Abstract
Manipulating biology through chemistry is older than the discipline of chemistry itself. Traditionally, selectivity of oral or intravenous drugs relied on preferential drug-receptor binding. A novel approach exploiting image-guided techniques to impart spatial selectivity opens up a wide range of new possibilities for study and manipulation of biology. Motivated initially by the poor prognosis for solid tumors such as liver cancer, we demonstrate the use of an extreme form of in vivo chemistry for targeted delivery of 2-propylpentanoyl chloride in a swine model. The ensuing reaction in tissue devitalizes it by multiple mechanisms with lasting effects and, critically, demonstrates very low systemic exposure compared to controls. This work points toward a new, powerful strategy for investigating the interface between chemistry and biology in vivo.
Keywords: hepatocellular carcinoma, liver cancer, transarterial chemoembolization, covalent modification, image-guided chemistry, thermoembolization


1. Introduction
Treating solid tumors is an ongoing challenge. Cancer originating in the liver (hepatocellular carcinoma or HCC) remains a particularly complex problem despite advances in technology and medicine in the past few decades. Incidence is increasing and overall survival remains poor, with HCC being the third most common cause of cancer mortality worldwide and causing nearly 1 million deaths annually. , After many disappointing clinical trials, advances in medical therapies have finally begun to turn the tide but much room remains for improvement. − Unfortunately, few patients are candidates for potentially curative surgery or liver transplantation by the time they are diagnosed. When a large tumor or multiple tumors are present, a minimally invasive procedure referred to as transarterial chemoembolization (TACE) is often brought to bear. In this procedure, a small catheter is advanced under imaging guidance to a location slightly upstream in the vascular bed supplying a tumor to exploit the predominantly arterial blood supply of a tumor. From this position, doxorubicin is delivered as an emulsion and the blood flow to the area is blocked with an embolic material. In contrast to oral or intravenous delivery routes that distribute a drug throughout the body repeatedly, in TACE the drug is largely retained in the target area for an extended period of time and at a substantially higher concentration than seen in intravenous chemotherapy. This strategy results in lower systemic toxicity from the drug than would otherwise be expected. A survival benefit is well-established, , but it has plateaued and the procedure is rarely curative. When explanted HCC specimens that have been treated by TACE are examined at pathology, viable tumor is present in the majority of cases. −
We sought to address incomplete treatment by viewing the problem through a chemical biology lens, developing a fundamentally new method we call thermoembolization. Interpreted broadly, we envisioned that this could involve directed application of chemistry in vivo using image guided techniques similar to those used in TACE. In the present report, we hypothesized that selective delivery of a suitable electrophile to a precisely targeted vascular bed would result in a hydrolysis reaction with targeted effects in the desired region yet minimal systemic exposure, depicted in Figure . We report here on the effects in vivo and persistence of the material after 7 days in tissue using 2-propylpentanoyl chloride (VPACl) in a swine model. This compound also functions as a prodrug of valproate (VPA), a well-known drug which has antitumor activity in addition to its extensive use in neurologic conditions. To understand and quantify potential whole-body exposure from thermoembolization we measured systemic plasma levels of VPA following the experimental procedure and compared the results to positive controls.
1.

Hydrolysis of 2-propylpentanoyl chloride on reaction with water in tissue yields 2 equiv of acid and simultaneously releases heat energy.
2. Methods
2.1. Animal Care
The study was performed under a protocol approved by the institutional animal care and use committee (00001478-RN03 approval date 8/20/24). Animals (n = 3) were housed in accordance with institutional policy. Induction was accomplished with tiletamine hydrochloride and zolazepam intramuscular injection (Zoetis US, Parsippany, NJ), followed by intubation and maintenance of general anesthesia with 2–4% isoflurane. At the conclusion of the study, euthanasia was accomplished with an intravenous dose of phenytoin and pentobarbital.
2.2. Reagents
2-Propylpentanoyl chloride 98% (CAS 2936-08-5) and valproic acid (CAS 99-66-1) analytical grade (Millipore-Sigma, St. Louis, MO) were used as supplied. Immediately prior to use, the required amount was prepared as a solution in ethiodized oil (Lipiodol, Guerbet, Princeton NJ) at a concentration of 1 mol/L.
2.3. Imaging
Computed tomography (CT) imaging was performed on a Siemens Definition Edge scanner (Siemens Healthineers, Forchheim, Germany) and fluoroscopy was accomplished with a linked Artis-Q unit for digital subtraction angiography (DSA). Scans were obtained preprocedure, immediately postprocedure, and at 7 days postprocedure. Iodinated contrast (Visipaque 320, GE Healthcare, Milwaukee, WI) was given intravenously or intraarterially as appropriate. Image reconstruction was performed at 0.5 mm and 3 mm slice thickness. Postprocessing for multiplanar reformats was performed using Osirix MD v14.0.1 (Pixmeo, Geneva, Switzerland).
2.4. Angiography and Thermoembolization Procedure
A 5F introducer sheath was placed in the common femoral artery and through this access a 5F reverse curve catheter was negotiated to the celiac artery for digital subtraction angiography at up to 7 frames per second. Through this base catheter a 2.8F Renegade Hi-Flo microcatheter and Fathom 0.018 guidewire (Boston Scientific, Marlborough MA) were negotiated more distally in the hepatic arterial tree to the level of the target lobar supply within the liver. A baseline angiogram was obtained, after which the thermoembolization solution (1 mol/L 2-propylpentanoyl chloride in ethiodized oil) was delivered in small aliquots under direct visualization. The procedure was terminated when either the full amount (approximately 600 μL) had been delivered or stasis was achieved. A completion angiogram was then obtained followed by a noncontrast CT scan to document distribution of the ethiodized oil. CT hepatic arteriography was performed via the microcatheter positioned in the proximal common hepatic artery using 3 mL per sec/18 mL total/2 s inj. delay.
2.5. Pathology
At 7 days post treatment, following a final CT scan and euthanasia, en bloc removal of the liver was performed. Gross specimen photography and CT of the liver was performed, and sections were taken for fixation in 10% neutral buffered formalin solution. After paraffin embedding and sectioning, samples were processed for histopathologic analysis. Hematoxylin/eosin and immunohistochemistry/immunofluorescence stains were performed per standard protocols.
2.6. Analytical Methods
Serial blood samples were obtained via remote site to prevent contamination that would confound the results (common femoral vein). Samples were drawn beginning 2 min prior to delivery and at time points of 0, 2, 5, 10, 15, 20, 45, 60, and 120 min after the delivery was completed or after intravenous bolus injection. As a positive internal control in each animal, valproate sodium injection, USP 100 mg/mL (Athenex, Buffalo NY) was administered via ear vein 7 days after the thermoembolization procedure as a bolus at 20 mg/kg with blood samples drawn as before. Data were reported as averages from the three animals. Plasma samples were prepared for mass spectrometric analysis by protein precipitation using methanol to denature plasma proteins. Denatured proteins were removed from sample by centrifugation. Clear plasma supernatant was analyzed by flow injection using a Sciex 5500+ mass spectrometer (Sciex, Toronto, Canada). Valproic acid was detected using a mass transition of m/z 142.65 > 142.65 in electrospray negative ionization mode. Quantification of valproic acid was done by comparison of sample peak area to a reference standard regression curve prepared from analytical grade valproic acid added to protein precipitated porcine plasma. A Phenomenex Kinetex C18 column (Phenomenex, Torrance, CA) was used to focus sample injections.
3. Results
3.1. Laboratory Studies
Baseline and 7-day blood samples were obtained. All major analytes, including complete blood count and comprehensive metabolic panel (electrolytes, renal and hepatic panel), were compared and remained within normal limits (Supporting Materials).
3.2. Imaging
See Figures and .
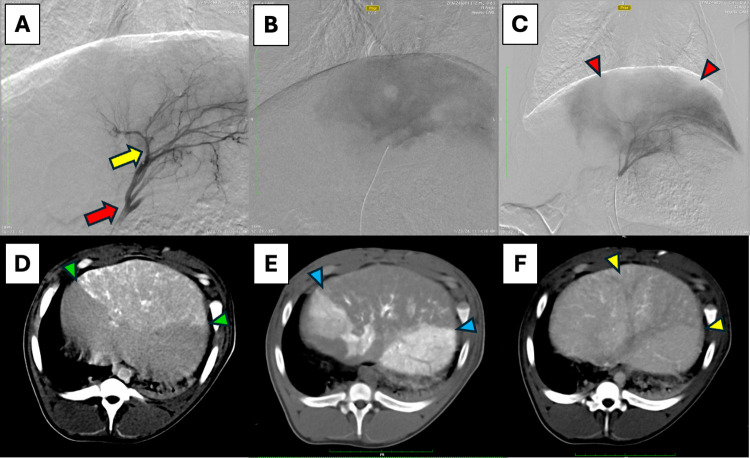
2.
In vivo procedural imaging of thermoembolization using digital subtraction angiography (DSA) and computed tomography (CT): (A) DSA mapping of left hepatic artery, red arrow–tip of microcatheter, yellow arrow–tip of microcatheter in following magnified views. (B) DSA in late capillary phase showing blush of contrast in treated area (note: clear circular area is a draining hepatic vein en face devoid of contrast). (C) DSA in late capillary phase of same region post-treatment showing the nonperfused segment (absence of blush) denoted by red arrowheads surrounded by perfused tissue with contrast. (D) CT without contrast, immediately post procedure revealing the treated area with ethiodized oil/VPACl solution appearing as white and light gray areas (green arrowheads). (E) CT with contrast directly injected in the hepatic artery clearly shows enhancement in the surrounding perfused areas but not in the treated section (blue arrowheads). (F) CT with intravenous contrast in late portal venous phase showing differential portal perfusion in the treated area (yellow arrowheads) relative to surrounding liver.
3.
Imaging at 7 days post treatment to assess persistence of material in tissue. (A) CT without contrast showing residual ethiodized oil solution as whiter areas (yellow arrow) in the treated area of the liver. (B) CT of explanted liver clearly showing lobar distribution of the thermoembolization solution appearing as white against the gray backdrop of the untreated liver (green arrow).
3.3. Plasma Levels
See Figure .
4.
Graph of plasma levels for valproate over 2 h, front-weighted. Thermoembolization (blue) shows a much lower and later peak concentration of VPA which rapidly returns to near baseline. After 7 days, the positive control of an intravenous bolus shows a rapid rise as expected. Over the course of the 2 h sampling window the level is reduced by approximately 50% but the procedure was terminated and animals were euthanized before levels returned to baseline.
3.4. Histopathology
See Figure .
5.
Liver Pathology of VPACl Thermoembolization in a Pig Model. (A,B) Images showing multifocal to coalescing coagulative necrosis in the left medial liver lobe. (C) Image showing coagulative hepatic necrosis on the cut section. (D) Photomicrograph showing multifocal to coalescing areas of coagulative necrosis, the largest of which measures 6478 μm × 2972 μm (H&E, 0.5×), (E) Photomicrograph showing focally extensive area of chronic hepatocellular necrosis and fibrosis. (F) Photomicrograph showing lytic necrosis in central region of chronic lesion (H&E, 40×). (G) Photomicrograph showing peripheral fibrosis and early neovascularization with infiltration by low to moderate numbers of lymphocytes, macrophages, and neutrophils. (H) Photomicrograph showing focal area of acute coagulative hepatocellular necrosis with hemorrhage. (I) Photomicrograph showing a central area of hemorrhage in area of hepatocellular necrosis. (J) Photomicrograph showing karyolysis and infiltration by abundant viable and degenerate neutrophils at the periphery of an area of acute hepatocellular coagulative necrosis (H&E, 40×).
4. Discussion
Several requirements must be met for a candidate electrophile to be suitable for delivery through a microcatheter in vivo. First, the reagent should be appropriately reactive but not too reactive with nucleophiles including water and tissues. Second, from a pharmacokinetic standpoint, a compound should have adequate solubility in the delivery vehicle, in this case ethiodized oil. An important property for controlled delivery and for quantitation with CT imaging is that the delivery solution must be visible in real time at fluoroscopy. Ethiodized oil fulfills this requirement as it contains covalently bound iodine, rendering the liquid radiopaque. Third, the compound must not self-react due to internal incompatibilities inherent in structure.
In addition to the above general requirements, the selection of 2-propylpentanoyl chloride would offer several more advantages if our hypothesis of hydrolysis to valproate in tissues is correct. Antitumor activity of valproate across multiple cancer cell types has been shown, with the most evidence in support of inhibition of histone deactylases (HDACs). − Among its varied effects, VPA is known to inhibit class I (1, 2, 3, 8) and class IIa (4, 5, 7, 9) HDACs. VPA also benefits from considerable familiarity and availability in medicine. It is an FDA approved drug and is on the WHO list of essential medicines for anticonvulsant, mood disorder, and migraine therapies. It has predicted safety in the proposed use, side effects are well understood, and because it is widely used and widely available, it is readily assayed with established protocols.
An example case is shown in Figure with DSA done in 3 stages for the procedure. First we show the mapping of the target vascular bed in a lobe of the liver at an early time point, in which only the vessels are opacified by contrast. This is followed by an image taken several seconds later when the microcatheter has been navigated more distally into the lobar artery. The contrast has flowed from the vessels to the capillary level, and a diffuse blush in the tissue appears before washing away. The final DSA image was obtained after delivery of the thermoembolization solution, at the same catheter position and a corresponding time point to visualize the blush. A filling defect can be appreciated in the treated section and is manifest as an area that does not show the expected blush. The disruption of blood flow in the treated area caused by thermoembolization means that contrast is excluded from the area. Similarly, immediately after the procedure a CT scan was obtained, this time without contrast. Due to the radiopaque property of the ethiodized oil, the treated area is revealed as a wedge-shaped area in white and shades of light gray. A subsequent CT scan obtained shortly after contrast was injected through the microcatheter in the artery just upstream of the area reveals a large perfusion defect compared to the contrast flowing antegrade through the surrounding untreated tissues. Finally, a delayed portal venous phase image from a scan in which contrast was given intravenously reveals again the wedge-shaped defect. Importantly, this defect illustrates that the arterial delivery is also impeding portal venous flow in addition to the disruption on the arterial side. The dual blood supply of the liver has therefore been impacted significantly by the thermoembolization procedure.
Prior reports ,− had shown persistence of thermoembolization solutions in tissues for up to 24 h, but whether the material might wash out into the body over time or not was unknown. If this were to occur, it would decrease effectiveness and increase potential for toxicity beyond the desired targeted area. In this report, we show the material remained in place for the duration of the 7-day study. Figure shows this to particularly good effect in the CT images obtained on day 7 in vivo without contrast and of the explanted specimen that was subsequently scanned ex vivo. These encouraging results and the degree of coagulative necrosis revealed by histopathology suggest that the treated areas were permanently devitalized. The data further argue for a posited reservoir function for a drug in the treated tissue in much the same manner as in TACE, that would diffuse outward over time to provide an extended benefit around the margins where recurrence would be most likely.
Observed plasma levels of valproate are noteworthy for several reasons. As shown in Figure the peak level of VPA in plasma from thermoembolization (averaged at each time point across the 3 subjects, see supporting material) was quite low, peaking at 3–4 μg/mL and returning rapidly to baseline. This is much lower than the control IV dose given 1 week later, which peaked at 42 μg/mL and was still elevated at 18 μg/mL when sampling was terminated 2h after delivery and the animals were euthanized. The increase in concentration observed in thermoembolization was blunted compared to the IV dose, which was abrupt as would be anticipated from bolus administration directly into the circulation. No symptoms or signs of toxicity specific to VPA were observed. This is to be expected given that only a single dose was administered and because of the disruption of perfusion resulting from the treatment. Recently a much higher dose of 150 mg/kg IV × 2 within 5h was reported to be well tolerated in a swine model of resuscitation after trauma. To provide context, the therapeutic range in humans is 50–100 μg/mL and the maximum tolerated dose in healthy human subjects of a single dose of intravenous valproic acid is reported as 140 mg/kg.
Screening programs for early detection in patients at risk for HCC are established, but in spite of this the disease continues to increase in both incidence and mortality worldwide. Major underlying causes of HCC include viral hepatitis, alcohol use, and metabolic fatty liver disease. These conditions affect the overall health of the liver and lessen its capacity to withstand insult. Accordingly, the degree of compromise in liver function for a given patient must be borne in mind to avoid decompensation and complications arising from intervention. Compounding the situation further, HCC also spans a range of genetic mutations. − Individual tumors may be less likely to respond to many targeted drugs if the mutation profile is a poor match for the drug target. With such diversity in causes and in the mutational status, a nonspecific method may contribute a decided advantage. Although thermoembolization with an acid chloride as the electrophile is not expected to be selective for a specific receptor or functional group, the spatial selectivity for controlled delivery within the body can be quite precise.
The tissue damage observed at histopathology was primarily coagulative necrosis with a relatively narrow margin as seen in Figure . The radius of the damage bears further comment as it was quite extensive, reaching hundreds and frequently thousands of microns beyond what was presumably the vessel lumen where the reagent was delivered. This distance compares very favorably against the radius of cytotoxic levels for doxorubicin in TACE as discussed further below. The most accurate studies in this regard used quantitative microspectrofluoroscopy to investigate a variant of TACE (drug-eluting beads, presumably a best-case scenario). Using this technique and beads loaded with doxorubicin, a concentration of 1 μM was observed to extend 100–200 μm outward from the bead surface. Worth noting is that the concentration at this distance is in the IC50 range for several cancer cell lines rather than complete cell kill. This contrasts sharply with the complete necrosis observed in the present study. Histologic images showed that thermoembolization caused complete cytoskeletal disruption up to the margin.
With respect to the observed damage, historical context provides some additional insight. For many years, small HCC was treated with chemical ablation under image guidance. The majority of the experience was accrued with direct intratumoral injection of ethanol as a protein denaturant. Later reports emerged indicating that 50% acetic acid was also very effective at causing local tissue coagulation. Both acetic acid and valproic acid are weak aliphatic carboxylic acids, and the pK a values are quite similar at 4.7–4.8. This precedent suggests that one of the likely mechanisms for cell death in the present study is due to valproic acid generated in situ (the hydrolysis product of the acid chloride) acting as a denaturant. Hydrolysis should also result in localized generation of an equivalent of HCl, further contributing to acidic denaturation in surrounding tissues. We propose that the observed results arise from synergy between acidic denaturation, the exotherm from hydrolysis of an acid chloride, and ischemia due to vessel blockade.
5. Conclusions
We conclude that thermoembolization with VPACl has potential to provide an effective, durable, and highly selective strategy for targeted therapy with very minimal systemic exposure. Further work with a larger sample size will be needed to validate these findings. Based on the results, application in tumor models and a more detailed interrogation of molecular aspects and tissue concentrations of reaction products such as VPA are warranted. The relative contributions from acidic, thermal, and ischemic components are as yet unclear and will require further study. Additional work will be needed for technical optimization regarding the dose delivered and in development of protocols that ensure complete coverage. Perhaps most importantly, however, the way now appears open for studies across a wide spectrum of reactivity using different electrophiles already familiar from covalent chemistry with specific functional group selectivity. It should therefore be possible to expand this strategy to noncytotoxic and nonembolic applications at the interface of chemistry and biology. Combining image-guided methods with chemistry may provide a powerful new way to manipulate biological systems in vivo in the broader field of chemical biology.
Supplementary Material
Acknowledgments
Funding acknowledgements: MD Anderson Bridge Fund, Dunn Foundation, CCSG.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.4c01456.
Laboratory values (electrolytes, liver function, complete blood counts), and plasma valproate levels of each animal for all time points over 2 h for thermoembolization and for IV positive control of sodium valproate 7 days later (PDF)
Combined tables CBC and Chem16 (XLSX)
Plasma level raw data and Graph (XLSX)
The authors declare no competing financial interest.
References
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., Jemal A.. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- Vogel A., Meyer T., Sapisochin G., Salem R., Saborowski A.. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- Kudo M., Finn R. S., Galle P. R., Zhu A. X., Ducreux M., Cheng A. L., Ikeda M., Tsuchiya K., Aoki K. I., Jia J.. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer. 2023;12(3):238–250. doi: 10.1159/000528272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquijo-Ponce J. J., Alventosa-Mateu C., Latorre-Sanchez M., Castello-Miralles I., Diago M.. Present and future of new systemic therapies for early and intermediate stages of hepatocellular carcinoma. World J. Gastroenterol. 2024;30(19):2512–2522. doi: 10.3748/wjg.v30.i19.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan J. D., Kennedy E. B., Abou-Alfa G. K., Beal E., Finn R. S., Gade T. P., Goff L., Gupta S., Guy J., Hoang H. T.. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024;42:02745. doi: 10.1200/JCO.23.02745. [DOI] [PubMed] [Google Scholar]
- Llovet J. M., Real M. I., Montaña X., Planas R., Coll S., Aponte J., Ayuso C., Sala M., Muchart J., Solà R.. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Lo C. M., Ngan H., Tso W. K., Liu C. L., Lam C. M., Poon R. T., Fan S. T., Wong J.. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- Mosenthal M., Adams W., Cotler S., Ding X., Borge M., Malamis A., Lee D., Thomas T., Jawahar A., Amin P.. et al. Locoregional Therapies for Hepatocellular Carcinoma prior to Liver Transplant: Comparative Pathologic Necrosis, Radiologic Response, and Recurrence. J. Vasc. Interv. Radiol. 2024;35(4):506–514. doi: 10.1016/j.jvir.2023.12.009. [DOI] [PubMed] [Google Scholar]
- Sarwar A., Bonder A., Hassan L., Malik M. S., Novack V., Curry M., Ahmed M.. Factors Associated With Complete Pathologic Necrosis of Hepatocellular Carcinoma on Explant Evaluation After Locoregional Therapy: A National Analysis Using the UNOS Database. AJR Am. J. Roentgenol. 2023;220(5):727–735. doi: 10.2214/AJR.22.28385. [DOI] [PubMed] [Google Scholar]
- Toskich B., Vidal L. L., Olson M. T., Lewis J. T., LeGout J. D., Sella D. M., Montazeri S. A., Devcic Z., Lewis A. R., Frey G. T.. Pathologic Response of Hepatocellular Carcinoma Treated with Yttrium-90 Glass Microsphere Radiation Segmentectomy Prior to Liver Transplantation: A Validation Study. J. Vasc. Interv. Radiol. 2021;32(4):518–526e511. doi: 10.1016/j.jvir.2020.12.019. [DOI] [PubMed] [Google Scholar]
- Sen A., Troncoso P., Venkatesan A., Pagel M. D., Nijkamp J. A., He Y., Lesage A. C., Woodland M., Brock K. K.. Correlation of in-vivo imaging with histopathology: A review. Eur. J. Radiol. 2021;144:109964. doi: 10.1016/j.ejrad.2021.109964. [DOI] [PubMed] [Google Scholar]
- Li C., Wang M. D., Lu L., Wu H., Yu J. J., Zhang W. G., Pawlik T. M., Zhang Y. M., Zhou Y. H., Gu W. M.. et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (>/= 10 cm): a multicenter propensity matching analysis. Hepatol Int. 2019;13(6):736–747. doi: 10.1007/s12072-019-09981-0. [DOI] [PubMed] [Google Scholar]
- Dioguardi Burgio M., Ronot M., Bruno O., Francoz C., Paradis V., Castera L., Durand F., Soubrane O., Vilgrain V.. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016;22(11):1491–1500. doi: 10.1002/lt.24615. [DOI] [PubMed] [Google Scholar]
- Beal, E. W. ; Dittmar, K. M. ; Hanje, A. J. ; Michaels, A. J. ; Conteh, L. ; Davidson, G. ; Black, S. M. ; Bloomston, P. M. ; Dillhoff, M. E. ; Schmidt, C. R. . Pretransplant Locoregional Therapy for Hepatocellular Carcinoma: Evaluation of Explant Pathology and Overall Survival. Front . Oncol. 2016, 6. DOI: 10.3389/fonc.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette C. T., Osorio R. C., Stark J., Fok B., Boktour M. R., Guy J., Rhee J., Osorio R. W.. Conventional TACE and drug-eluting bead TACE as locoregional therapy before orthotopic liver transplantation: comparison of explant pathologic response. Transplantation. 2014;98(7):781–787. doi: 10.1097/TP.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Guo C., Baluya D. L., Thompson E. A., Whitley E. M., Cressman E. N. K.. Correlation of molecular and morphologic effects of thermoembolization in a swine model using mass spectrometry imaging. J. Mass Spectrom. 2020;55(4):e4477. doi: 10.1002/jms.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawruszak, A. ; Halasa, M. ; Okon, E. ; Kukula-Koch, W. ; Stepulak, A. . Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers (Basel) 2021, 13 (14). DOI: 3409 10.3390/cancers13143409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M., Kavoosi F.. Effect of Valproic Acid on the Class I Histone Deacetylase 1, 2 and 3, Tumor Suppressor Genes p21WAF1/CIP1 and p53, and Intrinsic Mitochondrial Apoptotic Pathway, Pro- (Bax, Bak, and Bim) and anti- (Bcl-2, Bcl-xL, and Mcl-1) Apoptotic Genes Expression, Cell Viability, and Apoptosis Induction in Hepatocellular Carcinoma HepG2 Cell Line. Asian Pac. J. Cancer Prev. 2021;22(S1):89–95. doi: 10.31557/APJCP.2021.22.S1.89. [DOI] [PubMed] [Google Scholar]
- Gurvich N., Tsygankova O. M., Meinkoth J. L., Klein P. S.. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64(3):1079–1086. doi: 10.1158/0008-5472.CAN-03-0799. [DOI] [PubMed] [Google Scholar]
- Machado M. C., Bellodi-Privato M., Kubrusly M. S., Molan N. A., Tharcisio T. Jr., de Oliveira E. R., D’Albuquerque L. A.. Valproic acid inhibits human hepatocellular cancer cells growth in vitro and in vivo. J. Exp. Ther. Oncol. 2011;9(2):85–92. [PubMed] [Google Scholar]
- Stolley, D. L. ; Crouch, A. C. ; Ozkan, A. ; Seeley, E. H. ; Whitley, E. M. ; Rylander, M. N. ; Cressman, E. N. K. . Combining Chemistry and Engineering for Hepatocellular Carcinoma: Nano-Scale and Smaller Therapies. Pharmaceutics 2020, 12 (12). DOI: 1243 10.3390/pharmaceutics12121243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman, E. N. K. ; Guo, C. X. . Feasibility study using tissue as reagent for cancer therapy: endovascular ablation via thermochemistry. Convergent Science Physical Oncology 2018, 4 (2). DOI: ARTN 25003 025003 10.1088/2057-1739/aab905. [DOI] [Google Scholar]
- Cressman E. N. K., Guo C.. First In Vivo Test of Thermoembolization: Turning Tissue Against Itself Using Transcatheter Chemistry in a Porcine Model. Cardiovasc. Intervent. Radiol. 2018;41(10):1611–1617. doi: 10.1007/s00270-018-2003-3. [DOI] [PubMed] [Google Scholar]
- Cressman E. N. K., Guo C., Karbasian N.. Image-guided chemistry altering biology: An in vivo study of thermoembolization. PLoS One. 2018;13(7):e0200471. doi: 10.1371/journal.pone.0200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Ho J. W., Keeney-Bonthrone T. P., Pai M. P., Wen B., Ober R. A., Dimonte D., Chtraklin K., Joaquin T. A., Latif Z.. Prolonging the therapeutic window for valproic acid treatment in a swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2023;95(5):657–663. doi: 10.1097/TA.0000000000004022. [DOI] [PubMed] [Google Scholar]
- Patsalos P. N., Spencer E. P., Berry D. J.. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018;40(5):526–548. doi: 10.1097/FTD.0000000000000546. [DOI] [PubMed] [Google Scholar]
- Georgoff P. E., Nikolian V. C., Bonham T., Pai M. P., Tafatia C., Halaweish I., To K., Watcharotone K., Parameswaran A., Luo R.. et al. Safety and Tolerability of Intravenous Valproic Acid in Healthy Subjects: A Phase I Dose-Escalation Trial. Clin. Pharmacokinet. 2018;57(2):209–219. doi: 10.1007/s40262-017-0553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z. J., Tsilimigras D. I., Ruff S. M., Mohseni A., Kamel I. R., Cloyd J. M., Pawlik T. M.. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023;158(4):410–420. doi: 10.1001/jamasurg.2022.7989. [DOI] [PubMed] [Google Scholar]
- Rich N. E.. Changing Epidemiology of Hepatocellular Carcinoma Within the United States and Worldwide. Surg. Oncol. Clin. N. Am. 2024;33(1):1–12. doi: 10.1016/j.soc.2023.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepo E., Caruso S., Yang J., Imbeaud S., Couchy G., Bayard Q., Letouze E., Ganne-Carrie N., Moreno C., Oussalah A.. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. 2022;23(1):161–171. doi: 10.1016/S1470-2045(21)00603-3. [DOI] [PubMed] [Google Scholar]
- Beaufrere A., Caruso S., Calderaro J., Pote N., Bijot J. C., Couchy G., Cauchy F., Vilgrain V., Zucman-Rossi J., Paradis V.. Gene expression signature as a surrogate marker of microvascular invasion on routine hepatocellular carcinoma biopsies. J. Hepatol. 2022;76(2):343–352. doi: 10.1016/j.jhep.2021.09.034. [DOI] [PubMed] [Google Scholar]
- Meunier L., Hirsch T. Z., Caruso S., Imbeaud S., Bayard Q., Roehrig A., Couchy G., Nault J. C., Llovet J. M., Blanc J. F.. DNA Methylation Signatures Reveal the Diversity of Processes Remodeling Hepatocellular Carcinoma Methylomes. Hepatology. 2021;74(2):816–834. doi: 10.1002/hep.31796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault J. C., Martin Y., Caruso S., Hirsch T. Z., Bayard Q., Calderaro J., Charpy C., Copie-Bergman C., Ziol M., Bioulac-Sage P.. et al. Clinical Impact of Genomic Diversity From Early to Advanced Hepatocellular Carcinoma. Hepatology. 2020;71(1):164–182. doi: 10.1002/hep.30811. [DOI] [PubMed] [Google Scholar]
- Calderaro J., Couchy G., Imbeaud S., Amaddeo G., Letouze E., Blanc J. F., Laurent C., Hajji Y., Azoulay D., Bioulac-Sage P.. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017;67(4):727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Safri F., Nguyen R., Zerehpooshnesfchi S., George J., Qiao L.. Heterogeneity of hepatocellular carcinoma: from mechanisms to clinical implications. Cancer Gene Ther. 2024;31(8):1105–1112. doi: 10.1038/s41417-024-00764-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namur J., Wassef M., Millot J. M., Lewis A. L., Manfait M., Laurent A.. Drug-eluting beads for liver embolization: concentration of doxorubicin in tissue and in beads in a pig model. J. Vasc. Interv. Radiol. 2010;21(2):259–267. doi: 10.1016/j.jvir.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Livraghi T., Giorgio A., Marin G., Salmi A., de Sio I., Bolondi L., Pompili M., Brunello F., Lazzaroni S., Torzilli G.. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197(1):101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- Liang H. L., Yang C. F., Pan H. B., Lai K. H., Cheng J. S., Lo G. H., Chen C. K., Lai P. H.. Small hepatocellular carcinoma: safety and efficacy of single high-dose percutaneous acetic acid injection for treatment. Radiology. 2000;214(3):769–774. doi: 10.1148/radiology.214.3.r00mr06769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.