Abstract
Background
Inhalant insect allergens are widely implicated as common triggers of respiratory allergies, but little is known about cross-reactivity between cockroaches and other insect allergens. This study mainly investigates sensitization profiles and cross-reactivity between cockroach allergens and moth allergens to provide insights into the clinical management of cockroach-allergic patients.
Methods
A total of 386 suspected cockroach-sensitized patients were enrolled. Sensitization rates were determined using IgE testing for common airborne allergens. Cross-reactivity was evaluated using IgE inhibition assays, immunoblotting, and basophil activation tests. Age-related sensitization patterns were analyzed to assess variability in immune response across different demographics.
Results
A high frequency of silk moth was observed in cockroach-sensitized patients, with sensitization rates to German cockroach and silk moth allergens at 81.09% and 81.61%, respectively, and a high co-sensitization rate (54.66%). Age analysis revealed similar peak sensitization of German cockroach and silk moth in school-age children and young adults, with a lower prevalence in the elderly. Additionally, cockroach crude extract can inhibit the IgE-binding of cockroach-sensitized patients' sera and silk moth crude extract, while various moths can activate basophils as well.
Conclusions
This study highlights the similar age-related sensitization patterns between German cockroach and silk moth, differing from those observed with house dust mites. Besides, cross-reactivity between different cockroach and moth allergens was confirmed through significant IgE inhibition, multiple sequences alignment of shared epitopes and basophil activation tests. These findings highlight the need to consider cross-reactivity between cockroach and moth allergens in clinical evaluations.
Keywords: Cockroach allergy, Silk moth, Cross-reactivity, Insect allergen, Blattella germanica (German cockroach)
Introduction
Allergic diseases are among the top health concerns of the 21st century, as identified by the World Health Organization (WHO). These conditions affect 10%–40% of the global population, and more than 4 billion people will suffer from allergic disorders by 2050, according to the World Allergy Organization (WAO).1 Cockroaches, a significant airborne allergen in China, are responsible for triggering year-round allergic reactions, with sensitization rates between 6.0% and 30.0% among affected individuals.2 Various species of cockroaches are common in different regions, particularly in southern and eastern coastal areas, due to favorable climate conditions.3
The cockroaches that pose potential concerns for human health are primarily domestic species. These insects fall under the class Insecta, the subclass Pterygota (winged insects), and the order Blattodea, encompassing the superfamilies Blaberoidea and Blattoidea.4 Studies indicate that cross-reactivity between different cockroach species, such as the Periplaneta americana (American cockroach), Blattella germanica (German cockroach), Blattella asahinai (Asian cockroach), and Blatta orientalis (Oriental cockroach).5 Additionally, the presence of “pan-allergens” leads to cross-reactivity between cockroaches and other arthropods, including crustaceans (such as shrimp, crabs, and lobsters), insects (such as silkworms and butterflies), arachnids (such as mites), and mollusks (such as oysters, scallops, and clams).6, 7, 8, 9 Most existing studies on cross-reactivity have concentrated on cockroaches and crustaceans or arachnids, while limited attention has been given to cross-reactivity between cockroaches and other insects, such as silk moths. Silk moths are important sources of inhalant allergens, both indoors and outdoors. Their wings are coated with scales that can easily detach and become airborne. When inhaled by sensitive individuals, these scales may trigger respiratory allergy symptoms.10 Besides, silk moths remain an important economic product in East and Southeast Asia, including rural areas in China and India.11, 12, 13 Patients with allergic respiratory diseases may experience symptoms when exposed to allergens in their environment or workplace. Silkworm pupae, a primary by-product, are especially popular in the diets of southern China, where they have become part of a distinct culinary culture. Also, silkworm pupae are also known to cause food allergies, which can lead to severe allergic reactions.14, 15, 16, 17 Therefore, clinicians must differentiate between co-sensitization and cross-sensitization when both cockroaches and other animal allergens test positive skin prick tests (SPT) and/or serum-specific IgE tests. Determining whether a patient reacts to multiple allergens or cross-reactivity is at play due to shared pan-allergens can significantly impact treatment decisions.
This study aims to explore the sensitization profiles of cockroach-allergic individuals and their cross-reactivity with other insect allergens, thereby offering new insights into the role of insect allergens in allergic diseases. These findings provide valuable information for the early diagnosis and clinical management of allergic diseases and lay the groundwork for more targeted immunotherapy strategies in the future.
Methods and materials
Patients and study design
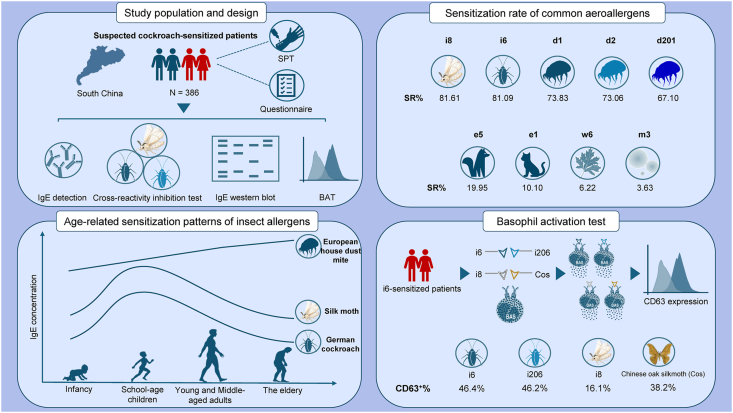
This study included 386 allergic rhinitis and/or asthma patients suspected of being sensitized to cockroaches, all recruited from the Allergy Clinic at the First Affiliated Hospital of Guangzhou Medical University between January 2022 and December 2023. All patients underwent a SPT by German cockroach (I701, 1000 μg/mL, Inmunotek) and filling in a self-assessment questionnaire (Supplementary File S1). The study design was presented in Fig. 1. All participants provided informed consent, and the study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University.
Fig. 1.
Graphical abstract.
The study identifies significant cross-reactivity between cockroach and moth allergens in cockroach-allergic patients, demonstrating shared sensitization patterns, age-related differences, and strong IgE inhibition. These findings emphasize the importance of addressing co-exposure to insect allergens in clinical management and suggest targeted strategies for improved allergy diagnosis and treatment. Abbreviation: d1 (Dermatophagoides pteronyssinus), d2 (Dermatophagoides farinae), d201 (Blomia tropicalis), e1 (Felis domesticus), e5 (Canis familiaris), m3 (Aspergillus fumigatus), w6 (Artemisia vulgaris), i6 (Blattella germanica), i8 (Bombyx mori) and i206 (Periplaneta americana)
Sera-specific IgE (sIgE) detection
We evaluated sensitization to 9 common airborne allergens prevalent in southern China among all cockroach-sensitized participants: Dermatophagoides pteronyssinus (D. pteronyssinus, d1), Dermatophagoides farinae (D. farinae, d2), Blomia tropicalis (B. tropicalis, d201), Felis domesticus (F. domesticus, e1), Canis familiaris (C. familiaris, e5), Aspergillus fumigatus (A. fumigatus, m3), Artemisia vulgaris (A. vulgaris, w6), Blattella germanica (B. germanica, i6) and Bombyx mori (B. mori, i8). The concentration of sIgE was detected by using the ImunoCAP (Thermo Fisher Scientific, USA) system. Based on RAST classification, levels of sIgE were quantitatively categorized into 6 levels: Level 0, <0.35 kU/L; Level 1, 0.35–0.70 kU/L; Level 2: 0.70–3.50 kU/L; Level 3: 3.50–17.50 kU/L; Level 4: 17.50–50.00 kU/L; Level 5: 50.00–100.00 kU/L and Level 6: ≥100.00 kU/L.18
Preparation of allergen crude extract
After euthanizing by carbon dioxide gas, the frozen cockroach/silk moth/Chinese oak silkmoth (Antheraea pernyi)/termites (Macrotermes barneyi Light) (30 g) was crushed in liquid nitrogen to prepare the whole-body extract. The allergen crude extract was homogenized and extracted at 4 °C with slow, agitation in a phosphate-buffered saline (pH 7.2) containing 1/1000 volume of protease inhibitor overnight. After centrifugation at 4 °C at 10,000 g for 30 min, the extracts were filtered through a syringe filter (0.22 μm), followed by quantitative detection of protein concentration using Pierce™ BCA Protein Assay Kit. Store at −20 °C for future use.
Cross-reactivity inhibition test
Allergen crude extracts were purchased from GREER® Laboratories Inc and dissolved with appropriate phosphate buffered saline (PBS), including German cockroach and American cockroach. While homemade silk moth was crushed and subsequently dissolved in PBS for crude extract preparation. First, we conducted silk-moth-sIgE detection in all cockroach-sensitized patients and 16 silk-moth-positive sera were selected randomly for further research. Next, silk-moth-postitive sera were mixed with the German cockroach or American cockroach (Periplaneta americana) crude extract in equal volume and preincubated at 37 °C for 1 h, followed by sIgE detection. Results were shown as the percentage of inhibition, calculated as follows. Inhibition rate (%) = (sIgE concentration before inhibition - sIgE concentration after inhibition)/sIgE concentration before inhibition × 100%.
Immunoblotting
20 μg of silk moth crude extract was loaded in each lane after diluted in loading buffer and boiled in 100 °C for 5 min. Allergen crude extract was separated by the 15% precast-SDS-polyacrylamide gel (Beyotime, China), and transferred to PVDF membranes (Millipore, USA). Membranes were blocked with 5% BSA and incubated overnight with mixed silk-moth-positive sera under different treatments, followed by HRP-conjugated secondary antibodies incubation at 37 °C for 1 h. Immunoblots were visualized with ECL (Beyotime, China). Each lane represents different inhibitors incubated with the mixed silk-moth-positive sera at 37 °C for 2 h in advance. Lane 1 represents the control condition without any inhibition. While the inhibitor in lane 2–5 was German cockroach, American cockroach, Chinese oak silkmoth and termite allergen crude extract, respectively.
Basophil activation test (BAT)
Fresh EDTA-anticoagulated blood (2 mL) was collected from 3 non-allergic healthy donors and 3 German-cockroach-sensitized patients (IgE-positive for Blattella germanica but IgE-negative for silk moths and other evaluated allergens, as shown in Supplementary Table S1). The BAT was performed using the Flow CAST® kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland) following the manufacturer's protocol. Anti-FcƐRI antibodies (PC1) and PBS serve as the positive and negative controls, respectively. Basophil activation was assessed by measuring the expression level of CD63 on at least 200 basophils, which were analyzed using a FACS Canto II flow cytometer (BD Biosciences, USA) and FlowJo V10 software (TreeStar). Four different insect crude extracts (German cockroach, American cockroach, silk moth and Chinese oak silkmoth) were diluted with PBS at a final concentration of 20 μg/μL as a potential allergen stimulant and incubated with 100 μL whole blood at 37 °C for 15 min respectively. Basophils were identified based on the following criteria: SSClow, CCR3+, and CD63+. The Stimulation Index (SI) was calculated as the ratio of allergen-activated basophils to spontaneously activated basophils (PBS).
Statistical analysis
Quantitative variables were described as mean and standard deviation (mean ± S.D.), while qualitative variables were expressed as frequency or percentage. Data was analyzed by SPSS v. 22 software (IBM Corp., USA). Charts were plotted by GraphPad Prism v. 7 (San Diego, USA) and R v. 4.2.1(Core Team 2022). A p-value <0.05 was regarded as statistically significant. Significance displayed in figures as follows: ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Results
Characteristics of study participants
This study included 386 patients suspected of being sensitized to cockroaches. The participants were recruited from the Allergy Clinic at the First Affiliated Hospital of Guangzhou Medical University between January 2022 and December 2023, with a median age of 32 years. Of these, 224 (58.00%) and 162 (42.00%) patients were male and female respectively. Detailed demographic and clinical characteristics were presented in Table 1.
Table 1.
Baseline characteristics of participants
| Characteristics | Total cohort |
|---|---|
| Patients, no. | 386 |
| Gender, no. (%) | |
| Male | 224 (58.00) |
| Female | 162 (42.00) |
| Age, (mean ± SD) | 32.00 (14.00, 48.00) |
| Allergic diseases, no. (%) | |
| Bronchial asthma | |
| Yes | 197 |
| No | 136 |
| Unknown | 53 |
| Allergic rhinitis | |
| Yes | 162 |
| No | 151 |
| Unknown | 73 |
| Total IgE (tIgE), no. (%) | |
| x ≤ 200 | 105 (27.20) |
| 200 < x ≤ 500 | 94 (24.35) |
| 500 < x ≤ 1000 | 59 (15.28) |
| >1000 | 72 (18.65) |
| Not available | 56 (14.51) |
| sIgE (mean ± SD) of German cockroach | 3.51 ± 6.94 |
| sIgE level of German cockroach, no. (%) | |
| Negative | 73 (18.91) |
| Levle 1 | 52 (13.47) |
| Levle 2 | 179 (46.37) |
| Levle 3 | 70 (18.13) |
| Levle 4 | 10 (2.59) |
| Levle 5 | 2 (0.52) |
| sIgE (mean ± SD) of silk moth | 5.88 ± 10.25 |
| sIgE level of silk moth, no. (%) | |
| Negative | 71 (18.39) |
| Levle 1 | 33 (8.55) |
| Levle 2 | 138 (35.75) |
| Levle 3 | 118 (30.57) |
| Levle 4 | 23 (5.96) |
| Levle 5 | 3 (0.78) |
Sensitization rates to common aeroallergens in cockroach-sensitized patients
Results showed that 81.09% (313/386) of the patients were sensitized to cockroaches, with an average cockroach-specific IgE level of 3.51 kU/L. The majority (46.37%, 179/386) had IgE concentrations at Level 2. Besides, allergen D. pteronyssinus exhibits the highest sensitization rate of 73.83%, followed by d2 (73.06%) and d201 (67.10%). Co-sensitization rates between German cockroach (B. germanica) and d1 (D. pteronyssinus), d2 (D. farinae), and d201 (B. tropicalis) were 91.69%, 92.67%, and 88.98%, respectively, consistent with the predominance of mites in mainland China. Sensitization rates for e1 (F. domesticus), e5 (C. familiaris), m3 (A. fumigatus), and w6 (A. vulgaris) were below 20%, with most IgE levels at Level 1 (Table 2).
Table 2.
Sensitization rates to common aeroallergens in cockroach-sensitized patients.
| Allergen | d1 | d2 | d201 | e1 | e5 | m3 | w6 | i6 | i8 |
|---|---|---|---|---|---|---|---|---|---|
| Negative | 101 | 104 | 127 | 347 | 309 | 372 | 362 | 73 | 71 |
| Level 1 | 39 | 24 | 45 | 16 | 26 | 9 | 17 | 52 | 33 |
| Level 2 | 45 | 64 | 67 | 12 | 22 | 3 | 5 | 179 | 138 |
| Level 3 | 66 | 54 | 54 | 4 | 14 | 1 | 2 | 70 | 118 |
| Level 4 | 30 | 39 | 48 | 3 | 7 | 1 | 0 | 10 | 23 |
| Level 5 | 42 | 39 | 27 | 3 | 6 | 0 | 0 | 2 | 3 |
| Level 6 | 63 | 62 | 18 | 1 | 2 | 0 | 0 | 0 | 0 |
| Sensitization rate (%) | 73.83 | 73.06 | 67.10 | 10.10 | 19.95 | 3.63 | 6.22 | 81.09 | 81.61 |
Abbreviation: d1 (Dermatophagoides pteronyssinus), d2 (Dermatophagoides farinae), d201 (Blomia tropicalis), e1 (Felis domesticus), e5 (Canis familiaris), m3 (Aspergillus fumigatus), w6 (Artemisia vulgaris), i6 (Blattella germanica), i8 (Bombyx mori)
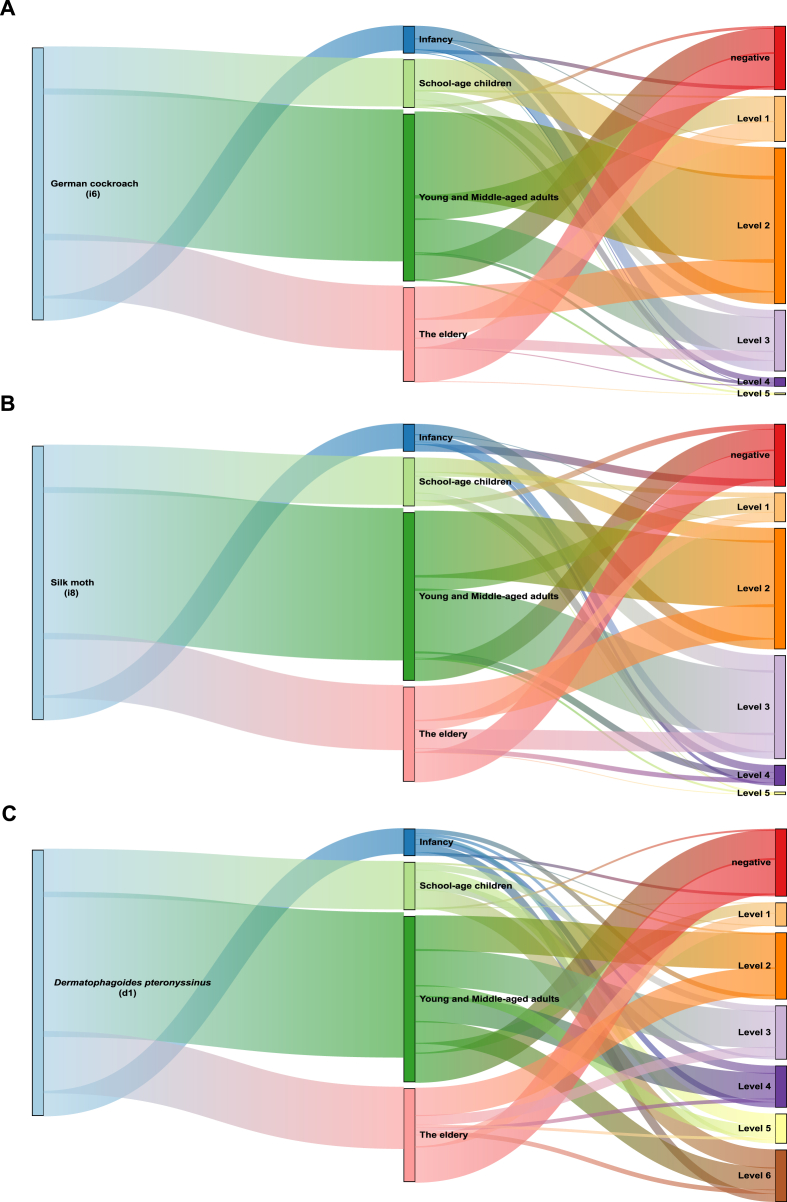
Analysis of age-related sensitization patterns of insect allergens
The Sankey diagram illustrates the sensitization differences among German cockroach/silk moth/D. pteronyssinus-sensitized patients across various age groups, showing the distribution across different IgE sensitization levels. Fig. 2A showed that German cockroach sensitization peaks in school-age children and young to middle-aged adults, particularly at “Level 2” and “Level 3”, while the elderly generally exhibit sensitization levels at “Level 1” and “Level 2”. The highest sensitization levels (“Level 4” and “Level 5”) were rarely observed in all age patients, indicating that this group experiences lower intense German cockroach sensitization. The average IgE levels for German cockroach remain relatively stable across the age groups, with values around 3.41–3.54 kU/L in the younger age groups and slightly lower (3.34 ± 6.80) in the 50+ age group. The highest sensitization rate (94.55%) was seen in the 6,14 age group, followed by aged 0–6 years old (87.10%), 14–50 age group (85.42%), and 50,∞ age group (64.81%). Sensitization to German cockroach was most prevalent in the 14,50 age group, with a total positive rate (PR%) of 42.49%.
Fig. 2.
Analysis of age-related sensitization patterns of insect allergens.
Sankey diagrams show the flow of sensitization and distribution from age-related trends to the IgE level of 3 insect allergens, including German cockroach (A), silk moth (B) and Dermatophagoides pteronyssinus (C). The width of the arrows corresponds to the proportion of sensitized patients, with thicker arrows indicating stronger associations
Remarkably, patients sensitized to German cockroach and/or silk moth displayed a similar age sensitization difference. Among silk-moth-sensitized patients, school-age children and young to middle-aged adults have the highest levels of sensitization, particularly concentrated in “Level 2” and “Level 3”. Sensitization levels were primarily at “Level 2” across all age groups, except for school-age children, who predominantly show “Level 3” sensitization. This suggested that the severity of silk moth sensitization peaks in school-age children and young to middle-aged adults, with a tendency for more intense reactions in these groups compared to infants and the elderly. This pattern may indicate a higher exposure or immune responsiveness to silk moth in these age ranges. However, at the same age/stage, serum IgE levels of silk moth in allergic patients were significantly higher than those of German cockroach. The highest mean IgE level for silk moth was seen in the 6,14 age group (5.93 ± 10.31 kU/L), with the lowest in the 50,∞ age group (5.61 ± 9.93 kU/L). The age group 6,14 has the highest sensitization rate for silk moth (89.09%), followed by 14,50 age group (87.50%), 0,6 age group (74.19%), and 50,∞ age group (69.44%). Similar to German cockroach, the 14,50 age group shows the highest prevalence with a total PR% of 43.52% (Fig. 2B).
Sensitization to D. pteronyssinus generally peaks in school-age children and young to middle-aged adults. Higher levels, such as “Level 5” to “Level 6”, appear more frequently in infancy and school-age children, whereas young and middle-aged adults and elderly individuals display lower sensitization levels, mostly “Level 2” or at “Level 3”. This pattern could reflect an age-related modulation of immune response to D. pteronyssinus allergens. IgE levels for D. pteronyssinus increase with age, from 18.28 ± 27.48 kU/L in the 0,6 age group to 29.80 ± 38.29 kU/L in the 50+ group. The 6,14 age group has the highest sensitization rate for D. pteronyssinus (96.36%), followed by 0,6 age group (90.32%), 14,50 age group (82.81%), and 50,∞ age group (62.96%). Like German cockroach and silk moth, D. pteronyssinus sensitization was most prevalent in the 14,50 age group, with a total PR% of 41.19% (Fig. 2C).
Across all 3 allergens (German cockroach, silk moth, D. pteronyssinus), the 6,14 age group generally exhibits the highest sensitization rates. The 14,50 group has the highest total PR% for all allergens, indicating the greatest overall prevalence of sensitization in this age range. IgE levels for D. pteronyssinus notably increase with age, while German cockroach and silk moth show more stability across different age groups. The 50+ age group generally shows lower sensitization rates across all allergens compared to younger age groups.
Co-sensitization and cross-reactivity between cockroaches and silk moths (Bombyx mori)
Previous studies indicated a correlation between IgE levels for cockroaches and silk moth's sensitization.19 To investigate this further, we assessed silk-moth-IgE in all cockroach-sensitized patients. Remarkably, allergens German cockroach and silk moth show a similar sensitization rate and average IgE level among these subjects, with most of the number in IgE concentration in level 2. The results showed that 81.61% (315/386) of participants were sensitized to silk moth, with an average IgE level of 5.88 ± 10.25 kU/L, compared to 3.51 ± 6.94 kU/L for German cockroach (Table 1). In addition, IgE levels for German cockroach and silk moth showed a 54.66% concordance, suggesting a high likelihood of co-sensitization or cross-reactivity (Supplementary Table S2). Spearman correlation analysis revealed a moderate positive correlation (R2 = 0.7168, p < 0.05) between German cockroach-specific IgE levels and silk moth-specific IgE levels, indicating a proportional increase in silk moth-specific IgE with rising German cockroach-specific IgE levels. Cross-reactivity inhibition tests with cockroach allergens (German cockroach and American cockroach) and silk moth revealed that preincubation with German cockroach and American cockroach reduced silk-moth-IgE levels by 20%–90%. Notably, American cockroach had a higher average inhibition rate (78.90%, Fig. 3B) compared to German cockroach (50.52%, Fig. 3A). Sequence alignment indicated that tropomyosin (Bla g 7, Per a 7, Bomb m 3) and arginine kinase (Bla g 9, Per a 9, Bomb m 1) showed high sequence conservation among these species, with 87.72% and 86.24% amino acid identity, respectively, supporting the hypothesis of conserved, cross-reactive IgE epitopes in cockroach and silk moth species (Supplementary File S2).
Fig. 3.
Evaluation of cross-reactivity between cockroaches and moths by sera cross-inhibition assay and immunoblotting.
The graph shows the percentage of IgE inhibition in the serum of cockroach-sensitized patients when exposed to German cockroach (A) and American cockroach (B) insect allergens. Higher inhibition rates indicate stronger cross-reactivity. (C) M: marker, Lane 1: without inhibitor (control lane), Lane 2: Preincubated with Chinese oak silkmoth crude extract, Lane 3: Preincubated with American cockroach crude extract, Lane 4: Preincubated with German cockroach crude extract, Lane 5: Preincubated with termite crude extract
Immunoblotting results demonstrated differences in IgE binding between silk moth crude extract and silk-moth-IgE-positive sera under various inhibitory conditions. In the control lane (Lane 1), without inhibition, prominent protein bands appeared at approximately 50 kDa and 100 kDa, indicating high levels of allergenic components in the silk moth extract. Sera preincubated with American cockroach or German cockroach showed almost complete absence of these bands, suggesting strong inhibition of IgE binding by cockroach allergens. In contrast, under the Chinese oak silkmoth inhibition condition, allergen protein bands are still visible, but there is a reduction in both the intensity and number of bands compared to Lane 1. Lane 5, using termites extract, showed several distinct bands above 50 kDa, implying that IgE binding to silk moth proteins was not entirely inhibited and may even be enhanced in some cases. Each inhibitory condition revealed unique IgE-allergen binding patterns, underscoring the varied affinities and cross-reactive effects of these allergens. This analysis laid a foundation for further exploration of their roles in allergic reactions (Fig. 3C).
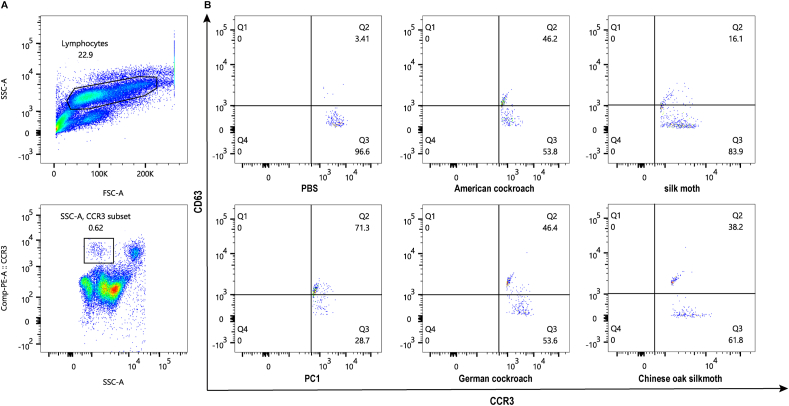
Cockroach-sensitized patients’ basophils can be stimulated by cockroaches and moths
To assess the clinical relevance and cross-reactivity of these insect allergens, basophils were collected from 3 cockroach-sensitized patients and exposed to German cockroach, American cockroach, silk moth and Chinese oak silkmoth allergens. Further characterization of the CCR3-positive subset is shown in the SSC-A versus CCR3 plot, with SSClow, CCR3+ cells representing the basophils (Fig. 4A). Upon stimulation, the proportion of cells expressing both CD63 and CCR3 (Q2 quadrant) varied significantly. Notably, stimulation with German cockroach or American cockroach led to an increase in double-positive basophils by around 46%, exhibiting similar basophil activation capacities. In contrast, distinct responses were evident across different moths. Silk moth and Chinese oak silkmoth treatments elicited substantial activation, with 16.1% and 38.2% of cells, respectively, falling in the Q2 quadrant, highlighting their potent stimulatory effects and distinct allergenicity on basophils activation (Fig. 4B). This finding further supported the hypothesis of cross-reactivity between cockroach and moth allergens, implying distinct insect allergen components potentially relevant to immune response modulation in allergic or inflammatory contexts.
Fig. 4.
Basophils activation test.
(A) Flow cytometry gating strategy for basophil subsets; (B) The x-axis represents CCR3, while the y-axis represents CD63. Activated basophils are in the Q2 quadrant. Anti-FcƐRI antibodies (PC1) and PBS serve as the positive and negative controls, respectively
Discussion
Cockroaches are common indoor allergens worldwide and are significant contributors to the onset of allergic diseases.20 Typically, sensitization occurs when patients inhale allergens released by cockroaches into the environment, leading to IgE production. Potential sources of cockroach allergens in the environment include the insect's body, exoskeletons (from molting), secretions (such as saliva), egg sacs, and feces. A multicenter epidemiological survey of allergens in China showed that the sensitization rate to cockroaches among patients with allergic symptoms was 24.5%, second only to dust mites, with significant regional variation. The highest sensitization rate was observed in the southwest region, while the lowest was in the northeast.21 In this study, which tested common inhalant allergens in 386 suspected cockroach-sensitized patients, we found that the sensitization rate to German cockroaches was as high as 81%, similar to that of silk moths. This finding may be related to the silkworm farming industry in southern China and the dietary habit of consuming silkworm pupae, consistent with the previous findings.10
Furthermore, although the study cohort consisted of suspected cockroach-sensitized patients, an interesting observation was that, among patients with similar IgE levels, the average IgE concentration of silk moth was generally higher than that in German cockroach. This may be related to structural characteristics of allergens, environmental exposure frequency, and the immune system's response to the allergens. First, exposure to silk moths is more frequent in southern regions where silkworm culture is prevalent, and silkworm allergens can sensitize individuals not only via inhalation but also through gastrointestinal ingestion, leading to higher IgE levels. Additionally, silkworm pupae are often processed by high-temperature frying or deep-frying, which could alter the structure of allergens or expose hidden epitopes. Furthermore, regional differences in the species of cockroaches, and variations in allergen structures between species could lead to differences in sensitization rates and IgE levels among patients from different regions. The main indoor cockroach species include Blattella germanica, Periplaneta americana, and Periplaneta fuliginosa.22 In China, German cockroach and American cockroach are the predominant species in the southern and northern regions, respectively. In this study, only the IgE levels for German cockroach were measured, and the IgE levels for American cockroach were not included.
Several “pan-allergens” from cockroaches have been identified as molecules with allergic activity and potential clinical relevance, such as tropomyosin (Bla g 7, Per a 7) and arginine kinase (Bla g 9, Per a 9).23 Therefore, clinical practice requires differentiation between cockroach allergens and those from other common arthropods (dust mites, shrimp, crabs), annelids (parasites), mollusks (shellfish), and insects (silkworm moths, butterflies, ants) to identify cross-reactivity.24,25 Given the high homology (approximately 80%) of tropomyosin among invertebrates, most studies have focused on identifying key allergenic components responsible for cross-reactivity between cockroaches and dust mites or shrimp,26 with fewer studies addressing cross-reactivity between cockroaches and other insects.
In this study, we observed a correlation between cockroach IgE levels and silk moth sensitization, with an IgE level concordance of 54.66%. Previous studies have primarily been cross-sectional, based on which we further explored cross-reactivity between cockroaches (German cockroach and American cockroach) and silk moths (silk moth and Chinese oak silkmoth). Apart from performing sequence alignment of the common allergen components, we also provided evidence from both humoral and cellular immunity perspectives. Inhibition tests showed that the average inhibition rate of American-cockroach-IgE was significantly higher than that of German cockroach, indirectly suggesting a higher likelihood of sensitization to American cockroach, which is consistent with the observation that, in patients with the same IgE level, silk moth exhibited higher IgE levels than German cockroach. We randomly selected fifty German-cockroach-IgE-positive patients to perform American-cockroach-IgE testing, and the results showed that 80% of patients were sensitized to American cockroach, indicating that co-sensitization between German cockroach and American cockroach is highly prevalent. Serum IgE inhibition tests and immunoblotting confirmed that the allergic reaction in silk-moth-IgE-positive patients was primarily induced by cross-reactivity between cockroaches and silk moths, and both German cockroach and American cockroach extracts could inhibit this reaction. Basophil activation tests showed that, in addition to American cockroach, both silk moth and Chinese oak silkmoth could activate basophils in German-cockroach-sensitized patients, further supporting cross-reactivity between cockroaches and silk moths. Cockroach-sensitized patients should be cautious of silk moths triggering allergic asthma and rhinitis in daily life, such as silk moth secretions, metabolites, scales, and body debris may induce such reactions. Notably, in this study, for the first time, we specifically evaluated the effect of various outdoor moth extracts (Pangrapta, Sinna extrema, Spilosoma, and Axyiia putris) on basophils from cockroach-sensitized patients. These moths were capable of activating basophils to varying degrees, upregulating CD63 expression.
Moreover, we explored the differences in insect allergen sensitization across age groups. These differences may be influenced by various factors, including immune system development, allergen exposure, immune tolerance, and genetic susceptibility.27,28 Infants and school-age children are more prone to develop IgE sensitization to allergens. During this stage, the immune system lacks a fully developed immune tolerance mechanism, making it more likely to produce exaggerated immune responses to environmental allergens, resulting in higher IgE levels.29 As individuals age, their immune systems mature and establish tolerance to common environmental allergens, which may make elderly individuals less likely to experience strong allergic reactions upon exposure to allergens. Infants and school-age children are more likely to be in indoor environments such as homes, schools, and communities, leading to more frequent exposure to cockroaches or other insects, thus increasing the likelihood of immune system reactions to these allergens. In addition, compared to adults, children have a faster breathing rate and proportionately increase the intake of air per kilogram of body weight, thus receiving a higher dose.30 Interestingly, D. pteronyssinus allergen (d1) shows distinct age-related sensitization differences when compared to German cockroach and silk moth. In contrast to cockroach and silkworm allergens, dust mite allergens are more complex and highly cross-reactive. According to the WHO/IUIS (International Union of Immunological Societies) database (http://www.allergen.org), 41 allergenic components of mites have been identified, far exceeding the number of allergens found in cockroaches or silk moths.31 Dust mite allergens are widely present in the environment, and long-term or frequent exposure can lead to sustained stimulation of IgE production, maintaining high levels of IgE in patients.
Several limitations of this study should be noted. First, the study participants were all from southern China, where exposure to cockroaches and silk moths is relatively common. Therefore, the study sample has regional limitations and may not represent the allergic status of cockroach-sensitized individuals in other regions or countries. Future studies should include patients from other geographic areas for comparison, particularly between southern and northern regions, as the species and sensitization rates of cockroach allergens differ. Second, due to limitations in testing reagents, we did not measure American-cockroach-IgE in the enrolled patients. Future studies could supplement this test to further clarify the cross-reactions between different species of cockroaches and between cockroaches and silk moths. Additionally, a small proportion of the study participants were selected based on self-reported allergic symptoms without performing SPT. Although specific IgE testing for cockroaches was conducted afterward, the inclusion of patients may have introduced bias. It is possible that individuals with allergic reactions caused by other factors (such as environmental factors or other allergens) were included, which could lead to an overestimation of cross-reactivity and co-sensitization. Lastly, the number of silk-moth-positive serum samples was limited, and increasing the sample size for inhibition tests could enhance the reliability of the results. Despite our findings suggesting cross-reactivity between cockroaches and silk moths and confirming this cross-reaction via IgE inhibition tests and immunoblotting, the specific molecular mechanisms of cross-reactivity remain unclear. Therefore, to verify true allergic sensitization or cross-reactivity, future studies should be conducted based on component-resolved diagnostics.
Conclusions
This study reveals similar age-related sensitization patterns between German cockroach and silk moth, contrasting with the distinct patterns observed for D. pteronyssinus. Cross-reactivity between cockroach and moth allergens is suggested by IgE inhibition, sequence homology, and basophil activation assays. These findings provide scientific evidence for the cross-reaction between cockroaches and silk moths and offer new perspectives for the clinical management of cockroach-sensitized patients.
Availability of data and materials
The data that supports the findings of this study are available on reasonable request from the corresponding author.
Ethics approval and consent to participate
The study was in accordance with the Declaration of Helsinki and approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (No. 20240020). All authors consented to participate in this work and approved the final version of the manuscript.
Fundings
This study was supported by the Clinical and Epidemiological Research Project of State Key Laboratory of Respiratory Disease (SKLRD-L-202505), Guangzhou Medical University (GMUCR2024-01009, GMUCR2025-02009), Plan on enhancing scientific research in Guangzhou Medical University (2025SRP017) and Guangdong Zhong Nanshan Medical Foundation (20240015).
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We express sincere gratitude to Professor Sheng Li from South China Normal University for his support in providing lab-raised cockroaches (Blattella germanica and Periplaneta american) in this study. We would like to thank the Biobank for Respiratory Disease at the National Clinical Research Center for Respiratory Disease (BRD-NCRCRD, Guangzhou, Southern China) for sample preservation.
Footnotes
Full list of author information is available at the end of the article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2025.101057.
Contributor Information
Wenting Luo, Email: 348307379@qq.com.
Baoqing Sun, Email: sunbaoqing@vip.163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Simons F.E., Ebisawa M., Sanchez-Borges M., et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar M., Gupta R.K., Kumar R., et al. Cockroach exposure and its allergy sensitization in asthma patients. Monaldi Arch Chest Dis. 2021;91(3) doi: 10.4081/monaldi.2021.1685. [DOI] [PubMed] [Google Scholar]
- 3.Pomes A., Mueller G.A., Randall T.A., et al. New insights into cockroach allergens. Curr Allergy Asthma Rep. 2017;17(4):25. doi: 10.1007/s11882-017-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han W., Qiu L., Zhang J., et al. Phylogenetic reconstruction of Corydioidea (Dictyoptera: Blattodea) provides new insights on the placement of Latindiinae and supports the proposal of the new subfamily Ctenoneurinae. Syst Entomol. 2023;49(1):156–172. doi: 10.1111/syen.12610. [DOI] [Google Scholar]
- 5.Helm R.M., Squillace D.L., Jones R.T., et al. Shared allergenic activity in Asian (Blattella asahinai), German (Blattella germanica), American (Periplaneta americana), and Oriental (Blatta orientalis) cockroach species. Int Arch Allergy Appl Immunol. 1990;92(2):154–161. doi: 10.1159/000235207. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J.L., Zheng X.H., Luo W.T., et al. [From exposure to control: the application of allergen component-resolved diagnosis in the clinical management of cockroach allergies. Zhonghua Yu Fang Yi Xue Za Zhi. 2024;58(7):1103–1112. doi: 10.3760/cma.j.cn112150-20240416-00312. [DOI] [PubMed] [Google Scholar]
- 7.Thivalapill N., Andy-Nweye A.B., Bilaver L.A., et al. Sensitization to house dust mite and cockroach may mediate the racial difference in shellfish allergy. Pediatr Allergy Immunol. 2022;33(8) doi: 10.1111/pai.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong L., Huang C.H., Lee B.W. Shellfish and house dust mite allergies: is the link tropomyosin? Allergy Asthma Immunol Res. 2016;8(2):101–106. doi: 10.4168/aair.2016.8.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugraha R., Kamath S.D., Johnston E., et al. Conservation analysis of B-Cell allergen epitopes to predict clinical cross-reactivity between shellfish and inhalant invertebrate allergens. Front Immunol. 2019;10:2676. doi: 10.3389/fimmu.2019.02676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araujo L.M.L., Souza C., Zanchin N.I.T., et al. Identification of the major allergenic proteins from silkworm moth (Bombyx mori) involved in respiratory allergic diseases. Allergol Immunopathol (Madr) 2020;48(6):597–602. doi: 10.1016/j.aller.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang S.H., You Z.Y., Ye L.P., et al. Quantitative proteomic and transcriptomic analyses of molecular mechanisms associated with low silk production in silkworm Bombyx mori. J Proteome Res. 2014;13(2):735–751. doi: 10.1021/pr4008333. [DOI] [PubMed] [Google Scholar]
- 12.Yang R., Zhao X., Kuang Z., et al. Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology. J Food Agric Environ. 2013;11(1):952–956. [Google Scholar]
- 13.Araujo L.M., Rosario Filho N.A., Riedi C.A. Respiratory allergy to moth: the importance of sensitization to Bombyx mori in children with asthma and rhinitis. J Pediatr. 2014;90(2):176–181. doi: 10.1016/j.jped.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Ji K.M., Zhan Z.K., Chen J.J., et al. Anaphylactic shock caused by silkworm pupa consumption in China. Allergy. 2008;63(10):1407–1408. doi: 10.1111/j.1398-9995.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X., Li L., Kuang Z., et al. Proteomic and immunological identification of two new allergens from silkworm (Bombyx mori L.) pupae. Cent Eur J Immunol. 2015;40(1):30–34. doi: 10.5114/ceji.2015.50830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y., Chen X.M., Zhao M., et al. Edible insects in China: utilization and prospects. Insect Sci. 2018;25(2):184–198. doi: 10.1111/1744-7917.12449. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y., Huang M., Xu Y., et al. Enzymatic hydrolysis of silkworm pupa and its allergenicity evaluation by animal model with different immunization routes. Food Sci Hum Wellness. 2023;12(3):774–782. doi: 10.1016/j.fshw.2022.09.011. [DOI] [Google Scholar]
- 18.Zhang J., Luo W., Li G., et al. Patterns of aeroallergen sensitization in asthma patients identified by latent class analysis: a cross-sectional study in China. Clin Transl Allergy. 2023;13(7) doi: 10.1002/clt2.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun B., Zheng P., Wei N., et al. Co-sensitization to silkworm moth (Bombyx mori) and 9 inhalant allergens among allergic patients in Guangzhou, Southern China. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0094776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomes A., Chapman M.D., Wunschmann S. Indoor allergens and allergic respiratory disease. Curr Allergy Asthma Rep. 2016;16(6):43. doi: 10.1007/s11882-016-0622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo W., Wang D., Zhang T., et al. Prevalence patterns of allergen sensitization by region, gender, age, and season among patients with allergic symptoms in mainland China: a four-year multicenter study. Allergy. 2021;76(2):589–593. doi: 10.1111/all.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q., Liu M., Liu T., et al. An evolutionary game study of cockroach control strategies in residential households. Sci Rep. 2023;13(1):7342. doi: 10.1038/s41598-023-33561-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder M., Mahler V., Hayek B., et al. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001;167(9):5470–5477. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 24.Martinez D., Fang L., Meza-Torres C., et al. Toward consensus epitopes B and T of tropomyosin involved in cross-reactivity across diverse allergens: an in silico study. Biomedicines. 2024;12(4) doi: 10.3390/biomedicines12040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao B., Chen A.H., Zhang Y.Y., et al. Complete mitochondrial genomes of two cockroaches, Blattella germanica and Periplaneta americana, and the phylogenetic position of termites. Curr Genet. 2012;58(2):65–77. doi: 10.1007/s00294-012-0365-7. [DOI] [PubMed] [Google Scholar]
- 26.Jeong K.Y., Hwang H., Lee J., et al. Allergenic characterization of tropomyosin from the dusky brown cockroach, Periplaneta fuliginosa. Clin Diagn Lab Immunol. 2004;11(4):680–685. doi: 10.1128/CDLI.11.4.680-685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pointner L., Bethanis A., Thaler M., et al. Initiating pollen sensitization - complex source, complex mechanisms. Clin Transl Allergy. 2020;10:36. doi: 10.1186/s13601-020-00341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamad Zainal N.H., Mohd Nor N.H., Saat A., et al. Childhood allergy susceptibility: the role of the immune system development in the in-utero period. Hum Immunol. 2022;83(5):437–446. doi: 10.1016/j.humimm.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Dharma C., Lefebvre D.L., Tran M.M., et al. Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy. 2018;48(1):48–59. doi: 10.1111/cea.13063. [DOI] [PubMed] [Google Scholar]
- 30.Agency UEP . 2011. Exposure Factors Handbook: 2011 Edition (Final Report)https://www.epa.gov/expobox/about-exposure-factors-handbook [EB/OL]. [Google Scholar]
- 31.Pomes A., Davies J.M., Gadermaier G., et al. WHO/IUIS allergen nomenclature: providing a common language. Mol Immunol. 2018;100:3–13. doi: 10.1016/j.molimm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available on reasonable request from the corresponding author.