Abstract
Intravenously infusible nanoparticles to control bleeding have shown promise in rodents, but translation into preclinical models has been challenging as many of these nanoparticle approaches have resulted in infusion responses and adverse outcomes in large animal trauma models. We developed a hemostatic nanoparticle technology that was screened to avoid one component of the infusion response: complement activation. We administered these hemostatic nanoparticles, control nanoparticles, or saline volume controls in a porcine polytrauma model. While the hemostatic nanoparticles promoted clotting as marked by a decrease in prothrombin time and both the hemostatic nanoparticles and controls did not active complement, in a subset of the animals, hard thrombi were found in uninjured tissues in both the hemostatic and control nanoparticle groups. Using data science methods that allow one to work across heterogeneous data sets, we found that the presence of these thrombi correlated with changes in IL-6, INF-alpha, lymphocytes, and neutrophils. While these findings might suggest that this formulation would not be a safe one for translation for trauma, they provide guidance for developing screening tools to make nanoparticle formulations in the complex milieux of trauma as well as for therapeutic interventions more broadly. This is important as we look to translate intravenously administered nanoparticle formulations for therapies, particularly considering the vascular changes seen in a subset of patients following COVID-19. We need to understand adverse events like thrombi more completely and screen for these events early to make nanomaterials as safe and effective as possible.
Keywords: Hemorrhage, inflammation, IL-6, neutrophils, lymphocytes, TNF-alpha, hemostasis
Graphical Abstract

Summary:
The administration of both hemostatic and control nanoparticles based on PEGylated polylactide triggers thrombi in both a naïve porcine model as well as in a porcine model of trauma, and these thrombi correlate with changes in cytokines and immune cells that should be considered for future intravenously infusible nanoparticle therapies.
Body of Paper
Traumatic injury is the leading cause of death in men, women, and children between the ages of 1 and 46 worldwide 1, and blood loss is the primary cause of death at acute time points post-injury in both civilian 2 and battlefield trauma 3. While rapid intervention to achieve hemostasis is imperative to minimize mortality associated with severe trauma 4, 5, pressure dressings and absorbent topical agents are currently the only hemostats available for field use. We need therapies that can be administered in the field to control bleeding for non-compressible junctional and torso internal injuries6. To address this need, many groups have developed intravenously infusible nanomaterials7–11.
While many agents have shown promise in small animal models, translating the work to large animal models has proven exceptionally difficult in great part because of complement activation to intravenously infused agents, including both cell-derived and nanomaterial systems.12–16 Complement activation is extremely rapid and triggers vasodilation which significantly hinders the hemostatic effect of any technology. Unfortunately, approximately 10% of humans exhibit the same significant response during infusions of the liposomal formulation of doxorubicin (DOXIL)17 as well as infusions of biologics.18 These complement responses have been observed by us and others and are particularly challenging in the context of infusible treatments for trauma given the urgency and time-sensitive nature of hemostatic treatments. Slow infusions can reduce infusion responses19, 20, but a slow infusion rate would risk unmitigated hemorrhage before the hemostatic agent is delivered in patients with major hemorrhage.
We have been developing and testing hemostatic nanoparticles that can be infused intravenously to stop bleeding and improve survival in several of rodent models of trauma21–25. However, in a porcine model of trauma, we saw that these particles triggered complement activation and vasodilation, leading to a rapid drop in blood pressure, blood gas changes, and, ultimately, increased bleeding26. We found that by modulating the charge on these hemostatic nanoparticles, we could reduce this complement response and halt bleeding in the large animal model, but at the highest doses, we still saw exacerbation of bleeding26. This motivated us to develop a screening assay for complement activation with nanoparticles that was more sensitive than the ones most commonly described in the field27. Using this assay, we identified a hemostatic nanoparticle formulation with two molecular weights of PEG that led to no measurable complement activation in vitro28. Based on this, we sought to determine the safety and efficacy of these hemostatic nanoparticles in a large animal model of trauma, a critical step in translating nanoparticles for trauma applications.
In this work, we tested these hemostatic nanoparticles, control nanoparticles, or saline in a porcine liver injury model and assessed their impact on bleeding, survival, and immune responses following administration. We also tested the nanoparticles and controls in naïve uninjured animals to parse out the impact of the nanoparticles from the trauma as trauma independently leads to significant changes in inflammatory markers. We applied data science methods to look for correlations between outcomes and biological parameters, including physiological data, cytokines, and immune cells, to understand the impact of the infusion of nanoparticles on immune responses beyond complement responses. This work provides a foundation for understanding the complex role of infusion responses and approaches to mitigate their effect.
Results/Discussion
Nanoparticle Design and Characterization
Based on our previous work screening nanoparticles, we used PLA-PEG nanoparticles consisting of a blend of PLA-PEG with a PEG molecular weight of 5000 Da (75 wt%) and a PLA-PEG with a PEG molecular weight of 3400 Da (25 wt%) as the platform for the nanoparticles in this study. We have previously shown that this blend is highly PEGylated (92%)28 and leads to no measurable complement activation in vitro, as marked by changes in C5a28. To make these nanoparticles hemostatic, we conjugated the GRGDS peptide to the PLA-PEG nanoparticles using EDC/NHS chemistry following our previous work21. The GRGDS peptide binds with the glycoprotein IIb/IIIa receptor on activated platelets, and this binding can be inhibited by blocking the glycoprotein IIb/IIIa receptor which, in turn, blocks the hemostatic capacity of the PLA-PEG-GRGDS system21. Therefore, we have two nanoparticles in this work: control nanoparticles based on PLA-PEG and hemostatic nanoparticles based on PLA-PEG with GRGDS conjugated following the nanoparticle fabrication procedure.
Each batch of nanoparticles was characterized for size, zeta potential, and peptide content. The average values across batches are in Figure 1, and the detailed values for each batch are in Supplementary Tables 1 and 2.
Figure 1:

Nanoparticle characterization for large animal studies. (A) Schematic of the PLA-PEG based nanoparticles functionalized with GRGDS for the studies. (B) SEM micrograph of PLA-PEG nanoparticles. (C) TEM micrograph of PLA-PEG nanoparticle showing the PEG corona. (D) Table of properties of PLA-PEG control and PLA-PEG-GRGDS hemostatic nanoparticles.
The average hydrodynamic diameter and zeta potential in 10 mM KCl solution of each batch were determined using dynamic light scattering (DLS). The amount of peptide was quantified using the orthopthaladehyde (OPA) assay. The presence of the peptide increases the hydrodynamic diameter of the hNPs versus the cNPs, thus impacting the DLS values slightly, as has been seen previously 21. However, SEM of both the control and hemostatic nanoparticles showed no differences in the size of the core (Figure 1B). TEM of nanoparticles showed what may be the PEG corona of the nanoparticles (Figure 1C). We have seen this previously28, but only at the highest degrees of PEGylation.
While the nanoparticle formulation was chosen because it showed no measurable change in complement in vitro28, we confirmed the lack of change in each batch before administration in vivo (Supplementary figure 1). It should be noted that there is some batch-to-batch variability in the size of the nanoparticles (Supplementary table 1.) However, we did not see an impact of the size variation on complement activation or in vivo outcomes, as described in more detail below.
Administration of the Nanoparticles in a Porcine Model of Trauma
All animal procedures were approved by the 711th HPW/RHD JBSA-Fort Sam Houston Institutional Animal Care and Use Committee (IACUC) in compliance with all applicable Federal regulations governing the protection of animals in research.
Following sedation, animals were intubated, and sedation was maintained on 1–3% isoflurane. A midline laparotomy was performed to expose the spleen and liver. A splenectomy was performed. 30% total blood was collected from a controlled femoral artery bleed in a blood donor bag containing the anticoagulant citrate phosphate dextrose adenine solution (CPDA) at a 1:10 ratio for subsequent use in resuscitation. A standardized liver injury was created as previously described29. Briefly, to create this injury, the liver was isolated, and a ring clamp was placed on the lower aspect of the left lobe. A standardized liver laceration was created using the ring clamp as a guide, followed by removal of the lacerated lobe (approximately 25% of the liver lobe mass). The liver was allowed to spontaneously bleed in an uncontrolled manner for 5 minutes, with blood being removed via intraperitoneal suction and weight/volume quantified at one-minute intervals throughout hemorrhage and treatment phases. Figure 2A provides a timeline of all the events involved in the procedure.
Figure 2:

(A) Timeline for trauma model. The key parts include the hemorrhage followed by treatment or controls at T0, whole blood infusions between T30 and T60 to maintain blood pressure, and closure at T60. (error bars=SD, n=6 per group) (B) Average cumulative blood loss across the groups, saline, control nanoparticles (cNPs), and hemostatic nanoparticles (hNPs). There are no significant differences in blood loss over the time period studied for any of the groups. (C) In our previous work, we found the first ten minutes post-administration were critical for complement activation, which typically presents as greater blood loss due to the release of the vasodilators, C3a and C5a. Two animals in the hNP group show greater blood loss following the administration of the nanoparticles. (D) Likewise, two of the animals in the control group, cNP4 and cNP5, showed what looks like a small change in blood loss rate following nanoparticle administration, but the data are less clear, with a greater blood loss rate overall before the particle administration. (E) Blood loss for saline in the first 10 minutes. The saline2 animal shows an upturn in blood loss following saline administration. (F) The prothrombin time (PT) is lower for the hNPs than for controls which suggests that the hNPs are hemostatic and reduce the time for blood to clot. (*p<0.05, error bars=SD)
At T=0, the treatment or controls were administered. Nanoparticles were suspended in 30 ml saline at 2 mg/kg and given as a bolus. At the 30-minute timepoint, autologous whole blood (collected shed blood) was given as needed to maintain a systolic blood pressure (SBP) of 90±5 mmHg. At T=60 minutes, the liver injury was packed, and the abdomen was closed. Animals received maintenance fluids in the form of lactated Ringer’s and were monitored under anesthesia until T=180 min, when animals were humanely euthanized.
Blood Loss and Physiological Parameters
There were no significant differences in blood loss for any of the groups (Fig. 2B). Our previous work found that early blood loss data is important for assessing complement-mediated infusion reactions.26 Complement activation leads to an increase in the rate of blood loss due to increases in the vasodilators C3a and C5a. There is a slight upturn in the hemostatic particle animals, hNP2 and hNP4 post-particle administration (Fig 2C). In contrast, in the control nanoparticle group, while cNP4 and cNP5 bleed more than the other control nanoparticle animals, there is no upturn in the blood loss post-particle administration (Fig 2D). Likewise, there are no upturns in the saline group (Fig 2E). Supplementary figure 2 shows the individual animals’ blood loss curves to help understand these responses. This upturn could be due to the increase in the vasodilators C3a and C5a due to complement activation, but looking at blood loss, alone does not give a complete picture of complement activation.
A second important consideration regarding infusion reactions is changes in heart rate (HR) and mean arterial pressure (MAP) in concert with the change in blood loss rate 26. The HR for the saline-treated animals was constant without spikes except in two animals following saline administration (Supplementary Figure 3A). Saline does not trigger complement, so the HR changes are due to other factors in these animals. In the control group, cNP4 and cNP5 show changes in HR, but several other animals show substantial changes as well without a change in blood loss rate. In the hemostatic nanoparticle group, hNP2 and hNP4 show changes in HR as does hNP7 which survived to the end of the experiment and showed no change in blood loss rate upon particle administration. The MAPs for the saline-treated animals were very consistent throughout the experiment. The MAP for the cNP4 and cNP5 does not exhibit spikes but the animals died shortly after particle administration. However, there were small changes in the MAPs for the other cNP animals. Likewise, the MAP did not exhibit changes or spikes for hNP2 or hNP4 but did show small changes for other hNPs.
The HR and MAP results coupled with the increased bleeding in a subset of animals that received nanoparticles suggest that if complement activation is occurring, the impact is modest compared to those typically seen with complement activation following nanoparticle administration.26 However, the nanoparticles were administered to animals undergoing a significant trauma which could mask the effects of the nanoparticle administration. To rule out the impact of trauma, we looked at the impact of particle administration on a small set of naïve animals. Similar results to those seen in animals experiencing trauma were seen in the naïve animals with some changes in HR and MAP in the animals that received either control or hemostatic nanoparticles (Supplementary Figure 4.) To understand whether there were changes in complement or other molecules following nanoparticle administration, we performed ELISAs on serum isolated at 15-minute intervals throughout the study.
Platelet Activating Factor (PAF) and Complement
Platelet activating factor (PAF) has been seen to trigger adverse physiologic effects following nanoparticle infusion including vasodilation and increased vascular permeability 30. To investigate the role of PAF, we performed ELISAs on serum from the blood draws that were performed at 15-minute intervals. None of the serum samples across animals or timepoints had detectable PAF. The limits of the PAF ELISA assay are 0.156 ng/ml.
While the HR and MAP data suggest that complement is either not activated or is activated at very modest levels, we performed ELISAs on the serum samples from the 15-minute intervals for the animals that exhibited increases in blood loss following particle administration. Supplementary figure 5 shows the complement results. If complement is activated, C3 should decrease and C3a should increase. In Supplementary Figure 5A, we see that the saline animal, for which there is no complement activation by blood loss or physiological data (Supplementary Figure 5C) shows some variation in C3 over time, but the changes are small compared to the 2–4-fold changes expected for complement activation. The changes seen in the saline animal are the same or greater than those seen for C3 in any of the animals that received nanoparticles. Likewise, the changes seen in C3a which should increase if complement is activated, are greater for the saline animal than any of the nanoparticle-animals (Supplementary Figure 5B). These data, taken with the physiological data suggest that complement activation is not occurring with the bolus administration of nanoparticles in this model.
Coagulation
Blood was collected at 15-minute intervals from the start of the study through the first 60 minutes and complete blood counts as well as coagulation tests were performed via the STAGO coagulation analyzer, ROTEM. The platelet counts were statistically identical for all the groups throughout the study (Supplementary Figure 6.) The hNPs led to a significant reduction in prothrombin time (PT) compared to either the saline or cNP groups at T=45 and 60 minutes (Figure 2F). This finding indicates that globally, the hemostatic nanoparticles effectively reduce time to clot formation beyond normal, adaptive physiologic hemostatic intrinsic to the animal. The half-life of the nanoparticles is relatively short, on the order of minutes31. Thus, it is not surprising that the impact of the nanoparticles is not seen at timepoints past T60. It is not surprising that the reduction is not fully resolved until the T=45-minute timepoint. There is a trend towards reduction before this, but the trend is not significant.
We found a similar trend towards a reduced PT in uninjured naïve animals (Supplementary Figure 7), although it must again be noted that the groups were very small (n=3). While the hemostatic impact is not evident in the blood loss data in Fig. 2B, that could be a function of the dose with a higher dose showing a more significant impact coupled with the fact that some of the animals that received particles had complications (Fig. 3).
Figure 3:

(A) Survival curves for treatments in the injured arm of the study. (n=6 per group) (B) Survival curves for the naïve arm of the study (n=3 per group) (C) Table of animals, treatments and clots as characterized by gross pathology and histology. Clots occurred in the brain, lungs, and kidneys of a subset of the animals receiving particle formulations. Animals with stars (*) died.
There are no differences in the partial thromboplastin time (PTT) or fibrinogen for any of the treatments in either the injured (Supplementary Figure 8) or naïve animals (Supplementary Figure 9). Likewise, no differences in d-dimer for any of the groups in the injured or naïve animals was seen. PTT measures clotting time based on the intrinsic pathway in contrast to PT which is clotting time based on the extrinsic pathway triggered by tissue factor. Since the particles are designed to reduce bleeding by forming bridges akin to fibrinogen, they should not impact PTT, and so it reassuring to see that there are no changes in PTT with particle administration32. ROTEM results follow similar patterns to the STAGO coagulation results for PTT with no significant differences between groups or over time (Supplementary Figure 10).
While the reduction in PT with no changes in other coagulation parameters are promising data, administration of nanoparticles in this study was associated with reduced survival in both the injured (Figure 3A) and naïve arms of the study (Figure 3B). Furthermore, pathology revealed that several of the animals that received particles exhibited clots in uninjured tissues including the lungs, kidneys, and brain (Figure 3C), perhaps indicating an abnormal systemic thrombotic or embolic phenomenon.
Survival and Clot Formation
All the animals in the saline group survived, but a subset in the control nanoparticle and hemostatic nanoparticle groups died within the first 30 minutes following particle administration. The first observation is that the animals died of hemorrhage. Animals that died bled more (13+/−3.6 ml/kg) and those that lived (8+/−2.6 ml/kg). At T=0 when particles were administered, the group that survived exhibited similar blood loss on average to the group that died, but the cumulative blood loss varied significantly. The group that survived bled 1.4+/−1 ml/kg and the group that died bled 1.9+/−1.4 ml/kg. In only two animals, hNP2 and hNP4, was blood loss at T=0 low (0.3 and 0.4 ml/kg respectively.) In all the other animals that died, early cumulative blood loss was between 2.3 and 3.4 ml/kg. Excessive blood loss is lethal. However, there are animals that survived with blood loss equal to or greater than animals that died, indicating that hemorrhage alone may not have been the cause of the mortality. For example, animal hNP4 bled 9.3 ml/kg at death, but there are animals that bled more and yet survived (two saline animals and hNP6, Supplementary Table 3.) Typically, if blood loss is the cause of death, there will be a tipping point, a volume as a function of body weight, past which all animals would be expected to die. The fact that some animals bled less than others and died suggests that cumulative blood loss alone is not the entire answer for these early deaths.
If blood loss is not the entire answer, pathology becomes especially critical. In the gross pathology report, there were animals that had clots in a range of tissues (brain, kidneys, and lungs) that were hard nodules (Supplementary Figure 11). Histology confirmed that these hard nodules were not typical postmortem clotting but were thrombi that occurred during the study. While these clots were seen in multiple tissues, every animal with the hard nodules had them had these thrombi in the caudal lungs which is fed by the blood flow return from the heart. In contrast, no such thrombi were seen in the saline animals as described by the pathology reports based on gross pathology and the histological grading done by a pathologist blinded to the treatments. Representative hematoxylin and eosin (H&E) images of a saline animal cranial lung (Supplementary Figure 11C) and of lungs with thrombi (Supplementary Figure 11C–F) show examples of what appear to be thrombi at 40X magnification as marked by arrows. These H&E images also show extensive congestion in the lungs of these animals.
These thrombi were seen in both the control nanoparticle and hemostatic nanoparticle groups but not seen in the saline group. In both the control nanoparticle and hemostatic nanoparticle groups, these clots were seen in a similar number of animals and tissues. The clots do not appear to correlate with the presence of the GRGDS peptide on the nanoparticles that differentiates the hemostatic nanoparticles from the control nanoparticles (Figure 3C.) The pathology suggests that these thrombi are a significant adverse event that may have contributed to the lethality seen in the nanoparticle groups. It should be noted that the pathological and histological grading was done by a pathologist blinded to the treatments and based on typical protocols for grading pathology and histological sections. Thrombi were not found in saline animals.
Looking at higher resolution specifically for thrombi led to the identification of microthrombi in the lungs of two animals with either control or hemostatic nanoparticles (Supplementary Figure 11G–H) that had not been identified previously based on gross pathology and grading of the H&E sections at lower magnification. Finding microthrombi is not entirely surprising. The secondary review was specifically looking for examples of thrombi in the lungs at higher magnification than previously used for the histological grading. These two animals were not included in the clot group for the correlation analysis because they did not present with pathological or histological findings consistent with thrombi during the initial review, but we note them to emphasize that the definition of thrombi used in the correlation analyses and subsequent analyses, while based on standard histopathological analyses, may not include every microthrombus present in the tissue.
Similar thrombi were seen in the naïve arm of the study in both the control and hemostatic nanoparticle groups suggesting that the trauma is not essential to trigger the formation of these hard nodule clots (Supplementary Table 4.) Understanding what correlates with these thrombi is important to understanding how the nanoparticles impact outcomes.
One of the questions one might ask is whether the variation in size of the nanoparticles as measured by DLS (Supplementary Table 1) contributed to these outcomes. Supplementary table 5 shows the average and standard deviation for all the nanoparticles as well as for those associated with clots and those associated with death. An ANOVA for both the cNPs and hNPs suggests there are no differences in size of nanoparticles involved in clots or survival.
Biodistribution across both injured and naïve animals showed similar patterns with no significant differences between animals with clots and animals without clots (Supplementary Figure 12.)
Data Science to Determine Parameters that Correlate with Lethality and Clot Formation
The critical question that arises from this, then, is what correlates with the clots? To investigate this, we performed a series of analyses using data science methods. Specifically, we used feature selection and random forest algorithms to identify features that are most predictive about the clots and survivability. While feature selection and random forests help us show the relationship to survivability and clots, the canonical correlation depicts the cluster if features which are correlated and the variables that are unique from the overlapping features.
Multiple forms of correlation analysis were used with the Luminex data, cell counts, clotting results, blood loss, and survival to determine what features correlated most often with the formation of clots and with survival. There was strong overlap of the factors influencing clots and survival even though there is partial overlap with animals. TNF-alpha, INF-alpha, IL-8, neutrophils, and lymphocyte changes correlated with survival and IL-6 correlated with clotting using a canonical correlation (Supplementary Figure 13C and D). We also performed feature selection using the machine learning algorithm, Random Forest. This is an ensemble algorithm which is comprised of multiple decision trees. Decision trees create splits which maximize the reduction in impurity of a node for classification of an outcome feature (clot or survival). By calculating the mean decrease in impurity for each feature across all trees we can know that feature’s importance from our given dataset. Feature importance techniques assign a score to independent features according to the role each feature plays in predicting the dependent feature/ target feature. Using this method, we found that Average_IL6 is listed as the top feature when we consider ‘clot’ as the target feature whereas for the experiment where ‘survival’ is the target feature we noticed that Average_Neut (neutrophils) is listed as the top feature (figure 4A and B). Thus, across both canonical correlation and random forests we saw high correlation for IL-6 with clotting (figure 4B and Supplementary Figure 13D) and similar feature correlation for survivability (Figure 4C and Supplementary Figure 13C). Importantly, we saw similar trends in the naïve groups for important features (Supplementary Figure 13B) suggesting that these features are important for thrombi even in the absence of trauma which further suggests that the nanoparticles are the critical factor. It should be noted that since these are large animal studies, the animal numbers are small for both the trauma and naïve arms of the study. Nonetheless, there is substantial heterogeneity in the data. This, coupled with multiple correlation analyses lends credence to the importance of the features that repeatedly showed high correlation with the clot and survival outcomes.
Figure 4:

Multiple data science methods were used to look for correlations between survival and parameters measured in the study (cytokines, cells, and blood loss) and clot formation. A set of features rose to the top for all the feature selection methods that correlated with both survival and clot formation. These included IL-6, TNF-alpha, neutrophils, lymphocytes, IL-8, and INF-alpha. (A) Features of importance for survival. (B) Features of importance for clot formation.
In all correlation analyses, IL-6 was one of the most common features with a strong robustness regarding correlation. IL-6 increased in all groups over time with the fold change in IL-6 increasing the most for the hemostatic nanoparticle group (Supplementary Figure 14). A similar increase was seen in the naïve arm of the experiments primarily driven by one animal that showed a substantial spike towards T=120 minutes (Supplementary Figure 15.)
Likewise, animals exhibited a significant increase in IL-6 values towards the end of the experiment in the injured arm whether they had clots or not with animals who did not show clots showing, on average, higher values (Figure 5A). At the earliest timepoints, animals with clots did show an increase at T=15 minutes (inset to Figure 5A).
Figure 5:
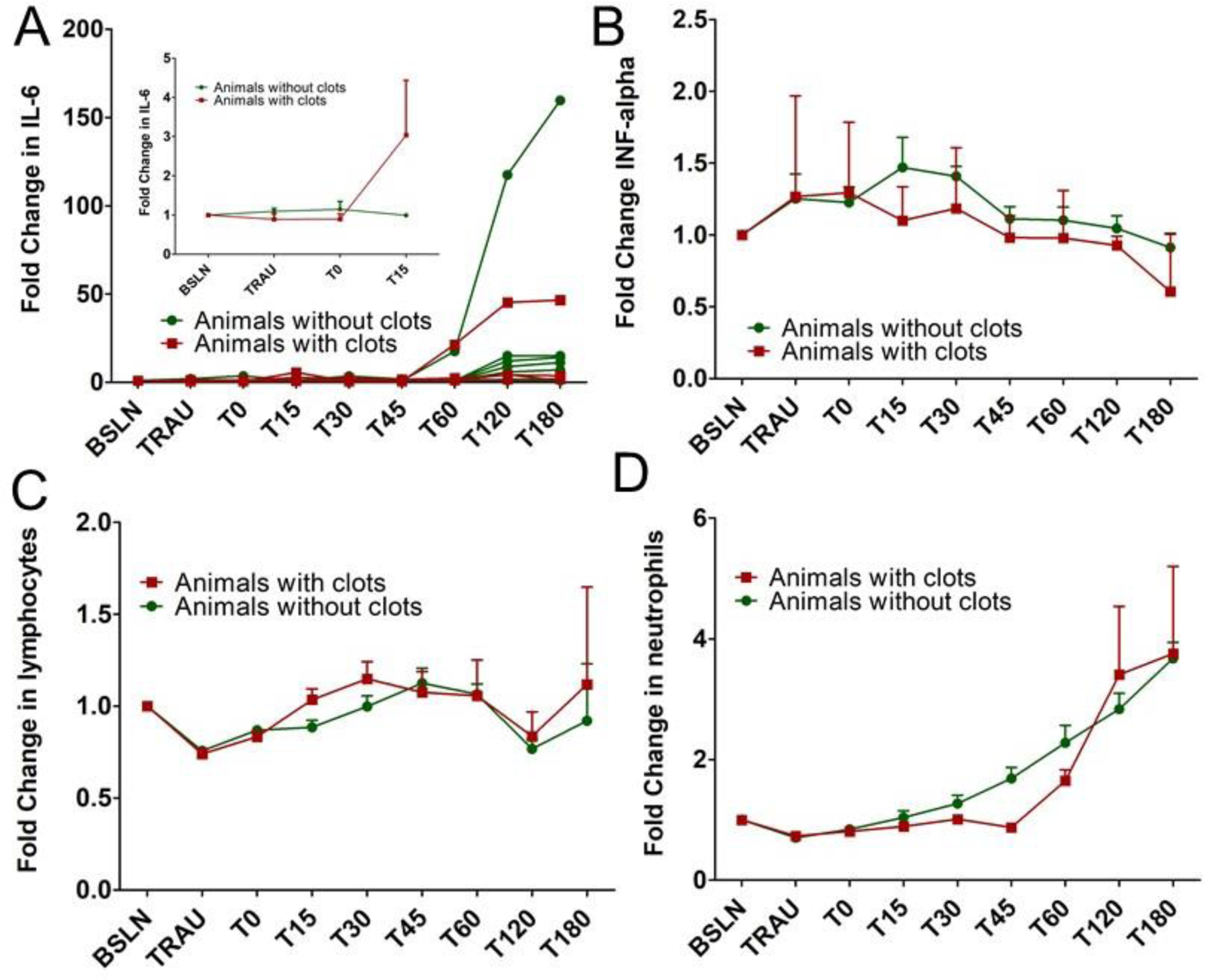
(A) Fold change in IL-6 over time as a function of whether clots were found postmortem. Curves for individual animals are plotted to show the variation in responses along with the trends. 15 minutes post treatment, animals who exhibited clots also showed an elevation in their IL-6 levels. (B) Fold change in the inflammatory cytokine, INF-alpha. 15 minutes post treatment, animals that exhibited clots showed a small decrease in INF-alpha whereas animals without clots showed an elevation in the INF-alpha level. (C) Lymphocytes increased more 15 minutes post treatment for animals with clots than for those without. (D) Neutrophiles increased more for animals without clots than for those with clots. It should be noted that no blood draws were made postmortem. (n=6 and error bars=SD for A-D)
Both TNF-alpha and IL-8 showed up in many of the correlations for clot formation, but their values showed no clear trends with regards to the fold change in values. The data sets used are small and can be driven by outlier values which seems to be the case with IL-8 that exhibited a few very high values (Supplementary Figure 16). TNF-alpha moved around a great deal making patterns and trends unclear (Supplementary Figure 17).
In looking through the top correlates with clot formation or survival, the four parameters that stood out were IL-6, INF-alpha, lymphocytes, and neutrophils. The early trends in IL-6 suggest that an increase correlates with clot formation (Figure 5A). That is not surprising since an increase in IL-6 has been correlated with clot formation in deep vein thrombosis 33–35 as well as COVID-19 36. One of the questions that is raised is whether there are early changes in IL-6 that may correlate with an infusion response. IL-6 does increase in this study for animals that exhibited clots over the first 15 minutes, but this observation is based on a very small data set. The data sets are extremely small since the clots are only seen in a subset of the animals that received nanoparticles but in both the control and hemostatic groups in similar numbers (n=2 for the control group and n=3 for the hemostatic group). However, it would be wise to look at early IL-6 changes in future studies since the increase may be important for understanding potentially adverse outcomes that may be related to nanoparticle infusions.
Focusing on the first 30 minutes post particle administration when infusion responses are often seen, we observe that INF-alpha increases following treatment in animals without clots but stays constant or decreases slightly for animals with clots. Increases in INF-alpha has been correlated with thrombi in several models of disease 37–39 which makes the fact that the increase correlates with animals that did not exhibit clots surprising although at the longer timepoints INF-alpha decreased in all the animals studied (Figure 5B). Lymphocytes increased more 15 minutes post treatment for animals with clots than for those without (Figure 5C). In contrast, over early timepoints, the number of neutrophils were lower for animals with clots than those without (Figure 5D). The changes in these values were not seen from the naïve arm of the study, but the naïve groups were extremely small (n=3 per group for n=9 total with two animals exhibiting clots, Supplementary Figure 15). This follows the trend that there were no changes seen in naïve animals, with respect to IL-6 in first 15 min (Supplementary Figure 15.) Of course, the caveat with all of this is that the animal numbers are small. While the heterogeneity, multiple methods, and repeated correlations suggest that these factors have high correlation with the clot and survival findings, it is important to be cautious about drawing conclusions from the specific changes in this small data set. Instead, we present this data not to over interpret the findings but to provide a basis for understanding the underlying data that correlates with the outcomes regarding clots and survival following nanoparticle infusions in this work.
As noted above, the same factors that correlate with survival correlate with clot formation even though there is only partial overlap between the animals that die and those with clots. Said another way, the group that died included both animals with clots and animals without clots. Nonetheless, looking at survival/death, IL-6 decreased slightly for animals that died, but the blood draw was immediately before death and other processes may confound the results. Neutrophils were also lower for animals that died immediately before death (Supplementary Figure 19) thus following the same trend seen with clot formation.
Parameters that Correlate and In Vitro Screening
The PEGylated polyester nanoparticles used in this study correlated with negative outcomes independent of whether they had the GRGDS peptide or not (both the hemostatic nanoparticles and control nanoparticles). Not all animals that received polyester nanoparticles had negative outcomes (death and/or clots) but a subset did. In contrast, the vehicle control group did not exhibit similar negative outcomes. While we did not see complement activation in vivo, there were signs of inflammatory processes that correlated with the firm clots and negative outcomes.
By looking at the data via correlation analysis and coupling that with an analysis of the findings, it appears that changes in IL-6, INF-alpha, lymphocytes, and neutrophils correlate with negative outcomes in vivo with nanoparticles. To gain more insight into the role polyester nanoparticles may be playing in vivo, nanoparticles or controls were incubated with heparinized whole blood and a cytokine array assay was performed.
The cytokines represented above are those that showed positive spots in the array (Figure 6). This method is pseudo-quantitative and leads to intensities rather than absolute values of cytokines. No spots were seen for IL-6 or for TNF-alpha, and the kit does not include INF-alpha. Nonetheless, there are points of connection to the in vivo findings that are important to explore. C5/C5a is positive for zymosan which is expected because zymosan is a positive control for complement. The levels for the control and hemostatic nanocapsules are the same as the negative control, MEM. The hemostatic nanoparticles but not the control nanoparticles, MEM, or zymosan are positive for CXCL12/SDF-1. SDF-1 is known to upregulate TNF-alpha 40. Likewise, the hemostatic nanoparticles but not the control nanoparticles, MEM or zymosan are positive for IL-1ra/IL-1F3. The Interleukin-1 receptor antagonist (IL-1ra) can reduce acute inflammation following trauma 41 and dampen inflammation in diseases such as rheumatoid arthritis 42. Neutrophils are known to release IL-1ra, IL-16, and MIF when undergoing cell death 43. IL-16 is lower for zymosan, and both the hemostatic and control nanoparticles compared to MEM. Interleukin-16 (IL-16) is a chemoattractant factor 44 released in response to mitogens, antigens, or vasoactive amines such as histamine or serotonin 44. IL-16 induces upregulation of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-alpha) 45. Downregulation of IL-16, as is seen here, is associated with a reduction in lymphocyte migration 46. Macrophage migration inhibitory factor (MIF) activates neutrophils 47. The downregulation of MIF in the nanoparticle groups is significant and may lead to modulation of the immune response that, especially in the presence of trauma, leads to a reduction in neutrophils, which we saw correlated with worse outcomes following particle administration (Figure 5D).
Figure 6:

Cytokine profiler array. Change in cytokines for heparinized human whole blood incubated with control and hemostatic nanoparticles. Change in complement protein C5/C5a was most significant for zymosan based on Chi-square analysis. (error bars=SD)
We designed the study to focus on blood loss with complete survival of the saline group. As blood loss becomes more severe and patients progress towards death, there are several factors that occur including trauma induced coagulopathy and the lethal triad 48–50. While these are extremely important to model and understand, they significantly increase the complexity of the model and assessments. Therefore, we chose a more conservative bleeding model as a first step in assessing the safety and efficacy of these nanocapsules.
We tested the hemostatic nanoparticles, control nanoparticles, or saline in a porcine liver injury model, and examined the impact of these nanoparticles on blood loss, physiological outcomes, coagulation parameters, immune cell responses, and cytokine arrays. We also included a naïve arm of the study in which we administered our hemostatic nanoparticles, control nanoparticles, or saline in the absence of a trauma to determine whether off-target effects were the result of a combination of the particles and trauma or a result of the particles alone. While we found that the hemostatic nanoparticles did positively impact coagulation parameters, there were off-target effects leading to non-specific clotting. We applied a series of approaches from data science to understand the correlates with these clotting events as well as survival to determine the off-target mechanism triggered by these polyester nanoparticles.
The ultimate goal of our work is to lay the groundwork for treatments for severe bleeding in the context of trauma. Stopping bleeding in rodents with nanotechnologies has been achieved with several different approaches 7, 21, 31, 51–54 even in the context of thrombocytopenic conditions 54. But, stopping bleeding in clinically translatable large animal models in a safe manner has proven very challenging. We have known for some time that complement activation was a hurdle 20, 26, 55, 56. In this work, we looked at nanoparticles that do not activate complement 28. While these particles did not activate complement in vitro or in vivo, their administration unmasked other challenges that must be addressed.
The animal numbers in this study are small which can lead to challenges in having the data to power results, but it is clear that early changes in IL-6, INF-alpha, lymphocytes, and neutrophils are potentially significant and should be considered carefully in designing and testing nanomaterials to treat trauma and nanomaterials for intravenous administration more broadly. We know that IL-6 correlates with worse outcomes in trauma 57, but what we are seeing with regards to thrombi is not limited to trauma. The hard nodule clots were seen in the naïve groups that received particles as well, and data science to look for correlations found similar patterns with the naïve arm of the study as were seen in the injured arm of the study. Thus, the findings that polyester-based, PEGylated nanoparticles can cause serious, adverse events goes beyond the complex milieux of trauma.
How can we begin to develop screening systems to look at the safety of nanoparticles for translation based on these findings? We have previously seen that incubation of polyester nanoparticles with endothelial cells leads to a decrease in IL-6 28 similar to what is seen in the naïve arm of the study. In vitro screens like this play an important role in understanding how nanoparticles impact outcomes. There are already elegant assays to look at the interactions between neutrophils and microparticles 58–61 as well as lymphocytes and micro/nanoparticles 62–64 that can be leveraged to understand the mechanisms involved in these interactions and screen for safety for nanotechnologies.
One of the surprising findings in the work was that we saw a reduction in neutrophils in animals that exhibited thrombi. Neutrophils are associated with increases in coagulation factors including PTT and can promote clotting 65. However, we saw no changes in PTT and, in fact, fewer neutrophils in early times post particle administration correlated with thrombi in this model. In contrast, we saw an increase in lymphocytes. Thus, the neutrophil to lymphocyte ratio (NLR) was decreasing at early timepoints following administration of particles. Again, this contrasts with the elevated NLR that has been seen associated with clot formation both in COVID-19 and in patients with admission to the ER with cardiopulmonary distress 66–68. Based on the wealth of literature and small changes in the findings, while neutrophils and lymphocytes are clearly important in the thrombi, the changes in these cells following nanoparticle administration are small, and confidence in suggesting how they change and how that impacts outcomes is small. Follow on studies looking at neutrophil and lymphocyte interactions with nanoparticles will be an important part of understanding these interactions.
This is a pilot study, and it is possible that the limited number of animals could lead to results that are not fully replicated at scale, but the key point is that the changes in cytokines, lymphocytes, and neutrophils are important to screen for in vitro moving forward with nanomaterials to ensure safety.
One of the caveats that must always be considered when dealing with porcine models is that their innate immune and thrombotic responses are often more intense than humans’ responses. Infusion reactions tend to be more intense in porcine models than those seen in the clinic 69, 70. Such responses are often magnified by the speed of the infusion which in this study was a bolus. Bolus infusions are preferred when managing trauma, but they may magnify the impact of nanomaterials given systemically. Furthermore, swine appear to have earlier and more intense activation of pro-thrombotic mechanisms compared to humans and have limited thrombolysis capacity 71, 72. Nonetheless, the findings here point to potential adverse events that must be studied and considered when developing intravenously infusible nanomedicines.
Ultimately, if the goal is to control bleeding in models of trauma, the challenge goes beyond stopping bleeding. Acutely bleeding trauma patients are well-known to also be hyper-coagulable from systemic activation of pro-coagulant mechanisms. Furthermore, trauma patients can rapidly switch between hypocoagulable and hypercoagulable depending on intrinsic physiologic factors, external factors such as the rate and extent of blood loss, and environmental factors such hypothermia 5, 50. Thus, pharmacologic or technology solutions that only stop bleeding could increase complications or even worsen outcomes. These conditions include disseminated intravascular coagulation (DIC) 73, trauma-induced coagulopathy (TIC) 74, 75 and traumatic brain injury (TBI) 76–78.
Triggered by trauma as well as sepsis, DIC leads to inflammatory reactions, endothelial dysfunction, and generalized thrombosis causing organ damage and failure and poor patient outcomes 73. With DIC, a patient can transition rapidly from a hypocoagulable to hypercoagulable state. In trauma patients, the complex interplay of tissue injury and hemorrhagic shock combine to create TIC 79, also known as acute traumatic coagulopathy. TIC occurs rapidly in 20–35% of severely injured trauma patients 80 when protein C is activated and inhibits the clotting cascade through cleaving factors Va and VIIIa. Factor Va is part of the prothrombinase complex that cleaves prothrombin into thrombin, a critical component for hemostasis. Activated protein C also accelerates fibrinolysis and leads to platelet dysfunction 81, 82. Rapid surgical intervention is essential to stop the contribution of ongoing blood loss to the cycle of TIC 83, 84.
The complexity of these challenges is further exacerbated by TIC-associated hypercoagulability, potentially a result of endothelial damage leading to a reduction in tissue-type plasminogen activator (tPA) 85–87. Finally, the extent of hemorrhaging in TBI has been correlated with the degree of functional deficits following central nervous system trauma in humans88, 89 and with the extent of injury in rodent models 88, 90. Again, the challenge with halting bleeding is that TBI patients can rapidly switch back and forth between hypocoagulable and hypercoagulable states 76–78, 91, and while research is ongoing, predicting the transitions is extremely difficult 92, 93. In addition, TBI brain microparticles can induce systemic coagulation including pulmonary emboli 94.
Conclusions
Thus, as we look to develop therapies to manage bleeding, we have a high bar to meet. The therapies must be safe and avoid adverse events in an extremely complex environment. The need is there to tackle this challenge, though, with trauma and uncontrolled bleeding being the leading cause of death of young people. This work elucidates some of the cellular and molecular responses that should be considered in developing technologies to control bleeding.
The complexity of administering nanomedicines intravenously may be changing as well. The potential impact of COVID-19 on endothelial dysfunction, clotting disorders, and thrombi 95–97. As we translate infusible nanotechnologies in patients who have had COVID-19, we may find that the safety profile for these patients has changed. Being able to screen nanotechnologies at early stages for cytokine and immune cell responses has the potential to lay a framework for safer nanomedicines more broadly, beyond the complex milieux of trauma.
Methods/Experimental
Materials
Poly(lactic acid)-b-poly(ethylene glycol) block copolymer was synthesized using L-lactide from Polysciences Inc. (Warrington, PA), heterobifunctional poly(ethylene glycol) with 5000 Da and 3400Da molecular weight from Laysan Biosciences (Arab, AL), and D-lactide from Purac Biomaterials (Corbion, Amsterdam, Netherlands). All solvents used were of ACS grade and obtained from Fisher Scientific.
We monitored Complement protein C5a using a C5a human ELISA duo kit (DY2037) obtained from R&D Systems (Minneapolis, MN). Zymosan was obtained from Sigma Aldrich. Dulbecco’s phosphate-buffered saline (without phosphate and magnesium) was obtained from Fisher Scientific. We measured cytokine levels in vitro using Proteome Profiler Human Cytokine Array Kit (R and D Systems, catalog number ARY005B). For in vitro assays, as blood matrix, we used heparinized human blood and complement protected human serum from Innovative Research Inc. (Novi, MI).
Synthesis and characterization of nanoparticles
Block copolymers of Poly(lactic acid)-b-poly(ethylene glycol) with either 3400 or 5000Da PEG macroinitiators was synthesized through the ring-opening polymerization reaction.21, 98 The nanoparticles were prepared using poly(L-lactic acid)-b-poly(ethylene glycol) (PLLA-b-PEG5000) and Poly(D-lactic acid)-b-poly(ethylene glycol) (PDLA-b-PEG3400) through nanoprecipitation. The numbers denote the molecular weight of the PEG macroinitiator, i.e., 3400Da and 5000Da. The peptide GRGDS was conjugated through NHS/EDC conjugation to PLLA-b-PEG5000.21
The ratio for the polymers is 3:1 by weight. 90 mg of PLLA-b-PEG5000 and 30mg of PDLA-b-PEG3400 was used for making the control and hemostatic nanoparticles. The polymer was dissolved in the organic phase, i.e., Tetrahydrofuran (THF), at a concentration of 20mg/ml was added dropwise to double the phosphate-buffered saline’s volumetric amount and stir hardened to form the nanoparticles. After stir-hardening for 3 hours, excess THF was removed by exposing to air, and poloxamer was added as the stabilizer. The nanoparticles were then collected through centrifugation at 4000XG for 10 minutes at 4°C and resuspended in phosphate-buffered saline. The particles were characterized through dynamic light scattering for determining the hydrodynamic diameter and the zeta-potential for nanoparticles in dilute potassium chloride solution (10mM). The extent of PEGylation was determined through 1H-NMR as well. The control nanoparticles were prepared in similar manner, but instead of peptide conjugated PLLA-b-PEG5000-GRGDS, the block copolymer PLLA-b-PEG5000 was used.
Conjugation of GRGDS to PLLA-b-PEG through NHS/EDC Coupling
The peptide of interest, GRGDS, was conjugated to the block copolymer PLLA-b-PEG5000 through NHS/EDC conjugation.1 Utilizing the Carboxyl end of the bifunctional PEG, the NHS/EDC based conjugation leads to coupling with the amine end of the peptide GRGDS. First, 300g of the block copolymer PLA-b-PEG is dissolved in 4mL DCM. To that, 12 mg NHS and 10 mg EDC dissolved in DMSO, is added to form an amine-reactive NHS-ester.2 The reaction is allowed to proceed for 60 minutes, and then excess DCM is removed by exposure to air. The polymer is precipitated in methanol and collected by centrifugation at 4000rpm for 5 minutes and lyophilized overnight. The lyophilized intermediate product is dissolved in 3mL of DCM and to that, 8mg of the peptide dissolved in 1.5mL DMSO is added. The reaction is allowed to proceed for 24 hours, and then the excess DCM is removed by exposure to air, and the polymer is precipitated in methanol. The precipitated PLLA-b-PEG-GRGS is the collected through centrifugation at 4000rpm for 5 minutes and lyophilized.
Measuring peptide density using OPA assay
The presence of peptide in the nanoparticles was confirmed through an orthopthaladehyde (OPA) assay, which leads to fluorescence in the presence of free amines. 200uL of OPA reagent is added to 20uL of nanoparticles dissolved in DMSO and mixed well. The fluorescence is recorded immediately after 15 minutes of incubation in the dark using an excitation wavelength of 340 nm and emission wavelength of 455 nm.
Handling of blood products
For generating the responses in vitro, the heparinized human whole blood and complement protected human serum used was obtained from Innovative Research Inc. (Novi, MI). In case of whole blood, blood was collected in heparinized collection tubes from single donor and shipped using the same collection vial to avoid exposure to multiple surface and lead to unwanted complement activation. Blood was handled with care, stored, and shipped at 4°C, and only mixed gently, and stored on ice while running the assay.
Evaluation of changes in complement protein in vitro
The change in complement protein C5a was quantified following previously established protocol. 27 The blood matrix was incubated with nanoparticles suspended in Dulbecco’s PBS (without calcium and magnesium). As a positive control, zymosan was used. The dosage used for the nanoparticles and zymosan was 0.25mg/ml in serum. The samples were incubated at 37 for 45 minutes and then centrifuged at 4000g for 5 minutes to separate the nanoparticles. The plasma was aliquoted in clean tubes and stored on ice until assay was carried out.
Ethical Approval and Accreditation
The study protocol was reviewed and approved by the 711th HPW/RHD JBSA-Fort Sam Houston Institutional Animal Care and Use Committee (IACUC) in compliance with all applicable Federal regulations governing the protection of animals in research. All procedures were performed in facilities accredited by the AAALAC international.
Power Analysis
We designed the experiment to looks for differences in hemorrhage rather than survival. Because this was a large animal porcine study, we used a resource equation method to power the study. This approach is considered appropriate when designing resource-intensive large animal studies and/or those involve potential ethical concerns. In this approach, Error = Total number of animals − Total number of groups with the ideal target for Error (E) being between 10 and 20. In our experimental design, E=18–3=15 with n=6 per group. Conversely, using a conventional power analysis, assuming that reducing blood loss by 0.3 liters in pigs would be significant based on previous work where the standard deviation in blood loss was 0.2 liters, we would find that we need 7 pigs per group. Thus, the calculations are close, but considering the negative outcomes, the complexity of trauma models in large animals, and the resources, we focused on the resource equation method for calculating sample size and ended the study with 6 animals per group.
Animal Subjects and Pre-operative Preparation
Thirty male swine (Sus Scrofa domesticus) weighing 35–60 kilograms were randomized and blinded into two injury arms each containing three treatment groups: 1) Injured Arm 1.a) vehicle control (normal saline, 0.9% NaCl) (n=6); 1.b) 2 mg/kg control nanoparticles (n=6); or 1.c) 2 mg/kg hemostatic nanoparticles (n=7) or 2) Uninjured Arm 2.a) vehicle control (normal saline, 0.9% NaCl) (n=3); 2.b) 2 mg/kg control nanoparticles (n=3); or 2.c) 2 mg/kg hemostatic nanoparticles (n=3).
Animals were sedated with Telazol (6.0 mg/kg; Fort Dodge Animal Health, Overland Park, KS, USA), pre-medicated with an analgesic (Buprenex 0.01 mg/kg; Reckitt & Colman Pharmaceuticals Inc., Richmond, VA, USA), and intubated with anesthesia maintained on 1–3% isoflurane. Core body temperature was monitored via a rectal temperature probe and maintained between 36.0–38.0°C.
Catheter Placement, Laparotomy and Splenectomy
Four catheters were placed percutaneously under ultrasound guidance. Briefly, two 8 Fr catheter (Arrow, Morrisville, NC, USA) was placed in the jugular veins for fluid infusion and treatment administration and central venous pressure monitoring (CVP). Femoral arteries were cannulated with either a 5 Fr catheter (Cook Medical, Bloomington, IN, USA) for blood pressure monitoring (MAP), or an 8 Fr catheter (Arrow, Morrisville, NC, USA) for blood collection and sampling.
A midline laparotomy was performed to expose the spleen and liver. The left upper quadrant of the spleen was identified and mobilized to the midline. The splenic vein and artery were clamped off. All surgical procedures were performed under aseptic techniques.
Whole Blood Collection
Before injury, 30% total blood was collected from a controlled femoral bleed in a blood donor bag containing anticoagulant citrate phosphate dextrose adenine solution (CPDA) at a 1:10 ratio for subsequent use in resuscitation.
Injury and Hemorrhage
For the Injured Arm, a standardized grade III liver injury was created as previously described by Gurney J., et al. 29, 99 Briefly, the liver was isolated and a ring clamp placed over the lower left lobe, ~50% in width and ~2.0 inches from the apex, adjusting for relative size of the liver and weight of the pig. The clamp was closed, and an 11 blade was used to lacerate the lobe from the top of the clamp through the remaining width, marking the beginning of hemorrhage. After 1 minute the clamp was removed and the rest of the tissue excised, resulting in ~25% lobectomy. Excised tissue was measured and weighted. The liver was allowed to spontaneous bleed for 5 minutes, with blood being removed via intraperitoneal suction and weight/volume quantified at 1-minute intervals through hemorrhage and treatment phases.
Treatment, Resuscitation, and Euthanasia
Treatment was initiated at T=0 min via a 30 mL bolus of either 1) 0.9% NaCl, 2) 2 mg/kg control nanoparticles, or 3) 2 mg/kg hemostatic nanoparticles. Additional fluids were postponed for 30 minutes at which point whole blood was given as needed to maintain a systolic blood pressure (SBP) of 90±5 mmHg. Initial whole blood transfusion was preceded with 23% calcium gluconate solution (Vedco, Saint Joseph, MO, USA) at 1 mL/200mL based off the 30% whole blood bleed.
At the end of treatment (T=60 min) liver injuries were packed, closed, and animals received maintenance fluids in the form of lactated Ringer’s and were monitored under anesthesia until T=180 min. Swine were humanely euthanized at T=180 min with pentobarbital sodium and phenytoin sodium (Euthasol®, 390 mg/mL, Virbac Corporation, Fort Worth, TX, USA)
Blood Draws and Laboratory Analysis
Whole blood was collected as BSLN, TRAU, T=0, 15, 30, 45, 60, 120, and 180 min. No blood draws were made postmortem. Arterial blood gas parameters were assessed utilizing a GEM® Premier 4000 (Instrumentation Laboratory, Bedford, MA, USA). Complete blood counts, basic metabolic panels, and liver associated enzymes were evaluated on ProCyte Dx Hematology and Catalyst One Chemistry Analyzers (IDEXX Laboratories, Inc., Westbrook, ME, USA).
Whole blood viscoelastic clotting properties were evaluated by rotational thromboelastometry (ROTEM) (ROTEM® Delta system, TEM Systems Inc., Durham, NC, USA). ROTEM analyses included evaluation of extrinsic coagulation pathway function in the absence (ExTEM), presence of the platelet inhibitor, cytochalasin D (FibTEM), and presence of fibrinolysis inhibitor, aprotinin (ApTEM) including: clotting time (CT), amplitude 10 minutes after CT (A10), amplitude 20 minutes after CT (A20), clot formation time (CFT), maximum clot firmness (MCF), alpha-angle (α), lysis index 30 minutes after CT (LI30), lysis index 60 minutes after CT (LI60) and maximum lysis (ML).
Concentrations of coagulation factors were evaluated using STAGO® STA Compact® (Diagnistica Stago Inc., Parsippany, NJ, USA). STAGO® analysis included prothrombin (PT) and partial thromboplastin (PTT) times, and concentration levels of d-dimer, fibrinogen, von Willabrand Factor (vWF), and antithrombin (ATIII).
EDTA plasma was separated from remaining whole blood samples and divided into aliquots for subsequent Luminex analysis which included: interferon (INF) INFγ, INFα, interleukin (IL) IL-1β, IL-10, IL-12p40, IL-4, IL-6, IL-8, and tumor necrosis factor alpha (TNFα) (Invitrogen Cytokine and Chemokine 9-Plex Porcine ProcartaPlex Panel 1, ThermoFisher Scientific, Waltham, MA, USA). Cytokine and chemokine concentrations were quantified using the Bio-Plex 200 Luminex system (Biorad, Hercules, CA, USA).
Nanoparticle Bioaccumulation and Biodistribution
During necropsy 1cm cubes of tissues were collected and flash frozen for subsequent nanoparticle biodistribution analysis which included: liver, kidney, cranial lung (left and right), caudal lung (left and right), frontal cortex, occipital cortex, cerebellum, hippocampus, mesenteric lymph nodes, and gastrohepatic lymph nodes. The 1 cm cubes of tissue were kept at −80 C until biodistribution was performed.
Preparing Tissue Samples
Each 1 cm cube (one per tissue from the center of the tissue) was rinsed with DI water 3 times. Samples were placed into pre-weighed 50mL tubes and placed at −80C for 2 hours to freeze. Samples were then lyophilized. Following lyophilization, each sample was weighed to determine dry weight. The samples were transferred into homogenizing tubes and homogenized (Precellys 24; 6500 rpm, 2 cycles, 20 seconds per cycle, 5 second wait time between cycles). Samples were resuspended in 1 mL acetonitrile and homogenization was repeated. Samples were then kept at 37 C for 48 hours. After incubation, the samples were centrifuged at 13.3 g for 10 minutes. Supernatant was transferred to eppendorf tubes. The samples were diluted 1:10 with ACN and read using the plate reader at an excitation of 387 nm and emission of 470 nm (Molecular Devices, Spectramax M2). A standard curve of C6 in ACN was used to determine the concentration of C6 and the amount per mg of tissue. Knowing the loading of C6 in the nanoparticles, the concentration of nanoparticles per mg of tissue was determined.
Tissue Preparation and Pathology
Immediately following euthanasia, full necropsies were performed by veterinarian pathologists for gross and microscopic anatomic evaluation on each animal. Lung tissues were collected for histopathologic evaluation. These tissues were fixed in 10% neutral buffered formalin; paraffin embedded, cross-sections were cut at 5µm, and stained with hematoxylin and eosin (H&E). A pathologist blinded to the treatments reviewed and scored the pathology and histological sections.
Complement Assays and PAF Assay on Serum Samples from In vivo study
Serum samples were stored until use. Serum samples were aliquoted and only used once to avoid freeze-thaw cycles. Samples were taken from the storage condition of −80 immediately before the ELISA assay was carried out. They were placed in 37 C for few seconds to thaw immediately. Four different dilutions were used. Undiluted, 1:1, 1:50, 1:1000. The standards were also prepared following the kit instructions. PAF was quantified using the pig specific kit from LifeSpan BioSciences, Inc. (Seattle, WA, Catalog no. LS-F37444). C3 and C3a were Quantified using Abcam kits we had used previously with pigs (Abcam ab157705 and ab133037, respectively) 26.
Generating immune response in vitro and quantification through cytokine array
Nanoparticles resuspended in Minimum essential medium (MEM) were added to human heparinized whole blood to reach a final concentration of 0.25mg/ml. The ratio of blood to the nanoparticle in MEM was 5:1. The blood was gently pipetted with the nanoparticle resuspension and incubated for 45 minutes at 37 °C in a rotating shaker. Immediately after incubation, samples were placed on ice and then centrifuged at 4000G for 5 minutes. The supernatant plasma was collected in clean microcentrifuge tubes. For the cytokine array panel, 200µl of plasma was used to prepare samples following the protocol, and subsequent steps given in the protocol were followed for the human cytokine array panel (R&D Systems). The final image was taken using a Bio-Rad imager. For further analysis, ImageJ was used. Integrated density gives the sum of total pixels within a region. Following this method, each dot blot within the membrane was quantified for each sample The levels of the detected cytokines were expressed as a percentage of the pixel density determined for the reference spot.100
Data and Statistical Analysis
Statistical analyses were performed using Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as mean±SD. Data with baseline discrepancies were normalized to baseline before statistical analysis. Single time point analyses were performed by one-way ANOVA using post-hoc Tukey and multiple timepoint analyses were analyzed by mixed-effects analysis using post-hoc Tukey for intra-analysis and Dunnet for inter-analysis.
Data science
We carried out canonical correlation analysis experiments both in R Studio and Python. Packages exist in R Studio and Python which create a visualization showing highly correlated variables. Independent attributes that are highly correlated to the target attribute are present near the target attribute.
Multi-view learning:
Multi-view learning is a domain where we identify relations between related datasets. This technique aids in identifying important attributes towards the target attributes. Multi-view learning includes various types of techniques. Few among these popular techniques are canonical correlation analysis and matrix factorization.
Canonical Correlation Analysis (CCA):
We carried out canonical correlation analysis experiment on the porcine data. Canonical correlation analysis gives us a better idea of correlations that exist between two datasets. Canonical correlation analysis consists of two core parts, namely identifying canonical variables and canonical correlation among variables.
Supplementary Material
Supplementary information including figures, tables and data regarding the study are available online at ACS. This supplementary information includes details regarding every batch of nanoparticles synthesized and used in this work, blood loss data, physiological data, coagulation results, cell counts and cytokines for both injured and naïve pigs, and histological findings. Figures regarding correlation analysis findings are also included.
Acknowledgements
We would like to thank Nidhi Naik and Tobias Coombs for their help synthesizing and characterizing the particles for the study. This work was supported by the AIMM Research award (DOD) (Award Number# W81XWH1820061) and by NIH R56 Grant (Project# 1R56NS100732–01)
References
- 1.Krug EG; Sharma GK; Lozano R, The global burden of injuries. Am J Public Health 2000, 90 (4), 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauaia A; Moore FA; Moore EE; Moser KS; Brennan R; Read RA; Pons PT, Epidemiology of trauma deaths: a reassessment. Journal Of Trauma-injury Infection And Critical Care 1995, 38, 185–193. [DOI] [PubMed] [Google Scholar]
- 3.Champion HR; Bellamy RF; Roberts CP; Leppaniemi A, A profile of combat injury. J Trauma 2003, 54 (5 Suppl), S13–9. [DOI] [PubMed] [Google Scholar]
- 4.Regel G; Stalp M; Lehmann U; Seekamp A, Prehospital care, importance of early intervention on outcome. Acta anaesthesiologica Scandinavica. Supplementum 1997, 110, 71–76. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JW, Hemorrhagic Shock. N Engl J Med 2018, 378 (4), 370–379. [DOI] [PubMed] [Google Scholar]
- 6.Cap AP; Cannon JW; Reade MC, Synthetic blood and blood products for combat casualty care and beyond. J Trauma Acute Care Surg 2021, 91 (2S Suppl 2), S26–s32. [DOI] [PubMed] [Google Scholar]
- 7.Anselmo AC; Modery-Pawlowski CL; Menegatti S; Kumar S; Vogus DR; Tian LL; Chen M; Squires TM; Sen Gupta A; Mitragotri S, Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014, 8 (11), 11243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylis JR; Yeon JH; Thomson MH; Kazerooni A; Wang X; John AE St; Lim EB; Chien D; Lee A; Zhang JQ; Piret JM; Machan LS; Burke TF; White NJ; Kastrup CJ, Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Science advances 2015, 1 (9), e1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gkikas M; Peponis T; Mesar T; Hong C; Avery RK; Roussakis E; Yoo HJ; Parakh A; Patino M; Sahani DV; Watkins MT; Oklu R; Evans CL; Albadawi H; Velmahos G; Olsen BD, Systemically Administered Hemostatic Nanoparticles for Identification and Treatment of Internal Bleeding. ACS Biomater Sci Eng 2019, 5 (5), 2563–2576. [DOI] [PubMed] [Google Scholar]
- 10.Brown AC; Stabenfeldt SE; Ahn B; Hannan RT; Dhada KS; Herman ES; Stefanelli V; Guzzetta N; Alexeev A; Lam WA; Lyon LA; Barker TH, Ultrasoft microgels displaying emergent platelet-like behaviours. Nature materials 2014, 13 (12), 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Moure J; Maisha N; Lavik EB; Cannon J, The chemistry of lyophilized blood products. Bioconjug Chem 2018, 29 (7), 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghimi SM; Wibroe PP; Helvig SY; Farhangrazi ZS; Hunter AC, Genomic perspectives in inter-individual adverse responses following nanomedicine administration: The way forward. Adv Drug Deliv Rev 2012, 64 (13), 1385–93. [DOI] [PubMed] [Google Scholar]
- 13.Thasneem YM; Sajeesh S; Sharma CP, Glucosylated polymeric nanoparticles: a sweetened approach against blood compatibility paradox. Colloids Surf B Biointerfaces 2013, 108, 337–44. [DOI] [PubMed] [Google Scholar]
- 14.Rampton D; Folkersen J; Fishbane S; Hedenus M; Howaldt S; Locatelli F; Patni S; Szebeni J; Weiss G, Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014, 99 (11), 1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornaguera C; Caldero G; Mitjans M; Vinardell MP; Solans C; Vauthier C, Interactions of PLGA nanoparticles with blood components: protein adsorption, coagulation, activation of the complement system and hemolysis studies. Nanoscale 2015, 7 (14), 6045–58. [DOI] [PubMed] [Google Scholar]
- 16.Li Y; Fung J; Lin F, Local Inhibition of Complement Improves Mesenchymal Stem Cell Viability and Function After Administration. Mol Ther 2016, 24 (9), 1665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goram AL; Richmond PL, Pegylated liposomal doxorubicin: tolerability and toxicity. Pharmacotherapy 2001, 21 (6), 751–63. [DOI] [PubMed] [Google Scholar]
- 18.Janssen BJ; Huizinga EG; Raaijmakers HC; Roos A; Daha MR; Nilsson-Ekdahl K; Nilsson B; Gros P, Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437 (7058), 505–11. [DOI] [PubMed] [Google Scholar]
- 19.Dawson K; Moran M; Guindon K; Wan H, Managing Infusion-Related Reactions for Patients With Chronic Lymphocytic Leukemia Receiving Obinutuzumab. Clin J Oncol Nurs 2016, 20 (2), E41–8. [DOI] [PubMed] [Google Scholar]
- 20.Fulop T; Kozma GT; Vashegyi I; Meszaros T; Rosivall L; Urbanics R; Storm G; Metselaar JM; Szebeni J, Liposome-induced hypersensitivity reactions: Risk reduction by design of safe infusion protocols in pigs. J Control Release 2019, 309, 333–338. [DOI] [PubMed] [Google Scholar]
- 21.Lashof-Sullivan M; Holland M; Groynom R; Campbell D; Shoffstall A; Lavik E, Hemostatic Nanoparticles Improve Survival Following Blunt Trauma Even after 1 Week Incubation at 50 ( degrees )C. ACS Biomater Sci Eng 2016, 2 (3), 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbard WB; Lashof-Sullivan MM; Lavik EB; VandeVord PJ, Steroid-Loaded Hemostatic Nanoparticles Combat Lung Injury after Blast Trauma. ACS Macro Lett 2015, 4 (4), 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lashof-Sullivan MM; Shoffstall E; Atkins KT; Keane N; Bir C; VandeVord P; Lavik EB, Intravenously administered nanoparticles increase survival following blast trauma. Proc Natl Acad Sci U S A 2014, 111 (28), 10293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoffstall AJ; Everhart LM; Varley ME; Soehnlen ES; Shick AM; Ustin JS; Lavik EB, Tuning ligand density on intravenous hemostatic nanoparticles dramatically increases survival following blunt trauma. Biomacromolecules 2013, 14 (8), 2790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoffstall AJ; Atkins KT; Groynom RE; Varley ME; Everhart LM; Lashof-Sullivan MM; Martyn-Dow B; Butler RS; Ustin JS; Lavik EB, Intravenous hemostatic nanoparticles increase survival following blunt trauma injury. Biomacromolecules 2012, 13 (11), 3850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onwukwe C; Maisha N; Holland M; Varley M; Groynom R; Hickman D; Uppal N; Shoffstall A; Ustin J; Lavik E, Engineering Intravenously Administered Nanoparticles to Reduce Infusion Reaction and Stop Bleeding in a Large Animal Model of Trauma. Bioconjug Chem 2018, 29 (7), 2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisha N; Coombs T; Lavik E, Development of a Sensitive Assay to Screen Nanoparticles in vitro for Complement Activation. ACS Biomater Sci Eng 2020, 6 (9), 4903–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisha N; Naik N; Okesola M; Coombs T; Zilberberg R; Pandala N; Lavik E, Engineering PEGylated Polyester Nanoparticles to Reduce Complement-Mediated Infusion Reaction. Bioconjug Chem 2021. [DOI] [PubMed] [Google Scholar]
- 29.Gurney J; Philbin N; Rice J; Arnaud F; Dong F; Wulster-Radcliffe M; Pearce LB; Kaplan L; McCarron R; Freilich D, A Hemoglobin Based Oxygen Carrier, Bovine Polymerized Hemoglobin (HBOC-201) versus Hetastarch (HEX) in an Uncontrolled Liver Injury Hemorrhagic Shock Swine Model with Delayed Evacuation. The Journal of Trauma: Injury, Infection, and Critical Care 2004, 57, 726–738. [DOI] [PubMed] [Google Scholar]
- 30.Jackson MA; Patel SS; Yu F; Cottam MA; Glass EB; Hoogenboezem EN; Fletcher RB; Dollinger BR; Patil P; Liu DD; Kelly IB; Bedingfield SK; King AR; Miles RE; Hasty AM; Giorgio TD; Duvall CL, Kupffer cell release of platelet activating factor drives dose limiting toxicities of nucleic acid nanocarriers. Biomaterials 2021, 268, 120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertram JP; Williams CA; Robinson R; Segal SS; Flynn NT; Lavik EB, Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med 2009, 1 (11), 11ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapkota B; Shrestha SK; Poudel S, Association of activated partial thromboplastin time and fibrinogen level in patients with type II diabetes mellitus. BMC research notes 2013, 6, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmon CT, Possible involvement of cytokines in diffuse intravascular coagulation and thrombosis. Bailliere’s best practice & research. Clinical haematology 1999, 12 (3), 343–59. [DOI] [PubMed] [Google Scholar]
- 34.Grignani G; Maiolo A, Cytokines and hemostasis. Haematologica 2000, 85 (9), 967–72. [PubMed] [Google Scholar]
- 35.Stouthard JM; Levi M; Hack CE; Veenhof CH; Romijn HA; Sauerwein HP; van der Poll T, Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost 1996, 76 (5), 738–42. [PubMed] [Google Scholar]
- 36.Eslamifar Z; Behzadifard M; Soleimani M; Behzadifard S, Coagulation abnormalities in SARS-CoV-2 infection: overexpression tissue factor. Thrombosis journal 2020, 18 (1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faille D; Lamrani L; Loyau S; Huisse MG; Bourrienne MC; Alkhaier S; Cassinat B; Boulaftali Y; Debus J; Jandrot-Perrus M; Chomienne C; Dosquet C; Ajzenberg N, Interferon Alpha Therapy Increases Pro-Thrombotic Biomarkers in Patients with Myeloproliferative Neoplasms. Cancers (Basel) 2020, 12 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Focosi D; Franchini M; Pirofski LA; Burnouf T; Fairweather D; Joyner MJ; Casadevall A, COVID-19 Convalescent Plasma Is More than Neutralizing Antibodies: A Narrative Review of Potential Beneficial and Detrimental Co-Factors. Viruses 2021, 13 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y; Wang J; Liu C; Su L; Zhang D; Fan J; Yang Y; Xiao M; Xie J; Xu Y; Li Y; Zhang S, IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Molecular medicine (Cambridge, Mass.) 2020, 26 (1), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han Y; He T; Huang DR; Pardo CA; Ransohoff RM, TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest 2001, 108 (3), 425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates AG; Jogia T; Gillespie ER; Couch Y; Ruitenberg MJ; Anthony DC, Acute IL-1RA treatment suppresses the peripheral and central inflammatory response to spinal cord injury. Journal of neuroinflammation 2021, 18 (1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arend WP, The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002, 13 (4–5), 323–40. [DOI] [PubMed] [Google Scholar]
- 43.Roth S; Agthe M; Eickhoff S; Möller S; Karsten CM; Borregaard N; Solbach W; Laskay T, Secondary necrotic neutrophils release interleukin-16C and macrophage migration inhibitory factor from stores in the cytosol. Cell Death Discov 2015, 1, 15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruikshank WW; Kornfeld H; Center DM, Interleukin-16. Journal of leukocyte biology 2000, 67 (6), 757–66. [DOI] [PubMed] [Google Scholar]
- 45.Mathy NL; Scheuer W; Lanzendörfer M; Honold K; Ambrosius D; Norley S; Kurth R, Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000, 100 (1), 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerhard R; Queisser S; Tatge H; Meyer G; Dittrich-Breiholz O; Kracht M; Feng H; Just I, Down-regulation of interleukin-16 in human mast cells HMC-1 by Clostridium difficile toxins A and B. Naunyn Schmiedebergs Arch Pharmacol 2011, 383 (3), 285–95. [DOI] [PubMed] [Google Scholar]
- 47.Schindler L; Smyth LCD; Bernhagen J; Hampton MB; Dickerhof N, Macrophage migration inhibitory factor (MIF) enhances hypochlorous acid production in phagocytic neutrophils. Redox biology 2021, 41, 101946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett CD; Hsu AT; Ellson CD; B, Y. M.; Kong YW; Greenwood JD; Dhara S; Neal MD; Sperry JL; Park MS; Cohen MJ; Zuckerbraun BS; Yaffe MB, Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clinical and experimental immunology 2018, 194 (1), 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peralta R; Thani HA; Rizoli S, Coagulopathy in the surgical patient: trauma-induced and drug-induced coagulopathies. Curr Opin Crit Care 2019, 25 (6), 668–674. [DOI] [PubMed] [Google Scholar]
- 50.Moore EE; Moore HB; Kornblith LZ; Neal MD; Hoffman M; Mutch NJ; Schöchl H; Hunt BJ; Sauaia A, Trauma-induced coagulopathy. Nature reviews. Disease primers 2021, 7 (1), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baylis JR; Chan KY; Kastrup CJ, Halting hemorrhage with self-propelling particles and local drug delivery. Thromb Res 2016, 141 Suppl 2, S36–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubbard WB; Lashof-Sullivan M; Greenberg S; Norris C; Eck J; Lavik E; VandeVord P, Hemostatic nanoparticles increase survival, mitigate neuropathology and alleviate anxiety in a rodent blast trauma model. Scientific reports 2018, 8 (1), 10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo T; Hao S; Chen X; Wang J; Yang Q; Wang Y; Weng Y; Wei H; Zhou J; Wang B, Development and assessment of kerateine nanoparticles for use as a hemostatic agent. Materials science & engineering. C, Materials for biological applications 2016, 63, 352–8. [DOI] [PubMed] [Google Scholar]
- 54.Okamura Y; Takeoka S; Eto K; Maekawa I; Fujie T; Maruyama H; Ikeda Y; Handa M, Development of fibrinogen γ-chain peptide-coated, adenosine diphosphate-encapsulated liposomes as a synthetic platelet substitute. Journal of Thrombosis and Haemostasis 2009, 7, 470–477. [DOI] [PubMed] [Google Scholar]
- 55.Jackman JA; Meszaros T; Fulop T; Urbanics R; Szebeni J; Cho NJ, Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: foundation of a validatable immune toxicity model. Nanomedicine 2016, 12 (4), 933–943. [DOI] [PubMed] [Google Scholar]
- 56.Szebeni J, Mechanism of nanoparticle-induced hypersensitivity in pigs: complement or not complement? Drug Discov Today 2018, 23 (3), 487–492. [DOI] [PubMed] [Google Scholar]
- 57.Brown D; Namas RA; Almahmoud K; Zaaqoq A; Sarkar J; Barclay DA; Yin J; Ghuma A; Abboud A; Constantine G; Nieman G; Zamora R; Chang SC; Billiar TR; Vodovotz Y, Trauma in silico: Individual-specific mathematical models and virtual clinical populations. Sci Transl Med 2015, 7 (285), 285ra61. [DOI] [PubMed] [Google Scholar]
- 58.Fromen CA; Kelley WJ; Fish MB; Adili R; Noble J; Hoenerhoff MJ; Holinstat M; Eniola-Adefeso O, Neutrophil-Particle Interactions in Blood Circulation Drive Particle Clearance and Alter Neutrophil Responses in Acute Inflammation. ACS Nano 2017, 11 (11), 10797–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatfield SM; Thieblemont N; Witko-Sarsat V, Expanding Neutrophil Horizons: New Concepts in Inflammation. J Innate Immun 2018, 10 (5–6), 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelley WJ; Fromen CA; Lopez-Cazares G; Eniola-Adefeso O, PEGylation of model drug carriers enhances phagocytosis by primary human neutrophils. Acta Biomater 2018, 79, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelley WJ; Onyskiw PJ; Fromen CA; Eniola-Adefeso O, Model Particulate Drug Carriers Modulate Leukocyte Adhesion in Human Blood Flows. ACS Biomater Sci Eng 2019, 5 (12), 6530–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zolnik BS; González-Fernández A; Sadrieh N; Dobrovolskaia MA, Nanoparticles and the immune system. Endocrinology 2010, 151 (2), 458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Akiva E; Est Witte S; Meyer RA; Rhodes KR; Green JJ, Polymeric micro- and nanoparticles for immune modulation. Biomaterials science 2018, 7 (1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ayer M; Burri O; Guiet R; Seitz A; Kaba E; Engelhardt B; Klok HA, Biotin-NeutrAvidin Mediated Immobilization of Polymer Micro- and Nanoparticles on T Lymphocytes. Bioconjug Chem 2021, 32 (3), 541–552. [DOI] [PubMed] [Google Scholar]
- 65.Ruf W; Ruggeri ZM, Neutrophils release brakes of coagulation. Nat Med 2010, 16 (8), 851–2. [DOI] [PubMed] [Google Scholar]
- 66.Fu J; Kong J; Wang W; Wu M; Yao L; Wang Z; Jin J; Wu D; Yu X, The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb Res 2020, 192, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terpos E; Ntanasis-Stathopoulos I; Elalamy I; Kastritis E; Sergentanis TN; Politou M; Psaltopoulou T; Gerotziafas G; Dimopoulos MA, Hematological findings and complications of COVID-19. American journal of hematology 2020, 95 (7), 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao J; Liu K; Li S; Gao Y; Zhao L; Liu H; Fang H; Song B; Xu Y, Neutrophil-to-lymphocyte Ratio Predicts the Outcome of Cerebral Venous Thrombosis. Curr Neurovasc Res 2021, 18 (2), 204–210. [DOI] [PubMed] [Google Scholar]
- 69.Moghimi SM, Nanomedicine safety in preclinical and clinical development: focus on idiosyncratic injection/infusion reactions. Drug Discov Today 2018, 23 (5), 1034–1042. [DOI] [PubMed] [Google Scholar]
- 70.Skotland T, Injection of nanoparticles into cloven-hoof animals: Asking for trouble. Theranostics 2017, 7 (19), 4877–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sondeen JL; de Guzman R; Amy Polykratis I; Dale Prince M; Hernandez O; Cap AP; Dubick MA, Comparison between human and porcine thromboelastograph parameters in response to ex-vivo changes to platelets, plasma, and red blood cells. Blood Coagul Fibrinolysis 2013, 24 (8), 818–29. [DOI] [PubMed] [Google Scholar]
- 72.Tarandovskiy ID; Shin HKH; Baek JH; Karnaukhova E; Buehler PW, Interspecies comparison of simultaneous thrombin and plasmin generation. Scientific reports 2020, 10 (1), 3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gando S; Levi M; Toh CH, Disseminated intravascular coagulation. Nature reviews. Disease primers 2016, 2, 16037. [DOI] [PubMed] [Google Scholar]
- 74.Cardenas JC; Wade CE; Holcomb JB, Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol 2014, 21 (5), 404–9. [DOI] [PubMed] [Google Scholar]
- 75.Innerhofer P; Fries D; Mittermayr M; Innerhofer N; von Langen D; Hell T; Gruber G; Schmid S; Friesenecker B; Lorenz IH; Strohle M; Rastner V; Trubsbach S; Raab H; Treml B; Wally D; Treichl B; Mayr A; Kranewitter C; Oswald E, Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. The Lancet. Haematology 2017, 4 (6), e258–e271. [DOI] [PubMed] [Google Scholar]
- 76.Samuels JM; Moore EE; Silliman CC; Banerjee A; Cohen MJ; Ghasabyan A; Chandler J; Coleman JR; Sauaia A, Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg 2019, 86 (4), 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King DR; Cohn SM; Proctor KG, Changes in intracranial pressure, coagulation, and neurologic outcome after resuscitation from experimental traumatic brain injury with hetastarch. Surgery 2004, 136 (2), 355–63. [DOI] [PubMed] [Google Scholar]
- 78.Kumar MA, Coagulopathy associated with traumatic brain injury. Curr Neurol Neurosci Rep 2013, 13 (11), 391. [DOI] [PubMed] [Google Scholar]
- 79.Chang R; Cardenas JC; Wade CE; Holcomb JB, Advances in the understanding of trauma-induced coagulopathy. Blood 2016, 128 (8), 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hess JR; Brohi K; Dutton RP; Hauser CJ; Holcomb JB; Kluger Y; Mackway-Jones K; Parr MJ; Rizoli SB; Yukioka T; others, The coagulopathy of trauma: a review of mechanisms. Journal of Trauma-Injury, Infection, and Critical Care 2008, 65 (4), 748–754. [DOI] [PubMed] [Google Scholar]
- 81.Bajzar L; Jain N; Wang P; Walker JB, Thrombin activatable fibrinolysis inhibitor: not just an inhibitor of fibrinolysis. Crit Care Med 2004, 32 (5 Suppl), S320–4. [DOI] [PubMed] [Google Scholar]
- 82.Brohi K; Cohen MJ; Davenport RA, Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 2007, 13 (6), 680–5. [DOI] [PubMed] [Google Scholar]
- 83.Spahn DR; Bouillon B; Cerny V; Coats TJ; Duranteau J; Fernandez-Mondejar E; Filipescu D; Hunt BJ; Komadina R; Nardi G; Neugebauer E; Ozier Y; Riddez L; Schultz A; Vincent JL; Rossaint R, Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013, 17 (2), R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossaint R; Bouillon B; Cerny V; Coats TJ; Duranteau J; Fernandez-Mondejar E; Filipescu D; Hunt BJ; Komadina R; Nardi G; Neugebauer EA; Ozier Y; Riddez L; Schultz A; Vincent JL; Spahn DR, The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016, 20, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sumislawski JJ; Kornblith LZ; Conroy AS; Callcut RA; Cohen MJ, Dynamic coagulability after injury: Is delaying venous thromboembolism chemoprophylaxis worth the wait? J Trauma Acute Care Surg 2018, 85 (5), 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antovic A, Screening haemostasis--looking for global assays: the Overall Haemostasis Potential (OHP) method--a possible tool for laboratory investigation of global haemostasis in both hypo- and hypercoagulable conditions. Current vascular pharmacology 2008, 6 (3), 173–85. [DOI] [PubMed] [Google Scholar]
- 87.Duque P; Mora L; Levy JH; Schöchl H, Pathophysiological Response to Trauma-Induced Coagulopathy: A Comprehensive Review. Anesth Analg 2020, 130 (3), 654–664. [DOI] [PubMed] [Google Scholar]
- 88.Ma X; Cheng Y; Garcia R; Haorah J, Hemorrhage Associated Mechanisms of Neuroinflammation in Experimental Traumatic Brain Injury. J Neuroimmune Pharmacol 2020, 15 (2), 181–195. [DOI] [PubMed] [Google Scholar]
- 89.McMillan TM; Teasdale GM, Death rate is increased for at least 7 years after head injury: a prospective study. Brain 2007, 130 (Pt 10), 2520–7. [DOI] [PubMed] [Google Scholar]
- 90.Ma X; Aravind A; Pfister BJ; Chandra N; Haorah J, Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol Neurobiol 2019, 56 (8), 5332–5345. [DOI] [PubMed] [Google Scholar]
- 91.Cannon JW; Dias JD; Kumar MA; Walsh M; Thomas SG; Cotton BA; Schuster JM; Evans SL; Schreiber MA; Adam EH; Zacharowski K; Hartmann J; Schöchl H; Kaplan LJ, Use of Thromboelastography in the Evaluation and Management of Patients With Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit Care Explor 2021, 3 (9), e0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Esnault P; Mathais Q; D’Aranda E; Montcriol A; Cardinale M; Cungi PJ; Goutorbe P; Joubert C; Dagain A; Meaudre E, Ability of Fibrin Monomers to Predict Progressive Hemorrhagic Injury in Patients with Severe Traumatic Brain Injury. Neurocrit Care 2020, 33 (1), 182–195. [DOI] [PubMed] [Google Scholar]
- 93.Tur Martínez J; Petrone P; Axelrad A; Marini CP, Comparison between thromboelastography and conventional coagulation test: Should we abandon conventional coagulation tests in polytrauma patients? Cirugia espanola 2018, 96 (7), 443–449. [DOI] [PubMed] [Google Scholar]
- 94.Tian Y; Salsbery B; Wang M; Yuan H; Yang J; Zhao Z; Wu X; Zhang Y; Konkle BA; Thiagarajan P; Li M; Zhang J; Dong JF, Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 2015, 125 (13), 2151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ackermann M; Verleden SE; Kuehnel M; Haverich A; Welte T; Laenger F; Vanstapel A; Werlein C; Stark H; Tzankov A; Li WW; Li VW; Mentzer SJ; Jonigk D, Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383 (2), 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fogarty H; Townsend L; Morrin H; Ahmad A; Comerford C; Karampini E; Englert H; Byrne M; Bergin C; O’Sullivan JM; Martin-Loeches I; Nadarajan P; Bannan C; Mallon PW; Curley GF; Preston RJS; Rehill AM; McGonagle D; Ni Cheallaigh C; Baker RI; Renné T; Ward SE; O’Donnell JS, Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 2021, 19 (10), 2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iba T; Connors JM; Levy JH, The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflammation research : official journal of the European Histamine Research Society ... [et al.] 2020, 69 (12), 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Connor EF; Nyce GW; Myers M; Möck A; Hedrick JL, First Example of N-Heterocyclic Carbenes as Catalysts for Living Polymerization: Organocatalytic Ring-Opening Polymerization of Cyclic Esters. Journal of the American Chemical Society 2002, 124, 914–915. [DOI] [PubMed] [Google Scholar]
- 99.Philbin N; Rice J; Gurney J; McGwin G; Arnaud F; Dong F; Johnson T; Flournoy WS; Ahlers S; Pearce LB; McCarron R; Freilich D, A hemoglobin-based oxygen carrier, bovine polymerized hemoglobin (HBOC-201) versus hetastarch (HEX) in a moderate severity hemorrhagic shock swine model with delayed evacuation. Resuscitation 2005, 66, 367–378. [DOI] [PubMed] [Google Scholar]
- 100.Lategan K; Alghadi H; Bayati M; de Cortalezzi MF; Pool E, Effects of Graphene Oxide Nanoparticles on the Immune System Biomarkers Produced by RAW 264.7 and Human Whole Blood Cell Cultures. Nanomaterials (Basel, Switzerland) 2018, 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


