and DR. A significant association was found between the likelihood of DR and the PINP levels, diminished PINP level was in connection to an elevated risk of DR and remained significantly associated after controlling for a few confounding factors. When patients with T2DM present with either of microvascular complications and osteoporosis, more attention needs to be paid to the patient’s disease status, especially in patients of higher age and longer duration of diabetes mellitus.
DM is a major metabolic disease threatening human health, and microvascular complications of diabetes (MVCs) are vascular complications of diabetes characterized by dysfunction of the systemic multi-organ microvascular system.10 The prevalence of DR tended to increase steeply with age,11 and the development of DR strongly correlates with different risk factors, such as hypertension and diabetes duration.12,13 The onset and severity of DR vary widely and cannot be fully explained by known risk factors, it is crucial to find additional biomarkers to predict the risk of DR or assess treatment response.14 Previous studies have demonstrated that T2DM is accompanied by a lower bone turnover state. DR is a common MVC that has been elucidated to be associated with bone health and may lead to an increased risk of fall-related fractures in patients in the developmental stage.15–17 The healthy bone metabolism is maintained by a balance of dynamic turnover processes between osteoblasts and osteoclasts. Bone turnover markers (BTMs) are metabolites of the bone turnover process and key indicators for assessing bone metabolism. A previous study found that PINP levels were similarly found to be more reduced in diabetics with MVCs than in diabetics without MVCs, suggesting that the development of MVC may be associated with the evolution of T2DM-induced defects in bone metabolism.16 In a matched case-control study, researchers found that individuals with T2DM exhibited lower bone formation markers, such as osteocalcin (OC) and PINP, and it was hypothesized that the reduction in PINP may reflect microvascular dysfunction in patients with T2DM.18 Wei et al found that there was a closely associated of bone turnover markers (BTMs) with diabetic kidney disease (DKD) in elderly patients with T2DM, suggesting that maintaining optimal BTMs levels from an early age may be associated with a reduced future risk of DKD development.19 Consistency with the above studies, our study demonstrated the relationship between the bone turnover marker PINP and the risk of developing DR. Lower levels of PINP were accompanied by a higher risk of DR, and the bone formation marker PINP could be adopted as an exemplary predictor of DR. Moreover, age and diabetes duration were positively associated with the risk of DR. The ROC curves demonstrated a higher diagnostic value of PINP in combination with age and the duration of diabetes for the diagnosis of DR, which is of practical significance for the early clinical screening of potential patients with DR.
Bone remodeling is a complex and highly anabolic-dependent physiological process that centers on the dynamic balance between bone resorption and bone formation. Normal metabolism and renewal of bone tissue require precise synergistic action of osteoblasts, osteoclasts, and other cells, as well as adequate oxygen supply and nutrient-rich support. However, T2DM patients are often associated with abnormal energy metabolism and microcirculation disorders. Abnormal energy metabolism interferes with cellular energy supply and metabolic activities, affecting the function of osteoblasts and osteoclasts, and thus disrupting the balance between bone resorption and bone formation. Microcirculatory disorders can lead to insufficient local blood perfusion in bone tissue, which can block the transportation of oxygen and nutrients and inhibit the metabolic activities of osteoblasts. This dual effect may significantly exacerbate the imbalance in bone metabolism, leading to a slowing of the bone conversion process.20 BTMs are metabolites generated during bone turnover and serve as crucial indicators for the assessment of bone metabolism.21 PINP circulates with minimal daily fluctuations and exhibits remarkable stability at room temperature, remaining unaffected by dietary influences. This characteristic positions it as the premier marker for bone formation.22 Normal or high levels of serum PINP suggest a normal or slightly faster rate of type I collagen synthesis and an active bone turnover rate that maintains homeostasis of the bone’s internal environment. Bone cytokines are in a state of low conversion rate, which destroys capabilities of bone marrow stromal stem cells, leading to abnormal bone metabolism and affecting bone vascular distribution. The main manifestation is the deformation, atrophy or even fracture of the micro-vessels supplying the bone trabeculae, the thickening of the basement membrane around the blood vessels, which may even affect the microcirculatory system in many parts of the body.23,24 T2DM patients are often accompanied by a higher risk of microvascular complications and risk of bone metabolic diseases due to hyperglycemia, hyperinsulinemia, accumulation of advanced glycosylation end products (AGEs), and metabolic disorders.25 Our study demonstrated that higher levels of PINP are a protective factor against DR, therefore, when patients with T2DM present with either of microvascular complications and osteoporosis, more attention needs to be paid to the patient’s disease status, especially in patients of higher age and longer duration of diabetes mellitus.
Our research has certain limitations. The sample size of this study is small, it is indispensable to future validate by expanding the sample size and including multicenter data to improve the generalizability and reliability of our findings. Since this is a cross-sectional study, establishing a causal relationship is not possible. In addition, the mechanism of correlation between PINP and DR also needs to be explored in depth through basic research to clarify the effect of PINP on DR and even systemic microvasculature.
Conclusion
DR is associated with lower bone turnover status, compared to non-DR individuals, and DR patients had lower levels of the bone formation marker PINP. Additionally, PINP is a predictor for the development of DR. Thus, it is essential to strengthen the monitoring of bone metabolism in patients with diabetes-associated microangiopathy. Simultaneously, timely diagnosis and prevention of possible microvascular complications in diabetic patients who have already developed bone abnormalities can contribute to effective treatment and comprehensive management of patients with T2DM.
Acknowledgments
Guanhua Chen, Yuan Zhang, and Weimin Wang are co-first authors for this study. We would like to thank all participants of the study for their cooperation and support.
Funding Statement
This work was supported by the National Natural Science Foundation of China Grant Awards (82374554) and Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2024-LCYJ-ZXY-02).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Declaration
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. Due to the retrospective nature of the study, the medical research ethics committee of the Nanjing Drum Tower Hospital waived the need to obtain informed consent, and we committed absolute confidentiality of patient data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):117–124. doi: 10.2174/1570161117666190502103733 [DOI] [PubMed] [Google Scholar]
- 3.Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi: 10.1016/j.ophtha.2021.04.027 [DOI] [PubMed] [Google Scholar]
- 4.Hou X, Wang L, Zhu D, et al. Prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy in adults with diabetes in China. Nat Commun. 2023;14(1):4296. doi: 10.1038/s41467-023-39864-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CE, Coleman CM. Impact of diabetes mellitus on bone health. Int J Mol Sci. 2019;20(19):4873. doi: 10.3390/ijms20194873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheu A, White CP, Center JR. Bone metabolism in diabetes: a clinician’s guide to understanding the bone-glucose interplay. Diabetologia. 2024;67(8):1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. [DOI] [PubMed] [Google Scholar]
- 8.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 10.Yu MG, Gordin D, Fu J, Park K, Li Q, King GL. Protective factors and the pathogenesis of complications in diabetes. Endocr Rev. 2024;45(2):227–252. doi: 10.1210/endrev/bnad030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010803. doi: 10.7189/jogh.08.010803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perais J, Agarwal R, Evans JR, et al. Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database Syst Rev. 2023;2023(2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellor J, Jeyam A, Beulens JWJ, et al. Role of systemic factors in improving the prognosis of diabetic retinal disease and predicting response to diabetic retinopathy treatment. Ophthalmol Sci. 2024;4(4):100494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studnicka J, Nemcansky J, Vyslouzilova D, Ernest J, Nemec P. Diabetic retinopathy - diagnostics and treatment guidelines. Cesk Slov Oftalmol. 2023;79(5):238–247. [DOI] [PubMed] [Google Scholar]
- 15.Lim Y, Chun S, Lee JH, et al. Association of bone mineral density and diabetic retinopathy in diabetic subjects: the 2008-2011 Korea national health and nutrition examination survey. Osteoporos Int. 2016;27(7):2249–2257. doi: 10.1007/s00198-016-3527-5 [DOI] [PubMed] [Google Scholar]
- 16.An Y, Liu S, Wang W, et al. Low serum levels of bone turnover markers are associated with the presence and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. J Diabetes. 2021;13(2):111–123. doi: 10.1111/1753-0407.13089 [DOI] [PubMed] [Google Scholar]
- 17.Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes. 2016;65(7):1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y, Hou X, Nie Q, et al. Association of bone turnover markers with type 2 diabetes mellitus and microvascular complications: a matched case-control study. Diabetes Metab Syndr Obes. 2023;16:1177–1192. doi: 10.2147/DMSO.S400285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei S, Pan X, Wei J. Relationship between bone turnover markers and renal disease in elderly patients with type 2 diabetes: a cross-sectional study. BMC Endocr Disord. 2024;24(1):179. doi: 10.1186/s12902-024-01698-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Zheng X, Zhang Y, et al. The endocrine role of bone: novel functions of bone-derived cytokines. Biochem Pharmacol. 2021;183:114308. doi: 10.1016/j.bcp.2020.114308 [DOI] [PubMed] [Google Scholar]
- 21.Piccoli A, Cannata F, Strollo R, et al. Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res. 2020;35(12):2415–2422. doi: 10.1002/jbmr.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross RD, Deng Y, Fang R, Frisch NB, Jacobs JJ, Sumner DR. Discovery of biomarkers to identify peri-implant osteolysis before radiographic diagnosis. J Orthop Res. 2018;36(10):2754–2761. doi: 10.1002/jor.24044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Li D, Yan Y, Yang Q, Li L, Zha Y. Microvascular permeability and texture analysis of bone marrow in diabetic rabbits with critical limb ischemia based on dynamic contrast-enhanced magnetic resonance imaging. J Diabetes Investig. 2024;15(5):584–593. doi: 10.1111/jdi.14145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usiskin IM, Mitchell GF, Bouxsein ML, Liu CT, Kiel DP, Samelson EJ. Vascular function and skeletal fragility: a study of tonometry, brachial hemodynamics, and bone microarchitecture. J Bone Miner Res. 2024;39(7):906–917. doi: 10.1093/jbmr/zjae071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol. 2022;13:1077669. doi: 10.3389/fendo.2022.1077669 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.