Abstract
Detection of measles-specific immunoglobulin M (IgM) has become the standard diagnostic method for laboratory confirmation of measles. In outbreaks, the interpretation of an IgM-positive result can be complicated when persons with suspected measles receive a dose of measles vaccine as part of outbreak control measures. This investigation evaluated the decay of measles-specific IgM antibodies 1 to 4 months after primary vaccination with measles, mumps, and rubella vaccine (MMRII). Serum samples were obtained from 536 infants vaccinated when they were 15 months old as part of a study to assess primary and secondary measles vaccine failure. Sixty serum specimens per week were selected from specimens collected between 4 and 9 weeks after MMRII vaccination; all 176 available serum specimens collected between 10 and ≥16 weeks were included. Specimens were tested for the presence of measles-specific IgM by an antibody-capture enzyme immunoassay. The proportion of IgM-positive specimens dropped from 73% at 4 weeks after vaccination to 52% at 5 weeks after vaccination and then declined to 7% by 8 weeks after vaccination. Less than 10% of children remained IgM positive between 9 and 11 weeks. An IgM-negative result helps rule out the diagnosis of measles in a person with suspected infection and a history of recent vaccination. The interpretation of a positive IgM result from a person with a clinically suspected case of measles and a recent history of measles vaccination (especially within 8 weeks) is problematic, and the diagnosis of measles should be based on epidemiologic linkage to a confirmed case or on detection of wild-type measles virus.
In the United States, the surveillance definition of a confirmed case of measles is a clinically compatible illness (fever of >101°F, generalized rash for ≥3 days, and either cough, coryza, or conjunctivitis) plus either an epidemiologic linkage to a confirmed case or laboratory confirmation of recent measles infection (2). As measles vaccination coverage increases and the number of large outbreaks declines, the case definition is increasingly met through laboratory confirmation rather than an epidemiologic linkage. Laboratory confirmation is commonly done by detecting the presence of measles-specific immunoglobulin M (IgM) antibodies in the sera of persons with clinically suspected measles. Measles-specific IgM can usually be detected reliably between 3 and 28 days after a rash appears in persons with suspected measles by using an IgM-capture enzyme immunoassay (EIA) (5). In outbreak settings, however, persons with suspected measles may have recently received a dose of measles vaccine as part of outbreak control measures, making a positive IgM result difficult to interpret. Currently, the Centers for Disease Control and Prevention (CDC) recommends that a positive IgM result from a person who has received measles vaccine between 6 and 45 days prior to testing cannot be interpreted (8). However, the timing of the decline of IgM antibodies after measles vaccination has not been well established (7). In this report, we describe the decay of measles-specific IgM antibodies 1 to 4 months after primary vaccination with measles, mumps, and rubella vaccine (MMRII).
MATERIALS AND METHODS
Preliminary study.
For this report, we identified sera from two previous studies. In the first study, conducted between June 1992 and April 1993, children 12 to 18 months old received MMRII at the time of enrollment and varicella vaccine 6 weeks later. Serum from each child was drawn at 0, 6, and 12 weeks after primary vaccination with MMRII. We used these sera to identify a pattern in the decay of measles-specific IgM antibodies over time and to provide data to plan the primary study.
Primary study.
The original purpose of the primary study, conducted at two sites (A and B), was to evaluate primary and secondary vaccine failure after vaccination with MMRII (1). Serum samples were collected from 15- to 18-month-old children before primary vaccination with MMRII and 1 to 4 months, 3 years, and 5 years after vaccination. At site A, most of the first postvaccination blood samples were collected between 4 and 9 weeks after vaccination, while the postvaccination blood samples at study site B were collected later. Because findings from the preliminary study suggested that the most rapid IgM decay began before week 6, we used serum samples from site A for the primary study.
Available serum samples were obtained from 536 infants from site A vaccinated between January 1991 and December 1992. Sixty serum specimens per week were selected from specimens collected between 4 and 9 weeks after MMRII vaccination; all 176 available serum specimens collected between 10 and ≥16 weeks were included. For the samples collected between 4 and 9 weeks after vaccination, samples were selected to be as close to the nominal collection time as possible. All samples collected on the target day after vaccination (e.g., day 28 for week 4) were selected for testing, followed by those collected within 1 day of the target date, then by those collected within 2 days, and so forth (Table 1), until 60 specimens per week were identified. If more specimens than needed were available, the required number of samples were selected at random.
TABLE 1.
Timing of specimen collection for primary study by week after vaccination
| Wk after vaccination | No. of specimens collecteda
|
Total no. of specimens | |||
|---|---|---|---|---|---|
| ± 0 days | ± 1 day | ± 2 days | ± 3 days | ||
| 4 | 24 | 36 | 0 | 0 | 60 |
| 5 | 27 | 33 | 0 | 0 | 60 |
| 6 | 28 | 32 | 0 | 0 | 60 |
| 7 | 27 | 33 | 0 | 0 | 60 |
| 8 | 18 | 28 | 14 | 0 | 60 |
| 9 | 17 | 31 | 12 | 0 | 60 |
| 10 | 10 | 11 | 10 | 8 | 39 |
| 11 | 7 | 11 | 4 | 9 | 31 |
| 12 | 2 | 6 | 5 | 4 | 17 |
| 13 | 7 | 6 | 5 | 9 | 27 |
| 14 | 7 | 8 | 2 | 3 | 20 |
| 15 | 4 | 12 | 7 | 2 | 25 |
| ≥16 | NAb | NA | NA | NA | 17 |
| Total | 178 | 247 | 59 | 35 | 536 |
Number of specimens collected within the indicated number of days of the target date for specimen collection for different lengths of time after vaccination.
NA, not applicable.
The specimens from study site A had originally been frozen at −20°C shortly after collection and shipped to CDC on dry ice. Upon arrival at CDC, they were thawed and tested for the presence of measles-specific IgG and refrozen at −20°C until testing for the present study was conducted in 1997.
EIA testing.
As part of the original vaccine trials, testing for measles-specific IgG antibodies was performed by an indirect EIA described previously (6). For the preliminary and primary studies, serum samples were tested for the presence of measles-specific IgM antibodies by a previously described IgM-capture EIA (6). Briefly, microtiter plates were coated with goat anti-human IgM antibodies diluted in phosphate-buffered saline (PBS). The plates were incubated for 1 h at 37°C and then washed. Next, serum diluted 1:200 in PBS with 0.5% gelatin and 0.15% Tween 20 (PBS-GT) was added to four consecutive wells, followed by incubation for 1 h at 37°C and then washing. Baculovirus-measles virus nucleoprotein or sf9-uninfected cell control lysate diluted in PBS-GT with 4% normal goat serum and 0.3% sodium deoxycholate was added to duplicate cells, and the plates were incubated for 2 h at 37°C and washed. The plates were then incubated with biotinylated monoclonal antibody (83VIIKK2) in PBS-GT for 1 h at 37°C and washed. Streptavidin-peroxidase in PBS-GT was added, and the plates were incubated for 20 min and washed again. Tetramethylbenzidine substrate was added for 15 min, and the reaction was stopped by acidification. Optical densities for antigen-positive and -negative wells were then determined photometrically.
IgM-capture EIA results were expressed as the average difference in measured optical density values between duplicate wells of positive antigen (P) and negative tissue culture control antigen (N) for each serum specimen (P-N). A positive cutoff value was defined as a P-N value of ≥0.10 and a P/N ratio of ≥3. A borderline value was defined as either (i) 0.09 ≤ P-N < 0.10 and P/N ≥ 3 or (ii) P-N ≥ 0.10 and 2 ≤ P/N < 3.
RESULTS
Preliminary study.
Serum specimens from the second blood collection were available for testing for IgM antibodies from 62 children (median age, 15.8 months; age range, 12.3 to 24.2 months). These specimens were collected a median of 6.6 weeks after MMRII vaccination (range, 6 to 11 weeks). Overall, 21% (13 of 62) of children were IgM positive, 6.5% (4 of 62) had borderline IgM results, and 72.6% (45 of 62) were IgM negative. Serum from the third blood draw, collected a median of 13 weeks after vaccination (range, 12 to 18 weeks), was available for 60 children. For the third blood draw, 10% (6 of 60) were IgM positive, 1.7% (1 of 60) had borderline IgM results, and 88.3% (53 of 60) were IgM negative.
Primary study.
The median age of children included in the analysis was 15.2 months (range, 14.5 to 19.2 months; age information was missing for five children). Two hundred ninety-seven (56%) of 532 children were male (gender was unknown for 4 children). Five hundred twenty-eight of 531 (99.4%) samples tested were IgG positive for measles. Table 1 shows the timing of sample collection after vaccination.
Figure 1a illustrates the proportion of samples that were IgM positive by week after vaccination. The proportion that was IgM positive dropped from 73% at 4 weeks after vaccination to 52% at 5 weeks after vaccination and then declined to 7% by 8 weeks after vaccination. Figure 1b shows the optical density (P-N) value for each sample plotted against week after vaccination. Most samples were IgM negative by 8 weeks, with a small percentage of children exhibiting low levels of IgM positivity for approximately 4 more weeks. However, as seen in Table 1, fewer samples were available for testing beginning with week 10, making the estimates beyond this time less precise.
FIG. 1.
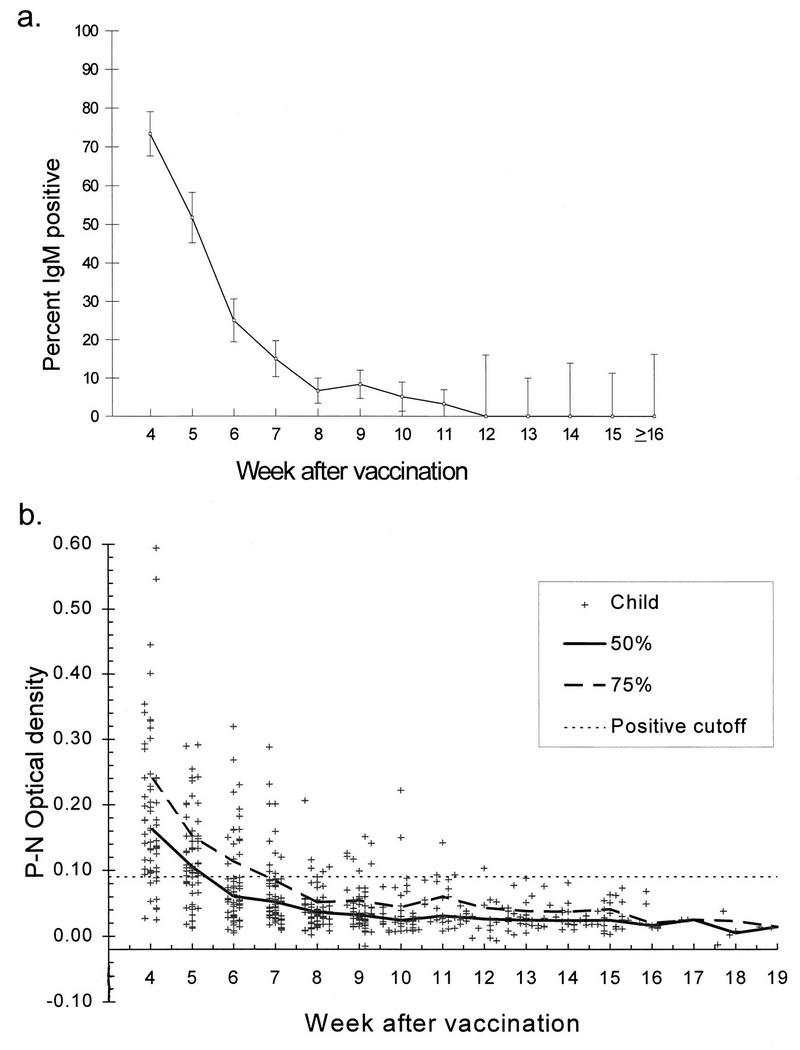
(a) Percentage of specimens that were IgM positive over time (weeks after MMRII vaccination). Vertical lines represent 95% confidence boundaries. A week was defined as a multiple of 7 ± 3 days (e.g., week 10 = 67 to 73 days). (b) Difference in optical densities of positive and negative (P-N) wells for each child by week after MMRII vaccination. The heavy solid and dashed lines show the 50th and 75th percentiles, respectively, for each week as defined above. The horizontal dashed line represents the cutoff P-N value of 0.10, which was used in combination with the P/N ratio to determine whether the specimen was considered IgM positive, borderline, or negative.
Comparison of the two studies.
The proportion of children with IgM-positive results 6 to 7 weeks after vaccination in the two studies was similar; 25% (13 of 53) of samples in the first study were IgM positive compared with 20% (24 of 120) of samples in the second study (P = 0.50 by Mantel-Haenszel chi-square test). However, at 12 to 15 weeks, the rate of IgM positivity in the first study (10%) was significantly higher than the rate of IgM positivity in the second study (0%) (P = 0.002 by Mantel-Haenszel chi-square test, controlling for week).
DISCUSSION
The results of the primary study illustrate that the proportion of vaccine recipients with detectable IgM antibodies declines rapidly between 4 and 8 weeks after vaccination. By 8 to 12 weeks, 10% or less of samples remained IgM positive. The proportions of children who were IgM positive at 12 to 15 weeks differed for the two studies for reasons that are not clear. Data from both studies indicate that most IgM has disappeared by 8 weeks and that a small percentage (10% or less) of persons may remain IgM positive for an additional 1 to 2 months.
The finding that only 73% of the vaccinees were IgM positive (8% borderline) at 4 weeks was unexpected. Earlier data from Erdman and colleagues (4) using the IgM-capture EIA demonstrated that 97% children were IgM positive 3 weeks after vaccination. IgM antibodies may peak at 3 weeks and be declining at 4 weeks. A study is currently under way to test this hypothesis, and early data suggest that this is the case (3). If so, the interval of highest sensitivity for detecting measles-specific IgM antibodies after primary vaccination may be very narrow. Even if this is the case, it should still be possible to determine seroconversion by using specimens tested for the presence of both measles-specific IgM and IgG antibodies; persons who are IgM negative at 4 weeks or more after vaccination should have developed measles-specific IgG by this time. In this study, 99.4% samples were IgG positive.
The results of this study should help health professionals interpret an IgM test result from a person with clinically suspected measles who has been recently vaccinated. A negative IgM result can help exclude measles as a diagnosis. However, a positive result from a recently vaccinated person is not diagnostic of a wild-type measles virus infection. Moreover, the magnitude of the IgM response does not help to distinguish between vaccine and wild-type measles virus infection. Our results provide some guidelines on the period during which vaccine-associated IgM is likely to be present. This information can be used in conjunction with the clinical and epidemiologic information in making a final interpretation of the result. For example, a positive IgM result from a person with suspected measles known to have been exposed 2 weeks earlier but who had been vaccinated 8 weeks earlier is most likely to reflect a wild-type measles virus infection. On the other hand, a positive result from a person who develops a rash 5 weeks after measles vaccination may be difficult to interpret; in this case, one cannot distinguish wild-type infection from vaccine infection based on IgM results, and a diagnosis of measles should be based on an epidemiologic linkage to a confirmed case or on the detection of wild-type measles virus.
ACKNOWLEDGMENTS
We thank the following persons for their contributions: Janet Heath and Alissa Murray for laboratory testing, John O’Connor for editorial support, Steve Redd for critical review of the manuscript, and Sheila Burns for coordinating study participants.
REFERENCES
- 1.Atkinson W, Markowitz L, Baughman A, Sullivan B, Nordin J, Heath J, Erdman D, Nelson A, Patricia P. Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. Response to measles revaccination among children who fail to respond to the first dose of measles vaccine, abstr. 421; p. 181. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46RR-10:1–64. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Unpublished data.
- 4.Erdman D, Anderson L, Adams D, Stewart J, Markowitz L, Bellini W. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helfand R F, Heath J L, Anderson L J, Maes E F, Guris D, Bellini W J. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis. 1997;175:195–199. doi: 10.1093/infdis/175.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Hummel K, Erdman D, Heath J, Bellini W. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz L E, Katz S L. Measles vaccine. In: Plotkin S A, Mortimer E A, editors. Vaccines. W. B. Philadelphia, Pa: Saunders; 1994. pp. 229–276. [Google Scholar]
- 8.Redd S C, Wharton M, Helfand R. Manual for the surveillance of vaccine-preventable diseases. Atlanta, Ga: Centers for Disease Control and Prevention; 1996. Measles; pp. 2:50–2:67. [Google Scholar]


