Abstract
Study Design
Systematic review and meta-analysis.
Objective
A systematic review and meta-analysis of comparative studies was performed to compare the fusion rates, functional outcomes, and complications between Titanium-Coated Polyetheretherketone (TiPEEK) and polyetheretherketone (PEEK) cages.
Methods
Four databases were systematically searched according to PRISMA. Adult patients who underwent one- or two-level lumbar fusion with TiPEEK or PEEK cages were included in the study. Studies that reported radiographic fusion and functional or complication outcomes were also included. Study quality was assessed using the Cochrane Risk of Bias tool and MINORS criteria. The meta-analysis was performed using Review Manager 5.4. Heterogeneity was assessed using I2, and random effects were used to analyze the heterogeneity.
Results
8 studies (n = 670) were analyzed. TiPEEK showed a significantly higher overall fusion rate (OR 1.83, 95% CI: 1.18-2.83). TiPEEK cages presented significantly higher fusion rates at 6 months (OR 2.52, 95% CI: 1.11 to 5.72), but there were no significant differences at 12 months (OR 1.33, 95% CI: 0.65 to 2.73). No differences were observed in the global ODI (SMD -0.04, 95% CI: −0.15-0.06). There were no significant differences regarding overall subsidence (OR 0.72, 95% CI: 0.48 to 1.07), screw complications (OR 1.25, 95% CI: 0.30-5.27) or reoperations (OR 0.61, 95% CI: 0.11-3.37).
Conclusions
The results from this study suggest that TiPEEK cages may demonstrate earlier fusion as compared to PEEK cages, particularly at 6 months. However, the functional outcomes and safety profiles were comparable.
Keywords: spinal fusion, titanium, coated, polyetheretherketone, PEEK, meta-analysis
Introduction
Spinal arthrodesis is the current mainstay in the treatment of degenerative conditions of the spine. Successful bony fusion is generally correlated with improved clinical outcomes and reduced implant-related complications.1,2 Whilst spinal instrumentation, including interbody and posterior implants, provide mechanical stability in the immediate post-operative phase, the attainment of a solid bony fusion plays a significant role in limiting motion and load sharing. 3 The choice of implant material is an important consideration in spinal fusion surgeries. Titanium and polyetheretherketone (PEEK) are the 2 principal options that each have advantages and limitations. Titanium is traditionally favored because of its proven osteointegrative properties and corrosion resistance. 4 However, its bulk modulus mismatch with bone can lead to stress shielding effects, such as bone resorption around the implant interface. 4 The high radiodensity of titanium also impedes a clear imaging-based assessment of fusion status postoperatively. 4 To address these shortcomings, PEEK emerged as an alternative cage material during the 1990s. When reinforced with carbon fibers, PEEK’s mechanical properties more closely mimic those of bone. 4 This allows for improved load transfer and reduced stress shielding potential compared to other more rigid materials. As a radiolucent polymer, PEEK also permits uncomplicated radiographic fusion monitoring. 4 Although osteointegration may be poorer for unmodified PEEK than for titanium due to differences in surface chemistry, modification techniques show promise in enhancing osteoblast response at the implant surface. 5 Continued research on advancing PEEK material properties aims to better approximate native bone. 2 Recently, titanium-coated PEEK cages have combined the features of both materials.6,7 Titanium coating imparts mechanical integrity resembling bone mechanics while maintaining PEEK’s radiolucency. The improved biocompatibility of titanium is also hypothesized to augment osteointegration compared with uncoated PEEK. 4 However, studies have drawn attention to the potential fracture and loss of coatings due to damage and wear during impaction insertion which yield particles in the size range to allow phagocytosis.8,9
Some studies have reported higher fusion rates with Ti-coated PEEK (TiPEEK) cages. Hasegawa et al and Singhatanadgige et al observed superior fusion with TiPEEK vs uncoated PEEK at the 6-month follow up.6,7 Similarly, Willems et al. found that nanocoated titanium PEEK yielded positive results compared to uncoated PEEK, with fusion superiority evident at 6 months postoperatively. 10 However, Rickert et al and Schnake et al detected no intergroup differences at later timepoints points (12-24 months).11,12 A previous meta-analysis concluded that there were no differences in the fusion rates between TiPEEK and PEEK. 13 However, that meta-analysis has some limitations. Specifically, it only included 4 studies and did not account for all available follow-up time points in published reports. Patient numbers were drawn from the initial cohorts rather than the final sample sizes remaining at the study endpoints. Insufficient data were provided on factors such as surgical approach, fusion levels, and graft material used, all of which could influence outcomes. 13
The aim of this systematic review and meta-analysis was to provide an updated comparative analysis of titanium-coated PEEK (TiPEEK) and uncoated PEEK interbody cages. Specifically, we sought to compare fusion rates, functional outcomes, and complications between the 2 materials based on all available randomized and observational evidence.
Methods
Eligibility Criteria
This systematic review and meta-analysis was registered with PROSPERO (CRD42024516982) and conducted according to PRISMA guidelines. 14 An inclusive PICOS framework was employed in which adult patients undergoing one- or two-level lumbar interbody fusion comprised the population (P). The intervention group (I) was treated with titanium-coated PEEK cages, referred to as the “experimental” group or TiPEEK group. The comparison was made against a control group (C) using uncoated PEEK interbody cages, designated as the PEEK group. The primary outcomes (O) of interest were the radiographic fusion rate and patient-reported functional status/quality of life. Only comparative studies (S), specifically randomized controlled trials or comparative cohort studies, were eligible for inclusion to directly assess the outcomes between the experimental and control groups. Studies were excluded if they involved pediatric populations, as bone healing differs from that in adults, duplicated data, case reports/commentaries lacking comparison, or studies that could not be appropriately pooled for meta-analysis owing to varying follow-up intervals or incomplete outcome reporting preventing meaningful data synthesis.
Information Sources
A systematic search of the literature was conducted using multiple databases including PubMed, EMBASE, SCOPUS, and the Cochrane Library. The reference lists of the included studies were manually searched to identify any additional references. No restrictions were imposed on publication date or language. Database searches were supplemented by hand-searching and contacting study authors to obtain missing outcome data.
Search Methods for Identification of Studies
A sensitive search strategy was developed to identify all relevant comparative studies. Medical subject headings, title/abstract keywords, and free-text terms related to PEEK interbody devices, titanium coatings, and spinal fusion outcomes were combined. Preliminary searches tested variations in word order and spelling to maximize the capture of eligible research. The final search string combined synonyms and closely related terms to provide a comprehensive coverage (Supplemental File 1). Two independent reviewers screened all titles and abstracts based on an exhaustive literature search according to pre-specified eligibility criteria. Any discrepancies in the initial selection were resolved through a discussion with a third reviewer. The full texts of potentially relevant reports underwent dual independent assessments for final inclusion.
Data Extraction and Data Items
Data extraction was conducted independently in duplicate to minimize bias or error. Discrepancies were addressed through consensus discussion with the involvement of a third reviewer when needed. The following baseline characteristics were extracted from each included study: study, region, period, follow-up, study design, number of patients, mean age, number of females, conflict of interest, and funding. Variables regarding surgery were also collected, including the surgical procedure, bone graft, type of cage, and etiologies. The primary outcomes extracted were fusion rate and patient-reported outcome measures (PROMs). The PROMs that could be compared were the Oswestry Disability Index (ODI), visual analog scale (VAS) for low back pain (BP) and leg pain (LP), and EQ-5D. In addition, complications compared by at least 2 studies, such as cage subsidence, screw loosening/malposition/break, and revisions, were collected.
Assessment of Risk of Bias in Included Studies
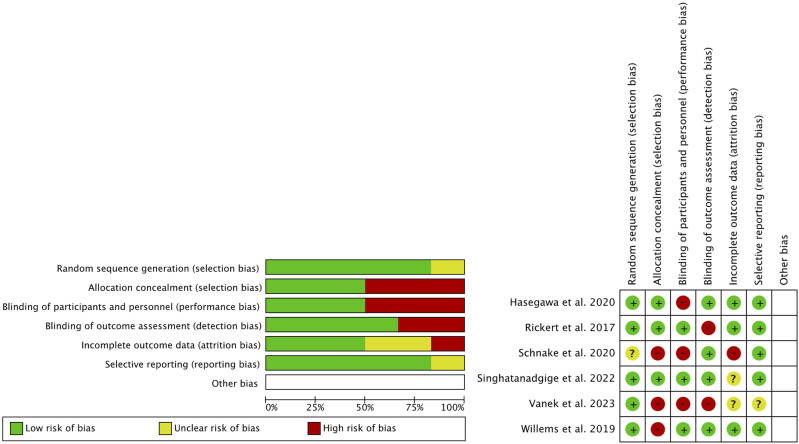
The methodological quality and risk of bias of the included randomized controlled trials were independently evaluated by 2 reviewers using the Cochrane Collaboration’s risk of bias tool, as implemented in the Review Manager software (Version 5.4, The Cochrane Collaboration, 2020). This tool systematically assesses 6 key domains related to bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. For each domain across all included trials, explicit prespecified criteria were used to assign ratings of low, high, or unclear risk of bias. These evaluations, along with the supporting rationale, are presented in Figure 1 and Supplemental File 2 to ensure transparent reporting and to allow critical appraisal. Within the forest plots, the risk of bias grading for each trial was also noted for the relevant outcomes to facilitate the interpretation of the results.
Figure 1.
Risk of bias (green = low risk; red = high risk; yellow = unknown).
The methodological quality of the observational studies was independently evaluated by 2 reviewers using the Methodological Index for Non-Randomized Studies (MINORS) criteria (Supplemental Table 1). This instrument contains items to evaluate key methodological aspects such as study design, patient selection, outcome measures, and follow-up. Scoring ranges from 0-16 for non-comparative studies, and 0-24 for comparative designs. For non-comparative studies, those scoring 0-4 were considered very low quality, 5-7 as low quality, 8-12 as fair quality, and 13-16 as high quality. Comparative studies scoring 0-6 were deemed very low-quality, 7-10 as low quality, 11-15 as fair quality, and 16-24 as high quality. Any discrepancies in the quality assessment scoring between the 2 reviewers were discussed to reach a consensus. 15
Assessment of Results
Meta-analyses were conducted using Review Manager. For dichotomous outcomes, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to evaluate the group differences. Continuous measures were analyzed using the mean differences (MDs), standardized mean differences (SMDs), and 95% CIs. Heterogeneity across studies was formally tested using both the chi-squared statistic and the I2 index, which describes the percentage of variability beyond chance. I2 values of 25%, 50%, and 75% represented low, moderate, and high degrees of inconsistency, respectively. A fixed-effects model was adopted if the heterogeneity was negligible, whereas a random-effects model was applied for more prominent between-study variability (I2 ≥ 50%). WebPlotDigitizer (Version 4.5, Free Software Foundation, Inx., Boston, MA, USA) was utilized to digitize the figures for numerical abstraction when necessary. Guidelines from the Cochrane Handbook were followed to address missing or incomplete outcome reporting. 16
Risk of Bias Across the Studies
The possibility of a publication bias was formally evaluated. A scatter plot of each study’s effect size against its precision, was generated to visually assess asymmetry, which could arise from failure to publish small negative studies.
Additional Analyses
Pre-specified subgroup meta-analyses according to follow-up duration were conducted to further explore potential heterogeneity.
Leave-one-out sensitivity analyses also evaluated the impact of each study by iteratively removing the highest-weighted trial and recalculating the pooled estimates to assess potential outliers. Additional sensitivity analyses controlled for variables, such as study design, conflicts of interest, funding source, surgical procedure details, evaluator influence, and outcome definitions.
The certainty of evidence for the primary outcomes was systematically evaluated using GRADE, 17 assessing the risk of bias, consistency, directness, precision, and potential publication bias. Limitations in study design and conduct, inconsistent results, indirectness of evidence, imprecision from wide confidence intervals or small sample sizes, and suspicion of publication bias could all downgrade certainty.
Results
Study Selection
An electronic database search retrieved 266 articles. Duplicates, case reports, letters, reviews, and non-comparative study designs (N = 61) were first removed, leaving 205 titles and abstracts to screen. Two independent reviewers then evaluated the eligibility, excluding 193 publications that did not directly compare titanium-coated vs uncoated PEEK interbody cages, involved other material comparisons, or lacked PEEK. This resulted in the assessment of 12 full-text articles for inclusion. Upon in-depth review, 7 studies were excluded for not meeting the eligibility criteria for comparing titanium-coated vs uncoated PEEK cages. The screening process identified 5 studies for inclusion. The reference lists were also manually searched, yielding an additional 3 studies that satisfied the criteria. Any disagreements between reviewers at any stage were resolved through discussion. In total, 8 comparative studies deemed appropriate for the review question were included in the meta-analysis, as summarized in the PRISMA flow diagram (Figure 2).6,7,10-12,18-20
Figure 2.
Study selection flow diagram (preferred reporting items for systematic reviews and meta-analyses).
Study Characteristics
Table 1 presents the main characteristics of the studies included. Eight studies were included, with a total of 670 patients (304 in the experimental group and 366 in the control group). The mean age in the experimental group ranged from 50.0 to 67.7 years, while in the control group, it ranged from 51.5 to 68.6 years. Six studies were RCTs, while the other 2 were retrospective cohort studies. The follow-up period ranged from 6 to 24 months. Among the studies that reported sex, 65.3% were women in the experimental group and 54.4% were women in the control group. Regarding surgery, 3 studies were PLIF, 4 were TLIF (2 were MIS), and one study included both PLIF and TLIF. Six studies were at a single level and 2 studies had one or 2 levels. The bone graft, cage type, and etiologies are shown in Table 2. The definitions of fusion and observer experience are presented in Supplemental Table 2.
Table 1.
Baseline Data of the Eight Included Studies.
| Study | Region | Period | Follow-Up (Months) | Study Design | n (TiPEEK/PEEK) | Age (TiPEEK/PEEK) | Female (TiPEEK/PEEK) | COI | Funding |
|---|---|---|---|---|---|---|---|---|---|
| Hasegawa et al. (2020) 6 | Japan | NS | 12 | RCT | 69/80 | 67.4/67.0 | 31/34 | NS | Yes |
| Rickert et al. (2017) 11 | Germany | 2012 | 12 | RCT | 20/20 | 67.7/68.3 | 14/14 | NS | Yes |
| Sakaura et al. (2018) 18 | Japan | Before 2015 | 6 | Retrospective cohort | 36/92 | NS | NS | No | NS |
| Schnake et al. (2020) 12 | Germany | NS | 24 | RCT | 27/28 | 50.6/52.9 | 21/15 | Yes | Yes |
| Singhatanadgige et al. (2022) 7 | Thailand | 2018 to 2020 | 12 | RCT | 41/41 | 62.7/64.1 | 28/26 | No | No |
| Vanek et al. (2023) 19 | Czech Republic | 2017 to 2021 | 24 | RCT | 40/41 | NS | 33/23 | Yes | Yes |
| Willems et al. (2019) 10 | Belgium | 2013 to 2014 | 12 | RCT | 44/37 | 50.0/51.5 | 27/16 | NS | Yes |
| Yao et al. (2023) 20 | Taiwan | 2015 to 2017 | 24 | Retrospective cohort | 27/27 | 67.9/68.6 | 21/21 | No | No |
NS, not specified; RCT, randomized clinical trial.
Table 2.
Surgery Procedures and Etiologies.
| Study | Surgical Procedure | Bone Graft | Type of Cage | Etiologies |
|---|---|---|---|---|
| Hasegawa et al. (2020) 6 | Single-level PLIF or TLIF | Local bone | NS | Degenerative spondylolisthesis, degenerative scoliosis, lumbar spinal canal stenosis, lumbar radiculopathy, lumbar herniation, isthmic spondylolisthesis |
| Rickert et al. (2017) 11 | One or two level TLIF | Local bone + bone substitutes ((hydroxyapatite 65% + beta-tricalcium phosphate 35%; mectabone, medacta) |
Ti-PEEK: MectaLIF Ti-PEEK, medacta International SA, PEEK: MectaLIF PEEK | Degenerative disk disease, spinal stenosi, isthmic/low dysplastic spondylolisthesis |
| Sakaura et al. (2018) 18 | Single-level CBT-PLIF | Local bone | NS | Degenerative lumbar spondylolisthesis |
| Schnake et al. (2020) 12 | Single level PLIF | Local bone | Medacta International SA, Ti-PEEK: Coated wave, medtronic, PEEK: Wave, medtronic | Degenerative spondylolisthesis, advanced degenerative disk disease, lumbar spinal canal stenosis |
| Singhatanadgige et al. (2022) 7 | One or 2 MI-TLIF | Autograft (from the laminectomy step) and demineralized bone matrix (demineralized bone matrix; GRAFTON, Medtronic, Minneapolis, Minnesota, USA) |
(CAPSTONE PEEK cage or CAPSTONE PTC TiPEEK cage; Medtronic, Minneapolis, Minnesota, USA |
Spondylolisthesis, spinal canal stenosis, herniated nucleus pulposus |
| Vanek et al. (2023) 19 | One-level TLIF | Local bone | Bananashaped PEEK and TiPEEK cages (TSPACE®, Aesculap AG, Aesculap, Germany) were inserted. The TiPEEK implant is composed of PEEK core mantled with plasmapore coating (Ti6AL4V/ISO5832-3) |
Degenerative, isthmic olisthesis, low back pain |
| Willems et al. (2019) 10 | One level PLIF | Local bone | Ti-PEEK: TSC, orthobion; PEEK: reCreo, orthobion | Degenerative spondylolisthesis, postnucleotomy syndrome, degenerative disk disease, spondylolisthesis, spinal canal stenosis |
| Yao et al. (2023) 20 | One level MI-TLIF | Autologous morselized bone graft from a local bone and 1 cc demineralized bone matrix (OsteoSelect® DBM putty, bacterin International, Inc., belgrade, MT, USA) |
PEEK cage (Rainboo® lumbar cage, A-SPINE, United Orthopedic corporation, Taiwan) or Ti/PEEK composite cage (Combo® lumbar cage, A-SPINE, United orthopedic Corporation, Taiwan) |
Spinal stenosis, degenerative, spondylolisthesis, isthmic spondylolisthesis |
CBT, cortical bone trajectory; DBM, demineralized bone matrix; LIF, lumbar interbody fusion; MI, minimally invasive; NS, not specified; PLIF, posterolateral lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion.
Risk of Bias
The quality of the studies is shown in Figure 1 and Supplemental Table 1. The quality of RCTs was moderate. Randomization sequence and selective reporting showed a low risk of bias. Attrition bias and blinding of outcomes showed greater controversy in terms of the risk of bias. Finally, the allocation concealment and blinding of participants and personnel showed a higher risk of bias. On the other hand, the quality of the cohort studies was high for one study and fair for the other.
Fusion
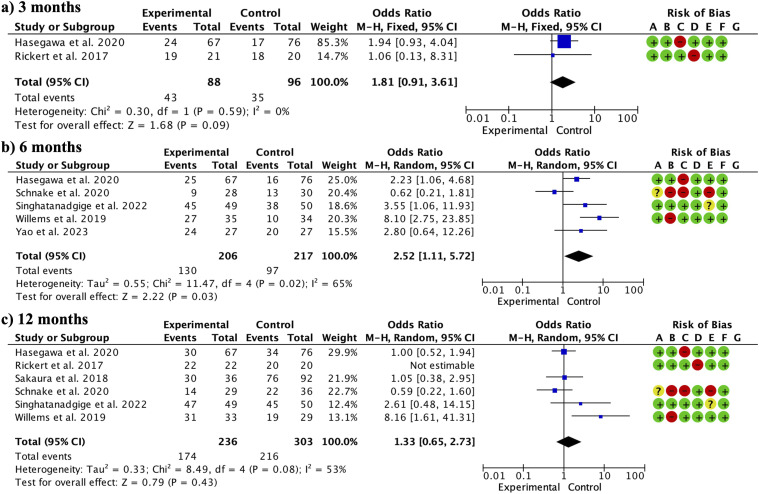
Regarding the fusion rate, the overall fusion rate (without considering the different follow-up periods) was significantly higher in the TiPEEK group (OR 1.83, 95% CI: 1.18 to 2.83; participants = 664; studies = 8; I2 = 53%). When subgroups were made, at 3 months, there were no significant differences between groups (OR 1.81, 95% CI: 0.91 to 3.61; participants = 184; studies = 2; I2 = 0%) (Figure 3a). TiPEEK cages presented significantly higher fusion rates at 6 months (OR 2.52, 95% CI: 1.11 to 5.72; participants = 423; studies = 5; I2 = 65%) (Figure 3b). There were no significant differences between the groups at 12 months (OR 1.33, 95% CI: 0.65 to 2.73; participants = 539; studies = 6; I2 = 53%) (Figure 3c).
Figure 3.
Forest plot showing the comparison between TiPEEK cages vs PEEK cages. TiPEEK cages showed a higher overall fusion rate. When divided into subgroups, TiPEEK cages showed significantly higher fusion rates specifically at 6 months.
Table 3 shows the sensitivity analysis considering the confounding variables. By study design, RCTs showed significant differences favoring TiPEEK (OR 1.89, 95% CI: 1.15 to 3.11) compared with cohort studies (OR 1.47, 95% CI: 0.63 to 3.41) which did not show differences. Studies without declared conflict of interest showed significant differences favoring TiPEEK (OR 1.91, 95% CI: 1.49 to 2.44) compared to those declaring conflict (OR 0.89, 95% CI: 0.49 to 1.61). Studies receiving funding showed no significant differences (OR 1.74, 95% CI: 0.99 to 3.06). Studies without funding also showed no significant differences (OR 1.55, 95% CI: 0.97 to 2.48). Study quality showed that studies with a lower risk of bias had significant differences favoring TiPEEK cages (OR 2.50, 95% CI: 1.52 to 4.12) compared to those with a higher risk, which did not show differences (OR 0.93, 95% CI: 0.56 to 1.55). Regarding the procedure type, studies including only PLIF (OR 1.62, 95% CI: 1.03 to 2.56), only TLIF (OR 2.48, 95% CI: 1.33 to 4.61), and both techniques (OR 1.68, 95% CI: 1.27 to 2.22) showed significant differences favoring TiPEEK. Regarding number of fused levels, studies including only single level fusion (OR 1.75, 95% CI: 1.05 to 2.89) and those including one or 2 levels (OR 2.64, 95% CI: 1.10 to 6.31) showed significant differences favoring TiPEEK. Regarding graft, studies including local bone plus bone substitute (OR 2.68, 95% CI: 1.26 to 5.6) showed significant differences favoring TiPEEK. And those including local bone only, showed no significant differences (OR 1.69, 95% CI: 0.99 to 2.89). Regarding examiner type, independent observers of the study showed no significant differences (OR 1.80, 95% CI: 0.99 to 3.26). When the examiner was not independent from the study there were significant differences favoring TiPEEK cages (OR 1.95, 95% CI: 1.07 to 3.58). Due to significant heterogeneity, cage type, fusion definition or etiologies could not be compared in this way.
Table 3.
Sensitivity Analysis Considering Confounding Variables.
| Effect Size | Fixed-Effect Model (MD/SMD 95% CI) | I2 (%) |
|---|---|---|
| Study design | ||
| RCTs | OR 1.89, 95% CI: 1.15 to 3.11 | 59 |
| Cohort studies | OR 1.47, 95% CI: 0.63 to 3.41 | 12 |
| Conflict of interests | ||
| No | OR 1.91, 95% CI: 1.49 to 2.44 | 41 |
| Yes | OR .89, 95% CI: 0.49 to 1.61 | 40 |
| Funding | ||
| No | OR 1.55, 95% CI: 0.97 to 2.48 | 31 |
| Yes | OR 1.74, 95% CI: 0.99 to 3.06 | 65 |
| Study quality | ||
| Lower risk of bias | OR 2.50, 95% CI: 1.52 to 4.12 | 46 |
| Higher risk of bias | OR .93, 95% CI: 0.56 to 1.55 | 12 |
| Procedure type | ||
| PLIF | a OR 1.62, 95% CI: 1.03 to 2.56 | 80 |
| TLIF | OR 2.48, 95% CI: 1.33 to 4.61 | 0 |
| PLIF + TLIF | OR 1.68, 95% CI: 1.27 to 2.22 | 22 |
| n fused levels | ||
| Single level | OR 1.75, 95% CI: 1.05 to 2.89 | 62 |
| One or 2 levels | OR 2.64, 95% CI: 1.10 to 6.31 | 0 |
| Graft | ||
| Local bone + bone substitute | OR 2.68, 95% CI: 1.26 to 5.6 | 0 |
| Local bone | OR 1.69, 95% CI: 0.99 to 2.89 | 66 |
| Examiner | ||
| Independent from the study | OR 1.80, 95% CI: 0.99 to 3.26 | 69 |
| From the study | OR 1.95, 95% CI: 1.07 to 3.58 | 0 |
aRandom effect model; PLIF, posterior lumbar interbody fusión; TLIF, transforaminal lumbar interbody fusión; RCT, randomized clinical trial.
PROMs
The ODI did not show significant differences between the groups globally (SMD: −.04, 95% CI: −0.15, 0.06; participants = 461; studies = 20; I2 = 0%) (Figure 4). There were no significant differences at 3 months (SMD −.11, 95% CI: −0.31 to 0.09; participants = 372; studies = 5; I2 = 0%), 6 months (SMD −.05, 95% CI: −0.24 to 0.13; participants = 453; studies = 6; I2 = 0%), 12 months (SMD .06, 95% CI: −0.12 to 0.25; participants = 448; studies = 6; I2 = 0%), or 24 months (SMD −.13, 95% CI −0.42 to 0.15; participants = 190; studies = 3; I2 = 0%).
Figure 4.
Forest plot showing there were no differences between TiPEEK and PEEK cages regarding the ODI. There were no differences when subgroups were analyzed at different follow-up times.
The VAS BP scale did not show significant differences globally (MD −0.14, 95% CI: −0.36 to 0.09; participants = 312; studies = 17; I2 = 0%) (Figure 5). There were also no significant differences at 3 months (MD −0.15, 95% CI: −0.68 to 0.38; participants = 229; studies = 4; I2 = 17%), 6 months (MD −0.36, 95% CI: −0.77 to 0.06; participants = 310; studies = 5; I2 = 0%), 12 months (MD 0.13, 95% CI: −0.26 to 0.52; participants = 305; studies = 5; I2 = 36%), or 24 months (MD −0.26, 95% CI: −0.79, 0.27; participants = 190; studies = 3; I2 = 0%).
Figure 5.
Forest plot showing the results of VAS back pain between the 2 groups. There were no differences globally or when subgroups were made for different follow-up times.
The EQ-5D did not show significant global differences (SMD .08, 95% CI: −0.11 to 0.27; participants = 439; studies = 8; I2 = 0%). There were also no significant differences at 3 months (SMD .13, 95% CI: −0.27 to 0.54; participants = 94; studies = 2; I2 = 0%), 6 months (SMD .15, 95% CI: −0.15 to 0.44; participants = 175; studies = 3; I2 = 0%), or 12 months (SMD −.01, 95% CI: −0.31, 0.29; participants = 170; studies = 3; I2 = 0%). The VAS LP could only be assessed at 12-14 months and did not show significant differences between the groups (MD .16, 95% CI: −0.90 to 1.22; participants = 195; studies = 3; I2 = 71%).
Complications
The complications analyzed were cage subsidence, screw loosening/malposition/break, and revision. There were no significant differences in the overall cage subsidence (without considering the different follow-up periods) (OR .72, 95% CI: 0.48 to 1.07; participants = 406; studies = 6; I2 = 34%) (Figure 6). There were no significant differences at 3 months (OR 1.79, 95% CI: 0.60 to 5.33; participants = 140; studies = 1; I2 = 0%), 6 months (OR .63, 95% CI: 0.36 to 1.11; participants = 296; studies = 3; I2 = 0%), or 12 months (OR 1.06, 95% CI: 0.44 to 2.56; participants = 241; studies = 3; I2 = 0%). At 24 months, only one study reported a significantly lower incidence of cage subsidence in the TiPEEK group (OR .14, 95% CI: 0.03 to 0.70; participants = 81; studies = 1; I2 = 0%). There were no significant differences for screw complications (OR 1.25, 95% CI: 0.30 to 5.27; participants = 230; studies = 3; I2 = 0%) or for the incidence of reoperations (OR 0.61, 95% CI: 0.11 to 3.37; participants = 223; studies = 4; I2 = 0%).
Figure 6.
Forest plot showing there were no differences between TiPEEK and PEEK cages regarding the risk of cage subsidence.
Publication Bias
Formal assessment of publication bias was performed by visual inspection of a funnel plot showing the effect size (x-axis) of each study against its precision (y-axis) (Figure 7). In the absence of publication bias arising from the non-reporting of small negative trials, the funnel plot forms a symmetrical inverted cone shape, as less precise studies with wider confidence intervals spread out more at the bottom. Most studies clustered closely around the pooled mean effect and distributed themselves in a reasonably symmetrical fashion around it, with outliers attributable to heterogeneity between studies rather than bias. While no objective test was available, the shape of the plot did not suggest substantial omission of data from small trials on either side of the measure of effect that could notably skew the spread distribution. Therefore, although no methodology could completely rule out selective reporting, inspection of the constructed funnel plot did not provide convincing evidence of a high risk of reporting bias that would significantly distort the overall review conclusion.
Figure 7.
Funnel plot showing there was publication bias regarding overall fusion rate.
GRADE
The quality of evidence and recommendations was systematically evaluated using GRADE for 3 key outcomes (Table 4): Oswestry Disability Index (ODI), Visual Analog Scale (VAS) back pain, and rate of fusion. For the ODI and VAS, there were no serious concerns regarding the risk of bias, inconsistency, imprecision, or publication bias. The evidence was deemed highly certain despite some concerns about indirectness from variability in patient populations and procedures across studies. The fused data also exhibited no serious methodological biases. However, the precision was rated down owing to wide confidence intervals, enlarging the uncertainty in the effect estimate. Despite this, an overall high to moderate certainty classification was achieved.
Table 4.
GRADE Assessment of the Quality of the Evidence and the Strength of the Recommendations.
| Certainty Assessment | No of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Clinical | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| ODI | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Serious a | Not serious | Undetected | 729 | 734 | - | SMD 0.04 lower (0.15 lower to 0.06 higher) | ⨁⨁⨁⨁ High |
IMPORTANT |
| VASbp | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Serious a | Not serious | Undetected | 528 | 506 | - | MD 0.14 lower (0.36 lower to 0.09 higher) | ⨁⨁⨁⨁ High |
IMPORTANT |
| Fusion | ||||||||||||
| 8 | Randomised trials | Not serious | Not serious | Serious a | Serious b | Undetected | - | - | OR 1.83 (1.18 to 2.83) | 137 more per 1000 (from 40 more to 218 more) | ⨁⨁⨁◯ Moderado |
CRITICAL |
CI, confidence interval; MD, mean difference; OR, odds ratio; SMD, standardised mean difference.
aDifferences in study designs, etiologies, surgical treatment received.
bWide confidence intervals.
Discussion
This updated meta-analysis suggests that TiPEEK interbody cages may be associated with somewhat higher fusion rates than uncoated PEEK at 3 and 6 months, although these findings are based on a limited number of studies and should be interpreted with caution. No significant difference in fusion rates were observed for the 2 cage groups at 12 months. No significant differences were detected between the cage types for any patient-reported outcome, including the ODI, VAS back and leg pain scores, and EQ-5D scores. The rates of complications such as subsidence, screw issues, and revisions did not significantly differ. Overall, clinical outcomes were generally comparable between cages, whereas radiographic fusion appeared to improve with titanium-coated PEEK implants in the short term.
Regarding the examiner’s fusion assessment, it was not possible to measure the effect of this variable given that many studies did not specify the type of professional performing the radiographic evaluation. Therefore, it is possible to extract weak associations only through a visual analysis of the funnel plots, although with little statistical consistency. Most of the included studies used computed tomography for fusion assessment. Compared with other investigations, these studies reported lower bone consolidation rates,6,12,18 possibly because they required higher radiographic standards in the definition of complete bone fusion.21,22 The most frequent follow-up periods were 6 and 12 months, postoperatively. Only 2 studies extended the evaluation to 24 months. Therefore, no conclusions on fusion could be made for follow-up beyond 12-months post-op. The use of bone graft material may also influence fusion outcomes that are independent of the cage type. Studies using local autologous bone plus bone substitutes showed higher overall fusion in the TiPEEK group at 6 months (63.1% TiPEEK vs 44.7%). Reported substitutes include 65% hydroxyapatite plus beta-tricalcium phosphate11,20 and a demineralized bone matrix.7,20 A recent meta-analysis concluded hydroxyapatite shows similar results to local bone. 23 Another systematic review also did not show divergence between demineralized bone matrix and local bone or other grafts. 24 Likewise, a comparative meta-analysis between various bone substitutes reported higher fusion rates with exclusive use of local autologous bone. 25 These data suggest the need to develop bone substitutes capable of further improving bone consolidation indices. At 3- and 6-months follow-up, a significantly higher bone consolidation rate was observed in the group with titanium-coated PEEK cages, indicating better osteoconductivity and initial bone kinetics with this material. As suggested by Hasewa et al in their discussion, it is foreseeable that these patients will recover their functionality earlier or return to their jobs sooner. 6 However, in this meta-analysis, no differences were identified regarding quality-of-life scale outcomes, although some isolated studies analyzed this clinical variable. Likewise, a few studies have reported additional parameters, such as patient satisfaction or work reincorporation. At the physiological level, it has been found that the main mechanism by which titanium-coated PEEK cages improve osteoconductivity is as follows: (1) titanium promotes bone formation by positively interacting with osteoblasts, improving bone-implant bonding, and (2) it has greater biocompatibility, superficial hydrophilicity, and cellular response compared to uncoated PEEK, which facilitates bone integration.26,27 The potential drawbacks associated with TiPEEK, especially the inflammatory responses that may occur, require more comprehensive exploration. 28 Although titanium is generally considered biocompatible and well-tolerated, it has been observed to potentially induce low-grade inflammatory reactions in some cases. This issue merits closer scrutiny, as chronic inflammation could undermine the long-term stability and success of the implant by adversely affecting tissue healing and integration. 28 Moreover, titanium implants pose additional challenges such as long-term degradation failures and allergic reactions in some patients.29,30 Furthermore, titanium can create artifacts in imaging tests, complicating the evaluation of fusion and assessment of the surrounding tissues. 31 An important complication, delamination, has not been thoroughly studied. A study examined the wear and delamination of titanium-coated PEEK cages and surface-etched titanium cages during the insertion process. The results revealed that the PEEK-coated cages exhibited coating wear and detached particles, whereas the surface-etched titanium cages showed no signs of wear or particle release. This suggests that the surface-etched titanium cages may be more resistant to wear, and delamination compared to the coated PEEK cages. 32 Another study showed that porous PEEK devices maintained their structure and had minimal damage, while titanium-coated PEEK devices experienced substantial coating loss during impaction. These findings suggest that although surface modifications enhance osseointegration, they may be vulnerable to damage. 33
Risk factors such as obesity, smoking, and diabetes have been associated with lower consolidation rates.34-36 However, few studies have performed adjusted analyses that considered these potential confounding variables. Vanek et al. observed worse fusion rates in obese patients with TiPEEK cages. 19 Further studies using multivariate models are required. Minimally invasive approaches could not be adequately assessed because of the lack of research on this topic.
Willems et al. compared titanium-coated PEEK cages (TiPEEK), calcium phosphate-coated PEEK cages (CaP PEEK), and uncoated PEEK cages and found fusion rates at 12 months of follow-up of 93.9% and 88%, and lower for the uncoated ones, respectively, without significant differences between the first 2. 10 One of the main benefits of calcium phosphate (Ca-P) coating is to improve osteoconductivity and osseointegration, increase bone growth along the porous surface of the implant compared to uncoated implants, and significantly increase the area of bone growth within the implant, with greater bone-implant bonding in the porous junction regions. 37 Willems also evaluated nanostructured PEEK coatings, which offer advantages such as requiring a thickness order of magnitude thinner than conventional thermal coatings, causing less wear. 10 It improves osteoconductivity similar to traditional coatings, allowing preservation of PEEK’s radiolucency and elasticity properties of PEEK in a less invasive manner by minimally altering its mechanical properties. 10
While the 6-month fusion rates observed with coated cages were statistically significant, the broader clinical implications of these findings merit further scrutiny, particularly in light of the lack of accompanying improvements in functional outcomes. Other factors may have a pronounced influence on postsurgical outcomes. Variables such as the severity of the condition before surgery, existing comorbidities, patient adherence to rehabilitation protocols, and psychosocial factors affecting pain perception could significantly affect a patient’s functional recovery. Additionally, the duration of follow-up in the reviewed studies was predominantly limited to 6- or 12-months post-surgery, with only a few studies extending up to 24 months. This limited timeframe restricts the ability to draw definitive conclusions regarding the long-term status of bone fusion and its impact on clinical outcomes. The preliminary data suggest that both groups, those with and without coated cages, experience significant relief from preoperative symptoms; however, the absence of long-term follow-up prevents a robust comparison of fusion rates and long-term functional status.
For cage subsidence specifically, while one individual study found lower rates with TiPEEK, 19 other studies may have been underpowered on their own to detect differences given the typically low subsidence incidence. Larger sample sizes across studies may enable the detection of subgroup effects. Regarding screw problems and reoperations, similar issues around modest sample sizes limit the ability to compare relatively uncommon adverse events apply. For both complications, shorter follow-up durations may have precluded the full manifestation of any divergence prior to the study endpoints.
In examining the long-term complications associated with different cage materials in spinal surgeries, variable outcomes were noted across studies regarding rigidity and the risk of adjacent segment degeneration. Biomechanical studies have indicated that interbody fusion using PEEK cages may exhibit greater rigidity, potentially increasing the risk of stress on adjacent segments compared with porous titanium cages. 38 This phenomenon may contribute to adjacent segment degeneration, which is a significant concern for long-term spinal health. However, in a long-term study comparing titanium and PEEK cages in the treatment of multilevel cervical spondylotic myelopathy, the incidence of subsidence was significantly higher in the titanium group after 7 years. Despite these findings, direct long-term comparative results, specifically for TiPEEK and traditional PEEK cages, remain limited.39,40
However, given that titanium is only applied as a thin coating, it is crucial to consider whether this modification significantly contributes to the overall mechanical stiffness of the implant. It should be acknowledged that a thin titanium coating is unlikely to affect the bulk biomechanical properties of the implant. The primary purpose of titanium coating is to enhance the biological properties, specifically to facilitate better integration and adherence with surrounding bone tissue, rather than to tune structural stiffness.
Limitations
Some of the main limitations of the present study include the relatively small sample size of the included studies. This restricts the ability to perform subgroup analyses and adjust multivariate models by robustly considering confounding variables. Similarly, some studies did not precisely specify the number of patients evaluated at each follow-up point. Another limitation was that several studies did not provide details about the vertebral segments included in the measurements; therefore, in some cases, the results corresponded to total fusion and were not broken down by level. Despite conducting a formal analysis of the graft materials used, there were few studies within each subgroup analyzed, and most used local bone, which limited our ability to consistently explore other options. The influence of these materials on fusion rates and overall success could confound the results, as different combinations and graft materials might independently affect the outcomes regardless of the cage type used. This variability introduces a significant factor that could influence the interpretation of the effectiveness across different implant types. There was also heterogeneity in data presentation, as Hasegawa et al and Vanek et al provided segregated results that required independent meta-analyses following Cochrane guidelines.3,15 In a study by Hasegawa et al, 3 providing data on fusion rates for both intent-to-treat and per-protocol analyses, the per-protocol analysis branch was selected as a conservative approach. Although the intent-to-treat results were similar, testing them with Review Manager did not alter the direction of the outcomes. Other aspects to consider were heterogeneity in patients’ baseline characteristics, fusion definition used, and experience of radiological evaluators between studies. Regarding the complications, there was a limited number of studies, with some outcomes being supported by fewer than 3 studies. This limited dataset restricts our ability to make comprehensive and definitive conclusions about these complications. Finally, it was not possible to consider the economic aspects of these 2 interventions. It is important for future studies to evaluate this information to assess the cost-effectiveness of the 2 procedures.
Conclusion
In conclusion, the results of this meta-analysis suggest that TiPEEK cages fuse earlier, showing a higher fusion rate at 6 months, as compared to uncoated PEEK cages. No significant differences were found in functional outcomes, quality of life or complications between the 2 groups.
Supplemental Material
Supplemental Material for Titanium-Coated Polyetheretherketone Cages Versus Uncoated Polyetheretherketone Cages for Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis by Gonzalo Mariscal, Praveer Vyas, Boyle Cheng, J. J. Chris Arts, Thomay-Claire A. Hoelen, Chen Xu, and Christopher D. Chaput in Global Spine Journal.
Supplemental Material for Titanium-Coated Polyetheretherketone Cages Versus Uncoated Polyetheretherketone Cages for Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis by Gonzalo Mariscal, Praveer Vyas, Boyle Cheng, J. J. Chris Arts, Thomay-Claire A. Hoelen, Chen Xu, and Christopher D. Chaput in Global Spine Journal.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mariscal G. is a consultant for Cerapedics and received compensation in connection with completing these analyses. Chaput C. D. and Hoelen T. A. received consulting fees from Cerapedics unrelated to this work. Arts J. J. C. is an advisory board member for Cerapedics. Cerapedics did not influence and was not involved with the methods, analysis, or outcomes reported. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Cerapedics Inc.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Gonzalo Mariscal https://orcid.org/0000-0002-5166-198X
Thomay-Claire A. Hoelen https://orcid.org/0000-0001-9944-1831
References
- 1.Adogwa O, Parker SL, Shau D, et al. Long-term outcomes of revision fusion for lumbar pseudarthrosis: clinical article. J Neurosurg Spine. 2011;15:393-398. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Bridwell KH, Lenke LG, Rinella AS, Edwards C. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine. 2005;30:468-474. [DOI] [PubMed] [Google Scholar]
- 3.Bono CM, Khandha A, Vadapalli S, Holekamp S, Goel VK, Garfin SR. Residual sagittal motion after lumbar fusion: a finite element analysis with implications on radiographic flexion-extension criteria. Spine. 2007;32(4):417-422. doi: 10.1097/01.brs.0000255201.74795.20 [DOI] [PubMed] [Google Scholar]
- 4.Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81-89. doi: 10.1111/os.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu D, Lei X, Zhu H. Modification of polyetheretherketone (PEEK) physical features to improve osteointegration. J Zhejiang Univ - Sci B. 2022;23(3):189-203. doi: 10.1631/jzus.B2100622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa T, Ushirozako H, Shigeto E, et al. The titanium-coated PEEK cage maintains better bone fusion with the endplate than the PEEK cage 6 months after PLIF surgery: a multicenter, prospective, randomized study. Spine. 2020;45(15):E892-E902. doi: 10.1097/BRS.0000000000003464 [DOI] [PubMed] [Google Scholar]
- 7.Singhatanadgige W, Tangchitcharoen N, Kerr SJ, et al. A comparison of polyetheretherketone and titanium-coated polyetheretherketone in minimally invasive transforaminal lumbar interbody fusion: a randomized clinical trial. World Neurosurg. 2022;168:e471-e479. doi: 10.1016/j.wneu.2022.10.006 [DOI] [PubMed] [Google Scholar]
- 8.Kienle A, Graf N, Wilke HJ. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016;16(2):235-242. doi: 10.1016/j.spinee.2015.09.038 [DOI] [PubMed] [Google Scholar]
- 9.Torstrick FB, Klosterhoff BS, Westerlund LE, et al. Impaction durability of porous polyether-ether-ketone (PEEK) and titanium-coated PEEK interbody fusion devices. Spine J. 2018;18(5):857-865. doi: 10.1016/j.spinee.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Willems K, Lauweryns P, Verleye G, van Goethem J. Randomized controlled trial of posterior lumbar interbody fusion with Ti- and CaP-nanocoated polyetheretherketone cages: comparative study of the 1-year radiological and clinical outcome. Internet J Spine Surg. 2019;13(6):575-587. doi: 10.14444/6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickert M, Fleege C, Tarhan T, et al. Transforaminal lumbar interbody fusion using polyetheretherketone oblique cages with and without a titanium coating: a randomised clinical pilot study. Bone Joint Lett J. 2017;99-B(10):1366-1372. doi: 10.1302/0301-620X.99B10.BJJ-2016-1292.R2 [DOI] [PubMed] [Google Scholar]
- 12.Schnake KJ, Fleiter N, Hoffmann C, et al. PLIF surgery with titanium-coated PEEK or uncoated PEEK cages: a prospective randomised clinical and radiological study. Eur Spine J. 2021;30(1):114-121. doi: 10.1007/s00586-020-06642-x [DOI] [PubMed] [Google Scholar]
- 13.Lv ZT, Xu Y, Cao B, et al. Titanium-coated PEEK versus uncoated PEEK cages in lumbar interbody fusion: a systematic review and meta-analysis of randomized controlled trial. Clin Spine Surg. 2023;36(5):198-209. doi: 10.1097/BSD.0000000000001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2019. [Google Scholar]
- 17.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173-183. doi: 10.1016/j.jclinepi.2012.08.001. Erratum in: J Clin Epidemiol. 2015 Apr;68(4):475. [DOI] [PubMed] [Google Scholar]
- 18.Sakaura H, Ohnishi A, Yamagishi A, Ohwada T. Early fusion status after posterior lumbar interbody fusion with cortical bone trajectory screw fixation: a comparison of titanium-coated polyetheretherketone cages and carbon polyetheretherketone cages. Asian Spine J. 2019;13(2):248-253. doi: 10.31616/asj.2018.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanek P, Svoboda N, Bradac O, Malik J, Kaiser R, Netuka D. Clinical and radiological results of TLIF surgery with titanium-coated PEEK or uncoated PEEK cages: a prospective single-centre randomised study. Eur Spine J. 2024;33(1):332-338. doi: 10.1007/s00586-023-07947-3 [DOI] [PubMed] [Google Scholar]
- 20.Yao YC, Chou PH, Lin HH, Wang ST, Chang MC. Outcome of Ti/PEEK versus PEEK cages in minimally invasive transforaminal lumbar interbody fusion. Glob Spine J. 2023;13(2):472-478. doi: 10.1177/21925682211000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disk height in stand-alone and instrumented extreme lateral interbody fusion (XLIF). Clin Spine Surg. 2017;30(2):E90-E98. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang X, Sun Y, et al. Safety analysis of two anterior lateral lumbar interbody fusions at the initial stage of learning curve. World Neurosurg. 2019;127:e901-e909. [DOI] [PubMed] [Google Scholar]
- 23.Liu XJ, Zhu QS, Sun HF, et al. The clinical efficacy of hydroxyapatite and its composites in spinal reconstruction: a meta-analysis. Eur Rev Med Pharmacol Sci. 2022;26(13):4614-4624. doi: 10.26355/eurrev_202207_29183 [DOI] [PubMed] [Google Scholar]
- 24.Zadegan SA, Abedi A, Jazayeri SB, Vaccaro AR, Rahimi-Movaghar V. Demineralized bone matrix in anterior cervical discectomy and fusion: a systematic review. Eur Spine J. 2017;26(4):958-974. doi: 10.1007/s00586-016-4858-9 [DOI] [PubMed] [Google Scholar]
- 25.Tavares WM, de França SA, Paiva WS, Teixeira MJ. A systematic review and meta-analysis of fusion rate enhancements and bone graft options for spine surgery. Sci Rep. 2022;12(1):7546. doi: 10.1038/s41598-022-11551-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han CM, Lee EJ, Kim HE, et al. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials. 2010;31(13):3465-3470. doi: 10.1016/j.biomaterials.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 27.Walsh WR, Pelletier MH, Christou C, He J, Vizesi F, Boden SD. The in vivo response to a novel Ti coating compared with polyether ether ketone: evaluation of the periphery and inner surfaces of an implant. Spine J. 2018;18(7):1231-1240. doi: 10.1016/j.spinee.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 28.Wang JC, Yu WD, Sandhu HS, Betts F, Bhuta S, Delamarter RB. Metal debris from titanium spinal implants. Spine. 1999;24(9):899-903. doi: 10.1097/00007632-199905010-00011 [DOI] [PubMed] [Google Scholar]
- 29.Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine. 2015;40(6):399-404. doi: 10.1097/BRS.0000000000000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Xi Y, Sun B, et al. Suspected allergy to titanium after anterior cervical discectomy and fusion using a zero-P device: a case report. Br J Neurosurg. 2023;37(4):916-920. doi: 10.1080/02688697.2020.1718605 [DOI] [PubMed] [Google Scholar]
- 31.Carreon LY, Glassman SD, Schwender JD, Subach BR, Gornet MF, Ohno S. Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages. Spine J. 2008;8(6):998-1002. doi: 10.1016/j.spinee.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Kahaer A, Maimaiti A, Guo H, Rexiti P. Complication, fusion, and revision rate in the lumbar cortical bone trajectory and pedicle screw fixation techniques: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18(1):382. doi: 10.1186/s13018-023-03820-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheatley BM, Nappo KE, Christensen DL, Holman AM, Brooks DI, Potter BK. Effect of NSAIDs on bone healing rates: a meta-analysis. J Am Acad Orthop Surg. 2019;27(7):e330-e336. doi: 10.5435/JAAOS-D-17-00727 [DOI] [PubMed] [Google Scholar]
- 34.Phan K, Rogers P, Rao PJ, Mobbs RJ. Influence of obesity on complications, clinical outcome, and subsidence after anterior lumbar interbody fusion (ALIF): prospective observational study. World Neurosurg. 2017;107:334-341. doi: 10.1016/j.wneu.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 35.Andersen T, Christensen FB, Laursen M, Høy K, Hansen ES, Bünger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26(23):2623-2628. doi: 10.1097/00007632-200112010-00018 [DOI] [PubMed] [Google Scholar]
- 36.Moazzeni K, Kazemi KA, Khanmohammad R, Eslamian M, Rostami M, Faghih-Jouibari M. Comparison of surgical outcome between diabetic versus nondiabetic patients after lumbar fusion. Internet J Spine Surg. 2018;12(4):528-532. doi: 10.14444/5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen HQ, Deporter DA, Pilliar RM, Valiquette N, Yakubovich R. The effect of sol-gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials. 2004;25(5):865-876. doi: 10.1016/s0142-9612(03)00607-0 [DOI] [PubMed] [Google Scholar]
- 38.Adl Amini D, Moser M, Oezel L, et al. Early outcomes of three-dimensional-printed porous titanium versus polyetheretherketone cage implantation for stand-alone lateral lumbar interbody fusion in the treatment of symptomatic adjacent segment degeneration. World Neurosurg. 2022;162:e14-e20. doi: 10.1016/j.wneu.2021.11.122 [DOI] [PubMed] [Google Scholar]
- 39.Mesbah M, Barkaoui A. Is pedicle-based hybrid stabilization (PBHS) protecting posterior lumbar fixation from adjacent-segment failure? Finite element analysis and comparison of different systems. Orthop Traumatol Surg Res. 2021;107(7):103038. doi: 10.1016/j.otsr.2021.103038 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J. 2013;22(7):1539-1546. doi: 10.1007/s00586-013-2772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Titanium-Coated Polyetheretherketone Cages Versus Uncoated Polyetheretherketone Cages for Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis by Gonzalo Mariscal, Praveer Vyas, Boyle Cheng, J. J. Chris Arts, Thomay-Claire A. Hoelen, Chen Xu, and Christopher D. Chaput in Global Spine Journal.
Supplemental Material for Titanium-Coated Polyetheretherketone Cages Versus Uncoated Polyetheretherketone Cages for Lumbar Spinal Fusion: A Systematic Review and Meta-Analysis by Gonzalo Mariscal, Praveer Vyas, Boyle Cheng, J. J. Chris Arts, Thomay-Claire A. Hoelen, Chen Xu, and Christopher D. Chaput in Global Spine Journal.