Abstract
Neuropsychiatric disorders are widespread and serious and have a detrimental effect on the patient’s physical and mental health. The aim of the present study was to find the protective effect of Rubus ellipticus in epilepsy by maximal electroshock seizure, pentylenetetrazol, and related depressive behavior by Forced Swim Test and Tail Suspension Test. The animals were given ethanol extract of R. ellipticus (EERE) (100, 200, or 400 mg/kg) doses for 10 days. On every 5th day, seizure monitoring was performed (day 5 and day 10), followed by depressive behavior monitoring by evaluating immobility time. It was revealed that EERE treatment caused antidepressive-like behavior as a comorbid antiepileptogenic effect. This study will provide compelling evidence to support further clinical trials to grow this medicinal plant in the management of epileptogenic and associated debilitating behavioral comorbidities.
Keywords: Comorbidities, depression, epilepsy, ethanol extract of Rubus ellipticus, Rubus ellipticus
Introduction
Epilepsy is a chronic neurobiological, cognitive, and psychological brain disorder characterized by a persistent predisposition to produce seizures unprovoked by any immediate central nervous system (CNS) insult.[1] An annual occurrence of epilepsy includes a high number of people in developing countries and a prevalence of 1–10 cases/1000 people, in which children and the elderly have the highest rates.[2] Acute CNS insults may also cause epileptic seizures (structural, systemic, toxic, or metabolic). These seizures (acutely symptomatic or provoked) are meant to be acute manifestations of the provocation.[3] When the underlying cause has been eliminated or the acute period has passed, the condition does not recur.[4] Regular antiepileptic drug (AED) therapy or the most frequent seizure occurring during the last 5 years are also signs of active epilepsy.[5] Sudden unexpected death in epilepsy is an unexpected death, with or without proof of a seizure, with no toxicological or anatomic cause of death.[6]
Rubus ellipticus (family: Rosaceae) is valued as a medicinal herb in traditional Tibetan medicine, including use as a renal tonic, an antidiuretic, an antitumor, and a wound healer, as well as in the management of neuropathy, epilepsy, Parkinson’s disease, and depression.[7] It is rich in polyphenols, alkaloids, terpenes, and minerals. Its roots contain ursolic acid, acuminatic acid, and rubuside.[8] Leaves have pentacyclic acid, elliptic acid, kaempferol, quercetin, phenolic acid, and triterpenes. Flowers contain rutin, quercetin, kaemferol, and rubuside. Its fruits have minerals such as rutin, ursolic acid, rubuside, and kaemferol.[9]
Materials and Methods
Collection and authentication
R. ellipticus whole plant was collected from the roadside of Lohaghat, Uttarakhand, India, in December 2019. Dr. Anjula Pandey, Principal Scientist, National Bureau of Plant Genetic Resources, Pusa Institute of Agriculture, New Delhi, a Voucher (specimen No: AC-7/2020) is preserved.
Experimental animals
Swiss albino mice were randomly selected for antiepileptic activity and antidepressive activity. These mice of 25–30 g of body weight were obtained from Central Animal House of Noida Institute of Engineering and Technology (Pharmacy Institute), Greater Noida. All parameters were carefully observed as per the CCSEA guidelines with protocol number IAEC/NIET/2019/01/18.
Preparation of Rubus ellipticus extract
The whole plant of R. ellipticus was shade-dried and crushed by the mechanical grinder. The crushed air-dried powdered material was taken and treated with ethanol solvent (1000 mL) in soxhlet apparatus and extracted until brown color disappear. At 55°C–65°C, the temperature was maintained.
Pharmacological activity
Behavioral study
The behavioral studies of the ethanol extract of R. ellipticus (EERE) were performed on mice at doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg. These tests should nonetheless be considered only as an initial screen for neuromuscular impairment. Motor activity and muscle relaxant activity using the rota rod test[10] and locomotor activity using the actophotometer.[11]
Pentylenetetrazole induced convulsions
Induced seizures as a consequence of pentylenetetrazol (PTZ) and the responses of EERE were evaluated for latency, onset, and duration of tonic-clonic seizures.[12]
Maximal electroshock induced convulsions
Six groups of mice (n = 8–12) were formed. The first group was a negative control, while the second group was standard, phenytoin (25 mg/kg i.p.) next three groups (EERE 100 mg/kg, EERE 200 mg/kg, and EERE 400 mg/kg), the test treatment was given 15 days before the electrical stimulation. The mice were placed in clear plastic boxes immediately after electroconvulsive shock pulses of 50 mA–60 Hz for 0.2 s were administered to epilepsy animals via auricular electrodes and monitored for tonic convulsion and latency, extension, flexion, tonic, and stupor were analyzed.[13]
Tail suspension test
The experimental protocol was the same as above except the second group, which was referred to as standard (Imipramine), i.e. 10 mg/kg i.p. With the assistance of adhesive tape, animals were placed approximately 1 cm from the tip of the tail, and each mouse was suspended from the edge of a 58 cm high table top. The duration of immobility was measured over a 5-min period.[14]
Forced swim test
The experimental protocol was the same as above. Increased immobility time reflects a depressed state.[15]
Results
The percentage yield calculated by the following formula was found to be 7.2% w/w on a dry basis.
Muscle relaxant activity
The data showed that EERE 200 and EERE 400 had the same significant effect as diazepam (2 mg/kg) (*** P < 0.001) in comparison to the negative control on the latency caused by the fall of the experimental animal from the rotarod apparatus [Figure 1].
Figure 1.
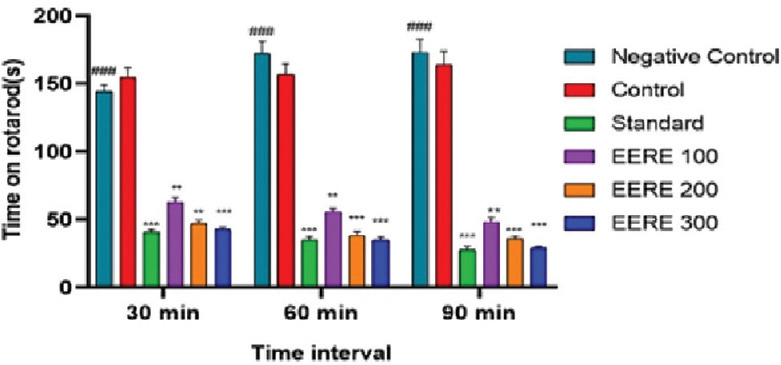
Effect of ethanol extract of Rubus ellipticus (100–400 mg/kg; p.o.) and diazepam (2 mg/kg; i.p.) on the time course of the rota rod test in mice. Data are presented as mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to negative control group analyzed by Graph Pad Prism 9.0 (GraphPad Prism 9.0 is developed by GraphPad Software, Inc. The company is headquartered in San Diego, California, USA) (two-way ANOVA followed by Tukey’s multiple post hoc test). ###Negative control group which is required to compare its data with that of the treatment groups EERE: Ethanol extract of Rubus ellipticus
Locomotive activity
Locomotive activity is considered an awareness index, and the spontaneous reduction in baseline activity results in anxiety reduction. When calculated, it showed that there was a significant (*P < 0.05, **P < 0.01, and ***P < 0.001) decrease in the locomotor score in the case of animals treated with the extract of R. ellipticus (100 mg/kg, 200 mg/kg, and 400 mg/kg) and diazepam (4 mg/kg) as compared to the negative control group [Figure 2].
Figure 2.
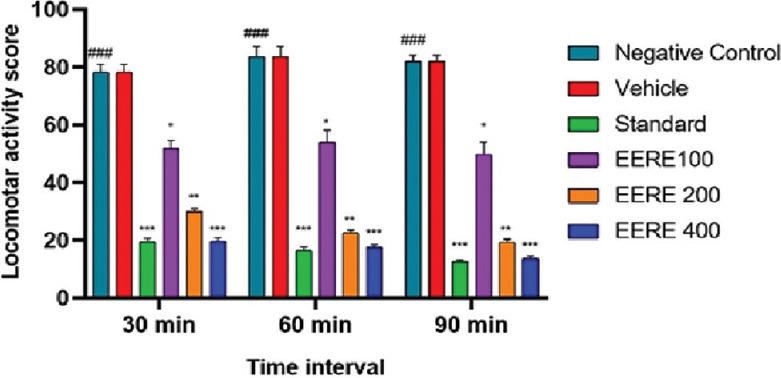
Effect OD ethanol extract of Rubus ellipticus (100–400 mg/kg; p.o.) and diazepam (2 mg/kg; i.p.) on the time course of the locomotor activity score in mice. Data are presented as mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to negative control group analysed by Graph Pad Prism 9.0 (two-way ANOVA followed by Tukey’s multiple post hoc test). ###Negative control group and its significance is that it helps to ensure the reliability and validity of the study results, draw more accurate conclusions. EERE: Ethanol extract of Rubus ellipticus
Anticonvulsive activity
Pentylenetetrazol induced model
The EERE showed significant antiepileptic activity toward PTZ-induced seizures. Pretreatment (100–400 mg/kg) showed a significant delay in the onset and duration of tonic-clonic seizures compared with the disease group (negative control). Furthermore, the EERE extract provided 83 and 90% protection at 400 mg/kg against mortality, whereas the standard drug (diazepam, 4 mg/kg; i.p.) completely abolished convulsion.
Maximal electroshock seizure model
EERE at all the doses produced profound anticonvulsant effects against the maximal electroshock seizure-induced tonic seizure phase in mice. Therefore, the findings of this activity showed that EERE 400 and EERE 200 were significantly effective to prevent animals from grandmal type of seizures including the further continuation of seizures [Figure 3].
Figure 3.
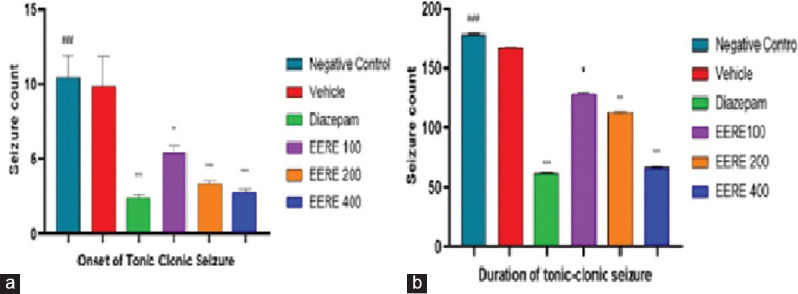
Effect of ethanol extract of Rubus ellipticus (100–400 mg/kg; p.o.) and diazepam (4 mg/kg; i.p.) on seizure monitoring episode I analyzing onset and latency of seizure in Pentylenetetrazol (PTZ) induced convulsive seizure expressed as mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to negative control group (PTZ) analyzed by Graph Pad Prism 9.0 (one-way ANOVA followed by Barlett’s post hoc test) n = 12. ###Negative control group and its significance is that it helps to ensure the reliability and validity of the study results, draw more accurate conclusions. (a) Onset of tonic-clonic seizure and (b) Duration of tonic-clonic seizure EERE: Ethanol extract of Rubus ellipticus
Behavior’s studies
Tail suspension test
The study elaborated the action of short-term dosing of EERE (100–400 mg/kg; p.o.) and the classical antidepressant drug imipramine (10 mg/kg; p.o.) on mice behaviors in the Tail Suspension Test in different interval of time after the seizure monitoring episodes on day 5 and day 10. Administration of EERE (100–400 mg/kg; p.o.) pretreated prior 10 days before initiating the experiment. It significantly reduced the motionless episodes of treated mice by maximum when compared to the negative control group, indicating prominent antidepressant-like activity.
Behavior-depresssive episode by forced swim test
It depicted the effect of acute administration of EERE (100–400 mg/kg, p.o.) and the antidepressant drug imipramine (10 mg/kg) on mice behaviors in the Forced Swim Test (FST) in time-dependent manner after the seizure monitoring Episode I and II. Analysis of ANOVA showed that at all the doses of EERE, a significant down in the motionless time and increased swimming time of mice in the FST was observed.
Discussion
The classical antidepressants are more effective in the treatment of most depressive disorders. During the treatment of depression, patients do not show any response to mood elevation until 2–3 weeks after the beginning of the treatment. Therefore, there is an urgent need for research in the areas of AEDs involving bioactive from herbal sources that may produce effective antiepileptic and antidepressant activities with the least adverse effects. Traditional medicinal herbs used in the management of CNS disorders have been proven to be effective since the ancient era.[16]
R. ellipticus has a potential therapeutic activity for neurodegenerative and neuropsychological disorders. Hence, this study was aimed at investigating the dose-dependent activity of R. ellipticus for the combined management of epilepsy and comorbid depression. As noted, the mice were classified as depressed and immobile. Immobility time was increased in the negative control and vehicle-treated groups (***P < 0.001). According to the present study, the severity and frequency of epileptic seizures showed a potential protective effect on epilepsy-induced depression. It was observed that R. ellipticus was more effective and showed a high significant value at high doses (400 mg/kg) and 200 mg/kg than at lower doses (100 mg/kg). Kaempferol, quercetin, and rutin are present in R. ellipticus, which is reported to have significant antioxidant properties and is responsible for the management of CNS comorbid disorders. It has already been reported that flavonoids showed significant antidepressant activity, especially flavonols such as rutin, quercetin, and kaempferol. The above-mentioned bioactive constituents are responsible for reversing oxidative stress-induced decreased hippocampal brain-derived neurotrophic factor and cAMP response element binding protein (pCREB)is cyclic adenosine mono phosphate response element-binding protein expression in the brain, and the kynurenine pathway has also been reported as the major possible reason for the association of depression with epilepsy, mainly due to tryptophan metabolism susceptibility. Thus, flavonoids are also reported to modulate PI3K/AKT, protein kinase C, and mitogen-activated protein kinase pathways, which further leads to the suppression of apoptosis, neuronal survival, synaptic plasticity, and long-term potentiation; hence, they can be used experimentally to manage cognitive impairments and depressive behavior.[16]
Conclusion
The present study justifies the folklore use of R. ellipticus. It was found that the anticonvulsant activity along with the depressive behavior comorbid ameliorative impact is mainly due to the flavonoid-rich fraction of R. ellipticus whole plant extract. The large quantity of quercetin and kaempferol present in the plant extract might be responsible for the biological activity chosen in the present research work. Future preclinical and clinical studies are warranted to provide more scientific support for the R. ellipticus plant and its phytoflavonoids for the better management of epilepsy and associated psychological disorders.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
References
- 1.Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54:185–91. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- 2.Tao K, Wang X. The comorbidity of epilepsy and depression: Diagnosis and treatment. Expert Rev Neurother. 2016;16:1321–33. doi: 10.1080/14737175.2016.1204233. [DOI] [PubMed] [Google Scholar]
- 3.Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–5. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 4.Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50:1102–8. doi: 10.1111/j.1528-1167.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Hesdorffer Dale C, Emma B, Cascino Gregory D, Hauser Allen W. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Rev Neurol Argent. 2009;50:1102–8. doi: 10.1111/j.1528-1167.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, et al. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–50. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham J, Woodhead M. Wild Crop Relatives: Genomic and Breeding Resources. Springer; Berlin: 2011. Rubus; pp. 179–96. [Google Scholar]
- 8.Prakash KC. Prioritization of cultivated and wild edibles by local people in the Uttaranchal hills of Indian Himalaya. Indian J Tradit Knowledge. 2007;6:239–44. [Google Scholar]
- 9.Suluh N, Andi S, Imam MS. Morphological characteristics of Indonesian Rubus flowers. Biodiversitas Journal of Biological Diversity. 2021;22:38–45. [Google Scholar]
- 10.Bertagna NB, Dos Santos PG, Queiroz RM, Fernandes GJ, Cruz FC, Miguel TT. Involvement of the ventral, but not dorsal, hippocampus in anxiety-like behaviors in mice exposed to the elevated plus maze: Participation of CRF1 receptor and PKA pathway. Pharmacol Rep. 2021;73:57–72. doi: 10.1007/s43440-020-00182-3. [DOI] [PubMed] [Google Scholar]
- 11.Łuszczki JJ, Lepiech J, Zagaja M, Wróblewska-Łuczka P, Florek-Łuszczki M, Bojar H, et al. Anticonvulsant and neurotoxic effects of a novel 1,2,4-triazole-3-thione derivative (TPF-34) and its isobolographic interaction profile with classical antiepileptic drugs in mice. Pharmacol Rep. 2020;72:87–95. doi: 10.1007/s43440-019-00044-7. [DOI] [PubMed] [Google Scholar]
- 12.Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: A comparative analysis with s.c. PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44. doi: 10.1016/j.seizure.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Łuszczki JJ, Podgórska D, Kozińska J, Jankiewicz M, Plewa Z, Kominek M, et al. Polygonogram with isobolographic synergy for three-drug combinations of phenobarbital with second-generation antiepileptic drugs in the tonic-clonic seizure model in mice. Pharmacol Rep. 2021;73:111–21. doi: 10.1007/s43440-020-00164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murtala AA, Akindele AJ. Anticonvulsant, muscle relaxant and in-vitro antioxidant activities of hydroethanol leaf extract of Newbouldia laevis Seem. (Bignoniaceae) in mice. Univ Lagos J Basic Med Sci. 2021;6:38–45. [Google Scholar]
- 15.Pucilowski O, Overstreet DH. Effect of chronic antidepressant treatment on responses to apomorphine in selectively bred rat strains. Brain Res Bull. 1993;32:471–5. doi: 10.1016/0361-9230(93)90293-k. [DOI] [PubMed] [Google Scholar]
- 16.Mazarati AM, Pineda E, Shin D, Tio D, Taylor AN, Sankar R. Comorbidity between epilepsy and depression: Role of hippocampal interleukin-1beta. Neurobiol Dis. 2010;37:461–7. doi: 10.1016/j.nbd.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


