Abstract
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease involving multiple pathophysiological mechanisms, such as insulin resistance and β-cell dysfunction. Recently, the emergence of precision medicine has ushered in new ideas and strategies for the treatment of T2DM. Studies have indicated that alterations in gut microbiota and changes in multiple micro ribonucleic acids (miRNAs) are closely associated with T2DM, suggesting that regulating miRNAs and gut microbiota may become novel therapeutic targets for T2DM. In addition, the modulation of mitophagy, regulation of glucagon-like peptide-1(GLP-1) secretion, and the application of immune cell exosomes have also demonstrated significant therapeutic potential. Furthermore, regulating inhibiting serine phosphorylation, reducing proinsulin synthesis, adjusting central nervous system function, modulating transcription factors MondoA and ChREBP, adjusting Omega-3 fatty acid levels, and regulating the mammalian target of rapamycin (mTOR) signaling pathway are also considered promising therapeutic targets. The rise of precision medicine has provided a plethora of possible targets for the treatment of T2DM, spanning a wide range of areas from epigenetic modifications to gut microbiota, immune regulation, and metabolic pathways. Future research should further explore the clinical feasibility and safety of these targeted therapies while developing personalized treatment plans to improve outcomes for T2DM patients. This article highlights the latest discoveries of molecular pharmacological targets that may play a role in the pathogenesis of T2DM over the past 5 years. In addition, from the perspective of precision medicine, this review explores potential therapeutic targets for T2DM and evaluates emerging treatment strategies and drug development pathways.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-025-02682-5.
Keywords: Type 2 diabetes mellitus, Pathophysiological, Mechanistic targets, Therapeutic targets, Drug, Drug development, Metabolism, Precision medicine
Introduction
T2DM, characterized by persistently elevated blood glucose levels, is increasingly recognized as a complex cardiorenal metabolic disease [1, 2]. It also represents a significant public health issue, being the fastest-growing metabolic disease in the world [3], affecting approximately 10% of the global population [4]. Globally, the number of diabetics has doubled in the past three decades, with one in every eleven adults worldwide suffering from diabetes, 90% of whom have T2DM. The prevalence of T2DM continues to rise globally, posing a worldwide health challenge [5]. There are regional disparities in T2DM prevalence, with higher rates in developed countries [6] but a more rapid growth in developing nations [7].Furthermore, the onset of T2DM is closely associated with multiple risk factors. Obesity, often accompanied by hyperinsulinemia and insulin resistance, is one of the primary risk factors for T2DM [8]. Typically, T2DM manifests in adulthood, especially among middle-aged and older populations. However, there has been a recent surge in the diagnosis of T2DM among adolescents and children [9], which is strongly correlated with the increasing obesity rates in this demographic [10]. Genetic factors also play a significant role in T2DM, with a higher incidence observed in families with a clustering tendency [11]. In addition, other metabolic syndromes such as hyperthyroidism or hypothyroidism [12], Cushing's syndrome [13], hypertension [14], and others [15] may also elevate the risk of T2DM. In summary, the epidemiology of T2DM is highly complex, influenced by a multitude of factors. Therefore, effective prevention and control of T2DM are paramount. This requires the implementation of comprehensive, multi-dimensional intervention strategies, including promoting healthy eating habits, enhancing physical activity, improving public health services, and disseminating health education. These measures are key to reducing T2DM incidence and alleviating its societal and health burdens. Concerted efforts from the entire society are essential to effectively address this increasingly critical public health challenge. Currently, conventional clinical treatments for T2DM have several limitations, including individual differences in the efficacy of first-line medications, side effects such as hypoglycemia, the inability to reverse the pathological mechanisms of T2DM (such as insulin resistance and β-cell dysfunction), and insufficient management of long-term complications. In this context, the application of precision medicine in this field appears to be both necessary and urgent.
Highlights
The review summarizes the latest discoveries in therapeutic targets for T2DM over the past 5 years. It discusses molecular pharmacological targets and drug development progress related to T2DM. From a precision medicine perspective, the study identifies and evaluates potential therapeutic targets for T2DM. Special emphasis is placed on the therapeutic potential and drug development of miRNA regulation, the gut microbiome, NK cells, and GLP-1. In addition, the review explores emerging drug development strategies and future research directions.
The current precision medicine initiative defines precision medicine as"an emerging approach for disease treatment and prevention that takes into account individual variations in genes, environment, and lifestyle."This approach promotes more accurate treatment and prevention strategies tailored to each individual, rather than a one-size-fits-all method, where disease treatment and prevention strategies are developed for universal use [16]. In the past 5 years, significant progress has been made in research on therapeutic targets for T2DM. Large-scale genomics and metabolomics studies have revealed multiple new therapeutic targets, providing novel insights for drug development. These targets span various domains, including epigenetic modifications, gut microbiota, immune modulation, and metabolic pathways. In particular, through in-depth analyses of the relationships between T2DM, gut microbiota, and epigenetic modifications, researchers have discovered the integrated regulatory effects of gut microbiota and epigenetic modifications on glucose metabolism, insulin resistance, and inflammatory responses. These findings offer rich resources for developing new treatment strategies. The development of treatment targets for T2DM should consider its complex etiology, including multiple risk factors, such as obesity, insulin resistance, genetic predisposition, and other metabolic disorders. Therefore, treatment strategies should adopt a comprehensive approach, focusing on improving insulin sensitivity, regulating insulin secretion, and managing metabolic disturbances. As the incidence of T2DM rises among adolescents and children, early intervention and personalized treatment are particularly important, with precision medicine playing a crucial role in tailoring therapeutic plans. Furthermore, given the close association between T2DM and complications such as cardiovascular and renal diseases, drug development should not only focus on blood glucose control but also on multi-benefit outcomes, such as cardioprotective and nephroprotective effects. With the global rise in T2DM prevalence, particularly in developing countries, treatment target development needs to consider regional differences and design solutions suited to various populations. Ultimately, treatment approaches should combine pharmacological interventions with lifestyle modifications, fostering a synergistic effect through precision therapy and health management to improve the overall prevention and control of T2DM. However, despite notable achievements in basic research, the effective translation of these newly discovered targets into clinical treatment remains an urgent issue. The perspective of precision medicine presents new possibilities for this translational process, emphasizing the customization of treatment plans based on individuals'genetic backgrounds, metabolic profiles, and lifestyles [17]. Precision medicine in the field of T2DM enables personalized risk prediction, precise disease classification, and individualized pharmacological treatment. By leveraging genomics, metabolomics, and gut microbiome research, along with pharmacogenomics and artificial intelligence (AI) technologies, it facilitates optimized drug selection, insulin management, and blood glucose monitoring, thereby enhancing therapeutic efficacy while minimizing adverse effects. Despite challenges related to data privacy, cost, and healthcare integration, ongoing technological advancements will further expand the role of precision medicine in T2DM management. In the future, within the framework of precision medicine, we should further explore the clinical feasibility and safety of new target-based treatment strategies, evaluating the efficacy and side effects of different targeted therapies for patients. In addition, there is a need to promote clinical trials to validate the applicability of various treatment regimens to different patient subpopulations. This will provide T2DM patients with more effective and safer treatment options, ultimately improving their quality of life and treatment outcomes [18].
Research progress on therapeutic targets for T2DM in the past 5 years
Search strategy
The search of PubMed was conducted on 1 October 2024 since 1 January 2019, and included the broad terms ‘T2DM[Title/Abstract]’,‘type 2 diabetes mellitus’[Title/Abstract]’, and ‘Therapeutic targets’[Title/Abstract]’,‘Mechanistic Targets’. Only peer reviewed English language publications were considered. The inclusion criteria included: a clinical randomized controlled trial (RCT), animal experiment study, literature reviews, retrospective analyses and meta-analyses; the exclusion criteria included: non-RCT, case analyses, and guidelines. The specific search strategy can be found in the supplementary materials.
Targets related to the regulation of gut microbiota
Research has found a significant reduction in the number of butyrate-producing bacteria in patients with T2DM and its precursors. Butyrate is produced in the body through the metabolic process of these bacteria, making butyrate a potential therapeutic target for T2DM [19]. Akkermansia muciniphila, a commensal bacterium negatively correlated with obesity and T2DM, can alleviate high-fat diet-induced obesity and T2DM by inducing metabolic changes and exerting anti-inflammatory effects. It also alters the composition of gut microbiota, thus being considered a potential therapeutic target for T2DM [20]. Both butyrate and Akkermansia muciniphila contribute to improving gut barrier integrity, thereby alleviating inflammation and enhancing insulin sensitivity to regulate metabolism. In addition, they play a crucial role in shaping gut microbiota diversity, influencing overall microbial composition and function. Their synergistic effects highlight the potential of targeting gut microbiota as a therapeutic strategy for metabolic disorders such as T2DM. The main feature of T2DM is insulin resistance, which is often a result of the host's inflammatory response. Omega-3 fatty acids can regulate T2DM by modulating the diversity and richness of gut microbiota and inhibiting inflammatory pathways, making them another potential therapeutic target for T2DM [21]. In addition, there is increasing evidence suggesting that the gut microbiota, as a second genome, can serve as an attractive target to improve the efficacy and safety of medications [22].
Targets related to the regulation of epigenetic modifications
Epigenetic modifications, including DNA/RNA methylation, histone acetylation, and miRNA expression, when dysregulated, can induce T2DM by promoting several underlying factors of diseases, such as obesity, insulin resistance, beta-cell dysfunction, mitochondrial dysfunction, and cellular senescence. Lifestyle choices, including diet and exercise, also contribute to either increasing or decreasing the risk of T2DM through epigenetic modifications [23]. Therefore, targeted therapies aimed at epigenetic modifications in T2DM hold significant therapeutic potential. Among these, miRNAs are closely associated with T2DM [24]. In patients with T2DM, 16 upregulated and 32 downregulated miRNAs have been identified. Through an analysis of these two miRNA profiles, seven intersecting miRNAs were discovered, exhibiting opposite changes. These seven miRNAs and their target genes are closely related to T2DM, suggesting that they may be potential targets for regulating T2DM [25]. Other studies have shown that gene ablation of miRNA-22 contributes to metabolic remodeling, increases energy expenditure, and promotes the browning of white adipose tissue, indicating that regulating miRNA-22 could be a therapeutic target for treating obesity and other metabolic disorders [26]. In addition, a study found that M2-polarized BMDM-derived exosomes (M2Exos) can improve insulin sensitivity in vitro and in vivo. miR-690 is highly expressed in M2Exos, suggesting that miR-690 may become a new insulin-sensitizing target for the treatment of T2DM [27]. Furthermore, miRNAs carried by extracellular vesicles (EV-miRNAs) are believed to mediate cross-organ and cross-cellular communication, playing a crucial role in the pathophysiology of T2DM and offering insights and hope for diabetes-related biomarkers, as well as the possibility of serving as therapeutic targets [28]. Plasma samples from female T2DM patients showed significantly reduced levels of miR-10a/b-5p, estrogen, and insulin receptors, but elevated insulin levels. Estrogen protects against insulin resistance in female T2DM via the miR-10a/b-5p/NCOR2 pathway, indicating the potential of miR-10a/b-5p restoration as a therapeutic target in the management of female T2DM [29]. The relationship between miR-182-5p and systemic glucose homeostasis and hepatic lipid metabolism is most closely associated in both humans and mice. Under conditions of miR-182-5p overexpression, its target gene, LRP6, remains lowly expressed in the livers of obese T2DM patients and mice. This suggests that miR-182-5p inhibits LRP6 and promotes adipogenesis while impairing glucose homeostasis. Therefore, inhibiting miR-182-5p could be a potential therapeutic target for the treatment of obesity-driven T2DM [30]. A total of 24 differentially expressed miRNAs were detected in ZDF rats, among which miR-34a-5p and miR-452-5p showed the greatest upregulation and downregulation, respectively. This indicates that miR-34a-5p and miR-452-5p are novel regulators of pancreatic endocrine dysfunction [31]. There are numerous such reports, fully illustrating the significant role of miRNAs in the occurrence, development of T2DM, and its relationship with insulin secretion and insulin resistance. Future research should comprehensively evaluate various miRNAs as potential therapeutic targets for the prevention and treatment of T2DM[32].
Targets associated with the regulation of mitophagy
Mitophagy emerges as a promising therapeutic target for T2DM, suggesting that mitophagy inducers should be considered as potential and novel therapeutic agents for the treatment of T2DM [33]. According to a recent study in April 2024, enhancing autophagic activity through mechanisms targeting the activation of AMP-activated protein kinase (AMPK) and sirtuin-1 signaling pathways or the inhibition of mTOR complex 1 signaling pathway can effectively improve insulin resistance and balance glucolipid metabolism in key tissues, such as the hypothalamus, skeletal muscle, liver, and adipose tissue [34]. Other research findings indicate that targeted therapy aimed at appropriately regulating mitophagy mediated by the mitochondrial autophagy receptor FUNDC1 in white adipose tissue (WAT) can enhance mitochondrial turnover in adipocytes, potentially improving systemic insulin sensitivity and metabolic homeostasis [35]. Furthermore, the miR-27-3p-Miro1 axis, identified as a novel regulatory mechanism of mitophagy, represents a potential new therapeutic target for the development of lipotoxicity-related T2DM [36]. In summary, mitophagy plays a crucial role in maintaining mitochondrial homeostasis, reducing oxidative stress, suppressing inflammation, and preserving insulin signaling. Impaired mitophagy can lead to mitochondrial dysfunction, excessive ROS (Reactive oxygen species) production, elevated inflammatory factors, and disrupted insulin signaling, ultimately contributing to insulin resistance. Enhancing mitophagy may serve as a potential strategy for improving insulin resistance.
Other potential therapeutic targets
According to a recent study in April 2024, in models of insulin resistance and T2DM, the binding of insulin to the insulin receptor (IR) triggers the phosphorylation of multiple IR and IRS-1 tyrosines, eliciting numerous cellular responses. Serine phosphorylation within the IRS-1 PIR domain disrupts the interaction between IRS-1 and IR, thereby reducing the sensitivity of insulin signaling and leading to insulin resistance. Therefore, inhibiting serine phosphorylation may represent a therapeutic target for T2DM [37]. Natural killer-derived exosomes containing miR-1249-3p significantly induce cellular insulin sensitivity and reduce inflammation, thus alleviating insulin resistance. Mechanistically, exosomal miR-1249-3p directly targets SKOR1 to regulate the formation of the ternary complex SMAD6/MYD88/SMURF1, which mediates glucose homeostasis by inhibiting the TLR4/NF-κB signaling pathway, providing a series of potential therapeutic targets for T2DM [38].
GLP-1 stimulates insulin secretion in a glucose-dependent manner, inhibits glucagon secretion, reduces appetite, and slows gastric emptying. Modulating endogenous GLP-1 can mimic the beneficial effects of bariatric surgery on food intake, body weight, and blood glucose levels [39]. In patients with T2DM, this incretin effect is impaired, leading to a relative deficiency of GLP-1. Based on this, GLP-1 has become an important target molecule for T2DM treatment [40]. Furthermore, targeting enteroendocrine L cells, which secrete GLP-1 and other incretins, represents a new and promising approach that can provide direct and indirect synergistic therapeutic effects for T2DM [41].
Microparticles (MPs) are considered major players in the pathogenesis of many systemic inflammatory diseases and may serve as potentially useful biomarkers of disease activity, not only having prognostic value but also possibly acting as new therapeutic targets for T2DM [42].
In the early stages of T2DM, both insulin synthesis and basal insulin secretion are elevated, often seen as a compensation for increased peripheral insulin resistance in the liver, muscle, and adipose tissue. The increased synthesis of proinsulin is thought to be the main direct cause of pre- and early stage T2DM beta-cell endoplasmic reticulum stress. Acutely reducing proinsulin synthesis can alleviate beta-cell endoplasmic reticulum stress and promote beta-cell proliferation, thus having the potential to become a new therapeutic target [43].
Endothelial dysfunction is an early marker of T2DM development and plays a significant role in the progression of the disease. It includes dysregulation of the endothelium's ability to produce and release vasoactive mediators, considered an initial feature of impaired vascular function under conditions of obesity and other insulin resistance states. Therefore, modulating endothelial function to restore the production and release of vasoactive mediators is an important target for preventing T2DM [44].
Insulin resistance in T2DM increases the activation of Forkhead box O1 (FoxO1) in metabolic tissues, including the liver, skeletal muscle, adipose tissue, pancreas, and brain. Targeting optimal tissues to activate or inhibit FoxO1 and developing tissue-specific delivery strategies for FoxO1 therapeutics can effectively treat T2DM while minimizing adverse side effects [45].
Mammalian target of mTOR is a key regulator of cell metabolism and growth. Substantial evidence suggests that two mTOR-mediated signaling schemes, mTORC1-p70S6 kinase 1 (S6 K1) and mTORC2-protein kinase B (AKT), play crucial roles in insulin sensitivity, and their dysfunction contributes to the development of T2DM. Inhibiting mTOR has potential applications in T2DM treatment [46].
Transcription factors such as MondoA (MLX-interacting protein) and the carbohydrate response element-binding protein (ChREBP) have been confirmed as central mediators of glucose sensing in multiple metabolic organs, playing a significant role in maintaining glucose homeostasis. Under chronic nutritional overload conditions, MondoA/ChREBP dysregulation can lead to metabolic disorders, such as insulin resistance (IR) and T2DM. MondoA/ChREBP represents a potential target for treating T2DM and its complications [47].
Obesity and T2DM are closely related to hypothalamic pathology. A neuronal subpopulation in the ventromedial hypothalamus normally coordinates counter-regulatory responses to glucose deficiency but abnormally promotes elevated BDLG in T2DM. In rodent models of T2DM, the inactivation of these neurons leads to insulin-independent hypoglycemia. Future therapeutic strategies and drug development should focus on the central nervous system regulation of both insulin-dependent and insulin-independent glucose processing, representing a vast untapped potential for current T2DM treatments [48].
Precision medicine and emerging drug development pathways
The management of blood glucose in T2DM involves lifestyle interventions and pharmacological therapies, with the latter being the most common approach. Driven by precision medicine, the development of T2DM drugs has shifted from the traditional “one-size-fits-all” model to individualized and targeted therapies [49]. As our understanding of the pathophysiological mechanisms of T2DM deepens, new drug research and development are increasingly focusing on individual differences in microbiota, genes, metabolism, and other factors. Based on recent advancements in therapeutic targets, emerging drug development pathways can be categorized into the following four types.
Gut microbiota
The focus of gut microbial therapy strategies is to shape the gut microbiota to improve the progression of T2DM [50]. Probiotics, prebiotics, synbiotics, and postbiotics (PPSP) are powerful modulators of the gut microbiota. By regulating the gut microbiota, PPSP exerts beneficial control over metabolic diseases, such as obesity and T2DM. In addition, PPSP indirectly improves metabolic diseases by regulating gut microbial metabolites, inhibiting the production of lipopolysaccharide and trimethylamine oxide, and reducing the bile acid pool. Numerous clinical trials have demonstrated the effects of PPSP on metabolic diseases; however, the optimized formulations of synthetic preparations and the specific efficacy of postbiotic preparations in humans deserve further exploration [51]. Furthermore, repeated fecal microbiota transplantation (FMT) can enhance the level and duration of implanted microbiota in obese T2DM patients. Combining lifestyle intervention with FMT can induce more favorable changes in the recipient's microbiota, improve lipid levels, and benefit T2DM, as well as reduce liver stiffness [52]. However, FMT still faces challenges in procedural standardization and long-term safety. FMT requires consistency in donor screening, stool processing, and delivery methods to minimize individual variability and potential risks. In addition, in terms of long-term safety, factors such as potential pathogen transmission, persistent alterations in the gut microbiota, and immune system responses may pose unpredictable health risks. A study in Japan found that Blautia wexlerae is an effective beneficial gut bacterium with anti-inflammatory properties and the ability to improve the host's intestinal environment and lipid metabolism. Oral administration of Blautia wexlerae can improve obesity and T2DM through metabolic remodeling of the gut microbiota [20]. Recent research indicates that T2DM is associated with a reduction in butyrate-producing bacteria, sparking interest in exploring the therapeutic potential of butyrate for T2DM. Probiotics, next-generation probiotics, and fiber supplements may be successful strategies to increase butyrate-producing bacteria and improve hyperglycemia and insulin resistance [19]. In summary, guided by precision medicine, altering the gut microbiota and its metabolites represents an emerging and potential drug therapy strategy for the treatment of T2DM.
Epigenetic modifications
MiRNAs represent a new class of drug targets due to their extensive involvement in numerous cellular metabolic processes and disease regulation, including in adipocytes. Novel miRNA therapeutics targeting adipose tissue ultimately aim to overcome metabolic disorders [53]. Experimental evidence suggests that weekly subcutaneous injections of various miRNA-22-3p antagonists significantly reduce fat mass, improve insulin sensitivity, and lower circulating glucose and cholesterol levels. miRNA-22-3p antagonists could potentially become an effective treatment for human obesity and T2DM characterized by lipotoxicity and insulin resistance [54].
Furthermore, basic research indicates that miRNA-132 is upregulated in T2DM mice. After 24 h of injecting antagomirs-132 into mice, the expression of miRNA-132 in the islets decreased, blood glucose levels dropped, and insulin secretion increased. Antagomirs show promise as therapeutic agents to regulate islet miRNA levels, thereby improving β-cell function, enhancing insulin secretion, and lowering blood glucose [55]. In addition, antagomirs exhibit great potential for clinical translation. Through chemical modifications and targeted ligand design, antagomirs can precisely regulate disease-associated miRNAs without affecting normal physiological functions. To enhance stability and reduce off-target effects, various delivery strategies, including lipid nanoparticles (LNPs), polymeric carriers, and chemical modifications such as 2'-O-methylation, have been developed to ensure effective cellular uptake and therapeutic action. However, the clinical translation of miRNA antagonists remains in its early stages, with challenges such as delivery efficiency and tissue specificity, immunogenicity, metabolic stability, and long-term safety still posing significant barriers. In the future, advancements in delivery technologies, novel modification strategies, and precision medicine approaches may help overcome these obstacles, enabling miRNA antagonists to play a greater role in personalized medicine. Multiple clinical trials have also demonstrated that metformin and pioglitazone regulate various miRNAs in T2DM patients, helping to explain the mechanisms by which these drugs lower blood glucose and reduce the risk of complications in T2DM [56].
In addition, maturity-onset diabetes of the young (GCK–MODY), a specific form of T2DM, is caused by heterozygous inactivating mutations in glucokinase (GK, gene symbol GCK) and impaired glucose sensing. The novel allosteric GK activator, dozagrel, is a dual-action glucokinase activator. In vitro, dozagrel directly reduces the glucose half-saturation concentration of wild-type GK and exhibits varying degrees of selectivity for different types of GK mutants. Dozagrel can directly restore enzymatic activity to selective GK mutants and enhance wild-type GK activity, thereby correcting the primary defect of glucose sensitivity in GCK–MODY [57]. In summary, epigenetics, based on multi-omics studies, such as genomics, transcriptomics, and epigenomics, offers a novel therapeutic strategy for regulating genes.
GLP-1 receptor agonists
GLP-1 receptor agonists (GLP-1RAs) represent a subclass of"incretin-based therapies (IBT)". GLP-1RAs are classified into short-acting and long-acting based on their pharmacokinetic and pharmacodynamic characteristics. Exenatide and lixisenatide are short-acting GLP-1RAs with a half-life of 2–4 h, primarily affecting and reducing postprandial hyperglycemia. Long-acting GLP-1RAs include extended-release exenatide, liraglutide, semaglutide, and dulaglutide. In addition, a novel drug, tirzepatide, functions as a dual GIP and GLP-1 receptor agonist, primarily improving basal hyperglycemia [40]. A new GLP-1-gastrin dual agonist, ZP3022, has demonstrated improved glycemic control in db/db mice, promoted an increase in β-cell quantity and quality [58], facilitated the reprogramming of pancreatic exocrine cells into β-cells [59], and had a significant impact on β-cell recovery in db/db mice [60]. Furthermore, various multi-target hybrid peptide receptor agonists, such as acylated GLP-1/gastrin/xenin hybrid peptide receptors tri-agonists [61], glucagon/GLP-1 receptor co-agonists [62], GLP-1/glucagon (GCG)/CCK2 receptors tri-agonists [63], and peptide-based selective GLP-1/cholecystokinin receptors co-agonists [64], offer broad prospects for the treatment of T2DM.
NK cells
T2DM exerts a negative impact on the immune system, resulting in decreased activity of natural killer (NK) cells. Vitamin D has been proven to modulate both innate and adaptive immune cells. According to a recent study in July 2024, dietary supplementation with vitamin D3 can enhance NK cell activity in T2DM mice, thereby improving the occurrence and progression of T2DM [65]. Currently, research on immune system-related drug targets in the treatment of T2DM is still in its infancy, yet NK cells are playing an increasingly important role in the pathophysiology of T2DM [66]. Studies have found that NK cells not only participate in immune surveillance but may also influence the occurrence and development of T2DM by regulating insulin sensitivity and suppressing inflammatory responses. NK cells are a crucial component of the innate immune system, playing a key role in eliminating virus-infected and tumor cells. In addition, NK cells can enhance insulin sensitivity; however, aberrant activation of NK cells may exacerbate low-grade chronic inflammation by secreting pro-inflammatory cytokines such as TNF-α, ultimately contributing to insulin resistance. Despite their significant roles in immune surveillance and metabolic regulation, the clinical translation of NK cell therapy faces multiple challenges. These include difficulties in NK cell expansion and persistence, loss of targeting specificity due to immune evasion in certain tumor or disease microenvironments, and potential risks, such as cytokine storm, rejection, and host adaptation issues. In the future, advancements in technology may facilitate broader applications of NK cell therapy in cancer immunotherapy and metabolic disease interventions.
Future research directions and therapeutic options
The Precision Medicine Initiative defines precision medicine as"an emerging approach for disease treatment and prevention that takes into account individual differences in genes, environment, and lifestyle for each person."This approach will facilitate more accurate treatment and prevention strategies, moving away from a one-size-fits-all method, where disease treatment and prevention strategies are formulated for general use [16]. From the perspective of precision medicine, potential future therapeutic targets and drug development pathways for T2DM may focus on the following aspects.
Gut microbiota and their metabolites
Gut microbiota and their metabolites play a critical role in the pathogenesis of T2DM. Increasing studies have shown that gut microbiota is closely related to the metabolic state of the host, especially in terms of insulin resistance and chronic low-grade inflammatory responses. From the perspective of precision medicine, differences in individual gut microbiota can provide new insights for personalized intervention in T2DM. Research has found that specific gut microbiota and their metabolites (such as short-chain fatty acids, secondary bile acids, etc.) can affect insulin signaling, regulate intestinal barrier function, and systemic inflammatory responses [67]. In addition, intervention methods such as probiotics, prebiotics, and microbiota transplantation can regulate the composition of gut microbiota, thereby promoting the restoration of metabolic homeostasis and improving β-cell dysfunction [68]. This precision intervention strategy based on gut microbiota provides new possibilities for personalized treatment of T2DM. Future research should further explore targets and drug treatments related to gut microbiota, providing a new perspective and strategy for developing personalized treatment plans.
Genomics and epigenetics
Through genomic sequencing and analysis, specific genetic variations that affect the onset of T2DM can be identified. For example, certain gene mutations may lead to abnormal insulin secretion or insulin resistance. By analyzing genetic information, personalized treatment plans can be developed for patients, optimizing blood glucose control and reducing the probability of complications. In addition, T2DM affects gene function through epigenetic modifications, thereby influencing the pathogenesis of T2DM [3]. There is also a strong interaction between genes and epigenetic modifications, which seems to jointly affect T2DM. Future research should deeply explore the interaction mechanisms between these genes and epigenetic modifications, and develop new therapeutic targets and biomarkers to improve the prediction, prevention, and personalized treatment of T2DM and its complications. This research approach based on precision medicine is expected to bring more targeted and effective intervention strategies to patients with T2DM [69]. With the advancement of multi-omics integration technology, the combination of genomics, transcriptomics, proteomics, metabolomics, and microbiomics enables a more comprehensive understanding of the pathogenesis of T2DM. This approach facilitates the identification of novel therapeutic targets and accelerates drug development, paving the way for breakthroughs in personalized treatment and prevention of T2DM. Moreover, bioinformatics plays a crucial role in identifying genetic mutations, analyzing epigenetic regulation, and integrating multi-omics data in T2DM research. With advancements in AI and big data analytics, bioinformatics is expected to further drive precision-targeted therapies and personalized intervention strategies, offering new insights into the prevention and treatment of T2DM.
Pharmacogenetics
Pharmacogenetics is a branch of genetics that studies how the genome affects an individual's response to drugs, treatment outcomes, and the incidence of adverse reactions. With the deepening of the concept of precision medicine, especially in the clinical practice of T2DM, utilizing patients'genetic information will become a key strategy to prevent disease progression [70]. By identifying specific genes related to drug metabolism, doctors can develop personalized treatment plans for each patient, thereby determining the most effective and safe drug selection. This can not only improve treatment effects but also significantly reduce the risk of adverse reactions, thereby reducing the economic burden on patients and the pressure on the global health system. The progress of pharmacogenetics provides new possibilities for the personalized management of T2DM, contributing to more precise medical intervention [71].
Mental health management
Numerous studies have shown that psychological stress also affects the occurrence and prognosis of T2DM, so maintaining good mental health is also crucial for diabetes management [72]. Precision medicine provides a new perspective for the management of T2DM, focusing not only on the physiological mechanisms of the disease but also covering multi-dimensional factors, such as psychology, behavior, and society. Through personalized intervention programs, precision medicine can help patients achieve better health outcomes in diabetes education and reducing psychological stress, and reduce the risk of T2DM-related complications. Mental health and metabolic status are mutually influential. In T2DM management, integrating psychological assessment with metabolic monitoring helps optimize personalized treatment strategies, enhance patient adherence, and improve long-term health outcomes. In the future, through multidisciplinary collaboration and precision medicine, this comprehensive management approach will provide more effective solutions for the treatment of metabolic disorders. Therefore, combining the concept of precision medicine with mental health and the treatment of T2DM is an important development direction for future diabetes management.
Artificial intelligence and big data analysis
Data-driven precision medicine provides innovative means for the prediction and management of T2DM. With the development of big data technology and AI, the medical industry can more accurately identify and predict the risk of T2DM. By integrating data from multiple dimensions, such as genomics, metabolomics, lifestyle, and environment, AI can not only analyze individual genetic susceptibility and potential metabolic disorders but also conduct a comprehensive dynamic monitoring of patients'health status, thereby achieving early warning and personalized treatment [73].
Meanwhile, the application of digital health platforms and wearable devices is also rapidly developing. T2DM patients can monitor their blood glucose, exercise, diet, and other data in real-time through mobile apps or smart wearable devices. These devices can combine patients'daily health behaviors with clinical data, providing doctors with more comprehensive information, optimizing treatment plans, and supporting remote management. Through this intelligent health management approach, it not only improves the accuracy of treatment but also helps patients achieve more efficient self-management, reducing complications caused by improper control. This data-driven precision medicine model can improve the prevention effect of T2DM to a greater extent, delay disease progression, and even help prevent the occurrence of the disease, ultimately improving the overall quality of life of patients[74].
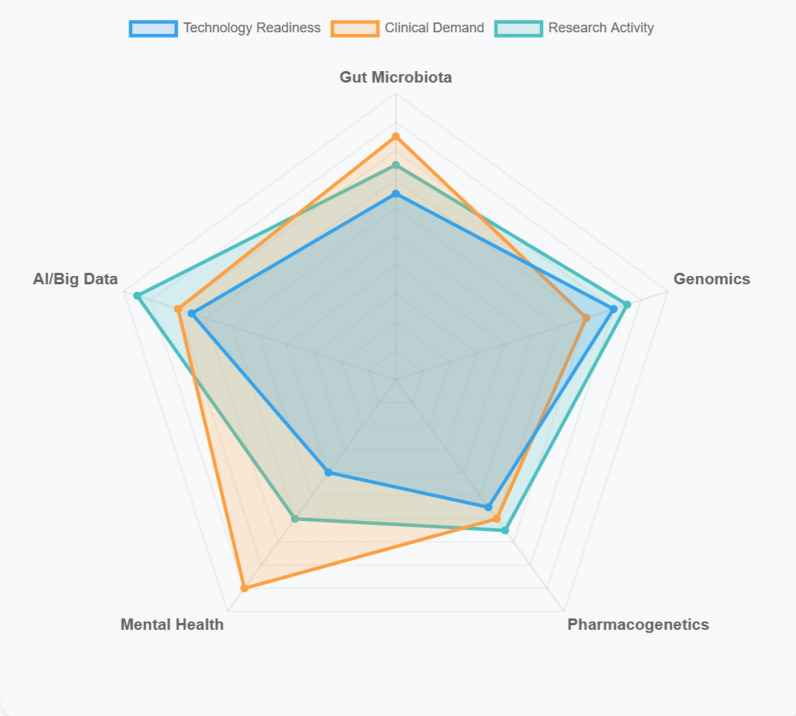
Furthermore, to accurately demonstrate the differing potential of future therapeutic targets and drug development pathways for T2DM in terms of technological maturity, clinical demand, and research interest, a radar chart can be used to present a multi-dimensional comparison of these indicators, as shown in Fig. 1.
Fig. 1.
Different potential of future therapeutic targets and drug development pathways for T2DM
Discussion
Rapid advancements in medical science, human biology, data science, and technology have provided a fresh perspective for the research and treatment of T2DM. These progressions enable us to gain a deeper understanding of the diversity, epidemiological characteristics, and treatment responses among different populations of T2DM, thereby updating our understanding of the"T2DM"phenotype. Global research on T2DM has gradually uncovered variations in its manifestations, and there are significant differences in prevalence among different regions and racial groups. These disparities impact the diagnostic and therapeutic strategies for T2DM [16]. Future therapeutic approaches will place greater emphasis on individual differences, optimizing treatment outcomes and minimizing side effects. Selecting treatment targets and drug development for T2DM based on glycemic benefits and guided by precision medicine methodologies holds significant potential, but it is crucial to ensure equity and clinical effectiveness. Treatment selection algorithms should be validated on data sets from diverse geographic and ethnic backgrounds, addressing disparities in treatment access. More global cost-effectiveness data is needed, and generic alternatives should be provided to reduce prices. As new evidence emerges, future algorithms should incorporate more customized variables, with priority given to verifying their clinical effectiveness in practical applications [75].
The novel therapeutic targets for T2DM encompass multiple aspects, including insulin sensitivity, β-cell protection, and energy metabolism regulation. With advancements in gene editing, targeted drug delivery, and AI-driven drug screening technologies, the clinical translation of these targets holds promise for developing more precise and effective treatment strategies, ultimately offering improved personalized interventions for patients. However, the implementation of precision medicine strategies in T2DM faces multiple challenges, particularly in the discovery of therapeutic targets and drug development. First, T2DM is a highly heterogeneous disease with significant individual differences in genetic and environmental factors, complicating the design of precision therapies. The identification of potential targets often relies on large-scale genomic and transcriptomic data analysis, but the clinical relevance and mechanisms of these targets are frequently unclear, and their validation process is fraught with uncertainty. Moreover, the development of precision medicine drugs entails long development cycles and high costs, with the added complexity of managing multiple comorbidities. In addition, precision therapies must not only integrate genetic information but also account for the interaction with environmental factors, further complicating treatment strategies. The clinical dissemination and application of such treatments also face practical barriers, such as inadequate resources in primary healthcare and low patient acceptance. Finally, the implementation of precision medicine relies on vast multidimensional data, yet issues related to data integration and privacy protection remain pressing challenges. Therefore, despite some progress in precision medicine research for T2DM, widespread clinical application and the realization of personalized treatment still require overcoming these multifaceted obstacles.
Precision medicine plays a crucial role in the prevention of T2DM. By leveraging multi-omics technologies, including genomics, proteomics, and metabolomics, along with big data and artificial intelligence, precision medicine enables individualized and targeted disease prevention while optimizing intervention pathways. Based on different genetic backgrounds and metabolic characteristics, precision medicine facilitates the development of personalized lifestyle modification strategies. In addition, by integrating digital health tools, it enables dynamic monitoring of T2DM-related biomarkers, thereby achieving early warning and intervention. Compared to traditional universal prevention strategies, precision medicine transforms T2DM prevention into an individualized and dynamic management approach, significantly enhancing preventive efficacy. In the future, guided by the principles of precision medicine, T2DM prevention strategies will become more intelligent and forward-looking, paving the way for more effective early intervention and health management.
Conclusion
T2DM is a complex metabolic chronic disease characterized by insulin resistance and elevated blood glucose levels, which are almost irreversible once diagnosed. Overall, the molecular mechanisms underlying the development of T2DM and its progression to irreversible organ damage remain elusive (76). As research on the pathophysiological mechanisms of T2DM deepens, some recently discovered signals and molecular pathways have emerged as important targets for new T2DM therapies. In the future, we should explore precision medical treatment targets for T2DM from multiple dimensions, including gut microbiota, genetic and epigenetic modifications, metabolic abnormalities, mitochondrial dysfunction and autophagy, immune system dysregulation, environmental factors, and lifestyle habits. By combining patients'genetic characteristics, environmental conditions, and individual differences in lifestyle, we can develop personalized blood glucose control strategies to select the most suitable treatment methods, thereby achieving optimized therapeutic outcomes.
Supplementary Information
Acknowledgements
All authors made a significant contribution to the work reported.XT and LW conceived the idea for this study. XC and LZ organized the outline and drafted the manuscript. XZ and HS provided a few studies, ideas, and some revised opinions. KZ and ZL participated in the study design and helped draft the manuscript. WW and XG contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Author contributions
All authors made a significant contribution to the work reported.XT and LW conceived the idea for this study. XC and LZ organized the outline and drafted the manuscript. XZ and HS provided a few studies, ideas, and some revised opinions. KZ and ZL participated in the study design and helped draft the manuscript. WW and XG contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Fundamental Research Funds for the China Academy of Chinese Medical Sciences (BJZYYB-2023–28).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xu Zhai, Email: zhaixu@mail.cintcm.ac.cn.
Huaqiang Shao, Email: Shq1969@163.com.
References
- 1.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400(10365):1803–20. [DOI] [PubMed] [Google Scholar]
- 2.Gurung RL, Zheng H, Lee BTK, Liu S, Liu JJ, Chan C, et al. Proteomics profiling and association with cardiorenal complications in type 2 diabetes subtypes in Asian population. Diabetes Res Clin Pract. 2024;214: 111790. [DOI] [PubMed] [Google Scholar]
- 3.Ling C, Bacos K, Rönn T. Epigenetics of type 2 diabetes mellitus and weight change - a tool for precision medicine? Nat Rev Endocrinol. 2022;18(7):433–48. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021. 10.1172/JCI142243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillon NJ, Loos RJF, Marshall SM, Zierath JR. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell. 2021;184(6):1530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodall R, Alazawi A, Hughes W, Bravis V, Salciccioli JD, Marshall DC, et al. Trends in type 2 diabetes mellitus disease burden in European Union countries between 1990 and 2019. Sci Rep. 2021;11(1):15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–8. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. [DOI] [PubMed] [Google Scholar]
- 9.Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus—implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16(6):321–31. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekaran P, Weiskirchen R. The role of obesity in type 2 diabetes mellitus-an overview. Int J Mol Sci. 2024;25(3):1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–10. [DOI] [PubMed] [Google Scholar]
- 13.Krarup T, Krarup T, Hagen C. Do patients with type 2 diabetes mellitus have an increased prevalence of Cushing’s syndrome? Diabetes Metab Res Rev. 2012;28(3):219–27. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A, Node K. Pathogenic connection between hypertension and type 2 diabetes: how do they mutually affect each other? Hypertens Res. 2022;45(11):1840–2. [DOI] [PubMed] [Google Scholar]
- 15.Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, et al. Precision medicine in diabetes: a Consensus report from the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia. 2020;63(9):1671–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad RB, Groop L. Precision medicine in type 2 diabetes. J Intern Med. 2019;285(1):40–8. [DOI] [PubMed] [Google Scholar]
- 18.Aiming for equitable precision medicine in diabetes. Nat Med. 2022;28(11):2223. [DOI] [PubMed]
- 19.Arora T, Tremaroli V. Therapeutic potential of butyrate for treatment of type 2 diabetes. Front Endocrinol. 2021;12: 761834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosomi K, Saito M, Park J, Murakami H, Shibata N, Ando M, et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat Commun. 2022;13(1):4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar M, Pal N, Sharma P, Kumawat M, Sarma DK, Nabi B, et al. Omega-3 fatty acids and their interaction with the gut microbiome in the prevention and amelioration of type-2 diabetes. Nutrients. 2022;14(9):1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia L, Huang S, Sun B, Shang Y, Zhu C. Pharmacomicrobiomics and type 2 diabetes mellitus: a novel perspective towards possible treatment. Front Endocrinol. 2023;14:1149256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jazieh C, Arabi TZ, Asim Z, Sabbah BN, Alsaud AW, Alkattan K, et al. Unraveling the epigenetic fabric of type 2 diabetes mellitus: pathogenic mechanisms and therapeutic implications. Front Endocrinol. 2024;15:1295967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker DC, Wan M, Lohman K, Hou L, Nguyen AT, Ding J, et al. Monocyte miRNAs are associated with type 2 diabetes. Diabetes. 2022;71(4):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu G, Gao H, Dong Z, Jiang S, Hu R, Wang C. Change profiles and functional targets of microRNAs in type 2 diabetes mellitus patients with obesity. Diabetes Metab J. 2023;47(4):559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri A, Desai BN, Fagoonee S, Cotton CA, Nguyen PK, et al. MicroRNA-22 is a key regulator of lipid and metabolic homeostasis. Int J Mol Sci. 2023;24(16):12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021;33(4):781-90.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao Y, Gu T, Zhang Z, Wu T, Wang J, Bi Y. The role of miRNAs carried by extracellular vesicles in type 2 diabetes and its complications. J Diabetes. 2023;15(10):838–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha SE, Singh R, Jin B, Baek G, Jorgensen BG, Zogg H, et al. miR-10a/b-5p-NCOR2 regulates insulin-resistant diabetes in female mice. Int J Mol Sci. 2024;25(18):10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause C, Britsemmer JH, Bernecker M, Molenaar A, Taege N, Lopez-Alcantara N, et al. Liver microRNA transcriptome reveals miR-182 as link between type 2 diabetes and fatty liver disease in obesity. Elife. 2024. 10.7554/eLife.92075.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su T, Hou J, Liu T, Dai P, Qin L, Ding L, et al. MiR-34a-5p and miR-452-5p: the novel regulators of pancreatic endocrine dysfunction in diabetic zucker rats? Int J Med Sci. 2021;18(14):3171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kariuki D, Aouizerat BE, Asam K, Kanaya AM, Zhang L, Florez JC, et al. MicroRNA biomarkers target genes and pathways associated with type 2 diabetes. Diabetes Res Clin Pract. 2023;203: 110868. [DOI] [PubMed] [Google Scholar]
- 33.Shan Z, Fa WH, Tian CR, Yuan CS, Jie N. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging. 2022;14(6):2902–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Ding W, Chen Z, Lai K, Liu Y. The role of autophagy in insulin resistance and glucolipid metabolism and potential use of autophagy modulating natural products in the treatment of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2024;40(1): e3762. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Wang Y, Li W, Chen H, Du L, Liu D, et al. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy. 2019;15(11):1882–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JM, Li X, Chan LWC, Hu R, Zheng T, Li H, et al. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 2023;66(12):2368–86. [DOI] [PubMed] [Google Scholar]
- 37.Woo JR, Bae SH, Wales TE, Engen JR, Lee J, Jang H, et al. The serine phosphorylations in the IRS-1 PIR domain abrogate IRS-1 and IR interaction. Proc Natl Acad Sci USA. 2024;121(17): e2401716121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Li M, Chen L, Bian H, Chen X, Zheng H, et al. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct Target Ther. 2021;6(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226–37. [DOI] [PubMed] [Google Scholar]
- 40.DeMarsilis A, Reddy N, Boutari C, Filippaios A, Sternthal E, Katsiki N, et al. Pharmacotherapy of type 2 diabetes: an update and future directions. Metabolism. 2022;137: 155332. [DOI] [PubMed] [Google Scholar]
- 41.Lok KH, Wareham NJ, Nair RS, How CW, Chuah LH. Revisiting the concept of incretin and enteroendocrine L-cells as type 2 diabetes mellitus treatment. Pharmacol Res. 2022;180: 106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maphumulo SC, Pretorius E. Role of circulating microparticles in type 2 diabetes mellitus: implications for pathological clotting. Semin Thromb Hemost. 2022;48(2):188–205. [DOI] [PubMed] [Google Scholar]
- 43.Yong J, Johnson JD, Arvan P, Han J, Kaufman RJ. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coco C, Sgarra L, Potenza MA, Nacci C, Pasculli B, Barbano R, et al. Can epigenetics of endothelial dysfunction represent the key to precision medicine in type 2 diabetes mellitus? Int J Mol Sci. 2019;20(12):2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teaney NA, Cyr NE. FoxO1 as a tissue-specific therapeutic target for type 2 diabetes. Front Endocrinol. 2023;14:1286838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Zhang Z, Wang D, Jiang Y, Liu Y. Targeting mTOR signaling in type 2 diabetes mellitus and diabetes complications. Curr Drug Targets. 2022;23(7):692–710. [DOI] [PubMed] [Google Scholar]
- 47.Song Z, Yang H, Zhou L, Yang F. Glucose-sensing transcription factor mondoA/ChREBP as targets for type 2 diabetes: opportunities and challenges. Int J Mol Sci. 2019;20(20):5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzadeh Z, Faber CL, Schwartz MW. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Annu Rev Pharmacol Toxicol. 2022;62:55–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engwa GA, Nweke FN, Karngong GN, Afiukwa CA, Nwagu KE. Understanding the pathogenesis, therapeutic targets/drug action and pharmacogenetics of type 2 diabetes: is there a future for personalised medicine? Endocr Metab Immune Disord Drug Targets. 2020;20(10):1569–89. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Zhang J, Cheng Y, Zhu M, Xiao Z, Ruan G, et al. Gut microbiota: A new target for T2DM prevention and treatment. Front Endocrinol. 2022;13: 958218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, et al. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients. 2021;13(9):3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng SC, Xu Z, Mak JWY, Yang K, Liu Q, Zuo T, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716–23. [DOI] [PubMed] [Google Scholar]
- 53.Kornmueller K, Amri EZ, Scheideler M, Prassl R. Delivery of miRNAs to the adipose organ for metabolic health. Adv Drug Deliv Rev. 2022;181: 114110. [DOI] [PubMed] [Google Scholar]
- 54.Thibonnier M, Esau C. Metabolic benefits of microRNA-22 inhibition. Nucleic Acid Ther. 2020;30(2):104–16. [DOI] [PubMed] [Google Scholar]
- 55.Bijkerk R, Esguerra JLS, Ellenbroek JH, Au YW, Hanegraaf MAJ, de Koning EJ, et al. In Vivo silencing of microRNA-132 reduces blood glucose and improves insulin secretion. Nucleic Acid Ther. 2019;29(2):67–72. [DOI] [PubMed] [Google Scholar]
- 56.Lewis KA, Stroebel BM, Zhang L, Aouizerat B, Mattis AN, Flowers E. MicroRNAs associated with metformin treatment in the diabetes prevention program. Int J Mol Sci. 2024;25(11):5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chow E, Wang K, Lim CKP, Tsoi STF, Fan B, Poon E, et al. Dorzagliatin, a dual-acting glucokinase activator, increases insulin secretion and glucose sensitivity in glucokinase maturity-onset diabetes of the young and recent-onset type 2 diabetes. Diabetes. 2023;72(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fosgerau K, Jessen L, Lind Tolborg J, Østerlund T, Schæffer Larsen K, Rolsted K, et al. The novel GLP-1-gastrin dual agonist, ZP3022, increases β-cell mass and prevents diabetes in db/db mice. Diabetes Obes Metab. 2013;15(1):62–71. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki S, Miyatsuka T, Matsuoka TA, Takahara M, Yamamoto Y, Yasuda T, et al. Activation of GLP-1 and gastrin signalling induces in vivo reprogramming of pancreatic exocrine cells into beta cells in mice. Diabetologia. 2015;58(11):2582–91. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Fu J, Zhou F, Yang Q, Wang J, Feng H, et al. Stapled and xenopus glucagon-like peptide 1 (GLP-1)-based dual GLP-1/gastrin receptor agonists with improved metabolic benefits in rodent models of obesity and diabetes. J Med Chem. 2020;63(21):12595–613. [DOI] [PubMed] [Google Scholar]
- 61.Hasib A, Ng MT, Tanday N, Craig SL, Gault VA, Flatt PR, et al. Exendin-4(Lys(27) PAL)/gastrin/xenin-8-Gln: a novel acylated GLP-1/gastrin/xenin hybrid peptide that improves metabolic status in obese-diabetic (ob/ob) mice. Diabetes Metab Res Rev. 2019;35(3): e3106. [DOI] [PubMed] [Google Scholar]
- 62.Blüher M, Rosenstock J, Hoefler J, Manuel R, Hennige AM. Dose-response effects on HbA(1c) and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia. 2024;67(3):470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao S, Yan Z, Du Y, Li Z, Tang C, Jing L, et al. A GLP-1/glucagon (GCG)/CCK(2) receptors tri-agonist provides new therapy for obesity and diabetes. Br J Pharmacol. 2022;179(17):4360–77. [DOI] [PubMed] [Google Scholar]
- 64.Zhou F, Song P, Tang X, Yang Q, Zhou S, Xu R, et al. Discovery of once-weekly, peptide-based selective GLP-1 and cholecystokinin 2 receptors co-agonizts. Peptides. 2022;153: 170811. [DOI] [PubMed] [Google Scholar]
- 65.Oh M, Jung S, Kim YA, Lee GY, Han SN. Dietary vitamin D(3) supplementation enhances splenic NK cell activity in healthy and diabetic male mice. Nutr Res. 2024;127:144–55. [DOI] [PubMed] [Google Scholar]
- 66.Mxinwa V, Dludla PV, Nyambuya TM, Mokgalaboni K, Mazibuko-Mbeje SE, Nkambule BB. Natural killer cell levels in adults living with type 2 diabetes: a systematic review and meta-analysis of clinical studies. BMC Immunol. 2020;21(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du L, Li Q, Yi H, Kuang T, Tang Y, Fan G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed Pharmacother. 2022;149: 112839. [DOI] [PubMed] [Google Scholar]
- 68.Zhai L, Wu J, Lam YY, Kwan HY, Bian ZX, Wong HLX. Gut-microbial metabolites, probiotics and their roles in type 2 diabetes. Int J Mol Sci. 2021;22(23):12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ling C. Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes. J Intern Med. 2020;288(2):158–67. [DOI] [PubMed] [Google Scholar]
- 70.Teerawattanapong N, Srisawat L, Narkdontri T, Yenchitsomanus PT, Tangjittipokin W, Plengvidhya N. The effects of transcription factor 7-like 2 rs7903146 and paired box 4 rs2233580 variants associated with type 2 diabetes on the therapeutic efficacy of hypoglycemic agents. Heliyon. 2024;10(5): e27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mannino GC, Andreozzi F, Sesti G. Pharmacogenetics of type 2 diabetes mellitus, the route toward tailored medicine. Diabetes Metab Res Rev. 2019;35(3): e3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skinner TC. Psychological barriers. Eur J Endocrinol. 2004;151 Suppl 2:T13–7; discussion T29–30. [DOI] [PubMed]
- 73.Mosquera-Lopez C, Jacobs PG. Digital twins and artificial intelligence in metabolic disease research. Trends Endocrinol Metab. 2024;35(6):549–57. [DOI] [PubMed] [Google Scholar]
- 74.Huang YJ, Chen CH, Yang HC. AI-enhanced integration of genetic and medical imaging data for risk assessment of Type 2 diabetes. Nat Commun. 2024;15(1):4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.The Lancet Digital H. Equitable precision medicine for type 2 diabetes. Lancet Digit Health. 2022; 4(12): e850. [DOI] [PubMed]
- 76.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.