Abstract
Purpose:
To develop and evaluate a deep learning (DL)-based rapid image reconstruction and motion correction technique for high-resolution Cartesian first-pass myocardial perfusion imaging at 3 Tesla with whole-heart coverage for both single-slice (SS) and simultaneous multi-slice (SMS) acquisitions.
Methods:
3D physics-driven unrolled network architectures were utilized for the reconstruction of high-resolution Cartesian perfusion imaging. The SS and SMS MB=2 networks were trained from 135 slices from 20 subjects. Structural similarity index (SSIM), peak signal-to-noise ratio (PSNR), and normalized root mean square error (NRMSE) were assessed, and prospective images were blindly graded by 2 experienced cardiologists (5, excellent; 1 poor). For respiratory motion correction, a 2D U-Net based motion corrected network was proposed, and the temporal fidelity and second-order derivative were calculated to assess the performance of the motion correction.
Results:
Excellent performance was demonstrated in the proposed technique with high SSIM, PSNR and low NRMSE. Image quality scores were 4.3 [4.3, 4.4], 4.5 [4.4, 4.6], 4.3 [4.3, 4.4], and 4.5 [4.3, 4.5] for SS DL and SS L1-SENSE, MB=2 DL and MB=2 SMS-L1-SENSE, respectively, showing no statistically significant difference (p>0.05 for SS and SMS) between (SMS)-L1-SENSE and the proposed DL technique. The network inference time was around 4 seconds per dynamic perfusion series with 40 frames while the time of (SMS)-L1-SENSE with GPU acceleration was approximately 30 minutes.
Conclusion:
The proposed DL-based image reconstruction and motion correction technique enabled rapid and high-quality reconstruction for SS and SMS MB=2 high-resolution Cartesian first-pass perfusion imaging at 3 T.
1 |. INTRODUCTION
Coronary artery disease (CAD) is the most prevalent heart disease in the United States1–5 and remains a major public health concern. First-pass contrast-enhanced cardiac magnetic resonance (CMR) perfusion imaging is a non-invasive and non-radioactive technique which has proven to be a valuable tool for evaluating patients with known or suspected CAD1–4. To provide an accurate assessment of perfusion in the myocardium, it is helpful to acquire multiple slices to achieve whole-heart coverage5. For first-pass perfusion imaging, a contrast agent is injected, and images are acquired over the subsequent 40–60 heart beats. As images are acquired in each heartbeat during the first pass of the contrast agent, the data for each frame must be collected in less than 500–600 ms to support the expected increase in heart rate in patients undergoing adenosine stress requiring highly efficient data acquisition strategies. However, this typically requires undersampling in the k-space domain which can result in undesirable aliasing artifacts in the image domain. Specifically, for SMS imaging, residual aliasing artifacts, noise amplification and leakage artifacts6–10 could potentially deteriorate image quality.
Most clinically available techniques for first-pass perfusion imaging have utilized Cartesian sampling11. However, compared to non-Cartesian imaging strategies such as spiral imaging, Cartesian imaging has relatively low acquisition efficiency limiting the spatial coverage (3–4 slices). Additionally, Cartesian perfusion images are susceptible to the dark-rim artifact, especially when acquired with low spatiotemporal resolution12. To increase spatiotemporal resolution, highly undersampled Cartesian perfusion pulse sequences have been proposed, but the higher acceleration rates necessitate more sophisticated iterative reconstructions such as compressed sensing (CS) which have longer reconstruction times than standard parallel imaging techniques. To overcome the limitation of spatial coverage, multiple investigators have proposed using SMS perfusion imaging techniques to enable whole-heart coverage13,14 without additional cost of signal-to-noise ratio (SNR). For cine, parametric mapping, and late gadolinium enhancement, SMS could lead to fewer breath-hold to cover the whole heart. However, this this comes at the cost of additional acceleration in the slice dimension further exacerbating aliasing in the image domain.
Furthermore, respiratory motion correction (MOCO) improves qualitative assessment of the perfusion images and enables pixel-wise quantification6,15–17. Due to the dynamic variance of contrast, MOCO for perfusion imaging can be challenging. To overcome this, our group has developed a simple and robust MOCO technique for perfusion imaging and proposed to utilize principle component analysis along temporal dimension to reduce the contrast variance, showing promising performance for both Cartesian17 and spiral6 perfusion imaging. However, this technique is time consuming and limited to affine transformation. Related works by Scannell at al15 and Xue at al16 utilized robust PCA and Karhunen-Loève transform to reduce the effect of dynamic variance of contrast agent on the registration. However, these methods are iterative and computationally expensive. Recently, DL-based motion correction approaches18 have been proposed to automatically estimate deformation field between image pairs by self-supervised learning. Furthermore, this approach is rapid and takes two random images in a dynamic perfusion series as an image pair to the input of the model, and thus training pairs may contain different image contrast and the model thus learns to be robust to contrast variations during the training. Overall, a DL-based rapid and accurate motion correction which could correct respiratory motion after DL-based rapid image reconstruction is desired to further shorten the total time of image processing.
Thus, a faster image reconstruction and motion correction technique, instead of an iterative Cartesian image reconstruction, is essential to facilitate clinical translation. Recently, DL-based data-driven and physics-driven image reconstruction techniques have been proposed to accelerate the image reconstruction process. For data-driven approaches19–22, denoising networks in the image space are trained in an end-to-end fashion. Compared with data-driven reconstruction network, physics-based reconstruction methods23–25 incorporate data fidelity and may help preserve fine features of the images. Several previously proposed image reconstruction techniques for Cartesian imaging incorporated data fidelity24,26–28. Demirel et al7 demonstrated the application of physics-driven deep learning reconstruction on SMS perfusion imaging with uniform sampling patterns. This technique required a spatially dependent signal-intensity informed encoding operator prior to passing the images through the network to avoid slice leakage effects, which requires a calibration from single-slice data from either split-slice-GRAPPA reconstruction or pre-acquired data and the calculation process could be optimized with GPU-based hardware so that the reconstruction could be real-time. There has been limited application variable density undersampling patterns for deep learning reconstruction for CMR perfusion imaging, which could lessen coherent slice leakage artifacts. We hypothesized that a variable density poisson disk undersampling pattern combined with SMS would enable high quality perfusion image reconstruction without the need for a spatially dependent signal-intensity informed encoding operator
In this work, we sought to develop a fully automatic DL-based image reconstruction and respiratory motion correction technique for Cartesian SS and SMS high-resolution perfusion imaging to provide rapid and high-quality reconstruction, advancing the clinical workflow (Figure 1).
Figure 1.
The proposed deep learning-based image reconstruction and respiratory motion correction pipeline for high-resolution Cartesian perfusion imaging. (A) shows the proposed workflow aiming to provide immediate feedback right after the scan. (B) shows the proposed reconstruction network, which is a ResNet-based denoising structure with four repetitive blocks that fit the compressed sensing-based denoising reconstruction process. (C) shows the proposed motion correction network. The numbers above each layer denote the number of kernels at each layer, and the corresponding image shape at each layer is also labelled.
2 |. METHODS
1. Pulse Sequences and Data Acquisition
The Cartesian single slice (SS) and SMS perfusion sequence utilized a Poisson-disc sampling patterns along phase encoding and temporal direction ( direction)29 with a fixed number of lines (8 lines in this study) in the center for each dynamic frame and a net in-plane acceleration factor of 4. For SMS imaging, a CAIPIRINHA phase modulation factor of 2 was adopted30, resulting in a total of acceleration factor of 8. The detailed acquisition strategy and parameters are listed in Supporting Information Part 2 and Table S1.
Resting perfusion images from 10 healthy volunteers and 10 clinical patients undergoing clinically ordered CMR studies with gadolinium (Gd)-based contrast agents (Gadoteric acid - Gadoterate meglumine; Dotarem Guerbet or Clariscan GE Healthcare) were included. Written informed consent was obtained from all subjects using protocols approved by the University of Virginia Health Sciences Research Institutional Review Board. Imaging was performed on 3 T MRI scanners (MAGNETOM Prisma/Skyra; Siemens Healthineers, Erlangen, Germany). Subjects were asked to hold their breath as long as possible followed by shallow breathing during the acquisition of perfusion images over 50 to 60 heartbeats. 10 healthy subjects underwent Cartesian perfusion imaging during two separate gadolinium injections separated by a 20-minute washout period and the 10 clinical volunteers underwent 1 perfusion scan during their clinically ordered study. Overall, the studies in volunteers and patients resulted in 30 perfusion datasets for analysis.
For SS acquisition, 3 slices were acquired for each subject due to the limitation of the available acquisition window in each heartbeat. While for SMS MB=2 acquisitions, 3 phase-modulated slices (6 slices) were acquired, enabling whole-heart coverage.
2. CS-based Cartesian (SMS)-L1-SENSE Reconstruction
For perfusion imaging, due to the unavailability of the fully sampled data, a state-of-art CS-based image reconstruction technique - (SMS)-L1-SENSE served as the reference for the network training. The (SMS-)L1-SENSE reconstruction method is an extension of the SS k-t SPARSE-SENSE reconstruction31 and SMS perfusion imaging6, and the details can be found in Supporting Information Part 3.
3. Deep learning-based Image Reconstruction Network
Figure 1–B shows the proposed physics-driven unrolled image reconstruction network consisting of several denoising modules, and each module has a ResNet-based32 denoiser and data fidelity update. Four repetitive residual blocks with five 3D convolutional layers in each denoising module and 32 kernels in each layer were implemented, which is the maximum denoising capacity allowed in our GPU (40 GB memory) due to the limited GPU memory. Each 3D input data (40-frame dynamic perfusion series) were fed through the complex ResNet-based32 denoising network with subsequent data fidelity enforcement. Complex-valued convolution was conducted along concatenated real and imaginary values in two channels as described by Cole et al33, and the output of the final layer was summed with the noisy input, inherently enforcing the denoising task by network.
To comprehensively evaluate the performance of the proposed image reconstruction network, we also assessed the performance of utilizing shared weights and non-shared weights in each denoising block (Supporting Information Part 1–A), and the utilization of the data fidelity (Supporting Information Part 1–B).
4. Deep learning-based Motion Correction Network
A 2D U-Net was utilized to estimate the deformation field between two frames in a series (Figure 1–C)18. The inputs are two random image pairs reconstructed using (SMS)-L1-SENSE in each dynamic perfusion series. The deformation field is estimated between image pairs and then applied on the moving image to minimize the difference to target image. This is a self-supervised approach where the network estimated the non-linear deformation between image pairs. To have robustness to contrast changes, image pairs were chosen randomly during network training, and the first 10 frames where the noisy images may exist were excluded as the respiratory motion typically occurs in the later stage of perfusion acquisition due to the failure of long breath holding. Furthermore, registration is challenging prior to the arrival of contrast to the heart.
As perfusion images contain dynamically varying contrast, cross correlation, which is insensitive to contrast variation, is utilized as the loss function. The smoothness of the deformation field is also incorporated in the loss function to enforce abrupt drifting of the deformation field18.
5. Experimental Setup
The SS and SMS reconstruction networks were trained separately. The inputs to the network were single-channel complex-valued image series after inverse Fourier transform and coil combination34. The details of pre-processing can be found in Supporting Information Part 4. 30 SS slices from 10 subjects and 60 SMS slices from 10 subjects were used to train the SS and SMS networks, respectively. Another 15 SS slices from 5 subjects and 30 SMS slices from 5 subjects were used for testing. Each network was trained with a batch size of 1 using the standard ADAM optimizer with hyperparameters =0.9, =0.999, =10−8 and a learning rate of 0.0001. All of the trainings were conducted for 150 epochs by minimizing an 1 loss: mean absolute error (MAE) of the reconstructed images to the reference (SMS)-L1-SENSE images. MAE was calculated using the magnitude images of the final reconstructions. The choice of this 1 loss function was based on our initial experiments that demonstrated improved SSIM for 1 loss as compared to 2 loss, which was consistent with other studies comparing loss functions for image reconstruction and restoration35,36.
For training the MOCO network, the data from the reconstruction network was used: 30 SS slices from 10 subjects and 60 SMS slices from 10 subjects were used to train the SS and SMS networks, respectively. Another 15 SS slices from 5 subjects and 30 SMS slices from 5 subjects were used for testing. The network was also trained with a batch size of 20 image pairs using the standard ADAM optimizer with a learning rate of 0.0001. The training could be conducted with a larger batch size, but no improvement was noticed. All of the trainings were conducted for 500 epochs by minimizing the cross-correlation loss.
Both training and testing were conducted on a NVIDIA Tesla A100 GPU (40 GB memory), and the (SMS)-L1-SENSE reconstruction was conducted on a server with an Intel i7–7700K CPU (4.20 GHz), a NVIDIA Tesla A100 GPU and 128 GB memory.
6. Image Analysis
To evaluate the performance of the reconstruction network, a separate 15 slices from 5 subjects that were prospectively acquired using the SS pulse sequence was used. For SMS MB=2 networks, another 18 slices from 3 subjects with prospective SMS MB=2 acquisitions were used for testing. Structural similarity index (SSIM), peak signal-to-noise ratio (PSNR) and normalized root mean square error (NRMSE) were assessed for prospective single-slice data. For both prospectively acquired SS and SMS MB=2 data, two experienced cardiologists blindly graded images reconstructed using the proposed networks and the CS-based (SMS-)L1-SENSE (5, excellent; 1, poor).
To evaluate the performance of the MOCO network, the second-order derivative of the voxel-wise time-intensity values were computed to analyze the temporal smoothness15. The second-order derivative is defined as the difference of the image intensity difference along temporal dimension, which characterizes the temporal smoothness of dynamic images15. The time of image reconstruction and MOCO per slice (40 dynamic frames) was also evaluated.
3 |. Results
High image reconstruction quality and MOCO performance was achieved using the proposed technique for both SS and SMS MB=2 acquisitions. The Shapiro-Wilk test showed that the SSIM, PSNR and NRMSE for each network structure were not normally distributed, therefore the median and IQR values were used to represent the data. Supporting Information Table S2 showed the image quality assessment with respect to SSIM, PSNR and NRMSE for both prospective SS and SMS MB=2 data.
Based on the analysis of shared weights and data fidelity in Supporting Information Part 1–A (Supporting Information Figure S1) and 1–B (Supporting Information Figure S2), for the visual image quality assessment study we used a network with shared weights for each denoiser, and data fidelity enforcement. Both single slice acquisitions and SMS MB=2 acquisitions were assessed by cardiologists using results from these network settings.
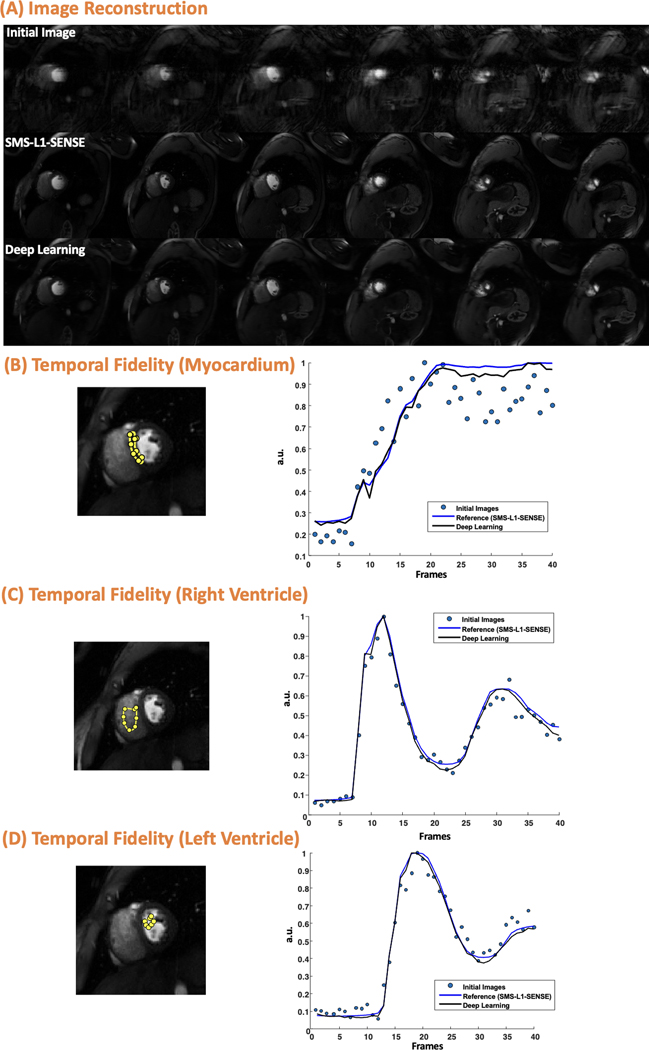
Figure 2 shows an example case from a healthy volunteer undergoing clinical Cartesian single-slice perfusion imaging with an image quality score of 4 and 4 for L1-SENSE and DL image reconstruction, respectively, demonstrating the high image quality of the proposed method. The corresponding perfusion video using DL reconstruction and L1-SENSE reconstruction without motion correction can be found in Supporting Information Video S1 (top row for DL, bottom row for L1-SENSE).
Figure 2.
Cartesian perfusion imaging from a healthy volunteer underwent SS acquisition. The image is reconstructed using the inverse Fast Fourier transform (initial image), compressed sensing (reference) and the proposed deep learning method. Excellent image quality was demonstrated using the proposed image reconstruction network. This case was reconstructed using L1-SENSE and deep learning had an image quality score of 4 and 4, respectively (5, excellent; 1, poor). (B), (C) and (D) shows the temporal fidelity of the signal intensities at myocardium, right ventricle, and left ventricle in the middle slice.
Figure 3 shows an example case from a healthy volunteer undergoing clinical Cartesian SMS MB=2 perfusion imaging with an image quality score of 4 and 4 for SMS-L1-SENSE and DL reconstruction, respectively, demonstrating the good performance of the proposed image reconstruction method. The corresponding perfusion video using DL reconstruction and SMS-L1-SENSE without motion correction can be found in Supporting Information Video S2 and S3, respectively.
Figure 3.
SMS MB=2 Cartesian perfusion imaging from a healthy volunteer with 6 slices reconstructed using the proposed reconstruction network. Good image quality was demonstrated using the proposed image reconstruction network. This case reconstructed using CS-based SMS-L1-SENSE and deep learning (DL) had an image quality score of 4 and 4, respectively (5, excellent; 1, poor). (B), (C) and (D)shows the temporal fidelity of the signal intensities at myocardium, right ventricle, and left ventricle in one of the middle slices shown in (A).
Figure 4 shows the MOCO performance. Temporal fidelity shows excellent performance of the proposed technique. The second-order derivative of SS and SMS before and after MOCO were 0.25 vs. 0.22 and 0.26 vs.0.23, respectively, demonstrating better temporal smoothness after MOCO. The corresponding perfusion video using DL reconstruction before and after motion correction can be found in Supporting Information Video S1 and S4, respectively.
Figure 4.
Evaluation of motion correction (MOCO) for example cases with SS (A) and SMS MB=2 (B) acquisitions. Temporal fidelity for the dashed line pointed in a middle frame demonstrate the excellent performance of the proposed technique.
Figure 5 presents the image quality scores from two experienced cardiologists (5, excellent; 1, poor). The SS and SMS MB=2 images were reconstructed using the networks that had the best performance and the CS-based (SMS-)L1-SENSE. Image quality scores were 4.3 [4.3, 4.4], 4.5 [4.4, 4.6], 4.3 [4.3, 4.4], and 4.5 [4.3, 4.5] for SS DL and SS L1-SENSE, MB=2 DL and MB=2 SMS-L1-SENSE, respectively. For both SS and SMS MB=2, the Wilcoxon signed-rank test showed that there was no statistically significant difference (p>0.05) between the proposed DL and (SMS-)L1-SENSE reconstructions.
Figure 5.
Image quality score for each acquisition method. Images were blindly graded by 2 experienced cardiologists (5, excellent; 1, poor). The scores shown are the average score from 2 cardiologists. There was no statistically significant difference (p>0.05 for SS and SMS) between (SMS)-L1-SENSE and the proposed DL technique.
The reconstruction and MOCO time using the proposed DL technique is significantly shorter than that of the reference (SMS-)L1-SENSE technique. The image reconstruction and motion correction time were around 3 seconds and 1 second per dynamic series, respectively, while the reconstruction time of using (SMS-)L1-SENSE with 30 iterations was around 30 minutes per slice, demonstrating a rapid and high-quality image reconstruction performance.
4 |. Discussion
We demonstrated high-quality high-resolution Cartesian first-pass myocardial perfusion imaging for both SS (3 spatial slices) and SMS MB=2 acquisitions (6 spatial slices) at 3 T and images can be reconstructed using the proposed rapid DL-based image reconstruction and MOCO technique.
In this study, we used a variable density Possion-disc sampling pattern for the phase encoding lines which has previously demonstrated superior performance with respect to uniform sampling for CS reconstruction37. Similar to CS-based image reconstruction, the DL-based image reconstruction process for variable-density sampled data is a image-based denoising process. DL-denoising has demonstrated the potential to achieve better denoising performance than CS-based denoisers.26,37. Prior work by Wang et al38 also demonstrated superior performance of DL-based image reconstructions by using variable-density sampling patterns for Cartesian imaging with cascade network proposed by Schlemper et al24. Specifically, for Cartesian perfusion imaging with uniform sampling patterns, the temporal signal intensity could be distorted in DL-based image reconstructions7, which impedes the quantification of dynamic perfusion imaging. However, in this work, signal intensity distortion could be avoided by using variable density-based sampling patterns. Further experiments such as sampling the same subject with and without variable density and exploring the signal-informed multi-coil encoding operator could potentially provide a more comprehensive analysis of this problem.
The model we adopted used shared weights among different denoising modules. Compared with networks trained using different weights for each module, utilizing shared weights could reduce the number of trainable parameters significantly and save the GPU memory cost while achieving comparable image reconstruction as the network without shared weights. The utilization of data fidelity enhanced the image reconstruction performance and preserved fine details. In this work, the proximal gradient decent method was utilized to conduct the data fidelity update. Optimization of the data fidelity update such as by utilizing conjugate gradient descent could potentially further improve the performance23, at the expense of a longer reconstruction time.
After rapid image reconstruction, the proposed MOCO network could rapidly register the image to the target frame to reduce respiratory motion. Compared with the traditional motion correction techniques for perfusion imaging15, DL-based technique is rapid (around 1 second) so that results could be provided online, or utilized as a pre-processing technique for automatic quantification of perfusion. Separating the motion correction and image reconstruction into separate networks provides flexibility of the network training and reduces the memory consumption, especially for dynamic SMS perfusion imaging. While the proposed method significantly reduces respiratory motion, in cases with significant through-plane motion, residual motion may still exist.
This study also has several limitations. Firstly, the coil compression in pre-processing steps for a single slice took around 5 seconds and the image reconstruction and MOCO took around 4 seconds. The optimization of the pre-processing by utilizing high-performance computers and parallel processing could further shorten the time of pre-processing. Optimization of the data fidelity step could further shorten the inference time. Exploration of DL-based image reconstruction and motion correction approach for SMS imaging is still of interest. Moreover, given the fact that it is impossible to acquire the fully sampled data for perfusion imaging with whole-heart coverage, a state-of-art CS-based image reconstruction method (SMS-L1-SENSE) was considered as the reference in the network training process. For high acceleration rates where SMS-L1-SENSE may not produce high quality images, a supervised deep learning approach may not be feasible, especially for SMS imaging with the additional in-plane acceleration. Alternatively, self-supervised approaches7,37 could potentially be applied on reconstructions with high acceleration factors to overcome the limitation of initial image quality. Further optimization of the acquisition strategy could improve both the CS reconstructed images and the DL-based image reconstructions.
5 |. Conclusion
The proposed DL-based image reconstruction and motion correction technique enabled rapid and high-quality reconstruction for SS and SMS MB=2 high-resolution Cartesian first-pass perfusion imaging at 3 T. Further validation will be required in patients undergoing stress CMR to assess the clinical value.
Supplementary Material
Supporting Information Table S1. Detailed acquisition parameters for the Cartesian high-resolution perfusion imaging.
Supporting Information Table S2. Summary of the image quality assessment reconstructed by deep learning.
Supporting Information Figure S1. Comparison of image reconstruction network using shared weights and non-shared weights for each denoising module.
Supporting Information Figure S2. Comparison of image reconstruction network with and without data fidelity.
Supporting Information Video S1. Perfusion video from a healthy volunteer using SS acquisition and DL reconstruction (top row) and L1-SENSE reconstruction (bottom row) without motion correction.
Supporting Information Video S2. Whole-heart perfusion movie from a healthy volunteer using SMS MB=2 acquisition and DL reconstruction.
Supporting Information Video S3. Whole-heart perfusion movie from a healthy volunteer using SMS MB=2 acquisition and SMS-L1-SENSE reconstruction.
Supporting Information Video S4. Perfusion video from a healthy volunteer as shown in Video S1 using SS acquisition and DL reconstruction with motion correction.
6 |. Acknowledgements
The authors would like to acknowledge the help of our study coordinators and nurses Caroline Flournoy PhD, Sara Prince RN and Jayne Missel RN, and our research CMR technologists Jamie Lynn Weathersbee, RT(R)MR and Jose M. Reyes, RT(R)MR.
Funding Sources:
Wallace H. Coulter Foundation Grant and NIH R01 HL131919
9 |. Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
10 | References
- 1.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. The Lancet. 2012;379(9814):453–460. doi: 10.1016/S0140-6736(11)61335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salerno M, Beller GA. Noninvasive Assessment of Myocardial Perfusion. Circ Cardiovasc Imaging. 2009;2(5):412–424. doi: 10.1161/CIRCIMAGING.109.854893 [DOI] [PubMed] [Google Scholar]
- 3.Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic Performance of Noninvasive Myocardial Perfusion Imaging Using Single-Photon Emission Computed Tomography, Cardiac Magnetic Resonance, and Positron Emission Tomography Imaging for the Detection of Obstructive Coronary Artery Disease: A Meta-Analysis. J Am Coll Cardiol. 2012;59(19):1719–1728. doi: 10.1016/j.jacc.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 4.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic Value of Stress Cardiac Magnetic Resonance Imaging in Patients With Known or Suspected Coronary Artery Disease: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2013;62(9):826–838. doi: 10.1016/j.jacc.2013.03.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9–e119. doi: 10.1161/CIR.0000000000001168 [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Yang Y, Weller DS, et al. High spatial resolution spiral first-pass myocardial perfusion imaging with whole-heart coverage at 3 T. Magn Reson Med. 2021;86(2):648–662. doi: 10.1002/mrm.28701 [DOI] [PubMed] [Google Scholar]
- 7.Demirel OB, Yaman B, Shenoy C, Moeller S, Weingärtner S, Akçakaya M. Signal intensity informed multi-coil encoding operator for physics-guided deep learning reconstruction of highly accelerated myocardial perfusion CMR. Magn Reson Med. 2023;89(1):308–321. doi: 10.1002/mrm.29453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Meyer CH, Epstein FH, Kramer CM, Salerno M. Whole-heart spiral simultaneous multi-slice first-pass myocardial perfusion imaging. Magn Reson Med. 2019;81(2):852–862. doi: 10.1002/mrm.27412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques: SMS Imaging. Magn Reson Med. 2016;75(1):63–81. doi: 10.1002/mrm.25897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moeller S. Simultaneous Multi-Slice Methods. In: Proceedings of the ISMRM 20th Annual Scientific Sessions, Melbourne, Australia, 2012. ; 2012. [Google Scholar]
- 11.Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43. doi: 10.1186/s12968-017-0355-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bella EVRD Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54(5):1295–1299. doi: 10.1002/mrm.20666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElroy S, Ferrazzi G, Nazir MS, et al. Combined simultaneous multislice bSSFP and compressed sensing for first-pass myocardial perfusion at 1.5 T with high spatial resolution and coverage. Magn Reson Med. 2020;84(6):3103–3116. doi: 10.1002/mrm.28345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demirel ÖB, Weingärtner S, Moeller S, Akçakaya M. Improved Simultaneous Multi-Slice Imaging for Perfusion Cardiac MRI Using Outer Volume Suppression and Regularized Reconstruction. In: 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI). ; 2020:1954–1957. doi: 10.1109/ISBI45749.2020.9098326 [DOI] [Google Scholar]
- 15.Scannell CM, Villa ADM, Lee J, Breeuwer M, Chiribiri A. Robust Non-Rigid Motion Compensation of Free-Breathing Myocardial Perfusion MRI Data. IEEE Trans Med Imaging. 2019;38(8):1812–1820. doi: 10.1109/TMI.2019.2897044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue H, Brown LAE, Nielles-Vallespin S, Plein S, Kellman P. Automatic in-line quantitative myocardial perfusion mapping: Processing algorithm and implementation. Magn Reson Med. 2020;83(2):712–730. doi: 10.1002/mrm.27954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R, Huang W, Yang Y, et al. Simple motion correction strategy reduces respiratory-induced motion artifacts for k-t accelerated and compressed-sensing cardiovascular magnetic resonance perfusion imaging. J Cardiovasc Magn Reson. 2018;20(1). doi: 10.1186/s12968-018-0427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balakrishnan G, Zhao A, Sabuncu MR, Guttag J, Dalca AV. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans Med Imaging. 2019;38(8):1788–1800. doi: 10.1109/TMI.2019.2897538 [DOI] [PubMed] [Google Scholar]
- 19.Fan L, Shen D, Haji-Valizadeh H, et al. Rapid dealiasing of undersampled, non-Cartesian cardiac perfusion images using U-net. NMR Biomed. 2020;33(5):e4239. doi: 10.1002/nbm.4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Weller DS, Kramer CM, Salerno M. DEep learning-based rapid Spiral Image REconstruction (DESIRE) for high-resolution spiral first-pass myocardial perfusion imaging. NMR Biomed. 2022;35(5):e4661. doi: 10.1002/nbm.4661 [DOI] [PubMed] [Google Scholar]
- 21.Le J, Tian Y, Mendes J, et al. Deep Learning for Radial Myocardial Perfusion Reconstruction using 3D residual booster U-Nets. In: Proceedings of the 28th Annual Meeting of ISMRM, 2020. p 2095. [Google Scholar]
- 22.Wang J, Awad M, Zhou R, et al. High-resolution spiral real-time cardiac cine imaging with deep learning-based rapid image reconstruction and quantification. NMR Biomed. 2024;37(2):e5051. doi: 10.1002/nbm.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal HK, Mani MP, Jacob M. MoDL: Model-Based Deep Learning Architecture for Inverse Problems. IEEE Trans Med Imaging. 2019;38(2):394–405. doi: 10.1109/TMI.2018.2865356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlemper J, Caballero J, Hajnal JV, Price AN, Rueckert D. A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction. IEEE Trans Med Imaging. 2018;37(2):491–503. doi: 10.1109/TMI.2017.2760978 [DOI] [PubMed] [Google Scholar]
- 25.Hammernik K, Klatzer T, Kobler E, et al. Learning a variational network for reconstruction of accelerated MRI data: Learning a Variational Network for Reconstruction of Accelerated MRI Data. Magn Reson Med. 2018;79(6):3055–3071. doi: 10.1002/mrm.26977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandino CM, Lai P, Vasanawala SS, Cheng JY. Accelerating cardiac cine MRI using a deep learning-based ESPIRiT reconstruction. Magn Reson Med. 2021;85(1):152–167. doi: 10.1002/mrm.28420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Küstner T, Fuin N, Hammernik K, et al. CINENet: deep learning-based 3D cardiac CINE MRI reconstruction with multi-coil complex-valued 4D spatio-temporal convolutions. Sci Rep. 2020;10(1):13710. doi: 10.1038/s41598-020-70551-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammernik K, Schlemper J, Qin C, Duan J, Summers RM, Rueckert D. Σ-net: Systematic Evaluation of Iterative Deep Neural Networks for Fast Parallel MR Image Reconstruction. ArXiv191209278 Cs Eess. December 2019. http://arxiv.org/abs/1912.09278. Accessed November 24, 2020.
- 29.Chen X, Salerno M, Yang Y, Epstein FH. Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: Block low-rank sparsity with motion-guidance (BLOSM): BLOSM: Block Low-rank Sparsity with Motion-guidance. Magn Reson Med. 2014;72(4):1028–1038. doi: 10.1002/mrm.25018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53(3):684–691. doi: 10.1002/mrm.20401 [DOI] [PubMed] [Google Scholar]
- 31.Feng L, Srichai MB, Lim RP, et al. Highly accelerated real-time cardiac cine MRI using k–t SPARSE-SENSE. Magn Reson Med. 2013;70(1):64–74. doi: 10.1002/mrm.24440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He K, Zhang X, Ren S, Sun J. Deep Residual Learning for Image Recognition. December 2015. http://arxiv.org/abs/1512.03385. Accessed November 3, 2022.
- 33.Cole E, Cheng J, Pauly J, Vasanawala S. Analysis of deep complex-valued convolutional neural networks for MRI reconstruction and phase-focused applications. Magn Reson Med. 2021;86(2):1093–1109. doi: 10.1002/mrm.28733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43(5):682–690. doi: [DOI] [PubMed] [Google Scholar]
- 35.Ghodrati V, Shao J, Bydder M, et al. MR image reconstruction using deep learning: evaluation of network structure and loss functions. Quant Imaging Med Surg. 2019;9(9):1516527–1511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Gallo O, Frosio I, Kautz J. Loss Functions for Image Restoration With Neural Networks. IEEE Trans Comput Imaging. 2017;3(1):47–57. doi: 10.1109/TCI.2016.2644865 [DOI] [Google Scholar]
- 37.Yaman B, Hosseini SAH, Moeller S, Ellermann J, Uğurbil K, Akçakaya M. Self-supervised learning of physics-guided reconstruction neural networks without fully sampled reference data. Magn Reson Med. 2020;84(6):3172–3191. doi: 10.1002/mrm.28378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Yang Y, Feng X, Weller DS, Salerno M. Analysis of Sampling Strategies for Convolutional Neural Network Based Cardiac Magnetic Resonance Image Reconstruction. In: Proceedings of the ISMRM 27th Annual Meeting and Exhibition, Montreal, Quebec, Canada, 2019, p. 2155. ; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1. Detailed acquisition parameters for the Cartesian high-resolution perfusion imaging.
Supporting Information Table S2. Summary of the image quality assessment reconstructed by deep learning.
Supporting Information Figure S1. Comparison of image reconstruction network using shared weights and non-shared weights for each denoising module.
Supporting Information Figure S2. Comparison of image reconstruction network with and without data fidelity.
Supporting Information Video S1. Perfusion video from a healthy volunteer using SS acquisition and DL reconstruction (top row) and L1-SENSE reconstruction (bottom row) without motion correction.
Supporting Information Video S2. Whole-heart perfusion movie from a healthy volunteer using SMS MB=2 acquisition and DL reconstruction.
Supporting Information Video S3. Whole-heart perfusion movie from a healthy volunteer using SMS MB=2 acquisition and SMS-L1-SENSE reconstruction.
Supporting Information Video S4. Perfusion video from a healthy volunteer as shown in Video S1 using SS acquisition and DL reconstruction with motion correction.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.