Abstract
Background
Lyme disease, caused by Borrelia burgdorferi sensu lato (s.l.), is the most common vector-borne disease in the Northern Hemisphere, with Ixodes ticks as its primary vectors. However, many patients do not recall tick bites, fueling speculation about alternative transmission routes, particularly via mosquito bites. This belief is reinforced by studies reporting Borrelia presence in mosquitoes. This study evaluates whether three mosquito species can acquire, maintain, and transmit Borrelia spirochetes.
Methods
Mosquitoes (Aedes aegypti, Culex quinquefasciatus, and Culex pipiens biotype molestus) were fed on Borrelia-infected mice to assess pathogen acquisition. Additional experiments involved ex vivo feeding on Borrelia-enriched blood to examine spirochete persistence in the mosquito gut. The potential for mechanical transmission was tested by simulating interrupted feeding between infected and naive hosts. The role of trypsin in Borrelia survival and infectivity was also investigated.
Results
Mosquitoes exhibited low efficiency in acquiring Borrelia from infected hosts. Spirochetes artificially introduced through ex vivo blood meals were rapidly eliminated during digestion, primarily due to trypsin activity. Inhibition of trypsin prolonged spirochete persistence and infectivity in the mosquito gut. Mechanical transmission experiments revealed no evidence of Borrelia transmission from infected to naive hosts.
Conclusions
Our findings demonstrate that mosquitoes lack the biological capacity to efficiently acquire and maintain B. burgdorferi s.l. spirochetes and are unable to transmit them through natural or mechanical means. This study provides compelling evidence against mosquito-borne transmission of Lyme disease and reinforces Ixodes ticks as the sole competent vectors, which is crucial for targeted public health interventions and accurate risk communication.
Graphical Abstract
Keywords: Lyme disease, Borreliosis, Mosquito, Tick, Borrelia, Transmission
Background
Lyme disease (borreliosis) is the most prevalent vector-borne disease in Europe and the United States, representing a significant public health concern in the Northern Hemisphere. The disease is primarily caused by spirochetes from the Borrelia burgdorferi sensu lato (s.l.) complex. In the United States, B. burgdorferi sensu stricto (s.s.) Johnson et al., 1984 is the predominant species, whereas in Europe, the most common species associated with human infections are Borrelia afzelii Canica et al., 1993, Borrelia garinii Baranton et al., 1992, and B. burgdorferi s.s. Borrelia spirochetes are maintained in nature through an enzootic cycle involving small vertebrates, primarily rodents and birds, with humans serving as incidental hosts [1].
Ticks of the genus Ixodes are widely recognized as the primary vectors of Lyme disease. However, many patients diagnosed with the disease do not recall being bitten by a tick [2, 3]. In a retrospective cohort study, only 56 of 210 (26.7%) Canadian Lyme disease patients reported a tick bite [4]. In another study, 1070 forestry workers from Ukraine were tested using enzyme-linked immunosorbent assay (ELISA) for specific B. burgdorferi immunoglobulin M (IgM) and IgG antibodies and were surveyed regarding their history of tick bites. Among those who did not recall any tick bites, 27.0% tested seropositive [5]. Similarly, data from the MyLymeData online patient registry revealed that 59% of 3903 US patients clinically diagnosed with Lyme disease either did not recall or were unsure about a tick bite [6]. This is primarily due to the painless nature of tick bites and the small size of nymphal ticks, which are responsible for most human cases of Lyme disease and often go unnoticed. In contrast, people are more likely to recall the painful bites of other blood-feeding insects, such as mosquitoes, deer flies, horse flies, and black flies. As a result, people frequently associate their Lyme disease infection with previous insect bites, perpetuating the belief that these insects can transmit the pathogen. This belief is further reinforced by numerous studies reporting the detection of Borrelia spirochetes in arthropods other than ticks, including mosquitoes [7–11], raising questions about their vector competence. However, the mere detection of Borrelia in mosquitoes does not necessarily indicate their ability to transmit the pathogen. To date, experimental evidence confirming or refuting the role of mosquitoes in transmitting Lyme disease spirochetes remains lacking.
This study investigates whether mosquitoes are capable of transmitting Lyme disease by evaluating their ability to acquire, maintain, and transmit B. burgdorferi, B. afzelii, and B. garinii spirochetes. Our findings provide critical insights into the vector competence of mosquitoes, addressing public health concerns and contributing to a deeper understanding of Lyme disease ecology.
Methods
Borrelia spirochetes and laboratory animals
Infectious and low-passage strains of B. afzelii CB43 [12, 13], B. burgdorferi s.s. N40 (isolate obtained from Prof. Joppe W.R. Hovius, Amsterdam University Medical Center, Netherlands), and B. garinii WSLB 8096/1 (isolate obtained from Dr. Jiří Nepeřený, Bioveta, Czech Republic) were grown in BSK-H medium (Sigma-Aldrich, St. Louis, MO, USA) at 33 °C. A low-passage strain of the relapsing fever spirochete Borrelia duttonii Novy & Knapp, 1906, strain 1120 K3 (origin, Congo) [14], was grown in mBSK medium supplemented with 10% rabbit serum at 35 °C [15]. For both mouse injections and acquisition experiments using artificial membrane feeders, spirochetes were counted using dark-field microscopy.
The mosquito colonies were obtained from the Laboratory of Molecular Biology and Physiology of Mosquitoes, Institute of Parasitology, Biology Centre, Czech Academy of Sciences (Aedes aegypti Linnaeus, 1762) and from the Faculty of Science, Charles University in Prague (Culex quinquefasciatus Say, 1823 and Culex pipiens biotype molestus Forskal, 1775). The mosquitoes were kept in 20 × 20 × 20 cm nylon nets in an incubator with constant conditions of 28 °C, 80% humidity and 16 h:8 h light/dark photoperiod. The mosquitoes had permanent access to 10% sucrose. The mosquito larvae were fed aquarium fish food (Culex spp: TetraMin flakes; Ae. aegypti: TabiMin tablets, Tetra, Melle, Germany). Female mosquitoes were used for experiments 1 week after eclosion. Ixodes ricinus Linnaeus, 1758 ticks were obtained from the Institute of Parasitology, Biology Centre, Czech Academy of Sciences. They were maintained under controlled conditions (temperature 24 °C, 95% humidity, 15 h:9 h light/dark photoperiod). Borrelia afzelii- and B. burgdorferi s.s.-infected nymphs were prepared as described previously [12]. C3H/HeN mice (Jackson Laboratory, Bar Harbor, ME, USA) were used for mosquito feeding, as well as Borrelia acquisition and transmission experiments.
All laboratory animals were treated in accordance with the Animal Protection Law of the Czech Republic no. 246/1992 Sb., ethics approval no. 25/2020. The study was approved by the Institute of Parasitology, Biology Centre CAS and the Central Committee for Animal Welfare, Czech Republic (Protocol No. 25/2020).
PCR detection and quantification of Borrelia
DNA was extracted using the NucleoSpin Tissue kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s protocol. Spirochete detection in mosquitoes and murine tissues was performed by a nested polymerase chain reaction (PCR) targeting a 222-base-pair (bp) fragment of the 23S rRNA gene. Quantitative real-time PCR (qPCR) was employed to quantify the total spirochete load in mosquitoes by amplifying a 154-bp fragment of the flagellin gene. The PCR and qPCR amplification conditions followed previously described protocols [12].
Mouse acquisition experiments
Six-week-old female C3H/HeN mice were infected by subcutaneous injection of 105 B. afzelii or B. burgdorferi s.s. spirochetes per mouse. The presence of spirochetes in ear biopsies was verified by PCR 3 weeks post-injection. The Borrelia-positive mouse was then anesthetized and placed into the mosquito net, allowing Ae. aegypti, Cx. quinquefasciatus, and Cx. pipiens biotype molestus mosquitoes to feed ad libitum on the mouse.
Eight-week-old female C3H/HeN mice were inoculated intraperitoneally and subcutaneously with 105 B. duttonii spirochetes per mouse. On days 3 and 4 post-inoculation, 5 μl of blood from tail snips was placed on glass slides to confirm the presence of spirochetes by dark-field microscopy. Once spirochetemia was assured, the mice were used to feed Ae. aegypti.
Twenty fully engorged mosquitoes were collected from each experimental group and tested for the presence of spirochetes by PCR.
Acquisition experiments on artificial membrane feeders
The 3D-printed feeders were designed de novo in FreeCAD 0.20 based on previously published nano-feeders [16] and printed on a Creality Halot Sky (CL-89) 3D printer with low-odor rigid resin (Creality, Shenzhen, China), with the following modifications: (i) the blood reservoir capacity was increased to 1 ml, (ii) the feeding area was expanded to 2.4 cm2, (iii) the blood filling port was shaped to tightly accommodate a 1 ml pipette tip, and (iv) the warm water circulation connectors were made compatible with 5 mm internal diameter silicone tubing.
A total of 3.5 × 107 B. afzelii, B. burgdorferi s.s., and B. garinii spirochetes were resuspended in 1 ml blood (mouse blood for B. afzelii and B. burgdorferi s.s.; and chicken blood for B. garinii) supplemented with ATP (1 mM) and injected into the feeding unit. The blood temperature was maintained at 37 °C. Prior to feeding, Ae. aegypti and Cx. quinquefasciatus mosquitoes were deprived of sugar for 12 h and then transferred to feeding units using an aspirator. Mosquitoes were allowed to feed until fully satiated. At 0, 24, 48, 72, and 96 h post-feeding, mosquitoes were euthanized by snap-freezing, and the presence of spirochetes in whole-body homogenates was assessed using nested PCR and quantified by qPCR. For each mosquito species and time point combination, 20 mosquitoes were tested.
Borrelia viability and infectivity experiments
Female Ae. aegypti and Cx. quinquefasciatus were fed on membrane feeding units containing mouse blood spiked with B. afzelii spirochetes. Whole-body homogenates were prepared from mosquitoes at 0, 24, 48, and 72 h post-feeding and subcutaneously injected into C3H/HeN mice (five mice/group, one mosquito/mouse). Four weeks after injection, infection in ear, heart, and bladder tissues was determined by nested PCR.
Effect of trypsin on Borrelia survival
Female mosquitoes were fed on membrane feeding units containing mouse blood spiked with B. afzelii spirochetes and soybean trypsin inhibitor (SBTI) at a concentration of 2 mg/ml for Cx. quinquefasciatus and 1 mg/ml for Ae. aegypti. Mosquitoes were allowed to feed until fully satiated. At 0, 24, 48, 72, and 96 h post-feeding, mosquitoes were euthanized by snap-freezing, and the presence of spirochetes in whole-body homogenates was assessed using nested PCR and quantified by qPCR. For each mosquito species and time point combination, 20 mosquitoes were tested. The results were compared to untreated control mosquitoes.
To assess the direct impact of trypsin on Borrelia viability, cultures of B. afzelii (3.5 × 107 spirochetes/ml) were treated with trypsin (300 ng/ml). Control cultures were treated with heat-inactivated trypsin (10 min at 95 °C). The viability of B. afzelii spirochetes was evaluated after 48 h.
Transmission experiments
Female Ae. aegypti and Cx. quinquefasciatus mosquitoes were fed on membrane feeding units containing mouse blood spiked with B. afzelii or B. burgdorferi s.s. spirochetes. After digestion and egg-laying, the females were fed on naive mice (four mice/group, 15 mosquitoes/mouse). Immediately after feeding, DNA was extracted from fully engorged mosquitoes. Four weeks after the second feeding, ear, skin, bladder, and heart biopsies were collected and the presence of Borrelia spirochetes in mouse tissue and mosquito samples was determined by nested PCR.
Mechanical transmission experiments
Female Ae. aegypti and Cx. quinquefasciatus mosquitoes were fed on membrane feeding units containing mouse blood spiked with B. afzelii or B. burgdorferi s.s. spirochetes, interrupted mid-feeding, and then immediately placed on naive mice to resume their feeding (four mice/group, 18–20 mosquitoes/mouse). After feeding, DNA was extracted from the collected mosquitoes. Four weeks post-feeding, mouse tissues were collected as described above. The presence of Borrelia spirochetes in mouse tissue and mosquito samples was determined by nested PCR.
Statistics and software
Data were analyzed using GraphPad Prism 10, and an unpaired Student t-test was used to assess statistical significance. A P-value of less than 0.05 was considered statistically significant. The error bars in the graphs represent the standard errors of the means.
Results
Mosquitoes rarely acquire Borrelia from infected host
To investigate whether mosquitoes can acquire Borrelia spirochetes from infected hosts, female Ae. aegypti, Cx. quinquefasciatus, and Cx. pipiens biotype molestus mosquitoes were fed on mice infected with B. afzelii or B. burgdorferi s.s. PCR analysis showed that 15% of Ae. aegypti (3/20) fed on B. burgdorferi s.s.-infected mice tested positive, whereas no spirochetes were detected in Ae. aegypti that fed on mice infected with B. afzelii, or in Cx. quinquefasciatus or Cx. pipiens biotype molestus fed on mice infected with either Borrelia species (Table 1).
Table 1.
Acquisition of Borrelia spirochetes by mosquitoes
| B. afzelii | B. burgdorferi | B. duttonii | |
|---|---|---|---|
| Ae. aegypti | 0% (0/20) | 15% (3/20) | 100% (20/20) |
| Cx. quinquefasciatus | 0% (0/20) | 0% (0/20) | ND |
| Cx. pipiens molestus | 0% (0/20) | 0% (0/20) | ND |
Ae. aegypti, Cx. quinquefasciatus, and Cx. pipiens biotype molestus females were fed on mice infected with B. afzelii or B. burgdorferi s.s. Twenty fully engorged mosquitoes were collected from each experimental group and tested for the presence of spirochetes using PCR. As a control for spirochete acquisition, Ae. aegypti females were fed on mice infected with B. duttonii. ND not done
In a control experiment using B. duttonii, a Borrelia species naturally present in the bloodstream, all Ae. aegypti (20/20) tested positive for spirochetes 24 h post-feeding (Table 1).
These findings indicate that, unlike ticks, mosquitoes lack mechanisms to actively attract Borrelia to their feeding sites. Instead, the spirochetes must already be present in the bloodstream for potential mosquito ingestion. Overall, these results indicate a low probability of mosquitoes acquiring the Lyme disease pathogen under natural conditions.
Borrelia spirochetes are quickly eliminated during mosquito digestion
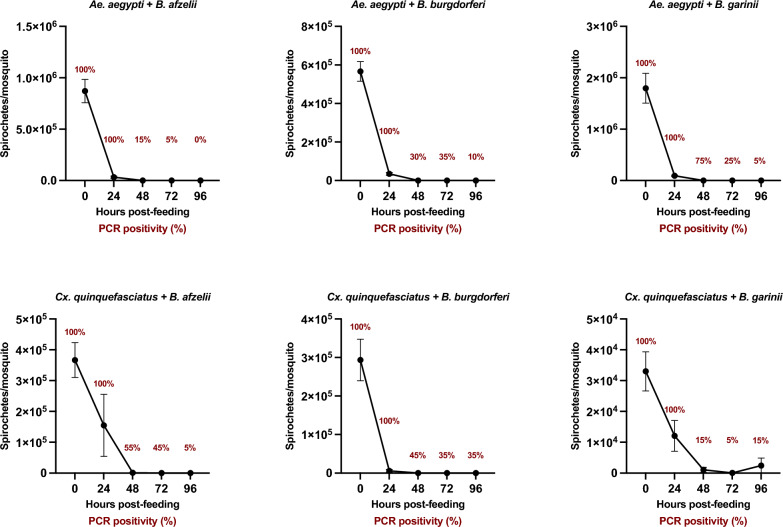
Previous experiments demonstrated that mosquitoes can only rarely acquire Borrelia spirochetes. We further investigated the fate of Borrelia spirochetes in mosquitoes. Aedes aegypti and Cx. quinquefasciatus were fed on membrane feeding units containing mouse blood spiked with B. afzelii, B. burgdorferi s.s., or B. garinii. Spirochetes were detected and quantified by PCR and qPCR at 0, 24, 48, 72, and 96 h post-feeding.
Spirochetes were successfully introduced into mosquitoes via artificial feeding, with 100% efficacy. By 24 h, spirochete DNA remained detectable in all mosquitoes but gradually disappeared over subsequent time points. In Cx. quinquefasciatus, spirochete decline was slower than in Ae. aegypti. At 96 h, 5%, 35%, and 15% of Cx. quinquefasciatus and 0%, 10%, and 5% of Ae. aegypti were PCR-positive for B. afzelii, B. burgdorferi s.s., and B. garinii, respectively (Fig. 1).
Fig. 1.
Decline in Borrelia spirochete numbers in mosquitoes during post-blood meal digestion. The temporal dynamics of Borrelia spirochete levels in Ae. aegypti and Cx. quinquefasciatus following ingestion of mouse blood spiked with B. afzelii, B. burgdorferi s.s., or B. garinii. Spirochetes were detected using PCR and quantified by qPCR at 0, 24, 48, 72, and 96 h post-feeding. Error bars represent the standard error of the mean
A rapid decline in spirochete numbers post-feeding was confirmed by qPCR. Within 24 h, median spirochete counts in Ae. aegypti decreased 58-, 17-, and 23-fold for B. afzelii, B. burgdorferi s.s., and B. garinii, respectively, with near-total elimination by 96 h (two mosquitoes remained positive for B. burgdorferi s.s. and one for B. garinii). In Cx. quinquefasciatus, declines at 24 h were 8-, 213-, and fourfold, and 5/30 mosquitoes remained positive at 96 h (three for B. burgdorferi s.s. and two for B. garinii) (Fig. 1).
These results confirm that ingested Borrelia spirochetes are rapidly eliminated in the mosquito gut.
Trypsin promotes Borrelia clearance in mosquitoes
The previous experiment showed that Borrelia spirochetes are efficiently eliminated from the mosquito gut after a blood meal. Midgut trypsin plays a key role in the digestion of blood components between blood meals.
To gain deeper insight into the role of extracellular digestion in Borrelia survival, we next examined whether trypsin activity influences the viability of Borrelia spirochetes within the mosquito gut. Aedes aegypti and Cx. quinquefasciatus were fed B. afzelii-spiked blood with or without soybean trypsin inhibitor (SBTI). Trypsin inactivation prolonged B. afzelii persistence in both mosquito species. At 72 h post-feeding, spirochetes were detected in 100% (20/20) of trypsin-inactivated Ae. aegypti, compared to 5% (1/20) of control mosquitoes (Fig. 2A). Similarly, B. afzelii persisted longer in Cx. quinquefasciatus mosquitoes with inactivated trypsin. At 72 h post-feeding, 85% (17/20) of SBTI-treated Cx. quinquefasciatus mosquitoes were positive, compared to 45% (9/20) of control mosquitoes (Fig. 2B).
Fig. 2.
Persistence of B. afzelii in trypsin-inhibited mosquitoes. A Aedes aegypti and B Cx. quinquefasciatus were fed on mouse blood spiked with B. afzelii in the presence (SBTI) or absence (control) of soybean trypsin inhibitor. The presence of spirochetes in mosquitoes was assessed using PCR at 24, 48, 72, and 96 h post-feeding.
In vitro assays confirmed the direct effect of trypsin on B. afzelii. Cultures treated with active trypsin showed ~ 97% spirochete death at 48 h, compared to ~ 34% with heat-inactivated trypsin. These findings establish trypsin as a critical factor in Borrelia clearance.
Borrelia lose infectivity during mosquito digestion
The infectivity of B. afzelii during digestion was assessed using homogenates of fed mosquitoes. All mice (5/5) injected with Ae. aegypti homogenates at 0 h post-feeding became infected. At 24 h post-feeding, spirochetes remained infectious for 4/5 mice, while mice injected with homogenates at 48 and 72 h post-feeding tested negative. Similarly, 5/5, 4/5, 2/5, and 0/5 mice injected with Cx. quinquefasciatus homogenates at 0, 24, 48, and 72 h post-feeding, respectively, developed infections.
Trypsin inhibition extended spirochete infectivity. At 72 h, 3/5 mice injected with homogenates from trypsin-inhibited Cx. quinquefasciatus developed infections, compared to 0/5 in controls (Table 2).
Table 2.
Infectivity of B. afzelii during mosquito digestion
| Ae. aegypti | Cx. quinquefasciatus | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | |
| Control | 5/5 | 4/5 | 0/5 | 0/5 | 5/5 | 4/5 | 2/5 | 0/5 |
| SBTI | 5/5 | ND | 1/5 | 0/5 | 5/5 | ND | 3/5 | 3/5 |
These results indicate that Borrelia spirochetes survive in the mosquito gut for up to 48 h but lose their infectivity thereafter.
Aedes aegypti and Cx. quinquefasciatus were fed on mouse blood spiked with B. afzelii in the presence (SBTI) or absence (control) of soybean trypsin inhibitor. Homogenates of mosquitoes collected at 0, 24, 48, and 72 h post-feeding were injected subcutaneously into mice (five mice/group). Infection in murine tissues was assessed using nested PCR 4 weeks after injection.
Mosquitoes cannot naturally transmit Borrelia
We further tested whether Borrelia spirochetes, if surviving mosquito digestion, could be transmitted during subsequent feeding. To model this, Ae. aegypti and Cx. quinquefasciatus were fed B. afzelii- or B. burgdorferi s.s.-spiked blood, allowed to digest and oviposit, and then fed on naive mice.
No spirochetes were detected in any mice (0/4) exposed to mosquitoes, or in the fully fed mosquitoes collected post-feeding (Table 3A), confirming that Borrelia cannot survive in mosquitoes between blood meals. In contrast, all (4/4) control mice exposed to infected I. ricinus ticks became infected, validating the efficiency of tick transmission under identical experimental conditions.
Table 3.
Transmission of Borrelia spirochetes by feeding of infected mosquitoes or I. ricinus nymphs
| (A) Natural transmission | (B) Mechanical transmission | |||
|---|---|---|---|---|
| B. afzelii | B. burgdorferi | B. afzelii | B. burgdorferi | |
| Ae. aegypti | 0/4 | 0/4 | 0/4 | 0/4 |
| Cx. quinquefasciatus | 0/4 | 0/4 | 0/4 | 0/4 |
| I. ricinus | 4/4 | 4/4 | ND | ND |
(A) Borrelia transmission via natural feeding. (B) Borrelia transmission via interrupted feeding. Numbers represent the number of infected mice/total number of experimental mice in each group. ND not done
Borrelia cannot be transmitted mechanically by mosquitoes
Next, we tested whether interrupted feeding could result in mechanical transmission of Borrelia spirochetes. This hypothesis suggests that mosquitoes interrupted while feeding on an infected host could transmit spirochetes adhered to their mouthparts to a new host during subsequent feeding. Aedes aegypti and Cx. quinquefasciatus were fed on B. afzelii- or B. burgdorferi s.s.-spiked blood, interrupted mid-meal, and immediately placed on naive mice to resume feeding.
No B. afzelii or B. burgdorferi s.s. infections were detected in the exposed mice (0/4), although all mosquitoes collected post-feeding tested positive for spirochetes (Table 3B).
These findings confirm that mosquitoes cannot transmit Borrelia spirochetes via mechanical or natural routes.
Discussion
In recent years, there has been growing interest and concern regarding the potential role of mosquitoes in the transmission of Lyme disease. While ticks have long been recognized as the primary vectors for B. burgdorferi s.l., the causative agent of Lyme disease, emerging evidence suggests that other arthropods, including mosquitoes, may also play a role in the transmission cycle. The presence of spirochetes in mosquitoes was first reported in 1907 by Jaffé, who microscopically observed a spirochete in Culex sp. [17]. Magnarelli and Anderson later detected B. burgdorferi s.s. by indirect fluorescent-antibody staining in Aedes mosquitoes (with a prevalence of up to 11.1%), three species of horse flies (up to 14.3%), and four species of deer flies (up to 10.5%) [18]. More recently, Melaun et al. detected DNA from B. afzelii, Borrelia bavariensis Margos et al., 2013, and B. garinii in 10 mosquito species from four different genera: Aedes (incl. Ochlerotatus), Culiseta, and Culex [11]. However, the mere detection of Borrelia in mosquitoes does not necessarily imply their ability to effectively transmit the pathogen to humans or other vertebrate hosts. In this study, we experimentally tested the ability of three mosquito species to transmit Lyme disease spirochetes.
Vector competence is the inherent capability of an organism to acquire, maintain, and transmit a specific pathogen. Ticks of the genus Ixodes, the competent vectors of Lyme disease, fulfil all these requirements. They remain attached to a host for an extended period, typically spanning several days, creating favorable conditions for the acquisition of B. burgdorferi s.l. During this time, spirochetes migrate from the host tissues into the feeding cavity that forms in the skin and are then ingested by the tick [12, 19]. In contrast, mosquitoes feed from blood capillaries and do so much faster than ticks, typically within seconds to minutes [20]. This rapid feeding behavior limits their ability to effectively acquire spirochetes, as demonstrated in our study, where only 15% of Ae. aegypti mosquitoes feeding on B. burgdorferi s.s.-infected mice tested positive for spirochetes. In addition, no spirochetes were detected in Ae. aegypti exposed to B. afzelii-infected mice, or in Cx. quinquefasciatus or Cx. pipiens biotype molestus mosquitoes feeding on mice infected with either B. afzelii or B. burgdorferi s.s.
In contrast, 100% of mosquitoes feeding on B. duttonii-infected mice ingested the spirochetes. Borrelia duttonii, the causative agent of tick-borne relapsing fever, is transmitted by the argasid tick Ornithodoros moubata Murray, 1877. Unlike ixodid ticks, argasid ticks feed rapidly (in under 10 min), making their feeding behavior more similar to that of mosquitoes. Borrelia duttonii spirochetes are well adapted to this rapid feeding. Compared to B. burgdorferi s.l., which are rarely found in the blood [21], B. duttonii are present in high concentrations in the blood during the febrile phase of the disease (up to 107 spirochetes/ml) [22].
Overall, these findings indicate that mosquitoes have a low probability of being infected with B. burgdorferi s.l., likely due to the adaptation of these spirochetes to the slow feeding behavior of ixodid ticks and their absence in the blood.
The next biological factor influencing vector competence is the ability to maintain pathogens between blood meals. Ixodes ticks are highly competent in maintaining B. burgdorferi s.l. spirochetes. Ixodes larvae initially acquire a small number of Borrelia spirochetes, which colonize and multiply in the tick midgut post-repletion [12, 19]. After molting into nymphs, the spirochetes adhere to the midgut epithelial cells [23] and remain there until the next feeding. Borrelia burgdorferi s.l. spirochetes have evolved mechanisms to survive in the nutrient-poor tick gut during long intervals between blood meals.
Little is known about the competence of mosquitoes to maintain B. burgdorferi s.l. spirochetes. Magnarelli et al. demonstrated that B. burgdorferi s.s. can survive briefly in Aedes mosquitoes after feeding on spirochete-spiked bovine blood. However, the spirochetes were quickly eliminated and rarely detectable in Ae. aegypti and Ae. triseriatus Say, 1823 up to 14 days post-feeding [24].
In our study, artificial membrane feeding efficiently introduced Borrelia spirochetes into the mosquito gut. However, the spirochetes were rapidly cleared during the post-feeding period. This clearance was driven by the digestion mechanisms in the mosquito midgut. Although both mosquitoes and ticks feed on blood, they have developed different digestive mechanisms. Tick digestion is intracellular. Once blood is ingested, it is absorbed into midgut cells where lysosomes degrade blood components using multiple proteolytic enzymes [25]. In mosquitoes, however, digestion is extracellular. Blood enters the mosquito midgut, which is the primary site of digestion. The blood is degraded by digestive enzymes secreted by the midgut epithelium. Trypsin plays a central role in this process [26]. In our experiments, inactivation of trypsin significantly prolonged the persistence of spirochetes in the midgut of mosquitoes, highlighting the importance of extracellular digestion in preventing Borrelia maintenance.
The final requirement of the vector competence is the ability to transmit the pathogen to a new host during subsequent feeding. Studies on ticks have shown that the infectivity of Borrelia spirochetes depends on differential gene expression stimulated by feeding [12, 27]. Furthermore, to establish an infection in the host, Borrelia spirochetes must evade the tick midgut and migrate to the feeding lesion. These processes typically require 24–48 h [12].
Unlike ticks, mosquitoes only need a few minutes to complete a blood meal [20]. This short feeding period limits the time available for Borrelia spirochetes to transition from a non-infectious to a vertebrate-infective state. In addition, Borrelia would need to exit the mosquito midgut, traverse the hemocoel, and reach the salivary glands. This process would require additional time and multiple adaptations, including evasion of immune defenses in the mosquito hemolymph, receptor-mediated infection of the salivary glands, and other mechanisms that typically evolve over long periods of coevolution [28]. Supporting this hypothesis, a transmission experiment demonstrated that Ae. aegypti and Cx. quinquefasciatus mosquitoes experimentally infected with B. afzelii and B. burgdorferi s.s. spirochetes were unable to transmit the infection to naive mice.
Mechanical transmission has also been considered as a potential route for mosquito-mediated Borrelia transmission. In this scenario, a mosquito would feed on an infected host, be disturbed, and then complete feeding on another host, mechanically transferring the spirochetes adhering to its mouthparts to the second host. However, an experiment in which mosquitoes were half-fed on mouse blood spiked with B. afzelii or B. burgdorferi s.s. spirochetes, and then allowed to complete feeding on naive mice, ruled out this possibility. Despite the presence of spirochetes in the mosquitoes, there was no transmission to the second host.
Conclusions
Our study reinforces the consensus that mosquitoes cannot serve as competent vectors for Lyme disease spirochetes, underscoring the exclusive role of ixodid ticks in transmitting this pathogen. Specifically, (1) mosquitoes are unable to acquire sufficient quantities of spirochetes during feeding; (2) ingested spirochetes are efficiently eliminated during blood meal digestion, primarily due to the activity of trypsin; and (3) mosquitoes cannot serve as either natural or mechanical vectors for Borrelia spirochetes. Our findings highlight the need for careful interpretation when evaluating the presence of Borrelia in non-tick arthropods. Attributing vectorial capacity to mosquitoes based solely on pathogen detection risks failing to capture the complex biological and ecological requirements for Lyme disease transmission.
Acknowledgements
We acknowledge the excellent technical assistance provided by Adéla Palusová, Jan Erhart, and Zuzana Vavrušková from the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences.
Author contributions
MP, IR, FN, OH, JP, JV, MN and RŠ planned the experiments. MP, KK, RV, HS, DH, KV, SŠ, RR and RŠ carried out the laboratory-based experimental work. RŠ designed the study and drafted the main manuscript text, assisted by MP. KV, IR, RR, FN, OH, JP, JV and MN critically revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was primarily supported by the Ministry of Health of the Czech Republic, grant no. NU20-05–00396 to RS. Additional support was provided by the Czech Science Foundation, grants no. 22-30920S to RS, 22-21244S to MN, and 25-16064S to OH; Ministry of Health of the Czech Republic, grant no. NU21-05–00143 to IR and SŠ as well as by grant R21 AI167849 from the National Institutes of Health-NIAID, USA, to FGN. JV and KV were supported by INTER-COST-LUC23, project no. LUC23151.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marques AR, Strle F, Wormser GP. Comparison of Lyme Disease in the United States and Europe. Emerg Infect Dis. 2021;27:2017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigrovic LE, Neville DN, Balamuth F, Bennett JE, Levas MN, Garro AC. A minority of children diagnosed with Lyme disease recall a preceding tick bite. Ticks Tick Borne Dis. 2019;10:694–6. 10.1016/J.TTBDIS.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Perthame E, Chartier L, George JC, Varloud M, Ferquel E, Choumet V. Case presentation and management of Lyme disease patients: a 9-year retrospective analysis in France. Front Med (Lausanne). 2024. 10.3389/FMED.2023.1296486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogerson AG, Lloyd VK. Lyme disease patient outcomes and experiences; a retrospective cohort study. Healthcare (Basel). 2020. 10.3390/HEALTHCARE8030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreychyn M, Shkilna M, Tokarskyy O, Ivakhiv O, Smahlii Z, Korda M. Not bitten by Ixodes ticks or bitten without symptoms, why still to worry? Cent Eur J Public Health. 2024;32:173–7. 10.21101/CEJPH.A8114. [DOI] [PubMed] [Google Scholar]
- 6.Johnson L, Shapiro M, Mankoff J. Removing the mask of average treatment effects in chronic Lyme disease research using big data and subgroup analysis. Healthcare (Basel). 2018. 10.3390/HEALTHCARE6040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosik-Bogacka D, Bukowska K, Kuźna-Grygiel W. Detection of Borrelia burgdorferi sensu lato in mosquitoes (Culicidae) in recreational areas of the city of Szczecin. Ann Agric Environ Med. 2002;9:55–7. [PubMed] [Google Scholar]
- 8.Zákovská A, Nejedla P, Holíková A, Dendis M. Positive findings of Borrelia burgdorferi in Culex (Culex) pipiens pipiens larvae in the surrounding of Brno city determined by the PCR method. Ann Agric Environ Med. 2003;9:257–9. [PubMed] [Google Scholar]
- 9.Halouzka J, Postic D, Hubálek Z. Isolation of the spirochaete Borrelia afzelii from the mosquito Aedes vexans in the Czech Republic. Med Vet Entomol. 1998;12:103–5. 10.1046/J.1365-2915.1998.00086.X. [DOI] [PubMed] [Google Scholar]
- 10.Magnarelli LA, Anderson JF, Barbour AG, Magnarelli LA, Anderson JF. The etiologic agent of Lyme disease in deer flies, horse flies, and mosquitoes. J Infect Dis. 1986;154:355–8. 10.1093/INFDIS/154.2.355. [DOI] [PubMed] [Google Scholar]
- 11.Melaun C, Zotzmann S, Santaella VG, Werblow A, Zumkowski-Xylander H, Kraiczy P, et al. Occurrence of Borrelia burgdorferi s.l. in different genera of mosquitoes (Culicidae) in Central Europe. Ticks Tick Borne Dis. 2016;7:256–63. [DOI] [PubMed] [Google Scholar]
- 12.Pospisilova T, Urbanova V, Hes O, Kopacek P, Hajdusek O, Sima R. Tracking of Borrelia afzelii transmission from infected Ixodes ricinus nymphs to mice. Infect Immun. 2019. 10.1128/IAI.00896-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stĕpánová-Tresová G, Kopecký J, Kuthejlová M. Identification of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in Ixodes ricinus ticks from southern Bohemia using monoclonal antibodies. Zentralbl Bakteriol. 2000;289:797–806. [DOI] [PubMed] [Google Scholar]
- 14.Thein M, Bunikis I, Denker K, Larsson C, Cutler S, Drancourt M, et al. Oms38 is the first identified pore-forming protein in the outer membrane of relapsing fever spirochetes. J Bacteriol. 2008;190:7035–42. 10.1128/JB.00818-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson-Litteken CD, Guo W, Hogland BA, Ratliff CT, McFadden L, Fullerton MS, et al. Development and validation of systems for genetic manipulation of the Old World tick-borne relapsing fever spirochete Borrelia duttonii. PLoS Negl Trop Dis. 2024;18:e0012348. 10.1371/JOURNAL.PNTD.0012348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graumans W, Heutink R, Van Gemert GJ, Van De Vegte-Bolmer M, Bousema T, Collins KA. A mosquito feeding assay to examine Plasmodium transmission to mosquitoes using small blood volumes in 3D printed nano-feeders. Parasit Vectors. 2020. 10.1186/S13071-020-04269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffé J. Spirochaeta culicis nov. spec. Arch F Protist 1907;9:100–7.
- 18.Magnarelli LA, Anderson JF. Ticks and biting insects infected with the etiologic agent of Lyme disease Borrelia burgdorferi. J Clin Microbiol. 1988;26:1482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg. 1990;42:352–7. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Martin I, Williams AE, Calvo E. Determination of Mosquito Probing and Feeding Time to Evaluate Mosquito Blood Feeding. Cold Spring Harb Protoc 2023;2023:pdb.top107659. 10.1101/PDB.TOP107659. [DOI] [PMC free article] [PubMed]
- 21.Aase A, Hajdusek O, Øines Ø, Quarsten H, Wilhelmsson P, Herstad TK, et al. Validate or falsify: lessons learned from a microscopy method claimed to be useful for detecting Borrelia and Babesia organisms in human blood. Infect Dis (Lond). 2016;48:411–9. 10.3109/23744235.2016.1144931. [DOI] [PubMed] [Google Scholar]
- 22.Barbour AG. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol. 1990;44:155–71. 10.1146/ANNUREV.MI.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 23.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–68. 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Magnarelli LA, Freier JE, Anderson JF. Experimental infections of mosquitoes with Borrelia burgdorferi, the etiologic agent of lyme disease. J Infect Dis. 1987;156:694–5. 10.1093/INFDIS/156.4.694. [DOI] [PubMed] [Google Scholar]
- 25.Sojka D, Franta Z, Horn M, Caffrey CR, Mareš M, Kopáček P. New insights into the machinery of blood digestion by ticks. Trends Parasitol. 2013;29:276–85. 10.1016/j.pt.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Borovsky D. Biosynthesis and control of mosquito gut proteases. IUBMB Life. 2003;55:435–41. 10.1080/15216540310001597721. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barillas-Mury C, Ribeiro JMC, Valenzuela JG. Understanding pathogen survival and transmission by arthropod vectors to prevent human disease. Science. 2022. 10.1126/SCIENCE.ABC2757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.