Abstract
Nucleolar small RNA (snoRNA), as a class of non-coding RNAs, play a crucial role in eukaryotic cells. They are widely involved in post-transcriptional modifications of ribosomal RNAs, including methylation and pseudouridylation, precisely regulating the process of ribosome biogenesis, ensuring the integrity of ribosome structure and function, and thereby guaranteeing the accuracy and efficiency of protein synthesis. snoRNAs not only maintain cell growth, proliferation, and differentiation under normal physiological conditions but also have abnormal expression closely associated with various diseases, such as cancer, cardiovascular diseases and neurodegenerative diseases. In recent years, with the innovation of research techniques, there has been a deeper exploration of the biosynthesis pathways, functional mechanisms of snoRNAs, and their potential value in disease diagnosis and treatment. This review comprehensively summarizes the structural characteristics, classification systems, biological functions, and disease associations of snoRNAs, and looks forward to future research directions, aiming to provide a systematic reference for further exploration of the mysteries of snoRNAs in related fields.
Keywords: Nucleolar small RNAs, Cancer, Cardiovascular diseases, Neurodegenerative diseases
Introduction
Intranuclear small nucleolar RNA (snoRNA), discovered in the late 1960s, originates from the small ncRNA of archaea and is named for its presence mainly in the nucleolus. Like other non-coding RNA(ncRNA), snoRNA has no translation function; The main difference is that snoRNA contains specific nucleic acid sequences and spatial structures, and is mainly involved in the modification of other RNA, such as ribosomal RNA (rRNA) methylation and pseuduridylization [1, 2]. Recently, with the development of high-throughput genomics technology, researchers have discovered many new snoRNA members and revealed their multiple functional within the cell [3]. SnoRNA is not only limited to traditional RNA modifications, but also involved in regulating gene expression, interacting with multiple binding partners, and playing important regulatory roles within cells. The classification and function of snoRNA are also expanding. It has been shown that snoRNA can affect protein function and accessibility by interacting with other RNA molecules. Moreover, the expression patterns of snoRNA show complex regulatory relationships in different tissues, suggesting that they may play a more important role in cell biology. For example, certain snoRNA are enriched in specific tissues, showing a correlation of expression with their host genes [4, 5].
In recent years, more and more studies have shown that snoRNA not only plays a key role in RNA biosynthesis, but also plays an important role in the occurrence and development of various human diseases. For example, abnormal expression of snoRNA is closely related to various pathological states such as cancer, cardiovascular diseases(CVDs), and neurodegenerative diseases(NDs) [6]. In cancer research, snoRNA’s function is increasingly being recognized. Studies have found that the expression level of some snoRNA in tumor cells is significantly changed, which may affect the occurrence and progression of tumors [7]. In addition, snoRNA derived small RNA (sdRNA) have also been found to regulate gene expression, which provides a new perspective for understanding snoRNA’s role in cancer. Another important research direction is snoRNA’s interaction with other ncRNA. For example, the relationship between long non-coding RNA (lncRNA) and snoRNA is considered to be one of the important mechanisms regulating gene expression. This interaction may play a role in neurological disorders such as schizophrenia, affecting the function of neurons and the health of synapses [4, 8]. In addition, genomic variation in snoRNA has also attracted the attention of researchers. Systematic analyses have shown that single nucleotide variation at snoRNA sites may significantly affect its structure and function, thus playing a role in disease [9]. These findings highlight the complexity and diversity of snoRNA in disease mechanisms.
SnoRNA’s research in disease is progressing rapidly, revealing its importance in cell biology and pathophysiology. Therefore, this paper will delve into the function of snoRNA and its role in disease, providing a new perspective for our understanding of cell biology and disease mechanisms, and further exploring the function of snoRNA and its possibility as a potential therapeutic target.
Basic structure of snoRNA
SnoRNA is a highly conserved and abundant class of ncRNA, typically ranging in length from 60 to 300 nucleotides. They are predominantly localized in the nucleolus and exhibit metabolic stability. SnoRNA interact with various core proteins to form ribonucleoprotein (RNP) complexes, which guide modifications on target RNA through complementary base pairing [10]. Based on their structure and function, snoRNA are classified into two major groups: C/D box snoRNA and H/ACA box snoRNA (Fig. 1). C/D box snoRNAs are typically 60 to 90 nucleotides long and are composed of two key sequence elements: the C box (5’rugauga3’) located at the 5’ end and the D box (5’cuga3’) at the 3’ end. These ends include 4–6 nucleotides of reverse complementary sequences, forming a single hairpin structure. C/D box snoRNAs act as recognition sites for specific proteins, such as nucleolar protein 56 (NOP56), NOP58, small nucleolar ribonucleoprotein 13 (SNU13), and the methyltransferase fibrillarin [11–13]. When these proteins bind to C/D box snoRNAs, they create a scaffold-like structure that facilitates interactions with other proteins to carry out various biological functions. Most C/D box snoRNAs also possess an upstream segment exceeding 21 nucleotides, termed an antisense snoRNA, which binds to target RNA via complementary base pairing. This binding directs methylation at specific 2’-O-hydroxyl sites, promoting 2’-O-ribose methylation modifications on rRNA and transfer RNA (tRNA) at designated positions [2]. H/ACA box snoRNAs are longer, ranging from 120 to 140 nucleotides, and exhibit a distinct secondary structure called the “hairpin-hinge-hairpin-tail” structure. The hinge region, consisting of a single-stranded nucleic acid, bridges the two hairpin structures. Both the hinge and tail regions contain conserved motifs: the H (ACA guide sequence) motif and the ACA (Amino Acid) motif, respectively. These motifs enable the formation of a double-hairpin structure [14]. Within the pseudouridine pockets of these hairpins, antisense snoRNAs bind specific target sites. When associated with glycine-arginine-rich protein 1, non-histone chromosomal protein 2, NOP10, and dyskeratosis congenita 1, they form homologous snoRNP complexes, guiding the pseudouridylation of target RNA. Most H/ACA box snoRNAs direct pseudouridylation modifications at specific sites within rRNA and small nuclear RNA (snRNA) in eukaryotes, converting uridine into pseudouridine [15–17]. Interestingly, mammalian SNORAs share H/ACA motifs with human telomerase RNA, suggesting a link between H/ACA-like domains and cellular proliferation [18]. These two classes of snoRNAs differ in their cellular roles and expression patterns while displaying high evolutionary conservation across organisms [19]. MRP RNA is an exceptionally unique type of snoRNA, distinct from other snoRNA in both quantity and function. Only one molecule of MRP RNA exists in the cell, and it associates with nine proteins to form the MRP RNA complex. This complex participates in the processing of 5.8S rRNA, mitochondrial DNA replication, contributing to the maturation of the 5’ ends of tRNA [20]. Additionally, a considerable number of snoRNA with unknown functions, referred to as orphan snoRNA, have been identified [21].
Fig. 1.
Basic structure of snoRNA. SnoRNA has two basic structures, C/D box snoRNA (A) and H/ACA box snoRNA (B), which interact with a variety of core proteins to form ribonucleoprotein complexes (C, D) and guide target RNA modification through complementary base pairing
Biological function of snoRNA
rRNA processing
One of the most prominent functions of snoRNA is to guide the chemical modification and processing of rRNA. By forming complexes with specific RNPs, snoRNA direct two primary types of chemical modifications at specific sites on rRNA [22]: The first is 2’-O-Methylation, guided by C/D box snoRNA, this modification occurs through base pairing with target rRNA at specific nucleotide positions, directing methyltransferases to add 2’-O-methyl groups. This modification enhances the stability of rRNA and facilitates the functional assembly of ribosomes. For example, SNOR28.1 directs 2’-O-ribose methylation at G2396 of 25 S rRNA, promoting pre-rRNA processing, which is crucial for the proper functioning of ribosomes [23]. Pseudouridylation is directed by H/ACA box snoRNA, this modification converts uridine into pseudouridine, thereby improving the chemical stability of rRNA and strengthening its interaction with ribosomal proteins. These modifications are essential for the proper folding of rRNA, the assembly of ribosomal subunits, and the execution of ribosomal functions [24]. In addition to chemical modifications, snoRNA also play a role in processing rRNA. They guide the cleavage and maturation of precursor rRNA to ensure the accurate production of mature rRNA, which forms the functional ribosomal subunits (40 S and 60 S). This process lays the foundation for ribosome assembly and efficient protein synthesis.
Post-transcriptional regulation of messenger RNA (mRNA)
Studies have shown that the Snord88 family of snoRNA contains a segment of approximately 20 nucleotides located downstream of the D’-box, known as the M-box, which exhibits high complementarity to endogenous pre-mRNA sequences [25]. This indicates that snoRNA are involved in the regulation of mRNA splicing. Some snoRNA may interact with precursor mRNA to influence the selection of splicing sites, thereby altering gene expression profiles. Additionally, experiment has demonstrated that snoRNA-mediated pseudouridylation can occur on mRNA targets, highlighting the role of snoRNA in the post-transcriptional modification of mRNA [26]. The U/A-rich SNORD50A suppresses mRNA 3’ processing by disrupting the interaction between Fip1 and the poly(A) site, altering alternative polyadenylation profiles and affecting the transcript levels of specific genes [27]. snoRNA is involved in the transport of mRNA from the nucleus to the cytoplasm. By binding to mRNA, they protect mRNA from nuclease degradation, ensuring its stability and maintaining an appropriate expression level. In addition, snoRNA can affect the structure of mRNA or the binding efficiency of the translation initiation complex, thereby regulating translation activity [28, 29].
SnRNA and tRNA modifications
SnoRNA also play a role in the chemical modification of snRNA, particularly the U-family snRNA. For example, snoRNA-guided 2’-O-methylation is essential for the formation of U6 snRNP. These modifications are critical for the functionality of snRNA within the spliceosome. Through specific 2’-O-methylation or pseudouridylation, snoRNA enhance the stability of snRNA and their binding capacity to splicing factors, thereby improving the efficiency and precision of the spliceosome [30, 31]. Additionally, snoRNA are involved in the post-transcriptional modification of tRNA, such as guiding pseudouridylation or methylation. These modifications stabilize the structure of tRNA, increase their binding efficiency to aminoacyl-tRNA synthetases, and enhance their functionality during translation, promoting the accurate synthesis of polypeptide chains. A snoRNA-tRNA interaction network has been found to be essential for global tRNA modifications, including as 2’-O-methylation and other kinds of modifications. For example, tRNA fragments, a major class of regulatory RNA, are globally upregulated when fibrillarin, a 2’-O-methyltransferase regulated by snoRNA, is lost [25, 32].
Ribosome assembly
Ribosome assembly process begins with RNA polymerase I transcribing rDNA in the nucleolus to produce precursor rRNA, which is assembled into an 80–90 S small particle. As ribosomal proteins are incorporated, the structure of the precursor rRNA undergoes reorganization and specific nucleotide modifications. These modifications typically occur in specific regions of the rRNA, such as within stem-loop structures, which play crucial roles in ribosome function. Finally, the mature ribosomal subunits are formed through cleavage. Through these modification and processing steps, snoRNA help optimize the structure and function of ribosomes, thereby enhancing the efficiency and accuracy of protein synthesis [33](Fig. 2).
Fig. 2.
Biological function of snoRNA. Small nucleolar RNA guides the chemical modification and processing of rRNA, transcription from cDNA to mRNA, post-transcriptional regulation of mRNA, modification of snRNA and tRNA, as well as ribosome assembly through 2 ‘-o methylation and pseuduridine
Oxidative stress
It has been reported that doxorubicin (Dox)-induced oxidative stress can cause C/D-box snoRNA to shuttle from the nucleus to the cytoplasm. H/ACA-box snoRNA and scaRNA have also been detected accumulating in the cytoplasm, albeit at lower levels, indicating that snoRNA play an important role in cellular oxidative stress [34]. SnoRNA can bind to mRNA, affecting its stability and translation efficiency, thereby regulating the expression of antioxidant genes. For example, knocking out SNORA73 in vivo can prevent hepatic steatosis, lipid-induced oxidative stress, and inflammation [35]. Additionally, snoRNA are involved in the modification of mitochondria-related RNA, influencing mitochondrial function and participating in the formation of stress granules. Experimental evidence has demonstrated that Rpl13a snoRNA have dual functions: guiding 2’-O-ribose methylation of 18 S and 28 S rRNA, and acting as oxidative stress enhancers, exerting effects in distal tissues [36].
snoRNA and cancer
SnoRNA not only participate in the modification of RNA but also play diverse roles in regulating the cell cycle, proliferation, and apoptosis, particularly in tumor cells [37, 38]. The expression levels of snoRNA are closely associated with the occurrence and progression of various cancers. Some snoRNA are considered oncogenic factors, while others may act as tumor suppressors. For example, SNORA21 has been identified as an oncogenic snoRNA in colorectal cancer (CRC), with its expression closely linked to patient prognosis. Inhibition of SNORA21 results in reduced tumor cell proliferation and invasion [39]. Snord104 is upregulated in endometrial cancer and promotes tumor growth by regulating the 2’-O-methylation of PARP1 [40]. Conversely, SNORA52 is downregulated in hepatocellular carcinoma (HCC) and is associated with clinical features of the disease, highlighting its potential value as a biomarker [41]. In prostate cancer, sdRNA-D19b and sdRNA-A24 have been found to downregulate tumor suppressor genes, thereby promoting cancer cell proliferation and metastasis [42]. Additionally, sdRNA also plays significant roles in cancer. These sdRNA can interact with ribosomes during translation, potentially inhibiting protein synthesis and thereby affecting tumor cell growth and metastatic ability [43]. These findings highlight the complex roles of snoRNA in tumor initiation and progression, suggesting that they could serve as novel therapeutic targets for cancer [33]. As our understanding of the mechanisms underlying snoRNA functions in cancer deepens, further research on the involvement of snoRNA in cancer signaling pathways is expected. Such studies could provide a theoretical foundation for precision cancer therapies in the future.
Signaling pathways
P53
The p53 protein is a crucial tumor suppressor that regulates the cell cycle, apoptosis, and genomic stability. Dysregulation of the p53 signaling pathway is a common feature in many cancers, and studies have shown that snoRNA can influence the stability and activity of p53, thereby affecting cancer cell proliferation and survival. In endometrial cancer, SNORD15B has been identified as an oncogene that promotes tumor growth by targeting the TRIM25/p53 complex, preventing the nuclear translocation of p53 and thus inhibiting its tumor-suppressive function. This highlights the potential of snoRNA as therapeutic targets in cancers with impaired p53 signaling [44]. In lung adenocarcinoma, DDB1 And CUL4 Associated Factor 13 (DCAF13) has been shown to suppress the p53 signaling pathway by promoting p53 ubiquitination and degradation, a process facilitated by snoRNA-mediated mechanisms [45]. Additionally, in CRC, the circSLC6A6/miR-1265/ C2 Calcium Dependent Domain Containing 4 A (C2CD4A) axis, which incorporates snoRNA components, has been found to inhibit the p53 signaling pathway, thereby promoting cancer growth. This axis represents a complex regulatory network where snoRNA contribute to modulating p53 activity and influencing cancer progression [46]. The regulation of p53 by snoRNA is also evident in breast cancer, where miR-375, regulated by enhancer of zeste homolog 2 (EZH2), targets fork head box O1 (FOXO1), a downstream activator of the p53 signaling pathway, thereby promoting cancer progression [47]. This interaction underscores the intricate relationship between snoRNA and the p53 signaling pathway in cancer biology. Certain snoRNA may enhance the tumor-suppressive function of p53 by stabilizing p53 mRNA through methylation modifications [48]. These findings emphasize the dual roles of snoRNA in cancer, with potential therapeutic implications in targeting p53-associated pathways.
Phosphatidylinositol-3-kinase (PI3K) / protein kinase B (AKT) signaling pathway
A key target for the creation of anti-cancer medications is the PI3K/AKT signaling system, which is marked by aberrant activation in a variety of malignancies [49]. For example, increased PI3K/AKT axis activation is a common occurrence in colorectal cancer (CRC) and is directly linked to treatment resistance, invasion, migration, and proliferation of tumor cells [50]. The regulation of the PI3K/AKT pathway is not limited to cancer but also involves the pathological mechanisms of other diseases. In atherosclerosis, this pathway plays a role by modulating cellular inflammatory responses and oxidative stress [51]. In bladder cancer, tumor necrosis factor receptor superfamily, member 11b (TNFRSF11B) promotes tumor progression through the PI3K/AKT pathway [52]. Research suggests that snoRNA can influence tumor cell proliferation, migration, and apoptosis by modulating the PI3K/AKT pathway (Table 1). Therefore, further exploration of the interactions between snoRNA and the PI3K/AKT signaling pathway may provide novel targets and strategies for the treatment of cancers and other related diseases.
Table 1.
SnoRNA affects tumors by regulating the PI3K/AKT signaling pathway
| SnoRNA | Regulation (+/—) | Cancer | Reference |
|---|---|---|---|
| ACA11 | + | Hepatocellular carcinoma | [53] |
| SCARNA12 | + | Colorectal cancer | [54] |
| SNHG1 | + | Squamous cell carcinoma of Head and neck | [55] |
| + | Nasopharyngeal carcinoma | [55] | |
| + | Carcinoma of bladder | [56] | |
| + | Pancreatic ductal Adenocarcinoma | [57] | |
| SNHG4 | + | Gastric cancer | [58] |
| SNHG7 | + | Thyroid cancer | [59] |
| - | Adenocarcinoma of lung | [60] | |
| SNHG16 | + | Hepatocellular carcinoma | [61] |
| + | Acute myeloid leukemia | [62] | |
| + | Glioma | [63] | |
| + | Neuroblastoma | [64] | |
| SNHG17 | + | Melanoma | [65] |
| SNHG20 | + | Glioblastoma | [66] |
| SNHG22 | + | Epithelial ovarian cancer | [67] |
| SNHG25 | + | Prostate cancer | [68] |
| SNHG29 | + | Chronic myeloid leukemia | [69] |
| SNORA23 | - | Hepatocellular carcinoma | [70] |
| SNORD44 | - | Breast cancer | [71] |
| - | Squamous cell carcinoma of head and neck | [71] | |
| SNORA47 | + | non-small cell lung cancer | [72] |
| SNORD60 | + | Endometrial cancer | [73] |
| SNORD126 | + | Hepatocellular carcinoma | [74] |
| + | Colorectal cancer | [74] |
Ras-mitogen-activated protein kinase (RAS/MAPK) signaling pathway
The RAS/MAPK signaling pathway is a crucial regulator of cell proliferation, differentiation, and survival. RAS proteins, as small GTPases, play a key role in signal transduction, with the rapidly accelerated fibrosarcoma (RAF)- mitogen-activated protein (MEK)-MAPK module being one of the primary downstream pathways of RAS signaling. Dysregulation of this pathway is closely associated with the development of various cancers [75]. SnoRNA can influence cancer cell growth and metastasis by regulating different nodes of this signaling pathway. For instance, SNORA71A is overexpressed in non-small cell lung cancer (NSCLC) tissues, and its upregulation is significantly associated with reduced survival rates in NSCLC patients. Knockdown of SNORA71A induces G0/G1 arrest, inhibits cell invasion and migration, suppresses epithelial-mesenchymal transition (EMT), and decreases phosphorylation of MEK and extracellular regulated protein kinases 1/2 (ERK1/2) in the MAPK/ERK signaling pathway, making it a potential therapeutic target and prognostic biomarker for NSCLC [76]. SNORD113-1 exhibits tumor-suppressive effects in HCC by inhibiting the phosphorylation of ERK1/2 in the MAPK/ERK pathway and SMAD2/3 in the transforming growth factor-β (TGF-β) pathway. Its expression is significantly associated with patient survival, making it a potential diagnostic and therapeutic target for HCC. Similarly, SNHG14 knockdown inhibits triple negative breast cancer (TNBC) progression by regulating the ERK/MAPK signaling pathway, providing evidence for its potential as a therapeutic target for TNBC [77, 78]. Additionally, snoRNA may regulate the RAS/MAPK signaling pathway through interactions with other ncRNA. For example, SNHG12 is upregulated in Temozolomide (TMZ)-resistant glioblastoma cells and tissues. By binding to miR-129-5p, it leads to the upregulation of MAPK1 and E2F transcription factor 7(E2F7), contributing to TMZ resistance in glioblastoma cells, suggesting SNHG12 as a potential therapeutic target to overcome TMZ resistance and improve chemotherapy efficacy. In glioblastoma, SNHG5 exerts oncogenic effects by activating the p38/MAPK signaling pathway [79, 80]. SNHG12 also promotes E2F7 expression by binding to miR-129-5p, activating the MAPK/ERK pathway, inducing apoptosis of human peritoneal mesothelial cells, and affecting gastric cancer progression [81]. SNHG4 is closely associated with miR-154, miR-206, E2F transcription factors, and the MAPK/ERK and mTOR signaling pathways, playing a role in HCC formation by regulating tumor-related pathways [82]. Elevated SNHG4 expression in GC tissues correlates with TNM stage, tumor size, and metastatic status. GO analysis suggests SNHG4 involvement in cell-cell adhesion, while KEGG enrichment analysis links SNHG4 to gastric cancer signaling pathways. ELAV like RNA binding protein 1 and insulin-like growth factor (IGF) binding protein 2, which target SNHG4, participate in PI3K-Akt-mTOR and ERK-MAPK signaling pathways [58]. Exploring the diverse functions of snoRNA in different cancer types can aid in developing anti-cancer drugs targeting the RAS/MAPK signaling pathway and provide new insights into cancer diagnosis and treatment.
Wnt/β-catenin
The regulation of the Wnt/β-catenin signaling pathway is closely associated with the metastatic and invasive properties of tumor cells. Studies have shown that snoRNA can influence tumor stem cell self-renewal and tumor progression by modulating the Wnt/β-catenin pathway [83, 84]. For example, in HCC, the expression of SNHG16, SNORD76, and SnoU2_19 in HCC tissues, compared to corresponding non-tumor tissues, induces G0/G1 cell cycle arrest and apoptosis, inhibiting cell proliferation. These snoRNA regulate the development of HCC via the Wnt/β-catenin signaling pathway and serve as prognostic biomarkers for HCC patients [85, 86]. Knockdown of SNHG5 also suppresses HCC cell proliferation and cancer stem cell-like characteristics. SNHG5 plays a key role in promoting HCC cell proliferation and tumor stem cell-like properties by modulating UPF1 and the Wnt/β-catenin pathway [84]. In oral squamous cell carcinoma (OSCC), knockdown of the SNHG3 gene significantly inhibits OSCC cell proliferation and migration [87]. In papillary thyroid carcinoma (PTC), SNHG12 is highly expressed in tissues and cells, and both in vivo and in vitro experiments have demonstrated that SNHG12 promotes PTC cell proliferation and metastasis by affecting the Wnt/β-catenin signaling pathway. In bladder cancer, inhibition of SNHG20 suppresses tumor progression by inhibiting the activation of the Wnt/β-catenin signaling pathway [88]. Similarly, SNHG20 acts as an oncogenic lncRNA in ovarian cancer, promoting cell growth and activating the Wnt/β-catenin signaling pathway [89]. In CRC, SNORD1C functions as an oncogene by promoting cell proliferation, migration, and invasion while inhibiting apoptosis. Knockdown of SNORD1C reduces the enrichment of the Wnt signaling pathway and pluripotent stem cell regulatory pathways, decreases chemoresistance, and inhibits CRC progression. These findings suggest the potential for targeting SNORD1C in clinical applications during CRC treatment [90]. In addition, snoRNA can also affect the expression and function of microRNA (miRNA) through interaction with other ncRNA, such as binding with miRNA, and further regulate the Wnt/β-catenin signaling pathway, thus affecting the occurrence and development of cancer (Table 2).
Table 2.
SnoRNA binds to MiRNA and regulates tumor development by influencing Wnt/β-catenin
| SnoRNA | MiRNA | Cancer | Reference |
|---|---|---|---|
| SNHG1 | miR-140-5p | Non-small cell lung cancer | [91] |
| miR-577 | Osteosarcoma | [92] | |
| miR-489-3p | Acute myeloid leukemia | [93] | |
| miR-101-3p | Colorectal cancer | [94] | |
| SNHG3 | miR-340-5p | Squamous cell carcinoma of larynx | [95] |
| SNHG4 | miR-98-5p | Ovarian cancer | [96] |
| miR-490 | Gastric cancer | [58] | |
| SNHG5 | miR-26a-5p | Liver cancer | [97] |
| SNHG6 | miR-15a | Nephroblastoma | [98] |
| miR-101-3p | Colorectal cancer | [99] | |
| SNHG7 | mir-181 | Adenocarcinoma of lung | [100] |
| miR-425 | Hepatocellular carcinoma | [101] | |
| miR-5095 | Glioblastoma | [102] | |
| SNHG11 | miR-4436a | Lung cancer | [103] |
| SNHG16 | miR-140-5p | Squamous cell carcinoma of the throat | [104] |
| miR-338-3p | Neuroblastoma | [64] | |
| SNHG17 | miR-384 | Oral squamous cell carcinoma | [105] |
| SNHG22 | miR-361-3p | Gastric cancer | [106] |
Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) signaling pathway
The JAK/STAT signaling pathway plays a crucial role in cell proliferation, differentiation, and apoptosis. In lung cancer, abnormal activation of the JAK/STAT pathway is closely related to tumor cell proliferation and immune evasion [107]. The JAK/STAT signaling pathway can be regulated by snoRNA The expression of SNHG15, through dysregulation of the JAK-STAT 6 pathway, exerts an anti-inflammatory effect in stroke-induced immunosuppression, suggesting its potential as a novel biomarker and therapeutic target for stroke-associated infections [108]. Additionally, the role of snoRNA in CRC has drawn attention. SNORA28 has been shown to promote cell proliferation and radio resistance in CRC, with its mechanism involving activation of the STAT3 signaling pathway [109]. In esophageal squamous cell carcinoma (ESCC), overexpression of SNHG20 enhances cell proliferation, migration, invasion, and EMT while inhibiting apoptosis. SNHG20 regulates the expression of ataxia-telangiectasia mutated kinase (ATM), p-JAK1/2, and programmed death-ligand 1 (PD-L1). Upregulation of ATM reverses the inhibitory effect of SNHG20 knockdown on ESCC progression, demonstrating that SNHG20 promotes ESCC growth and metastasis through the ATM-JAK-PD-L1 pathway and may serve as an effective therapeutic target for ESCC [110]. In summary, snoRNA play a significant role in the development and progression of various cancers by modulating the JAK/STAT signaling pathway. These findings offer new perspectives and potential targets for cancer diagnosis and treatment [111].
Hippo signaling pathway
The Hippo signaling system is a conserved signal transduction mechanism that controls stemness, apoptosis, and cell proliferation to maintain tissue homeostasis and organ size. The preservation of tissue structure and cellular equilibrium depends critically on key elements of the Hippo pathway, including transcriptional co-activator with PDZ-binding motif (TAZ), yes-associated protein (YAP), macrophage stimulating 1 (MST1), large tumor suppressor kinase 1 (LATS1), and LATS2 [112]. Dysregulation of the Hippo pathway contributes to tumor progression and the development of secondary cancers. lncRNA interact with the Hippo pathway, promoting malignant cancer traits such as proliferation, metastasis, recurrence, and resistance to cancer therapies. LncRNA can regulate or be regulated by the Hippo pathway, forming feedback loops that make Hippo-related lncRNA significant biomarkers and therapeutic targets in human cancers [113]. SNHG5 has been identified as an oncogene in various human cancers. Liver fibrosis experiments demonstrate that SNHG5 interacts with the neurofibromatosis type2 (NF2) protein, and blocking this interaction effectively prevents SNHG5 from inhibiting the EMT process and Hippo signaling, suggesting that SNHG5 acts as a regulator of activated hepatic stellate cells by modulating NF2 and the Hippo pathway [114]. A tumor-promoting snoRNA called SNHG9 suppresses the Hippo pathway and promotes the production of LATS1 droplets. By interacting with the C-terminal domain of LATS1, SNHG9 and its related phosphatidic acid (PA) facilitate LATS1 phase separation and prevent LATS1-mediated YAP phosphorylation. The growth of xenograft breast tumors is suppressed by SNHG9 loss, indicating a new regulatory function for SNHG9 in signal transduction and cancer development [115]. In gastric cancer, SNHG1 binds to miR-195-5p, directly targeting YAP1 and influencing tumor cell proliferation and metastasis through the Hippo signaling pathway [116]. In hepatocellular carcinoma, SNORD88B anchors Wemer syndrome protein to the nucleolus, facilitating the interaction between XRCC5 and the STK4 promoter, thereby suppressing STK4 transcription and inactivating the Hippo signaling pathway. Knocking out SNORD88B inhibits liver tumor formation in mice, offering a potential therapeutic target for liver cancer [117]. Dysregulation of the Hippo signaling pathway frequently occurs in various human cancers, making it an ideal molecular target for cancer therapy. The regulatory involvement of snoRNA provides additional opportunities for therapeutic interventions.
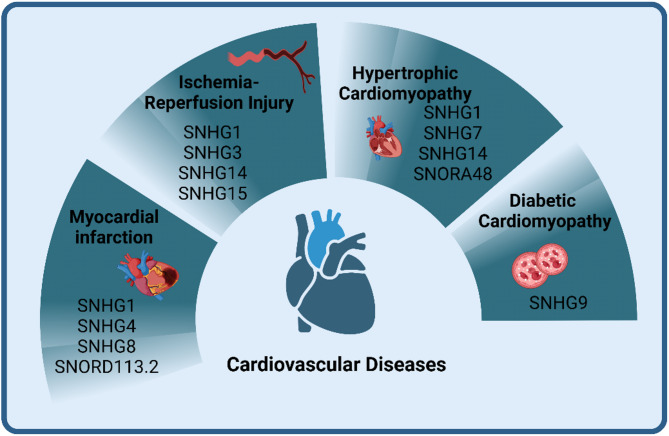
snoRNA and CVDs
CVDs are the leading causes of death and disability worldwide, and the role of snoRNA, in CVDs is gaining increasing attention. Studies have shown that snoRNA guide the post-transcriptional modification and maturation of small nuclear RNA and rRNA, highlighting their potential role in CVDs, particularly in heart development and functional maintenance [118]. Although the exact mechanisms remain unclear, the importance of snoRNA in CVDs is increasingly recognized. For instance, snoRNA play diverse roles in heart development and congenital heart diseases and are dynamically regulated in conditions such as cardiomyopathy, coronary artery disease, Myocardial Infarction (MI), cardiac fibrosis, and heart failure [119]. Overall, the role of snoRNA in CVDs represents an emerging field of research. Current studies are gradually uncovering the specific mechanisms by which snoRNA contribute to cardiovascular health and disease. Future discoveries in this area may provide novel strategies and approaches for the diagnosis and treatment of CVDs (Fig. 3).
Fig. 3.
snoRNA and CVDs. Different snoRNA are involved in cardiovascular diseases, such as myocardial infarction, ischemia-reperfusion, hypertrophic cardiomyopathy, and dilated cardiomyopathy
Role of snorna in cardiac development and function
During heart development, snoRNA influence the morphology and function of the heart by regulating gene expression and signaling pathways. In terms of gene expression, a global downregulation of snoRNA expression has been observed in patients with congenital heart disease, resembling the expression profile in the developing heart. Studies have revealed that snoRNA not only maintain RNA stability and function through guiding RNA modifications but also promote the secretion of mRNA encoding secretory proteins via interactions with signal recognition particles (SRPs). Additionally, snoRNA interact with ribosomal proteins to regulate ribosome biogenesis, thereby affecting cell proliferation, differentiation, and physiological functions such as cardiac contractility, electrophysiological properties, and cellular metabolism [120, 121]. Furthermore, snoRNA can participate in the fine regulation of gene expression during heart development by interacting with other ncRNA [122]. They may also influence heart development by regulating associated signaling pathways. For example, snoRNA can modulate the Notch1/c-Myc pathway, promoting the self-renewal and migration abilities of cardiac stem cells, which in turn affects heart development and function [123]. These results shed fresh light on how snoRNA functions in the development of the heart and could lead to the discovery of new targets for heart disease diagnostics and treatment.
SnoRNA regulates CVDs
Hypertrophic cardiomyopathy (HCM)
Cardiac hypertrophy (CH) is a common cardiac condition closely linked to heart failure. Reports indicate that protocadherin 17 (PCDH17) exacerbates MI, and snoRNA SNHG14 can increase PCDH17 levels by binding to miR-322-5p and miR-384-5p, highlighting the regulatory role of the SNHG14/miR-322-5p/miR-384-5p/PCDH17 axis in CH [124]. Heart failure caused by pathological CH is a leading cause of mortality. SNHG1 has been shown to inhibit cardiomyocyte apoptosis and is upregulated during CH in vivo (aortic banding treatment) and in vitro (phenylephrine treatment). Experimental evidence reveals that SNHG1 alleviates cardiomyocyte hypertrophy by promoting HMGA1 expression through binding to miR-15a-5p, suggesting a novel protective mechanism of SNHG1 in cardiomyocyte hypertrophy [125]. SNHG7, previously identified as an oncogene in human cancers, has been found to promote Ang II-induced CH by stabilizing SDAD1 mRNA, suggesting that SNHG7 is a novel regulator of CH [126]. Mutations in myosin-binding protein C (encoded by the MYBPC3 gene) are associated with HCM. Using black fruit flies as a model, flies carrying MYBPC3 missense mutations (A31P and R820W) exhibited cardiovascular defects, including altered heart rate and reduced exercise endurance. Transcriptomic analysis revealed significant downregulation of genes encoding snoRNA in exercise-stressed female flies carrying mutant alleles compared to those with wild-type alleles [127]. Studies also show altered snoRNA release in HCM, such as SNORA48, which is predicted to regulate post-transcriptional modifications and splicing. These represent common pathways in HCM mutations, supporting the potential of targeting snoRNA as therapeutic approaches for managing common HCM phenotypes [128, 129].
Dilated cardiomyopathy (DCM)
DCM is a leading indication for heart transplantation and a common cause of heart failure. SNHG9 has been identified as a potential biomarker for DCM. It shows excellent performance in distinguishing DCM from normal controls, as well as between stage III and stage I/II DCM, based on the New York Heart Association classification. In a Dox-induced DCM mouse model, serum SNHG9 expression levels were found to be upregulated and negatively correlated with cardiac function. Moreover, AAV-9-mediated SNHG9 knockout alleviated cardiac injury in the Dox-induced mouse model. In summary, current findings suggest that SNHG9 is a novel regulatory factor in the progression of DCM, providing potential insights for diagnosis and therapeutic intervention [130].
MI
Acute myocardial infarction (AMI) is a severe CVDs that can lead to congestive heart failure. Fibrosis is a hallmark of MI. To better understand the signaling mechanisms regulating protective reductions in fibrosis, RNA sequencing of nhCFs treated with hCBSC-dEXO revealed a decrease in snoRNA, which are known to be associated with ribosomal stability [131]. SNHG 1 expression is elevated in human and mouse fetal hearts as well as in MI hearts, particularly in cardiomyocytes. Overexpression of SNHG 1 promotes CM proliferation, angiogenesis, and inhibits CM apoptosis post-MI, resulting in improved cardiac function. Through a positive feedback loop between c-Myc and the maintenance of PI3K/Akt signaling activity, SNHG 1 efficiently stimulates CM growth and improves cardiac function following MI. This implies that Snhg1 is a potentially effective cardiac regeneration method for the treatment of MI heart failure [132]. SNHG4 expression is downregulated in hypoxia-induced H9c2 cells and MI rat models. Overexpression of SNHG4 enhances cell viability, suppresses apoptosis and inflammatory responses, reduces MI and fibrosis areas, alleviates pathological changes, and improves cardiac function. These findings demonstrate that SNHG4 mitigates MI through the miR-148b-3p/DUSP1 axis, providing a potential therapeutic target [133]. SNHG8, a lncRNA with physiological roles in epithelial and muscle satellite cells, regulates MI pathogenesis through the SNHG8/miRNA molecular axis and NF-κB signaling pathway [134]. Additionally, SNHG8 promotes hypoxia-induced apoptosis in NMCMs partially mediated by periostin. Vascular endothelial growth factor A (VEGFA) interacts with miR-203-3p and is involved in SNHG8-mediated AML cell apoptosis and angiogenesis. This reveals that SNHG8 counteracts the effects of miR-203-3p on periostin and VEGFA-induced AMI progression [135]. The expression profile of 14q32 snoRNA exhibits high vascular specificity and is distinctly regulated throughout the human vasculature. Notably, its expression is upregulated during CVDs, including SNORD113.2 [136].
Ischemia-reperfusion (I/R)
Myocardial I/R injury is a severe complication of AMI. SNHG1 protects the myocardium by activating the PI3K/Akt signaling pathway through the lncRNA SNHG1/miR-450b-5p/IGF1 axis, effectively decreasing apoptosis and oxidative stress in AC16 cells subjected to I/R injury [137]. Moreover, SNHG1 plays a novel protective role in promoting cell proliferation, tube formation, and the expression of VEGF, VE-cadherin, and MMP2 via the HIF-1α/VEGF signaling pathway, aiding in the management of cardiac I/R injury [138]. miRNA also play a role in the pathogenesis of myocardial I/R injury. For instance, miR-330-5p alleviates cerebral I/R injury and regulates myocardial damage. SNHG3, as the upstream lncRNA of miR-330-5p, suppresses miR-330-5p expression and mitigates I/R injury in vitro via the SNHG3/miR-330-5p axis, targeting the ERK/p38 signaling pathway through HSD11B1 [139]. Moreover, SNHG14 expression is significantly upregulated during I/R, demonstrating good sensitivity and specificity, and can serve as a biomarker for I/R diagnosis in conjunction with miR-1206 [140]. SNHG15 regulates I/R-induced cardiomyocyte apoptosis by modulating the TLR4/NF-κB signaling pathway through miR-335-3p [141].
snoRNA and NDs
The role of snoRNA in NDs has increasingly captured the attention of researchers, as their functional diversity and the complexity of their mechanisms are gradually being unveiled. Studies indicate that snoRNA can interact with different binding partners to influence protein function and accessibility, thus playing a central role in key cellular processes such as gene expression regulation [3]. SnoRNA are also associated with protein misfolding and aggregation in NDs, processes that are hallmark features of many such conditions [142]. Specifically, the roles of snoRNA in NDs such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) have been widely studied, with snoRNA influencing disease progression by regulating neuronal gene expression and protein translation [143]. Furthermore, snoRNA may participate in the pathological processes of NDs by affecting pathways such as neuroinflammation and oxidative stress. The role of snoRNA in these diseases extends beyond their intracellular functions, as they can also serve as potential biomarkers for early diagnosis and the assessment of treatment efficacy [144]. These findings provide new perspectives on snoRNA research in NDs and open possibilities for developing new therapeutic strategies (Fig. 4).
Fig. 4.
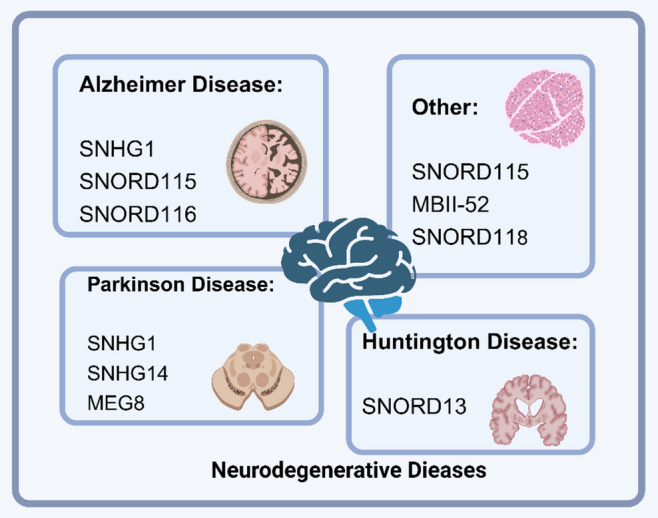
snoRNA and NDs. Different snoRNA are involved in cardiovascular diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and other
SnoRNA and the development of the nervous system
Originally, snoRNA were thought to primarily be involved in the modification of rRNA, but increasing evidence suggests they have more complex roles in the nervous system. A study revealed that maternal alcohol consumption during pregnancy modifies C/D box RNA levels in fetal brain cells, influencing neurobehavioral outcomes like cognitive function, anxiety, attention deficit hyperactivity disorder, and mood disorders by disrupting snoRNA levels [145]. First, snoRNA expression in the nervous system is tissue- and cell-type specific. Studies have shown significant differences in the abundance and functions of snoRNA across different tissues, with specific enrichment observed in the brain and reproductive tissues [5]. This distinct expression pattern indicates that snoRNA could be critical in the development and functioning of the nervous system. The interactions between snoRNA and other RNA molecules, such as mRNA, also highlight their non-canonical functions in the nervous system. For example, SNORA73 interacts with mRNA encoding secretory and membrane proteins, promoting their secretion [121]. This non-canonical function suggests that snoRNA may influence the nervous system’s function by regulating protein translation and secretion. Additionally, during the development of the nervous system, snoRNA levels are regulated during stem cell differentiation, which may fine-tune ribosomal function by altering pseudouridylation, thereby affecting neural development [146]. Finally, the functional diversity and complexity of snoRNA are also reflected in their interactions with other ncRNA. SnoRNA can impact gene expression by regulating alternative splicing and chromatin structure, further expanding their roles in the nervous system [147].
SnoRNA regulates NDs
AD
AD is a long-term neurodegenerative condition defined by gradual memory decline and cognitive dysfunction, accompanied by the accumulation of Tau aggregates. Studies have shown that cytoplasmic and nuclear Tau aggregates in cell cultures and mouse brains are enriched with snRNA and snoRNA [148]. Mitochondrial dysfunction can also act as a primary or secondary factor in promoting disease progression, as programmed cell death and efficient energy production depend on normal mitochondrial function. SnoRNA may play a critical role in ensuring the proper functioning of nuclear and mitochondrial genes associated with mitochondria. Modulating snoRNA levels could be a therapeutic approach to mitigate and reduce the severity of AD [149]. Most snoRNA have potential roles in the etiology of AD, acting as biomarkers and regulating Tau phosphorylation, TGF-β receptor function, or Aβ metabolism [150]. The in vitro AD cell models, SNHG1 regulates Aβ25-35-induced neuronal injury and exhibits neuroprotective effects by acting as a competing endogenous RNA (ceRNA) for miR-137 and inhibiting kringle containing transmembrane protein 1 (KREMEN1) upon knockdown [151]. Knockdown of SNHG1 has been shown to enhance DNA methylation via the PI3K/Akt signaling pathway, reducing Tau phosphorylation levels in the SAMP8 AD mouse model and alleviating brain damage caused by p-Tau accumulation [152]. Additionally, SNHG1 deletion improves learning and memory deficits in rats infused with Aβ1–42 fibrils, ameliorates cognitive dysfunction, and suppresses hippocampal inflammation and apoptosis by regulating the specificity protein 1 (Sp1)/SNHG1/miR-361-3p axis [153]. SNHG1 expression is upregulated by Aβ and downregulated by resveratrol, with its knockdown counteracting the harmful effects of Aβ on cells [154]. SNORD115 and SNORD116 exhibit high discriminatory ability between AD samples and normal controls, making them potential plasma biomarkers for AD [155].
PD
PD is a neurodegenerative condition marked by the degeneration of dopaminergic neurons and the abnormal accumulation of α-synuclein, a defining characteristic of the disorder. SnoRNA influence PD pathology by regulating the expression and aggregation of α-synuclein through interactions with mRNA [156]. They may also participate in PD regulation by affecting other related genes, such as the synuclein alpha (SNCA) gene [157]. Additionally, snoRNA may have non-canonical intracellular functions relevant to PD. Studies have shown that snoRNA interact with the 7SL RNA in SRPs, promoting protein secretion, suggesting a role in intracellular transport and secretion [121]. SnoRNA may also form complex regulatory networks with other ncRNA, such as circular RNA (circRNA) and miRNA, influencing PD pathology. For instance, SNHG1 is elevated in brain samples from PD patients and in MPP+-treated SH-SY5Y cells. Knockdown of SNHG1 inhibits LPS-induced BV2 microglial activation and inflammation, suggesting that SNHG1 promotes neuroinflammation in PD via the miR-7/ nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) pathway [158]. SNHG1 also acts as a pathogenic factor by promoting α-synuclein aggregation and toxicity through the miR-15b-5p/ siah E3 ubiquitin protein ligase 1 (SIAH1) axis, shedding light on mechanisms underlying Lewy body formation and dopaminergic neuron loss in PD [159]. Upregulation of lncRNA SNHG1 in human dopaminergic SH-SY5Y cells promotes MPP+-induced cytotoxicity and ROS production via the miR-15b-5p/ glycogen synthase kinase-3β (GSK3β) axis [160]. In contrast, reducing SNHG1 expression decreases MPP+-induced levels of light chain 3 (LC3)-II, an autophagy marker, and mitigates cytotoxicity via the miR-221/222/p27/mTOR pathway, emphasizing its potential as a therapeutic target for neuroprotection and PD treatment [161]. In MPTP-induced PD mouse models and MPP+-treated SK-N-SH cells, SNHG14 and nuclear factor of activated T cells 5 (NFAT5) are upregulated, while miR-375-3p is downregulated. Knockdown of SNHG14 or NFAT5, or overexpression of miR-375-3p, reverses MPP+-induced neuronal apoptosis, inflammation, and oxidative stress. SNHG14 promotes neuronal injury via the miR-375/NFAT5 axis [162]. Additionally, SNHG14 mediates miR-135b-5p/karyopherin subunit alpha 4 (KPNA4) signaling, aggravating MPP+-induced neuronal damage in PD cell models [163]. Knockdown of SNHG14 enhances cell viability, inhibits apoptosis, and reduces pro-inflammatory cytokine production in MPP+-stimulated SK-N-SH cells. Targeting the miR-214-3p/ Krüppel-like factor 4 (KLF4) axis, this study demonstrated SNHG14 knockdown’s protective effect against MPP+-induced cytotoxicity, providing a promising target for PD intervention [164]. Neuroinflammation plays a crucial role in PD pathogenesis. Maternally expressed gene 8 (MEG8), a regulator of inflammation, is upregulated in PD and serves as a potential diagnostic biomarker. MEG8 may alleviate PD-associated inflammatory damage via the miR-485-3p/F-box protein 45 (FBXO45) axis [165]. In summary, snoRNA play multifaceted roles in regulating PD, including gene expression, protein secretion, RNA processing, and interactions with other ncRNA. These findings offer new perspectives on the molecular mechanisms of PD and provide potential targets for developing novel therapeutic strategies.
Huntington’s disease (HD)
HD is an inherited neurodegenerative condition resulting from CAG repeat expansions in the huntingtin gene. Transcriptional dysregulation is a prominent feature of HD, with the dysfunction of various transcription factors and ncRNA considered a major contributor to the neurodegenerative process [166]. For example, SNORD13 levels are elevated in HD patients compared to controls. Interaction and pathway analyses of SNORD13 have revealed an enrichment of HD-associated factors, suggesting a potential disease-specific role for SNORD13 in HD [154]. SnoRNA may also regulate intracellular signaling and metabolic pathways through interactions with other ncRNA, thereby influencing cell survival and function [167]. Thus, investigating the role of snoRNA in HD not only aids in understanding the disease’s pathological mechanisms but may also provide insights for developing new therapeutic strategies.
Other
The heterogeneity of catatonia and catatonic symptoms involves nuclear dysfunction caused by abnormalities in the brain-specific non-coding miRNA SNORD115 gene (duplication or deletion). These abnormalities lead to various combinations of pathological biological dysfunctions in downstream pathways, such as autism spectrum disorder (ASD), schizophrenia, bipolar disorder, major depression, psychosis, hereditary diseases, and immune disorders [168]. The deletion of MBII-52 snoRNA is associated with Prader-Willi syndrome, a rare disorder characterized by hyperphagia and obesity [169]. Although ASD may not be classified as a neurodegenerative disease, RNA-seq studies have identified differential expression of multiple snoRNA genes in ASD [170]. Single nucleotide polymorphisms in the SNORD118 gene have recently been linked to a neurodegenerative disease involving leukoencephalopathy, calcifications, and cysts (LCC, also known as Labrune syndrome), although its molecular pathogenesis remains unclear [171]. In summary, the regulatory role of snoRNA in NDs is a complex and critical area of research. To better understand the precise mechanisms of snoRNA in various disorders and investigate their potential as therapeutic targets, more research is required.
Biomarkers and clinical diagnostic functions
In recent years, the role of snoRNA as biomarkers and tools for clinical diagnostics has garnered increasing attention. As biomarkers, snoRNA function in the following four areas: (a) Disease Association: Certain snoRNA show altered expression levels under disease conditions, making them valuable disease biomarkers. (b) Stability: SnoRNA are relatively stable in extracellular environments and resistant to degradation, making them suitable as biomarkers. (c) Tissue Specificity: Some snoRNA exhibit specific expression patterns in different tissues, aiding in disease localization. (d) Disease Progression: Changes in snoRNA expression may correlate with disease progression, helping monitor disease stages. For example, in CRC, One important oncogenic snoRNA that has been found to contribute significantly to the development of cancer and could be a prognostic biomarker is SNORA21 [39]. SNORD16 is upregulated in colon cancer, and its high expression is associated with poor prognosis, suggesting its potential as a diagnostic and prognostic marker for colon cancer [172]. In hepatocellular carcinoma, bioinformatics studies have identified a group of key snoRNA, including SNORA11, SNORD124, and SNORD46, which show potential for both diagnosis and prognosis [173]. SNORA14A’s function in tumor suppression has been demonstrated by its ability to inhibit cell growth and trigger apoptosis in hepatoblastoma [174]. In renal cell carcinoma, SNORD15A, SNORD35B, and SNORD60 have been identified as novel biomarkers, with significant upregulation in cancer tissues and stable presence in urinary sediment, highlighting their diagnostic potential [175]. Even in studies of osteoarthritis, serum snoRNA levels have been found to correlate with joint damage, suggesting that snoRNA can be used as a non-invasive biomarker for osteoarthritis progression [176]. Additionally, snoRNA show promise as biomarkers in cardiovascular and neurological diseases. Studies reveal that snoRNA abundance patterns are complex across different human tissues, suggesting diverse functional demands in various tissues [5].
Research on the potential uses of snoRNA in clinical diagnostics, such as cancer detection, disease subtyping, prognostic evaluation, and therapeutic targeting, is expanding. Studies show that snoRNA expression is abnormal in various cancers, with different disease subtypes potentially exhibiting distinct snoRNA expression profiles. This makes snoRNA valuable for precise disease classification. Additionally, snoRNA expression levels correlate with patient prognosis and can be used to evaluate treatment outcomes and predict patient survival. The abnormal expression of snoRNA may play a role in disease pathogenesis, making them promising therapeutic targets. In esophageal cancer (ESCA), snoRNA such as SNORA58, SNORA68, and SNORD93 in platelets are considered novel diagnostic biomarkers. These snoRNA are significantly upregulated in ESCA patients’ platelets and can effectively distinguish cancer patients from healthy controls [177]. SnoRNA also hold significant diagnostic and prognostic value in lung cancer. For example, NOP10-associated snoRNA drive the proliferation and migration of lung cancer cells [178]. In CRC, SNORA21 has been systematically identified as a key oncogenic snoRNA that plays a crucial role in cancer progression and may serve as an important therapeutic target [39]. Furthermore, in non-invasive testing, the stability and specificity of snoRNA make them ideal biomarkers. Unlike traditional tissue biopsies, snoRNA detection can be performed using bodily fluids such as blood, reducing patient discomfort while enhancing convenience and reproducibility [179]. For instance, studies have revealed significant differential expression of snoRNA in serum exosomes from NSCLC patients [180]. The detection method of snoRNA as a biomarker is also developing increasingly: for example, based on amplification: quantitative polymerase chain reaction (qRT-PCR), sequencing-based methods: next-generation sequencing (NGS), bioinformatics and computing tools: SnoRNA-specific databases (such as SNORic). In conclusion, snoRNA has shown broad application prospects in the field of biomarkers and clinical diagnosis, and with the development of science and technology, they are expected to play an important role in the future of precision medicine.
Research challenges and future directions
Challenges and solutions in snorna research
SnoRNA play a critical role in biological research, particularly in RNA modification and gene expression regulation. However, snoRNA research faces several challenges. First, the techniques for identifying and characterizing snoRNA targets remain underdeveloped. While snoRNA sequences and structures are relatively conserved, many potential snoRNA have yet to be discovered. Their tissue-specific expression, with some snoRNA active only in specific cell types or developmental stages, significantly limits a comprehensive understanding of their functions. Functionally, the roles of many snoRNA, especially atypical ones, are still unclear. Their interactions with other molecules and the specific mechanisms by which they operate in cells are poorly understood. Additionally, current bioinformatics tools have limitations in accurately predicting the precise locations and functions of snoRNA. Moreover, snoRNA are often low in abundance and unevenly distributed within cells, presenting experimental challenges for detection and manipulation.
To overcome these challenges, researchers have developed chemical crosslinking-based methods to comprehensively detect cellular RNA targets of snoRNA. This approach has revealed thousands of previously unidentified snoRNA-mRNA interactions in human cells and mouse brain tissues [121]. In evolutionary studies, annotating snoRNA has also proven challenging. By analyzing RNA sequencing data from Xenopus tropicalis (African clawed frog), researchers annotated nearly a complete set of snoRNA and compared them with snoRNA from human and other vertebrate genomes. This analysis identified frog-specific snoRNA and domains, uncovering the evolutionary diversity of RNA modifications [181]. At the same time, deep learning and machine learning algorithms are being employed to enhance snoRNA identification and prediction tools. Multi-omics data, including RNA sequencing and proteomics, are also integrated to predict and validate the presence of snoRNA. Gene editing technologies like CRISPR/Cas9 are used to knock out or knock down snoRNA, allowing researchers to observe changes in cellular or model organism phenotypes, thereby confirming snoRNA functions. Non-canonical functions of snoRNA are also emerging. For example, some snoRNA interact with 7SL RNA in SRPs, promoting the secretion of encoded proteins [121]. In bioinformatics, efforts are underway to construct more comprehensive snoRNA databases, integrating snoRNA information across various species. Advanced analysis software capable of processing complex RNA modification and interaction networks is also being developed. The relationship between snoRNA abundance and function across different tissues is another area of interest. RNA-Seq analysis of seven healthy human tissues revealed complex and diverse snoRNA abundance patterns, with varying relationships to host genes. This suggests that snoRNA are not merely housekeeping genes but also highly regulated, tissue-specific RNA [5]. In terms of experimental techniques, more sensitive RNA detection methods, such as single-cell RNA sequencing, are being developed to study snoRNA expression at the single-cell level. Specific antibodies or affinity tags are used to enrich and purify snoRNA and their interacting proteins. Additionally, interdisciplinary collaboration has emerged as a major innovation in recent years, fostering partnerships between bioinformatics, molecular biology, and computational science to address challenges in snoRNA research.
Future research directions and therapeutic strategies
In recent years, the advancement of high-throughput genomic technologies has led to significant progress in snoRNA research, revealing its critical roles in various biological processes. However, the complete functional spectrum of snoRNA remains to be fully elucidated, and future research directions and therapeutic strategies warrant further exploration. Key research areas will focus on uncovering the diversity of snoRNA, gaining deeper insights into their biological functions, exploring their associations with human diseases, analyzing their regulatory networks, and understanding their evolutionary conservation. Achieving these goals will require technological innovation and methodological advancements, including high-throughput screening technologies, the development of precise RNA analysis tools, and improved bioinformatics methods. These innovations will aid in identifying and characterizing novel snoRNA molecules and understanding their cellular roles in greater detail. Particularly in the context of snoRNA-disease associations, researchers have proposed a computational method known as iSnoDi-LSGT. This method leverages snoRNA sequence similarity and disease similarity as local constraints and uses network embedding techniques to extract topological features of snoRNA and diseases, effectively predicting previously unknown snoRNA-disease associations [182].
In terms of therapeutic strategies, snoRNA offer revolutionary potential in medicine. SnoRNA-based treatments, such as the design and development of small molecule drugs, can precisely regulate snoRNA functions, thereby influencing downstream RNA modification processes. Advances in gene editing technologies, such as the CRISPR-Cas system, may enable the correction of disease-causing snoRNA mutations. Additionally, drug development targeting RNA modifications and strategies utilizing snoRNA as therapeutic delivery vehicles hold promise for treating various diseases [183]. Personalized medicine will also rely heavily on a detailed analysis of individual snoRNA expression profiles. This will facilitate the development of more precise treatment plans, as monitoring snoRNA expression differences could help predict patient responses to specific drugs, guiding clinical decisions and improving therapeutic outcomes. In the domains of regenerative medicine and cell engineering, the applications of snoRNA are equally promising. By modulating snoRNA activity, stem cell functionality can be enhanced, and cellular differentiation can be promoted, representing a significant advancement for tissue engineering and regenerative medicine.
Conclusion
This article reviews the biological functions and therapeutic potential of snoRNA, providing new perspectives for understanding life processes in the field of biology while introducing innovative therapeutic strategies and interventions in medicine and biotechnology. With continued research advancements and technological development, snoRNA are expected to play an increasingly significant role in future scientific and medical practices, contributing to the improvement of human health.
Acknowledgements
HOME for Researchers and Bio Render.
Abbreviations
- AMI
Acute myocardial infarction
- AD
Alzheimer’s disease
- ATM
Ataxia-telangiectasia mutated kinase
- ASD
Autism spectrum disorder
- CH
Cardiac hypertrophy
- CVDs
Cardiovascular diseases
- circRNA
Circular RNA
- CRC
Colorectal cancer
- ceRNA
Competing endogenous RNA
- C2CD4A
C2 calcium dependent domain containing 4 A
- DCAF13
DDB1 and CUL4 associated factor 13
- DCM
Dilated cardiomyopathy
- Dox
Doxorubicin
- EZH2
Enhancer of zeste homolog 2
- EMT
Epithelial-mesenchymal transition
- ESCA
Esophageal cancer
- ESCC
Esophageal squamous cell carcinoma
- E2F7
E2F transcription factor 7
- FOXO1
Fork head box O1
- FBXO45
F-box protein 45
- GSK3β
Glycogen synthase kinase-3β
- HCC
Hepatocellular carcinoma
- HCM
Hypertrophic cardiomyopathy
- HD
Huntington’s disease
- IGF
Insulin-like growth factor
- JAK
Janus kinase
- KPNA4
Karyopherin subunit alpha 4
- KREMEN1
Kringle containing transmembrane protein 1
- KLF4
Krüppel-like factor 4
- LATS1
Large tumor suppressor kinase 1
- LC3
Light chain 3
- lncRNA
Long non-coding RNA
- MST1
Macrophage stimulating 1
- MEG8
Maternally expressed gene 8
- mRNA
Messenger RNA
- MEK
Mitogen-activated protein
- MI
Myocardial infarction
- NOP56
Nucleolar protein 56
- NLRP3
Nucleotide-binding oligomerization domain-like receptor protein 3
- NDs
Neurodegenerative diseases
- NF2
Neurofibromatosis type2
- NFAT5
Nuclear factor of activated T cells 5
- SNU13
Nucleolar ribonucleoprotein 13
- ncRNA
Non-coding RNA
- NSCLC
Non-small cell lung cancer
- OSCC
Oral squamous cell carcinoma
- PTC
Papillary thyroid carcinoma
- PD
Parkinson’s disease
- PTC
Papillary thyroid carcinoma
- PA
Phosphatidic acid
- PI3K
Phosphatidylinositol-3-kinase
- PD-L1
Programmed death-ligand 1
- AKT
Protein kinase B
- PCDH17
Protocadherin 17
- RNP
Ribonucleoprotein
- RAS/MAPK
Ras-mitogen-activated protein kinase
- RAF
Rapidly accelerated fibrosarcoma
- rRNA
Ribosomal RNA
- SIAH1
Siah E3 ubiquitin protein ligase 1
- SRPs
Signal recognition particles
- STAT
Signal transducer and activator of transcription
- snoRNA
Small molecule RNA
- snRNA
Small nuclear RNA
- sdRNA
Snorna derived small RNA
- Sp1
Specificity protein 1
- SNCA
Synuclein alpha
- TMZ
Temozolomide
- TAZ
Transcriptional co-activator with PDZ-binding motif
- tRNA
Transfer RNA
- TGF-β
Transforming growth factor-β
- TNBC
Triple negative breast cancer
- TNFRSF11B
Tumor necrosis factor receptor superfamily member 11b
- VEGFA
Vascular endothelial growth factor A
- YAP
Yes-associated protein
Author contributions
Yinghui Li wrote the manuscript. Xinzhe Chen and Shudan Xiao drew the tables and figures. Haoxuan Wang, Bo Li, MeiHua Zhang and Kun Wang revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82370291, 82070313); Qingdao Science and Technology Benefiting the People Demonstration Project (24-1-8-smjk-7-nsh); Major Basic Research Projects in Shandong Province (ZR2024ZD46).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinghui Li, Xinzhe Chen and Shudan Xiao are co-authors.
Contributor Information
MeiHua Zhang, Email: mhzhang0605@126.com.
Kun Wang, Email: wangk696@qdu.edu.cn.
References
- 1.Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26(17):3871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiss T. Small nucleolar rnas: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109(2):145–8. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron D, Fafard-Couture É, Scott MS. Small nucleolar rnas: continuing identification of novel members and increasing diversity of their molecular mechanisms of action. Biochem Soc Trans. 2020;48(2):645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falaleeva M, Stamm S. Processing of snornas as a new source of regulatory non-coding rnas: snorna fragments form a new class of functional RNAs. BioEssays News Rev Mol Cell Dev Biol. 2013;35(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fafard-Couture É, Bergeron D, Couture S, et al. Annotation of snorna abundance across human tissues reveals complex snorna-host gene relationships. Genome Biol. 2021;22(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao L, Wang J, Ju S, et al. Disorders and roles of tsrna, snorna, SnRNA and PiRNA in cancer. J Med Genet. 2022;59(7):623–31. [DOI] [PubMed] [Google Scholar]
- 7.McMahon M, Contreras A, Ruggero D. Small RNAs with big implications: new insights into H/ACA snorna function and their role in human disease. Wiley Interdiscip Rev RNA. 2015;6(2):173–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra P, Kumar S. Association of LncRNA with regulatory molecular factors in brain and their role in the pathophysiology of schizophrenia. Metab Brain Dis. 2021;36(5):849–58. [DOI] [PubMed] [Google Scholar]
- 9.Bhartiya D, Talwar J, Hasija Y, et al. Systematic curation and analysis of genomic variations and their potential functional consequences in snorna loci. Hum Mutat. 2012;33(10):E2367–2374. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell ES, Fournier MJ. The small nucleolar RNAs. Annu Rev Biochem. 1995;64:897–934. [DOI] [PubMed] [Google Scholar]
- 11.Samarsky DA, Fournier MJ, Singer RH, et al. The snorna box C/D motif directs nucleolar targeting and also couples snorna synthesis and localization. EMBO J. 1998;17(13):3747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoshnevis S, Dreggors RE, Hoffmann TFR, et al. A conserved Bcd1 interaction essential for box C/D SnoRNP biogenesis. J Biol Chem. 2019;294(48):18360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul A, Tiotiu D, Bragantini B, et al. Bcd1p controls RNA loading of the core protein Nop58 during C/D box SnoRNP biogenesis. Volume 25. RNA N Y N; 2019. pp. 496–506. 4. [DOI] [PMC free article] [PubMed]
- 14.Hamma T, Reichow SL, Varani G, et al. The Cbf5-Nop10 complex is a molecular bracket that organizes box H/ACA RNPs. Nat Struct Mol Biol. 2005;12(12):1101–7. [DOI] [PubMed] [Google Scholar]
- 15.Kiss T, Fayet-Lebaron E, Jády BE. Box H/ACA small ribonucleoproteins. Mol Cell. 2010;37(5):597–606. [DOI] [PubMed] [Google Scholar]
- 16.Ganot P, Bortolin ML, Kiss T. Site-specific Pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89(5):799–809. [DOI] [PubMed] [Google Scholar]
- 17.Yu YT, Meier UT. RNA-guided isomerization of uridine to pseudouridine–pseudouridylation. RNA Biol. 2014;11(12):1483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogacić V, Dragon F, Filipowicz W. Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol Cell Biol. 2000;20(23):9028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snornps: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3(3):397–414. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey JP, Tollervey D. Birth of the snornps: the evolution of RNase MRP and the eukaryotic pre-rRNA-processing system. Trends Biochem Sci. 1995;20(2):78–82. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Spensley M, Choi SK, et al. Plant U13 orthologues and orphan snornas identified by rnomics of RNA from Arabidopsis nucleoli. Nucleic Acids Res. 2010;38(9):3054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Yan Z, Zhang M, et al. SnoRNP is essential for thermospermine-mediated development in Arabidopsis thaliana. Sci China Life Sci. 2023;66(1):2–11. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Wang J, Wu S, et al. The small nucleolar RNA SnoR28 regulates plant growth and development by directing rRNA maturation. Plant Cell. 2022;34(11):4173–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Requena JM. The Leishmania ribosome: more than passive mRNA translating machinery. Trends Biochem Sci. 2024;49(9):754–6. [DOI] [PubMed] [Google Scholar]
- 25.Bratkovič T, Božič J, Rogelj B. Functional diversity of small nucleolar RNAs. Nucleic Acids Res. 2020;48(4):1627–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nir R, Hoernes TP, Muramatsu H, et al. A systematic dissection of determinants and consequences of snoRNA-guided pseudouridylation of human mRNA. Nucleic Acids Res. 2022;50(9):4900–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Shi J, Guo Y, et al. A snorna modulates mRNA 3’ end processing and regulates the expression of a subset of mRNAs. Nucleic Acids Res. 2017;45(15):8647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauhan W, Sudharshan Sjnull, Kafle S, et al. SnoRNAs: exploring their implication in human diseases. Int J Mol Sci. 2024;25(13):7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Chen LL. Long non-coding RNAs in the nucleolus: biogenesis, regulation, and function. Curr Opin Struct Biol. 2024;87:102866. [DOI] [PubMed] [Google Scholar]
- 30.Porat J, Slat VA, Rader SD, et al. The fission yeast Methyl phosphate capping enzyme Bmc1 guides 2’-O-methylation of the U6 SnRNA. Nucleic Acids Res. 2023;51(16):8805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Wang Y, Wang J, et al. Profiling of RNA ribose methylation in Arabidopsis thaliana. Nucleic Acids Res. 2021;49(7):4104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.M Z, K L, J B, et al. A snoRNA-tRNA modification network governs codon-biased cellular States. Proc Natl Acad Sci U S A, 2023;120(41). [DOI] [PMC free article] [PubMed]
- 33.Huang ZH, Du YP, Wen JT, et al. SnoRNAs: functions and mechanisms in biological processes, and roles in tumor pathophysiology. Cell Death Discov. 2022;8(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holley CL, Li MW, Scruggs BS, et al. Cytosolic accumulation of small nucleolar RNAs (snoRNAs) is dynamically regulated by NADPH oxidase. J Biol Chem. 2015;290(18):11741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sletten AC, Davidson JW, Yagabasan B, et al. Loss of SNORA73 reprograms cellular metabolism and protects against steatohepatitis. Nat Commun. 2021;12(1):5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimer JM, Lee J, Holley CL, et al. Long-range function of secreted small nucleolar RNAs that direct 2’-O-methylation. J Biol Chem. 2018;293(34):13284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yellamaty R, Sharma S. Critical cellular functions and mechanisms of action of the RNA helicase UAP56. J Mol Biol. 2024;436(12):168604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Sun S, Liu X, et al. U3 snorna inter-regulates with DDX21 in the perichromosomal region to control mitosis. Cell Death Dis. 2024;15(5):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida K, Toden S, Weng W, et al. SNORA21 - An oncogenic small nucleolar RNA, with a prognostic biomarker potential in human colorectal Cancer. EBioMedicine. 2017;22:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Chen X, Liu X, et al. C/D box small nucleolar RNA SNORD104 promotes endometrial cancer by regulating the 2’-O-methylation of PARP1. J Transl Med. 2022;20(1):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong W, Liu Y, Wang P, et al. U3 snoRNA-mediated degradation of ZBTB7A regulates aerobic Glycolysis in isocitrate dehydrogenase 1 wild-type glioblastoma cells. CNS Neurosci Ther. 2023;29(10):2811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coley AB, Stahly AN, Kasukurthi MV, et al. MicroRNA-like snoRNA-Derived RNAs (sdRNAs) promote Castration-Resistant prostate Cancer. Cells. 2022;11(8):1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mleczko AM, Machtel P, Walkowiak M, et al. Levels of SdRNAs in cytoplasm and their association with ribosomes are dependent upon stress conditions but independent from snorna expression. Sci Rep. 2019;9(1):18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen JT, Chen X, Liu X et al. Small Nucleolar RNA and C/D Box 15B Regulate the TRIM25/P53 Complex to Promote the Development of Endometrial Cancer. J Oncol, 2022;2022:7762708. [DOI] [PMC free article] [PubMed]
- 45.Wei S, Xing J, Chen J, et al. DCAF13 inhibits the p53 signaling pathway by promoting p53 ubiquitination modification in lung adenocarcinoma. J Exp Clin Cancer Res CR. 2024;43(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rong Z, Luo Z, Fu Z, et al. The novel circSLC6A6/miR-1265/C2CD4A axis promotes colorectal cancer growth by suppressing p53 signaling pathway. J Exp Clin Cancer Res CR. 2021;40(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan X, Shi A, Zou Y, et al. EZH2-Mediated microRNA-375 upregulation promotes progression of breast Cancer via the Inhibition of FOXO1 and the p53 signaling pathway. Front Genet. 2021;12:633756. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Sharma S, Marchand V, Motorin Y, et al. Identification of sites of 2’-O-methylation vulnerability in human ribosomal RNAs by systematic mapping. Sci Rep. 2017;7(1):11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murthy D, Attri KS, Singh PK. Phosphoinositide 3-Kinase signaling pathway in pancreatic ductal adenocarcinoma progression, pathogenesis, and therapeutics. Front Physiol. 2018;9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soleimani A, Rahmani F, Ferns GA, et al. Role of regulatory oncogenic or tumor suppressor MiRNAs of PI3K/AKT signaling Axis in the pathogenesis of colorectal Cancer. Curr Pharm Des. 2018;24(39):4605–10. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Li J, Lin S et al. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp Anim, 2021;70(4):488~497. [DOI] [PMC free article] [PubMed]
- 52.Deng H, Ning J, Ruan Y, et al. TNFRSF11B promotes the progression of bladder cancer through PI3K/AKT signaling pathway. Mol Cell Probes. 2024;78:101989. [DOI] [PubMed] [Google Scholar]
- 53.Wu L, Zheng J, Chen P, et al. Small nucleolar RNA ACA11 promotes proliferation, migration and invasion in hepatocellular carcinoma by targeting the PI3K/AKT signaling pathway. Biomed Pharmacother Biomedecine Pharmacother. 2017;90:705–12. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Liu X, Zhang W, et al. Oncogene SCARNA12 as a potential diagnostic biomarker for colorectal cancer. Mol Biomed. 2023;4(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Yang YP, Yi ML, et al. Exploring the expression of SNHG1 and its effect on the PI3K-AKT axis in nasopharyngeal cancer. Neoplasma. 2023;70(5):670–82. [DOI] [PubMed] [Google Scholar]
- 56.Q D, J C. SNHG1 promotes proliferation, migration and invasion of bladder cancer cells via the PI3K/AKT signaling pathway. Exp Ther Med, 2020;20(5). [DOI] [PMC free article] [PubMed]
- 57.Zhang Y, Zhang R, Luo G, et al. Long noncoding RNA SNHG1 promotes cell proliferation through PI3K/AKT signaling pathway in pancreatic ductal adenocarcinoma. J Cancer. 2018;9(15):2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pourghasem N, Ghorbanzadeh S, Nejatizadeh A. Expression and regulatory roles of small nucleolar RNA host gene 4 in gastric Cancer. Curr Protein Pept Sci. 2023;24(9):767–79. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Yu J, Xie R, et al. Roles of the SNHG7/microRNA–9–5p/DPP4 CeRNA network in the growth and 131I resistance of thyroid carcinoma cells through PI3K/Akt activation. Oncol Rep. 2021;45(4):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang K, Chen J, Li C, et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2022;526:142–54. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Qi Y, Huang Y, et al. Exosome-derived SNHG16 sponging miR-4500 activates HUVEC angiogenesis by targeting GALNT1 via PI3K/Akt/mTOR pathway in hepatocellular carcinoma. J Physiol Biochem. 2021;77(4):667–82. [DOI] [PubMed] [Google Scholar]
- 62.Shi M, Yang R, Lin J, et al. LncRNA-SNHG16 promotes proliferation and migration of acute myeloid leukemia cells via PTEN/PI3K/AKT axis through suppressing CELF2 protein. J Biosci. 2021;46:4. [PubMed] [Google Scholar]
- 63.Zhou XY, Liu H, Ding ZB, et al. LncRNA SNHG16 promotes glioma tumorigenicity through miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics. 2020;112(1):1021–9. [DOI] [PubMed] [Google Scholar]
- 64.Xu Z, Sun Y, Wang D, et al. SNHG16 promotes tumorigenesis and cisplatin resistance by regulating miR-338-3p/PLK4 pathway in neuroblastoma cells. Cancer Cell Int. 2020;20:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao H, Liu R, Sun X. STAT3-induced upregulation of LncRNA SNHG17 predicts a poor prognosis of melanoma and promotes cell proliferation and metastasis through regulating PI3K-AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23(18):8000–10. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Q, Gao S, Du Q, et al. Long non-coding RNA SNHG20 promotes bladder cancer via activating the Wnt/β-catenin signalling pathway. Int J Mol Med. 2018;42(5):2839–48. [DOI] [PubMed] [Google Scholar]
- 67.Zhang PF, Wu J, Luo JH, et al. SNHG22 overexpression indicates poor prognosis and induces chemotherapy resistance via the miR-2467/Gal-1 signaling pathway in epithelial ovarian carcinoma. Aging. 2019;11(19):8204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Ke S, Wang Q, et al. Analyzing roles of small nucleolar RNA host gene 25 from clinical, molecular target and tumor formation in prostate cancer. Exp Cell Res. 2023;429(2):113686. [DOI] [PubMed] [Google Scholar]
- 69.Feng X, Yang L, Liu X, et al. Long non-coding RNA small nucleolar RNA host gene 29 drives chronic myeloid leukemia progression via microRNA-483-3p/Casitas B-lineage lymphoma axis-mediated activation of the phosphoinositide 3-kinase/Akt pathway. Med Oncol Northwood Lond Engl. 2024;41(2):60. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Pang Y, Jia Y, et al. SNORA23 inhibits HCC tumorigenesis by impairing the 2’-O-ribose methylation level of 28S rRNA. Cancer Biol Med. 2021;19(1):104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan S, Wu Y, Wang Y, et al. An oncolytic adenovirus expressing SNORD44 and GAS5 exhibits antitumor effect in colorectal Cancer cells. Hum Gene Ther. 2017;28(8):690–700. [DOI] [PubMed] [Google Scholar]
- 72.H Y, L T. L Y, Knockdown of SNORA47 inhibits the tumorigenesis of NSCLC via mediation of PI3K/Akt signaling pathway. Front Oncol, 2021;11. [DOI] [PMC free article] [PubMed]
- 73.W W, X C, X L, et al. SNORD60 promotes the tumorigenesis and progression of endometrial cancer through binding PIK3CA and regulating PI3K/AKT/mTOR signaling pathway. Mol Carcinog, 2023;62(4). [DOI] [PubMed]
- 74.Fang X, Yang D, Luo H, et al. SNORD126 promotes HCC and CRC cell growth by activating the PI3K-AKT pathway through FGFR2. J Mol Cell Biol. 2017;9(3):243–55. [DOI] [PubMed] [Google Scholar]
- 75.Dillon M, Lopez A, Lin E, et al. Progress on ras/mapk signaling research and targeting in blood and solid cancers. Cancers. 2021;13(20):5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang G, Zeng Z, Sun W, et al. Small nucleolar RNA 71A promotes lung Cancer cell proliferation, migration and invasion via MAPK/ERK pathway. J Cancer. 2019;10(10):2261–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu G, Yang F, Ding CL, et al. Small nucleolar RNA 113-1 suppresses tumorigenesis in hepatocellular carcinoma. Mol Cancer. 2014;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang B, Xing AY, Li GX, et al. SNHG14 promotes triple-negative breast cancer cell proliferation, invasion, and chemoresistance by regulating the ERK/MAPK signaling pathway. IUBMB Life. 2024;76(12):1295–308. [DOI] [PubMed] [Google Scholar]
- 79.Lu C, Wei Y, Wang X, et al. DNA-methylation-mediated activating of LncRNA SNHG12 promotes Temozolomide resistance in glioblastoma. Mol Cancer. 2020;19(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Gong X, Huang M. YY1-Activated long noncoding RNA SNHG5 promotes glioblastoma cell proliferation through p38/MAPK signaling pathway. Cancer Biother Radiopharm. 2019;34(9):589–96. [DOI] [PubMed] [Google Scholar]
- 81.Zhang F, Guo C, Cao X, et al. Gastric cancer cell-derived extracellular vesicles elevate E2F7 expression and activate the MAPK/ERK signaling to promote peritoneal metastasis through the delivery of SNHG12. Cell Death Discov. 2022;8(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao J, Xiao C, Fong CJT, H, et al. Expression and regulatory network analysis of function of small nucleolar RNA host gene 4 in hepatocellular carcinoma. J Clin Transl Hepatol. 2022;10(2):297–307. [DOI] [PMC free article] [PubMed]
- 83.Liu P, Wang J, Du W, et al. LncRNA SNHG12 promotes proliferation and migration of hepatic progenitor cells via the Wnt/β-catenin pathway. Adv Clin Exp Med Off Organ Wroclaw Med Univ. 2023;32(9):1017–27. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Hu J, Guo D, et al. LncRNA SNHG5 promotes the proliferation and cancer stem cell-like properties of HCC by regulating UPF1 and Wnt-signaling pathway. Cancer Gene Ther. 2022;29(10):1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu L, Chang L, Wang H, et al. Clinical significance of C/D box small nucleolar RNA U76 as an oncogene and a prognostic biomarker in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2018;42(1):82–91. [DOI] [PubMed] [Google Scholar]
- 86.Shuwen H, Xi Y, Quan Q, et al. Can small nucleolar RNA be a novel molecular target for hepatocellular carcinoma? Gene. 2020;733:144384. [DOI] [PubMed] [Google Scholar]
- 87.Liu Z, Tao H. Small nucleolar RNA host gene 3 facilitates cell proliferation and migration in oral squamous cell carcinoma via targeting nuclear transcription factor Y subunit gamma. J Cell Biochem. 2020;121(3):2150–8. [DOI] [PubMed] [Google Scholar]
- 88.Gao XF, He HQ, Zhu XB et al. LncRNA SNHG20 promotes tumorigenesis and cancer stemness in glioblastoma via activating PI3K/Akt/mTOR signaling pathway. Neoplasma, 2019;66(4):532~542. [DOI] [PubMed]
- 89.He S, Zhao Y, Wang X, et al. Up-regulation of long non-coding RNA SNHG20 promotes ovarian cancer progression via Wnt/β-catenin signaling. Biosci Rep. 2018;38(1):BSR20170681. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Liu Y, Zhao C, Wang G, et al. SNORD1C maintains stemness and 5-FU resistance by activation of Wnt signaling pathway in colorectal cancer. Cell Death Discov. 2022;8(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi SL, Zhang ZH. Long non-coding RNA SNHG1 contributes to cisplatin resistance in non-small cell lung cancer by regulating miR-140-5p/Wnt/β-catenin pathway. Neoplasma. 2019;66(5):756–65. [DOI] [PubMed] [Google Scholar]
- 92.Jiang Z, Jiang C, Fang J. Up-regulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;495(1):238–45. [DOI] [PubMed] [Google Scholar]
- 93.Li C, Gao Q, Wang M, et al. LncRNA SNHG1 contributes to the regulation of acute myeloid leukemia cell growth by modulating miR-489-3p/SOX12/Wnt/β-catenin signaling. J Cell Physiol. 2021;236(1):653–63. [DOI] [PubMed] [Google Scholar]
- 94.Yang H, Wang S, Kang YJ, et al. Long non-coding RNA SNHG1 predicts a poor prognosis and promotes colon cancer tumorigenesis. Oncol Rep. 2018;40(1):261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.98 Kang R, Yao DF, Xu GZ, et al. The knockdown of SNHG3 inhibits the progression of laryngeal squamous cell carcinoma by miR-340-5p/YAP1 axis and Wnt/β-catenin pathway. Neoplasma. 2020;67(5):1094–105. [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Zhao S, Lv ZX, et al. Promoting action of long non-coding RNA small nucleolar RNA host gene 4 in ovarian cancer. Acta Biochim Pol. 2023;70(1):59–68. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Guo D, Zhao Y, et al. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3β signal pathway. Cell Death Dis. 2018;9(9):888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su L, Wu A, Zhang W, et al. Silencing long non-coding RNA SNHG6 restrains proliferation, migration and invasion of wilms’ tumour cell lines by regulating miR-15a. Artif Cells Nanomed Biotechnol. 2019;47(1):2670–7. [DOI] [PubMed] [Google Scholar]
- 99.Shao Q, Xu J, Deng R, et al. SNHG 6 promotes the progression of Colon and rectal adenocarcinoma via miR-101-3p and Wnt/β-catenin signaling pathway. BMC Gastroenterol. 2019;19(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pei YF, He Y, Hu LZ, et al. The crosstalk between lncRNA-SNHG7/miRNA-181/cbx7 modulates malignant character in lung adenocarcinoma. Am J Pathol. 2020;190(6):1343–54. [DOI] [PubMed] [Google Scholar]
- 101.Yao X, Liu C, Liu C, et al. LncRNA SNHG7 sponges miR-425 to promote proliferation, migration, and invasion of hepatic carcinoma cells via Wnt/β-catenin/EMT signalling pathway. Cell Biochem Funct. 2019;37(7):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ren J, Yang Y, Xue J, et al. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via Inhibition of miR-5095. Biochem Biophys Res Commun. 2018;496(2):712–8. [DOI] [PubMed] [Google Scholar]
- 103.Liu S, Yang N, Wang L, et al. LncRNA SNHG11 promotes lung cancer cell proliferation and migration via activation of Wnt/β-catenin signaling pathway. J Cell Physiol. 2020;235(10):7541–53. [DOI] [PubMed] [Google Scholar]
- 104.Wan L, Gu D, Li P. LncRNA SNHG16 promotes proliferation and migration in laryngeal squamous cell carcinoma via the miR-140-5p/NFAT5/Wnt/β-catenin pathway axis. Pathol Res Pract. 2022;229:153727. [DOI] [PubMed] [Google Scholar]
- 105.Qiao C, Qiao T, Yang S, et al. SNHG17/miR-384/ELF1 axis promotes cell growth by transcriptional regulation of CTNNB1 to activate Wnt/β-catenin pathway in oral squamous cell carcinoma. Cancer Gene Ther. 2022;29(1):122–32. [DOI] [PubMed] [Google Scholar]
- 106.Cui X, Zhang H, Chen T, et al. Long noncoding RNA SNHG22 induces cell migration, invasion, and angiogenesis of gastric Cancer cells via microRNA-361-3p/HMGA1/Wnt/β-Catenin Axis. Cancer Manag Res. 2020;12:12867–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu J, Li Y, Lv X. IL4I1 enhances PD-L1 expression through JAK/STAT signaling pathway in lung adenocarcinoma. Immunogenetics. 2023;75(1):17–25. [DOI] [PubMed] [Google Scholar]
- 108.Sun H, Li S, Xu Z, et al. SNHG15 is a negative regulator of inflammation by mediating TRAF2 ubiquitination in stroke-induced immunosuppression. J Neuroinflammation. 2022;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X, Zhang H, Fan Y, et al. SNORA28 promotes proliferation and radioresistance in colorectal Cancer cells through the STAT3 pathway by increasing H3K9 acetylation in the LIFR promoter. Adv Sci Weinh Baden-Wurtt Ger. 2024;11(32):e2405332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang C, Jiang F, Su C, et al. Upregulation of long noncoding RNA SNHG20 promotes cell growth and metastasis in esophageal squamous cell carcinoma via modulating ATM-JAK-PD-L1 pathway. J Cell Biochem. 2019;120(7):11642–50. [DOI] [PubMed] [Google Scholar]
- 111.Rah B, Rather RA, Bhat GR, et al. JAK/STAT signaling: molecular targets, therapeutic opportunities, and limitations of targeted inhibitions in solid malignancies. Front Pharmacol. 2022;13:821344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuracha MR, Radhakrishna U, Kuracha SV, et al. New horizons in Cancer progression and metastasis: Hippo signaling pathway. Biomedicines. 2024;12(11):2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang M, Xu T, Feng W, et al. Advances in Understanding the LncRNA-Mediated regulation of the Hippo pathway in Cancer. OncoTargets Ther. 2021;14:2397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang R, Zhan Y, Lang Z, et al. LncRNA-SNHG5 mediates activation of hepatic stellate cells by regulating NF2 and Hippo pathway. Commun Biol. 2024;7(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li RH, Tian T, Ge QW, et al. A phosphatidic acid-binding LncRNA SNHG9 facilitates LATS1 liquid-liquid phase separation to promote oncogenic YAP signaling. Cell Res. 2021;31(10):1088–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng F, Wang L, Yi S, et al. Long non-coding RNA SNHG1/microRNA-195-5p/Yes-associated protein axis affects the proliferation and metastasis of gastric cancer via the Hippo signaling pathway. Funct Integr Genomics. 2022;22(5):1043–55. [DOI] [PubMed] [Google Scholar]
- 117.Gu Y, Yi Z, Zhou Z, et al. SNORD88B-mediated WRN nucleolar trafficking drives self-renewal in liver cancer initiating cells and hepatocarcinogenesis. Nat Commun. 2024;15(1):6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chabronova A, Holmes TL, Hoang DM, et al. SnoRNAs in cardiovascular development, function, and disease. Trends Mol Med. 2024;30(6):562–78. [DOI] [PubMed] [Google Scholar]
- 119.Jagielski NP, Rai AK, Rajan KS, et al. A contemporary review of snornas in cardiovascular health: RNA modification and beyond. Mol Ther - Nucleic Acids. 2024;35(1):102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng Y, Wang S, Zhang H, et al. A non-canonical role for a small nucleolar RNA in ribosome biogenesis and senescence. Cell. 2024;187(17):4770–e478923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu B, Wu T, Miao BA et al. snorna-facilitated protein secretion revealed by transcriptome-wide snoRNA target identification. Cell, 2024;S0092-8674(24)01269-8. [DOI] [PMC free article] [PubMed]
- 122.Burnett LC, Hubner G, LeDuc CA et al. Loss of the imprinted, non-coding Snord116 gene cluster in the interval deleted in the Prader Willi syndrome results in murine neuronal and endocrine pancreatic developmental phenotypes. Hum Mol Genet, 2017;26(23):4606~4616. [DOI] [PMC free article] [PubMed]
- 123.Zhang L, Ma R, Gao M, et al. SNORA72 activates the Notch1/c-Myc pathway to promote stemness transformation of ovarian Cancer cells. Front Cell Dev Biol. 2020;8:583087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long Y, Wang L, Li Z. SP1-induced SNHG14 aggravates hypertrophic response in in vitro model of cardiac hypertrophy via up-regulation of PCDH17. J Cell Mol Med. 2020;24(13):7115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan SM, Li H, Shu Q, et al. LncRNA SNHG1 exerts a protective role in cardiomyocytes hypertrophy via targeting miR-15a-5p/HMGA1 axis. Cell Biol Int. 2020;44(4):1009–19. [DOI] [PubMed] [Google Scholar]
- 126.Jing J, Li L, Wang S, et al. Long non-coding RNA small nucleolar RNA host gene 7 facilitates cardiac hypertrophy via stabilization of SDA1 domain containing 1 mRNA. J Cell Biochem. 2019;120(9):15089–97. [DOI] [PubMed] [Google Scholar]
- 127.Tallo CA, Duncan LH, Yamamoto AH, et al. Heat shock proteins and small nucleolar RNAs are dysregulated in a Drosophila model for feline hypertrophic cardiomyopathy. G3 Bethesda Md. 2021;11(1):jkaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Witte F, Ruiz-Orera J, Mattioli CC, et al. A trans locus causes a ribosomopathy in hypertrophic hearts that affects mRNA translation in a protein length-dependent fashion. Genome Biol. 2021;22(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.James V, Nizamudeen ZA, Lea D, et al. Transcriptomic analysis of cardiomyocyte extracellular vesicles in hypertrophic cardiomyopathy reveals differential snorna cargo. Stem Cells Dev 2021. [DOI] [PMC free article] [PubMed]
- 130.Zhang F, Shi H, Xue H, et al. Up-regulated LncRNA SNHG9 mediates the pathogenesis of dilated cardiomyopathy via miR-326/EPHB3 axis. J Thromb Thrombolysis. 2023;55(4):634–48. [DOI] [PubMed] [Google Scholar]
- 131.Schena GJ, Murray EK, Hildebrand AN et al. Cortical bone stem cell-derived exosomes’ therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling. Am J Physiol Heart Circ Physiol, 2021;321(6):H1014~H1029. [DOI] [PMC free article] [PubMed]
- 132.Li M, Zheng H, Han Y, et al. LncRNA Snhg1-driven self-reinforcing regulatory network promoted cardiac regeneration and repair after myocardial infarction. Theranostics. 2021;11(19):9397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S, Cheng Z, Chen X et al. Long Noncoding RNA SNHG4 Attenuates the Injury of Myocardial Infarction via Regulating miR-148b-3p/DUSP1 Axis. Cardiovasc Ther, 2022;2022:1652315. [DOI] [PMC free article] [PubMed]
- 134.Ghafouri-Fard S, Harsij A, Hussen BM, et al. A review on the role of SNHG8 in human disorders. Pathol Res Pract. 2023;245:154458. [DOI] [PubMed] [Google Scholar]
- 135.Hu Y, Wang X, Ding F, et al. Periostin renders cardiomyocytes vulnerable to acute myocardial infarction via pro-apoptosis. ESC Heart Fail. 2022;9(2):977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Håkansson KEJ, Goossens EAC, Trompet S, et al. Genetic associations and regulation of expression indicate an independent role for 14q32 snornas in human cardiovascular disease. Cardiovasc Res. 2019;115(10):1519–32. [DOI] [PubMed] [Google Scholar]
- 137.Zhan J, Yin Q, Zhao P, et al. Role and mechanism of the LncRNA SNHG1/miR–450b–5p/IGF1 axis in the regulation of myocardial ischemia reperfusion injury. Mol Med Rep. 2022;25(5):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang S, Ren K, Li B et al. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol Cell Biochem, 2020;465(1~2):1~11. [DOI] [PubMed]
- 139.Bai X, Zhang J, Yang H, et al. SNHG3/miR-330-5p/HSD11B1 alleviates myocardial Ischemia-reperfusion injury by regulating the ERK/p38 signaling pathway. Protein Pept Lett. 2023;30(8):699–708. [DOI] [PubMed] [Google Scholar]
- 140.Abd El-Aziz A, El-Desouky MA, Shafei A, et al. Influence of Pentoxifylline on gene expression of PAG1/ miR-1206/ SNHG14 in ischemic heart disease. Biochem Biophys Rep. 2021;25:100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du H, Ding L, Zeng T, et al. LncRNA SNHG15 modulates Ischemia-Reperfusion injury in human AC16 cardiomyocytes depending on the regulation of the miR-335-3p/TLR4/NF-κB pathway. Int Heart J. 2022;63(3):578–90. [DOI] [PubMed] [Google Scholar]
- 142.Lázaro DF, Bellucci A, Brundin P, et al. Editorial: protein misfolding and spreading pathology in neurodegenerative diseases. Front Mol Neurosci. 2019;12:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sengupta S. Noncoding RNAs in protein clearance pathways: implications in neurodegenerative diseases. J Genet. 2017;96(1):203–10. [DOI] [PubMed] [Google Scholar]
- 144.149, Moreno-García L, López-Royo T, Calvo AC, et al. Competing endogenous RNA networks as biomarkers in neurodegenerative diseases. Int J Mol Sci. 2020;21(24):9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laufer BI, Mantha K, Kleiber ML, et al. Long-lasting alterations to DNA methylation and NcRNAs could underlie the effects of fetal alcohol exposure in mice. Dis Model Mech. 2013;6(4):977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCann KL, Kavari SL, Burkholder AB, et al. H/ACA snorna levels are regulated during stem cell differentiation. Nucleic Acids Res. 2020;48(15):8686–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bouchard-Bourelle P, Desjardins-Henri C, Mathurin-St-Pierre D, et al. SnoDB: an interactive database of human snorna sequences, abundance and interactions. Nucleic Acids Res. 2020;48D1:D220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lester E, Ooi FK, Bakkar N, et al. Tau aggregates are RNA-protein assemblies that mislocalize multiple nuclear speckle components. Neuron. 2021;109(10):1675–e16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abed S, Ebrahimi A, Fattahi F, et al. The role of Non-Coding RNAs in mitochondrial dysfunction of alzheimer’s disease. J Mol Neurosci MN. 2024;74(4):100. [DOI] [PubMed] [Google Scholar]
- 150.Lio CT, Kacprowski T, Klaedtke M, et al. Small RNA sequencing in the Tg4-42 mouse model suggests the involvement of snornas in the etiology of alzheimer’s disease. J Alzheimers Dis JAD. 2022;87(4):1671–81. [DOI] [PubMed] [Google Scholar]
- 151.Wang H, Lu B, Chen J. Knockdown of LncRNA SNHG1 attenuated Aβ25-35-inudced neuronal injury via regulating KREMEN1 by acting as a CeRNA of miR-137 in neuronal cells. Biochem Biophys Res Commun. 2019;518(3):438–44. [DOI] [PubMed] [Google Scholar]
- 152.Chen H, Zhang CJ, Zhao ZY, et al. Mechanisms underlying LncRNA SNHG1 regulation of alzheimer’s disease involve DNA methylation. J Toxicol Environ Health A. 2024;87(10):428–35. [DOI] [PubMed] [Google Scholar]
- 153.Gao Y, Zhang N, Lv C, et al. LncRNA SNHG1 knockdown alleviates Amyloid-β-Induced neuronal injury by regulating ZNF217 via sponging miR-361-3p in alzheimer’s disease. J Alzheimers Dis JAD. 2020;77(1):85–98. [DOI] [PubMed] [Google Scholar]
- 154.Romano S, Romano C, Peconi M, et al. Circulating U13 small nucleolar RNA as a potential biomarker in huntington’s disease: A pilot study. Int J Mol Sci. 2022;23(20):12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fitz NF, Wang J, Kamboh MI, et al. Small nucleolar RNAs in plasma extracellular vesicles and their discriminatory power as diagnostic biomarkers of alzheimer’s disease. Neurobiol Dis. 2021;159:105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thangavelu L, Moglad E, Afzal M, et al. Non-coding RNAs in parkinson’s disease: regulating SNCA and alpha-synuclein aggregation. Pathol Res Pract. 2024;261:155511. [DOI] [PubMed] [Google Scholar]
- 157.Hussain MS, Moglad E, Afzal M, et al. Autophagy-associated non-coding rnas: unraveling their impact on parkinson’s disease pathogenesis. CNS Neurosci Ther. 2024;30(5):e14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cao B, Wang T, Qu Q, et al. Long noncoding RNA SNHG1 promotes neuroinflammation in parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience. 2018;388:118–27. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y, Lian YJ, Ma YQ, et al. LncRNA SNHG1 promotes α-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology. 2018;68:212–21. [DOI] [PubMed] [Google Scholar]
- 160.Xie N, Qi J, Li S, et al. Upregulated LncRNA small nucleolar RNA host gene 1 promotes 1-methyl-4-phenylpyridinium ion-induced cytotoxicity and reactive oxygen species production through miR-15b-5p/GSK3β axis in human dopaminergic SH-SY5Y cells. J Cell Biochem. 2019;120(4):5790–801. [DOI] [PubMed] [Google Scholar]
- 161.Qian C, Ye Y, Mao H, et al. Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222 /p27/mTOR pathway in parkinson’s disease. Exp Cell Res. 2019;384(1):111614. [DOI] [PubMed] [Google Scholar]
- 162.Xu F, Bian N, Li X. SNHG14 elevates NFAT5 expression through sequestering miR-375-3p to promote MPP + -Induced neuronal apoptosis, inflammation, and oxidative stress in parkinson’s disease. Neurochem Res. 2024;49(5):1212–25. [DOI] [PubMed] [Google Scholar]
- 163.Yuan X, Wu Y, Lu L, et al. Long noncoding RNA SNHG14 knockdown exerts a neuroprotective role in MPP+-induced parkinson’s disease cell model through mediating miR-135b-5p/KPNA4 axis. Metab Brain Dis. 2022;37(7):2363–73. [DOI] [PubMed] [Google Scholar]
- 164.Zhou S, Zhang D, Guo J, et al. Knockdown of SNHG14 alleviates MPP+-Induced injury in the cell model of parkinson’s disease by targeting the miR-214-3p/KLF4 Axis. Front Neurosci. 2020;14:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin X, Tao T, He X, et al. LncRNA MEG8 ameliorates parkinson’s disease neuro-inflammation through miR-485-3p/FBXO45 axis. Acta Neurol Belg. 2024;124(2):549–57. [DOI] [PubMed] [Google Scholar]
- 166.N AP. C, P M, Transcriptional dysregulation in huntington’s disease: the role in pathogenesis and potency for Pharmacological targeting. Curr Med Chem, 2021;28(14). [DOI] [PubMed]
- 167.K C. S D, J C, Altered levels of long NcRNAs Meg3 and Neat1 in cell and animal models of huntington’s disease. RNA Biol, 2018;15(10). [DOI] [PMC free article] [PubMed]
- 168.Peter-Ross EM. Molecular hypotheses to explain the shared pathways and underlying Pathobiological causes in catatonia and in catatonic presentations in neuropsychiatric disorders. Med Hypotheses. 2018;113:54–64. [DOI] [PubMed] [Google Scholar]
- 169.Kishore S, Khanna A, Zhang Z, et al. The snorna MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Hum Mol Genet. 2010;19(7):1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wright C, Shin JH, Rajpurohit A, et al. Altered expression of Histamine signaling genes in autism spectrum disorder. Transl Psychiatry. 2017;7(5):e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ej M, Sj B. U8 variants on the brain: a small nucleolar RNA and human disease. RNA Biol, 2022;19(1). [DOI] [PMC free article] [PubMed]
- 172.He JY, Liu X, Qi ZH, et al. Small nucleolar RNA, C/D box 16 (SNORD16) acts as a potential prognostic biomarker in Colon cancer. Dose-Response Publ Int Hormesis Soc. 2020;18(2):1559325820917829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xie Q, Zhang D, Ye H, et al. Identification of key snornas serves as biomarkers for hepatocellular carcinoma by bioinformatics methods. Med (Baltim). 2022;101(39):e30813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu J, Mao S, Zhen N, et al. SNORA14A inhibits hepatoblastoma cell proliferation by regulating SDHB-mediated succinate metabolism. Cell Death Discov. 2023;9(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y, Shang X, Yu M, et al. A three-snoRNA signature: SNORD15A, SNORD35B and SNORD60 as novel biomarker for renal cell carcinoma. Cancer Cell Int. 2023;23(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang L, Yang M, Marks P, et al. Serum non-coding RNAs as biomarkers for osteoarthritis progression after ACL injury. Osteoarthritis Cartilage. 2012;20(12):1631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Q, Bi Z, Song X, et al. Tumor-educated platelet SNORA58, SNORA68 and SNORD93 as novel diagnostic biomarkers for esophageal cancer. Future Oncol Lond Engl. 2023;19(9):651–61. [DOI] [PubMed] [Google Scholar]
- 178.Cui C, Liu Y, Gerloff D, et al. NOP10 predicts lung cancer prognosis and its associated small nucleolar RNAs drive proliferation and migration. Oncogene. 2021;40(5):909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zakari S, Niels NK, Olagunju GV, et al. Emerging biomarkers for non-invasive diagnosis and treatment of cancer: a systematic review. Front Oncol. 2024;14:1405267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li L, Zhang Z, Xu W, et al. The diagnostic value of serum Exosomal SNORD116 and SNORA21 for NSCLC patients. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 2024. [DOI] [PubMed]
- 181.Deryusheva S, Talhouarne GJS, Gall JG. Lost and found: snorna annotation in the Xenopus genome and implications for evolutionary studies. Mol Biol Evol. 2020;37(1):149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang W, Liu B. iSnoDi-LSGT: identifying snoRNA-disease associations based on local similarity constraints and global topological constraints. RNA N Y N. 2022;28(12):1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ono M, Yamada K, Avolio F, et al. Analysis of human small nucleolar RNAs (snoRNA) and the development of snorna modulator of gene expression vectors. Mol Biol Cell. 2010;21(9):1569–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.