Abstract
Background
Aberrant angiogenesis is an important pathological feature of periodontitis, which is regulated by angiogenic paracrine factors derived from periodontal ligament stem cells (PDLSCs). We have previously demonstrated that ubiquitin C-terminal hydrolase L1 (UCHL1) was upregulated in PDLSCs from periodontitis patients, but its role in aberrant angiogenesis in periodontitis remains unclear.
Methods
PDLSCs were isolated from healthy individuals and periodontitis patients. To mimic inflammatory conditions in vitro, PDLSCs from healthy individuals were treated with tumor necrosis factor-alpha and interleukin-1beta. Human umbilical vein endothelial cells were cultured with conditioned media from PDLSCs, and their proliferation, migration, and tube formation were detected to evaluate the pro-angiogenic capacity of PDLSCs. Vascular endothelial growth factor A (VEGFA) and angiopoietin 1 (ANGPT1) levels were measured using RT-qPCR, Western blotting, Immunofluorescence, and enzyme-linked immunosorbent assay. UCHL1 was knocked down using shRNA to validate its function and regulation of Yes-associated protein (YAP) activity. Co-immunoprecipitation was used to verify the interaction of UCHL1 with hypoxia-inducible factor 1 alpha (HIF-1α), and chromatin immunoprecipitation was used to analyze the effect of HIF-1α on YAP transcription. UCHL1 inhibitor was administered in a periodontitis murine model to investigate its function in vivo.
Results
The pro-angiogenic capacity of PDLSCs from periodontitis patients was enhanced, accompanied by increased expressions of VEGFA and ANGPT1, which were positively correlated with UCHL1 expression. UCHL1 knockdown in PDLSCs abrogated the increased secretion of VEGFA and ANGPT1 and the enhanced pro-angiogenic capacity under inflammatory conditions. Mechanistically, UCHL1 functioned through facilitating YAP expression and nuclear translocation, a process that was mediated by the stabilization and activation of HIF-1α, which in turn promoted YAP transcription. In vivo inhibition of UCHL1 in a periodontitis murine model alleviated aberrant angiogenesis and reduced the expressions of VEGFA and ANGPT1, thus attenuating periodontitis progression by reducing lymphocyte infiltration, inflammatory cytokine levels, and alveolar bone resorption.
Conclusions
This study demonstrated that UCHL1 promoted the pro-angiogenic capacity of PDLSCs in periodontitis through the HIF-1α/YAP signaling, which provides insights into the pathogenesis of periodontitis and paves the way for novel treatment strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-025-04399-y.
Keywords: Periodontitis, Angiogenesis, Periodontal ligament stem cells, Ubiquitin C-terminal hydrolase L1, Yes-associated protein, Angiogenesis, Vascular endothelial growth factor A, Angiopoietin 1
Background
Periodontitis, a chronic inflammatory disease, is a public health problem worldwide. Chronic periodontal inflammation leads to the persistent destruction of periodontal tissues, which eventually results in tooth loss and reduced quality of life [1]. Aberrant angiogenesis is considered one of the significant manifestations of periodontitis [2]. It could lead to increased vascular permeability, thereby facilitating immune cell infiltration and pro-inflammatory mediator release [3], which contributes to the progression of periodontitis [4]. Hence, a better understanding of the mechanisms underlying aberrant angiogenesis is crucial for the therapeutic development of resolution for periodontitis.
Angiogenesis is robustly influenced by multiple factors, among which angiogenic paracrine factors produced by nonendothelial cells are critical regulators [5]. Recently, particular attention was attracted to a unique population of mesenchymal stem cells identified in the periodontal ligament (PDL), which were named periodontal ligament stem cells (PDLSCs) [6]. Apart from their basic properties of pluripotential capacity and regenerative potential [7], PDLSCs exhibit a robust capacity to modulate the surrounding niche and shape the periodontal microenvironment [8]. Accumulating evidence has identified the capacity of PDLSCs to promote aberrant angiogenesis by secreting paracrine mediators and thereby modulating endothelial cell behaviors in periodontitis-associated inflammation [9]. PDLSCs could respond to the inflammatory environment and increase the secretion of basic fibroblast growth factor (FGF), angiogenin, and vascular endothelial growth factor A (VEGFA), thus promoting aberrant angiogenesis [10, 11]. However, the mechanism underlying the enhanced pro-angiogenic capacity of PDLSCs in periodontitis remains obscure, suggesting a need for more studies in this field.
Ubiquitination and deubiquitination are post-translational modifications of proteins by covalent attachment and removal of ubiquitin to modulate the degradation of proteins, which participate in various cellular functions [12]. Ubiquitin C-terminal hydrolase L1 (UCHL1) belongs to deubiquitinating enzymes and removes ubiquitin from proteins to prevent their degradation [13]. We have previously revealed the upregulation of UCHL1 in inflamed PDLSCs and its negative role in alveolar bone regeneration during periodontitis [14]. Our previous study also demonstrated that UCHL1 modulated the paracrine function of mesenchymal stem cells, thereby regulating T cell proliferation and shaping the immune microenvironment [15]. Notably, UCHL1 has been implicated in angiogenesis. UCHL1 augmented VEGF signaling in renal cancer cells [16] and activated key pathways, hypoxia-inducible factor 1 alpha (HIF-1α) and Wnt/β-catenin pathway, to induce the secretion of pro-angiogenic factors [17–19]. However, whether UCHL1 regulates the paracrine pathway in PDLSCs to modulate aberrant angiogenesis in periodontitis remains unclear.
Yes-associated protein (YAP) is a transcriptional co-activator that regulates target gene transcription by translocating to the nucleus and interacting with transcription factors, thereby participating in various biological processes such as angiogenesis [20] and stem cell self-renewal [21]. While the involvement of YAP in periodontitis remains poorly understood, it has been reported that YAP activation contributes to inflammatory exacerbation in periodontitis through upregulating tumor necrosis factor-alpha (TNF-α) and interleukin-6 [22]. Current knowledge of YAP in periodontics mainly focuses on its regulatory roles in stem cell regeneration, paracrine modulation, and angiogenesis processes. For example, during alveolar bone regeneration, YAP could enhance the osteogenic differentiation of PDL cells [23] and promote osteovascularization [24]. Upon periodontal mechanical stress, YAP acts as a central mediator to promote PDLSC proliferation [25] and their secretion of osteoclastogenesis modulators [26], thus contributing to periodontal tissue remodeling. However, whether YAP regulates the paracrine function of PDLSCs in pathological angiogenesis during periodontitis remains unclear, and the precise regulation of YAP activity in the inflammatory context requires further investigation.
This study aimed to unveil the novel role of UCHL1 in modulating the pro-angiogenic capacity of PDLSCs in periodontitis. Firstly, the increased expressions of pro-angiogenic factors, VEGFA and angiopoietin 1 (ANGPT1), were examined in PDLSCs from periodontitis patients, and their correlation with UCHL1 expression was analyzed. Then UCHL1 was knocked down to identify its role in modulating the pro-angiogenic capacity of PDLSCs. Further, we investigated underlying mechanisms and found that UCHL1 exerted its function through HIF-1α/YAP signaling. Finally, a murine experimental periodontitis model was established, and the specific inhibitor targeting UCHL1 was administered in vivo to observe its effect on aberrant angiogenesis during the progression of periodontitis. These findings provide new insights into the understanding of periodontitis pathogenesis and pave the way for further investigation aimed at developing therapeutic approaches for periodontitis.
Methods
Human tissue sample collection
All the tissue samples were collected at the Department of Stomatology, Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The experimental procedures of human samples were approved by the Ethics Committee of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (KY[2019]068) and in accordance with the Declaration of Helsinki. Informed consent of the subjects involved in this study was obtained. Clinical parameters were used to determine the clinical diagnosis of periodontally healthy and periodontitis as previously described [27]. The detailed inclusion/exclusion criteria and the demographic data (Supplementary Table 1) are provided in the supplementary materials.
Healthy PDL tissues were collected from periodontally healthy donors (n = 8) who underwent extraction of third molar teeth because of nonfunction. Inflamed granulation tissues were collected from periodontitis patients diagnosed with stage III periodontitis (n = 8) who underwent open flap debridement surgery. Tissue samples were kept on ice and transferred immediately to the laboratory for further analysis.
The isolation of PDLSCs
PDLSCs derived from healthy individuals and PDLSCs derived from periodontitis patients were isolated as previously reported. Briefly, the healthy PDL tissues and inflammatory granulation tissues were cut into 1 mm3 small pieces and digested using dispase II (4 mg/mL, Sigma-Aldrich, St. Louis, MO, USA) and type I collagenase (3 mg/mL, Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 3 °C. Single-cell suspensions were generated by straining digested tissues through 70-μm cell strainers, placed into dishes, and cultured at 37 °C in 5% CO2 for 2 weeks to allow cells to grow. Homogenous populations of PDLSCs were obtained by the limiting dilution method, which generated single-cell-derived colonies of PDLSCs. To identify PDLSCs, surface markers, the ability of osteogenic differentiation, adipogenic differentiation, chondrogenic differentiation, and the colony-formation ability, were examined.
Cell culture
PDLSCs were cultured in α-MEM modification medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 2 mM glutamine (Gibco, Carlsbad, CA, USA), 1% Penicillin–Streptomycin (Gibco, Carlsbad, CA, USA). To mimic inflammatory conditions in vitro, culture media of PDLSCs was supplemented with TNF-α (2 ng/mL, PeproTech, Rocky Hill, NJ, USA) and interleukin-1beta (IL-1β) (5 ng/mL, PeproTech, Rocky Hill, NJ, USA). All the PDLSCs were used at the 2nd-5th passages. Human umbilical vein endothelial cells (HUVECs) were purchased from ALLCELLS (Shanghai, China) and cultured in Endothelial Cell Medium (ScienCell, Carlsbad, CA, USA).
Immunofluorescence
For immunofluorescence of frozen tissue sections, fresh tissue samples were rapidly frozen in liquid nitrogen and embedded in optimal cutting temperature compounds (Tissue-Tek, Sakura, Japan). The tissue blocks were sectioned at a thickness of 10 μm at − 25 °C using a Cryostat (Leica, Wetzlar, Germany). Tissue sections were then placed onto glass slides for further staining. For immunofluorescence of cells, cells cultured on glass coverslips were washed, and fixed with 4% paraformaldehyde. For the staining procedure, samples were permeabilized with 0.2% Triton X-100, blocked with 5% bovine serum albumin (BSA), and incubated with primary antibodies at 4 °C overnight. Then samples were washed and incubated with secondary antibodies including Alexa Flour conjugated 488 (Invitrogen, Carlsbad, California, USA) and Alexa Flour conjugated 555 (Invitrogen, Carlsbad, California, USA). DAPI (Cell Signaling Technology, Danvers, Massachusetts, USA) was used to counterstain nuclei according to the manufacturer’s instruction. The coverslips were finally mounted. Samples were observed under a laser scanning confocal microscope (SP8; Leica, Wetzlar, Germany). Primary antibodies used in this assay are listed in Supplementary Table 2. ImageJ software (X64, version 2.1.4) was used for quantification. Briefly, the mean fluorescence intensity was calculated from immunofluorescence images to assess protein expression levels. The nucleus/cytoplasm ratio was calculated to evaluate nuclear translocation.
Coculture of HUVECs with conditioned media from PDLSCs
PDLSCs were cultured in serum-containing media and treated with different reagents until cells reached 80% confluent. Then the serum-containing medium was replaced with serum-free α-MEM modification media. To obtain conditioned media from differently treated PDLSCs, PDLSCs were incubated in serum-free media for 72 h. Culture supernatant was harvested from PDLSCs, centrifuged at 4 °C at 1000 rpm for 5 min, filtered using 0.22 μm sterile syringe filters (Merck Millipore Ltd, Cork, Ireland), and stored at − 80 °C. To evaluate the pro-angiogenic capacity of PDLSCs, HUVECs were cocultured with conditioned media from PDLSCs and undergone further analysis.
Cell counting Kit-8 (CCK-8) assay
The CCK-8 (Dojindo, Kumamoto, Japan) was utilized to examine the viability changes of HUVECs under different treatments. Briefly, cells were inoculated onto 96‐well plates at a concentration of 5 × 103 cells/well and incubated for 24 h, 48 h, and 72 h. Then cells were incubated in 10% CCK‐8 solution diluted in culture media for another 3 h. After that, the absorbance of the mixture at 450 nm was recorded using a BioTek Synergy H1 microplate reader (Winooski, VT, USA).
Scratch wound healing assay
HUVECs were seeded onto 6‐well plates at a concentration of 3 × 105 cells/well and then incubated overnight. After cells completely adhered to plates, a sterile 200 μL pipette tip was applied to make scratches with clean borders on the edge. Then dislodged cells in wells were removed by gently washing with phosphate-buffered saline (PBS). Subsequently, HUVECs were incubated with conditioned media harvested from PDLSCs for 12 h. Images of migrated cells were captured using a microscope. Cell migration was determined quantitatively by measuring the area of scratches using ImageJ software (X64, version 2.1.4).
Tube formation assay
Matrigel Matrix (BD Biosciences, New Jersey, USA) was thawed at 4 °C overnight. 50 µl of Matrigel Matrix was loaded into per well of 96-well plates and allowed to gel at 37 °C for 30 min. Subsequently, the 80% confluent HUVECs were harvested, resuspended with conditioned media from PDLSCs, plated on top of gelled Matrigel Matrix (1.5 × 104 cells/well), and then incubated at 3 °C, 5% CO2 in the incubator for a period of 10 h. Tube formation was stained with 2 μM Calcein AM (Beyotime, Haimen, Jiangsu, China) solution diluted in PBS and observed using a ZOE™ Fluorescent Imaging Microscopy (Bio-Rad, Hercules, CA, USA). Quantification of tube formation was performed by measuring number of nodes, number of meshes, and total length using ImageJ software (X64, version 2.1.4).
Analysis of gene expression omnibus (GEO) dataset, GSE78074
The public dataset, GSE78074, was downloaded from GEO datasets of the National Center of Biotechnology Information. Gene expression data in GSE78074 were analyzed using Rstudio software (version 4.3.1). The expression levels of classical pro-angiogenic factors were examined [28], and the significantly upregulated genes of pro-angiogenic factors were presented using a heatmap. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of differentially expressed genes were identified from GSE78074.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol (Invitrogen, Carlsbad, California, USA) was used to extract total RNA of cell or tissue samples. Chloroform was added to samples at a ratio of 0.2:1, and the aqueous phase was separated by centrifugation at 12,000×g for 15 min. The RNA precipitation was obtained with an equal amount of isopropanol to the aqueous phase, and followed by a wash with 70% ethanol in RNase-free water, quality assessment, and reverse transcription into cDNA with PrimeScript™ RT Master Mix (Takara Bio, Otsu, Shiga, Japan). The mRNA levels were determined by real-time PCR using FastStart Universal SYBR Green Master Mix (Roche, Nutley, NJ, USA) according to the manufacturer’s instruction on the LightCycler ® 480 Real-Time PCR System (Roche, Indianapolis, IN, USA). Relative mRNA expression levels were normalized to the mRNA level of GAPDH. Primers used for real-time PCR analysis were synthesized by Sangon Biotech (Shanghai, China), and the sequences are presented in Supplementary Table 3.
Western blotting
Tissue lysates or cell lysates were prepared with radioimmunoprecipitation lysis buffer (Beyotime, Haimen, Jiangsu, China) containing 1% phenylmethylsulfonyl fluoride (Beyotime, Haimen, Jiangsu, China), and incubated on ice for 30 min. Concentrations of released protein were measured and corrected using the BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). Protein samples were then denatured at 100 °C for 5 min in 1 × sodium dodecyl sulfate (SDS) loading buffer (Takara Bio, Otsu, Shiga, Japan). After cooling on ice, protein samples were loaded onto SDS–polyacrylamide gels with equal amounts, and then wet transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% BSA in PBS for 1 h at room temperature, exposed to primary antibodies diluted in 5% BSA in PBS overnight at 4 °C, washed three times with PBS-Tween (0.1% v/v) (PBST), incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Danvers, Massachusetts, USA) diluted in PBST at room temperature for 1 h, and finally washed three times with PBST. Immunoreactive bands were visualized using chemiluminescence reagents (Millipore, Billerica, MA, USA) on the ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA). The blots were quantitatively analyzed using ImageJ software (X64, version 2.1.4), and GAPDH was used as a control. Primary antibodies used in this assay are listed in Supplementary Table 2.
Enzyme-linked immunosorbent assay (ELISA)
PDLSCs were cultured in serum-containing media and treated with different reagents until cells reached 80% confluent. Then the serum-containing media were replaced with serum-free α-MEM modification media and PDLSCs were further cultured for 72 h. Culture supernatant was harvested from PDLSCs, centrifuged at 4 °C at 1000 rpm for 5 min, filtered using 0.22 μm sterile syringe filters (Merck Millipore Ltd, Cork, Ireland), and stored at − 80 °C. To monitor the secretion of VEGFA and ANGPT1 in PDLSCs, the concentrations of VEGFA and ANGPT1 in the collected serum-free media were measured using a human VEGFA ELISA kit (Solarbio, Beijing, China) and a human ANGPT1 ELISA kit (Solarbio, Beijing, China) according to the manufacturer’s instruction.
Lentivirus packaging and cell transfection
To establish UCHL1-inhibited PDLSCs, lentivirus construction and production were performed. Briefly, the shRNA targeting human UCHL1 was cloned into the PLKO.1 puro plasmid (Addgene, Cambridge, USA) to construct lentivectors. Specific shRNAs to human UCHL1 or scramble control were pre-designed and synthesized (Sangon Biotech). The sequences are as follows: human UCHL1, 5′-GATCCCGGGTAGATGACAAGGTGAATCTCGAGATTCACCTTGTCATCTACCCGTTTTTG-3′; scrambled control: 5′-GATCCCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG-3′. For lentivirus production, HEK 293 T cells were transfected with the lentivectors utilizing Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA). High titer lentiviral stocks were then produced from the supernatant of HEK 293 T cells. To infect PDLSCs, lentiviruses expressing shRNA targeting UCHL1 or scrambled control lentiviruses were added to the culture medium supplemented with 8 μg/mL polybrene (Thermo Fisher Scientific, Rockford, IL, USA) for 24 h. Subsequently, PDLSCs resistant to puromycin (3 μg/ml) were selected at the 3rd passage and expanded at the 3rd-5th passages for further experiments.
Protein–protein interaction analysis
The Search Tool for the Retrieval of Interacting Genes (STRING) (https://string-db.org/,version 12.0), an online tool to analyze and predict protein–protein interactions [29], was queried for analyzing and predicting functional and physical interactions. The minimum required interaction score was set to medium confidence of > 0.4.
Co-immunoprecipitation (Co-IP)
To analyze the protein interactions, a Co-IP assay was performed in PDLSCs using the Biolinkedin Classic IP/Co-IP Kit (Biolinkedin, Shanghai, China). PDLSCs were treated with TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h and then lysed in IP lysis buffer containing 1% phenylmethylsulfonyl fluoride and 1% protease inhibitor cocktail. The cell lysate was incubated on ice for 30 min and centrifuged at 15,000 rpm for 30 min. After centrifugation, the supernatant of samples was collected, and part of the supernatant was taken as input. For each sample, the supernatant containing 1000 μg total protein was taken for the Co-IP assay. 10 μg of the antibody was added to each sample, and the mixture was incubated under constant rotation at 4 °C overnight to obtain antigen–antibody complexes. The samples added with protein A/G magnetic beads were incubated under constant rotation at room temperature for 2 h and then placed on a magnetic base. The magnetic bead-protein complexes were collected, washed with IP wash buffer, resuspended in 1 × SDS loading buffer, and denatured at 100 °C for 10 min. The antigen–antibody complexes were resolved on SDS–polyacrylamide gels, electroblotted onto polyvinylidene difluoride membrane, and immunoblotted. Antibodies used in this assay are listed in Supplementary Table 2.
Chromatin immunoprecipitation (ChIP)
PDLSCs were treated with or without TNF-α (2 ng/m) and IL-1β (5 ng/mL) for 72 h. ChIP assay was performed using the SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling Technology, Danvers, Massachusetts, USA) according to the manufacturer’s instructions. Briefly, PDLSCs were treated with 1% formaldehyde for cross-linking of DNA and protein. Glycine was used to quench the reaction. Micrococcal nuclease was used to digest DNA, and digestion was stopped by adding ethylenediaminetetraacetic acid. Then the lysate was subjected to a sonicator to break the nuclear membrane. Normal rabbit IgG (Cell Signaling Technology, Danvers, Massachusetts, USA) and the anti-HIF-1α (Abcam, Cambridge, MA, USA) antibody were added to the samples and incubated overnight at 4 °C. The DNA-antibody complexes were immunoprecipitated with protein G magnetic beads for 2 h at 4 °C. Then DNA was eluted from antibody/protein G magnetic beads, reversed cross-links, purified, and analyzed by agarose gel electrophoresis and RT-qPCR. The putative binding sites of HIF-1α in the YAP promoter were predicted using the JASPAR database (http://jaspar.genereg.net/). The most likely transcription factor binding site with the highest score was selected for further confirmation. The specific primers are listed in Supplementary Table 3. Antibodies used in this assay are listed in Supplementary Table 2.
Agarose gel electrophoresis
Agarose powder (0.4 g) was added to tris–acetate-ethylenediaminetetraacetic acid buffer (40 ml) to prepare 1% agarose gel. Heated gel was added with Gel-Red (Beyotime, Haimen, Jiangsu, China) and then placed to be solidified. RT-qPCR products added with DNA loading buffer (Beyotime, Haimen, Jiangsu, China) were loaded into the gel. The gel was run in tris–acetate-ethylenediaminetetraacetic acid buffer at 120 V for 25 min. Then DNA fragments were visualized using the Gel Doc™ EZ System (Bio-Rad, Hercules, CA, USA).
Establishment of murine periodontitis model
Male C57/BL6 mice aged 10 weeks were used to establish the periodontitis model. Mice were anesthetized with 2,2,2-Tribromoethanol (Macklin, Shanghai, China; 0.25 mg/g) given intraperitoneally, and a ligature (8–0) was placed around the maxillary first molar (M1) from day 0 to day 10 to induce periodontitis. Dimethyl sulfoxide (DMSO) or the inhibitor of UCHL1, LDN57444, was diluted in corn oil. DMSO or LDN57444 (0.4 mg/kg, Selleck, Houston, TX, USA) was injected intraperitoneally every two days during the induction of experimental periodontitis by ligation. Then mice were sacrificed by humane euthanasia with CO2 10 days after DMSO or LDN57444 treatment, and the maxillae were resected and collected for further analysis. Mice would be excluded if the ligature is not stable at the cervical of M1 (such as breakage or falling off) before the endpoint of the experiment, and there were no exclusions in this study. Our data showed that the means of bone resorption area are 1.020 mm2, 1.540 mm2, and 1.258 mm2 for the 3 groups, and the numbers of standard deviation are 0.1525, 0.2896, and 0.1497. Power was 0.90, and alpha was 0.05. The sample sizes were calculated to be 4, 4, and 4. Totally 24 mice were randomly divided into three groups: naïve group (n = 8), ligature group including DMSO (n = 8), and LDN57444 group (n = 8). Mice were housed in specific pathogen-free facilities at Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All animal experiments were complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines 2.0 and approved by Laboratory Animal Welfare and Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (RJ2024-197A).
Histological analysis in murine periodontitis model
Maxilla samples were fixed with 4% paraformaldehyde, decalcified using 10% ethylenediaminetetraacetic acid decalcification solution for 1 month, embedded in paraffin, and sectioned at 5 microns. Sections were deparaffinized and rehydrated by sequential immersion in xylene and grade alcohol series. Then the sections were subjected to hematoxylin & eosin (H&E) staining, immunohistochemistry staining, or immunofluorescence. For immunohistochemistry staining or immunofluorescence, sections underwent antigen retrieval by microwave processing, blocking, primary antibody (Supplementary Table 2) incubation, and horseradish peroxidase—diaminobenzidine colorimetric detection or fluorescent secondary antibodies incubation. The area occupied by blood vessels (CD31 positive) was used to evaluate blood vessel area. The expressions of VEGFA, ANGPT1, and YAP in gingival and PDL tissues were analyzed quantitatively using integrated optical density. To quantitatively analyze the infiltration of T cells and B cells, CD3-positive and CD20-positive cells in gingival and PDL tissues were counted, and the percentage of positive immunolabeled cells over the total cells was calculated.
Micro-computed tomography (micro-CT)
The bone microarchitecture of maxilla structure was scanned using high-resolution micro-CT system (Skyscan1172, Bruker, Belgium) at 50 kV acceleration voltage, 180 μA tube current, 475 ms exposure time, and 10 μm isometric resolution. Micro-CT 3-dimensional (3D) reconstructions and 2-dimensional (2D) sectional imaging were performed in all scanning planes using Mimics Medical 21.0 (Materialise, Plymouth, MI, USA). Bone resorption area (the area from the buccal cemento-enamel junction (CEJ) to alveolar bone crest (ABC)) of the maxillary M1 was measured in the 3D images using ImageJ software (X64, version 2.1.4). 2D images were processed with pseudocolor technology using Mimics Medical 21.0. Bone mineral density (BMD) and bone histomorphometry parameters (bone volume/tissue volume (BV/TV) and trabecular thickness (Tb.Th)) of maxillary alveolar bone surrounding M1 were measured from the most buccal aspect to the most palatal aspect of M1 root, from the most mesial aspect of M1 root to the most mesial aspect of the second molar (M2) root, and from the most apical aspect of M1 root to the most coronal part of alveolar bone crest using CTvol software (Bruker, Kontich, Belgium).
Statistical analysis
Significance of statistical differences between different groups was assessed using Mann–Whitney U test, one-way analysis of variance (ANOVA), or two-way ANOVA. Correlations were assessed using Spearman’s correlation analysis. All data in this study were reported as mean ± standard error of mean (S.E.M). P value < 0.05 would be interpreted as statistically significant. In the presented figures, P < 0.05, P < 0.01, P < 0.001, and not significant were indicated as “*, **, ***, and ns”, respectively. Statistical analyses were performed using GraphPad Prism 9.0.
Results
PDLSCs in periodontitis exhibited an enhanced pro-angiogenic capacity and increased expression levels of VEGFA and ANGPT1, which were positively correlated with UCHL1 expression
Increased angiogenesis is one of the most common manifestations of inflammation [5], which is confirmed in the inflamed granulation tissues from periodontitis patients, as indicated by the increased expression of the vascular marker, CD31 (Fig. 1A). Paracrine pathways mediated by PDLSCs are pivotal in angiogenesis [11]. Hence, we isolated PDLSCs from healthy individuals (H-PDLSCs) and periodontitis patients (P-PDLSCs) and identified them by surface markers (Supplementary Fig. 1A), osteogenic, adipogenic, and chondrogenic differentiation potential, and colony-forming ability (Supplementary Fig. 1B). Then conditioned media from H-PDLSCs or P-PDLSCs were collected to culture HUVECs (Supplementary Fig. 2A). Expectedly, coculture with conditioned media from P-PDLSCs significantly increased the proliferation (Supplementary Fig. 2B), migration (Supplementary Fig. 2C, D), and tube formation of HUVECs (Supplementary Fig. 2E, F), suggesting the enhanced pro-angiogenic capacity of P-PDLSCs.
Fig. 1.
The elevated expression level of UCHL1 was correlated with the elevated expression levels of VEGFA and ANGPT1 in P-PDLSCs. A Representative immunofluorescent images and the quantification of the immunofluorescence show CD31 expression in the PDL tissues from healthy individuals and the granulation tissues from periodontitis patients. B The heatmap displays gene expressions of upregulated pro-angiogenic factors in P-PDLSCs analyzed in GSE78074. C The mRNA expression levels of VEGFA, ANGPT1, VEGFB, VEGFC, and FGF1 in H-PDLSCs and P-PDLSCs were measured using RT-qPCR. D The mRNA expression levels of UCHL1 in H-PDLSCs and P-PDLSCs were measured using RT-qPCR. E The correlations of UCHL1 mRNA expression with VEGFA or ANGPT1 were analyzed using Spearman’s correlation analysis. F–K. The protein expression of VEGF, ANGPT1, and UCHL1 in the PDL tissues from healthy individuals and granulation tissues from periodontitis patients was evaluated using immunofluorescence. F Representative immunofluorescent images show the expressions of VEGFA. G Mean fluorescence intensity of VEGFA protein expression was calculated from immunofluorescence images. H Representative immunofluorescent images show the expressions of ANGPT1. I Mean fluorescence intensity of ANGPT1 protein expression was calculated from immunofluorescence images. J Representative immunofluorescent images show the expressions of UCHL1. K Mean fluorescence intensity of UCHL1 protein expression was calculated from immunofluorescence images. H represents the healthy group; P represents the periodontitis group. Statistical comparisons were analyzed using Mann–Whitney U test; Correlations were analyzed using Spearman’s correlation analysis; data are presented as mean ± S.E.M; ns indicates not significant, * indicates P < 0.05, and ** indicates P < 0.01
Subsequently, the expressions of pro-angiogenic factors in H-PDLSCs and P-PDLSCs were analyzed using the datasets, GSE78074. VEGFA, ANGPT1, VEGFB, VEGFC, and FGF1 were demonstrated to upregulate in P-PDLSCs (Fig. 1B), among which the upregulation of VEGFA and ANGPT1 was verified using RT-qPCR (Fig. 1C). The upregulated expression level of UCHL1 was also confirmed in P-PDLSCs (Fig. 1D), which has been demonstrated in our previous study [14]. Interestingly, we found that UCHL1 expression was positively correlated with VEGFA and ANGPT1 (Fig. 1E). Besides, immunofluorescence result validated that the upregulation of VEGFA and ANGPT1 (Fig. 1F–I) was accompanied by the upregulation of UCHL1 in inflamed granulation tissues from periodontitis patients (Fig. 1J, K). Overall, these results indicate that UCHL1 might be associated with VEGFA and ANGPT1 secretion, which contributed to the enhanced pro-angiogenic capacity of P-PDLSCs.
Knockdown of UCHL1 inhibited the secretion of VEGFA and ANGPT1 in PDLSCs under inflammatory conditions
To investigate the role of UCHL1 in modulating the pro-angiogenic capacity of PDLSCs, lentivirus short hairpin RNA targeting UCHL1 (shUCHL1) was used to knock down UCHL1 (lentivirus controls: shScramble) (Supplementary Fig. 3A-B). As indicated in Fig. 2A, UCHL1 knockdown significantly decreased the concentrations of VEGFA and ANGPT1 in the culture supernatant of P-PDLSCs. Accordingly, the mRNA and protein expression levels of VEGFA and ANGPT1 also decreased in shUCHL1 P-PDLSCs (Fig. 2B, C). Subsequently, shScramble and shUCHL1 H-PDLSCs were cultured in media supplemented with TNF-α and IL-1β to simulate PDLSCs under inflammatory conditions. Expectedly, H-PDLSCs exhibited an increase in the secretion of VEGFA and ANGPT1 upon TNF-α and IL-1β stimulation, while UCHL1 knockdown abrogated this increase (Fig. 2D). UCHL1 knockdown also disrupted the increased expression of VEGFA and ANGPT1 in H-PDLSCs under inflammatory conditions (Fig. 2E–H). Overall, these results suggested that UCHL1 facilitated the secretion of VEGFA and ANGPT1 in PDLSCs in periodontitis.
Fig. 2.
Knockdown of UCHL1 abrogated the increased levels of VEGFA and ANGPT1 in PDLSCs. PDLSCs were transfected with lentivirus controls (shScramble) or lentivirus shRNA targeted UCHL1 (shUCHL1). A–C shScramble and shUCHL1 P-PDLSCs were cultured for 72 h. A The concentration of VEGFA and ANGPT1 in the culture media of shScramble and shUCHL1 P-PDLSCs was measured using ELISA assay at 72 h post-culture with serum-free media. B The mRNA expression levels of VEGFA and ANGPT1 in shScramble and shUCHL1 P-PDLSCs were measured using RT-qPCR. C The protein expression levels of VEGFA, ANGPT1, UCHL1, and GAPDH in shScramble and shUCHL1 P-PDLSCs were measured using western blotting. Full-length blots are presented in Supplementary Fig. 4. D–G shScramble and shUCHL1 H-PDLSCs were treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. D The concentration of VEGFA and ANGPT1 in the culture media of shScramble and shUCHL1 H-PDLSCs was measured using ELISA assay. E The mRNA expression levels of VEGFA and ANGPT1 in shScramble and shUCHL1 H-PDLSCs were measured using RT-qPCR. F The protein expression levels of VEGFA, ANGPT1, UCHL1, and GAPDH in shScramble and shUCHL1 H-PDLSCs were measured using western blotting. Full-length blots are presented in Supplementary Fig. 5. G The protein expression levels of VEGFA and ANGPT1 in shScramble and shUCHL1 H-PDLSCs were measured using immunofluorescence. H Mean fluorescence intensity of VEGFA and ANGPT1 protein expression was calculated from immunofluorescence images. T represents TNF-α; I represents IL-1β. Statistical comparisons were analyzed using Mann–Whitney U test or two-way ANOVA; data are presented as mean ± S.E.M; * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001
UCHL1 knockdown impaired the pro-angiogenic capacity of PDLSCs under inflammatory conditions
Subsequently, the effect of UCHL1 on the pro-angiogenic capacity of PDLSCs was investigated by coculturing HUVECs with conditioned media from shScramble or shUCHL1 H-PDLSCs pretreated or not pretreated with TNF-α and IL-1β. (Fig. 3A). As indicated in Fig. 3B, HUVECs exhibited augmented proliferation when cocultured with conditioned media from TNF-α and IL-1β-pretreated H-PDLSCs, while UCHL1 knockdown partially reversed the increased proliferation of HUVECs. Similarly, UCHL1 knockdown abolished the enhanced migration of HUVECs cocultured with conditioned media from TNF-α and IL-1β-pretreated H-PDLSCs (Fig. 3C, D). Besides, using tube formation assay, more tubes with connected networks were observed when HUVECs were cocultured with conditioned media from TNF-α and IL-1β-pretreated H-PDLSCs, while UCHL1 knockdown disrupted the increased tube formation (Fig. 3E, F). Collectively, these results indicated that UCHL1 promoted the pro-angiogenic capacity of PDLSCs under inflammatory conditions.
Fig. 3.
Knockdown of UCHL1 enhanced the pro-angiogenic capacity of H-PDLSCs under inflammatory conditions. PDLSCs were transfected with lentivirus controls (shScramble) or lentivirus shRNA targeted UCHL1 (shUCHL1). shScramble and shUCHL1 H-PDLSCs were treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. A Schematic illustration shows the methods to coculture HUVECs with conditioned media from shScramble H-PDLSCs or shUCHL1 H-PDLSCs. B The proliferation activity of HUVECs was detected using CCK-8 assay 24 h, 48 h, and 72 h after the coculture with conditioned media from shScramble and shUCHL1 H-PDLSCs. C, D Representative images and the quantification results show the migration activity of HUVECs detected using scratch wound healing assay 0 h and 12 h post the coculture with conditioned media from shScramble and shUCHL1 H-PDLSCs. E Representative bright field and green field images show the tube formation of HUVECs 10 h after the coculture with conditioned media from shScramble and shUCHL1 H-PDLSCs. F Quantification of tube formation shows the number of nodes, number of meshes, and total length of HUVECs. T represents TNF-α; I represents IL-1β. Statistical comparisons were analyzed using Mann–Whitney U test or two-way ANOVA; data are presented as mean ± S.E.M; * indicates P < 0.05 ** indicates P < 0.01, and *** indicates P < 0.001
UCHL1 promoted YAP activity under inflammatory conditions
To explore signaling pathways involving UCHL1’s function in regulating the pro-angiogenic capacity of PDLSCs, differentially expressed genes in P-PDLSCs versus H-PDLSCs were analyzed in the datasets (GSE78074). KEGG enrichment analysis showed that Hippo signaling pathway, which was associated with the regulation of angiogenic factors, was the most significantly enriched in the KEGG map of environmental information processing (Fig. 4A). YAP and Tafazzin (TAZ) are key components in Hippo signaling pathway and can shuttle from cytoplasm to nucleus to trigger the transcription of the downstream gene [20]. By analyzing GSE78074, the upregulation of YAP and TAZ was validated in P-PDLSCs (Fig. 4B). Further, we found a significant and positive correlation between YAP and UCHL1 expressions, but the correlation between UCHL1 and TAZ was not significant (Fig. 4C). Additionally, the upregulation and nuclear translocation of YAP were confirmed in the inflamed granulation tissues from periodontitis patients (Fig. 4D, E).
Fig. 4.
Knockdown of UCHL1 abrogated the increased YAP activity of PDLSCs under inflammatory conditions. A-C. Gene expressions in H-PDLSCs and P-PDLSCs were evaluated by analyzing GSE78074. A KEGG analysis shows that in upregulated genes of P-PDLSCs, the top 10 significantly enriched signaling pathways in the map of environmental information processing, cellular processes, metabolism, genetic information processing, and organismal systems. B Line plots show the gene expressions of UCHL1, YAP, and TAZ in H-PDLSCs and P-PDLSCs analyzed in GSE78074. C The correlations of the gene expression level of UCHL1 with YAP and TAZ were analyzed using Spearman’s correlation analysis. D YAP expression in the PDL tissues from healthy individuals and granulation tissues from periodontitis patients was evaluated using immunofluorescence. E YAP nucleus/cytoplasm ratios in the PDL tissues from healthy individuals and granulation tissues from periodontitis patients were calculated from immunofluorescence images. F–I H-PDLSCs were transfected with lentivirus controls (shScramble) or lentivirus shRNA targeted UCHL1 (shUCHL1). shScramble and shUCHL1 H-PDLSCs were treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. F. The protein expression levels of YAP, p-YAP, TAZ, p-TAZ, UCHL1, and GAPDH in shScramble and shUCHL1 H-PDLSCs were measured using western blotting. Full-length blots are presented in Supplementary Figs. 6–7. G The expression and the location of YAP in shScramble and shUCHL1 H-PDLSCs were measured using immunofluorescence. H YAP nucleus/cytoplasm ratios immunofluorescence were calculated from immunofluorescence images. I The mRNA expression level of YAP in shScramble and shUCHL1 H-PDLSCs was measured using RT-qPCR. J H-PDLSCs were pretreated with or without Verteporfin (0.1 μM) for 24 h and then treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. The protein expression levels of VEGFA, ANGPT1, and GAPDH were measured using western blotting. Full-length blots are presented in Supplementary Fig. 8. H represents the healthy group; P represents the periodontitis group; T represents TNF-α; I represents IL-1β. Statistical comparisons were analyzed using two-way ANOVA; Correlations were analyzed using Spearman's correlation analysis; data are presented as mean ± S.E.M; ns indicates not significant, *indicates P < 0.05, and *** indicates P < 0.001
To further investigate whether UCHL1 is involved in YAP regulation under inflammatory conditions, UCHL1 was knocked down in H-PDLSCs treated with TNF-α and IL-1β. As shown in Fig. 4F, UCHL1 knockdown abolished the increased protein expression of YAP under inflammatory conditions, while its influence on TAZ expression was negligible. Immunofluorescence results revealed that UCHL1 knockdown disrupted the nuclear translocation of YAP upon TNF-α and IL-1β stimulation (Fig. 4G, H). YAP stability and subcellular localization can be regulated by its phosphorylation [20]. However, UCHL1 knockdown did not change the phosphorylation of YAP (Fig. 4F). Instead, UCHL1 knockdown abrogated the elevated mRNA expression levels of YAP in TNF-α and IL-1β-treated H-PDLSCs (Fig. 4I), indicating that YAP expression is primarily controlled at the transcription level. Finally, we inhibited YAP in H-PDLSCs with the specific inhibitor targeting YAP, Verteporfin, and found that Verteporfin abrogated the upregulation of VEGFA and ANGPT1 upon TNF-α and IL-1β stimulation (Fig. 4J). These data suggested that UCHL1 increased YAP expression and activity in PDLSCs under inflammatory conditions, thus facilitating the secretion of VEGFA and ANGPT1.
UCHL1 stabilized HIF-1α to promote YAP transcription
To further elucidate the molecular mechanism underlying the UCHL1-mediated upregulation and activation of YAP, the protein–protein interactions between UCHL1 and YAP were generated using the protein–protein interaction prediction database. Among the identified interactions, we focused on HIF-1α, a subunit of a heterodimeric transcription factor HIF-1, which has been reported to participate in the pathogenesis of periodontitis and the process of angiogenesis [30, 31]. As shown in Fig. 5A, UCHL1 might regulate YAP through HIF-1α. Then we investigated the influence of UCHL1 knockdown on HIF-1α. HIF-1α was upregulated in H-PDLSCs upon TNF-α and IL-1β-stimulation, while UCHL1 knockdown abolished the upregulation of HIF-1α (Fig. 5B). Further, the interaction between UCHL1 and HIF-1α in TNF-α and IL-1β-stimulated H-PDLSCs was confirmed (Fig. 5C), which indicated that UCHL1 could stabilize HIF-1α under inflammatory conditions. As a transcription factor, HIF-1α can translocate into the nucleus and bind to the hypoxia response element region of DNA, thus triggering the transcription of genes [32]. The results of immunofluorescence showed that UCHL1 knockdown disrupted the TNF-α and IL-1β-induced nuclear translocation of HIF-1α (Fig. 5D, E). These results, combined with the predicted interaction between HIF-1α and YAP, indicated that HIF-1α might regulate YAP transcription in the nucleus. Using ChIP-qPCR, we confirmed that HIF-1α could bind to the YAP promoter, and TNF-α and IL-1β stimulation significantly increased the enrichment of HIF-1α to the YAP promoter in H-PDLSCs (Fig. 5F, G). Taken together, these data demonstrated that UCHL1 regulated YAP transcription by increasing the stability and activity of HIF-1α.
Fig. 5.
UCHL1 promoted YAP transcription via stabilizing HIF-1α. A Interactions between UCHL1, HIF-1α, and YAP were analyzed using STRING. B H-PDLSCs were transfected with lentivirus controls (shScramble) or lentivirus shRNA targeted UCHL1 (shUCHL1). Then shScramble and shUCHL1 H-PDLSCs were treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. The protein expression levels of HIF-1α, UCHL1, and GAPDH in shScramble and shUCHL1 H-PDLSCs were measured using western blotting. Full-length blots are presented in Supplementary Fig. 9. C H-PDLSCs were treated with TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. Interactions between UCHL1 and HIF-1α in H-PDLSCs were analyzed using Co-IP and western blotting. Full-length blots are presented in Supplementary Figs. 10, 11. D shScramble and shUCHL1 H-PDLSCs were treated with or without TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. The expression and the location of HIF-1α in shScramble and shUCHL1 H-PDLSCs were measured using immunofluorescence. E HIF-1α nucleus/cytoplasm ratios were calculated from immunofluorescence images. F, G H-PDLSCs were treated with TNF-α (2 ng/ml) and IL-1β (5 ng/ml) for 72 h. YAP promoter activity in H-PDLSCs was analyzed using ChIP-RT-qPCR. IgG was used as a control. T represents TNF-α; I represents IL-1β. Statistical comparisons were analyzed using two-way ANOVA; data are presented as mean ± S.E.M; ** indicates P < 0.01, and *** indicates P < 0.001
In vivo inhibition of UCHL1 alleviated aberrant angiogenesis and attenuated periodontitis
To observe the effect of UCHL1 inhibition on aberrant angiogenesis during the progression of periodontitis, the specific inhibitor targeting UCHL1, LDN57444, was administered following ligation in a ligature-induced periodontitis experimental model (Fig. 6A). H&E staining showed the destruction of gingival tissues induced by ligation, as well as the inflammatory vessels surrounding the destruction (Fig. 6B). Immunohistochemical staining for CD31, the vascular marker, revealed the increased number of blood vessels in the PDL tissues of periodontitis mice, while LDN57444 treatment markedly reduced this increase (Fig. 6C), indicating that UCHL1 inhibition alleviated aberrant angiogenesis in periodontitis. Accordingly, LDN57444 abrogated the increased expression of the pro-angiogenic factors, VEGFA and ANGPT1, in the PDL tissues of periodontitis mice (Fig. 6D). Since we have demonstrated that UCHL1 facilitated the secretion of VEGFA and ANGPT1 by upregulating YAP transcription, the inhibitory effect of LDN57444 on both mRNA and protein expression of YAP in periodontitis mice was also verified (Fig. 6E, F). Besides, we also verified that LDN57444 abrogated the upregulation of HIF-1α protein in periodontitis, while exhibiting negligible effects on Hif1α mRNA expression (Fig. 6G, H). These data also support that UCHL1 stabilizes HIF1α protein to potentiate YAP transcriptional activation.
Fig. 6.
UCHL1 inhibition reduced aberrant angiogenesis, inflammation, and alveolar bone resorption in murine experimental periodontitis. A Schematic illustration shows that UCHL1 inhibitor LDN57444 was administered to mice for 10 days after the ligation. B Representative images of H&E staining show the destruction of the gingival tissues triggered by ligature (red arrows) and the blood vessels surrounding the ligature place (black arrows). C Representative images of immunohistochemical staining show the expression of CD31 and indicate blood vessels (black arrows) in the gingival and PDL tissues. The area occupied by blood vessels in the gingival and PDL tissues was statistically quantified. D Representative images of immunohistochemical staining show the expression of VEGFA and ANGPT1. The expression of VEGFA and ANGPT1 was statistically quantified. E The mRNA expression level of Yap in gingival tissues was measured using RT-qPCR. F Representative images of immunohistochemical staining show the expression of YAP. The expression of YAP was statistically quantified. G The mRNA expression level of Hif1a in gingival tissues was measured using RT-qPCR. H Representative images of immunohistochemical staining show the expression of HIF-1α. The expression of HIF-1α was statistically quantified. I Representative immunofluorescent images and the quantification of the immunofluorescence show CD3-positive T cells (red) and CD20-positive B cells (green) in the gingival and PDL tissues in mice. J The mRNA expression levels of Tnfa, Il1b, Il6, and Ifng in gingival tissues were measured using RT-qPCR. K Representative images of 3D reconstructions and 2D slices were performed using micro-CT. In 3D reconstructions, the red area between CEJ and ABC denotes bone resorption area. Gray and pseudocolored 2D images show alveolar bone resorption (red arrows) between the maxillary M1 and M2 and at M1 furcation sites in a sagittal and horizontal plane (color map: CT values were outlined using an RGB pseudocolor scale). L The bone resorption area between CEJ and ABC, bone mineral density, and bone histomorphometry parameters, BV/TV and Tb.Th, were quantitatively analyzed. H represents the healthy group; P represents the periodontitis group; P + LDN represents the LDN57444-treated periodontitis group. Statistical comparisons were analyzed using Mann–Whitney U test or one-way ANOVA; data are presented as mean ± S.E.M; ns indicates not significant, and * indicates P < 0.05
During the progression of periodontitis, aberrant angiogenesis enables lymphocyte infiltration, which promotes inflammatory cytokines release and thereby alveolar bone resorption [3]. As expected, the infiltration of T cells (CD3 positive) and B cells (CD20 positive) increased in periodontitis mice, while UCHL1 inhibition with LDN57444 abrogated this increase (Fig. 6I). LDN57444 also reduced the levels of inflammatory cytokines, Tnfa, Il1b, Il6, and Ifng (Fig. 6J) in the gingival tissues of periodontitis mice. Further, micro-CT reconstructions showed that LDN57444 treatment reduced alveolar bone resorption in periodontitis mice, which is indicated by smaller damage area between cementoenamel junction and alveolar bone crest and smoother surface on alveolar bone surrounding the root of the maxillary M1 (Fig. 6K, L). Sagittal and horizontal Sections showed that the LDN57444-treated periodontitis mice exhibited higher bone mass density in the alveolar bone between M1 and M2 and at the furcation sites of M1 (Fig. 6K, L). Besides, BV/TV and Tb.Th were significantly increased in LDN57444-treated periodontitis mice (Fig. 6L). Collectively, these results indicate that in vivo inhibition of UCHL1 alleviated aberrant angiogenesis and attenuated the progression of periodontitis.
Discussion
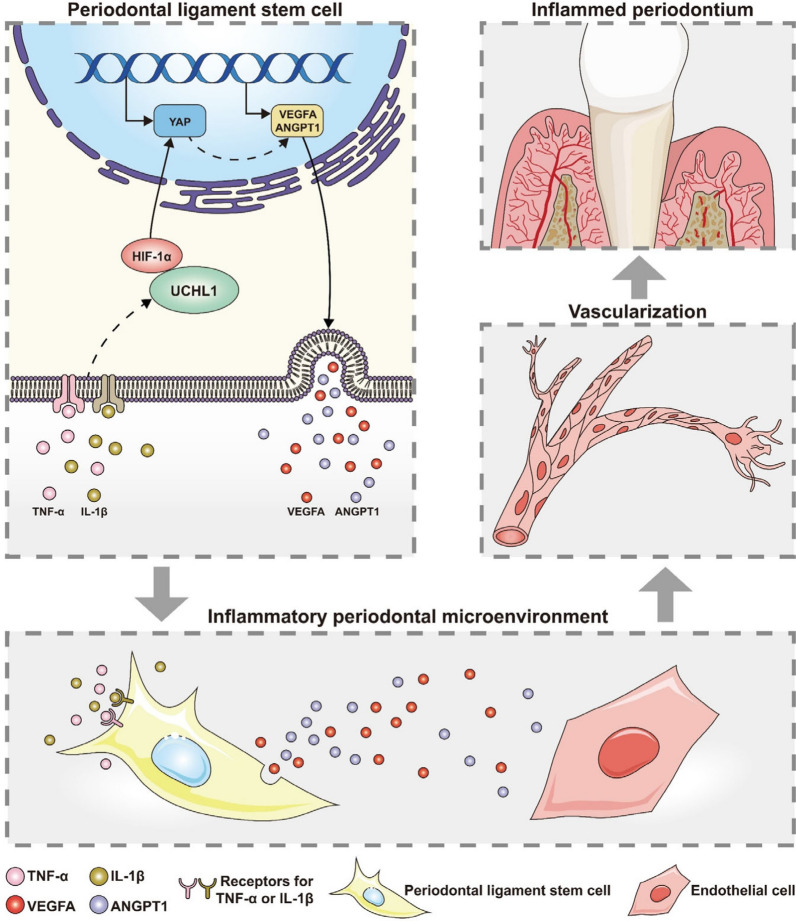
The current study revealed the pivotal function of UCHL1 in periodontitis to promote aberrant angiogenesis. Our findings demonstrated that UCHL1 promoted the pro-angiogenic capacity of PDLSCs in periodontitis by increasing the secretion of VEGFA and ANGPT1, which was mediated by the activation of YAP. Further, we found that UCHL1 upregulated and activated YAP by stabilizing and activating HIF-1α to boost YAP transcription (Fig. 7). Finally, in vivo inhibition of UCHL1 by LDN57444 in a murine periodontitis model alleviated aberrant angiogenesis and attenuated the progression of periodontitis. These findings shed light on the regulatory mechanism underlying the aberrant angiogenesis in periodontitis and provide an avenue for developing novel therapies for periodontitis.
Fig. 7.
Schematic diagram of the mechanism through which UCHL1 confers the pro-angiogenic capacity of PDLSCs in periodontitis. Under inflammatory conditions, the upregulated UCHL1 could stabilize and activate HIF-1α, thereby promoting YAP transcription, and resulting in the secretion of pro-angiogenic factors, VEGFA and ANGPT1 in PDLSCs. The increased pro-angiogenic capacity of PDLSCs subsequently promoted the angiogenesis of endothelial cells and thus played a role in periodontitis
Aberrant angiogenesis is a common feature in many inflammatory diseases and plays a pivotal role in inflammation. A considerable body of evidence has indicated the aberrant angiogenesis in periodontitis. More endothelial cells were found in periodontitis [10], and the expressions of pro-angiogenic factors, VEGF/VEGF receptor 2, were reported to progressively increase from mild to severe periodontal lesions [33]. These findings are in full agreement with our results, which indicated the enhanced vascularization and elevated levels of VEGFA and ANGPT1 in periodontitis. Further, our data demonstrated that in periodontitis, PDLSCs could promote the proliferation, migration, and tube formation of HUVECs by secreting VEGFA and ANGPT1. Combined with the previous studies that PDLSCs facilitated the angiogenesis of HUVECs under inflammatory conditions [10, 11], it is indicated that PDLSCs play a crucial role in facilitating aberrant angiogenesis in periodontitis.
This study highlights the role of UCHL1 in modulating paracrine signaling pathways by regulating the secretion of VEGFA and ANGPT1 in PDLSCs during angiogenesis. In addition to its role in paracrine pathways, UCHL1 has been demonstrated to promote angiogenesis by directly regulating the biological functions of endothelial cells. For example, UCHL1 was found to promote the angiogenesis of HUVECs by increasing VEGF-induced reactive oxygen species and activating VEGF receptor [34], thus facilitating the proliferation and migration of human choroidal or retinal endothelial cells. UCHL1 could potentiate transforming growth factor-beta signaling [35], which is indispensable for angiogenesis. Besides, our results (data not shown) also showed the elevation of UCHL1 expression in endothelial cells in inflamed granulation tissues compared with healthy PDL tissues. Therefore, it is possible that UCHL1 could regulate angiogenesis by modulating both the paracrine pathways and endothelial cell functions in periodontitis.
In this study, YAP mediated the mechanism through which UCHL1 regulated the secretion of VEGFA and ANGPT1. YAP is the key effector protein of Hippo signaling pathway and acts as a transcriptional coactivator when translocated to the nucleus [20]. Abundant evidence has revealed a pivotal role of YAP in facilitating the transcription of various pro-angiogenic factors under diverse pathophysiological conditions, such as retinal vessel development [36], mechanical stimuli [37], and reactive oxygen species [38]. This study focused on periodontal inflammation and found that UCHL1 upregulated YAP under inflammatory conditions. Emerging evidence has progressively elucidated the role of YAP in inflammatory diseases [39]. In atherosclerosis, IL-1β increased YAP stability and nuclear translocation, thereby increasing chemokine secretion in macrophages [40]. Hence, the important role of YAP in UCHL1-regulated angiogenesis is revealed during periodontal inflammation.
UCHL1 typically exerts its biological functions by regulating post-translational modifications of proteins [13]. However, this study demonstrated that UCHL1 did not regulate YAP expression by modulating the upstream kinases of YAP or through direct post-translational modification of YAP. Instead, UCHL1 regulated YAP expression by stabilizing HIF-1α, which translocated to the nucleus to promote YAP transcription. Previous research has demonstrated that UCHL1 served as a deubiquitinating enzyme to stabilize HIF-1α, thus protecting cardiomyocytes in myocardial infarction [17], which is consistent with our results. HIF-1α is known as a principal transcription factor of key genes involved in angiogenesis, such as VEGF and ANGPT [30]. Therefore, it is likely that the secretion of VEGFA and ANGPT1 in PDLSCs was modulated via both UCHL1/HIF-1α/YAP axis and UCHL1/HIF-1α axis in periodontitis. Of note, a considerable body of evidence has revealed that YAP could interact with HIF-1α in the nucleus and sustain the stability of HIF-1α protein [41, 42]. Therefore, it is likely that YAP, upregulated by HIF-1α, in turn stabilized HIF-1α and boosted its function in PDLSCs, which formed a positive feedback loop and thereby amplified the effect of UCHL1 on the pro-angiogenic capacity of PDLSCs. However, this hypothesis should be further confirmed.
Given that UCHL1 promoted the pro-angiogenic capacity of PDLSCs and aberrant angiogenesis is an essential pathological process during the progression of periodontitis, we focused on the progression period of periodontitis in the ligature-induced periodontitis model. As expected, in vivo inhibition of UCHL1 markedly alleviated aberrant angiogenesis during the progression of periodontitis, thereby decreasing lymphocyte infiltration, pro-inflammatory factor levels, and inflammatory bone resorption. Notably, in our previous study, in vivo inhibition of UCHL1 during the healing period of periodontitis enhanced alveolar bone repair and decreased pro-inflammatory factor levels [14]. Hence, it can be inferred that UCHL1 presumably played a negative role in periodontitis and might serve as a target for the treatment of periodontitis. Intriguingly, we noticed that UCHL1 inhibition reduced pro-inflammatory cytokine levels during both the progression and healing period of periodontitis [14]. It has been reported that UCHL1 inhibition decreased NLRP3-mediated IL-1β production in macrophages [43]. UCHL1 could facilitate the generation of MHC class I peptide complexes and antigen cross-presentation in dendritic cells [44], as well as the polarization of M1 macrophages [45]. In view of these studies, it is possible that UCHL1 might directly regulate the function of inflammatory cells in periodontitis.
Conclusions
This study highlighted the role of UCHL1 in aberrant angiogenesis in periodontitis. We demonstrated that UCHL1 promoted the pro-angiogenic capacity of PDLSCs by increasing the secretion of VEGFA and ANGPT1 in PDLSCs under inflammatory conditions. Then the molecular mechanism that UCHL1 functioned through increasing YAP transcription via stabilizing HIF-1α was depicted. Furthermore, in murine experimental periodontitis, UCHL1 inhibition alleviated aberrant angiogenesis and attenuated the progression of periodontitis. Hence, this study shed light on the mechanism of aberrant angiogenesis in periodontitis and provided an avenue for developing novel therapies for periodontitis.
Supplementary Information
Acknowledgements
The authors acknowledge the funding supported from National Natural Science Foundation of China [grant number 82401140, 52171075, 82271589]; the Non- profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number 2023-JKCS-14]; Science and Technology Commission of Shanghai Municipality [grant number 21DZ2294700], Opening Project of Shanghai Key Laboratory of Orthopaedic Implant [grant number KFKT2021001]; Incubating Program for National Program of Renji Hospital, School of Medicine, Shanghai Jiao Tong University [grant number RJTJ23-PY-052]. The authors also acknowledged the authors for sharing the public datasets GSE78074 used in this work. The authors declare that they have not use AI-generated work in this manuscript.
Abbreviations
- PDL
Periodontal ligament
- PDLSCs
Periodontal ligament stem cells
- FGF
Fibroblast growth factor
- VEGFA
Vascular endothelial growth factor A
- UCHL1
Ubiquitin C-terminal hydrolase L1
- HIF-1α
Hypoxia-inducible factor 1 alpha
- YAP
Yes-associated protein
- TNF-α
Tumor necrosis factor-alpha
- ANGPT1
Angiopoietin 1
- IL-1β
Interleukin-1beta
- HUVECs
Human umbilical vein endothelial cells
- BSA
Bovine serum albumin
- CCK-8
Cell Counting Kit-8
- PBS
Phosphate-buffered saline
- GEO
Gene expression omnibus
- KEGG
Kyoto encyclopedia of genes and genomes
- RT-qPCR
Reverse transcription-quantitative polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- PBST
PBS-Tween (0.1% v/v)
- ELISA
Enzyme-linked immunosorbent assay
- STRING
The search tool for the retrieval of interacting genes
- Co-IP
Co-immunoprecipitation
- ChIP
Chromatin immunoprecipitation
- DMSO
Dimethyl sulfoxide
- M1
The first molar
- H&E
Hematoxylin & eosin
- micro-CT
Micro-computed tomography
- 3D
3-Dimensional
- 2D
2-Dimensional
- CEJ
Cemento-enamel junction
- ABC
Alveolar bone crest
- BMD
Bone mineral density
- BV/TV
Bone volume/tissue volume
- Tb.Th
Trabecular thickness
- S.E.M
Standard error of mean
- H-PDLSCs
PDLSCs from healthy individuals
- P-PDLSCs
PDLSCs from periodontitis patients
- shUCHL1
Lentivirus short hairpin RNA targeting UCHL1
- shScramble
Lentivirus controls
- TAZ
Tafazzin
Author contributions
L.L. contributed to conception and design, acquisition, analysis, and interpretation, drafted manuscript, and critically revised manuscript; W.J.Y. contributed to acquisition, analysis, and interpretation, and critically revised manuscript; R.H.Y. contributed to design, acquisition, and critically revised manuscript; M.Z.L. contributed to analysis and interpretation, and critically revised manuscript; Y.J.S. contributed to acquisition, and critically revised manuscript; S.C.H. contributed to acquisition, and critically revised manuscript; G.L.L. contributed to acquisition and interpretation, and critically revised manuscript; J.Q.T. contributed to acquisition, and critically revised manuscript; Y.S. contributed to acquisition, and critically revised manuscript; Z.R.C. contributed to acquisition, and critically revised manuscript; M.J. contributed to design, analysis and interpretation, and critically revised manuscript; Y.T.G. contributed to conception and design, analysis, drafted manuscript, and critically revised manuscript; E.Y.L. contributed to conception, and critically revised manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Funding
The work was supported by National Natural Science Foundation of China [grant number 82401140, 52171075, 82271589]; the Non- profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number 2023-JKCS-14]; Science and Technology Commission of Shanghai Municipality [grant number 21DZ2294700], Opening Project of Shanghai Key Laboratory of Orthopaedic Implant [grant number KFKT2021001]; Incubating Program for National Program of Renji Hospital, School of Medicine, Shanghai Jiao Tong University [grant number RJTJ23-PY-052].
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request. The public GEO datasets (GSE78074) used in this research can be accessed via the following link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE78074.
Declarations
Ethics approval and consent to participate
The study titled “The Regulatory Mechanism of Protein Post-translational Modifications on the Functions of Periodontal Ligament Stem Cells in Periodontitis” was approved by Shanghai Jiao Tong University School of Medicine, Renji Hospital Ethics Committee, approval number “KY[2019]068” (Date of approval: 06/19/2019). HUVECs were purchased from ALLCELLS, which has confirmed that the ethical approval for collecting human cells was obtained, and the donors had signed informed consent (ALLCELLS, https://allcells.com/about-allcells/donor-facilities/). The animal study titled “The Mechanisms of UCHL1 to promote periodontitis by regulating inflammatory angiogenesis” was approved by Laboratory Animal Welfare and Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University; approval number: RJ2024-197A (Date of approval: 11/25/2024).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests" in this section.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Jin, Email: jinmin@renji.com.
Yuting Gu, Email: guyutingxm@126.com.
Eryi Lu, Email: lueryi222@outlook.com.
References
- 1.Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–60. [DOI] [PubMed] [Google Scholar]
- 2.Bonakdar MP, Barber PM, Newman HN. The vasculature in chronic adult periodontitis: a qualitative and quantitative study. J Periodontol. 1997;68(1):50–8. [DOI] [PubMed] [Google Scholar]
- 3.Jeong JH, Ojha U, Lee YM. Pathological angiogenesis and inflammation in tissues. Arch Pharm Res. 2021;44(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lira-Junior R, Figueredo CM, Bouskela E, Fischer RG. Severe chronic periodontitis is associated with endothelial and microvascular dysfunctions: a pilot study. J Periodontol. 2014;85(12):1648–57. [DOI] [PubMed] [Google Scholar]
- 5.Dudley AC, Griffioen AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. 2023;26(3):313–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–55. [DOI] [PubMed] [Google Scholar]
- 7.Adolpho LF, Lopes HB, Freitas GP, Weffort D, Campos Totoli GG, Loyola Barbosa AC, et al. Human periodontal ligament stem cells with distinct osteogenic potential induce bone formation in rat calvaria defects. Regen Med. 2022;17(6):341–53. [DOI] [PubMed] [Google Scholar]
- 8.Fawzy El-Sayed KM, Elahmady M, Adawi Z, Aboushadi N, Elnaggar A, Eid M, et al. The periodontal stem/progenitor cell inflammatory-regenerative cross talk: a new perspective. J Periodontal Res. 2019;54(2):81–94. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Deng M, Hao M, Tang J. Periodontal ligament stem cells in the periodontitis niche: inseparable interactions and mechanisms. J Leukoc Biol. 2021;110(3):565–76. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Shuai Y, Zhou F, Yin J, Hu J, Guo S, et al. PDLSCs regulate angiogenesis of periodontal ligaments via vegf transferred by exosomes in periodontitis. Int J Med Sci. 2020;17(5):558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W, An Y, An Y, Fei D, Wang Q. Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol. 2018;89(6):718–27. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Cheng Y, Zheng M, Yuan B, Wang Z, Li X, et al. Targeting the ubiquitination/deubiquitination process to regulate immune checkpoint pathways. Signal Transduct Target Ther. 2021;6(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mennerich D, Kubaichuk K, Kietzmann T. DUBs, hypoxia, and cancer. Trends Cancer. 2019;5(10):632–53. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Li S, Hu S, Yu W, Jiang B, Mao C, et al. UCHL1 impairs periodontal ligament stem cell osteogenesis in periodontitis. J Dent Res. 2023;102(1):61–71. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y, Ding X, Huang J, Xue M, Zhang J, Wang Q, et al. The deubiquitinating enzyme UCHL1 negatively regulates the immunosuppressive capacity and survival of multipotent mesenchymal stromal cells. Cell Death Dis. 2018;9(5):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J, Liu H, Shen Y, Ding J, He H, Mao S, et al. Deubiquitinase UCHL1 stabilizes KDM4B to augment VEGF signaling and confer bevacizumab resistance in clear cell renal cell carcinoma. Transl Oncol. 2024;45:101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng B, Wang X, Park KH, Lee KE, Kim J, Chen P, et al. UCHL1 protects against ischemic heart injury via activating HIF-1α signal pathway. Redox Biol. 2022;52:102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Luo W, Sun Y, Qiao Y, Zhang L, Zhao Z, et al. Wnt/β-catenin signaling mediated-UCH-L1 expression in podocytes of diabetic nephropathy. Int J Mol Sci. 2016;17(9):1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin K, Yu M, Fan J, Wang H, Zhao P, Zhao G, et al. Canonical and noncanonical Wnt signaling: multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024;11(1):103–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boopathy GTK, Hong W. Role of hippo pathway-YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol. 2019;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driskill JH, Pan D. Control of stem cell renewal and fate by YAP and TAZ. Nat Rev Mol Cell Biol. 2023;24(12):895–911. [DOI] [PubMed] [Google Scholar]
- 22.Pan W, Yang L, Li J, Xue L, Wei W, Ding H, et al. Traumatic occlusion aggravates bone loss during periodontitis and activates Hippo-YAP pathway. J Clin Periodontol. 2019;46(4):438–47. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Meng X, Piao Y, Dong S, Dong Q. METTL3 promotes the osteogenic differentiation of human periodontal ligament cells by increasing YAP activity via IGF2BP1 and YTHDF1-mediated m(6)A modification. J Periodontal Res. 2024;59(5):1017–30. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Wang H, Zhang H, Sun B, Wang Z. A novel mechanism of mscs responding to occlusal force for bone homeostasis. J Dent Res. 2024;103(6):642–51. [DOI] [PubMed] [Google Scholar]
- 25.Meng X, Zhu Y, Tan H, Daraqel B, Ming Y, Li X, et al. The cytoskeleton dynamics-dependent LINC complex in periodontal ligament stem cells transmits mechanical stress to the nuclear envelope and promotes YAP nuclear translocation. Stem Cell Res Ther. 2024;15(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan K, Wang J, Su X, Zheng Y, Li W. KAT6A/YAP/TEAD4 pathway modulates osteoclastogenesis by regulating the RANKL/OPG ratio on the compression side during orthodontic tooth movement. Prog Orthod. 2024;25(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Yu W, Zhang W, Li S, Hu S, Jiang B, et al. Expression profile of lipoxygenases in gingival tissues of human periodontitis. Oral Dis. 2021;27(3):567–76. [DOI] [PubMed] [Google Scholar]
- 28.Marech I, Leporini C, Ammendola M, Porcelli M, Gadaleta CD, Russo E, et al. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. 2016;380(1):216–26. [DOI] [PubMed] [Google Scholar]
- 29.Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(D1):D638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Invest. 2020;130(10):5074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MH, Wang YH, Sun BJ, Yu LM, Chen QQ, Han XX, et al. HIF-1α activator DMOG inhibits alveolar bone resorption in murine periodontitis by regulating macrophage polarization. Int Immunopharmacol. 2021;99:107901. [DOI] [PubMed] [Google Scholar]
- 32.Cowman SJ, Koh MY. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer. 2022;8(1):28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vladau M, Cimpean AM, Balica RA, Jitariu AA, Popovici RA, Raica M. VEGF/VEGFR2 axis in periodontal disease progression and angiogenesis: basic approach for a new therapeutic strategy. In Vivo. 2016;30(1):53–60. [PubMed] [Google Scholar]
- 34.Song IK, Kim HJ, Magesh V, Lee KJ. Ubiquitin C-terminal hydrolase-L1 plays a key role in angiogenesis by regulating hydrogen peroxide generated by NADPH oxidase 4. Biochem Biophys Res Commun. 2018;495(1):1567–72. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, González-Prieto R, Zhang M, Geurink PP, Kooij R, Iyengar PV, et al. Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFβ-induced breast cancer metastasis. Clin Cancer Res. 2020;26(6):1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. [DOI] [PubMed] [Google Scholar]
- 37.Hong SP, Yang MJ, Cho H, Park I, Bae H, Choe K, et al. Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi. Nat Commun. 2020;11(1):4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Zou R, Dai X, Wu X, Yuan F, Feng Y. YAP is critical to inflammation, endothelial-mesenchymal transition and subretinal fibrosis in experimental choroidal neovascularization. Exp Cell Res. 2022;417(2):113221. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Jin X, Ma J, Xiang B, Li X. YAP at the progression of inflammation. Front Cell Dev Biol. 2023;11:1204033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Yan M, Lv H, Wang B, Lv X, Zhang H, et al. Macrophage K63-Linked Ubiquitination of YAP Promotes Its Nuclear Localization and Exacerbates Atherosclerosis. Cell Rep. 2020;32(5):107990. [DOI] [PubMed] [Google Scholar]
- 41.Kashihara T, Mukai R, Oka SI, Zhai P, Nakada Y, Yang Z, et al. YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J Clin Invest. 2022. 10.1172/JCI150595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H, Qiu Z, Wang Y, Xi C, Zhang G, Sun Z, et al. HIF-1α/YAP signaling rewrites glucose/iodine metabolism program to promote papillary thyroid cancer progression. Int J Biol Sci. 2023;19(1):225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Z, Damianou A, Vendrell I, Jenkins E, Lassen FH, Washer SJ, et al. Proximity proteomics reveals UCH-L1 as an essential regulator of NLRP3-mediated IL-1β production in human macrophages and microglia. Cell Rep. 2024;43(5):114152. [DOI] [PubMed] [Google Scholar]
- 44.Reinicke AT, Raczkowski F, Mühlig M, Schmucker P, Lischke T, Reichelt J, et al. Deubiquitinating enzyme UCH-L1 promotes dendritic cell antigen cross-presentation by favoring recycling of MHC Class I molecules. J Immunol. 2019;203(7):1730–42. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, He S, Chen Y, Sheng J, Fu Y, Du X, et al. UCHL1 promoted polarization of M1 macrophages by regulating the PI3K/AKT signaling pathway. J Inflamm Res. 2022;15:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request. The public GEO datasets (GSE78074) used in this research can be accessed via the following link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE78074.