Abstract
Micronutrients play a crucial role in several metabolic processes including thyroid hormone metabolism and functions. The current study aimed to assess the associations between thyroid hormone levels and micronutrient status in a cohort of adolescents Afghan refugees residing in a refugee camp in Pakistan. A randomised, community based, cross-sectional study design was employed to recruit 206 adolescent (both male and female) Afghan refugees aged 10–19 years. Sociodemographic data, anthropometric assessments and blood samples were collected using standard methods. Serum vitamins, minerals and thyroid hormones levels were assessed using ELISA, electrochemiluminescence and inductively coupled plasma mass spectrometry (ICP-MS) respectively. Overall results showed the median levels of T3 and TSH were significantly higher (p < 0.05) in younger adolescents (10–14 years) compared to 15–18 year olds while T4 was significantly higher in boys compared to girls. Correlational analysis between serum micronutrients status (vitamin D, vitamin B12, ferritin, folate, zinc, copper, selenium) and thyroid hormones revealed significant relationships in different age groups. Overall, vitamin D exhibited a statistically significant positive correlation with T4 (r = 0.279) in the combined, younger (r = 0.277) and older (r = 0.319) age groups. In contrast, a statistically significant but negative correlation was observed when zinc levels were compared with T3 (r=-0.288) in the older age group and with T4 (r=-0.195) in the younger age group. In conclusion, micronutrients status, especially vitamin D and zinc, have important implications for thyroid health and thereby require close monitoring in any thyroid deficiency related disorders in vulnerable population such as refugees.
Clinical trial number: Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13044-025-00239-6.
Introduction
Micronutrient deficiencies are generally caused by inadequate intake of essential micronutrients (vitamins and minerals) and represent a major global public health burden. This problem is largely limited to developing nations and socioeconomically disadvantaged populations. Estimated reports from the World Health Organization (WHO) indicate that over 2 billion people worldwide suffer the physiological consequences of micronutrient insufficiency [1]. Micronutrient insufficiencies are widespread causes of suppressed immune function, disturbed metabolism, impaired physical and cognitive development in children, and increased risk of chronic conditions such as cancer and cardiovascular diseases [2]. Optimal metabolism in humans is predicated on adequate supplies of micronutrients that act as enzyme cofactors and structural components for macromolecules. One of the major human-health impacts of micronutrient availability is thyroid metabolism and function [3].
In humans, the thyroid gland plays an important role in growth and development through maintenance of homeostasis and by supporting the normal function of the cardiovascular, reproductive and nervous systems [4]. Thyroid function is primarily regulated by the hypothalamic-pituitary-thyroid axis through the action of thyroid-stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4). Thyroid hormones are crucial for cellular development, differentiation, growth, and regulation of protein, lipid and carbohydrate metabolism in nearly all tissues [5]. Thyroid hormone signaling is also essential for normal growth and maturation of organs such as the brain, lung, heart, skeletal muscle and bone. However, the production of inappropriate amounts of thyroid hormones results in hypothyroidism or hyperthyroidism which have devastating consequences on human health. Hyperthyroidism causes weight loss, heat intolerance and rapid heartbeat (tachycardia) while hypothyroidism is characterized by weight gain, feeling cold (cold intolerance), constipation, enlargement of the thyroid gland (goiter) and slowed metabolism (metabolic disruptions) [6]. Although the exact etiology and pathogenesis of thyroid disorders are not known, a role for micronutrients and trace elements is frequently suggested. Several micronutrients, especially trace elements, are found in higher concentration in the thyroid gland than in any other tissue in the body [7] and are essential for thyroid hormone synthesis, metabolism and function. Micronutrient status is also a key determinant of risk and severity of autoimmune thyroid disorders (AITD) as both micronutrient deficiency and excess can promote autoimmune attack on the thyroid gland. Indeed, epidemiological studies indicate an increased susceptibility to pathogenic thyroid dysfunction is linked to the dietary availability of the micronutrients iodine, iron, selenium, copper, zinc, and vitamins B12 and D [8].
Micronutrients, especially trace elements, are crucial to maintain thyroid metabolism and function. Mounting research evidence suggests that iron and iodine are closely related to thyroid metabolism and, as a result, iron deficiency is frequently associated with thyroid dysfunctions in humans [9]. Se deficiency has been repeatedly associated with increased risk of AITD, such as Graves’ disease and Hashimotos’s thyroiditis, and is also linked with exacerbated developmental hypothyroidism primarily caused by iodine deficiency [10]. In-vivo studies in animal models demonstrate that inadequate zinc levels impair thyroid hormone production, potentially causing hypothyroidism, developmental abnormalities and compromised immunity [11]. Thyroid hormones are also reported to modulate Zn homeostasis by regulating its intestinal absorption and renal reabsorption in rats [12]. In humans, the finding of a strong correlation between tissue levels of thyroid hormones and zinc suggests an intimate bidirectional relationship between the two [13]. Furthermore, zinc supplementation in humans results in a significant increase in plasma TSH, T3 and T4 levels [14].
Although the impact of micronutrients on thyroid functions has been frequently reported in the literature, such studies have been mostly focused on thyroid disease patients, children and pregnant women [15, 16]. Until now, little or no research investigating the relationship between micronutrient status and thyroid function has been conducted in populations that are at high risk of malnutrition, such as refugees. Refugee populations living in refugee camps, are particularly vulnerable to malnutrition due to factors like chronic food insecurity, limited access to diverse diets, and prolonged exposure to stress and adverse living conditions [17]. Consequently, malnutrition (especially deficiencies of essential micronutrients like iodine, selenium, and zinc) render these population at higher risk of endocrine and metabolic disorders, including thyroid dysfunction [18]. Therefore, this cross-sectional study aimed to investigate the associations between thyroid hormone levels and micronutrient status in a cohort of adolescent Afghan refugees residing in a refugee camp in Pakistan.
Methods
Study design and population
This cross-sectional study analysed 206 adolescent (both male and female) Afghan refugees (aged 10–19 years) residing in Khazana refugee camp, the largest refugee camp (5000 residents) in Peshawar, Pakistan. All participants shared similar regional backgrounds, dietary limitations and access to healthcare, making them an appropriate cohort for examining micronutrient levels in relation to thyroid function in a nutritionally vulnerable population. They were recruited by a non-probability, consecutive sampling method in a study assessing the nutritional status of apparently healthy adolescents [19]. Pregnant or lactating women, those physically or mentally handicapped and those who were on medication (antibiotics, nutritional supplements, pre and probiotics) at the time of data collection or in the past two months were excluded from the study. Participants with incomplete responses in the questionnaire data (n = 2), or missing plasma micronutrients data (n = 17) or thyroid hormone levels (n = 16) were also excluded from analysis yielding a final sample of 182 participants (91 boys and 91 girls). An information sheet containing the detailed procedures and purpose of the study in an easy to understand, local language (Pashto and Dari) was provided to all the participants before signing a written informed consent. Ethical and administrative approval for the study was provided by Khyber Medical University and the Peshawar Afghan Commissionerate (Peshawar), respectively.
Data and samples collection
Participant demographic and socioeconomic status information was collected by an interviewer-administered, structured questionnaire. Trained data collectors measured the height and weight of the participants using standard procedures. The body mass index (kg/m2) was calculated using the participants’ height and weight. Blood samples were drawn between 08:00 and 10:00 a.m. to account for diurnal variations in micronutrient and hormone levels. Plasma and serum were separated, transported to the main lab within 4 h and stored at -80 °C prior to analysis.
Laboratory assays
Complete blood counts were performed on freshly collected whole blood samples using a Sysmex automatic haematology analyzer (XP-100, Jalan Tukang, Singapore). Vitamin D status was assessed using a 25-OH vitamin D Diasorin radioimmunoassay ELISA kit (Euroimmun, Germany) while ferritin, vitamin B12 and folate were assessed using the Abbott Architect i2000 analyser (Abbott Diagnostics, Zug, Switzerland). Thyroid function tests were performed to measure the serum levels of total T3, T4 and thyroid stimulating hormone (TSH) using an Elecsys electrochemiluminescence based assay on a Cobas e 411 analyser (Roche Diagnostics, Germany). The levels of iron, zinc, selenium and copper were determined by reconstituting freeze-dried plasma samples in Milli-Q (18 MΩ) water and subjecting it to inductively coupled plasma mass spectrometry analysis (ICP-MS; Thermo Fisher Scientific iCAPQ, Bremen, Germany).
Statistical analysis
Age is reported as mean (SD) and age categories, educational status, family size and female household head educational status are reported as frequencies (percentage). This study used tertiles to categorize serum levels of T3, T4, and TSH to facilitate comparison across low, middle, and high levels of thyroid hormones. This tertile-based classification allows for assessing variations in micronutrient concentrations across different thyroid hormone ranges without assuming normality in data distribution, making it suitable for evaluating nonlinear relationships in micronutrient-thyroid interactions. Micronutrient concentrations are described as medians (Q1 and Q3) by tertiles for T3, T4 and TSH. Quantile regression was used to investigate association among thyroid hormones (T3, T4 and TSH).
Statistical analyses considered age, gender and BMI as demographic variables. Gender distribution was assessed across the tertile groups, which did not reveal statistically significant differences but indicated a trend. For clarity, analyses were adjusted to control for gender effects on micronutrient-thyroid associations, minimizing any potential confounding influence from gender differences within tertiles. Due to non-normal distribution of the micronutrient levels, the linear trends of these biomarkers across ordered categories of thyroid hormones (T3, T4 and TSH) were assessed using the Jonckheere Terpstra Test. Correlation between biomarkers and thyroid hormones were assessed using Spearman rank correlation. All data analysis was conducted using SAS Version 9.4.
Results
General characteristics of the study participants
General characteristics of the 206 study participants, 103 males and 103 females are presented in Table 1. Participants were divided into two age groups, 10–14 (63.7%) and 15–19 (36.3%) years. Background characteristics of the study participants were described previously and are thus provided here in supplementary Table S1.
Table 1.
Association of thyroid hormone levels with age, sex and BMI
| Characteristics | T3 | T4 | TSH |
|---|---|---|---|
| All ages | |||
| Age (Categories) | 0.22 (0.05, 0.43) * | 0.2 (-0.75, 0.81) | 0.46 (0.01, 0.74) * |
| Sex (Gender = 1) | 0.12 (-0.01, 0.26) | 1.29 (0.64, 1.94) * | 0.07 (-0.27, 0.34) |
| BMI (kg/m2) | 0.001 (-0.04, 0.03) | 0.1 (-0.08, 0.2) | 0.04 (-0.03, 0.11) |
| Age Group (10–14 Years) | |||
| Sex (Gender = 1) | 0.18 (0, 0.29) ** | 1.32 (0.36, 2.04) * | -0.11 (-0.47, 0.42) |
| BMI (kg/m2) | 0.02 (-0.04, 0.04) | 0.17 (-0.06, 0.37) | 0.08 (-0.04, 0.13) |
| Age Group (14–19 Years) | |||
| Sex (Gender = 1) | 0.16 (-0.33, 0.29) | 1.26 (0.15, 2.21) * | 0.29 (-0.05, 0.59) |
| BMI (kg/m2) | -0.01 (-0.07, 0.02) | 0.001 (-0.18, 0.19) | -0.01 (-0.09, 0.05) |
Coefficient estimates with 95% CI from quantile regression modeling median of T3, T4 and TSH to investigate association with age, sex and BMI. * p-value < 0.05, **p-value = 0.05
Association between demographic and nutritional status, and thyroid function biomarkers
We first assessed whether there is any relationship between demographic characteristics (age, gender), nutritional status (BMI) and median levels of thyroid hormones among the participants (Table 1). Overall, the results show that median levels of T3 and TSH are significantly higher in 10–14 years as compared to 15–18 years. Although no significant association was observed for median levels of T4 with age, T4 concentration was significantly higher in boys compared to girls. Stratified analysis by age group showed that T4 levels are significantly higher among boys in both age groups while T3 was high only in the younger adolescent group (10–14 years). However, no significant association was observed between thyroid hormones level and BMI (Table 1).
Association between plasma micronutrients levels and thyroid function biomarkers
To assess the association of selected micronutrients with thyroid function biomarkers, the median concentration of the micronutrients was divided into three equivalently sized tertiles based on serum T3, T4 and TSH levels. No statistically significant trend was observed between serum micronutrient levels across the three tertiles based on serum T3 levels (Table 2). However, stratified analysis by age showed a significantly decreasing trend in serum ferritin and zinc levels from across tertile 1.
Table 2.
Comparison of micronutrientstatus and T3 levels in adolescent Afghan refugees. Comparison was achieved by dividing participants into three equally-sized tertiles according to T3 levels
| Characteristics | T3 | ||||
|---|---|---|---|---|---|
| Overall | Tertile 1 | Tertile 2 | Tertile 3 | P-trend | |
| Median (Q1, Q3) of T3 | 1.05 (0.93, 1.16) | 1.42 (1.33, 1.51) | 1.79 (1.67, 2.06) | ||
| All age groups | |||||
| Ferritin (ng/mL) | 39 (27, 61.4) | 48.0 (29.5, 79.6) | 35.0 (24.6, 49.0) | 40.6 (26.54, 59.45) | 0.09 |
| Folate (ng/mL) | 4.4 (3.4, 6.1) | 4.6 (3.1, 6.6) | 4.3 (3.6, 5.8) | 4.2 (3.6, 6) | 0.82 |
| Vitamin B12 (pg/mL) | 219 (157, 348) | 228 (167, 392) | 220.0 (155.0, 349.0) | 205.0 (161.0, 300.0) | 0.29 |
| Vitamin D (ng/mL) | 22.3 (16.2, 27.5) | 20.4 (13.9, 25.1) | 25.3 (19.3, 28.9) | 21.5 (15.7, 26.9) | 0.17 |
| Zinc (µg/L) | 787 (619, 932) | 809 (637, 939) | 784.5 (623.8, 934.4) | 758.6 (593.7, 891.7) | 0.33 |
| Copper (µg/L) | 951 (791, 1147) | 936 (771, 1217) | 970.2 (827.1, 1134.6) | 951.4 (797.1, 1082.3) | 0.56 |
| Selenium (µg/L) | 71.1 (50.5, 91.3) | 72.4 (55.4, 93.3) | 75.2 (50.5, 96.1) | 62.9 (45.1, 84.2) | 0.10 |
| Age (10–14) | |||||
| Ferritin (ng/mL) | 37.7 (27.8, 51.6) | 39.4 (29.5, 60.3) | 35.5 (26.9, 44.7) | 40.7 (27.2, 59.5) | 0.95 |
| Folate (ng/mL) | 4.7 (3.6, 6.1) | 5.3 (4, 6.8) | 4.5 (3.625, 5.7) | 4 (3.6, 5.7) | 0.08 |
| Vitamin B12 (pg/mL) | 209 (155, 310) | 209.5 (157, 337) | 213.5 (155.5, 302.5) | 205.0 (154.0, 287.0) | 0.56 |
| Vitamin D (ng/mL) | 24.1 (7.3) | 22.9 (6.9) | 24.1 (7.5) | 25.0 (7.5) | 0.33 |
| Zinc (µg/L) | 789.6 (605.6, 934.4) | 773.2 (576.6, 900.7) | 798.4 (631.2, 933.7) | 789.6 (642.6, 949.4) | 0.46 |
| Copper (µg/L) | 992 (824.6, 1146.8) | 933.3 (765.7, 1188.6) | 1014.1 (837.4, 1146.4) | 987.7 (824.6, 1118.3) | 0.78 |
| Selenium (µg/L) | 72 (48.4, 91.4) | 73.4 (49.3, 102.5) | 70.5 (46.3, 95.5) | 68.5 (54.2, 86.2) | 0.48 |
| Age (15–18) | |||||
| Ferritin (ng/mL) | 48.3 (23.5, 79.8) | 66.1 (31.3, 105.4) | 34.1 (17.5, 57.3) | 35.2 (23.1, 56.5) | 0.04 |
| Folate (ng/mL) | 4 (3.1, 6.3) | 4 (2.85, 6.05) | 4 (3, 6.2) | 4.8 (3.6, 6.7) | 0.20 |
| Vitamin B12 (pg/mL) | 244 (170, 400) | 250.0 (174.5, 428.5) | 250.0 (148.0, 443.0) | 207.0 (175.0, 349.5) | 0.59 |
| Vitamin D (ng/mL) | 17.3 (12, 25.1) | 16.8 (12, 23.65) | 24.4 (14.9, 30.4) | 14.7 (11.35, 17.35) | 0.91 |
| Zinc (µg/L) | 784.5 (621.4, 905.2) | 838.3 (713.5, 969.1) | 697.2 (623.8, 1011.0) | 632.9 (539.5, 746.0) | 0.004 |
| Copper (µg/L) | 926.1(731.2, 1107.3) | 947.3 (773.6, 1235.0) | 941.1 (761.1, 975.4) | 817.1 (615.5, 991.4) | 0.09 |
| Selenium (µg/L) | 70.6 (51.5, 89.5) | 71.1 (55.6, 88.4) | 85.9 (53.0, 100.2) | 42.9 (37.7, 71.5) | 0.08 |
Mean and range of T3 levels in each tertile were: Tertile 1 (mean = 2.0 µg/dL; range = 1.5–2.5 µg/dL) Tertile 2 (mean = 3.0 µg/dL; range = 2.6–3.5 µg/dL) and Tertile 3 (mean = 4.0 µg/dL, range of 3.6–4.5 µg/dL). Significant differences are indicated in bold. A P-trend value of < 0.05 indicates that the the observed trend is significant (increase or decrease of the micronutrient indicator across the T3 tertials) and was determined using the Jonckheere-Terpstra test
The association of serum micronutrient levels with serum T4 levels is shown in Table 3. Vitamin D levels exhibit an increasing trend across T4 level tertiles 1–3. Vitamin D concentrations were higher in tertile 3 than tertiles 1 and 2, both in the combined age group and the older age group, but for the lower age group the tertile 3 levels were only higher than those of tertile 1. Vitamin D levels were also 1.3-fold higher in younger than older adolescents. An opposite trend was seen for zinc where levels were significantly (1.1-fold) higher for the younger age group in tertile 1 than in tertiles 2 and 3, and thus zinc levels display a clear negative correlation with T4 levels. In older adolescents (15–18 age group), selenium levels were significantly lower in tertile 1 compared to tertiles 2 and 3, and a positive correlation between selenium and T4 was apparent in this cohort.
Table 3.
Comparison of micronutrient status and serum T4 levels in adolescent Afghan refugees. Comparison was achieved by dividing participants into three equally-sized tertiles according to T4 levels
| Characteristics | T4 | ||||
|---|---|---|---|---|---|
| Overall | T1 | T2 | T3 | P-value | |
| Median (Q1, Q3) | 4.7(3.74,5.7) | 6.89(6.55,7.4) | 9.05(8.52,10.45) | ||
| All age groups | |||||
| Ferritin (ng/mL) | 39 (27, 61.4) | 39.1 (25.6, 62.6) | 37.7 (27.0, 59.5) | 39.4 (27.8, 57.3) | 0.92 |
| Folate (ng/mL) | 4.4 (3.4, 6.1) | 4.6 (3.6, 6.1) | 4.6 (3.4, 6.3) | 4.3 (3.3, 5.7) | 0.41 |
| Vitamin B12 (pg/mL) | 219 (157, 348) | 208.5 (159, 313) | 244.0 (150.0, 350.0) | 224.0 (158.0, 342.0) | 0.65 |
| Vitamin D (ng/mL) | 22.3 (16.2, 27.5) | 20.5 (15.0, 25.2) | 21.1 (13.9, 27.7) | 25.4 (20.0, 30.0) | 0.003 |
| Zinc (µg/L) | 787.1 (618.6, 932.1) | 805.5 (602.6, 1077.3) | 806.1 (639.5, 933.1) | 758.6 (593.7, 877.8) | 0.16 |
| Copper (µg/L) | 951.5 (790.8, 1146.7) | 928.5 (719.6, 1158.4) | 970.2 (828.1, 1153.3) | 956.3 (837.9, 1107.1) | 0.65 |
| Selenium (µg/L) | 71.1 (50.5, 91.3) | 69.4 (48.3, 95.7) | 69.9 (51.5, 84.4) | 73.7 (52.3, 95.0) | 0.53 |
| Age (10–14) | 13.1 (12.4, 13.8) | 13.1 (12.25, 13.85) | 13.1 (12.3, 13.7) | 13.1 (12.5, 13.7) | |
| Ferritin (ng/mL) | 37.7 (27.8, 51.6) | 35.8 (24.1, 51.4) | 37.4 (29.4, 52.1) | 39.4 (27.8, 54.7) | 0.64 |
| Folate (ng/mL) | 4.7 (3.6, 6.1) | 4.8 (3.6, 6.35) | 4.8 (3.6, 5.95) | 4.3 (3.6, 5.7) | 0.66 |
| Vitamin B12 (pg/mL) | 209 (155, 310) | 207.0 (155.5, 260.0) | 259.0 (148.0, 316.0) | 205.0 (156.0, 310.0) | 0.68 |
| Vitamin D (ng/mL) | 24.1 (7.3) | 22.0 (6.2) | 24.5 (7.8) | 25.8 (7.6) | 0.02 |
| Zinc (µg/L) | 789.6 (605.6, 934.4) | 884.0 (661.8, 1274.9) | 798.4 (630.1, 941.3) | 748.1 (593.7, 872.0) | 0.03 |
| Copper (µg/L) | 992 (824.6, 1146.8) | 971.6 (763.0, 1173.6) | 999.5 (839.2, 1132.3) | 974.6 (837.9, 1130.9) | 0.96 |
| Selenium (µg/L) | 72 (48.4, 91.4) | 78.9 (48.0, 109.7) | 61.9 (45.4, 79.9) | 72.5 (59.9, 87.5) | 0.49 |
| Age (15–18) | |||||
| Ferritin (ng/mL) | 48.3 (23.5, 79.8) | 60.7 (27.7, 92.2) | 48.3 (18.06, 63.7) | 44.3 (26.1, 82.6) | 0.48 |
| Folate (ng/mL) | 4 (3.1, 6.3) | 4.4 (3.3, 5.6) | 4 (3.3, 6.6) | 3.3 (2.6, 6.2) | 0.29 |
| Vitamin B12 (pg/mL) | 244 (170, 400) | 223.5 (178.5, 393.5) | 227.0 (170.0, 400.0) | 304.0 (170.0, 443.0) | 0.75 |
| Vitamin D (ng/mL) | 17.3 (12, 25.1) | 15.3 (12, 22.2) | 13.5 (11.5, 24.5) | 22.9 (17.3, 27.5) | 0.05 |
| Zinc (µg/L) | 784.5 (621.4, 905.2) | 724.7 (499.0, 838.3) | 821.9 (657.1, 909.1) | 783.8 (551.9, 941.0) | 0.36 |
| Copper (µg/L) | 926.1 (731.2, 1107.3) | 781.0 (630.7, 1081.0) | 937.7 (814.6, 1217.0) | 944.7 (731.2, 975.4) | 0.46 |
| Selenium (µg/L) | 70.6 (51.5, 89.5) | 58.2 (50.2, 71.1) | 75.3 (60.6, 93.3) | 84.9 (41.6, 111.1) | 0.02 |
Vitamin D levels were also significantly associated with TSH levels in the younger age group (Table 4). Lower vitamin D levels were correlated with lower TSH levels, which is opposite to the trend observed between vitamin D and T4.
Table 4.
Comparison of micronutrient status and TSH levels in Afghan adolescent refugees. Comparison was achieved by dividing participants into three equally-sized tertiles according to TSH levels
| Characteristics | TSH | ||||
|---|---|---|---|---|---|
| Overall | T1 | T2 | T3 | P-value | |
| Median | 1.18 (0.96,1.33) | 1.7 (1.54, 1.87) | 2.5 (2.28, 2.93) | ||
| All age groups | |||||
| Ferritin (ng/mL) | 39 (27, 61.4) | 40.0 (28.0, 62.1) | 44.0 (33.8, 67.8) | 31.7 (20.7, 52.7) | 0.14 |
| Folate (ng/mL) | 4.4 (3.4, 6.1) | 4.6 (3.4, 6.65) | 5 (3.6, 6.1) | 4 (3.5, 5.2) | 0.12 |
| Vitamin B12 (pg/mL) | 219 (157, 348) | 220.5 (174, 352.5) | 230.0 (165.0, 355.0) | 209.0 (145.0, 305.0) | 0.27 |
| Vitamin D (ng/mL) | 22.3 (16.2, 27.5) | 22.2 (16.35, 28.6) | 23.0 (14.8, 27.6) | 21.5 (17.1, 26.2) | 0.39 |
| Zinc (µg/L) | 787.1 (618.6, 932.1) | 780.1 (631.2, 899.6) | 748.1 (593.7, 877.8) | 811.8 (644.4, 956.4) | 0.42 |
| Copper (µg/L) | 951.5 (790.8, 1146.7) | 1019.4 (814.0, 1221.3) | 934.7 (761.1, 1077.9) | 960.3 (828.1, 1132.9) | 0.45 |
| Selenium (µg/L) | 71.1 (50.5, 91.3) | 74.0 (52.5, 96.1) | 67.5 (48.4, 86.2) | 71.0 (49.9, 86.4) | 0.47 |
| Age (10–14) | |||||
| Ferritin (ng/mL) | 37.7 (27.8, 51.6) | 40.7 (28.8, 62.0) | 40.7 (32.3, 51.2) | 32.5 (21.7, 49.9) | 0.08 |
| Folate (ng/mL) | 4.7 (3.6, 6.1) | 4.8 (3.6, 6.6) | 5.1 (4, 6.1) | 4.1 (3.6, 5.2) | 0.08 |
| Vitamin B12 (pg/mL) | 209 (155, 310) | 209.0 (176.0, 292.0) | 220.0 (157.0, 315.0) | 207.0 (145.0, 305.0) | 0.68 |
| Vitamin D (ng/mL) | 24.1 (19.3, 28.3) | 26.8 (21.1,31.3) | 24.7 (17.1,28.9) | 22.4 (18.4, 26.9) | 0.03 |
| Zinc (µg/L) | 789.6 (605.6, 934.4) | 798.3 (642.6, 933.1) | 724.9 (593.7, 884.0) | 803.4 (600.1, 956.4) | 0.77 |
| Copper (µg/L) | 992 (824.6, 1146.8) | 1102.0 (821.5, 1200.8) | 951.6 (837.9, 1118.3) | 963.5 (824.6, 1132.9) | 0.21 |
| Selenium (µg/L) | 72 (48.4, 91.4) | 78.5 (64.0, 97.6) | 63.2 (46.8, 86.9) | 68.2 (47.9, 86.3) | 0.07 |
| Age (15–18) | |||||
| Ferritin (ng/mL) | 48.3 (23.5, 79.8) | 37.5 (18.1, 62.2) | 63.6 (35.3, 88.1) | 23.0 (11.6, 130.0) | 0.55 |
| Folate (ng/mL) | 4 (3.1, 6.3) | 4.5 (3.1, 6.8) | 4.1 (2.6, 6.2) | 3.8 (3.1, 4.6) | 0.30 |
| Vitamin B12 (pg/mL) | 244 (170, 400) | 254.0 (172.0, 395.0) | 264.5 (179, 482) | 210.0 (147.0, 350.0) | 0.64 |
| Vitamin D (ng/mL) | 17.3 (12, 25.1) | 16.3 (12, 23.2) | 20.5 (12.6, 25.7) | 13.4 (11.2, 19.2) | 0.65 |
| Zinc (µg/L) | 784.5 (621.4, 905.2) | 754.2 (618.6, 885.4) | 793.6 (551.9, 876.0) | 878.9 (697.2, 969.1) | 0.33 |
| Copper (µg/L) | 926.1 (731.2, 1107.3) | 937.7 (751.5, 1241.1) | 804.3 (642.4, 975.4) | 955.2 (851.8, 1156.0) | 0.60 |
| Selenium (µg/L) | 70.6 (51.5, 89.5) | 61.4 (45.1, 89.0) | 71.7 (52.1, 85.9) | 80.3 (69.9, 95.8) | 0.15 |
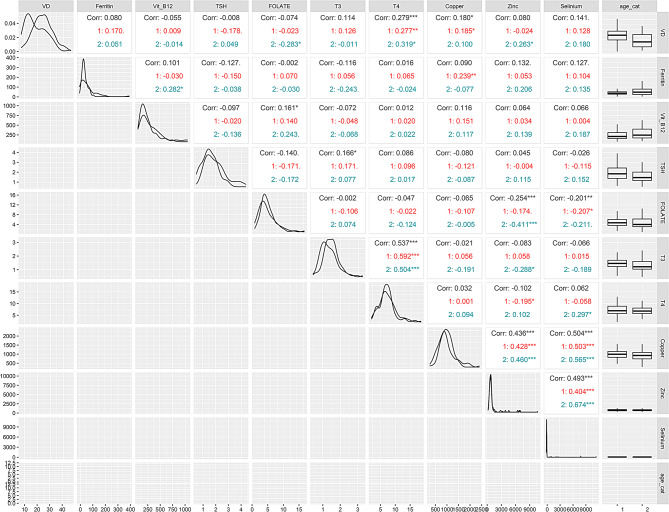
Analysis of the relationship between serum micronutrients and thyroid hormone levels, on a continuous scale (Fig. 1), indicates that vitamin D exhibits a statistically significant positive correlation with T4 (r = 0.279) in the combined, younger (r = 0.277) and older (r = 0.319) age groups. In contrast, a statistically significant but negative correlation was observed when zinc levels were compared with T3 (r=-0.288) in the older age group and with T4 (r=-0.195) in the younger age group.
Fig. 1.
Corretion coefficient, data distribution and scatter plot of the micronutrients with T3, T4 and TSH by gender (all genders). The upper right triangle present correlation coefficients and their statistical significance values are represented by asterisk. Black color represents significance of overall correlation while red and navy-blue color represent correlation with in the younger (age 10–14 years) and older (15–19 years) adolescents group respectively. * indicate P ≤ 0.05, ** indicate p ≤ 0.01 and *** indicate P ≤ 0.001.The diagonals indicate distribution of each parameter and lower triangular matrix indicate scatter plot. The right side column represent the box plot distribution of each parameter based on age groups while the lower horizontal row indicate histogram
Discussion
To our knowledge, this is the first study evaluating the association between thyroid hormone levels and micronutrient status in Afghan adolescent refugees. Thyroid dysfunction is a worldwide public health problem, the etiology of which is mostly unknown. Clinically, thyroid dysfunctions mainly involve subclinical thyroid function disorder and is identified by assessment of the levels of T3, T4 and TSH in serum along with other routine clinical investigations. In humans, thyroid metabolism depends on processes which are influenced by micronutrients status [8]. Although, iodine is the key micronutrient in thyroid hormone biosynthesis [20], other micronutrients are also involved in maintaining normal thyroid function [16]. For example, selenium (Se) is a constituent of three iodothyronine deiodinase enzymes that catalyze the conversion of prohormone thyroxine (T4) to the active hormone 3,3′,5-triiodothyronine (T3). Thus, Se plays a direct role in the metabolism and activation of thyroid hormones [21]. Zinc is another essential micronutrient with a well-established role in thyroid hormone biosynthesis, metabolism and signaling. Zn is an essential structural component and catalytic cofactor for numerous enzymes involved in thyroid hormone biosynthesis [22], playing a role in thyrotropin-releasing hormone (TRH) production. TRH stimulates release of thyroid stimulating hormone (TSH) from the pituitary, which in turn acts on the thyroid to promote thyroid hormone production [23]. Thus, micronutrient deficiency can exacerbate thyroid disorders [24], which is concerning given that almost one third of the global population suffer from deficiency in one or more micronutrients.
Our study indicates that there are age and gender dependent differences in thyroid hormone levels among adolescent Afghan refugees. Specifically, T3 and TSH were significantly higher in younger (10–14 years) than older adolescents (15–18 years) while T4 levels were significantly higher in boys compared to girls. These observed age-related changes in thyroid hormone levels align with findings from previous studies, such as those conducted on Danish (aged 6–18 years) and Indian (aged 6–17 years) cohorts of school age children, where the median values of free T3 and T4 decreased with increasing age [25, 26]. However, our cohort is distinct in being composed solely of refugees, a population that may experience unique environmental and nutritional challenges exacerbating these age-related changes. The age-related changes were more obvious in young children with convergence to adult like ranges after age 15 years and above. Similarly, higher levels of thyroid hormones in boys than girls, as found in our study, has also been reported previously [27]. It is important to note that these differences could be influenced by factors not fully explored in this study, such as variations in physical activity, diet and exposure to environmental stressors like chronic malnutrition or psychosocial stress, which are prevalent in refugee populations. However, it should be noted that the age and gender-based changes in thyroid hormones levels may also be influenced by other factors, such as ethnicity, body mass index and methodological approaches to measure thyroid parameters [28]. These variables are important for understanding the full spectrum of influences on thyroid function and should be considered when interpreting findings especially in a diverse and vulnerable population such as refugees.
The current study reports an association between selected micronutrients and thyroid function, as reported previously. For example, a positive association was observed between vitamin D and both TSH and T4 levels in younger adolescents aged 10–14 years. These findings are in concordance with previous studies reporting significantly low levels of TSH and T4 in vitamin D deficient individuals aged 12 to 18 years [29]. Vitamin D deficiency has been implicated in autoimmune thyroid disorders including Hashimoto’s thyroiditis and Graves`s disease [30]. Although, the exact mechanism by which vitamin D deficiency impairs thyroid functions is unknown, experimental evidence on a mouse model suggests that vitamin D exerts its effect by increasing the mRNA expression levels of the de-iodinase 2 gene, which encodes an enzyme necessary for conversion of T4 to T3 [31]. Other possible mechanisms include suppression of TSH-stimulated adenylyl cyclase activity and enhanced iodine uptake [32]. Several clinical trials have investigated the impact of vitamin D supplementation on thyroid function [33]. Much research has reported a significant reduction in anti-thyroid antibodies following vitamin D supplementation with little or no effect on thyroid hormones levels [30]. However, a larger clinical trial encompassing 11,017 participants (mean age 48 years, ranging from 18-95 years, and 58% female), receiving vitamin D supplementation for 12 months, reported decreases in serum TSH, anti-TPO, anti-TG and TG levels over time, and increases in serum FT3 and FT4, leading to reduction in hypothyroidism and thyroid autoimmune disorder [34].
Among the trace elements assessed, zinc exhibited a significant negative correlation with serum T3 and T4, and a positive, but non-significant, correlation with serum TSH levels. While this observation aligns with the previous findings [35], it is important to note that other studies (e.g., [36]) reported either no correlation or a positive correlation between zinc levels and thyroid hormones. These differences in findings highlight the need for further investigation into the specific mechanisms through which zinc interacts with thyroid hormones. Unlike the consistent effect observed for other trace elements, zinc’s influence on thyroid function appears to be complex and potentially context dependent. Zinc contributes to thyroid metabolism through its role in the synthesis of the thyrotropin-releasing hormone (TRH) in the hypothalamus, as cofactor for deiodinase I and II enzymes that help in conversion of T4 to T3, or as structural component of the T3 receptor [14, 37].
Additionally, our study showed a positive association between T4 status and selenium levels. Selenium is a critical component of the deiodinase enzymes, which are responsible for the conversion of thyroxine (T4) into the more active triiodothyronine (T3) [38]. It exerts its effects on thyroid metabolism seleno-proteins, a group of biologically active protein molecules involved in diverse processes including DNA synthesis and thyroid metabolism [10]. Previous research has described the importance of adequate selenium levels for optimal thyroid function, highlighting how selenium deficiency can impair the conversion process, potentially leading to altered thyroid hormone levels and subsequent metabolic disturbances [39]. Moreover, clinical research suggests that selenium deficiency is linked with a higher risk of elevated anti-thyroid antibody levels, while selenium supplementation has been shown to reduce thyroid peroxidase antibody levels [40, 41]. Selenium status has also been correlated with Grave’s disease, autoimmune hypothyroidism and thyroid cancer [42]. In the context of adolescent Afghan refugees, who are likely exposed to a variety of environmental stressors and nutritional deficiencies, our findings of a positive association between T4 status and selenium levels suggest that selenium may play a crucial role in maintaining thyroid function amidst these challenges.
Although our study is the first of its kind assessing the association between micronutrients status and thyroid function in vulnerable refugee population, it has some limitations. First, due to limited sample size and cross-sectional design, the study findings cannot be generalized to other refugee and host populations. Secondly, we could not collect dietary data and other lifestyle factors which may also be associated with micronutrients status and thyroid profile in this population. Nonetheless, the study provides important baseline data information regarding the potential impact of micronutrients on thyroid profile in a vulnerable population.
Conclusion
Overall, our study reports age and gender-based impacts of different micronutrients (both vitamins and minerals) on thyroid function in adolescent Afghan refugees. Of these, vitamin D and zinc are especially important as the serum level of these micronutrients is significantly correlated with thyroid hormone levels in this population. These findings highlight the importance of close monitoring and effective nutritional interventions to prevent thyroid related disorders in vulnerable population such as refugees.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Commissionerate Afghan Refugees Khyber Pakhtunkhwa for facilitating data collection and ground support. We would also like to thank all the study participants for their time and participation in the study.
Author contributions
S.S performed the experimental work, data analysis, drafted and edited the manuscript. M.S; N.S and S.C.A. were involved in supervising the research and editing the manuscript. K.I; M.S.K and H.A.A in data collection, analysis and writing and revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by a Seed Fund grant from the School of Biological Sciences (University of Reading) and supported by a BBSRC-DRINC grant (BB/N021800/1) to SCA. M.S. is recipient of Faculty Grant from Office of Research, Innovation and Commercialization (ORIC), Khyber Medical University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Board of the Khyber Medical University Pakistan vide letter number DIR/KMU-EB/PR/000766 dated 3 February 2020. Before recruitment, a written informed consent was obtained from all the participants and their parents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ritchie H, Roser M, Micronutrient, Deficiency. Our World in Data [Internet]. 2024 Feb 29 [cited 2024 May 12]; Available from: https://ourworldindata.org/micronutrient-deficiency
- 2.Calcaterra V, Verduci E, Milanta C, Agostinelli M, Todisco CF, Bona F, et al. Micronutrient deficiency in children and adolescents with Obesity—A. Narrative Rev Child (Basel). 2023;10(4):695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokokawa H, Morita Y, Hamada I, Ohta Y, Fukui N, Makino N, et al. Demographic and clinical characteristics of patients with zinc deficiency: analysis of a nationwide Japanese medical claims database. Sci Rep. 2024;14(1):2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamakawa H, Kato TS, Noh JY, Yuasa S, Kawamura A, Fukuda K, et al. Thyroid hormone plays an important role in cardiac function: from bench to bedside. Front Physiol. 2021;12:606931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahid MA, Ashraf MA, Sharma S, Physiology. Thyroid Hormone. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 May 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK500006/
- 6.Diab N, Daya NR, Juraschek SP, Martin SS, McEvoy JW, Schultheiß UT, et al. Prevalence and risk factors of thyroid dysfunction in older adults in the community. Sci Rep. 2019;9(1):13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Xue S, Zhang L, Chen G. Trace elements and the thyroid. Front Endocrinol [Internet]. 2022 Oct 24 [cited 2024 May 12];13. Available from: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2022.904889/full [DOI] [PMC free article] [PubMed]
- 8.Babiker A, Alawi A, Al Atawi M, Al Alwan I. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garofalo V, Condorelli RA, Cannarella R, Aversa A, Calogero AE, Vignera SL. Relationship between iron deficiency and thyroid function: A systematic review and Meta-Analysis. Nutrients. 2023;15(22):4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorini F, Sabatino L, Pingitore A, Vassalle C. Selenium: an element of life essential for thyroid function. Molecules. 2021;26(23):7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freake HC, Govoni KE, Guda K, Huang C, Zinn SA. Actions and interactions of thyroid hormone and zinc status in growing rats. J Nutr. 2001;131(4):1135–41. [DOI] [PubMed] [Google Scholar]
- 12.Prasad R, Kumar V, Kumar R, Singh KP. Thyroid hormones modulate zinc transport activity of rat intestinal and renal brush-border membrane. Am J Physiol. 1999;276(4):E774–782. [DOI] [PubMed] [Google Scholar]
- 13.Ye Y, Li Y, Ma Q, Li Y, Zeng H, Luo Y et al. Association of multiple blood metals with thyroid function in general adults: A cross– sectional study. Front Endocrinol [Internet]. 2023 Mar 27 [cited 2024 May 12];14. Available from: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2023.1134208/full [DOI] [PMC free article] [PubMed]
- 14.Mahmoodianfard S, Vafa M, Golgiri F, Khoshniat M, Gohari M, Solati Z, et al. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: A randomized Double-Blind controlled trial. J Am Coll Nutr. 2015;34(5):391–9. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Bansal R, Shergill HK, Garg P. Prevalence of thyroid dysfunction in pregnancy and its association with feto-maternal outcomes: A prospective observational study from a tertiary care Institute in Northern India. Clin Epidemiol Global Health. 2023;19:101201. [Google Scholar]
- 16.O’Kane SM, Mulhern MS, Pourshahidi LK, Strain JJ, Yeates AJ. Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutr Rev. 2018;76(6):418–31. [DOI] [PubMed] [Google Scholar]
- 17.Skinner A, Tester-Jones MC, Carrieri D. Undernutrition among children living in refugee camps: a systematic review of prevalence. BMJ Open. 2023;13(6):e070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Health of refugees and migrants - Practices in addressing the health needs of refugees and migrants: WHO African Region [Internet]. 2018 [cited 2024 Nov 13]. Available from: https://www.who.int/publications/i/item/health-of-refugees-and-migrants---practices-in-addressing-the-health-needs-of-refugees-and-migrants-who-african-region
- 19.Saeedullah A, Khan MS, Andrews SC, Iqbal K, Ul-Haq Z, Qadir SA et al. Nutritional Status of Adolescent Afghan Refugees Living in Peshawar, Pakistan. Nutrients [Internet]. 2021;13(9). Available from: https://www.mdpi.com/2072-6643/13/9/3072 [DOI] [PMC free article] [PubMed]
- 20.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–95. [DOI] [PubMed] [Google Scholar]
- 21.Lossow K, Renko K, Schwarz M, Schomburg L, Schwerdtle T, Kipp AP. The nutritional supply of iodine and selenium affects thyroid hormone axis related endpoints in mice. Nutrients. 2021;13(11):3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severo JS, Morais JBS, de Freitas TEC, Andrade ALP, Feitosa MM, Fontenelle LC, et al. The role of zinc in thyroid hormones metabolism. Int J Vitam Nutr Res. 2019;89(1–2):80–8. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Huang W, Worthington S, Drabik P, Osman R, Gershengorn MC. A model of inverse agonist action at Thyrotropin-Releasing hormone receptor type 1: role of a conserved Tryptophan in helix 6. Mol Pharmacol. 2004;66(5):1192–200. [DOI] [PubMed] [Google Scholar]
- 24.Hess SY. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab. 2010;24(1):117–32. [DOI] [PubMed] [Google Scholar]
- 25.Gunapalasingham G, Frithioff-Bøjsøe C, Lund MAV, Hedley PL, Fonvig CE, Dahl M, et al. Reference values for fasting serum concentrations of thyroid-stimulating hormone and thyroid hormones in healthy Danish/North-European white children and adolescents. Scand J Clin Lab Invest. 2019;79(1–2):129–35. [DOI] [PubMed] [Google Scholar]
- 26.Marwaha RK, Tandon N, Desai AK, Kanwar R, Aggarwal R, Sastry A, et al. Reference range of thyroid hormones in healthy school-age children: country-wide data from India. Clin Biochem. 2010;43(1–2):51–6. [DOI] [PubMed] [Google Scholar]
- 27.Yao C, Wu M, Liu M, Chen X, Zhu H, Xiong C, et al. Age- and sex-specific reference intervals for thyroid hormones in a Chinese pediatrics: a prospective observational study of 1,279 healthy children. Translational Pediatr. 2021;10(10):2479488–2472488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor PN, Lansdown A, Witczak J, Khan R, Rees A, Dayan CM, et al. Age-related variation in thyroid function– a narrative review highlighting important implications for research and clinical practice. Thyroid Res. 2023;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donayeva A, Kulzhanova D, Amanzholkyzy A, Abdelazim IA, Abilov T, Baubekov Z, et al. Relationship between vitamin D and adolescents’ hypothyroidism– a cross-sectional study. Prz Menopauzalny. 2023;22(4):186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babić Leko M, Jureško I, Rozić I, Pleić N, Gunjača I, Zemunik T. Vitamin D and the thyroid: A critical review of the current evidence. Int J Mol Sci. 2023;24(4):3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alrefaie Z, Awad H. Effect of vitamin D3 on thyroid function and de-iodinase 2 expression in diabetic rats. Arch Physiol Biochem. 2015;121(5):206–9. [DOI] [PubMed] [Google Scholar]
- 32.Berg JP, Liane KM, Bjørhovde SB, Bjøro T, Torjesen PA, Haug E. Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J Steroid Biochem Mol Biol. 1994;50(3–4):145–50. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Chen X, Qian X, Shao S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis—A meta-analysis of randomized controlled trials. Clin Pharm Therapeu. 2022;47(6):767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirhosseini N, Brunel L, Muscogiuri G, Kimball S. Physiological serum 25-hydroxyvitamin D concentrations are associated with improved thyroid function-observations from a community-based program. Endocrine. 2017;58(3):563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunier N, Beattie JH, Ciarapica D, O’Connor JM, Andriollo-Sanchez M, Taras A, et al. Basal metabolic rate and thyroid hormones of late-middle-aged and older human subjects: the ZENITH study. Eur J Clin Nutr. 2005;59(Suppl 2):S53–57. [DOI] [PubMed] [Google Scholar]
- 36.Jain RB. Thyroid function and serum copper, selenium, and zinc in general U.S. Population. Biol Trace Elem Res. 2014;159(1–3):87–98. [DOI] [PubMed] [Google Scholar]
- 37.Baltaci AK, Mogulkoc R, Belviranli M. L-thyroxine-induced hyperthyroidism affects elements and zinc in rats. Bratisl Lek Listy. 2013;114(3):125–8. [DOI] [PubMed] [Google Scholar]
- 38.Sabatino L, Vassalle C, Del Seppia C, Iervasi G. Deiodinases and the three types of thyroid hormone deiodination reactions. Endocrinol Metab (Seoul). 2021;36(5):952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura M, Melo M, Carrilho F. Selenium and thyroid disease: from pathophysiology to treatment. Int J Endocrinol. 2017;2017:1297658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani G, Isidori AM, Moretti C, Di Dato C, Greco E, Ciolli P, et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: results of the SERENA study, a randomized, double-blind, placebo-controlled trial. Endocrine. 2019;66(3):542–50. [DOI] [PubMed] [Google Scholar]
- 41.Rostami R, Nourooz-Zadeh S, Mohammadi A, Khalkhali HR, Ferns G, Nourooz-Zadeh J. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto’s thyroiditis. Antioxidants. 2020;9(11):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glattre E, Nygård JF, Aaseth J. Selenium and cancer prevention: observations and complexity. J Trace Elem Med Biol. 2012;26(2–3):168–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.