Abstract
Background
The recruitment-to-inflation (R/I) ratio is a new bedside tool with potential to evaluate lung recruitability by positive end-expiratory pressure (PEEP) but was only validated using pressure–volume curves and the optimal threshold separating low and high recruiters by PEEP is not precisely known. The aim of the study is to evaluate the diagnostic performance of the R/I ratio using computed tomography (CT) as a gold standard in patients with acute respiratory distress syndrome (ARDS).
Methods
This study is a single-center prospective observational study performed on adults ARDS patients under invasive mechanical ventilation. The R/I ratio was measured with the ventilator, and low-dose CT scans were acquired at PEEP 15 and 5 cmH2O to compute alveolar recruitment. Patients were classified as high recruiters if the recruited lung by PEEP was greater than 5% of lung weight on CT.
Results
Fifty patients were included, and eight patients were secondarily excluded (7/50 [14%] for suspected air leaks, and 1/50 [2%] for airway opening pressure > 15 cmH2O). Thirty-three patients [79%] were classified as low recruiters and 9 [21%] as high recruiters with CT.
The median R/I ratio was not significantly higher in high recruiters (0.67 [IQR: 0.57–0.74]), than in low recruiters (0.42 [IQR: 0.24–0.64]), p = 0.07. There was no significant correlation between the R/I ratio and the weight of the recruited lung on CT (R2 = 0.065; p = 0.10). The AUC of the R/I ratio to identify high recruiters was 0.70 (CI95%: [0.52–0.89]), with an optimal threshold amounting to 0.57. Sensitivity and specificity at this threshold were 78% [CI95%:44%-100%] and 67% [CI95%: 52%-82%], respectively. The false positive and false negative rates were 11/33 [33%] and 2/9 [22%], respectively, and all false negative patients had focal ARDS. Misclassification was mainly related to overestimation of PEEP volume by the ventilators, that was confirmed by a bench study for two out of three ventilators used in the study.
Conclusions
In a population of ARDS patients with low recruitment potential, the R/I ratio had a poor diagnostic performance to predict lung recruitability, but R/I ratio values below 0.57 reliably identified ARDS patients with low recruitability, provided they did not present focal ARDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-025-05453-0.
Keywords: Acute respiratory distress syndrome, Recruitment-to-inflation ratio, Computed tomography, Positive end expiratory pressure, Alveolar recruitment
Background
Acute respiratory distress syndrome (ARDS) management is based on mechanical ventilation with presumed protective settings using low tidal volume (VT), plateau pressure control below 30 cmH2O and positive end-expiratory pressure (PEEP) [1–3]. However, ARDS mortality remains high (between 40 and 50% [4]), and barotrauma is still observed in 10% to 15% of the patients [3, 5]. PEEP is a potentially modifiable factor independently associated with hospital mortality in observational studies [6]. However, randomized controlled trials have failed to identify whether lower or higher PEEP had unquestionable impact on ARDS mortality [7–9]. Personalization of PEEP setting using bedside tools such as standard respiratory mechanics or gas exchange variables is appealing, but their diagnostic performance is poor to identify lung recruitability by PEEP [10].
The recruitment-to-inflation ratio (R/I) ratio is a recent and ingenious tool [11] allowing evaluation of recruitability induced by PEEP at the bedside using standard intensive care unit (ICU) ventilators in ARDS patients. However, this technique has only been evaluated using pressure–volume curves as a reference and this may be problematic as the same physical variables (flow and pressure) are used with both techniques. Furthermore, the optimal threshold to identify lung recruitability has not been unambiguously addressed. As a consequence, the median R/I ratio value of the seminal study population (i.e., 0.50) is usually chosen to identify low or high recruiters by PEEP [11–13], making the measurement dependent to the case-mix of this seminal study. Furthermore, weak correlations between the R/I ratio and changes in lung aeration induced by PEEP assessed with lung ultrasound have been reported [14]. Finally, the R/I ratio computation is sensitive to air leaks [11] and adequate measurement of gas flow by ICU ventilators [15], and relies on the assumption of a linear pressure–volume relationship between PEEP 5 cmH2O (or airway opening pressure (AOP) if present) and PEEP 15 cmH2O [11].
Computed tomography (CT) has been used for decades to assess total regional lung volumes and aerated volumes in mechanically ventilated patients [16, 17]. Our group recently showed that intra and inter-observer measurements of lung aeration using CT are unbiased with a smallest detectable real difference lower than 10 mL in ARDS patients [18]. Taken together, these data suggests that CT is a precise and reliable method to assess regional aeration in ARDS patients.
We hypothesize that the study would confirm the reliability of the R/I ratio assessed on the ventilator (R/I ratioventilator) to identify high recruiters by PEEP and would help to identify a reliable threshold for the R/I ratioventilator to detect high recruiters.
Methods
Study aims
The primary aim of the study was to evaluate the diagnostic performance of the R/I ratioventilator to identify patients with high lung recruitability, using quantitative lung CT as a gold standard. The secondary aim of the study was to identify the optimal R/I ratioventilator threshold to detect high recruiters.
Study design and setting
We performed a single-center prospective observational study in an ICU located in an academic hospital. This study is an ancillary study of an ongoing prospective observational study (NCT06113276 registered on November 6 th, 2023). The study was conducted in accordance with the amended declaration of Helsinki and was approved by a local independent ethics committee (Comité Scientifique et Ethique des Hospices Civils de Lyon, #20_194). Consent for data utilization was obtained from the patients and/or their representatives. The study complies with the STARD criteria for reporting studies of diagnostic accuracy [19].
Patients
Eligible patients were consecutive patients aged 18 or older, under invasive mechanical ventilation, and ARDS according to the Berlin definition [20].
Exclusion criteria were ARDS or extracorporeal membrane oxygenation (ECMO) onset for more than 72 h, requirement for contrast agent injection during CT, no indication for CT according to the attending physician, contra-indication to transfer to the imaging facility (ratio of the arterial partial pressure of oxygen over inspired oxygen fraction (PaO2/FiO2) < 60 mmHg, mean arterial pressure < 65 mmHg, intracranial hypertension, or extreme obesity with body weight > 200 kg), inability to sustain a 10-s respiratory pause without respiratory effort, chronic obstructive pulmonary disease, pneumothorax or bronchopleural fistula, lung carcinoma, exacerbation of chronic interstitial pneumonia, presence of a chest metallic device resulting in artefacts within the lungs on CT, moribund patients, previous inclusion in present study, advanced directives to withhold or withdraw life-sustaining treatment, pregnancy, patient under a legal protective measure, opposition to study participation by patient or his/her relative.
Data collection
The following variables were recorded at inclusion: demographic and anthropometric data, date of ARDS onset, ARDS severity and risk factors, simplified acute physiology score (SAPS2 [21]) and SOFA [22] score, ICU ventilator brand, ventilatory settings, and arterial blood gas results.
Ventilatory settings at inclusion
Patients were ventilated with one of the following ventilators: EVITA-XL®, EVITA Infinity® V500 (Dräger, Lübeck, Germany), or CARESCAPE R860® (General Electric Healthcare, Madison, WI, USA). Non-ECMO patients were ventilated with VT 4 to 6 mL.kg−1 predicted body weight (PBW) [1] to keep plateau pressure of the respiratory system (PPlat,rs) < 30 cmH2O, with recommendation to use a PEEP-FiO2 table to adjust PEEP [1]. ECMO patients were ventilated in pressure-controlled mode targeting an airway driving pressure (ΔPrs) of 8 cmH2O, with PEEP adjusted to obtain a PPlat,rs between 20–25 cmH2O.
Respiratory mechanics measurements
Respiratory mechanics measurements were performed in the semi-recumbent position before patient transfer to the imaging facility. Total PEEP of the respiratory system (PEEPtot,rs) and PPlat,rs were measured at the end of 3-s end-expiratory and end-inspiratory pauses. In patients with an esophageal balloon already implanted (C7680U-Marquat, Boissy-St-Leger, France), the balloon was inflated with 2 mL of air and its proper position was assessed with an occlusion test [23], and the absence of esophageal pressure changes during gentle epigastric compressions. End-expiratory and end-inspiratory esophageal pressures were measured at the end of 3-s end-expiratory and end-inspiratory pauses. Transpulmonary total PEEP (PEEPtot,L) and transpulmonary plateau pressure (Pplat,L) were computed as airway pressures minus esophageal pressures. ΔPrs and transpulmonary driving pressure (ΔPL) were computed as PPlat,rs minus PEEPtot,rs, and PPlat,L minus PEEPtot,L, respectively. Respiratory system (Ers) and lung (EL) elastances were computed as ΔPrs and ΔPL divided by VT, respectively. The elastance of the chest wall was computed as Ers minus EL. Normalized Ers was computed as ΔPrs divided by VT expressed in ml.kg−1 PBW.
AOP and the R/I ratioventilator were measured as described by Chen et al. [11]. Briefly, the PEEP level was set to 15 cmH2O and the respiratory rate to 6 min−1 during 1 min without changing VT in volume-assisted control mode, and the expired VT was measured on the ventilator. PEEP was then set to 5 cmH2O during the inspiratory phase keeping the VT unchanged, and expired VT was measured on the ventilator immediately after PEEP decrease. Clinicians carefully checked meanwhile on the ventilator tracings that the pressure release to PEEP 5 cmH2O occurred immediately after inspiration, and PPlat,rs was subsequently measured at PEEP 5 cmH2O. A low-flow inflation curve was then performed on the ventilator, starting at PEEP 5 cmH2O and ending at 35 cmH2O or when reaching an inflation volume of 9 mL.kg−1 PBW whichever came first, using an inflation flow of 4L.min−1. AOP was identified as the pressure at which an abrupt change in slope occurred on the time-pressure curve displayed on the ventilator screen (after an initial phase of ultra-low compliance corresponding to the compliance of the respiratory circuit). In patients without occurrence of this phenomenon, AOP was set to the value of the set PEEP during the low-flow maneuver (i.e., 5 cmH2O). Patients with AOP > 15 cmH2O were secondarily excluded as this would make the R/I ratioventilator measurement unreliable.
Change in aerated lung volume between PEEP 5 and 15 cmH2O (PEEP volumeventilator) was computed as the difference between expired VT measured on the ventilator immediately after PEEP change from 15 to 5 cmH2O, and expired VT measured on the ventilator at PEEP 15 cmH2O.
Compliance of the respiratory system (Crs) at PEEP 5 cmH2O was computed as:
Predicted inflation of already aerated lung units at PEEP 5 cmH2O was computed as:
Recruitment assessed on the ventilator was computed as:
Compliance of the recruited lung was computed as:
Finally, the R/I ratioventilator was computed as:
CT measurements
Patients were switched to a transport ventilator (MONNAL T60—Air Liquide Medical Systems, Antony, France), using a Kocher clamp to transiently occlude the endotracheal tube to avoid lung derecruitment. Ventilator settings were kept unchanged during transport and imaging.
Low-dose CT acquisitions were performed in supine position with an iCT 256 or Ingenuity CT (Philips Healthcare, Eindhoven, The Netherlands) using the following settings: voltage 140 kVP, slice thickness 1 mm, matrix size 512 × 512. Field of view, pixel size and tube current–time product were adapted to each patient.
Four consecutive CT acquisitions were performed from lung apex to lung base during end-expiratory or end-inspiratory pauses: an end-expiratory and an end-inspiratory CT at the PEEP level set by the attending clinician, followed by an end-expiratory CT at PEEP 15 cmH2O and an end-expiratory CT at PEEP 5 cmH2O. The absence of respiratory efforts during the pauses was checked on the ventilator pressure–time curves. Image reconstruction was performed using a smooth filter (kernel B). The lungs were manually segmented using a CreaTools-based software [24], excluding pleural effusions, hilar and mediastinal structures. Segmented lung volumes were analyzed using dedicated MATLAB scripts (MathWorks, Natick, MA, USA).
Voxel tissue and gas fraction were computed as previously described [25, 26]. Tissue and gas volumes were computed as the product of their respective fractions multiplied by voxel volume multiplied by number of voxels within the segmented lung volume.
Lung voxels were classified into four compartments, according to their CT number: non-aerated (density between + 100 and − 100 Hounsfield units (HU)), poorly-aerated (density between − 101 and − 500 HU), normally-aerated (density between − 501 and − 900 HU), and hyper-aerated lung tissue (density ≤ − 901 HU). Total lung weight and weight of each compartment were estimated using lung tissue volume, assuming a tissue density of 1 g.mL−1 [16]. The weight of each compartment was standardized to total lung weight.
End-expiratory lung volume was computed on CT images acquired at end-expiration at both PEEP 5 (EELVCT,PEEP5) and 15 cmH2O (EELVCT,PEEP15) as the total volume of gas within the lungs.
Change in aerated lung volume between PEEP 5 and 15 cmH2O (PEEP volumeCT) was computed with CT as the difference between EELVCT,PEEP15 and EELV CT,PEEP5.
Lung tissue recruitment between PEEP 5 and 15 cmH2O was computed as the weight of the non-aerated compartment at PEEP 5 cmH2O minus its weight at PEEP 15 cmH2O and standardized to total lung weight [10]. Patients were classified as high recruiters if the recruited lung weight with a PEEP increase from 5 to 15 cmH2O was greater than 5% of total lung weight [18], as we recently showed that this was the minimum detectable difference in lung tissue recruitment with CT [18].
Three alternative methods to compute alveolar recruitment with CT were also assessed. Non- aerated and poorly-aerated tissue recruitment was computed as the weight of both non-aerated and poorly-aerated compartments at PEEP 5 cmH2O minus their weight at PEEP 15 cmH2O and standardized to total lung weight [10]. Gas recruitment was also computed with CT (ΔVrec,CT) using the method described by Paula et al. [27], which provides alveolar recruitment expressed in aerated volume. Finally, normalized gas recruitment was also computed as:
CT-measured inflation of already aerated units at PEEP5 was computed as the difference between PEEP volumeCT and ΔVrec,CT. Compliance of the recruited lung assessed with CT (Crec,CT) was computed as:
VT was assessed on CT (VTCT) by subtracting the volume of gas at end-inspiration and at end-expiration in segmented lungs. Patients in whom VT set on the ventilator differed from VTCT by more than 60 mL were secondarily excluded, as this is strongly evocative of air leaks that would make unreliable the measurement of the R/I ratioventilator.
Tidal hyperinflation was computed as the difference in hyper-aerated compartment volume at end-inspiration and end-expiration [28], and standardized to PBW.
Blinding
Assessors of the R/I ratioventilator and of lung recruitment with CT were blinded to the results of the other test.
Study endpoints
The primary endpoint of the study was the area under ROC curve (AUC) of the R/I ratioventilator to predict high recruitability identified with CT. The secondary endpoint was the optimal threshold of the R/I ratioventilator to predict high recruitability with CT.
Exploratory bench study
A bench study was conducted a posteriori to explore potential issues raised by the clinical study. The three ventilators used in the study were connected to an ASL 5000 breathing simulator (Ingmar Medical, Pittsburgh, PA, USA), after ventilator sensor calibration as recommended by manufacturers, and careful check of lack of leak. A proximal flow sensor (model 260,177, Hamilton medical, Bonaduz, Switzerland) and a proximal pressure sensor (Meritrans DTXPlus, Merit Medical Systems, South Jordan, UT, USA) were added at the Y-Piece level, and connected to an A/D card (MP 100, Biopac Systems, Santa Barbara, CA, USA). Signals were sampled at 200 Herz and analyzed with Acknowledge® software (Biopac Systems, Santa Barbara, CA, USA). External flow sensor was calibrated under NTPS condition with ten strokes of a 3-L super-syringe connected to the flow sensor, the pressure sensor and to an endotracheal tube, to correct for non-linear flow response using a 3rd order polynomial model [29], and for gas compression [29]. Volume was computed by integration of the flow signal.
Inspiratory and expiratory airway resistances were set at 10 cmH2O.L−1.s on the breathing simulator, and compliance was successively set at 10, 20, 40, 60 and 80 ml.cmH2O−1. The ventilators were set with the following settings: VT 400 ml, PEEP 15 cmH2O, respiratory rate 10 min−1, inspiratory flow 60 L.min−1, and inspiratory-to-expiratory ratio 1/2. In each experimental condition, and with each ventilator and the proximal flow sensor, the following measurements were performed in triplicate: expired VT at PEEP 15 cmH2O, expired VT during PEEP decrease from 15 to 5 cmH2O, and compliance at PEEP 5 cmH2O to mimic measurements performed to compute the R/I ratioventilator. PEEPvolume was computed as the difference between expired VT at PEEP 5 and 15 cmH2O.
Sample size and statistical analysis
We estimated that with a sample size of at least 17 patients with high lung recruitability on CT, the study would provide at worst a ± 0.15 precision for the CI95% of the AUC of the R/I ratioventilator, assuming an AUC of at least 0.8 and with a two-tailed hypothesis.
Statistical analyses were performed using R version 4.3.1 software [30] with the following packages: pROC [31], bootLR [32], sampling [33], cutpointr [34], and MethComp [35]. Median [interquartile range] and counts [percentages] are reported for quantitative and categorical variables, respectively. A p value below 0.05 was chosen for statistical significance.
Comparisons between groups of patients were performed with the Fisher’s exact test for categorical variables, and with the Wilcoxon rank sum test or the Kruskall-Wallis test for both continuous and ordinal variables. The statistical significance of the linear models was assessed with the F-test. Bias and limits of agreement were computed using the Bland and Altman technique [36]. In case of non-constant bias, bias and limits of agreement were computed from the posterior medians of a Monte-Carlo chain simulation [37].
Diagnostic performance of the R/I ratioventilator to predict high lung recruitability was assessed by computation of the AUC. CI95% for AUC was computed using the Delong method. The optimal cut-off point was computed by maximizing the Youden index. CI95% for optimal cut-off points, sensitivity and specificity were computed using bootstrapping and 1000 replicates.
Results
Population characteristics at inclusion
The study flow chart is presented in additional file 1. Fifty patients were included in the study between January 4, 2022, and November 12, 2024. The study was stopped prematurely, because of a low rate of high recruiters (see below). Eight patients were secondarily excluded (7/50 [14%] for suspected air leaks, and 1/50 [2%] for AOP > 15 cmH2O), leaving 42 patients for subsequent analyses. Patients secondarily excluded from the study presented with significantly higher PEEP, PPlat,rs and ΔPrs, as compared to included patients (additional file 2). No adverse events were reported when performing the R/I ratioventilator or CT scans.
Clinical characteristics on the day of inclusion are provided in Table 1, respiratory measurements and arterial blood gas in Table 2, and CT variables in Table 3. AOP amounted to 6 [5 – 7] cmH2O, and 25/42 patients [60%] presented an AOP greater than 5 cmH2O (additional file 3). The median R/I ratioventilator amounted to 0.45 [0.26–0.67], ranging from −0.59 to 1.33, with 3 patients/42 [7%] presenting negative values.
Table 1.
Patients’ characteristics on the day of inclusion
| Variables | Entire population (n = 42) | Low recruiters (n = 33) | High recruiters (n = 9) | p-value |
|---|---|---|---|---|
| Age – year | 61 [51–71] | 60 [50–70] | 65 [55–72] | NS |
| Sex male – no. (%) | 29 (69%) | 23 (70%) | 6 (67%) | NS |
| BMI – kg/m2 | 28 [25–32] | 27 [24–31] | 30 [26–32] | NS |
| SAPS2 score | 51 [41–61] | 50 [40–59] | 60 [49–67] | NS |
| SOFA score | 10 [7–12] | 10 [7–12] | 10 [8–12] | NS |
| Elapsed time from ARDS onset—day | 1 [0–2] | 2 [1, 2] | 0 [0–1] | < 0.01 |
| COVID-19 pneumonia – no. (%) | 10 (24%) | 6 (18%) | 4 (44%) | NS |
| ARDS risk factors – no. (%) † | NS | |||
| • Pneumonia | 33 (79%) | 26 (79%) | 7 (78%) | – |
| • Aspiration | 7 (17%) | 6 (18%) | 1 (11%) | – |
| • Abdominal sepsis | 3 (7%) | 3 (9%) | 0 (0%) | – |
| • Pancreatitis | 1 (2%) | 0 (0%) | 1 (11%) | –- |
| • Drowning | 2 (5%) | 2 (6%) | 0 (0%) | – |
| Immunodepression – no. (%) | 11 (26%) | 9 (27%) | 2 (22%) | NS |
| ARDS severity | NS | |||
|
• Mild • Moderate |
1 (2%) 12 (29%) |
1 (3%) 12 (36%) |
0 (0%) 0 (0%) |
– – |
| • Severe | 29 (69%) | 20 (61%) | 9 (100%) | – |
| Prone position * – no. (%) | 26 (62%) | 22 (67%) | 4 (44%) | NS |
| iNO * – no. (%) | 10 (24%) | 6 (18%) | 4 (44%) | NS |
| NMBA * – no. (%) | 39 (93%) | 30 (91%) | 9 (100%) | NS |
| ECMO – no. (%) | 2 (5%) | 2 (6%) | 0 (0%) | NS |
| RRT * – no. (%) | 5 (12%) | 4 (12%) | 1 (11%) | NS |
| Inotrope * – no. (%) | 4 (10%) | 2 (6%) | 2 (22%) | NS |
| Vasopressor * – no. (%) | 36 (86%) | 30 (91%) | 6 (67%) | NS |
Values are median [1st quartile; 3rd quartile], and count (percentage). Low and high recruiters were classified using computed tomography
†Total percentage exceeds 100% since multiple risk factors could be present in each patient
*in the 24 h preceding inclusion
ARDS denotes acute respiratory distress syndrome; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; iNO, inhaled nitric oxide; NMBA, neuromuscular blocking agents; NS, non-statistically significant; RRT, renal replacement therapy; and SAPS2, simplified acute physiology score 2
Bold p-values denotes statistical significance
Table 2.
Respiratory mechanics and arterial blood gas on the day of inclusion
| Variables | Entire population n = 42 | Low recruiters n = 33 | High recruiters n = 9 | p-value |
|---|---|---|---|---|
| ICU Ventilator † | NS | |||
| • EVITA XL | 21 (51%) | 16 (50%) | 5 (56%) | |
| • EVITA Infinity V500 | 13 (32%) | 10 (31%) | 3 (33%) | |
| • CARESCAPE R860 | 7 (17%) | 6 (19%) | 1 (11%) | |
| VT – mL.kg−1 PBW | 6.0 [5.9–6.0] | 6.0 [5.9–6.0] | 5.9 [5.9–6.0] | < 0.05 |
| RR – min−1 | 22 [20–28] | 22 [20–28] | 25 [20–30] | NS |
| PEEP – cmH2O | 5 [5–10] | 5 [5–8] | 10 [5–10] | NS |
| Pplat,rs – cmH2O | 17 [15–20] | 17 [15–20] | 19 [17–21] | NS |
| ΔPrs – cmH2O | 9 [8–11] | 9 [8–11] | 9 [8–10] | NS |
| Peso,end-expiratory – cmH2O | 9 [6–12] | 9 [6–11] | 13 [9–16] | < 0.05 |
| Ers – cmH2O.L−1 | 24 [20–31] | 24 [20–30] | 23 [20–32] | NS |
| Normalized Ers – cmH2O.mL−1.kg−1 PBW | 1.53 [1.35–1.86] | 1.54 [1.35–1.87] | 1.53 [1.43–1.68] | NS |
| EL – cmH2O.L−1 ‡ | 19 [12–24] | 18 [12–24] | 20 [11–26] | NS |
| ECW – cmH2O.L−1 | | 5 [3–7] | 4 [3–6] | 7 [6, 7] | NS |
| EL/Ers || | 0.80 [0.67–0.88] | 0.80 [0.68–0.89] | 0.80 [0.63–0.80] | NS |
| R/I ratioventilator | 0.45 [0.26–0.67] | 0.42 [0.24–0.64] | 0.67 [0.57–0.74] | NS |
| AOP – cmH2O | 6 [5–7] | 6 [5–7] | 5 [5, 6] | NS |
| AOP > 5 cmH2O | 25 (60%) | 22 (67%) | 3 (33%) | NS |
| PEEP volumeventilator – mL | 582 [474–706] | 593 [480–707] | 523 [349–681] | NS |
| pH | 7.36 [7.26–7.41] | 7.38 [7.32–7.41] | 7.22 [7.18–7.30] | < 0.01 |
| PaCO2 – mmHg | 43 [36–50] | 42 [35–49] | 50 [41–56] | NS |
| PaO2/FiO2 – mmHg * | 101 [89–165] | 104 [91–175] | 89 [84–93] | < 0.05 |
| Lactate – mmol.L−1 | 1.8 [1.4–2.6] | 1.7 [1.4–2.2] | 2.5 [1.5–4.5] | NS |
Values are median [1st quartile; 3rd quartile], and count (percentage). Low and high recruiters were classified using computed tomography
AOP denotes airway opening pressure; ΔPrs, driving pressure of the respiratory system; ECW, chest wall elastance; EL, lung elastance; Ers, elastance of the respiratory system; ICU, intensive care unit; NS, non-statistically significant; PaCO2,carbon dioxide partial pressure in arterial blood; PaO2/FiO2, ratio of the partial pressure of oxygen in arterial blood over the inspired fraction of oxygen; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PEEP volumeventilator, change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with the ventilator; Peso,end-expiratory, end-expiratory esophageal pressure; Pplat,rs, plateau pressure of the respiratory system; R/I ratioventilator, recruitment-to-inflation ratio assessed with the ventilator; RR, respiratory rate; and VT, tidal volume
† ICU ventilator brand was missing in 1 patients of the low recruiters group
‡ lung elastance was missing in 3 patients of the low recruiters group
| chest wall elastance was missing in 3 patients of the low recruiters group
|| EL/Ers was missing in 3 patients of the low recruiters group
* or ratio of PaO2 over fraction of oxygen of sweep gas flow on ECMO membrane in ECMO patients
Bold p-values denotes statistical significance
Table 3.
Computed tomography variables
| Variables | Entire population n = 42 | Low recruiters n = 33 | High recruiters n = 9 | p-value |
|---|---|---|---|---|
| Delay between respiratory mechanics assessment and CT – min* | 61 [45–109] | 61 [45–107] | 80 [41–109] | NS |
| ARDS morphology | NS | |||
| • Focal | 13 (31%) | 11 (33%) | 2 (22%) | |
| • Non focal | 29 (69%) | 22 (67%) | 7 (78%) | |
| Lung weight – g | 1,637 [1,354–1,972] | 1,608 [1,349–1,974] | 1,666 [1,400–1,874] | NS |
| VTCT – mL | 380 [319–425] | 394 [321–427] | 376 [300–421] | NS |
| Non-aerated lung at PEEP 5 cmH2O – % total lung weight | 51 [42–60] | 51 [38–57] | 58 [51–60] | NS |
| Poorly-aerated lung at PEEP 5 cmH2O – % total lung weight | 22 [15–31] | 19 [15–30] | 26 [21–31] | NS |
| Normally-aerated lung at PEEP 5 cmH2O – % total lung weight | 25 [15–34] | 30 [17–36] | 16 [10–18] | < 0.01 |
| Hyper-aerated lung at PEEP 5 cmH2O – % total lung weight | 0.1 [0.0–0.5] | 0.1 [0.0–0.6] | 0.0 [0.0–0.1] | NS |
| EELVCT at PEEP 5 cmH2O – mL | 1,253 [750–1,780] | 1,568 [835–1,871] | 861 [459–993] | NS |
| EELVCT at PEEP 15 cmH2O – mL | 1,777 [1,112–2,339] | 1,990 [1,125–2,555] | 1,444 [822–1,653] | NS |
| PEEP volumeCT – mL | 501 [321–581] | 484 [309–580] | 532 [363–590] | NS |
| Recruited lung weight – % total lung weight | 3 [1–4] | 2 [1–3] | 8 [7–9] | < 0.001 |
| Recruited lung volume – mL | 74 [34–99] | 44 [23–80] | 136 [99–170] | < 0.001 |
| Tidal hyperinflation – mL.kg−1 PBW | 1.01 [0.20–2.19] | 1.09 [0.24–2.25] | 0.45 [0.18–1.74] | NS |
Values are median [1st quartile; 3rd quartile], and count (percentage). Low and high recruiters were classified using computed tomography
ARDS denotes acute respiratory distress syndrome; CT, computed tomography; EELVCT, end-expiratory lung volume; NS, non-statistically significant; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PEEP volumeCT, change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with CT; and VTCT, tidal volume measured on computed tomography
* Delay between respiratory mechanics assessment and CT was missing in 1 patients of the low recruiters group
Bold p-values denotes statistical significance
Comparison between low and high recruiters (Table 1)
Thirty-three patients [79%] were classified as low recruiters and 9 [21%] as high recruiters on CT. There was no significant difference between groups, except that time from ARDS onset was significantly lower, VT was significantly lower (although marginally), end-expiratory esophageal pressure significantly higher, and both pH and PaO2/FiO2 significantly lower in the high recruiters group (Table 2). The R/I ratioventilator of high recruiters was non-significantly higher than the R/I ratioventilator of low recruiters (0.67 [0.57–0.74] vs. 0.42 [0.24–0.64]; p = 0.07; Table 2 and additional file 4).
Relationships between respiratory mechanics variables and CT-derived variables
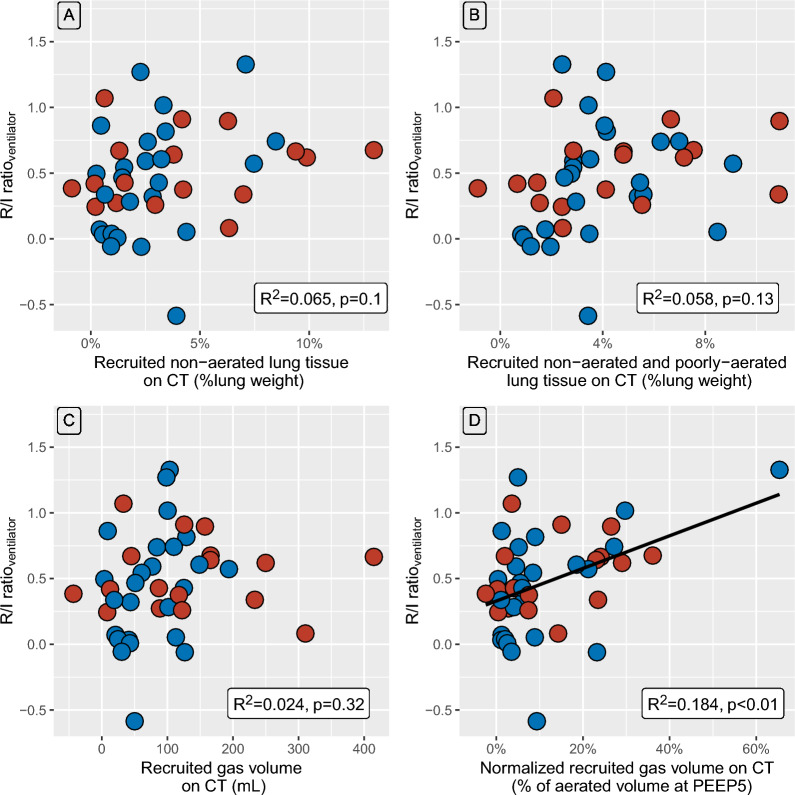
There was no statistically significant correlation between the R/I ratioventilator and the weight of the recruited non-aerated lung tissue on CT (Fig. 1A), or the weight of both non-aerated and poorly-aerated recruited lung tissue (Fig. 1B), or the recruited gas volume on CT (Fig. 1C). A slight correlation was identified between the R/I ratioventilator and normalized recruited gas volume on CT (R2 = 0.184; p < 0.01; Fig. 1D). Recruited tissue weight and recruited gas volume were strongly correlated (R2 = 0.599, p < 0.001, additional file 5).
Fig. 1.
Relationships between respiratory mechanics variables and CT-derived variables. Data points are individual values. Blue and red circles represent patients with or without airway opening pressure greater than 5 cm H2O, respectively, Black line is regression line. Panel A represents the relationship between R/I ratioventilator and recruited non-aerated lung tissue weight assessed on computed tomography. Panel B represents the relationship between R/I ratioventilator and both non-aerated and poorly aerated recruited lung tissue weight assessed on computed tomography. Panel C represents the relationship between R/I ratioventilator and recruited gas volume assessed on computed tomography. Panel D represents the relationship between R/I ratioventilator and normalized recruited gas volume assessed on computed tomography. CT denotes computed tomography; PEEP, positive end-expiratory pressure; R2, coefficient of determination of the linear regression; and R/I ratioventilator, recruitment-to-inflation ratio assessed with the ventilator
Diagnostic performance of the R/I ratioventilator to identify high recruiters
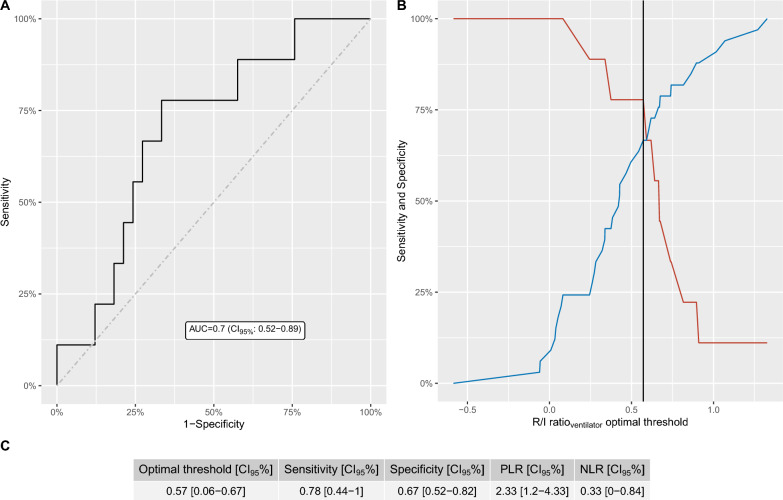
As presented in Fig. 2, the AUC of the R/I ratioventilator to identify high recruiters amounted to 0.70 (CI95%: [0.52–0.89]). The optimal threshold of the R/I ratioventilator to identify patients with high recruitability amounted to 0.57 [CI95%: 0.06–0.67]. Sensitivity and specificity at this threshold were 78% [CI95%: 44% – 100%] and 67% [CI95%: 52% – 82%], respectively (Fig. 2C). The false positive and false negative rates using the threshold of 0.57 were 11/33 [33%] and 2/9 [22%], respectively. Hyperinflation on CT as a function of true/false positive and true/false negative patients regarding recruitability assessed with the R/ratioventilator is provided in additional file 6.
Fig. 2.
Diagnostic performance of the recruitment-to-inflation ratio assessed with the ventilator to identify lung recruitability. Left panel: ROC curve (bold line), line of no-discrimination (dashed line) and area under the ROC curve (with its 95% confidence interval). Right panel: sensibility and specificity of the R/I ratioventilator as a function of the chosen threshold to identify high recruiters. Red and blue lines represent sensitivity and specificity, respectively. The black vertical line represents the optimal threshold assessed by the Youden index. AUC denotes area under the ROC curve; CI95%, 95% confidence interval.; and R/I ratioventilator the recruitment-to-inflation ratio assessed with the ventilator
Sources of bias related to R/I ratio components
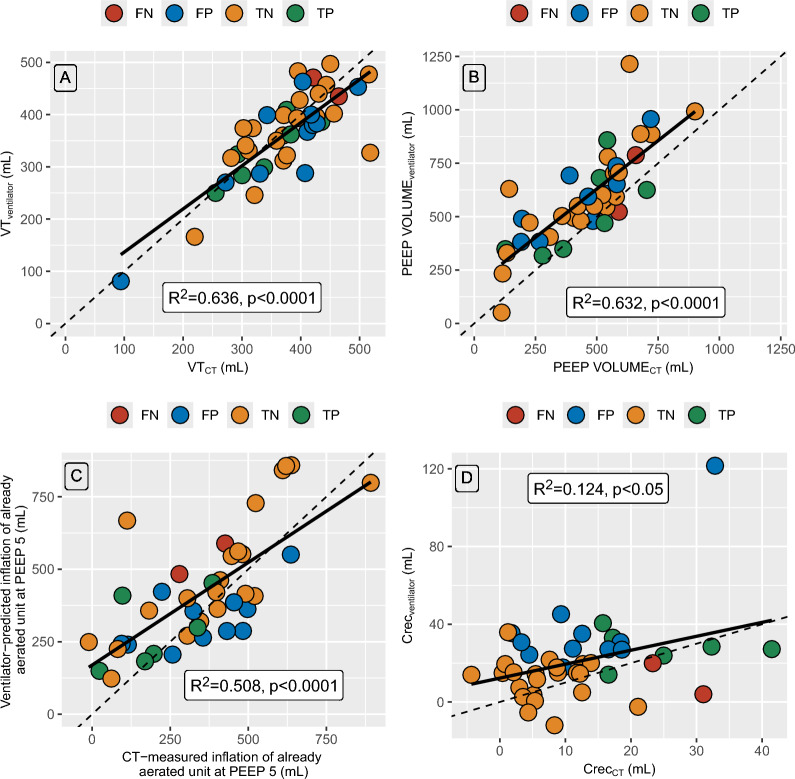
To investigate which component of the R/I ratioventilator was associated with misclassification compared to CT, we studied their relationships with their CT counterpart (Fig. 3). VT measured on the ventilator and with CT were strongly correlated (R2 = 0.636; p < 0.0001; Fig. 3A), with a bias amounting to − 11 ml (additional file 7). PEEP volumeventilator and PEEP volumeCT were also strongly correlated (R2 = 0.632; p < 0.0001; Fig. 3B), although PEEP volumeventilator systematically overestimated PEEP volumeCT with a bias amounting to 134 mL (additional file 7). The correlation between ventilator-predicted and CT-measured increase in lung volume of already aerated lung unit at PEEP 5 cmH2O was lower (R2 = 0.508; p < 0.0001), and further decreased when considering compliance of the recruited lung assessed with the ventilator or CT (R2 = 0.124; p < 0.05).
Fig. 3.
Relationships between each component of the R/I ratioventilator with its CT counterpart. Panel A. Tidal volume assessed on the ventilator vs. tidal volume assessed with CT Panel B. PEEP volume assessed on the ventilator vs. PEEP volume assessed with CT Panel C. Ventilator-predicted vs. CT measured inflation of already aerated lung units at PEEP 5 cmH2O. Panel D. Compliance of the recruited lung assessed by the ventilator and CT. Symbols are individual data points. Red symbols denote patients whose R/I ratioventilator was deemed as false negative by CT, blue symbols patients deemed as false positive, yellow patients deemed as true negative, and red symbols patients deemed as true positive. Continuous black lines are regression lines, and broken lines are lines of identity. CrecCT denotes compliance of the recruited lung assessed by CT, Crecventilator, compliance of the recruited lung assessed with the ventilator; CT, computed tomography; FN, false negative; FP, false positive; PEEP, positive end-expiratory pressure; PEEP volumeCT, change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with CT; PEEP volumeventilator, change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with the ventilator; R2, coefficient of determination; R/I ratioventilator, recruitment-to-inflation ratio assessed with the ventilator; and VT, tidal volume
Most patients considered as false positive with the R/I ratioventilator had their predicted increase in lung volume of already aerated lung units at PEEP 5 cmH2O underestimated by the ventilator, and their compliance of the recruited lung overestimated. There was no significant difference between the false positive and the true negative patients regarding any clinical, biological or CT variables (additional file 8).
Regarding the 2 false negative patients, both had focal ARDS, and they also differed from the 7 true positive patients by a lower amount of non-inflated lung and a higher amount of normally-inflated lung at both PEEP 5 and 15 cmH2O, explaining why their R/I ratioventilator was low as both recruitment and substantial inflation occurred simultaneously (additional file 9). CT scans of 4 representative patients are provided in Fig. 4, along with their corresponding R/I ratioventilator values.
Fig. 4.
CT images of four representative patients. Panel A to D display CT scans of four representative patients (2 high recruiters (panels A and B), and 2 low recruiters (panels C and D). Each panel represents CT images acquired at PEEP 5 cmH2O (first two columns) and 15 cmH2O (last two columns). At each PEEP level are presented on the left 3 raw CT images obtained at the aortic arch level, at the carina level and immediately above the diaphragmatic cupolas, and on the right corresponding parametric images with a color code depending on the voxel CT number defining four class of aeration. Each panel is associated with the classification status with the R/I ratio regarding lung recruitability (i.e., true positive, false positive, false negative, true negative), the R/I ratio measured with the ventilator and the amount of recruited lung assessed with CT. CT denotes computed tomography; PEEP, positive end-expiratory pressure; R/I ratio, recruitment-to-inflation ratio assessed with the ventilator; and TLW total lung weight
Exploratory sensitivity analyses
A first sensitivity analysis was conducted to identify whether alternative CT thresholds to define high recruiters could modify the study primary endpoint. Varying the CT threshold between 2.5 and 10% of total lung weight led to similar AUC values (Fig. 5). Another sensitivity analysis was performed to assess the diagnostic performance of the R/I ratioventilator using alternate definitions of recruitment with CT and yielded similar results (additional file 10). Finally, excluding the patient with a R/I ratioventilator below −0.5 (additional file 11), or excluding ECMO patients (additional file 12) yielded similar results.
Fig. 5.

Impact of CT threshold defining high recruiters on the diagnostic performance of the R/I ratioventilator. The dotted line represents the threshold used to define high recruitability with CT (i.e., 5% of total lung weight). AUC is the area under the ROC curve of the R/I ratioventilator at various CT thresholds. CT denotes computed tomography; and R/I ratioventilator, recruitment-to-inflation ratio assessed with the ventilator
Bench study
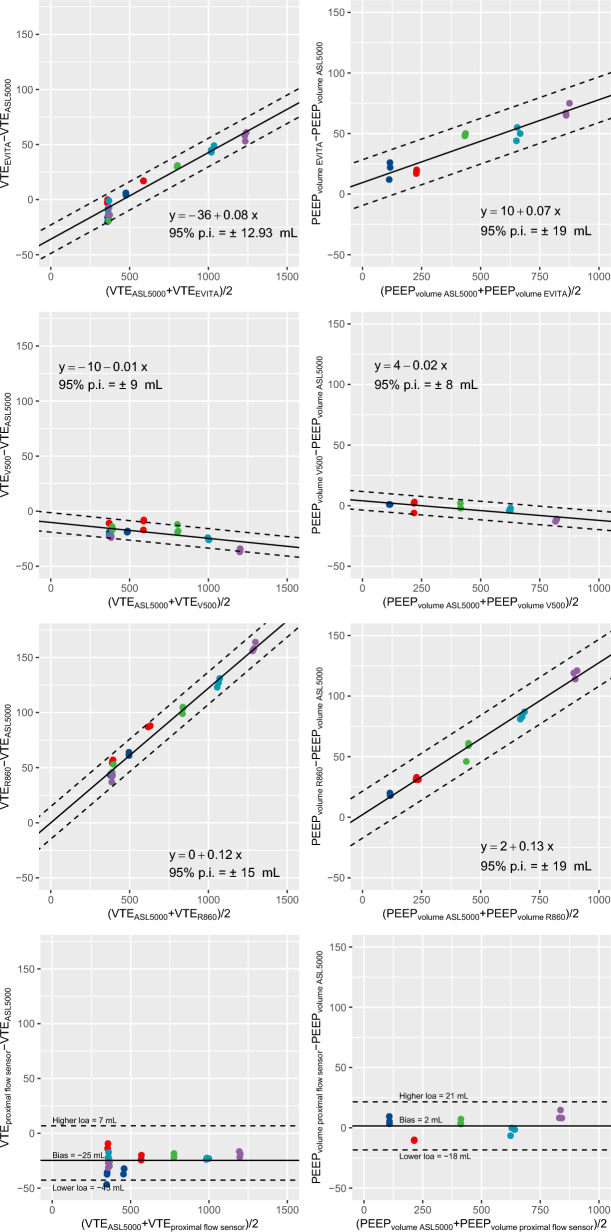
Two of the ventilators used in the study (EVITA-XL and CARESCAPE R860) overestimated expired VT, with a bias increasing at higher VT values (Fig. 6). Consequently, they overestimated PEEP volumeventilator with an increasing bias at higher PEEP volumeventilator values. The EVITA Infinity V500 ventilator and the Hamilton proximal flow sensor slightly underestimated expired VT, but their estimation of PEEPvolume was virtually unbiased (Fig. 6).
Fig. 6.
Bias and limits of agreement of expired VT and PEEP volume during the bench study. Left panels: expired tidal volume. Right panels: PEEP volume. Each symbol represents a concomitant measurement of expired tidal volume or PEEP volume measured with each of the three ventilators of the study and a proximal flow sensor, and the concomitant reference value measured on the breathing simulator. Continuous line and the two broken lines are the mean bias and 95% prediction interval limits of the bias between methods, respectively. Symbol color denotes compliance set on the breathing simulator at which tidal volumes were measured: compliance 10 ml.cmH2O−1 (dark blue), compliance 20 ml.cmH2O−1 (red), compliance 40 ml.cmH2O−1 (green), compliance 60 ml.cmH2O−1 (cyan), compliance 80 ml.cmH2O−1 (violet). 95% p.i. denotes 95% prediction interval of the bias between methods; ASL500, breathing simulator ASL 500; EVITA-XL, EVITA-XL ventilator; loa, limit of agreement; PEEP volume, change in aerated volume between PEEP 5 and 15 cmH2O; R860, CARESCAPE R860 ventilator; V500, Infinity V500 ventilator; VTE, expired tidal volume
Discussion
The main findings of the present study are the following: 1- the diagnostic performance of the R/I/ratio was poor to identify high recruiters, with an optimal threshold amounting to 0.57; 2- however, lower values of R/I ratio have an acceptable diagnostic performance for the identification of low recruiters; 3- the main reason for misclassification of recruitability by the R/I ratio was related to a relatively high rate of false positive (i.e., 33%), in relation with an overestimation of the PEEP volume by the ventilators, that was confirmed by a bench study for two out of three ventilators used in the present study.
External and internal validity
The non-aerated lung on CT at PEEP 5 amounted to approximately 50% of total lung weight in the present study, and was substantially higher than in the landmark study by Gattinoni’ s group [10], but similar to a large recent study on patients with similar ARDS severity published by the same group [38]. The large amount of derecruitment on CT in our study may be a consequence of the relatively low PEEP level at inclusion, and we cannot exclude that it may have impacted lung recruitability assessment by CT or by the R/I ratio, as recruitment is a time-dependent phenomenon [39].
The rate of focal ARDS in the present work was similar to previous studies, in which this pattern was observed in roughly one third of ARDS patients [40, 41]. Although focal ARDS are deemed less likely to be recruitable, 2/13 [15%] of the focal ARDS patients were high recruiters in our study, a value similar to the 22% rate of high recruiters observed in focal ARDS patients of a recent study on 168 ARDS patients [41]. However, the overall rate of high recruiters was substantially lower in our study (9/42 [21%], as compared to previous studies from Gattinoni’ s group using CT [10, 41] and methodological differences may explain this finding. While Gattinoni’ s group classified high recruiters by change of non-aerated lung weight between PEEP 5 and 45 cmH2O using the group median value as a threshold, we used a fixed threshold computed from the change of non-aerated lung weight between PEEP 5 and 15 cmH2O. This CT-derived recruitment threshold to identify high recruiters may be debatable, as this was derived from a previous study from our group [18], in which the 5% of total lung weight value was identified as the least significant change above measurement error. However, this cut-off remains congruent with the results of Gattinoni’ s group, who observed that the median recruitment was 9% of total lung weight, with 50% of the recruitment achieved between PEEP increase from 5 to 15 cmH2O (i.e. 4.5% of total lung weight) [10]. Finally, choosing alternate thresholds did not substantially modify the results of our study (Fig. 5). However, we cannot exclude that the lack of CT acquisition at PEEP 45 cmH2O may have contributed to the low rate of high recruiters in our study, although it should be noted that the R/I ratio was computed on the same PEEP range as recruitability assessed with CT in our study (i.e., 5 to 15 cmH2O).
The range of the R/I ratio in our study is similar to the one observed in the seminal study by Chen et al., while the rate of severe ARDS was substantially higher in the present study [11]. Three patients [7%] of the present study presented with negative values of R/I ratio, while this phenomenon was only observed in one patient [2%] in the study by Chen et al. [11]. This phenomenon occurs when the measured change in end-expiratory lung volume during PEEP decrease is less than the predicted one and is related to the mathematical coupling between recruitment and inflation components of the R/I ratioventilator. Aside from different case-mix and experimental setup, different ventilators were used in both studies and several factors may be involved in the occurrence of this phenomenon (see below). However, all three patients with a negative R/I ratio in our study were characterized as low recruiters on CT as shown in additional file 4, and hence have no deleterious impact on the computation of the diagnostic performance of the R/I ratio to identify high recruiters in our study.
Regarding the poor correlation between the R/I ratio and all the 4 methods assessing recruitment on CT (whether they were tissue-based, gas-based, with or without inclusion of poorly-inflated lung in their calculation), our results are strikingly different from those of Murgolo et al., who observed a strong correlation between the R/I ratio and both tissue and gas recruitment on CT in a highly recruitable model of experimental ARDS [42]. On the other hand, our findings are in line with Chiumello et al., who showed in ARDS patients, that recruitment measured by CT expressed either tissue-based, gas based, with or without inclusion of poorly-inflated lung, was unrelated to the recruitment measured using respiratory mechanics [43]. Manual delineation of both non- and poorly aerated lung [44] is an alternative, yet time-consuming, method to quantify recruitment on CT which may have yielded different results.
Finally, Del Sorbo et al., by computing a R/I ratio using computed tomography (using the same assumptions and computations as the “classical” R/I ratio) also identified a weak correlation between this parameter and PEEP-induced recruitment assessed with CT [45]. This poor correlation is however expected as the R/I ratio assesses the trade-off between recruitment and inflation (and hyperinflation), and hence is not only related to alveolar recruitment. Regarding the R/I ratio optimal threshold identified in the present study to classify patients as high recruiters, its value is relatively similar to the median R/I ratio value identified in the study by Chen et al. (0.57 in the former vs. 0.50 in the latter) [11], but substantially lower than the median R/I ratio (0.7) identified by Stevic et al. in a population of COVID-19 patients [14].
Factors involved in the poor diagnostic performance of the R/I ratio
Falsely negative R/I ratio occurred in 2/9 high recruiters patients, both being classified as focal ARDS, and their CT characteristics regarding lung aeration compartment provided evidence of the underlying mechanism. Indeed, high volume of the normally-aerated lung compartment at PEEP 5 cmH2O led to a substantial increase in this compartment’s inflation at higher PEEP (as its starting volume at PEEP 5 cmH2O was high), and led to low R/I ratio values despite high concomitant recruitment.
Regarding the high rate of false positives, our results are not unexpected as Cour et al. recently showed in a bench study that modern ICU ventilators (including those used in the present study) systematically overestimated R/I ratio when R/I ratio was set to 0, and most still overestimated R/I ratio for a R/I ratio set to 0.5 [15]. Several factors involved in the R/I ratio computation may explain this finding. First, an inability of the ventilator to measure expired VT at higher PEEP is unlikely, as VT measured on the ventilator and VT measured on CT were close to the line of identity (Fig. 3A). Second, an error in the computation of PEEP volume by the ventilator as compared to its CT counterpart is evident from our clinical data, as the former was systematically overestimated by 134 ml. As PEEP volumeventilator is computed as the difference between expired VT at PEEP 15 and PEEP 5 cmH2O, and as expired VT at PEEP 15 were probably unbiased (see above), overestimation of PEEP volumeventilator is a consequence of an overestimation of expired VT during PEEP decrease from 15 to 5 cmH2O. This was confirmed in our bench study in which two of the ventilators used in the present clinical study exhibited substantial increasing bias at higher tidal volumes (such as the ones measured during PEEP release during R/I ratioventilator computation). Interestingly, the use of an external flow sensor with a proper calibration to account for gas compression and non-linear response of the sensor at higher flow was able to provide unbiased estimates of PEEP volumeventilator. Third, an error in the computation of the predicted inflation of already aerated units at PEEP 5 cmH2O is also suspected from our data, as most false positive patients had a slight underestimation of this volume as compared to its measured CT counterpart. This may be related to an error during plateau pressure measurement at PEEP 5 cmH2O (operator related- or pressure sensors-related) or may be related to the lack of linearity of the pressure–volume curve between PEEP 5 (or AOP if present) and PEEP 15 cmH2O (an implicit assumption when computing the R/I ratio) in severe ARDS. Yet, we did not record pressure–volume curves in our study and are hence unable to evaluate this hypothesis. Finally, as both the numerator and denominator of the R/I ratio are mathematically coupled, all the above-mentioned errors tend to underestimate inflation and overestimate the recruitment part, leading to an overestimation of the R/I ratio, which explain the high rate of false positive patients.
Implications for clinical practice
Our study demonstrates that not all ICU ventilators are sufficiently reliable to measure the R/I ratioventilator. However, our data suggests that R/I ratio values greater than 0.57 may identify patients with high recruitability, although with a high rate of false positive patients. Additional functional tests of recruitability in this subpopulation of patients are still required to reliably personalize PEEP settings. On the other hand, our results suggest that R/I ratio values lower than 0.57 could exclude high recruitability, provided that patients do not present with focal ARDS on lung imaging. However, it should be stressed that the R/I ratio, by balancing recruitment over distension, may help modulate the PEEP level, by taking into account the potential harmful effects of excessive pressure on the lung parenchyma, particularly in patients with focal ARDS. Whether the R/I ratio, despite its limitations, has the potential to help individualize PEEP level and improve outcome is currently being evaluated in a large randomized controlled trial (NCT03963622). On the other hand, our results suggest that using a properly calibrated external flow sensor could improve the reliability of the measurement of the R/I ratioventilator, although this could hardly be translated in the clinical setting.
Study limitations and strengths
The present study has, however, several important limitations. First, the study is underpowered as the percentage of high recruiters is relatively low, and the planned sample size was not achieved. Consequently, the optimal R/I ratio threshold is imprecise and should be interpreted with caution. Second, the R/I ratio computation was not simultaneous to the CT-measured recruitment, and this could have biased the study. In this connection, derecruitment could have occurred during ventilator disconnection required for transport to the imaging facility, although occlusion of the endotracheal tube during the procedure should have limited this effect, and the low proportion of high recruiters in our study population does not support this hypothesis. Furthermore, the change of body position between respiratory mechanics and CT measurements (semi-recumbent to supine) may have also impacted their correlation. Third, we did not recalibrate the flow sensors of the ventilator immediately before the R/I ratio procedure (this was done at ICU admission), and we did not perform a 15-s inspiratory hold at a pressure greater than 30 cmH2O to ensure no gas leak occurred, but this could hardly be transferred to everyday clinical practice and our procedure mimic standard ICU management. Fourth, 143 patients [77%] out of 185 eligible patients were excluded from the present study, and a selection bias cannot be ruled out. Fifth, measurement of the R/I ratio was not replicated in our study, and this could have helped the detection of potential measurement errors. Finally, the choice of non-aerated lung tissue recruitment assessed with CT as the gold standard may be debatable (as opposed to recruited gas volume on CT), but both definitions did not substantially impact the diagnostic performance of the R/I ratio to predict lung recruitability.
Nevertheless, the strengths of our study are the following. First, the study is the first to evaluate the diagnostic performance of the R/I ratio with a reliable and independent gold standard. Second, the study provides a case-mix independent threshold value for the R/I ratio to identify patients with high recruitability. Third, we evaluated the reliability of the R/I ratio in the clinical context, while the seminal study was performed in experimental conditions, strengthening its generalizability. Fourth, we provide in the present study a comprehensive exploration of the underlying mechanisms explaining the lower-than-expected reliability of the R/I ratioventilator.
Conclusion
The R/I ratio had a poor diagnostic performance to predict lung recruitability when compared to CT used as a gold standard, at least partly in relation to unreliable measurement of expired tidal volume during PEEP release. However, R/I ratio values below 0.57 reliably identify ARDS patients with low recruitability, provided they do not present focal ARDS. The assessment of recruitability at PEEP 15 (vs. PEEP 45 cmH2O) with CT, the lung weight threshold used to identify recruitability with CT, and the low rate of high recruiters in the study population might have influenced these findings.
Supplementary Information
Acknowledgements
We thank all the patients, nurses and clinicians who generously donated their time during the study, and Loredana Baboi for her help in study coordination.
Abbreviations
- AOP
Airway opening pressure
- ARDS
Acute respiratory distress syndrome
- AUC
Area under the ROC curve
- CI95%
95% Confidence interval
- Crec,ventilator
Compliance of the recruited lung
- Crs
Compliance of the respiratory system
- CT
Computed tomography
- ΔPL
Transpulmonary driving pressure
- ΔPrs
Airway driving pressure
- ΔVrec,CT
Recruited volume assessed with computed tomography
- ∆Vrec,ventilator
Recruited volume assessed on the ventilator
- ECMO
Extracorporeal membrane oxygenation
- EELVCT,PEEP5
End-expiratory lung volume at PEEP 5 cmH2O assessed with computed tomography
- EELVCT,PEEP15
End-expiratory lung volume at PEEP 15 cmH2O assessed with computed tomography
- EL
Lung elastance
- Ers
Elastance of the respiratory system
- FiO2
Inspired oxygen fraction
- HU
Hounsfield unit
- ICU
Intensive care unit
- PaO2/FiO2
Ratio of the arterial partial pressure of oxygen over inspired oxygen fraction
- PBW
Predicted body weight
- PEEP
Positive end-expiratory pressure
- PEEPtot,L
Transpulmonary total PEEP
- PEEPtot,rs
Total PEEP of the respiratory system
- PEEP volumeventilator
Change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with the ventilator
- PEEP volumeCT
Change in aerated lung volume between PEEP 5 and 15 cmH2O assessed with computed tomography
- Pplat,L
Transpulmonary plateau pressure
- PPlat,rs
Plateau pressure of the respiratory system
- Predinf at PEEP5
Predicted inflation of already aerated lung units at PEEP 5 cmH2O
- R2
Coefficient of determination
- R/I
Recruitment-to-inflation ratio
- R/I ratioventilator
R/I ratio assessed on the ventilator
- SAPS2
Simplified acute physiology score 2
- VT
Tidal volume
- VTCT
Tidal volume assessed with computed tomography
Author contributions
JCR, L Bitker and FD made substantial contributions to study design, to data acquisition, study analysis, and interpretation of data, AND drafted the manuscript, AND approved the version to be published, AND agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. ER, ED, L Boussel, LP, and MO made substantial contributions to study design, to study analysis, and interpretation of data, AND revised the manuscript critically for important intellectual content, AND approved the version to be published, AND agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved GD, HY, MM, LC, MG, IN, RS, FB and YR made substantial contributions to data acquisition, study analysis, and interpretation of data, AND revised the manuscript critically for important intellectual content, AND approved the version to be published, AND agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study has no funding support.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. Further information is available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the amended declaration of Helsinki and was approved by a local independent ethics committee (Comité Scientifique et Ethique des Hospices Civils de Lyon, #20_194). Consent for data utilization was sought from all patients and/or their representatives.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Network ARDS, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. [DOI] [PubMed] [Google Scholar]
- 2.Qadir N, Sahetya S, Munshi L, Summers C, Abrams D, Beitler J, et al. An update on management of adult patients with acute respiratory distress syndrome: an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2024;209:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 5.Belletti A, Todaro G, Valsecchi G, Losiggio R, Palumbo D, Landoni G, et al. Barotrauma in coronavirus disease 2019 patients undergoing invasive mechanical ventilation: a systematic literature review. Crit Care Med. 2022;50:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–76. [DOI] [PubMed] [Google Scholar]
- 7.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. [DOI] [PubMed] [Google Scholar]
- 8.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55. [DOI] [PubMed] [Google Scholar]
- 9.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–45. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. a clinical trial. Am J Respir Crit Care Med. 2020;201:178–87. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez M, Pape SL, Arrivé F, Frat J-P, Thille AW, Coudroy R. Evolution of respiratory system compliance and potential for lung recruitment in COVID-19–induced acute respiratory distress syndrome. J Intensive Med. 2022;2:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier P-Y, Asfar P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. 2020;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevic N, Chatelain E, Dargent A, Argaud L, Cour M, Guérin C. Lung recruitability evaluated by recruitment-to-inflation ratio and lung ultrasound in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;203:1025–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cour M, Biscarrat C, Stevic N, Degivry F, Argaud L, Guérin C. Recruitment-to-inflation ratio measured with modern intensive care unit ventilators: how accurate is it? Crit Care. 2022;26:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Pesenti A, Bombino M, Baglioni S, Rivolta M, Rossi F, et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology. 1988;69:824–32. [DOI] [PubMed] [Google Scholar]
- 17.Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P. Regional distribution of gas and tissue in acute respiratory distress syndrome. II. physiological correlations and definition of an ARDS severity score. CT scan ARDS study group. Intensive Care Med. 2000;26:1046–56. [DOI] [PubMed] [Google Scholar]
- 18.Penarrubia L, Verstraete A, Orkisz M, Davila E, Boussel L, Yonis H, et al. Precision of CT-derived alveolar recruitment assessed by human observers and a machine learning algorithm in moderate and severe ARDS. Intensive Care Med Exp. 2023;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351: h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 21.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 23.Lanteri CJ, Kano S, Sly PD. Validation of esophageal pressure occlusion test after paralysis. Pediatr Pulmonol. 1994;17:56–62. [DOI] [PubMed] [Google Scholar]
- 24.Dávila Serrano EE, Guigues L, Roux JP, Cervenansky F, Camarasu-Pop S, Riveros Reyes JG, et al. Creatools: International Conference on Computer Vision and Graphics, ICCVG 2012. Computer Vision and Graphics - International Conference, ICCVG 2012, Proceedings. 2012; 55–62.
- 25.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–11. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. computed tomographic scan study. Am Rev Respir Dis. 1987;136:730–6. [DOI] [PubMed] [Google Scholar]
- 27.Paula LF, Wellman TJ, Winkler T, Spieth PM, Güldner A, Venegas JG, Vidal Melo MF. Regional tidal lung strain in mechanically ventilated normal lungs. J Appl Physiol. 2016;121:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–6. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Turner MJ, Yem JS, Baker AB. Calibration of pneumotachographs using a calibrated syringe. J Appl Physiol. 2003;95:571–6. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for statistical Computing; 2023. Available from: http://www.R-project.org
- 31.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marill KA, Chang Y, Wong KF, Friedman AB. Estimating negative likelihood ratio confidence when test sensitivity is 100%: A bootstrapping approach. Stat Methods Med Res. 2017;26:1936–48. [DOI] [PubMed] [Google Scholar]
- 33.Tillé Y, Matei A. sampling: Survey Sampling [Internet]. 2023. Available from: https://CRAN.R-project.org/package=sampling
- 34.Thiele C, Hirschfeld G. Cutpointr: improved estimation and validation of optimal cutpoints in R. J Stat Softw. 2021;98:1–27. [Google Scholar]
- 35.Carstensen B, Gurrin L, Ekstrom CT, Figurski M. MethComp: Analysis of Agreement in Method Comparison Studies [Internet]. 2024. Available from: http://CRAN.R-project.org/package=MethComp
- 36.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. [PubMed] [Google Scholar]
- 37.Carstensen B. Comparing clinical measurement methods: a practical guide (statistics in practice). 2nd ed. Chichester: Wiley; 2011. [Google Scholar]
- 38.Catozzi G, Pozzi T, Nocera D, Donati B, Giovanazzi S, Ghidoni V, et al. Rethinking ARDS classification: oxygenation impairment fails to predict VILI risk. Intensive Care Med. 2025;51:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert SP, DiRocco J, Allen GB, Bates JH, Lafollette R, Kubiak BD, et al. The role of time and pressure on alveolar recruitment. J Appl Physiol. 2009;106:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. consequences for lung morphology. CT Scan ARDS study group. Intensive Care Med. 2000;26:857–69. [DOI] [PubMed] [Google Scholar]
- 41.Coppola S, Pozzi T, Gurgitano M, Liguori A, Duka E, Bichi F, et al. Radiological pattern in ARDS patients: partitioned respiratory mechanics, gas exchange and lung recruitability. Ann Intensive Care. 2021;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murgolo F, Grieco DL, Spadaro S, Bartolomeo N, di Mussi R, Pisani L, et al. Recruitment-to-inflation ratio reflects the impact of peep on dynamic lung strain in a highly recruitable model of ARDS. Ann Intensive Care. 2024;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiumello D, Marino A, Brioni M, Cigada I, Menga F, Colombo A, et al. Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome. what is the relationship? Am J Respir Crit Care Med. 2016;193:1254–63. [DOI] [PubMed] [Google Scholar]
- 44.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ. Computed tomography assessment of positive end-expiratory pressure- induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1444–50. [DOI] [PubMed] [Google Scholar]
- 45.Del Sorbo L, Tisminetzky M, Chen L, Brochard L, Arellano D, Brito R, et al. Association of lung recruitment and change in recruitment-to-inflation ratio from supine to prone position in acute respiratory distress syndrome. Crit Care. 2023;27:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. Further information is available from the corresponding author upon reasonable request.