ABSTRACT
Early and timely intervention can restore normal fetal heart rhythm in cases of high‐grade atrioventricular block without structural abnormalities. However, the presence of complications like cardiomyopathy significantly worsens the prognosis, despite aggressive treatment. Multidisciplinary care and close monitoring are essential to optimize maternal and fetal outcomes.
Keywords: atrioventricular block, cardiology, case report, pediatrics
1. Introduction
High‐grade atrioventricular block (AVB) is a serious condition characterized by a significant delay or complete block of electrical impulses from the atria to the ventricles [1]. While typically diagnosed and managed in young and older adults, advances in prenatal cardiology have allowed for the early detection and intervention of such conditions during intrauterine life [2]. According to recent studies, the occurrence of AVB is estimated as 1 in 20,000 live births, which indicates the rarity of this condition [3, 4]. Meanwhile, it is known as a life‐threatening condition that can lead to fetal and post‐natal mortality [5].
Early detection through advanced imaging and fetal echocardiography, combined with innovative therapeutic approaches, such as fetal pacing or medical interventions, has opened new avenues for improving perinatal outcomes [6].
This study presents a comprehensive review of several cases, offering insights into the challenges, techniques, and successes associated with managing high‐grade AVB during pregnancy.
2. Case History/Examination
2.1. Case 1
A 27‐year‐old gravid 1, para 0, abort 0 pregnant woman with no history of specific illnesses presented to our clinic at 22 weeks of gestation due to a detected decrease in fetal heart rate. No other maternal or fetal risk factors were identified.
2.2. Differential Diagnosis, Investigations, and Treatment
Fetal echocardiography revealed no structural abnormalities of the fetal heart. However, spectral Doppler interrogation of the left ventricular outflow tract (ascending aorta) demonstrated a sustained ventricular rate of approximately 90–100 beats per minute, well below the normal fetal heart rate range of 110–160 bpm, confirming fetal bradycardia (Figure 1). To elucidate the mechanism, simultaneous Doppler sampling was then obtained in the left ventricular outflow tract, capturing both mitral inflow (atrial ‘A’ waves) and aortic outflow (ventricular ‘V’ waves). As shown in Figure 2, two discrete atrial flow peaks are seen for every single ventricular ejection, yielding an atrial‐to‐ventricular ratio of 2:1. This pattern is diagnostic of second‐degree (2:1) atrioventricular block, which explains the marked reduction in overall heart rate.
FIGURE 1.

Doppler spectral analysis of the fetal cardiac outflow tract demonstrates no significant bradycardia.
FIGURE 2.

Doppler spectral analysis of the fetal left ventricular outflow tract reveals atrioventricular dissociation. The atrial rate (A) is approximately twice the ventricular rate (V).
The patient was hospitalized, and treatment was initiated. Oral dexamethasone was prescribed for mother at a dose of 4 mg daily, with close monitoring of blood glucose and blood pressure. Intravenous immunoglobulin (IVIG) was administered at a dose of 1 g/kg as a single dose. Maternal blood samples were sent for antibody testing (anti RO ssa antibody and anti‐LA ssb antibody).
Two days after initiating treatment, follow‐up echocardiography demonstrated restoration of normal sinus rhythm and atrioventricular conduction. In Figure 3, simultaneous spectral Doppler sampling of mitral inflow (“A” waves) and aortic outflow (“V” waves) now shows a 1:1 A:V relationship, indicating that each atrial contraction is followed promptly by a ventricular contraction. Quantitative assessment in Figure 4 confirms a mean fetal heart rate of 145–150 beats per minute, well within the expected normal range, reflecting complete reversal of the previously noted AV block. Maternal antibody testing remained negative.
FIGURE 3.

Post‐medical treatment Doppler evaluation of the mitral and aortic valves demonstrates normal sinus rhythm with 1:1 atrioventricular conduction.
FIGURE 4.

Fetal spectral Doppler analysis following treatment indicates resolution of bradycardia, with a mean fetal heart rate (FHR) of 145–150 beats per minute.
The patient was discharged and advised to return for follow‐up visits. During subsequent follow‐ups, the fetal heart rhythm remained normal. From approximately 28 weeks of gestation, the dose of oral dexamethasone was gradually tapered and eventually discontinued. The pregnancy progressed without complications, culminating in a normal vaginal delivery at 39 weeks.
Postnatal examination of the neonate, including electrocardiography and transthoracic echocardiography, showed no evidence of conduction abnormalities. The electrocardiogram obtained shortly after birth revealed a normal sinus rhythm with a normal PR interval. Currently, at 2 months of age, the infant is in good clinical condition, with appropriate growth and no evidence of rhythm abnormalities (Figure 5).
FIGURE 5.
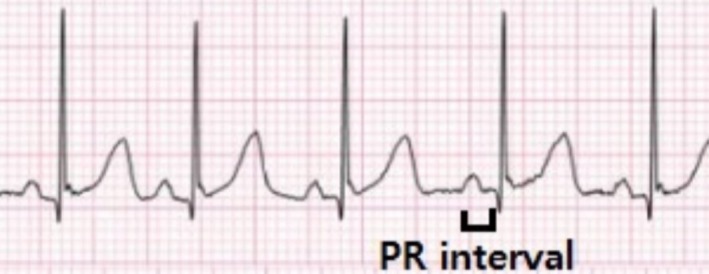
Lead II of the patient's electrocardiogram (ECG) shows normal sinus rhythm with a normal atrioventricular (PR) interval.
2.3. Case 2
A 36‐year‐old gravid 2, para 0, abort 0 pregnant woman, with one living child, presented at 27 weeks of gestation due to a decrease in fetal heart rate noted 1 week prior. She had no history of systemic disease. However, laboratory investigations revealed a positive anti‐Ro antibody.
2.4. Differential Diagnosis, Investigations and Treatment
Fetal echocardiography showed no structural abnormalities, but significant bradycardia was confirmed by Doppler spectral analysis (Figure 6). Simultaneous Doppler imaging of the fetal aorta and mitral valve revealed a clear pattern of atrioventricular dissociation, characterized by a consistent disparity between atrial and ventricular contractions. Specifically, the atrial rate was observed to be approximately twice the ventricular rate, indicating a 2:1 high‐grade atrioventricular block. The patient was admitted to the gynecology department, and treatment was initiated with oral dexamethasone at a dose of 4 mg daily. Several hours later, IVIG was administered at a dose of 1 g/kg single dose.
FIGURE 6.

Simultaneous Doppler spectral imaging of the fetal aorta and mitral valve reveals bradycardia with atrioventricular dissociation (block). The atrial rate is double the ventricular rate.
Within days of initiating treatment, a significant clinical response was observed, with the fetal heart rhythm normalizing to sinus rhythm at a normal rate (Figure 7). Subsequent follow‐ups confirmed that the fetal heart rate and rhythm remained normal. However, the patient did not return for long‐term follow‐up visits, and no additional information is available regarding her condition.
FIGURE 7.
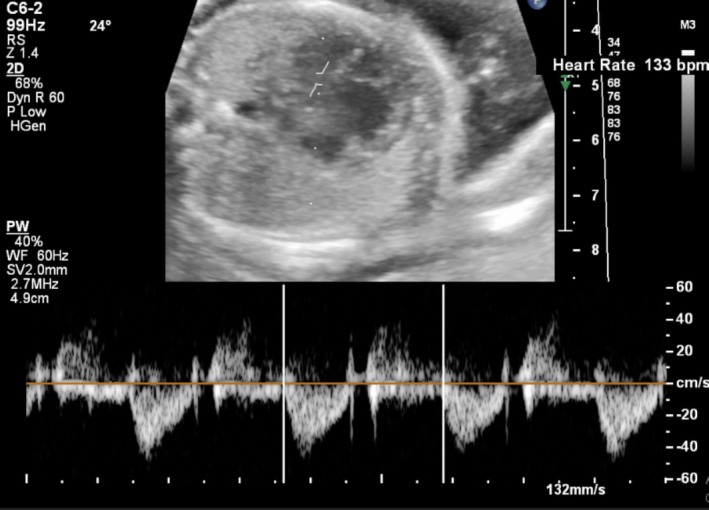
Post‐treatment Doppler spectral imaging of the left ventricular outflow tract demonstrates normalization of fetal heart rate and rhythm.
2.5. Case 3
A 28‐year‐old gravid 1, para 0, living child 0 pregnant woman with no history of systemic disease presented at 25 weeks of gestation, reporting reduced fetal movements. Ultrasound revealed fetal bradycardia, which was confirmed by fetal echocardiography performed at our center. Significant cardiomegaly, decreased cardiac contractility, and findings consistent with dilated cardiomyopathy were also noted.
A summary of the presented cases is provided in the Table S1.
2.6. Differential Diagnosis, Investigations and Treatment
The mother was admitted, and blood samples were obtained for antibody testing (anti‐Ro SSA and anti‐La SSB). Treatment with oral corticosteroids (dexamethasone at a dose of 6 mg/d/oral) was initiated, and IVIG was administered at a dose of 0.4 g/kg/d (5 days). Fetal condition was monitored daily via ultrasound during hospitalization.
Despite treatment, the fetal heart rate progressively declined. Approximately 1 week later, fetal ascites developed and continued to worsen. Tragically, the fetus passed away after 2 weeks.
Comprehensive functional assessment revealed significant cardiac compromise despite preserved structural anatomy. Spectral Doppler of the left ventricular outflow tract confirmed persistent fetal bradycardia, with a ventricular rate markedly below the normal 110–160 bpm range (Figure 8). Cross‐sectional thoracic imaging demonstrated cardiomegaly, evidenced by an increased cardiothoracic ratio exceeding established normative values (Figure 9). Color Doppler evaluation of the atrioventricular valves identified prominent regurgitant jets across both the tricuspid and mitral leaflets, indicating clinically significant valvular insufficiency (Figure 10). M‐mode interrogation of the ventricular walls revealed diminished left ventricular shortening fraction (LVSF) and reduced wall motion amplitude, consistent with impaired contractile function (Figure 11). Finally, spectral Doppler sampling of the main pulmonary artery corroborated severe bradycardia, showing prolonged intervals and underscoring the extent of conduction delay in utero.
FIGURE 8.

Doppler spectral analysis of the left ventricular outflow tract reveals fetal bradycardia.
FIGURE 9.

Axial view of the fetal thorax demonstrates cardiomegaly and an increased cardiothoracic ratio.
FIGURE 10.

Color Doppler imaging of the fetal atrioventricular valves reveals tricuspid regurgitation (TR) and/or mitral regurgitation (MR).
FIGURE 11.
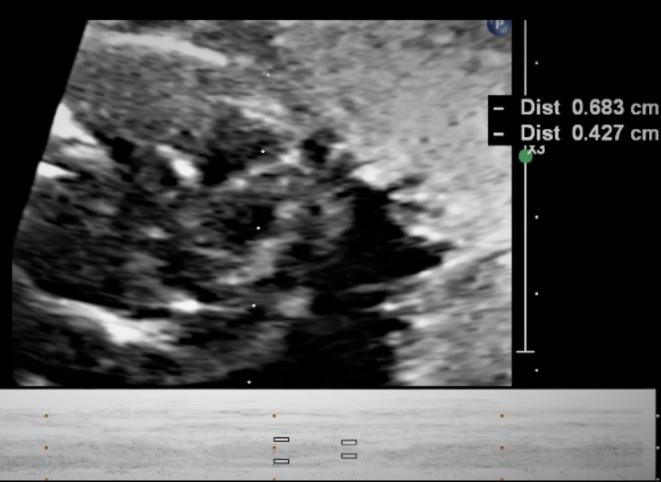
M‐mode imaging of both ventricles demonstrates left ventricular systolic dysfunction.
3. Literature Review
High‐grade atrioventricular (AV) block, a rare but serious condition in fetal medicine, is characterized by the failure of electrical signals to properly propagate between the atria and ventricles [3]. This disruption often leads to fetal bradycardia and, if untreated, can result in life‐threatening complications such as hydrops fetalis, heart failure, and intrauterine demise. Recent advancements in diagnostic modalities and therapeutic interventions have enhanced our ability to manage this condition, but significant challenges remain in understanding its pathogenesis and optimizing outcomes [7, 8].
3.1. Pathophysiology and Risk Factors
Fetal high‐grade AV block is most commonly associated with maternal autoimmune diseases, particularly systemic lupus erythematosus (SLE) and Sjögren's syndrome [9]. In these cases, maternal autoantibodies, specifically anti‐Ro/SSA and anti‐La/SSB antibodies, cross the placenta and cause immune‐mediated damage to the fetal cardiac conduction system. The exact mechanism involves the deposition of these antibodies in fetal cardiac tissues, leading to inflammation and fibrosis, particularly in the AV node [10]. Studies estimate that 1%–2% of fetuses exposed to these antibodies will develop CHB, with an increased recurrence risk of 15%–20% in subsequent pregnancies if a prior fetus was affected [11, 12].
3.2. Diagnostic Approaches
Timely diagnosis of fetal high‐grade AV block is crucial for effective management and improved outcomes. The gold standard for diagnosis is fetal echocardiography, supplemented by Doppler spectral analysis [13]. Key echocardiographic findings include bradycardia, AV dissociation, and a prolonged PR interval on M‐mode imaging. Doppler studies are particularly valuable in confirming atrial and ventricular dissociation by measuring atrial and ventricular contraction rates [14].
Advancements in three‐dimensional and speckle‐tracking echocardiography have further refined diagnostic capabilities, allowing for the detailed assessment of cardiac function and mechanics. These modalities are particularly useful in evaluating associated complications, such as cardiomyopathy or ventricular dysfunction, which can significantly impact prognosis [15].
3.3. Treatment Modalities
Management of fetal high‐grade AV block depends on the underlying etiology, degree of conduction impairment, and the presence of complications such as hydrops [3]. The cornerstone of treatment for autoimmune‐mediated AV block is maternal administration of corticosteroids, such as dexamethasone [16]. These medications aim to reduce inflammation and prevent further fibrosis of the conduction system. Evidence suggests that early initiation of corticosteroids may reverse first‐degree and some cases of second‐degree AV block, though their efficacy in reversing third‐degree block is limited [17].
Intravenous immunoglobulin (IVIG) has also been employed as an adjunct therapy, particularly in cases with significant maternal antibody titers [18]. The proposed mechanism involves neutralizing pathogenic antibodies and modulating the maternal immune response. However, clinical evidence regarding the efficacy of IVIG remains inconclusive, and its use is often case dependent [18, 19].
In cases of severe bradycardia or signs of fetal compromise, additional interventions may be necessary. Maternal administration of beta‐adrenergic agonists, such as terbutaline, can increase fetal ventricular rates and improve cardiac output. However, these agents carry potential maternal side effects, including tachycardia and hypotension, necessitating careful monitoring [20].
3.4. Prognosis and Outcomes
The prognosis of fetal high‐grade AV block varies widely depending on the degree of conduction impairment, timing of diagnosis, and the presence of associated complications. Fetuses with isolated second‐degree AV block, particularly those treated early, have a relatively favorable prognosis [3]. In contrast, third‐degree AV block is associated with significant morbidity and mortality, particularly when complicated by hydrops fetalis or cardiomyopathy [21].
Postnatal outcomes for neonates with high‐grade AV block are also variable. While some infants require pacemaker implantation shortly after birth, others may experience spontaneous resolution of conduction abnormalities [5]. Advances in neonatal care, including early pacemaker placement, have significantly improved survival rates and long‐term outcomes for affected neonates [22].
Conclusively, high‐grade AV block in the fetus presents unique diagnostic and therapeutic challenges. While advancements in fetal imaging and maternal immunotherapy have improved outcomes, significant gaps remain in our understanding of its pathogenesis and optimal management. This case series contributes to the existing literature by illustrating the clinical course, treatment strategies, and outcomes of three distinct cases. Continued research and interdisciplinary collaboration are essential to further enhance our ability to diagnose, treat, and ultimately prevent this potentially devastating condition.
4. Discussion
High‐grade atrioventricular block (AVB) during pregnancy is a rare but significant condition that poses considerable risks for fetal health [23]. This case series and literature review provide insights into the diagnosis, management, and outcomes of such cases, emphasizing the importance of early intervention and evolving treatment strategies. Each case highlights distinct clinical presentations and responses to various therapeutic approaches, underscoring the complexity of managing high‐grade AVB in utero.
4.1. Diagnostic Approaches
Intrauterine diagnosis of high‐grade AVB relies primarily on advanced imaging techniques, such as fetal echocardiography and Doppler spectral analysis. These modalities enable the detection of bradycardia, atrioventricular dissociation, and associated structural abnormalities. Timely diagnosis is critical for planning appropriate interventions, as early identification can significantly influence outcomes [24, 25].
4.2. Treatment Strategies
The management of high‐grade AVB during pregnancy focuses on mitigating the effects of fetal bradycardia and improving cardiac function. Corticosteroids, specifically dexamethasone, are the cornerstone of treatment for autoimmune‐mediated cases, as evidenced by Case 1 and Case 2. These cases demonstrated successful normalization of fetal heart rhythm following dexamethasone and, in some cases, the adjunctive use of intravenous immunoglobulin (IVIG). However, Case 3 highlights the limitations of current treatments, where progressive fetal compromise despite aggressive intervention ultimately led to a tragic outcome.
4.3. Challenges and Considerations
One of the primary challenges in managing high‐grade AVB is the variability in response to treatment. Factors such as the underlying etiology (autoimmune vs. structural) and the severity of the conduction block influence the success of interventions [1]. For example, Case 1 and Case 2 illustrate favorable outcomes with early and responsive interventions, whereas Case 3 underscores the limitations when fetal compromise advances despite intensive care.
Another challenge is the long‐term prognosis for neonates affected by high‐grade AVB. While some infants require postnatal pacing, advances in neonatal care have improved survival rates and long‐term outcomes [16]. However, the risk of complications, such as hydrops fetalis and cardiomyopathy, persists, necessitating ongoing research and innovative treatment options [26].
4.4. Emerging Research and Future Directions
The literature review reveals ongoing research into the pathophysiology of high‐grade AVB, with a focus on maternal antibodies and genetic predispositions. Emerging techniques, such as fetal cardiac MRI and minimally invasive interventions like in utero pacing, show promise for enhancing the precision of diagnosis and treatment. These advancements could offer more tailored therapeutic options, potentially improving outcomes for future cases.
5. Conclusion
The management of fetal high‐grade AVB during presents multifaceted challenges, requiring a multidisciplinary approach to ensure optimal outcomes. This case series, combined with a review of current literature, highlights both the successes and limitations of current strategies in the context of fetal AVB. Continued research and technological advancements will be essential in refining diagnostic and therapeutic approaches, ultimately striving for better outcomes for both mothers and their affected fetuses.
Limitations
The final outcome of the second case remains unknown due to insufficient follow‐up information regarding the patient's condition.
Author Contributions
Alireza Golbabaei: conceptualization, data curation, investigation, methodology, supervision, visualization, writing – original draft, writing – review and editing. Elham Sadat Alavi Moghaddam: conceptualization, data curation, methodology, supervision. Mahsa Naemi: conceptualization, supervision. Hooman Mohammad Talebi: conceptualization, data curation, software, supervision, writing – original draft, writing – review and editing.
Consent
A written informed consent was obtained from the patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Table S1.
Acknowledgments
We extend our heartfelt gratitude to all who contributed to this study.
Funding: The authors received no specific funding for this work.
Data Availability Statement
Data are available via sending a request email to the corresponding author.
References
- 1. Ata F., “Atrioventricular Block in Patients With Hyperthyroidism: A Narrative Review,” Journal of International Medical Research 52, no. 1 (2024): 03000605231223040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bokhari S. F. H., Faizan Sattar S. M., Mehboob U., et al., “Advancements in Prenatal Diagnosis and Management of Hypoplastic Left Heart Syndrome: A Multidisciplinary Approach and Future Directions,” World Journal of Cardiology 17, no. 3 (2025): 103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter L. E. and Simpson J. M., “Atrioventricular Block During Fetal Life,” Journal of the Saudi Heart Association 27, no. 3 (2015): 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadegh Fakhari M., Dorreh F., Ahangar Davood M., and Ghandi Y., “Thyroid Function in Children With Cyanotic and Non‐Cyanotic Congenital Heart Disease,” Turkish Archives of Pediatrics 58, no. 6 (2023): 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baruteau A.‐E., Pass R. H., Thambo J.‐B., et al., “Congenital and Childhood Atrioventricular Blocks: Pathophysiology and Contemporary Management,” European Journal of Pediatrics 175, no. 9 (2016): 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barber N. and Freud L., “Advances in Fetal Cardiac Imaging and Intervention,” CJC Pediatric and Congenital Heart Disease 3, no. 1 (2024): 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donofrio M. T., Moon‐Grady A. J., Hornberger L. K., et al., “Diagnosis and Treatment of Fetal Cardiac Disease,” Circulation 129, no. 21 (2014): 2183–2242. [DOI] [PubMed] [Google Scholar]
- 8. Veduta A., Panaitescu A. M., Ciobanu A. M., et al., “Treatment of Fetal Arrhythmias,” Journal of Clinical Medicine 10, no. 11 (2021): 2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan S., Anvekar P., Lohana P., Sheeraz Alam M., and Ali S. R., “Fetal Congenital Heart Block Associated With Maternal Primary Systemic Lupus Erythematosus and Sjogren's Syndrome,” Cureus 13, no. 9 (2021): e18036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Popescu M. R., Dudu A., Jurcut C., Ciobanu A. M., Zagrean A. M., and Panaitescu A. M., “A Broader Perspective on Anti‐Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review,” Diagnostics 10, no. 7 (2020): 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou K. Y. and Hua Y. M., “Autoimmune‐Associated Congenital Heart Block: A New Insight in Fetal Life,” Chinese Medical Journal 130, no. 23 (2017): 2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ambrosi A. and Wahren‐Herlenius M., “Congenital Heart Block: Evidence for a Pathogenic Role of Maternal Autoantibodies,” Arthritis Research & Therapy 14, no. 2 (2012): 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pruetz J. D., Miller J. C., Loeb G. E., Silka M. J., Bar‐Cohen Y., and Chmait R. H., “Prenatal Diagnosis and Management of Congenital Complete Heart Block,” Birth Defects Research 111, no. 8 (2019): 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manyari D. E., Ko P., Gulamhusein S., Boughner D. R., Kostuk W. J., and Klein G. J., “A Simple Echocardiographic Method to Detect Atrioventricular Dissociation. A Useful Aid in the Differential Diagnosis of Regular Tachycardia With Wide QRS Complexes,” Chest 81, no. 1 (1982): 67–73. [DOI] [PubMed] [Google Scholar]
- 15. Muraru D., Niero A., Rodriguez‐Zanella H., Cherata D., and Badano L., “Three‐Dimensional Speckle‐Tracking Echocardiography: Benefits and Limitations of Integrating Myocardial Mechanics With Three‐Dimensional Imaging,” Cardiovascular Diagnosis and Therapy 8, no. 1 (2018): 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mawad W., Hornberger L., Cuneo B., et al., “Outcome of Antibody‐Mediated Fetal Heart Disease With Standardized Anti‐Inflammatory Transplacental Treatment,” Journal of the American Heart Association 11, no. 3 (2022): e023000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clowse M. E. B., Eudy A. M., Kiernan E., et al., “The Prevention, Screening and Treatment of Congenital Heart Block From Neonatal Lupus: A Survey of Provider Practices,” Rheumatology 57, no. suppl_5 (2018): v9–v17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsaleem M., “Intravenous Immune Globulin Uses in the Fetus and Neonate: A Review,” Antibodies 9, no. 4 (2020): 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X., Cao W., and Li T., “High‐Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects,” Frontiers in Immunology 11 (2020): 1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner J. M., Morton M. J., Johnson K. A., O'Grady J. P., and Speroff L., “Terbutaline and Maternal Cardiac Function,” JAMA 246, no. 23 (1981): 2697–2701. [PubMed] [Google Scholar]
- 21. Lopes L. M., Tavares G. M. P., Damiano A. P., et al., “Perinatal Outcome of Fetal Atrioventricular Block,” Circulation 118, no. 12 (2008): 1268–1275. [DOI] [PubMed] [Google Scholar]
- 22. Zhao J., Huang Y., Lei L., et al., “Permanent Epicardial Pacing in Neonates and Infants Less Than 1 Year Old: 12‐Year Experience at a Single Center,” Translational Pediatrics 11, no. 6 (2022): 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang K., Xin J., Huang G., Wang X., and Yu H., “Pregnancy Maternal Fetal Outcomes Among Pregnancies Complicated With Atrioventricular Block,” BMC Pregnancy and Childbirth 22, no. 1 (2022): 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bravo‐Valenzuela N. J., Rocha L. A., Machado Nardozza L. M., and Araujo Júnior E., “Fetal Cardiac Arrhythmias: Current Evidence,” Annals of Pediatric Cardiology 11, no. 2 (2018): 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeno Y., Kiyomatsu Y., Rikitake N., et al., “Fetal Arrhythmias: Intrauterine Diagnosis, Treatment and Prognosis,” Acta Paediatrica Japonica: Overseas Edition 37, no. 4 (1995): 431–436. [DOI] [PubMed] [Google Scholar]
- 26. Strasburger J. F., Eckstein G., Butler M., Noffke P., and Wacker‐Gussmann A., “Fetal Arrhythmia Diagnosis and Pharmacologic Management,” Journal of Clinical Pharmacology 62, no. Suppl 1 (2022): S53–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
Data are available via sending a request email to the corresponding author.


