Abstract
Purpose
Prolonged postoperative ileus (PPOI) is associated with a lengthy hospital stay, risk of additional complications and substantial costs. Data regarding the incidence of PPOI following right extended colectomy (REC) with ileo-descending anastomosis is limited. This study aimed to compare the incidence of PPOI and evaluate risk factors for this condition among patients undergoing REC, left hemicolectomy (LC), and right hemicolectomy (RC) for colon carcinoma.
Methods
This retrospective, single-center cohort study included patients who underwent colectomy for cancer at our institution between January 2014 and January 2022. Outcome measures were postoperative time to first bowel movement and flatus, postoperative time to tolerate a solid low-residue diet, the need for nasogastric tube (NGT) decompression or total parenteral nutrition (TPN), and length of hospital stay (LOS).
Results
A total of 534 patients were included: 242 (45.3%) underwent LC, 207 (38.8%) RC, and 85 (15.9%) REC. There were no significant differences in surgical approach (laparoscopic vs. open), operative time, percentage of urgent surgeries, postoperative complication rates, or advanced T stages among the groups. Compared with LC and RC, REC was associated with a longer time to first flatus (p = 0.04), delayed tolerance of a solid diet (p < 0.001), and increased LOS (p < 0.001). Patients following REC were at a higher risk for the need of NGT decompression (p = 0.003). Analysis of covariance controlling for potential confounding variables identified REC as an independent risk factor for PPOI following surgery.
Conclusion
This study revealed a higher incidence of PPOI following REC for cancer compared to RC and LC.
Keywords: Prolonged postoperative ileus, Right extended colectomy, Length of hospital stay, Total parenteral nutrition, Nasogastric tube decompression
Introduction
Postoperative ileus is defined as a predictable, temporary reduction in gastrointestinal motility following abdominal surgery [1]. Typically, gut dysmotility resolves within 0–24 h for the small intestine, 24–48 h for the stomach, and 48–72 h for the colon [2, 3]. Prolonged postoperative ileus (PPOI), persisting beyond these time frames, might be (but is not definitely) a sign of severe underlying complication. Clinically, postoperative ileus presents as intolerance of oral feeding, abdominal distension, nausea or vomiting, diminished or absent bowel sounds, and delayed passage of flatus or bowel movements. Once diagnosed, treatment mainly involves supportive measures such as nasogastric tube (NGT) decompression, intravenous fluids repletion, and total parenteral nutrition (TPN) if required.
Inhibitory gastrointestinal tract reflexes, postoperative sympathetic hypersensitivity, local inflammation, and pharmacologic interactions, have all been reported as triggers for PPOI [2–6]. Patient characteristics and perioperative risk factors linked to PPOI include chronic pulmonary disease, male sex, smoking, surgery via open approach, prolonged operative time, and total opiate dosage [3, 7].
Never the less, once diagnosed, other causes of PPOI that might require further treatment should be excluded, such as intra-abdominal sepsis, anastomotic leak, and electrolyte imbalance. PPOI is associated with an increased rate of postoperative complications, prolonged hospital stay, and higher readmission rates, incurring estimated annual costs of $750 million in the USA [8–10].
Right extended colectomy (REC) for left-sided colonic and distal transverse colon tumors, first reported in 1985, offer significant technical advantages over left hemicolectomy (LC) or transverse segmental resections, despite resecting longer bowel segments [11]. Technically, REC utilizes a highly mobile segment of the bowel, the ileum, to transpose it toward the left colon and perform the ileo-colonic anastomosis without tension. Moreover, in cases of obstructing tumors of the left colon, REC simultaneously removes the cecum, which carries the highest risk of bowel perforation (according to Laplace law). Therefore, REC simultaneously allows for relief of intestinal obstruction, resection of the tumor, and restoration of bowel continuity with a tension-free anastomosis.
While postoperative recovery after colorectal surgery has been well studied, comparisons of PPOI incidence among patients undergoing right hemicolectomy (RC), REC, and LC are limited. Previous reports have shown a lower rate of PPOI in patients undergoing LC than in those undergoing RC [12–14]. Garfinkle et al. reported a 35% higher risk for PPOI in patients undergoing RC compared to those undergoing LC (OR = 1.35, 95%; CI: 1.25–1.47) among 40,636 patients from the American College of Surgeons National Surgical Quality Improvement Program database, with longer hospital stays and a higher 30-day readmission rate in the RC group [12]. Yuan et al. studied 94 consecutive patients undergoing elective colorectal resections with primary anastomosis and observed faster bowel function recovery in LC versus RC patients [13]. Grass et al. found lower PPOI incidence in LC compared to RC as well [14]. These studies all reported the different incidence of PPOI among patients undergoing RC versus LC, but none reported data on the incidence of PPOI following REC.
The long-term functional outcomes of RC and REC also warrant consideration. These procedures may lead to chronic watery stools due to reduced colonic water absorption or bile acid malabsorption. Phillips et al. reported that 50% of cancer survivors with Bristol stool types 6–7 had evidence of bile acid malabsorption [15]. Furthermore, resection of the ileocecal valve can promote small intestinal bacterial overgrowth and contribute to diarrhea [16–18]. Even limited resection of the terminal ileum may impair bile acid reabsorption, as shown by Kurien et al. [19].
This study aimed to compare the incidence of and evaluate risk factors for PPOI among patients undergoing REC, LC, and RC for colon carcinoma.
Materials and methods
Study design
In this single-center retrospective cohort study, we used a prospectively maintained database. Approval for this study was obtained from the Institutional Review Board of the Hadassah Hebrew University Medical Center (approval number HMO-0766–20), and the study was conducted in adherence to the principles of the Declaration of Helsinki. Patient records were anonymized and de-identified prior to analysis. The study is reported in line with the STROBE guidelines for observational studies.
Patient inclusion and exclusion
We included consecutive cancer patients who underwent colectomy with curative intent at the Hadassah Hebrew University Medical Center between January 2014 and January 2022. Eligible patients were cancer patients who underwent right-sided, left-sided, or right-extended colectomies, either elective or urgent.
Exclusion criteria were: (1) surgery for an indication other than cancer; (2) multi-organ resection; (3) mental disturbance; (4) stoma creation as a part of the procedure; and (5) age under 18.
Data collection
Demographic, pre-operative, operative, and short-term postoperative data were extracted from a prospectively maintained colorectal cancer database. The following variables were analyzed as potential risk factors for PPOI: patient demographics (gender, age), medical comorbidities and pre-operative data (American Society of Anesthesiologists class score, serum albumin levels, body mass index), operative data (resection type, surgical approach, operative time, anastomosis type), and postoperative outcomes (LOS, complications, TNM pathological stage). Postoperative morbidity, defined as any complication occurring during the hospital stay or within 30 postoperative days, was recorded using the Clavien-Dindo classification [20]. Anastomotic leakage was diagnosed radiologically, by computerized tomography (intra-abdominal collection, free fluid containing air bubbles or free contrast material adjacent to the anastomosis), or clinically (evidence of extravasation of bowel content or gas through a wound or drain) as proposed by the International Study Group of Rectal Cancer [21].
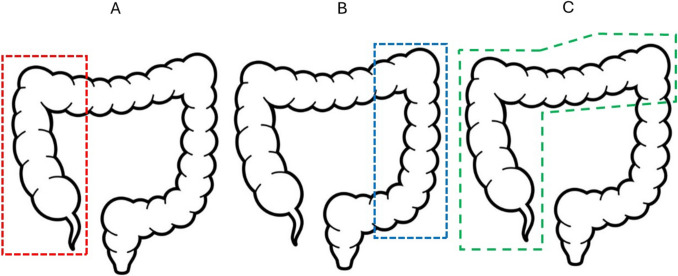
RC was defined as the resection of the terminal ileum and ascending colon followed by an extracorporeal stapled side-to-side functional end-to-end ileocolonic anastomosis for cecal, ascending, and hepatic flexure colon cancers. The ileocolic and the right branch of the middle colic vessels were ligated. LC was defined as the resection of any part of the descending or sigmoid colon followed by either an extracorporeal stapled side-to-side functional end-to-end colo-colonic anastomosis or an intracorporeal side-to-end colo-rectal anastomosis using a circular stapler, for splenic flexure, descending, sigmoid, or rectosigmoid junction colon cancers. The left colic and/or inferior mesenteric vessels were ligated. REC was defined as the resection of the terminal ileum and ascending, transverse, and proximal descending colon followed by an extracorporeal stapled side-to-side functional end-to-end ileocolonic anastomosis for transverse and splenic flexure colon cancers. The ileocolic, right colic, and middle colic vessels were ligated (Fig. 1).
Fig. 1.
Illustration of the three anatomical resections: A RC is the excision of the bowel within the red dotted line, including the terminal ileum and ascending colon followed by an ileo-colonic anastomosis; B LC is the excision of all or part of the bowel within the blue dotted line including the descending or sigmoid colon followed by a colo-colonic or colo-rectal anastomosis; C REC is the excision of the bowel within the green dotted line, including the terminal ileum and ascending, transverse, and proximal descending colon followed by an ileo-colonic anastomosis
All patients received a standardized perioperative management protocol. Preoperatively, all elective patients received a mechanical and antibiotic bowel preparation. Postoperative pain management was identical in all patients and included patient-controlled analgesia (PCA) with intravenous morphine for the first 48 h postoperatively, supplemented by intravenous dipyrone (1.0 g up to four times a day), intravenous paracetamol (1.0 g up to four times a day), and intravenous ketorolac (30 mg up to three times a day) as needed. After 48 h, patients were taken off the intravenous PCA treatment and were treated with oral Targin twice a day (oxycodone10 milligrams with naloxone controlled-release tablets) and dipyrone/paracetamol/ketorolac in the above-mentioned doses as needed, until discharge. A clear liquid diet was initiated on postoperative day one, followed by a solid low-residue diet on day two if bowel function recovered without abdominal distension, nausea, or vomiting. NGT was inserted for gastric decompression in cases of abdominal distention accompanied with non-resolving vomiting in the absence of flatus or bowel movement. TPN was used in cases where initiation of oral feeding was not possible until postoperative day 5–7 and according to the attending surgeon’s clinical decision.
Postoperative patient mobilization, chest physiotherapy, venous thromboembolism prophylaxis with sequential compression devices, low molecular weight heparin, and gastrointestinal prophylaxis with proton pump inhibitors were standard for all patients.
Study endpoints
Outcome measures were postoperative time to first bowel movement and flatus (documented based on patient reports during the surgeon’s rounds), postoperative time to tolerate solid low-residue diet, need for NGT decompression and/or TPN, and LOS.
Statistical analysis
Statistical analysis was conducted using Python’s statistical packages (e.g., statsmodels and scipy) by a professional statistician. Continuous data were presented as mean (standard deviation) or median (range or interquartile range), while categorical data were displayed as frequencies and percentages. The χ2 test was employed to examine the association between categorical variables. Quantitative data were compared using the Kruskal–Wallis (K-W) non-parametric analysis of variance (ANOVA) test. Additionally, an Analysis of Covariance (ANCOVA) test was performed to evaluate the effect of treatment while controlling for potential confounding variables. A p-value of 0.05 or less was considered statistically significant.
Results
A total of 534 colorectal cancer patients undergoing colectomy during the study period were included. Of these, 242 (45.3%) underwent LC, 207 (38.8%) underwent RC, and 85 (15.9%) underwent REC. Patient characteristics and perioperative outcomes are presented in Table 1. Although RC patients were older and had higher ASA scores (p < 0.001 and p = 0.002 respectively) compared to LC and REC patients, T stage was similar (p = 0.89). RC and REC patients were more likely to have perioperative hypoalbuminemia (p = 0.003).
Table 1.
Patient characteristics and perioperative data
| Group Variable |
REC (N = 85) |
RC (N = 207) |
LC (N = 242) |
p-value |
|---|---|---|---|---|
|
Age Years, mean ± std |
16.8 ± 61.1 | 69.7 ± 12.8 | 62.8 ± 13.1 | < 0.001 |
|
Gender, N (%) Females Males |
40 (47.1) 45 (52.9) |
105 (50.7) 102 (49.3) |
105 (43.4) 137 (56.6) |
0.3 |
|
BMI Kg/m2, mean ± std |
28.1 ± 5.7 | 28.0 ± 5.1 | 28.1 ± 4.9 | 0.857 |
|
ASA score, N (%) 1 2 3 |
20 (23.5) 52 (61.2) 13 (15.3) |
26 (12.6) 125 (60.4) 56 (27.0) |
63 (26.0) 133 (55.0) 46 (19.0) |
0.002 |
|
Preoperative Albumin gram/liter, mean ± std |
39.9 ± 5.3 | 39.6 ± 5.4 | 41.3 ± 4.7 | 0.003 |
|
Urgency, N (%) Yes No |
16 (18.8) 69 (81.2) |
25 (12.1) 182 (87.9) |
27 (11.2) 215 (88.8) |
0.146 |
|
Surgical approach, N (%) Open Laparoscopy Conversion to open surgery |
33 (38.8) 48 (56.5) 4 (4.7) |
52 (25.1) 147 (71.0) 8 (3.9) |
85 (35.1) 148 (61.2) 9 (3.7) |
0.086 |
|
Operative time Minutes, mean ± std |
157 ± 57.2 | 111 ± 32 | 188 ± 70.9 | < 0.001 |
|
Pathological stage, N (%) In-situ 1 2 3 4 |
0 (0) 13 (15.3) 35 (41.2) 24 (28.2) 13 (15.3) |
1 (0.5) 43 (20.8) 75 (36.2) 59 (28.5) 29 (14.0) |
1 (0.4) 54 (22.3) 81 (33.5) 71 (29.3) 35 (14.5) |
0.89 |
REC right extended hemicolectomy, RC right hemicolectomy, LC left hemicolectomy, Std Standard deviation, N Number, BMI Body Mass Index, ASA American Society of Anesthesiologists
There were no statistically significant differences in surgical approach (laparoscopic versus open) or the rate of urgent surgeries between the groups. Operative times were significantly longer in the LC group compared to RC and REC (p < 0.001).
Table 2 presents postoperative complication rates. REC patients experienced more anastomotic leaks than LC and RC patients (p = 0.04). No other significant differences in postoperative complication rate were found between the groups.
Table 2.
Postoperative complications
| Group | REC (N = 85) |
RC (N = 207) |
LC (N = 242) |
p-value |
|---|---|---|---|---|
| Variable | ||||
|
Complications according to Clavien-Dindo classification N (%) 1 2 3 4 5 |
12 (14.1) 19 (22.3) 1 (1.2) 1 (1.2) 0 (0) |
15 (7.2) 40 (19.3) 5 (2.4) 4 (1.9) 0 (0) |
14 (5.8) 31 (12.8) 6 (2.5) 2 (0.8) 1 (0.4) |
0.08 |
|
Anastomotic leak N (%) |
15 (17.6) | 21 (10.1) | 21 (8.7) | 0.04 |
REC right extended hemicolectomy, RC right hemicolectomy, LC left hemicolectomy, N Number
Compared with LC and RC, REC was associated with a longer time to first flatus (p = 0.04), delayed tolerance of solid diet (p < 0.001), and increased LOS (p < 0.001). Time to first bowel movement was significantly longer in REC patients compared to LC patients (p = 0.005) but similar to RC patients. REC patients also had a significantly higher risk for the need for NGT decompression postoperatively compared to RC and LC (p < 0.001), but a similar risk for the need for TPN. No differences were found between RC and LC in any of the outcome measures (Table 3).
Table 3.
Study endpoints
| Group | REC (N = 85) |
RC (N = 207) |
LC (N = 242) |
p-value |
|---|---|---|---|---|
| Variable | ||||
|
LOS Days, median (IQR) |
9 (8–13) | 7 (6–10) | 7 (6–10) |
REC vs RC: < 0.001 REC vs LC: < 0.001 RC vs LC: 1 |
|
TPN N (%) |
7 (8.2) | 11 (5.3) | 11 (4.5) | 0.454 |
|
NGT N (%) |
16 (18.8) | 15 (7.2) | 10 (4.1) |
REC vs RC: 0.003 REC vs LC: < 0.001 RC vs LC: 0.745 |
| First post-op flatus POD, median (IQR) | 4 (4–5) | 4 (3–5) | 4 (3–5) |
REC vs RC: 0.04 REC vs LC: 0.001 RC vs LC: 0.386 |
|
First post-op bowel movement POD, median (IQR) |
5 (4–6) | 5 (4–6) | 4 (3–6) |
REC vs RC: 0.113 REC vs LC: 0.005 RC vs LC: 0.531 |
|
Solid Diet POD, median (IQR) |
6 (5–8) | 5 (4–6) | 5 (4–6) |
REC vs RC: < 0.001 REC vs LC: < 0.001 RC vs LC: 0.23 |
REC right extended hemicolectomy, RC right hemicolectomy, LC left hemicolectomy, LOS length of hospital stay, N Number, IQR Interquartile Range, TPN need for Total Parenteral Nutrition, NGT need for Nasogastric Tube decompression, Post-op Postoperative, POD Post Operative Day
Other risk factors for PPOI included surgery via open approach and advanced T stage, which were found to be risk factors for a longer time to first bowel movement (p = 0.005 and p = 0.011 respectively), and older age, which was found to be a risk factor for a longer time to first flatus (p = 0.018). Open surgery (p = 0.023), old age (p = 0.007), advanced stage (p = 0.03) and anastomotic leak after surgery (p < 0.001) were all associated with longer time to tolerate solid low-residue diet and, as expected, longer LOS. Moreover, both open surgical approach and anastomotic leak were identified as significant predictors for the need for NGT decompression postoperatively (p = 0.048 and p = 0.014, respectively).
An ANCOVA analysis identified REC as an independent risk factor for PPOI across all outcome measures except for the first postoperative bowel movement. Additionally, analysis controlling for potential confounders (age, gender, BMI, ASA score, preoperative albumin level, surgical approach, tumor stage, operative time, and multivisceral resection) revealed that most of these variables did not significantly influence the outcomes. However, age was a significant factor affecting the length of postoperative hospital stay (p = 0.021), time to first postoperative flatus (p = 0.021), and time to tolerate diet (p = 0.031). Additionally, Surgery via open approach significantly impacted the time to first postoperative bowel movement (p = 0.006) and time to tolerate diet (p = 0.014), while tumor stage significantly affected the time to first postoperative bowel movement (p = 0.015). The detailed results of this analysis can be found in Table 4.
Table 4.
Prolonged post-operative ileus – Risk Factors
| Study Variable |
NGT (p-value) | First post-op bowel movement (p-value) |
First post-op flatus (p-value) |
Length of postoperative hospital stay (p-value) |
Solid Diet (p-value) |
|---|---|---|---|---|---|
| Surgery Group (REC vs RC vs LC) | 0.002 | 0.078 | 0.004 | < 0.001 | < 0.001 |
| Age at Diagnoses | 0.872 | 0.129 | 0.018 | 0.004 | 0.007 |
| Gender | 0.748 | 0.984 | 0.125 | 0.537 | 0.84 |
| Surgical approach (Open vs Laparoscopy vs Conversion to open surgery) | 0.048 | 0.005 | 0.073 | 0.089 | 0.023 |
| Preoperative Albumin | 0.135 | 0.824 | 0.842 | 0.399 | 0.539 |
| ASA score | 0.931 | 0.892 | 0.912 | 0.998 | 0.504 |
| Operative time | 0.343 | 0.079 | 0.144 | 0.111 | 0.957 |
| Additional resection of adjacent organs | 0.808 | 0.8 | 0.514 | 0.905 | 0.613 |
| BMI | 0.104 | 0.813 | 0.885 | 0.556 | 0.849 |
| Pathological stage | 0.0788 | 0.011 | 0.08 | 0.03 | 0.03 |
| Anastomosis leak | 0.014 | 0.74 | 0.564 | < 0.001 | < 0.001 |
NGT need for Nasogastric Tube decompression, REC right extended hemicolectomy, RC right hemicolectomy, LC left hemicolectomy, BMI Body Mass Index, ASA American Society of Anesthesiologists
Discussion
Although postoperative recovery following colorectal surgery has been well studied, few studies have directly studied PPOI following REC. Our study revealed a higher incidence of PPOI following REC compared to RC or LC. We found that REC was an independent risk factor for longer times to first flatus and bowel movement, for a longer time to tolerate a solid diet, and for the need for NGT decompression. Other risk factors for PPOI were advanced age and surgery via an open approach.
Previous studies comparing REC to LC have reported a higher proportion of urgent and open surgeries among REC patients but found no significant differences in short- or long-term postoperative outcomes [22, 23]. De’Angelis et al. compared outcomes of elective laparoscopic REC to LC in a matched case–control study of patients without metastatic disease and found similar postoperative outcomes and survival, although REC patients had significantly longer operative times [24]. Similarly, Beisani et al. conducted a multicenter study comparing elective REC to LC and found a higher overall morbidity rate, particularly ileus, among REC patients, but no significant differences in LOS, reoperation rates, or overall survival [25]. Wang et al. performed a meta-analysis of retrospective studies comparing four surgical approaches for splenic flexure tumors and, like our study, found that REC was associated with a higher risk of postoperative ileus (PPOI) [26].
Perrakis et al. explain that the rationale for extended resections is to address potential metastatic lymph nodes along the right gastroepiploic arcade, near the stomach’s greater curvature, over the pancreatic head, and along the inferior aspect of the pancreas [27]. However, studies suggest that the benefit of extensive resections may not justify the increased morbidity, as comparable R0 margin rates and oncologic outcomes can be achieved without such extensive procedures. In fact, extended resections are only truly necessary when there is clear tumor infiltration into nearby organs [28]. REC could sometimes be the procedure of choice in urgent surgeries to manage both the primary tumor and a compromised cecum, secondary to obstructing tumors. That might have led to a higher proportion of urgent procedures in our REC group compared to both the LC and RC groups. However, this difference did not reach statistical significance (p = 0.146), supporting the overall comparability and homogeneity of the three patient cohorts.
Recuzogullari et al. presented a nomogram of risk factors for ileus following colectomy, identifying “partial colectomy with removal of terminal ileum and ileocolostomy” as a formally acknowledged risk factor for ileus, entailing a relative risk (RR) of 1.218 (p = 0.003), compared to “partial colectomy with anastomosis” and “partial colectomy with low pelvic anastomosis,” which did not increase the risk of ileus (RR 0.992, p = 0.91) [29]. This finding highlights the slower postoperative recovery rate associated with colectomy with small-to-large bowel anastomosis. In the current study, ileocolostomy was associated with a higher rate of ileus only in the contest of REC, but not in RC.
The pathophysiological mechanisms underlying slower bowel function recovery following RC and/or REC remain unclear. Several mechanisms have been proposed including: Changes in rectosigmoid filling pattern [30, 31]; Bacterial translocation from the colon to the small intestine due to loss of the ileocecal valve [32, 33]; Elevated sympathetic activity from small bowel trauma during ileocolic anastomosis [34]; Variable parasympathetic innervation in the distal colon (pelvic ganglia) compared to the proximal colon (brainstem) which enhances postoperative bowel activity following LC [35] and increased bowel mobilization, manipulation, and traction when performing anastomoses in RC and REC [36]. Nevertheless, these proposed mechanisms do not necessarily explain the different risks for PPOI according to the anatomical resection.
Knowledge regarding differences of patient demographics and tumor variables between different anatomical locations of colon cancer, as well as different complication profiles and recovery patterns according to the extent of colectomy is growing. Despite this fact, preventive and treatment strategies for PPOI are still under debate. Furthermore, while various treatments for PPOI have been tested, their mechanisms of action remain unclear. Consequently, most Enhanced Recovery After Surgery protocols continue to take a non-specific approach regarding resection location and extent, as well as individual patient factors. An understanding of the specific mechanism of bowel recovery and motility pattern following different anatomical resections of the colon could enable personalized perioperative expectations and treatment.
Limitations
This study is limited by its retrospective nature, as well as by the heterogeneity in tumor location and choice of surgical procedure.
Conclusion
Functional bowel recovery is slower following REC compared to LC and RC for colon cancer, resulting in a longer hospital stay. In cases where an anatomical resection other than REC is oncologically acceptable, it should be considered. When REC is being performed, PPOI should be expected, and postoperative treatment should be planned accordingly.
Acknowledgements
The authors wish to thank Kineret Misgav and Ofer Feinstein for their insightful assistance with statistical analysis.
Author contribution
SYP, IM, AJP and NS conceptualized the study. SYP, RG, MAB, AJP and NS designed the study. SYP, AS, DP and SAS performed data acquisition. SYP, SAS, RG, MAB, IM, AJP and NS performed data analysis and interpretation. SYP and SAS prepared the tables. NS prepared the figure. SYP and NS wrote and revised the manuscript. All authors critically reviewed the manuscript and approved it.
Funding
This study did not receive any funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vather R, Trivedi S, Bissett I (2013) Defining postoperative ileus: Results of a systematic review and global survey. J Gastrointest Surgery 17:962–972. 10.1007/s11605-013-2148-y [DOI] [PubMed] [Google Scholar]
- 2.Bragg D, El-Sharkawy A, Psaltis E, Maxwell-Armstrong CA, Lobo DN (2015) Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr 34:367–376. 10.1016/j.clnu.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 3.Millan M, Biondo S, Fraccalvieri D, Frago R, Golda T, Kreisler E (2012) Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg 36:179–185. 10.1007/s00268-011-1339-5 [DOI] [PubMed] [Google Scholar]
- 4.Vather R, O’Grady G, Bissett IP, Dinning PG (2014) Postoperative ileus: mechanisms and future directions for research. Clin Exp Pharmacol Physiol 41:358–370. 10.1111/1440-1681.12220 [DOI] [PubMed] [Google Scholar]
- 5.van Bree SHW, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE (2012) New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol 9:675–683. 10.1038/nrgastro.2012.134 [DOI] [PubMed] [Google Scholar]
- 6.Glowka TR, Steinebach A, Stein K et al (2015) The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterol Motil 27:1038–1049. 10.1111/nmo.12584 [DOI] [PubMed] [Google Scholar]
- 7.Artinyan A, Nunoo-Mensah JW, Balasubramaniam S et al (2008) Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg 32:1495–1500. 10.1007/s00268-008-9491-2 [DOI] [PubMed] [Google Scholar]
- 8.Mao H, Milne TGE, O’Grady G, Vather R, Edlin R, Bissett I (2019) Prolonged postoperative ileus significantly increases the cost of inpatient stay for patients undergoing elective colorectal surgery: Results of a multivariate analysis of prospective data at a single institution. Dis Colon Rectum 62:631–637. 10.1097/DCR.0000000000001301 [DOI] [PubMed] [Google Scholar]
- 9.Tevis SE, Carchman EH, Foley EF, Harms BA, Heise CP, Kennedy GD (2015) Postoperative ileus-more than just prolonged length of stay? J Gastrointest Surg 19:1684–1690. 10.1007/s11605-015-2877-1 [DOI] [PubMed] [Google Scholar]
- 10.Livingston EH, Passaro EP Jr (1990) Postoperative ileus. Dig Dis Sci 35:121–132. 10.1007/BF01537233 [DOI] [PubMed] [Google Scholar]
- 11.Morgan WP, Jenkins N, Lewis P, Aubrey DA (1985) Management of obstructing carcinoma of the left colon by extended right hemicolectomy. Am J Surg 149:327–329. 10.1016/s0002-9610(85)80100-8 [DOI] [PubMed] [Google Scholar]
- 12.Garfinkle R, Al-Rashid F, Morin N et al (2020) Are right-sided colectomies for neoplastic disease at increased risk of primary postoperative ileus compared to left-sided colectomies? A coarsened exact matched analysis. Surg Endosc 34:5304–5311. 10.1007/s00464-019-07318-4 [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, O’Grady G, Milne T, Jaung R, Vather R, Bissett IP (2018) Prospective comparison of return of bowel function after left versus right colectomy. ANZ J Surg 88:E242–E247. 10.1111/ans.13823 [DOI] [PubMed] [Google Scholar]
- 14.Grass F, Lovely JK, Crippa J et al (2019) Comparison of recovery and outcome after left and right colectomy. Colorectal Dis 21:481–486. 10.1111/codi.14543 [DOI] [PubMed] [Google Scholar]
- 15.Phillips F, Muls AC, Lalji A, Andreyev HJN (2015) Are bile acid malabsorption and bile acid diarrhea an important cause of diarrhea complicating cancer therapy? Colorectal Dis 17:730–734. 10.1111/codi.12932 [DOI] [PubMed] [Google Scholar]
- 16.Eu KW, Lim SL, Seow-Choen F, Leong AF, Ho YH (1998) Clinical outcome and bowel function following total abdominal colectomy and ileorectal anastomosis in the Oriental population. Dis Colon Rectum 41:215–218. 10.1007/BF02238251 [DOI] [PubMed] [Google Scholar]
- 17.Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJN (2009) Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire. Clin Ther 31:2549–2558. 10.1016/j.clinthera.2009.11.027 [DOI] [PubMed] [Google Scholar]
- 18.Elphick DA, Chew TS, Higham SE, Bird N, Ahmad A, Sanders DS (2005) Small bowel bacterial overgrowth in symptomatic older people: can it be diagnosed earlier? Gerontology 51:396–401. 10.1159/000088704 [DOI] [PubMed] [Google Scholar]
- 19.Kurien M, Evans KE, Leeds JS, Hopper AD, Harris A, Sanders DS (2011) Bile acid malabsorption: an under-investigated differential diagnosis in patients presenting with diarrhea predominant irritable bowel syndrome type symptoms. Scand J Gastroenterol 46:818–822. 10.3109/00365521.2011.574728 [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 21.Rahbari NN, Weitz J, Hohenberger W et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351. 10.1016/j.surg.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 22.Odermatt M, Siddiqi N, Johns R et al (2014) Short- and long-term outcomes for patients with splenic flexure tumours treated by left versus extended right colectomy are comparable: a retrospective analysis. Surg Today 44:2045–2051. 10.1007/s00595-013-0803-2 [DOI] [PubMed] [Google Scholar]
- 23.Gravante G, Elshaer M, Parker R et al (2016) Extended right hemicolectomy and left hemicolectomy for colorectal cancers between the distal transverse and proximal descending colon. Ann R Coll Surg Engl 98:303–307. 10.1308/rcsann.2016.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de’Angelis N, Hain E, Disabato M, et al (2016) Laparoscopic extended right colectomy versus laparoscopic left colectomy for carcinoma of the splenic flexure: a matched case-control study. Int J Colorectal Dis 31:623–630. 10.1007/s00384-015-2469-2 [DOI] [PubMed] [Google Scholar]
- 25.Beisani M, Vallribera F, García A et al (2018) Subtotal colectomy versus left hemicolectomy for the elective treatment of splenic flexure colonic neoplasia. Am J Surg 216:251–254. 10.1016/j.amjsurg.2017.06.035 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Zheng Z, Chen M et al (2020) Subtotal colectomy, extended right hemicolectomy, left hemicolectomy, or splenic flexure colectomy for splenic flexure tumors: a network meta-analysis. Int J Colorectal Dis 36:311–322. 10.1007/s00384-020-03763-z [DOI] [PubMed] [Google Scholar]
- 27.Perrakis A, Weber K, Merkel S et al (2014) Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int J Colorectal Dis 29:1223–1229. 10.1007/s00384-014-1971-2 [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Pérez A, Brunetti F, Vitali GC, Abdalla S, Ris F, de’Angelis N, (2017) Surgical Treatment of Colon Cancer of the Splenic Flexure: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech 27:318–327. 10.1097/SLE.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 29.Rencuzogullari A, Benlice C, Costedio M, Remzi FH, Gorgun E (2017) Nomogram-derived prediction of postoperative ileus after colectomy: An assessment from nationwide procedure-targeted cohort. Am Surg 83:564–572. 10.1177/000313481708300620 [PubMed] [Google Scholar]
- 30.Lin AY, Du P, Dinning PG et al (2017) High-resolution anatomic correlation of cyclic motor patterns in the human colon: Evidence of a rectosigmoid brake. Am J Physiol Gastrointest Liver Physiol 312:508–515. 10.1152/ajpgi.00021.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo SHB, Bissett I, O’Grady G (2021) Variable gut function recovery after right vs. left colectomy may be due to rectosigmoid hyperactivity. Front Physiol 12: 635167. 10.3389/fphys.2021.635167. [DOI] [PMC free article] [PubMed]
- 32.Folaranmi S, Rakoczy G, Bruce J et al (2011) Ileocaecal valve: how important is it? Pediatr Surg Int 27:613–615. 10.1007/s00383-010-2841-9 [DOI] [PubMed] [Google Scholar]
- 33.Ibañez N, Abrisqueta J, Luján J, Hernández Q, Parrilla P (2017) Isoperistaltic versus antiperistaltic side-to-side anastomosis after right laparoscopic hemicolectomy for cancer (ISOVANTI) trial: study protocol for a randomised clinical trial. Int J Colorectal Dis 32:1349–1356. 10.1007/s00384-017-2840-6 [DOI] [PubMed] [Google Scholar]
- 34.Boeckxstaens GE, de Jonge WJ (2009) Neuroimmune mechanisms in postoperative ileus Gut 58:1300–1311. 10.1136/gut.2008.169250 [DOI] [PubMed] [Google Scholar]
- 35.Yorkshire Surgical Research Collaborative (2018) Multicentre observational study of gastrointestinal recovery after elective colorectal surgery. Colorectal Dis 20:536–544. 10.1111/codi.13949 [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Lu Z, Zheng Z, Cheng P, Zhou H, Wang X (2022) Comparison of short-term outcomes between totally laparoscopic right colectomy and laparoscopic-assisted right colectomy: a retrospective study in a single institution on 300 consecutive patients. Surg Endosc 36:176–184. 10.1007/s00464-020-08252-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.