Abstract
The gut is a target organ that functions as the “motor” of critical illness. In patients with critical illness, the disrupted gut microbiota following infection and injury could cause diarrhea, pneumonia, and systemic inflammation. For maintaining the gut microbiota, therapeutic approaches are required to modulate host responses and prevent systemic inflammation. Probiotics and synbiotics could maintain the gut microbiota and decrease not only the incidence of diarrhea but also that of ventilator‐associated pneumonia. The effects of probiotics/synbiotics differ with the type of bacteria and disease severity. Adverse effects of probiotics have been reported; therefore, the selection of safe and effective probiotics/synbiotics is warranted. Refractory diarrhea with prolonged dysbiosis may require a novel intestinal therapy, such as fecal microbiota transplantation, to alleviate gut dysbiosis.
Keywords: critically ill, dysbiosis, gut, ICU, microbiota
After severe injury, such as trauma, operation, burn, infection, shock, and bleeding, systemic inflammation progresses in various organs simultaneously. The gut is considered to have an important role in promoting systemic inflammation through bacterial translocation, intestinal lymphatic mediators, gut immunity, etc.

ROLE OF THE GUT IN CRITICAL ILLNESS
Severe trauma, burns, infections, and other major injuries to the body can induce Systemic Inflammatory Response Syndrome (SIRS), which can progress to multiple organ failure. 1 The gut is an important target organ following infection and injury. In particular, infection and injury could alter the gut microbiota, reduce gut immunity represented by IgA and other factors, cause bacterial translocation by disrupting the intestinal barrier, and induce the influx of inflammatory cytokines into the systemic circulation via the intestinal lymph. 2 In a mouse burn model, Escherichia coli was detected in the spleen and liver merely 5 min after injury. 3 Altered levels of intestinal tight junction proteins, such as decreased claudin‐5 and occludin levels and increased claudin‐2 levels, were found to change gut permeability in a cecal ligation puncture model. 4 In clinical research, bacterial translocation was noted in the mesenteric lymph nodes of 35% of patients who underwent hepatectomy for biliary cancer. 5 Disrupted tight junctions may facilitate the translocation of pathogenic bacteria from the intestines to other tissues and organs. Such intestinal dysfunction is thought to play a crucial role in the progression of systemic inflammatory response and subsequent systemic multiple organ failure. The gut thus serves as the “motor” of critical illness.
The systemic inflammatory response is common in the acute phase, in which the immune system is triggered by foreign substances, such as bacteria, or by self‐tissue damage from trauma, resulting in the activation of both inflammatory Th1‐type immune responses and anti‐inflammatory Th2‐type immune responses (Figure 1). 6 Prolonged systemic inflammation could also cause immunosuppression and increase the susceptibility to infection. Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PIICS) is a condition characterized by pro‐inflammatory and anti‐inflammatory responses. 7 On the pro‐inflammatory side, macrophages are mainly activated. On the anti‐inflammatory side, regulatory T cells or myeloid‐derived suppressor cells serve as compensatory mediators following infection and injury. 8 In this review, we focus on the gut microbiota of patients with critical illness.
FIGURE 1.
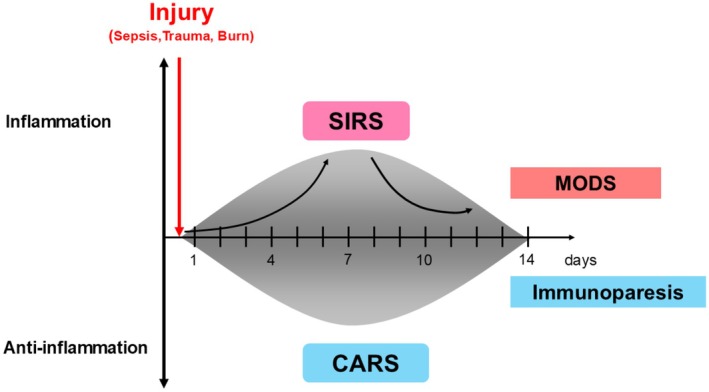
The concept of injury‐induced imbalances in the immune system. The host immune response induces inflammation following injury; this is known as Systemic Inflammatory Response Syndrome (SIRS). The immunosuppression coincidentally begins at the onset of inflammation; this is known as compensatory anti‐inflammatory response (CARS). If the SIRS and CARS state is prolonged, it progresses to a state known as multiple organ dysfunction syndrome (MODS). Progressive immunosuppression is known as Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PIICS). This pro‐inflammatory state is mainly driven by macrophages, neutrophils, and regulatory T cells (cited and edited from reference [1]).
DYSBIOSIS OF GUT MICROBIOTA AND ENVIRONMENT FOLLOWING INFECTION AND INJURY
Assessment of the gut microbiota by the culture method
The human gut microbiota is thought to contain more than 1000 microbial species and 100 trillion microbes. The predominant bacteria in the intestinal tract are Bacteroides and Bifidobacterium species, which are obligate anaerobes that can only grow in an anaerobic environment. The intestinal microbiota is associated with various diseases and conditions, such as obesity, gastrointestinal disease, allergy, liver cirrhosis, rheumatoid arthritis, cancer, heart failure, and autism. It also regulates the efficacy of anticancer drugs, such as immune checkpoint inhibitors. 9 In critically ill conditions, gut microbiota could be altered because of the present disease and the various kinds of treatments, such as antacids for bleeding prevention, catecholamines for blood pressure control, and oxygen via mechanical ventilation for respiratory failure. Especially, antibiotics for infection control could be one of the main factors to change gut microbiota 10 and lead to antibiotic‐associated diarrhea. 11
To quantitatively assess the gut microbiota, stool samples were collected from 25 critically ill patients in a previous study. The total number of commensal obligate anaerobes was significantly lower in the stool samples of critically ill patients than in those of healthy individuals (8.3 ± 2.3 vs. 10.5 ± 0.5 log10 colony‐forming units/g, p < 0.05). In particular, Bifidobacterium and Lactobacillus counts were reduced to 1/100–1000 of those in healthy individuals (Table 1). 12 Assessment of the organic acid contents in the stool samples revealed a significant decrease in short‐chain fatty acid (SCFA) levels (acetic acid, propionic acid, and butyric acid levels). However, the pH of the stool samples of critically ill patients was significantly higher than that of healthy individuals (7.4 ± 0.6 vs. 6.6 ± 0.3; Table 2). The total number of obligate anaerobes already decreased within 6 h of admission 13 and continued to be low over the following weeks. 14 The increased number of pathogenic bacteria and decreased number of obligate anaerobes were associated with bacteremia and mortality in critically ill patients. 15 These results reveal the deterioration of the gut microbiota with the progression of systemic inflammation.
TABLE 1.
Fecal flora in patients with severe SIRS.
| SIRS patients | Normal | |
|---|---|---|
| Total obligate anaerobes | 8.3 ± 2.3* | 10.5 ± 0.5 |
| Bacteroidaceae | 7.3 ± 3.0* | 10.1 ± 0.4 |
| Bifidobacterium | 4.8 ± 3.3* | 9.6 ± 0.7 |
| Clostridium | 2.1 ± 1.0 | 2.1 ± 0.7 |
| Veillonella | 3.1 ± 1.8* | 7.0 ± 1.2 |
| Total facultative anaerobes | 7.8 ± 1.4 | 7.5 ± 0.4 |
| Lactobacillus | 2.7 ± 1.5* | 5.0 ± 1.0 |
| Enterobacteriaceae | 4.1 ± 2.7* | 7.4 ± 0.8 |
| Enterococcus | 6.4 ± 2.5 | 7.0 ± 0.9 |
| Staphylococcus | 5.3 ± 1.7* | 2.7 ± 0.8 |
| Pseudomonas | 2.8 ± 1.4* | ND |
| Candida | 2.5 ± 1.0 | 2.0 ± 0.5 |
Note: Data are mean ± SD (Log10 counts/g feces).
Abbreviations: ND, not detected; SIRS, Systemic Inflammatory Response Syndrome.
p < 0.05 versus Normal.
TABLE 2.
Fecal organic acid concentrations and pH in patients with severe SIRS.
| SIRS patients | Normal | |
|---|---|---|
| Total organic acid | 30.3 ± 20.3* | 88.4 ± 21.2 |
| Succinic acid | 2.0 ± 2.5 | 0.9 ± 1.2 |
| Lactic acid | 3.8 ± 5.5 | 0.5 ± 0.3 |
| Formic acid | 1.7 ± 2.9 | 0.4 ± 0.3 |
| Acetic acid | 18.7 ± 15.9* | 50.8 ± 13.1 |
| Propionic acid | 2.5 ± 4.6* | 18.7 ± 6.8 |
| Isobutyric acid | 0.1 ± 0.5 | 1.1 ± 0.3 |
| Butyric acid | 0.9 ± 2.3* | 16.6 ± 6.7 |
| Isovaleric acid | 0.5 ± 1.9 | 1.4 ± 0.7 |
| Valeric acid | 0.1 ± 0.7 | 0.6 ± 0.4 |
| pH | 7.4 ± 0.6* | 6.6 ± 0.3 |
Note: Data are mean ± SD (μmol/g feces).
Abbreviation: SIRS, Systemic Inflammatory Response Syndrome.
p < 0.05 versus Normal.
Assessment of the gut microbiota by metagenomic analysis
While culture is the main method to determine the number of bacteria quantitatively, comprehensive metagenomic analysis using the 16S ribosomal RNA (16S rRNA) genes enables the analysis of hundreds of microbial taxa at the phylum, order, family, genus, and species levels. It can also be used to analyze unknown causative organisms. At the phylum level, Bacteroidetes (including the genus Bacteroides) and Firmicutes (including the genus Clostridium) were found to be predominant in the gut microbiota, followed by Proteobacteria (including Escherichia coli), Actinobacteria (including Bifidobacterium), and Fusobacteria. In the acute phase of the disease, the balance of the gut microbiota changed from that at the time of admission. On ICU admission, the gut microbiota differed significantly from that of healthy individuals (Figure 2A). At the genus level, the prevalence of Blautia, Clostridium, Faecalibacterium, and other genera decreased more significantly a week after admission. 16 On the other hand, the prevalence of the genus Enterococcus significantly increased in a week (Figure 2B). These losses of commensal microbiota, expansion of pathobionts, and loss of diversity are defined as gut dysbiosis. Dysbiosis is characterized by (i) loss of beneficial microbial organisms, (ii) expansion of pathobionts or potentially harmful microorganisms, and (iii) loss of overall microbial diversity. 17 The Bacteroidetes/Firmicutes ratio (B/F ratio) has been reported to be associated with mortality. 18 Obligate anaerobic bacteria, a major component of the gut microbiota, are the principal inhibitors of bacterial overgrowth and the translocation of E. coli and other potentially pathogenic bacteria. This phenomenon is known as colonization resistance. 19 Moreover, the gut microbiota plays a role in producing immune signals that affect host metabolism, immunity, and immune response to infection. 20 In one study, germ‐free mice exhibited a smaller mucus barrier, Peyer's patches, and lamina propria, lower IgA concentrations, and lower T‐cell and B‐cell counts than normal mice. 21 Regarding adaptive immunity, Th17 cells were induced by segmented filamentous bacteria. 22 Regulatory T cells were induced by 17 bacterial species, including those belonging to Clostridiales. 23 IFN‐γ‐producing CD8+ cells were induced by 11 bacterial species belonging to the phyla Bacteroidetes, Firmicutes, and Fusobacteria. 24 Thus, gut dysbiosis may lead to a loss of immune cells and immunity following infection and injury. 25
FIGURE 2.
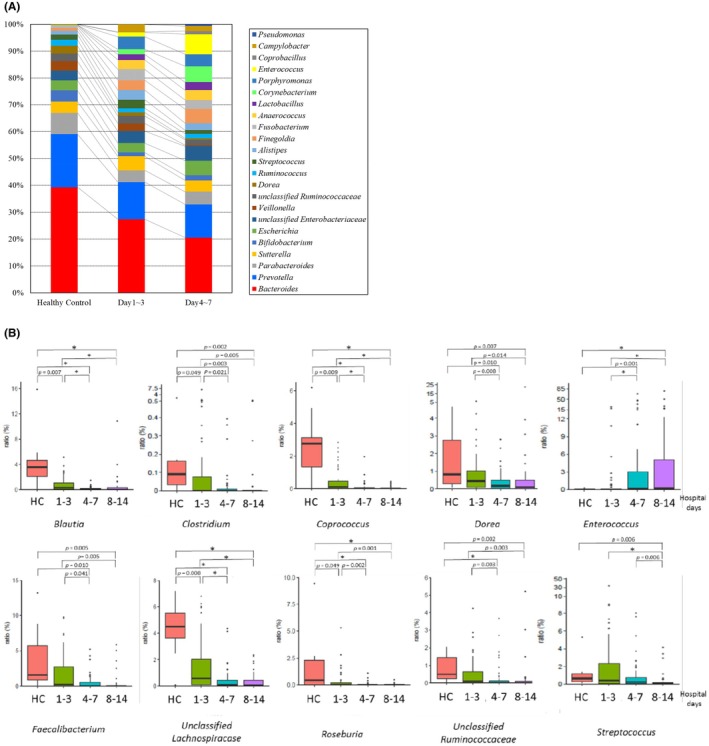
(A) Altered gut microbiota in ICU patients. Serial changes in the average proportions of genera in patients' fecal microbiota. Commensal bacteria (e.g., Bacteroidetes, Prevotella) decreased, whereas opportunistic bacteria (e.g., Enterococcus, Corynebacterium) increased (edited from reference [16]). (B) Serial changes in the fecal microbiota of ICU patients. The genera Blautia, Faecalibacterium, and Clostridium serially decreased in the fecal microbiota. On the other hand, the genus Enterococcus increased. An asterisk (*) indicates significant differences between the groups (cited and edited from reference [16]).
Commensal bacteria could therefore be essential to maintain homeostasis in the human body. The gut–lung axis perspective is important because intestinal dysbiosis is associated with increased pneumonia due to exacerbated inflammation. 26 Severe COVID‐19 patients also have gastrointestinal complications with decreased commensal gut microbiota and increased opportunistic bacteria. 27 , 28
Fecal gram staining as a bedside marker
Gram staining of endotracheal sputum targeting phagocytosed bacteria for antibacterial therapy has been reported to reduce the incidence of ventilator‐associated pneumonia and acute respiratory distress syndrome. 29 Fecal phagocytosed bacteria could also be a target for intestinal infection. 30
For gut dysbiosis, gram staining of fecal bacteria is also a quick bedside diagnostic marker (Figure 3). 31 If there are no phagocytosed bacteria, the Gram staining patterns can be classified into three types. In the diverse pattern, large numbers of multiple types of bacteria completely cover the field. In the single pattern, a specific type of bacterium or fungus predominantly covers the field. In the depleted pattern, most bacteria are diminished in the field. Alterations in the gut microbiota usually progress from a diverse pattern to a single pattern and then to a depleted pattern. In the case of a single or a depleted pattern, a therapeutic approach for microbiota reconstruction may be required using probiotics and synbiotics. Alternatively, fecal microbiota transplantation (FMT) may be required.
FIGURE 3.
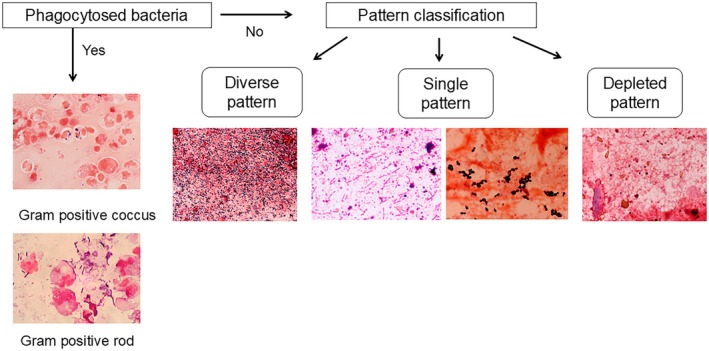
Pattern classification by fecal gram staining. If phagocytosed bacteria are detected, antibiotics can be administered in accordance with phagocytosed bacteria. If there are no phagocytosed bacteria, the Gram staining patterns are classified into three types with bacteria. In the diverse pattern, many types of bacteria cover the field. In the single pattern, a few types of bacteria predominantly cover the field. In the depleted pattern, most bacteria are depleted in the field. The dysbiosis progresses from a diverse pattern to a single pattern and from a single pattern to a depleted pattern.
Assessment of the gut environment by SCFAs and pH
Gut microbiota‐derived metabolites, especially SCFAs, could influence systemic inflammation. Acetate could improve intestinal defense and protect the host against lethal O157 infection. 32 Butyrate could induce the differentiation of colonic regulatory T cells. 33 In a fecal metabolomic analysis based on the cecal ligation puncture model, the levels of SCFAs, such as butyric acid, were found to decrease. 34 In clinical research, assessment of the organic acid contents in the stool samples revealed a significant decrease in SCFA levels (acetic acid, propionic acid, and butyric acid; Table 2). In addition, the pH of the stool samples of critically ill patients was significantly higher than that of healthy individuals (7.4 ± 0.6 vs. 6.6 ± 0.3).
Gastrointestinal pH also has a significant impact on bacterial flora, the absorption of vitamins and electrolytes, and the activity of digestive enzymes. 35 Nakahori et al. found that an increase in lactate levels and a decrease in propionate and acetate levels were significantly associated with mortality 36 An increase in fecal pH (>6.6) is significantly associated with increased mortality (odds ratio [OR], 2.46; 95% confidence interval [CI], 1.25–4.82) or the incidence of bacteremia (OR, 3.25; 95% CI, 1.67–6.30). 37 Fecal pH could be an important indicator of the gut environment. These results indicate that the deterioration of the gut microbiota and gut environment is associated with the prognosis of patients with critical illness.
TREATMENTS FOR GUT DYSBIOSIS IN CRITICALLY ILL PATIENTS
Probiotics and synbiotics
Probiotics are defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. 38 Probiotics, most commonly Lactobacillus and Bifidobacterium, 39 have been found to exert preventive effects in various diseases and conditions, such as acute diarrhea, antibiotic‐induced diarrhea, inflammatory bowel disease, and allergy. 40 Prebiotics are currently defined as nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon. 41 Synbiotics is a combination of probiotics and prebiotics. In the abdominal surgery setting, the perioperative administration of either probiotics or synbiotics was found to significantly reduce the risk of infectious complications following abdominal surgery. In a meta‐analysis involving 2723 patients, synbiotics exhibited a greater effect on postoperative infections than probiotics alone. 42 In the ICU setting, synbiotics increased the levels of both obligate and facultative anaerobes, such as Bifidobacterium and Lactobacillus, which could increase the SCFA levels and decrease the pH. 43 This virtuous circle could prevent infectious complications. In a meta‐analysis of ventilated patients in the ICU, the administration of probiotics reduced the incidence of diarrhea and ventilator‐associated pneumonia (VAP), duration of mechanical ventilation, length of ICU stay, and in‐hospital mortality. 44 , 45 Moreover, in patients with sepsis, the synbiotic group exhibited significantly greater levels of beneficial bacteria (Bifidobacterium and Lactobacillus) and acetate than the nonsynbiotic group. Synbiotics also lowered the incidence of diarrhea and VAP in ventilated patients with sepsis. 46 In a meta‐analysis of 281 patients in the trauma setting, the use of probiotics was associated with a decrease in the incidence of nosocomial infections. 47 These reports indicate that the administration of probiotics and synbiotics could maintain the gut microbiota balance and have preventive effects against infectious complications in critically ill patients.
The mechanisms of action of probiotics and synbiotics involve several factors, including gut microbiome modulation and barrier function: (a) Inhibition of Proteobacteria expansion: The aerobic condition consistently causes a shift of bacterial communities from obligate anaerobes to facultative anaerobes. 34 A dysbiotic expansion of facultative anaerobic bacteria, including Proteobacteria, could reduce the levels of SCFAs and form a dysfunctional colonic surface. 48 Probiotics/synbiotics could prevent the expansion of Proteobacteria and maintain gut homeostasis. (b) Bacterial inhibition by SCFAs: Acetate generated by the gut microbiota could inhibit pathogenic bacteria. A decrease in pH caused by probiotics/synbiotics could increase undissected acetate, resulting in further inhibition. (c) Tight junction strengthening: In an intestinal multidrug‐resistant Acinetobacter baumannii infection model, probiotics/synbiotics could maintain the levels of the tight‐junction‐related proteins claudin‐1, occludin, and ZO‐1 and serum diamine oxidase activity, a marker of intestinal integrity. 49 In biliary cancer patients, synbiotics could reduce the incidence of bacterial translocation in the mesenteric lymph nodes. 50 These factors could prevent bacterial translocation and infectious complications (Figure 4).
FIGURE 4.
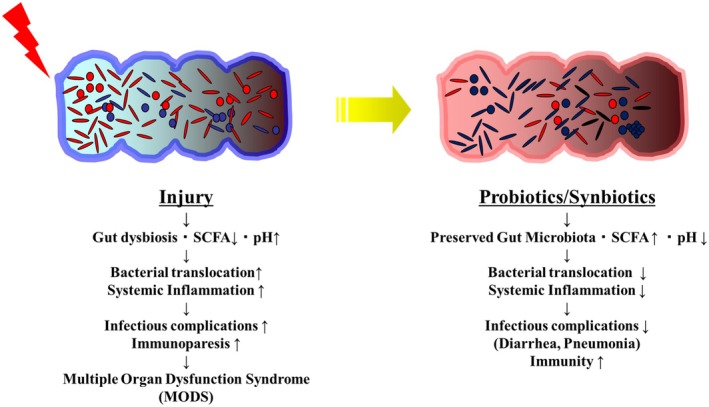
Potential infection prevention mechanism of probiotics/synbiotics. (Left) Severe injury deteriorates the gut microbiota and results in the progression of dysbiosis, thereby causing systemic inflammation, pneumonia, and multiple organ dysfunction syndrome. (Right) Probiotics/synbiotics could improve the gut microbiota and prevent infectious complications. SCFA, short‐chain fatty acids.
Not all types of probiotics are effective in critically ill patients. In vitro, even the same Lactobacillus strains exhibited different effects on endocarditis models. 51 In one randomized controlled trial, Lactobacillus had no significant effect on diarrhea and VAP. 52 Some probiotics could cause bacteremia and necrotizing enterocolitis. 53 , 54 These results indicate that not all probiotics are beneficial to the host. The effect and safety of probiotics differ with the type of gut microbiota, type of disease, and severity of disease. The type and number of bacteria should be elucidated to determine the appropriate effects of probiotics in critically ill patients.
Fecal microbiota transplantation
Antibiotic‐associated diarrhea (AAD) could cause dysbiosis following antibiotic administration. Antibiotics are mandatory drugs; therefore, the prevention of gut microbiota effects is an important topic. Clostridioides difficile infection (CDI) is a severe healthcare‐related infection characterized by AAD in the US. In refractory cases, FMT is recommended to reconstruct a normal gut microbiota. 55 FMT has been reported to be effective in not only CDI cases but also non‐CDI cases, such as inflammatory bowel disease, 56 graft versus host disease, 57 and refractory diarrhea. 58 In one study, AAD in the ICU was improved in 86.7% (13/15) after FMT. 59 Prolonged diarrhea with gut dysbiosis might be a good indication to restore gut microbiota and alleviate gastrointestinal symptoms in critically ill patients (jRCTs051220110). PIICS occurred in patients with prolonged stays in the ICU. Actually, 40% of patients following burn died after 30 days due to sepsis and pneumonia. 60 Gut dysbiosis prolonged 6 weeks after injury in the ICU. 14 As gut dysbiosis could associate with immunity, FMT might be a new solution to restore gut microbiota and prevent infection.
However, the screening of donors is crucial 61 and warranted because of ESBL infections for immunocompromised patients. 62 Safer screening and purification are required to manufacture fecal microbiota products.
Future intestinal therapy
Preventive effects of live biotherapeutic products on the gut microbiome or intestinal epithelium have been reported. In particular, oral microbiome therapy with live purified Firmicutes bacterial spores could reduce the risk of CDI recurrence with no adverse events. 63 The pharmacological efficacy of the oral microbiome and associated metabolites may restore the gut microbiota and reduce CD growth. Regarding other treatments, hydrogen exhibits antioxidant, anti‐inflammatory, and antiapoptotic effects. 64 Ikeda et al. found that the gastric administration of hydrogen water to a mouse peritonitis model significantly suppressed intestinal Enterobacteriaceae burst and improved survival. 65 High‐molecular‐weight polyethylene glycol, functioning as surrogate mucin, improved the survival rate in an intestinal Pseudomonas aeruginosa infection model. 66 Moreover, epidermal growth factor improved gut apoptosis and proliferation in a P. aeruginosa pneumonia mouse model. 67 With regard to tight junction proteins, claudin‐2 knockout mice upregulated intestinal permeability in a cecal ligation sepsis model. 68 Molecular targeted therapy with tight junction proteins may be considered for gut dysbiosis in the future.
In conclusion, the gut microbiota deteriorates in critical illnesses, such as trauma, burns, and sepsis. Gut dysbiosis reduces gut immunity and bacterial translocation, which could induce a systemic inflammatory response and cause multiple organ dysfunction. To prevent gut dysbiosis, intestinal treatments, such as the administration of probiotics/synbiotics, could maintain the gut microbiota and prevent infectious complications. A novel therapeutic approach involving the use of live biotherapeutic products and microbiological products may modulate the gut microbiota and environment.
CONFLICT OF INTEREST STATEMENT
Dr. Hiroshi Ogura is an Editorial Board member of AMS Journal and a co‐author of this article. Also, Dr. Jun Oda is the Editor‐in‐Chief of the journal and the co‐author of this article. To minimize bias, they were excluded from the peer‐review process and all editorial decisions related to the acceptance and publication of this article. Peer‐review was handled independently by AMS Journal editorial office and Dr. Yasuyuki Kuwagata as the Editor to minimize bias.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
The study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers 22H03174 and 25K02743).
Shimizu K, Ogura H, Oda J. Gut dysbiosis and its treatment in patients with critical illness. Acute Med Surg. 2025;12:e70068. 10.1002/ams2.70068
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Osuka A, Shigeno A, Matsuura H, Onishi S, Yoneda K. Systemic immune response of burns from the acute to chronic phase. Acute Med Surg. 2024;11:e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shimizu K, Ojima M, Ogura H. Gut microbiota and probiotics/synbiotics for modulation of immunity in critically ill patients. Nutrients. 2021;13:2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eaves‐Pyles T, Alexander JW. Rapid and prolonged impairment of gut barrier function after thermal injury in mice. Shock. 1998;9:95–100. [DOI] [PubMed] [Google Scholar]
- 4. Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizuno T, Yokoyama Y, Nishio H, Ebata T, Sugawara G, Asahara T, et al. Intraoperative bacterial translocation detected by bacterium‐specific ribosomal rna‐targeted reverse‐transcriptase polymerase chain reaction for the mesenteric lymph node strongly predicts postoperative infectious complications after major hepatectomy for biliary malignancies. Ann Surg. 2010;252:1013–1019. [DOI] [PubMed] [Google Scholar]
- 6. Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger‐‐damage control by the immune system. J Leukoc Biol. 2012;92:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chadda KR, Puthucheary Z. Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): a review of definitions, potential therapies, and research priorities. Br J Anaesth. 2024;132:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson RC, Shanahan ER, Scolyer RA, Long GV. Towards modulating the gut microbiota to enhance the efficacy of immune‐checkpoint inhibitors. Nat Rev Clin Oncol. 2023;20:697–715. [DOI] [PubMed] [Google Scholar]
- 10. Nagata N, Nishijima S, Miyoshi‐Akiyama T, Kojima Y, Kimura M, Aoki R, et al. Population‐level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology. 2022;163:1038–1052. [DOI] [PubMed] [Google Scholar]
- 11. Mekonnen SA, Merenstein D, Fraser CM, Marco ML. Molecular mechanisms of probiotic prevention of antibiotic‐associated diarrhea. Curr Opin Biotechnol. 2020;61:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126–133. [DOI] [PubMed] [Google Scholar]
- 13. Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56:2361–2365. [DOI] [PubMed] [Google Scholar]
- 14. Yamada T, Shimizu K, Ogura H, Asahara T, Nomoto K, Yamakawa K, et al. Rapid and sustained long‐term decrease of fecal short‐chain fatty acids in critically ill patients with systemic inflammatory response syndrome. JPEN J Parenter Enteral Nutr. 2015;39:569–577. [DOI] [PubMed] [Google Scholar]
- 15. Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ojima M, Shimizu K, Motooka D, Ishihara T, Nakamura S, Shintani A, et al. Gut Dysbiosis associated with antibiotics and disease severity and its relation to mortality in critically ill patients. Dig Dis Sci. 2022;67:2420–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. 2015;61:1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. [DOI] [PubMed] [Google Scholar]
- 21. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500:232–236. [DOI] [PubMed] [Google Scholar]
- 24. Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti‐cancer immunity. Nature. 2019;565:600–605. [DOI] [PubMed] [Google Scholar]
- 25. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. [DOI] [PubMed] [Google Scholar]
- 26. de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota modulation of the gut‐lung axis in COVID‐19. Front Immunol. 2021;12:635471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu K, Hirata H, Tokuhira N, Ueda A, Motooka D, Nakamura S, et al. A case of massive refractory diarrhea in a patient with COVID‐19. Acute Med Surg. 2022;9:e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimizu K, Hirata H, Tokuhira N, Motooka D, Nakamura S, Ueda A, et al. Dysbiosis of gut microbiota in patients with severe COVID‐19. Acute Med Surg. 2024;11:e923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsushima A, Tasaki O, Shimizu K, Tomono K, Ogura H, Shimazu T, et al. Preemptive antibiotic treatment based on gram staining reduced the incidence of ARDS in mechanically ventilated patients. J Trauma. 2008;65:309–315. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu K, Takahashi A, Motooka D, Nakamura S, Tomono K, Ogura H, et al. Fecal gram staining of phagocytosed bacteria to differentiate methicillin‐resistant Staphylococcus aureus: a case report. J Infect Chemother. 2020;26:1078–1081. [DOI] [PubMed] [Google Scholar]
- 31. Shimizu K, Ogura H, Tomono K, Tasaki O, Asahara T, Nomoto K, et al. Patterns of gram‐stained fecal flora as a quick diagnostic marker in patients with severe SIRS. Dig Dis Sci. 2011;56:1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. [DOI] [PubMed] [Google Scholar]
- 33. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- 34. Muratsu A, Ikeda M, Shimizu K, Kameoka S, Motooka D, Nakamura S, et al. Dynamic change of fecal microbiota and metabolomics in a polymicrobial murine sepsis model. Acute Med Surg. 2022;9:e770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- 36. Nakahori Y, Shimizu K, Ogura H, Asahara T, Osuka A, Yamano S, et al. Impact of fecal short‐chain fatty acids on prognosis in critically ill patients. Acute Med Surg. 2020;7:e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osuka A, Shimizu K, Ogura H, Tasaki O, Hamasaki T, Asahara T, et al. Prognostic impact of fecal pH in critically ill patients. Crit Care. 2012;16:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. FAO/WHO . Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina 1–4 October, 2001.
- 39. Wu XD, Liu MM, Liang X, Hu N, Huang W. Effects of perioperative supplementation with pro−/synbiotics on clinical outcomes in surgical patients: a meta‐analysis with trial sequential analysis of randomized controlled trials. Clin Nutr. 2018;37:505–515. [DOI] [PubMed] [Google Scholar]
- 40. Latif A, Shehzad A, Niazi S, Zahid A, Ashraf W, Iqbal MW, et al. Probiotics: mechanism of action, health benefits and their application in food industries. Front Microbiol. 2023;14:1216674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. [DOI] [PubMed] [Google Scholar]
- 42. Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta‐analysis of randomized controlled trials. Ann Surg. 2020;271:1036–1047. [DOI] [PubMed] [Google Scholar]
- 43. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, et al. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci. 2009;54:1071–1078. [DOI] [PubMed] [Google Scholar]
- 44. Shimizu K, Hirose T, Ogura H. Efficacy of probiotics in the prevention of diarrhea in ventilated critically ill ICU patients: meta‐analysis of randomized control trials. J Intensive Care. 2021;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta‐analysis of randomized control trials. J Intensive Care. 2020;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator‐associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gu WJ, Deng T, Gong YZ, Jing R, Liu JC. The effects of probiotics in early enteral nutrition on the outcomes of trauma: a meta‐analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2013;37:310–317. [DOI] [PubMed] [Google Scholar]
- 48. Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol. 2017;39:1–6. [DOI] [PubMed] [Google Scholar]
- 49. Asahara T, Takahashi A, Yuki N, Kaji R, Takahashi T, Nomoto K. Protective effect of a Synbiotic against multidrug‐resistant Acinetobacter baumannii in a murine infection model. Antimicrob Agents Chemother. 2016;60:3041–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokoyama Y, Nishigaki E, Abe T, Fukaya M, Asahara T, Nomoto K, et al. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy. Br J Surg. 2014;101:189–199. [DOI] [PubMed] [Google Scholar]
- 51. Asahara T, Takahashi M, Nomoto K, Takayama H, Onoue M, Morotomi M, et al. Assessment of safety of lactobacillus strains based on resistance to host innate defense mechanisms. Clin Diagn Lab Immunol. 2003;10:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator‐associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sato Y, Kujirai D, Emoto K, Yagami T, Yamada T, Izumi M, et al. Necrotizing enterocolitis associated with Clostridium butyricum in a Japanese man. Acute Med Surg. 2018;5:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimura M, Mizuma M, Nakagawa K, Aoki S, Miura T, Takadate T, et al. Probiotic‐related bacteremia after major hepatectomy for biliary cancer: a report of two cases. Surg Case Rep. 2021;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fujimoto K, Kimura Y, Allegretti JR, Yamamoto M, Zhang YZ, Katayama K, et al. Functional restoration of bacteriomes and viromes by fecal microbiota transplantation. Gastroenterology. 2021;160:2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okahara K, Ishikawa D, Nomura K, Ito S, Haga K, Takahashi M, et al. Matching between donors and ulcerative colitis patients is important for long‐term maintenance after fecal microbiota transplantation. J Clin Med. 2020;9:1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, et al. Fecal microbiota transplantation for patients with steroid‐resistant acute graft‐versus‐host disease of the gut. Blood. 2016;128:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wurm P, Spindelboeck W, Krause R, Plank J, Fuchs G, Bashir M, et al. Antibiotic‐associated apoptotic enterocolitis in the absence of a defined pathogen: the role of intestinal microbiota depletion. Crit Care Med. 2017;45:e600–e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dai M, Liu Y, Chen W, Buch H, Shan Y, Chang L, et al. Rescue fecal microbiota transplantation for antibiotic‐associated diarrhea in critically ill patients. Crit Care. 2019;23:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoneda K, Osuka A, Ohnishi S, Matsuura H, Oda J. The timing of death in burn patients. Acute Med Surg. 2024;11:e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kassam Z, Dubois N, Ramakrishna B, Ling K, Qazi T, Smith M, et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381:2070–2072. [DOI] [PubMed] [Google Scholar]
- 62. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug‐resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–2050. [DOI] [PubMed] [Google Scholar]
- 63. Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER‐109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386:220–229. [DOI] [PubMed] [Google Scholar]
- 64. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. [DOI] [PubMed] [Google Scholar]
- 65. Ikeda M, Shimizu K, Ogura H, Kurakawa T, Umemoto E, Motooka D, et al. Hydrogen‐rich saline regulates intestinal barrier dysfunction, dysbiosis, and bacterial translocation in a murine model of sepsis. Shock. 2018;50:640–647. [DOI] [PubMed] [Google Scholar]
- 66. Wu L, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, et al. High‐molecular‐weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa . Gastroenterology. 2004;126:488–498. [DOI] [PubMed] [Google Scholar]
- 67. Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, et al. Epidermal growth factor improves survival and prevents intestinal injury in a murine model of Pseudomonas aeruginosa pneumonia. Shock. 2011;36:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oami T, Abtahi S, Shimazui T, Chen CW, Sweat YY, Liang Z, et al. Claudin‐2 upregulation enhances intestinal permeability, immune activation, dysbiosis, and mortality in sepsis. Proc Natl Acad Sci USA. 2024;121:e2217877121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


