Abstract
Late-stage functionalization is an attractive strategy that allows chemists to bypass lengthy synthetic processes, facilitating the rapid generation of drug analogs with potentially enhanced pharmacokinetic and pharmacological properties. This study describes a novel approach for cross-dehydrogenative oxyalkylation, leveraging a unique γ-ray-enabled photoredox process to generate oxyalkyl radicals, followed by a Minisci-type addition in an aqueous solution. The metal- and oxidant-free aqueous conditions, coupled with excellent functional group compatibility, establish this method as a versatile protocol for the late-stage oxyalkylation of unprotected, structurally complex drug molecules. Notably, this method demonstrated improved pharmacokinetics in hydroxymethylated fibroblast activation protein inhibitor (FAPI) molecules, highlighting its potential to accelerate drug discovery efforts.
Keywords: ionizing radiation, minisci reaction, late-stage functionalization
Graphical abstract

Public summary
-
•
Metal- and oxidant-free oxidative cross-dehydrogenative coupling enabled by γ radiation in aqueous solution.
-
•
Aqueous γ-ray irradiation facilitates the generation of α-oxyalkyl radicals.
-
•
Late-stage functionalization of FDA-approved drugs with high functional group tolerance (e.g., −NH₂ and −COOH).
-
•
Enhanced pharmacokinetics of functionalized FAPI molecules demonstrated.
Introduction
One of the most fruitful strategies to discover a new drug is to start with an old drug.1 Late-stage functionalization (LSF) has emerged as a pivotal approach in drug discovery, facilitating the rapid establishment of structure-activity relationships and enabling the development of pharmaceutical compounds with improved on-target potency and enhanced physicochemical properties.2,3,4,5 However, the intrinsic complexity of drug molecules, with densely clustered polar functional groups (e.g., amines, alcohols, and N-heterocycles), presents challenges to achieving chemo-selective functionalization with sufficient functional group compatibility.3
Nitrogen-containing heteroarenes are essential scaffolds for a wide range of bioactive natural products and pharmaceuticals. Consequently, there is considerable interest in expanding their structural diversity through LSF.6,7,8 Among the strategies available, the cross-dehydrogenative coupling (CDC) reaction has emerged as one of the most sustainable and efficient methods for constructing C–C bonds, owing to its high step and atom economy.9,10,11,12,13,14,15,16,17
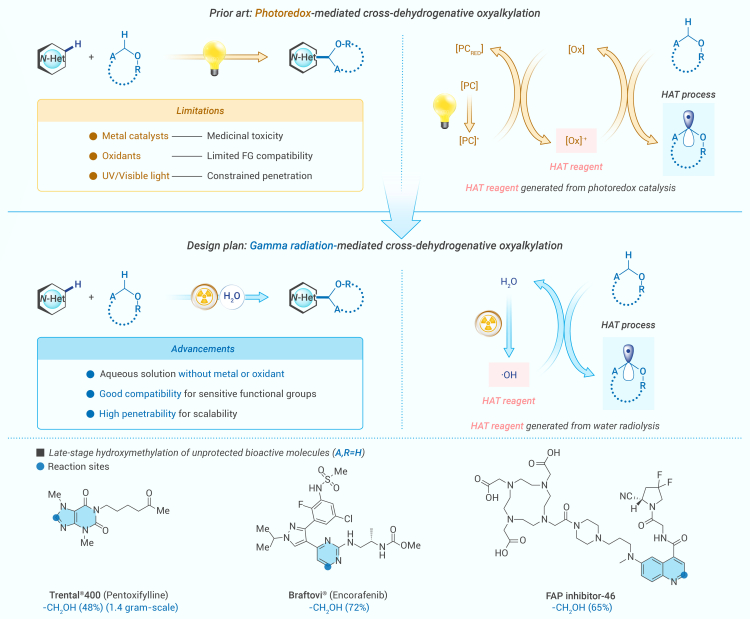
Photoredox catalysis has become a powerful tool for generating radicals, enabling numerous CDC reactions under mild conditions over the past decades.18,19,20,21,22,23,24 Notable progress includes the development of hydrogen atom transfer (HAT)-induced C–H activation, exemplified by the work of MacMillan and collaborators.25 By combining a metallaphotoredox catalyst with a peroxide, it is possible to generate an α-O carbon-centered radical (1) through hydrogen abstraction by a sulfate radical anion. This process is initiated by a single-electron transfer (SET) in a photocatalytic cycle, followed by the radical’s open-shell addition to an electron-deficient heterocycle (Scheme 1). Undesirably, metals pose challenges related to cost, environmental hazards, and health risks.26 Additionally, its limited applicability for LSF of pharmaceutical compounds typically arises from potential competition among heteroatoms or polar functional groups (such as −NH2, −COOH, etc.) with the metal catalysts for coordination.27 These factors render their implementation imperfect and inevitably problematic for pharmaceutical applications.3 Beyond metallaphotoredox catalysis, Lei and colleagues demonstrated that Selectfluor effectively facilitates oxidative cross-coupling between alcohols and heteroarenes.28 Metal-free photocatalytic strategies offer distinct advantages in pharmaceutical applications, particularly in minimizing issues related to metal toxicity and environmental impact.2,4,5,29 Nonetheless, the incorporation of equivalent oxidants typically introduces compromise to its functional group compatibility.30 To overcome these limitations, green chemistry principles emphasize the need for oxidant- and metal-free LSF methodologies that prioritize both sustainability and compatibility with sensitive functional groups.
Scheme 1.
Development of a novel photoredox strategy enabled by γ-radiation UV, ultraviolet; FG, functional groups; HAT, hydrogen atom transfer.
Radiolysis of water provides various reactive species, such as hydroxyl radicals (·OH), followed by a cascade of reactions that complete within 10−4 s.31,32,33,34 As the scavengers of radicals, alcohols or ethers react with ·OH at a relatively high rate (k = 9 × 108 M−1 s−1, CH3OH),35 producing α-O carbon-centered radicals.36,37,38 This radiation-induced process provides a metal- and oxidant-free pathway for radical formation. Unlike UV-visible light-mediated photoredox processes—where light energy is absorbed by photosensitizers—γ radiation predominantly transfers energy to solvent molecules under low substrate concentrations, selectively generating the desired reactive species while avoiding undesired radiolysis of reactants. To the best of our knowledge, a γ-radiation-mediated chemo-selective organic transformation with practical applications has not been developed yet. Herein, we introduce a γ-radiation-mediated cross-dehydrogenative oxyalkylation using α-O carbon-centered radicals generated from radiolysis (Scheme 1). This work expands the conventional use of ionizing radiation in organic synthesis, offering a novel approach that could inspire further interest among organic chemists.
Materials and methods
General procedure for hydroxymethylation of heteroarenes
Heteroarene (0.13 mmol), 2.0 g of methanol, and 8.0 g of sulfuric acid solution (1.6 mM in H2O) were charged to a Schlenk vial. The reaction mixture was degassed three times via a freeze-pump-thaw procedure, followed by exposure to 60Co γ-ray radiation at a dose rate of 30 Gy/min for 900 min at ambient temperature (20°C). Upon radiation completion, the reaction mixture was alkalized by saturated NaHCO3 aqueous solution until it reached pH 8.0. Then, the solvent was removed from under high vacuum, followed by being redissolved with a mixed solvent of CH2Cl2 and MeOH (1:1 v/v). The suspension was filtered to remove the solid salts, and the filtrate was dried over Na2SO4. Upon completion, the mixture was concentrated in vacuo, followed by further purification using preparative thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC) to afford the desired product.
General procedure for 1,4-dioxane α-heteroarylation
Heteroarene (0.13 mmol), 2.0 g of 1,4-dioxane, and 8.0 g of sulfuric acid solution (1.6 mM in H2O) were charged to a Schlenk vial. The reaction mixture was degassed three times via a freeze-pump-thaw procedure, followed by exposure to 60Co γ-ray radiation at a dose rate of 30 Gy/min for 900 min at ambient temperature (20°C). Upon radiation completion, the reaction mixture was alkalized by saturated NaHCO3 aqueous solution until it reached pH 8.0. Then, the solvent was removed from under high vacuum, followed by being redissolved with a mixed solvent of CH2Cl2 and MeOH (1:1 v/v). The suspension was filtered to remove the solid salts, and the filtrate was dried over Na2SO4. Upon completion, the mixture was concentrated in vacuo, followed by further purification using preparative TLC or HPLC to afford the desired product.
General procedure for alcohols/ethers α-heteroarylation (mmol scale, 4-methylquinoline as model substrate)
4-methylquinoline (19 mg, 0.13 mmol) and a specified quantity of alcohols or ethers were charged to a Schlenk vial and then were dissolved with H2SO4 solution (1.6 mM in H2O) to finally obtain a 10 mL reaction solution. The reaction mixture was degassed three times via a freeze-pump-thaw procedure, followed by exposure to 60Co γ-ray radiation at a dose rate of 30 Gy/min for 900 min at ambient temperature (20°C). Upon radiation completion, the reaction mixture was alkalized by saturated NaHCO3 aqueous solution until it reached pH 8. Then, the solvent was removed from under high vacuum (alcohols/ethers with high boiling points were washed away by brine twice), followed by being redissolved with a mixed solvent of CH2Cl2 and MeOH (1:1 v/v). The suspension was filtered to remove the solid salts, and the filtrate was dried over Na2SO4. Upon completion, the mixture was concentrated in vacuo, followed by further purification using preparative TLC or HPLC to afford the desired product.
General procedure for LSF of drugs
An N-heteroarene-based drug (0.13 mmol), 8.0 g of methanol, and 32.0 g of sulfuric acid solution (0.4 mM in H2O) were charged to a Schlenk vial. The reaction mixture was degassed three times via a freeze-pump-thaw procedure, followed by exposure to 60Co γ-ray radiation at a dose rate of 30 Gy/min for 250 min at ambient temperature (20°C). Upon radiation completion, the reaction mixture was alkalized by saturated NaHCO3 aqueous solution until it reached pH 8. Then, the solvent was removed from under high vacuum, followed by being redissolved with a mixture of DCM and MeOH (1:1 v/v). The suspension was filtered to remove the solid salts, and the filtrate was dried over Na2SO4. Upon completion, the mixture was concentrated in vacuo, followed by further purification using preparative TLC or HPLC to afford the desired product.
General procedure for hydroxymethylation of FAPIs
N-heteroarene-based fibroblast activation protein inhibitors (FAPIs; 10 μmol), 1.0 g of methanol, and 4.0 g of sulfuric acid solution (0.4 mM in H2O) were charged to a Schlenk vial. The reaction mixture was degassed three times via a freeze-pump-thaw procedure, followed by exposure to 60Co γ-ray radiation at a dose rate of 30 Gy/min for 60 min at ambient temperature (20°C). Upon radiation completion, the reaction mixture was alkalized by saturated NaHCO3 aqueous solution until it reached pH 8.0. Then, the solvent was removed from under high vacuum, followed by being redissolved with a mixture of H2O and MeOH (1:1 v/v). The suspension was filtered to remove the solid salts. Upon completion, the mixture was concentrated in vacuo, followed by further purification using preparative HPLC to afford the desired product.
18F radiolabeling
18F was produced from a proton cyclotron at Peking University. After production in a cyclotron, [18F]F− was transferred into the module and trapped on a Sep-Pak Plus QMA, preconditioned with 5 mL of 0.5 M sodium acetate buffer (pH 3.9) and 10 mL of water. Subsequently, [18F]F− (2.5 GBq) was eluted using 0.15 mL of 0.5 M sodium acetate buffer (pH 3.9). Then, 50 μL of eluted solution was added to a mixture of AlCl3 (20.0 nmol, 10 μL), sodium acetate buffer (0.2 M, 10 μL, pH 4.0), MeCN (30 μL), and precursor (5c/5f; 20.0 nmol) in 2 μL H2O. After being heated for 10 min at 100°C, the mixture was then cooled and diluted with 5 mL of deionized water. For purification, the cooled reaction mixture was passed through a pre-activated Sep-Pak Light C18 cartridge with deionized water to eliminate free radionuclides. The radiolabeled product was eluted using 0.50 mL of ethanol and then diluted with saline for subsequent experiments. If necessary, ethanol was removed by nitrogen blowing with a Termovap sample concentrator before dilution. The radiochemical yield (RCY) was assessed using a radioactivity meter, while the radiochemical purity (RCP) was determined by radio-HPLC.
Stability analysis of [18F]-AlF-NOTA-FAPI-04-HM
For in-serum stability, we incubated [18F]-AlF-NOTA-FAPI-04-HM (5.6 MBq/mL) in buffer (0.2 mL) with serum (0.2 mL) at 37°C. We then sampled 20 μL aliquots at each specified time point and stored them at −80°C before analysis. The supernatant was then subjected to analysis by radio-HPLC.
Cell-line-derived xenograft model construction
All mice were purchased from Beijing Vital River Laboratory Animal Technology. The animal experiments were performed under a protocol (CCME-LiuZB-2) approved by the ethics committee of Peking University. For cell-line-derived xenograft models, 6-week-old female Nu/Nu nude mice were implanted subcutaneously with 5 × 106 HT-1080-FAP cells at the forward right flanks. Mice were housed under a temperature- and humidity-controlled condition, and the room was on a 12-h light/dark cycle.
Small animal PET/CT imaging
All positron emission tomography (PET) scans were performed on a Mediso nanoScan PET 122S small animal PET/computed tomography (CT) imaging system. Mice were anesthetized with the isoflurane/O2 mixture (induction, 4%; maintenance, 2%). The static images were acquired at different time points after injection. PET data were reconstructed with a three-dimensional iterative algorithm (Tera-Tomo 3D). The regions of interest (ROIs) were delineated manually on the images, and the standard uptake value (SUV) of each ROI was calculated and used as the indicator for the quantification of radioactivity.
Ethical statement
All mouse studies were conducted following the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals (Ministry of Health, China) and the protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Peking University (IACUC ID: CCME-LiuZB-2).
Results and discussion
Optimization of the reaction conditions
To test our hypothesis, we took 4-methylquinoline (2) as the model substrate to investigate the radiation-induced N-heteroarene hydroxymethylation reaction. Considering the electrophilicity of a heterocycle, we chose sulfuric acid as the protonating agent to screen out the best concentration in the presence of 20 wt % MeOH and 1 mM of 2 under γ radiation for 75 min. As shown in Table 1, the rise of the concentration of methanol (0–60 wt %) results in the increase of the conversion ratio of 2 (Table S1, entries 1–5). Yet, the yield of 2a decreased to 72% (entry 2) when the concentration of methanol increased to 60 wt %. Hydroxy radicals, generated from the radiolysis of H2O, can rapidly react with CH3OH to yield ·CH2OH, which could attack electron-deficient N-heteroarenes nucleophilically. The optimal yield for 2a was observed at 20 wt % methanol, and higher concentrations led to increased undesired by-products, resulting in a yield decline of 2a. To enhance substrate electrophilicity, we explored various equivalents of sulfuric acid as an additive. The optimal equivalent was found to be 0.1 equiv, as higher acid concentrations led to an increase of undesired by-products (entry 3). We have screened other acids, such as acetic acid and trifluoroacetic acid, to investigate whether the counter anions would impact this reaction yield. As shown in entry 4 and Table S2, this radiation-induced hydroxymethylation works well with various acids. We have also found that increasing the reaction time from 10 to 100 min gave a higher conversion ratio of 2. The yield of 2a slightly decreased from 91% to 88% when increasing the reaction time from 75 to 100 min, which might be attributed to the undesired radiolysis of 2a (entry 5). At last, removing the air from the reaction system would notably increase the reaction yield from <10% to 91% (entry 6), as oxygen may quench the hydroxymethyl radicals.39
Table 1.
Investigation of reaction conditions
 | |||
|---|---|---|---|
| Entry | Variation from the standard conditions | Conversion (%)a | Yield (%)a |
| 1b | none | 96 | 91 |
| 2 | c(MeOH) changed to 60 wt % | >99 | 72 |
| 3 | w/o acid | 77 | 75 |
| 4 | changed to HOAc | 85 | 80 |
| 5 | reaction time changed to 100 min | 98 | 88 |
| 6 | ambient | <10 | <10 |
Yield was determined by HPLC with 2-methoxynaphthalene as an internal standard. Detection wavelength, λ = 315 nm.
The optimized condition: 2 (1 mM), CH3OH (20 wt %) in H2O, H2SO4 (0.1 equiv), Co-60 γ-ray (30 Gy/min, traced by Fricke dosimeter), freeze-pump-thaw degassing.
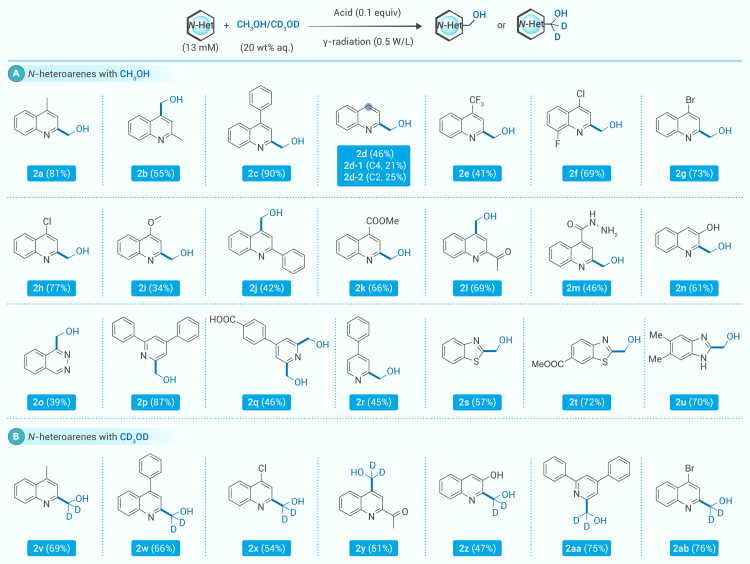
Substrate scope
With the optimized condition, we first evaluated the substrate scopes in the presence of methanol with various N-heteroarenes (Scheme 2). As the substrate concentration increased from 1 to 13 mM, we increased the absorbed radiation dose by 13 times. As shown in Scheme 2, quinolines with alkyl groups (2a and 2b), aryl groups (2b and 2c), and electron-withdrawing groups (e.g., methoxycarbonyl and acetyl) (2k and 2l) could be converted into the corresponding products in moderate to high yields. Similarly, quinolines with electron-donating groups (e.g., methoxy and hydroxyl) (2i and 2n) were converted into the corresponding products in moderate yields. The substrates with a trifluoromethyl group (2e) could also be tolerated, although a relatively moderate yield was obtained. The high functional group compatibility was shown by successful N-heteroarene hydroxymethylations with functional groups, including acid-sensitive hydrazide (2m) and base-sensitive carboxylic acid (2q and 2t). As the good functional handles for further diversification, aryl halides (F, Cl, and Br) (2f–2h) could also be tolerated in this strategy. Quinoxaline (2o) and substituted pyridines (2p–2r) were converted into the corresponding products in moderate yields. Additionally, benzothiazole and benzimidazoles with a variety of substituents (2s–2u) were found to be compatible with the coupling at relatively high yields.
Scheme 2.
Hydroxymethylation substrate scopes of heterocycles
Reaction conditions: (A) heterocycle substrates (0.52 mmol), methanol (8.0 g), and 1.6 mM H2SO4 (32.0 g) in a 50 mL Schlenk and (B) heterocycle substrates (0.31 mmol), CD3OD (8.0 g), and 1.6 mM H2SO4 (32.0 g) in a 50 mL Schlenk. The reaction mixtures were treated by freeze-pump-thaw degassing and then exposed to 60Co γ-ray radiation (30 Gy/min, traced by Fricke dosimeter) at 20°C for 15 h; isolated yield is given. More experimental details described in general procedure A in the supplemental materials and methods.
Given the effectiveness of generating ·CH₂OH through a HAT process under radiation, perdeuterated methanol (CD₃OD) was subjected to the reaction conditions. Due to the reduced radiation-chemical yield of ·CD₂OD under the same conditions, we lowered the concentrations of the N-heteroarene substrates to optimize the reaction. Quinolines with a variety of substituent groups were observed to convert into corresponding deuterium-containing hydroxymethylated derivatives at moderate to high yields (2v–2ab, 41%–76%).
Subsequently, we expanded the hydroxymethylation and examined 1,4-dioxane on N-heterocyclic substrates. As desired, this strategy works well on a variety of substituted quinolines and iso-quinolines (3 and 3a–3o). In addition, benzimidazole (3p), thiadiazol (3q, 3w, and 3z), oxadiazol (3r), and diazine (3u–3v) substrates can be converted to the corresponding products at moderate to high yields, demonstrating the good compatibility of oxidation-sensitive amines and S-heterocycles. A moderate yield was observed on an acridine substrate (3x) and a quinoxaline substrate (3s). Pyridines with a variety of substituents (3t and 3y) could be converted into the corresponding products in moderate to high yields. Of note, this radiation-induced coupling for 3 proceeded efficiently at gram scale (2.4 g, 94%), demonstrating that this strategy is promising for large-scale synthesis.
Besides methanol and 1,4-dioxane, we have also evaluated the other alcohols and ethers (Scheme 3). Ethanol could be converted into the corresponding heterocyclic products in a moderate yield (3aa). A variety of diols were converted into the corresponding heteroarene-coupled products at moderate to high yields, such as glycol (3ab), propane-1,3-diol (3ad), and butane-1,4-diol (3ac). Glycerol (3ae) was also found to be compatible with this radiation-induced Minisci-type coupling reaction. High site selectivity on the C-1 position of glycerol was observed, which can be attributed to the hindered C-2 position induced by adjacent hydroxymethyl groups. This steric hindrance makes it challenging for the C-2 position to couple with heteroarenes, thereby enhancing the selectivity of the C-1 position. In addition, a comparably good yield with tetrahydrofuran (THF) (3af) demonstrated that the other cyclic ethers might also be suitable substrates. As expected, this strategy works efficiently with a variety of macrocyclic ethers, such as cyclodecano-15-crown-5 (CD15C5) (3ag) and 18-crown-6 (18C6) (3ah), which may be a useful method to obtain functionalized crown-ether-based chelating agents. Of interest, a considerable amount of product 3ai was observed while oxetane was subjected to the reaction, which could be regarded as a result of the C–O bond cleavage under the reaction condition. We hypothesized that the C–O bond cleavage was presumably caused by the spin-center shift (SCS) process, owing to its high ring tension. The unexpected ring-opening process may offer a promising approach for synthesizing heteroarenes modified with primary alkanols.
Scheme 3.
Substrate scopes of heterocycles and alcohols/ethers
Reaction conditions: (A) heterocycle substrates (0.52 mmol), 1,4-dioxane (8.0 g), and 1.6–16 mM H2SO4 (32.0 g) in a 50 mL Schlenk vial and (B) 4-methylquinoline (74 mg, 0.52 mmol), alcohols or ethers (8.0 g), and 1.6 mM H2SO4 (32.0 g) in a 50 mL Schlenk vial. The reaction mixtures were treated by freeze-pump-thaw degassing and then exposed to 60Co γ-ray radiation (30 Gy/min, traced by Fricke dosimeter) at 20°C for 15 h; isolated yield is given.
∗See the supplemental information for the experimental details of the g-scale reaction.
∗∗15-C-5 (3.0 g) was added; isolated yield is given.
∗∗∗18-C-6 (1.5 g) was added; isolated yield is given.
LSF of drug molecules
Encouraged by the good compatibility with the broad scope of functional groups, such as the unprotected amino group, carboxyl group, and hydrazide group, we wondered whether this strategy would be applied to the LSF of drug molecules. As shown in Scheme 4, upon γ-ray irradiation, FDA-approved drug molecules with catalytic amounts of acid as the only additive provided good conversion to the corresponding hydroxymethylated products. The pyridine-ring-containing drug desloratadine was functionalized in a moderate yield (4a, 56%). Within the category of purine nucleoside antimetabolite medications, clofarabine (4b, 73%) can be effectively hydroxymethylated under these conditions even with its amino and hydroxyl groups unprotected. The quinoxaline-derived drug varenicline was functionalized in a moderate yield (4c, 57%) without any protecting group. Quinolines and iso-quinolines are some of the most common N-heterocycles in pharmaceutical molecules and the cores of quinine and fasudil. Pleasingly, γ-ray-irradiation-induced hydroxymethylation of these drugs proceeded efficiently (4f and 4m, yielding 38%–69%). It is noteworthy that the hydroxymethyl group was observed to be selectively coupled onto the C-1 position of the iso-quinoline ring in fasudil. This high selectivity can be attributed to the electron-deficient nature of the C-1 site. Pyrimidine-based drug molecules are completely suitable substrates for the hydroxymethylation reaction. Remarkably, even electron-rich heterocycles featuring a variety of unprotected reactive functional groups, such as amine or alkynyl groups, yielded functionalized drug molecules in excellent yields (4h and 4j, yielding 44%–72%). Purine-analog-based drugs that underwent γ-ray irradiation showed moderate to high yields and predictable selectivity (4d, 4e, 4g, 4i, and 4p–4r, yielding 47%–74%). An analog of theophylline with 2-chloroethyl substituted also reacts efficiently under these conditions (4l, 50%). Of note, the γ-ray-induced, gram-scale, late-stage hydroxymethylation of pentoxifylline was demonstrated to be feasible (4g, 1.4 g, 38%). Functioning as a phosphoinositide 3-kinase inhibitor for the treatment of specific blood cancers, idelalisib can be converted into the corresponding hydroxymethylated product at a moderate yield (4k, 52%). These transformations proceed in the presence of functionalities such as basic amines, alcohols, amides, and esters without the need for protecting groups. This may be a unique advantage compared to metal-mediated photocatalysis, as water absorbs most of the radiation energy and then generates radicals (e.g., ·OH) without the need for photoredox catalysts or oxidants.
Scheme 4.
Substrate scopes of FDA-approved drugs and bioactive molecules
Reaction conditions: heterocyclic drug substrates (0.13 mmol), methanol (8.0 g), and 0.4 mM H2SO4 (32.0 g) in a 50 mL Schlenk vial. The reaction mixtures were treated by freeze-pump-thaw degassing and then exposed to 60Co γ-ray radiation (30 Gy/min, traced by Fricke dosimeter) at 20°C for 4 h; isolated yield is given. See the supplemental information for experimental details.
Given the efficiency of this approach to generate α-O carbon-centered radical, we were interested in expanding the scope to other alkyl radicals. With the investigation of N-heteroarenes and alcohols/ethers, 1,4-dioxane was subjected to identical reaction conditions to LSF. Similar to the results from methanol, purine- and quinoline-containing drugs were converted into the corresponding hydroxymethylated products at relatively low to moderate yields (4m, 4u, and 4v, 20%–47%). When pyridine-containing Dp44mT was subjected to the reaction condition, the desired products with 4- and 6-substituted positions were observed (4s, 45% total), delivering the site diversity of functionalization for drug discovery.
LSF of FAPI molecules
FAP is a pan-cancer target and now the state of the art to develop radiopharmaceuticals. FAPIs have been of great success in developing imaging tracers.40,41 Yet, tracers conjugated with AlF-NOTA moieties exhibit relatively high lipophilicity, leading to undesirable nonspecific uptake in the gallbladder and intestines.40,42 In medicinal chemistry, incorporating the hydroxymethyl group (−CH2OH) can effectively modulate crucial physical properties such as log p and solubility.43 Nevertheless, a direct strategy for the LSF of FAPI molecules incorporating unprotected polar functional groups has not yet been reported. By wedding the rich medicinal potential of the hydroxymethyl group to the ubiquity of N-heteroarenes, the radiation-induced hydroxymethylation reaction has the potential to open up new vistas in drug development.
Given the broad substrate scope of drug molecules, the application of the radiation-induced hydroxymethylation reaction to FAPI molecules 5a–5c allowed the expedient preparations of 5d–5f in moderate yields (39%–65%) within 60 min (Scheme 5A). Furthermore, we took 5f as the model molecule to investigate its pharmacokinetics properties. The [18F]-AlF radiolabeling followed the detailed procedure in the supplemental information, and the radiolabeling yield of 5f was 58% (Scheme 5B). The RCP of [18F]-AlF-5f was over 99% (n > 10), and its specific activity was 22 GBq/μmol. The stability of [18F]-AlF-5f in serum was analyzed using radio-HPLC, as shown in Scheme 5C. The RCP of [18F]-AlF-5f was still over 90% after incubation in serum for 120 min.
Scheme 5.
Late-stage hydroxymethylation of FAPIs and pharmacokinetics studies of the functionalized FAPIs
(A) Radiation-induced late-stage hydroxymethylation of FAPI molecules.
(B) Radiolabeling of hydroxymethylated FAP inhibitors.
(C) Explore the stability of [18F]AlF-NOTA-FAPI-04-HM by radio-HPLC analysis. Top, the 254 nm UV absorption spectrum of [natF]AlF-NOTA-FAPI-04-HM. Middle, the radio-HPLC spectrum of [18F]AlF-NOTA-FAPI-04-HM before injection. Bottom, the radio-HPLC spectrum of [18F]AlF-NOTA-FAPI-04-HM incubated in serum for 120 min at 37°C.
(D) The quantitative analysis of [18F]AlF-NOTA-FAPI-04 (5c) and [18F]AlF-NOTA-FAPI-04-HM (5f) at 80 min post injection (n = 6/group). For gallbladder group, p = 0.0002 and for intestine group, p = 0.0022.
(E) A comparison PET/CT imaging assay of [18F]AlF-NOTA-FAPI-04 (5c) and [18F]AlF-NOTA-FAPI-04-HM (5f) in healthy mouse at 80 min post injection.
(F) A PET/CT imaging assay of [18F]AlF-NOTA-FAPI-04-HM (5f) in HT-1080-FAP tumor-bearing mice at 120 min post-injection.
Reaction conditions: FAPI substrates (0.03 mmol), methanol (1.0 g), and 0.4 mM H2SO4 (4.0 g) in a 10 mL Schlenk vial. The reaction mixtures were treated by freeze-pump-thaw degassing and then exposed to 60Co γ-ray radiation (30 Gy/min, traced by Fricke dosimeter) at 20°C for 60 min; isolated yield is given. See the supplemental information for experimental details.
To investigate the impact of the hydroxymethyl group on the pharmacokinetics and tumor uptake of [18F]-AlF-5f, we took [18F]-AlF-5c for comparison and performed a head-to-head PET/CT imaging of [18F]-AlF-5c and [18F]-AlF-5f in the same healthy Nu/Nu mice. As shown in Scheme 5D, the quantitative gallbladder and intestines SUVmean values of [18F]-AlF-5c are 3.55 ± 0.81 and 2.64 ± 0.42 g/mL at 80 min post injection, respectively. Compared to [18F]-AlF-5c, the nonspecific gallbladder and intestines uptakes of the hydroxymethylated analog [18F]-AlF-5f (2.01 ± 0.43 and 1.45 ± 0.59 g/mL at 80 min post injection, respectively) are significantly lower. The significant decrease in uptake could be attributed to the introduction of the hydroxymethyl group, which enhances the log p value of the FAPI molecules (Scheme 5E). As shown in Scheme 5F, for both [18F]-AlF-5c and [18F]-AlF-5f, the tumor was completely visible at 120 min after injection.
These optimized pharmacokinetics make 5f a promising PET imaging tracer with reduced nonspecific uptake, demonstrating that the radiation-induced LSF strategy holds significant potential for drug discovery.
Mechanistic considerations
It is known that water radiolysis provides various reactive species, such as hydrogen radical (·H), hydroxyl radical (·OH), and hydrated electron (e−aq), followed by a cascade of reactions that complete within 10−4 s.31,44 As the scavengers of radicals, methanol reacts with ·OH, mainly producing hydroxymethyl radicals (·CH2OH), a highly nucleophilic radical (nucleophilicity index ω− = 0.486).45,46 Furthermore, it is noteworthy that the substrate concentration (0.05–0.2 M in general) is remarkably lower than the solvent concentration (e.g., H2O, 55.6 M); therefore, the energy of radiation is predominantly absorbed by the solvent rather than the reactants, followed by the generation of reactive species, thereby circumventing the undesired radiolysis of the reactants, as shown in Scheme 6A.
Scheme 6.
Mechanistic considerations
(A) Comparison of mechanisms between photoredox process mediated by UV-visible near-infrared (UV-vis-NIR) light and γ radiation.
(B) Mechanism proposal for radiation-induced hydroxymethylation.
(C) Mechanistic experiments. A = H, alkyl.
∗Standard condition: reactant (1 mM), CH3OH (20 wt %) in H2O, H2SO4 (0.1 equiv), 20°C, 60Co γ-ray radiation, 2,250 Gy (30 Gy/min for 75 min), freeze-pump-thaw degassing.
PC, photocatalyst.
Exemplified by the reaction between 4-methylquinoline and alcohols/ethers, a proposed mechanism for the γ-ray-induced N-heteroarenes hydroxymethylation process is outlined in Scheme 6B. Reactive species generated from water radiolysis, such as ·H and ·OH (path a), would undergo the HAT process with methanol to yield ·CH2OH at a relatively high rate (k = 9 × 108 M−1 s−1)35 (path b). There may also be direct radiolysis of methanol, producing 2.7 ·CH2OH when absorbing 100 eV of radiation energy (0.63 mM ·CH2OH per 2,250 Gy) (path c).47 The radical ·CH2OH (6a) then took the nucleophilic addition to the protonated 2 in a Minisci-type pathway45,46 (path d) to yield the cation radical intermediate 6b, which subsequently undergoes the deprotonation process to yield the radical 6c (path e). In a major way, 6c can be converted into protonated hydroxymethylated adduct 6d through an oxidative process (path f).
We have also performed mechanistic experiments to further understand the chemical process of γ-ray-induced hydroxymethylation. As a radical-trapping reagent, 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) was subjected to the standard condition. The N-heteroarenes hydroxymethylation of methanol was suppressed when TEMPO was added, demonstrating that the heteroarylation might follow a radical pathway (Scheme 6C, i). Importantly, this reaction was shut down in the presence of blue light-emitting diode (LED) irradiation instead of γ irradiation (Scheme 6C, ii). Besides, the intermolecular kinetic isotope effect (KIE) experiment was undertaken in the presence of methanol or 1,4-dioxane. KIE values of 2.1 and 2.3, respectively, indicate that the cleavage of the α-O sp3 C−H bond is the rate-determining step for this pathway (Scheme 6C, iii).
Conclusion
As a proof of concept, we demonstrate that γ radiation can drive Minisci-type CDC reactions without the need for metal or oxidant. Its substrate scope and functional group compatibility were further demonstrated on several representative alcohols, ethers, heteroarenes, and medicinally relevant compounds. Mechanistic experiments were conducted to illustrate that the nucleophilic α-oxyalkyl radicals generated from γ radiation attack the electron-deficient heteroarenes to provide the corresponding products. Considering its efficiency and good compatibility with various functional groups, coupled with the remarkable penetration of ionizing radiation, this radiation-induced approach of functionalizing heteroarenes may have broad and promising applications in the field of medicinal chemistry.
Data and code availability
All data supporting the findings of this study are included in the article and its supplemental information.
Acknowledgments
This study was funded by the Ministry of Science and Technology of the People's Republic of China (2021YFA1601400), the National Natural Science Foundation of China (22225603 and 22441051), the New Cornerstone Science Foundation (The XPLORER PRIZE) and Changping Laboratory to Z.L., and the National Nature Science Foundation of China (22306005) to B.-S.M.. We are thankful for facility support from the Analytical Instrumentation Center of Peking University. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Z.L., C.-J.L., and W.L. conceived and designed the project. W.L., Jiahao Li, and Jianbin Li performed most of the experiments with input from B.-S.M. and L.S. Z.T. and Y.X. contributed to parts of the liquid chromatography quantitative experiments. M.X. and X.-Y.C. contributed to the radiolabeling experiments of FAPI molecules. All authors contributed to writing the manuscript and approved the final version.
Declaration of interests
The authors declare no conflicts of interest.
Published Online: January 22, 2025
Footnotes
It can be found online at https://doi.org/10.1016/j.xinn.2025.100809.
Contributor Information
Chao-Jun Li, Email: cj.li@mcgill.ca.
Zhibo Liu, Email: zbliu@pku.edu.cn.
Lead contact website
Supplemental information
References
- 1.Raju T.N. The Nobel chronicles. 1964: Konrad Bloch (b 1912) and Feodor Lynen (1911-79) Lancet. 1999;354:347. doi: 10.1016/s0140-6736(05)75261-2. [DOI] [PubMed] [Google Scholar]
- 2.Cernak T., Dykstra K.D., Tyagarajan S., et al. The medicinal chemist's toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016;45:546–576. doi: 10.1039/c5cs00628g. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore D.C., Castro L., Churcher I., et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018;10:383–394. doi: 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- 4.Gerry C.J., Schreiber S.L. Chemical probes and drug leads from advances in synthetic planning and methodology. Nat. Rev. Drug Discov. 2018;17:333–352. doi: 10.1038/nrd.2018.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom J., Brown D.G., Young R.J., et al. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018;17:709–727. doi: 10.1038/nrd.2018.116. [DOI] [PubMed] [Google Scholar]
- 6.Mermer A., Keles T., Sirin Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021;114 doi: 10.1016/j.bioorg.2021.105076. [DOI] [PubMed] [Google Scholar]
- 7.Pratley C., Fenner S., Murphy J.A. Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev. 2022;122:8181–8260. doi: 10.1021/acs.chemrev.1c00831. [DOI] [PubMed] [Google Scholar]
- 8.Kerru N., Gummidi L., Maddila S., et al. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules. 2020;25 doi: 10.3390/molecules25081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian T., Li Z., Li C.-J. Cross-dehydrogenative coupling: a sustainable reaction for C−C bond formations. Green Chem. 2021;23:6789–6862. doi: 10.1039/d1gc01871j. [DOI] [Google Scholar]
- 10.Li C.-J. Cross-Dehydrogenative Coupling (CDC): Exploring C−C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 11.Colgan A.C., Proctor R.S.J., Gibson D.C., et al. Hydrogen Atom Transfer Driven Enantioselective Minisci Reaction of Alcohols. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202200266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X., Zhang H., Tang N., et al. Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)−H bonds. Nat. Commun. 2018;9:3343. doi: 10.1038/s41467-018-05522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Tong J., Zhu Y., et al. Electrochemical Minisci reaction via HAT-driven α-C(sp3)–H functionalization of alcohols. Green Chem. 2022;24:8406–8411. doi: 10.1039/d2gc03156f. [DOI] [Google Scholar]
- 14.Ciszewski Ł.W., Gryko D. Pyridine N-oxides as HAT reagents for photochemical C−H functionalization of electron-deficient heteroarenes. Chem. Commun. 2022;58:10576–10579. doi: 10.1039/d2cc03772f. [DOI] [PubMed] [Google Scholar]
- 15.Li D.-S., Liu T., Hong Y., et al. Stop-Flow Microtubing Reactor-Assisted Visible Light-Induced Hydrogen-Evolution Cross Coupling of Heteroarenes with C(sp3)–H Bonds. ACS Catal. 2022;12:4473–4480. doi: 10.1021/acscatal.2c01087. [DOI] [Google Scholar]
- 16.Wu M., Wu Z., Ang H.T., et al. Enhanced Reactivity of Acridinium Perchlorate: Harnessing Redox Mediators for Trace Chloride Activation in Hydrogen Atom Transfer Photocatalysis. ACS Catal. 2024;14:9364–9373. doi: 10.1021/acscatal.4c01910. [DOI] [Google Scholar]
- 17.Liu T., Li T., Tea Z.Y., et al. Modular assembly of arenes, ethylene and heteroarenes for the synthesis of 1,2-arylheteroaryl ethanes. Nat. Chem. 2024;16:1705–1714. doi: 10.1038/s41557-024-01560-7. [DOI] [PubMed] [Google Scholar]
- 18.Proctor R.S.J., Phipps R.J. Recent Advances in Minisci-Type Reactions. Angew. Chem. Int. Ed. 2019;58:13666–13699. doi: 10.1002/anie.201900977. [DOI] [PubMed] [Google Scholar]
- 19.Bhakat M., Biswas P., Dey J., et al. Heteroarylation of Ethers, Amides, and Alcohols with Light and O2. Org. Lett. 2021;23:6886–6890. doi: 10.1021/acs.orglett.1c02440. [DOI] [PubMed] [Google Scholar]
- 20.Twilton J., Le C., Zhang P., et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017;1 doi: 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- 21.Prier C.K., Rankic D.A., MacMillan D.W.C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagdi A.K., Rahman M., Bhattacherjee D., et al. Visible light promoted cross-dehydrogenative coupling: a decade update. Green Chem. 2020;22:6632–6681. doi: 10.1039/d0gc02437f. [DOI] [Google Scholar]
- 23.Ohmatsu K., Ooi T. Catalytic acceptorless dehydrogenative coupling mediated by photoinduced hydrogen-atom transfer. Nat. Synth. 2023;2:209–216. doi: 10.1038/s44160-022-00195-1. [DOI] [Google Scholar]
- 24.Faisca Phillips A.M., C. Guedes da Silva M.d.F., Pombeiro A.J.L. New Trends in Enantioselective Cross-Dehydrogenative Coupling. Catalysts. 2020;10:529. doi: 10.3390/catal10050529. [DOI] [Google Scholar]
- 25.Jin J., MacMillan D.W.C. Direct alpha-arylation of ethers through the combination of photoredox-mediated C−H functionalization and the Minisci reaction. Angew. Chem. Int. Ed. 2015;54:1565–1569. doi: 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasso J.D., Castillo-Pazos D.J., Li C.-J. Green chemistry meets medicinal chemistry: a perspective on modern metal-free late-stage functionalization reactions. Chem. Soc. Rev. 2021;50:10955–10982. doi: 10.1039/d1cs00380a. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.J., Xu H., Kong W.J., et al. Overcoming the limitations of directed C-H functionalizations of heterocycles. Nature. 2014;515:389–393. doi: 10.1038/nature13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu L., Liu J., Liang X.A., et al. Visible light-induced direct alpha C−H functionalization of alcohols. Nat. Commun. 2019;10:467. doi: 10.1038/s41467-019-08413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Börgel J., Ritter T. Late-Stage Functionalization. Chem. 2020;6:1877–1887. doi: 10.1016/j.chempr.2020.07.007. [DOI] [Google Scholar]
- 30.Caron S., Dugger R.W., Ruggeri S.G., et al. Large-Scale Oxidations in the Pharmaceutical Industry. Chem. Rev. 2006;106:2943–2989. doi: 10.1021/cr040679f. [DOI] [PubMed] [Google Scholar]
- 31.Le Caër S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water. 2011;3:235–253. doi: 10.3390/w3010235. [DOI] [Google Scholar]
- 32.Duan D., Han Y., Tu Z., et al. Gadolinium Neutron Capture Reaction-Induced Nucleodynamic Therapy Potentiates Antitumor Immunity. CCS Chem. 2023;5:2589–2602. doi: 10.31635/ccschem.023.202202488. [DOI] [Google Scholar]
- 33.Mu B.-S., Xu Y., Tu Z., et al. Radiocatalytic ammonia synthesis from nitrogen and water. Natl. Sci. Rev. 2024;11 doi: 10.1093/nsr/nwae302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mu B.-S., Zhang Y., Peng M., et al. Radiocatalytic Synthesis of Acetic Acid from CH4 and CO2. Angew. Chem. Int. Ed. 2024;63 doi: 10.1002/anie.202407443. [DOI] [PubMed] [Google Scholar]
- 35.Buxton G.V., Greenstock C.L., Helman W.P., et al. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution. J. Phys. Chem. Ref. Data. 1988;17:513–886. doi: 10.1063/1.555805. [DOI] [Google Scholar]
- 36.Mao S.W., Kevan L. Electron paramagnetic resonance studies of spin trapping of the primary neutral radicals formed in γ-irradiated methanol. Chem. Phys. Lett. 1974;24:505–507. [Google Scholar]
- 37.Roux J.C., Baquey C., Sutton J. The radiolysis of binary ethanol-water mixtures. Int. J. Radiat. Phys. Chem. 1973;5:309–322. [Google Scholar]
- 38.Getoff N., Ritter A., Schwörer F., et al. Primary yields of CH3·O and ·CH2OH radicals resulting in the radiolysis of high purity methanol. Radiat. Phys. Chem. 1993;41:797–801. [Google Scholar]
- 39.Pagsberg P., Munk J., Sillesen A., et al. UV Spectrun and Kinetics of Hydroxymethyl Radicals. Chem. Phys. Lett. 1988;146:7. [Google Scholar]
- 40.Wei Y., Zheng J., Ma L., et al. [18F]AlF-NOTA-FAPI-04: FAP-targeting specificity, biodistribution, and PET/CT imaging of various cancers. Eur. J. Nucl. Med. Mol. Imag. 2022;49:2761–2773. doi: 10.1007/s00259-022-05758-0. [DOI] [PubMed] [Google Scholar]
- 41.Kratochwil C., Flechsig P., Lindner T., et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu K., Li J., Wang L., et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm. Sin. B. 2022;12:867–875. doi: 10.1016/j.apsb.2021.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos S.S., Gonzaga R.V., Scarim C.B., et al. Drug/Lead Compound Hydroxymethylation as a Simple Approach to Enhance Pharmacodynamic and Pharmacokinetic Properties. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.734983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alizadeh E., Sanche L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012;112:5578–5602. doi: 10.1021/cr300063r. [DOI] [PubMed] [Google Scholar]
- 45.De Vleeschouwer F., Van Speybroeck V., Waroquier M., et al. Electrophilicity and nucleophilicity index for radicals. Org. Lett. 2007;9:2721–2724. doi: 10.1021/ol071038k. [DOI] [PubMed] [Google Scholar]
- 46.Parsaee F., Senarathna M.C., Kannangara P.B., et al. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 2021;5:486–499. doi: 10.1038/s41570-021-00284-3. [DOI] [PubMed] [Google Scholar]
- 47.Spinks J.W., Woods R.J. An introduction to radiation chemistry. 3rd Edition. 1990. pp. 10–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are included in the article and its supplemental information.