Abstract
Background
Infectious endocarditis is an uncommon disease in dogs; however, its incidence and survival rates have increased owing to advances in the understanding of the disease and diagnostic techniques. For diagnosis, it is necessary to determine whether a dog suspected of being infected has any abnormalities that meet the modified Duke criteria. Staphylococcus spp., Streptococcus spp., and Escherichia coli are the most commonly isolated bacteria causing infective endocarditis in dogs, whereas the less commonly isolated bacteria include Pseudomonas spp. and Proteus spp.
Case presentation
A 5-year-old neutered male Maltese presented with lethargy, anorexia, anaemia, and pyrexia. A vegetative mass in the aortic valve was identified on echocardiography, and the possibility of endocarditis was considered. The dog’s fever, anorexia, and lethargy rapidly improved in response to the initial antibiotics and prednisolone, which was prescribed for a possible immune-mediated disorder. However, the dog’s condition deteriorated again after discontinuing antibiotics and tapering the prednisolone dose. During this period, Paenibacillus spp. was isolated from blood cultures. After prescribing antibiotics based on the sensitivity results and adding hydralazine to reduce afterload, the dog survived without recurrence of symptoms to date.
Conclusions
Paenibacillus spp. was identified as the causative agent of infectious endocarditis. A favourable prognosis can be expected if appropriate antibiotics in combination with medications that address the blood flow changes due to valve damage are used.
Keywords: Blood culture, Canine, Infectious endocarditis, Paenibacillus lautus, Heart failure
Background
Infectious endocarditis (IE) is an uncommon disease in dogs; however, its incidence and survival rates have increased owing to advances in the understanding of the disease and diagnostic techniques [1]. For the diagnosis, it is necessary to determine whether a dog suspected of having IE has any abnormalities that meet the modified Duke criteria [1]. The modified Duke criteria applicable in the field of veterinary medicine include major criteria, such as confirmation of vegetative lesions on echocardiography, and minor criteria, such as fever, as summarised in Table 1 [2–4]. A definitive diagnosis requires the presence of two or more major criteria, histological confirmation, or one major and two minor criteria [2]. A possible diagnosis requires the presence of one major and one minor criterion or three minor criteria [2]. IE is more prevalent in large-breed dogs, and gram-positive streptococci, staphylococci, and enterococci have been isolated as typical organisms [2–4].
Table 1.
Modified Duke criteria Met for the diagnosis of infective endocarditis in the present case
| Criteria | Specific findings |
|---|---|
| Major |
Echocardiographic findings consistent with infectious endocarditis Vegetative/erosive lesions Greater than trivial valvular insufficiency |
| Minor | Rectal temperature ≥ 39℃ |
| New or worsening heart murmur | |
| Blood cultures not meeting major criteria |
Paenibacillus species are gram-positive, endospore-forming bacteria that have not been reported to cause endocarditis in dogs [5]. Even in humans, Paenibacillus spp. do not commonly cause endocarditis, and only a few cases of endocarditis due to Paenibacillus spp. have been reported [5–7]. In these cases, there are differences in the location of the affected valve depending on the species, but most patients show successful recovery after antibiotic treatment and/or valve repair surgery [5–7].
The genus Paenibacillus comprises many species that contain compounds that promote the growth of plants such as pumpkin, rice, and switchgrass [8]. However, not all species are beneficial, and some are known to cause opportunistic infections in humans and honeybees [8, 9]. Several species have been suggested to exhibit pathogenic potential in vivo [10].
Herein, we describe the diagnosis and treatment of IE caused by Paenibacillus lautus in a small-breed dog. To the best of our knowledge, this is the first report of Paenibacillus spp. being identified as the causative agent of endocarditis in a dog.
Case presentation
A 5-year-old, 3.64 kg, neutered male Maltese was referred for evaluation of anaemia, anorexia, and fever. The patient showed signs of lameness 2–3 weeks prior to his first visit. Physical examination revealed fever (rectal temperature, 40.1 °C) and a grade III/VI diastolic murmur localised near the heart base. The mucous membranes were pale pink, and no pulse deficits were noted. The patient’s systolic blood pressure was 110 mmHg, measured using a noninvasive Doppler device. Bilateral medial patellar luxation was confirmed as grade 3, and dermatitis with erythema and alopecia were observed in the dorsal lumbar area. Cytology from the affected skin lesions revealed the presence of predominantly neutrophilic inflammation but no infectious agents.
Haematologic examination (ADVIA 2120i, Siemens Healthineers, Erlangen, Germany) revealed an increased white blood cell (WBC) count of 35.48 × 109/L (reference range: 5.2–13.9 × 109/L) and a decreased haematocrit (HCT) of 24.4% (reference range: 37.1–57.0%). A non-regenerative type of anaemia was present, with an absolute reticulocyte count of 26.8 × 109/L (reference range: 8.4–129.3 × 109/L). Serum chemistry findings were unremarkable, but the concentration of C-reactive protein (CRP) (Bionote, Hwaseong, Korea) was increased at 98.2 mg/L (reference range: 0–20 mg/L). Urinalysis revealed proteinuria with a urine protein to creatinine ratio of 0.70 (Catalyst ONE®, IDEXX, Westbrook, USA) (reference range: 0–0.2), whereas the urine sediment was inactive and urine culture yielded no growth. Thoracic radiography (VETTER-DX9, M4S CO., LTD, Gunpo, Korea) revealed a normal heart size (vertebral heart score, 9.9v) and a small amount of pleural effusion with a bronchointerstitial pattern throughout the lung fields (Fig. 1a). However, the amount of pleural effusion was too small to safely sample. To determine the cause of pleural effusion, we performed echocardiography (Aplio i700, Canon Medical System, 12 MHz sector probe, Otawara, Japan), which revealed a vegetative mass at the level of the aortic valve and aortic regurgitation with a velocity of 4.6 m/s (Fig. 2). Abdominal ultrasonography (Aplio i700, Canon Medical System, 18 MHz linear probe, Otawara, Japan), revealed no significant abnormalities associated with these symptoms. To investigate the cause of anaemia, a tick/vector comprehensive real-time polymerase chain reaction (PCR) panel (Postbio Co., Ltd., Guri, Korea) for various infectious agents, including Anaplasma, Ehrlichia, Babesia spp., and Bartonella spp., and blood smear assessment were carried out. Quantitative PCR analyses were performed using Agilent AriaMx (Agilent, Santa Clara, CA, USA), according to POSTBIO standard laboratory guidelines. The blood smear did not reveal the presence of spherocytes, inclusion bodies within the red blood cells, anisocytosis, or evidence of RBC agglutination. All PCR tests were negative. Due to the combination of lameness, fever, and skin lesions, the presence of antinuclear antibodies (ANA) using ANA-immunofluorescence assay (IDEXX Laboratories, Westbrook, USA) was assessed. There was no swelling of the peripheral joints.
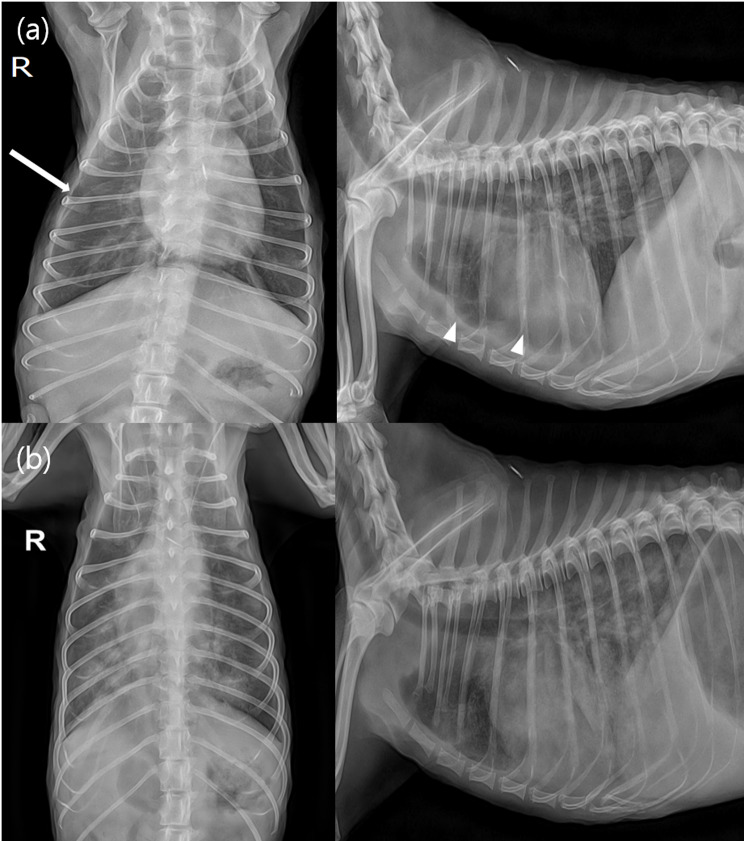
Fig. 1.
(a) Thoracic radiographs taken at the first visit. The white arrow and arrowheads indicate areas of pleural fissure lines. (b) Thoracic radiographs of the dog re-admitted 5 days after stopping antibiotics due to worsening condition. Note the severe, diffuse alveolar interstitial pattern in the lung field
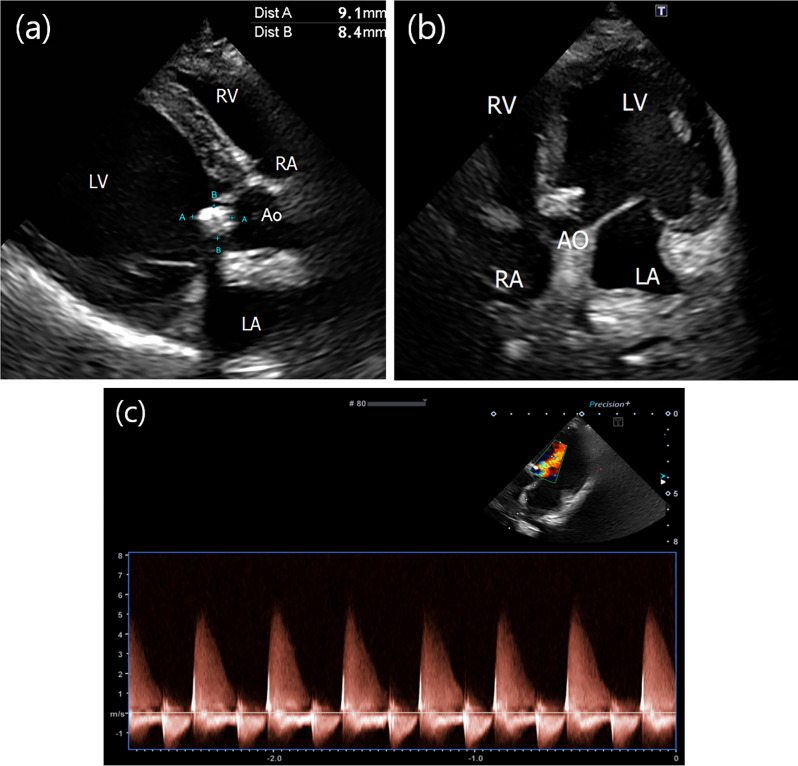
Fig. 2.
Echocardiography performed to differentiate the cause of pleural effusion. (a-b) A vegetative mass is observed at the level of the aortic valve. (c) Aortic regurgitation with a maximum reflux velocity of 4.6 m/s, expected as a result of vegetative mass, is confirmed
Based on these findings, the patient was hospitalised for treatment and monitoring. The fever resolved after the administration of intravenous (IV) fluids and antibiotics (ampicillin-sulbactam, 22 mg/kg IV q12h; Ubacilin®, Whan In Pharm Co. Ltd, Seoul, Korea). However, blood tests conducted 2 days after the initial visit showed that anaemia and CRP serum concentration had not improved despite the IV fluid administration and antibiotic treatment (HCT, 20.1%; WBC, 46.15 × 109/L; CRP, 107.5 mg/L). Therefore, pending the ANA results, prednisolone (Solondo tab, Yuhan Pharmacy, Seoul, Korea) was additionally prescribed at 1 mg/kg q12h considering the possibility of an immune-mediated disease. On the third day of hospitalisation, the dog was discharged with the following prescriptions: prednisolone 1 mg/kg PO every 12 h; amoxicillin-clavulanate (Yucla®, Yuhan Pharmacy, Seoul, Korea), 12.5 mg/kg PO every 12 h; and enrofloxacin (Baytril®, Bayer Animal Health, Seoul, Korea), 5 mg/kg PO every 24 h.
At the time of re-examination 2 days after discharge, the dog’s HCT was 22.7% and the CRP serum concentration (Bionote, Hwaseong, Korea) improved to 11.3 mg/L. Although the ANA test was confirmed to be negative on the day of follow-up, the same medications were prescribed considering the patient’s clinical response.
On the 14th day after the initial visit, the patient’s CBC and CRP serum concentration had improved (HCT, 29.6%; WBC, 23.98 × 109/L; CRP, < 10 mg/L). Furthermore, the patient’s anorexia had resolved and his rectal temperature was within the normal range.
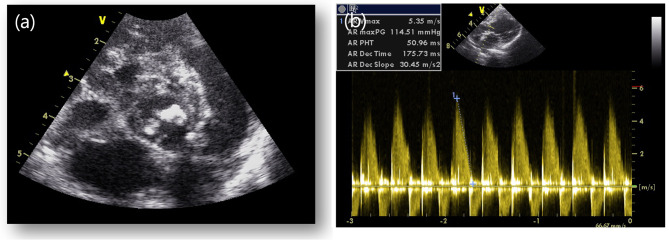
As the clinical symptoms resolved, the antibiotics were discontinued, and the dose of prednisolone was reduced to 0.75 mg/kg PO every 12 h. Five days after the discontinuation of antibiotics, the dog was readmitted due to anorexia and breathing difficulty. Physical examination revealed a rectal temperature of 39.4 °C, and thoracic radiography revealed a diffuse alveolar interstitial pattern, particularly in the caudal lung fields, indicating cardiogenic pulmonary oedema (Fig. 1b). CBC was repeated and revealed an HCT of 29.8%, which was not significantly different from that before antibiotic discontinuation; however, the WBC count was elevated to 44.04 K/µL. During this period, blood cultures were requested to determine whether infective endocarditis was the cause of the fever and pulmonary oedema. Blood was collected aseptically from the patient’s jugular vein into dedicated bottles provided by the laboratory for blood culture. Bacterial identification tests on cultured single colonies under anaerobic and aerobic conditions using MALDI-TOF (Vitek MS, Biomeriuex, France) revealed the presence of Paenibacillus lautus in the patient’s peripheral blood. N-terminal pro-B-type natriuretic peptide and canine cardiac troponin I serum concentrations (IDEXX Laboratories, Westbrook, USA) were elevated to 6853 pmol/L (reference range: 0–899 pmol/L) and 0.3 mcg/L (reference range: 0–0.1 mcg/L), respectively. Follow-up echocardiography performed to investigate the cause of clinical deterioration revealed a vegetative mass on the right coronary cusp (RCC) and posterior noncoronary cusps (NCC) of the aortic valve and prolapse of the RCC, causing severe diastolic regurgitation (Fig. 3a). The severity of aortic regurgitation was assessed based on the flail appearance of the aortic valve cusp, jet diameter, and pressure half time (Fig. 3b). Based on the echocardiographic findings, it was concluded that the RCC and NCC of the aortic valve were severely damaged due to changes caused by the infectious agent.
Fig. 3.
Echocardiographic images obtained after discontinuation of antibiotics. (a-b) The right coronary cusp (RC) and posterior noncoronary cusp (NC) of the aortic valve were thickened, and the RC was found to have prolapsed, resulting in severe diastolic regurgitation
Infectious endocarditis was diagnosed based on the results of the blood culture and the vegetative mass on the aortic valve on echocardiography.
Antibiotic treatment was restarted after the diagnosis. Antibiotics were initially administered intravenously during hospitalisation and were selected based on the sensitivity results and studies showing efficacy in previous veterinary IE cases [4] (ampicillin-sulbactam, 22 mg/kg IV q8h and enrofloxacin, 5 mg/kg IV q12h). The antibiotic susceptibility test showed susceptibility to enrofloxacin, amoxicillin-clavulanic acid, vancomycin, amikacin, and gentamicin, and resistance to doxycycline, clindamycin and cefazolin. Cardiogenic pulmonary oedema improved in response to constant rate infusion (CRI) of diuretics (furosemide, 1 mg/kg/hr) during hospitalisation but recurred after CRI discontinuation, possibly because of damage to the aortic valve caused by proliferation and destruction due to IE. During hospitalisation, an attempt was made to switch from CRI diuretics to oral administration (torsemide, 0.8 mg/kg/day) (Torsemid Tab, Boryung Pharmaceutical Co., Ltd., Seoul, Korea), but pulmonary oedema was not resolved with oral medication alone. Pulmonary oedema resolved following the addition of hydralazine 0.5 mg/kg q12h (Samjin Pharmaceutical, Seoul, Korea), due to improvement in the left ventricular outflow and afterload reduction. The dog was hospitalised for a total of 14 days after relapse; from the 9th day of hospitalisation, the dog was managed with the following oral medications: prednisolone, 0.75 mg/kg q12h; torsemide, 0.3 mg/kg q12h; hydralazine, 0.5 mg/kg q12h; enrofloxacin, 5 mg/kg q24h; amoxicillin-clavulanate, 12.5 mg/kg q12h; clopidogrel (Pravic, Sinilpharm, Seoul, Korea), 3 mg/kg q24h; and rivaroxaban (Xarelto, Bayer HealthCare, Essen, Germany), 1 mg/kg q24h.
To date, the dog has been successfully managed on an outpatient basis without rehospitalisation.
Discussion and conclusions
Paenibacillus spp. are soil-dwelling bacteria that contribute to the formulation of pesticides and antimicrobial peptides, but some Paenibacillus species have recently been identified as a cause of opportunistic infections in humans [8]. Cases of Paenibacillus spp. as the causative agent of endocarditis have rarely been reported, even in humans. Degeneration of the mitral valve or aortic valve due to infection caused by Paenibacillus spp. has been confirmed and treated with antibiotics and valve repair surgery in humans [5–7]. In veterinary medicine, the incidence and survival rates of IE have increased with an increased understanding of the disease and advances in diagnostic techniques [2]. Although the incidence and survival rates of dogs with IE are increasing, its diagnosis in veterinary medicine remains challenging. Most IE cases occur frequently in large-breed dogs, and the types of bacteria mainly identified are Staphylococcus spp., Streptococcus spp., and Escherichia coli [2]. Although Paenibacillus amyloyticus was recently reported as the causative agent in a dog with osteomyelitis [11], cases of endocarditis caused by Paenibacillus spp. have not been reported in dogs. To our knowledge, this is the first reported case and successful treatment of endocarditis caused by Paenibacillus spp. in a dog.
IE has different prognoses depending on the site in the heart that is primarily affected by the infectious agent; the aortic and mitral valves are the most common sites [12]. Aortic valve degeneration has a worse prognosis than mitral valve degeneration [4]. Symptoms of endocarditis include fever, lethargy, and dyspnoea due to pulmonary oedema associated with heart failure caused by valvular degeneration. IE is diagnosed when the requisite major/minor criteria of the modified Duke criteria are met. The dog in this case was diagnosed with IE because it met the major criterion of vegetative lesions on echocardiography and the minor criteria of fever and blood culture test results.
Specific antibiotic treatment guidelines for IE caused by Paenibacillus spp. are lacking, even in human medicine [5]. Regarding treatment in humans, antibiotics such as ciprofloxacin, clindamycin, ampicillin, and amoxicillin-clavulanate have been used on a case-by-case basis [5–7]. In our patient, due to lack of information and treatment guidelines for canine IE caused by Paenibacillus spp. in the veterinary field, we treated the dog based on the results of the antibiotic susceptibility tests for Paenibacillus spp.
Unlike other cases of IE in dogs with aortic valve degeneration that have extremely poor prognoses [4], in this case, various factors are thought to have contributed to the dog’s survival to date. Hydralazine was administered to reduce the afterload, based on the echocardiography findings. The addition of hydralazine to the treatment plan aimed at reducing the afterload, considering the aortic valve degeneration and blood regurgitation velocity in the left ventricular outflow tract. Hydralazine was chosen because it is a more potent direct-acting vasodilator for afterload reduction, compared to other drugs, such as amlodipine [13]. This decision resulted in a positive change in blood flow within the heart by reducing afterload, which enabled the treatment of endocarditis without hospitalisation, as pulmonary oedema did not recur even after discontinuation of furosemide CRI.
Furthermore, appropriate antibiotics based on sensitivity testing were used in this case.
Subsequent susceptibility test results confirmed that Paenibacillus lautus was susceptible to all the antibiotics administered initially. However, considering that pulmonary oedema did not resolve until hydralazine was added even after re-administration of antibiotics to which susceptibility was confirmed, it is possible that valve degeneration had progressed further during the antibiotic discontinuation period. Following antibiotic and hydralazine treatment, no relapse was observed.
Clopidogrel and rivaroxaban were administered based on a retrospective study that showed that antithrombotic drug use extended the survival of veterinary patients as well as studies on dual therapy in dogs and cats at high risk for thrombosis [2, 14, 15]. Although the preventive effects of dual therapy could not be confirmed in this case, it may have helped reduce the risk of sudden death due to thrombosis associated with vegetative lesions of the aortic valve.
A limitation of this case is that Paenibacillus lautus was isolated through blood culture at the time of symptom relapse after antibiotic treatment was discontinued and the patient was receiving immunosuppressive dose of prednisolone. Blood cultures were not performed prior to initial antibiotic treatment; therefore, it is possible that Paenibacillus spp. alone might not have been the cause of the aortic valve damage in this case. As shown in Fig. 1a, there was no pulmonary oedema at the initial visit and the amount of pleural effusion was insufficient to allow aspiration; however, pulmonary oedema developed as shown in Fig. 1b after the discontinuation of antibiotics. During this period the patient was diagnosed with IE based on the findings of vegetative lesions on the valve on echocardiography and blood culture. It is possible that if the antibiotics had not been discontinued, the patient’s valve damage may have been minimized, and aortic valve degeneration may not have progressed to the level of leading to pulmonary oedema, as shown in Fig. 1a.
Nevertheless, the diagnosis of this case and treatment course may be helpful in the management of dogs with severe aortic valve damage due to IE. In small breed dogs with severe aortic valve damage, afterload reduction with hydralazine and antithrombotic agents may be beneficial.
Acknowledgements
Not applicable.
Abbreviations
- IE
Infectious endocarditis
- WBC
White blood cell, CRP, C-reactive protein
- HCT
Haematocrit
- ANA
Antinuclear antibody
- SLE
Systemic lupus erythematosus
- IV
Intravenous
- CRI
Constant rate infusion
Author contributions
HKC contributed to conceptualization, investigation, resources, writing-original draft; SYJ and DSC contributed to writing-review and editing; WKY contributed to advice and provided drugs; WKY and YJH contributed to supervised the procedures; All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive any research support or other specific funding for this manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
No unnecessary tests were performed. The owner of this patient gave consent for all the procedures that were carried out.
Consent for publication
The patient’s owner gave consent for publishing this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Won-Kyoung Yoon, Email: harleyyoon@hanmail.net.
Yeon-Jung Hong, Email: vethong@hanmail.net.
References
- 1.Mahabadi AA, Mahmoud I, Dykun I, Totzeck M, Rath PM, Ruhparwar A, et al. Diagnostic value of the modified Duke criteria in suspected infective endocarditis—the PRO-ENDOCARDITIS study. Int J Infect Dis. 2021;104:556–61. [DOI] [PubMed] [Google Scholar]
- 2.Reagan KL, Visser LC, Epstein SE, Stern JA, Johnson LR. Outcome and prognostic factors in infective endocarditis in dogs: 113 cases (2005-2020). J Vet Intern Med. 2022;36:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilkenny E, Watson C, Dukes-McEwan J, Bode EF, Hezzell MJ, Payne JR, et al. Evaluation of serum cardiac troponin-I concentrations for diagnosis of infective endocarditis in dogs. J Vet Intern Med. 2021;35:2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald K. Infective endocarditis in dogs: diagnosis and therapy. Vet Clin North Am Small Anim Pract. 2010;40:665–84. [DOI] [PubMed] [Google Scholar]
- 5.Depta F, Pažitný M, Trebišovský M, Maďarová T, Deptová J. Infective endocarditis caused by Paenibacillus thiaminolyticus: a case report and review of literature. Eur Heart J Case Rep. 2023;7:ytad566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrand J, Hadou T, Selton-Suty C, Goehringer F, Sadoul N, Alauzet C, et al. Cardiac device-related endocarditis caused by Paenibacillus glucanolyticus. J Clin Microbiol. 2013;51:3439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho-Gomes AC, Nasir A, Mosca R, Mirza S, Kadir I. Intraoperative diagnosis of mitral valve endocarditis secondary to Paenibacillus provencensis. Ann R Coll Surg Engl. 2017;99:e54–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC. Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact. 2016;15:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sáez-Nieto JA, Medina-Pascual MJ, Carrasco G, Garrido N, Fernandez-Torres MA, Villalón P, et al. Paenibacillus spp. Isolated from human and environmental samples in Spain: detection of 11 new species. New Microbes New Infect. 2017;19:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celandroni F, Salvetti S, Gueye SA, Mazzantini D, Lupetti A, Senesi S, et al. Identification and pathogenic potential of clinical Bacillus and Paenibacillus isolates. PLoS ONE. 2016;11:e0152831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rampacci E, Sforna M, Dentini A, Di Matteo I, Lidano P, Capucci C, et al. Paenibacillus amylolyticus osteomyelitis in a poodle dog: case report and literature review. J Vet Diagn Invest. 2022;34:703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannoulopoulos G, Errington K. Case report and successful management of canine aortic endocarditis caused by Actinomyces neuii subsp. Anitratus (Winkia neuii subsp. anitrata). BMC Vet Res. 2022;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McComb MN, Chao JY, Ng TM. Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther. 2016;21:3–19. [DOI] [PubMed] [Google Scholar]
- 14.Lo ST, Walker AL, Georges CJ, Li RH, Stern JA. Dual therapy with clopidogrel and Rivaroxaban in cats with thromboembolic disease. J Feline Med Surg. 2022;24:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T, Uemura A, Tanaka R, Farag A, Mandour AS, Hamabe L, et al. Secondary right atrial thrombosis in three dogs: antithrombotics therapy and echocardiographic follow-up. Vet Med Sci. 2023;9:1973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.