Abstract
We have previously demonstrated that about one-third of patients with either Sjögren’s syndrome (SS) or systemic lupus erythematosus (SLE) react to human immunodeficiency virus (HIV) p24 core protein antigen without any evidence of exposure to, or infection with, HIV itself. Herein, we further characterize the specificity of this reaction using enzyme-linked immunosorbent assay to peptides representing fragments of p24. Characteristic epitope-specific profiles were seen for SS and SLE patients. SS patients had significantly increased responses to peptides F (p24 amino acids 69 to 86) and H (amino acids 101 to 111) and diminished reactivity to peptides A (amino acids 1 to 16) and P (amino acids 214 to 228). SLE patients had increased reactivity to peptides E (amino acids 61 to 76), H, and P. Utilization of peptide P hyporeactivity as the criterion to select for SS patients results in a screen that is moderately sensitive (64%) and specific (79.3%). Adding hyperreactivity to one other peptide (F or H) as an additional criterion yields an expected decrease in sensitivity (to 41%) while increasing specificity (to 93.1%). All sera-reactive peptides from regions of known structure of HIV p24 were located in the apex of the p24 molecule. Thus, the specificity of the peptide reactivities described here indicates a specific pattern of a nonrandom cross-reactivity between HIV type 1 p24 and autoimmune sera which may be partially syndrome specific. The future focus of our work will be to optimize assays of the peptide as diagnostic tools.
Our laboratories have been interested in probing the possibility that viral agents, especially retroviruses, play either an etiologic or contributory role in autoimmune diseases. Two sets of findings have led to this hypothesis. The first is that patients with systemic autoimmune diseases show reactivity to retroviral antigens without evidence of infection (2, 6, 21, 25, 26). The second is the isolation of either novel retroviral sequences or a new human retrovirus from such patients (5, 9, 12, 13, 18). Although the evidence to date is primarily circumstantial, it is highly suggestive of a connection between retroviruses and autoimmunity. The isolation of retrovirus-like particles (13) and the identification of novel exogenous retroviral sequences (16) from salivary glands in two separate laboratories reinforce this suggestion.
Autoimmune disease occurs when the body’s immune system breaks tolerance of a self-antigen and mounts an attack against that antigen. This response can be mediated by either the cellular or the humoral arm of the immune system. A major group of systemic autoimmune diseases has been termed connective tissue diseases and comprises such important representatives as rheumatoid arthritis, systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), polymyositis, and scleroderma. Specific association of reactivity with retroviral antigens or novel retroviral elements has been described for both SLE and SS.
The connective tissue diseases are classified on the basis of clinical criteria, rather than being defined by any unique etiology, eliciting or reactive antigen, or single specific diagnostic test. SS is an autoimmune disease primarily affecting older women. While SLE is diagnosed primarily in younger women, it is often associated with other connective tissue diseases, such as SS (22, 24), with considerable overlap of clinical signs and symptoms.
We have previously found that about one-third of SLE and SS patients specifically react to core antigens of human immunodeficiency virus (HIV) without any evidence of exposure to or infection with HIV itself (25, 26). The current diagnostic method for HIV infection consists of the two-step protocol of a preliminary enzyme-linked immunosorbent assay (ELISA) screen for antibodies against the virus, followed by Western blotting analysis of any positive sera. Following infection by HIV, the first viral molecule recognized by sera on this latter test is generally a protein of approximately 24 kDa (capsid [CA]). Sera which react only with p24 are termed indeterminate and are tested again at a later time to determine whether seroconversion to reactivity with other HIV proteins has occurred (3, 4, 14, 20). Sera must recognize p24 plus a glycoprotein of 41 or 120 kDa to be considered positive for HIV, and persons whose sera remain indeterminate are not considered to be infected with HIV (7, 8). It has been established by work in a number of laboratories that persons with systemic, but not organ-specific, autoimmue diseases are often among those in this nonconverting indeterminate group (9, 19, 25–27).
Since Western blots in our laboratories have indicated that approximately one-third of the sera from SS or SLE patients reacted with p24, we wished to further characterize the specificity of this reaction with peptide analogs representing fragments of the HIV p24 sequence. The work to be reported here had three experimental aims: (i) to determine the peptide specificity of the p24 reactivity; (ii) to determine whether any of the reactive peptides correspond to regions of HIV p24 that have sequence similarity to known autoantigens such as SS-B, Sm, and 70-kDa proteins; and (iii) to determine whether the peptide reactivities can distinguish indeterminate results from among SS and SLE groups.
The present data are a comparison of the reactivities against p24 and its peptides of a panel of sera from patients with SS or SLE with those of sera from healthy donors by both Western blotting and ELISA.
MATERIALS AND METHODS
Antigens.
The antigens consisted of 17 commercially available synthetic peptides of the HIV p24 protein, with some overlap among several of the sequences, covering the entire length of p24. Of particular interest were peptides with sequence similarities to known autoantigens (11): peptides E (amino acids 61 to 76) and F (amino acids 69 to 86), similar in sequence to the 70-kDa small nuclear ribonucleoprotein particle (snRNP) antigen; peptide G (amino acids 87 to 101), similar to Sm; peptides K (amino acids 135 to 155) and L (amino acids 153 to 172), similar to the SS-B autoantigen; and peptide P (amino acids 214 to 228), similar to SSB-La. The peptides, their designations in this work, and their sequences are shown in Table 1.
TABLE 1.
p24 peptides
| Amino acid number | Designation | Sequence |
|---|---|---|
| 1–16 | A | PIVQNIQGQMVHQAIS |
| 16–30 | B | SPRTLNAWKKVVEEK |
| 36–52 | C | VIPMFSALSEGATPQDL |
| 44–60 | D | SEGATPQDLNTMLNTVG |
| 61–76 | E | GHQAAMQMLKETINEE |
| 69–86 | F | LKETINEEAAEWDRVHPV |
| 87–101 | G | HAGPIAPGQMREPRG |
| 101–111 | H | GSDIAGTTSTL |
| 113–129 | I | EQIGWMTNNPPIPVGEI |
| 124–138 | J | IPVGEIYKRWIILGL |
| 138–155 | K | LNKIVRMYSPTSILDIRQ |
| 153–172 | L | IRQGPKEPFRDYVDRFYKTL |
| 170–188 | M | KTLRAEQASQEVKNWMTE |
| 187–205 | N | ETLLVQNANPDCKTILKAL |
| 202–219 | O | KALGPAATLEEMMTACQ |
| 214–228 | P | MMTACQGVGGPGHKA |
| 225–240 | Q | GHKARVLAEAMSQVTN |
Sera.
The panel of sera comprised samples from 38 healthy donors, 39 patients with clinically diagnosed SS, and 20 patients with SLE. Blood was collected from volunteers, allowed to clot, and centrifuged at 320 × g to obtain serum, and the serum was divided into 1-ml portions and stored at −20°C until used.
Western blots.
HIV Western blot strips (Cambridge-Biotech, Inc.) were produced by separating proteins in the HIV preparations by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferring the proteins to a nitrocellulose membrane. Strips of the nitrocellulose were exposed to sera from patients diagnosed with either SS or SLE, with known HIV-positive and -negative sera as controls. Specifically bound immunoglobulin G was visualized by standard Western blotting procedures, according to the manufacturer’s instructions. Reactivity was scored blindly by two independent observers as 4+, 3+, 2+, 1+, or 0.
ELISA.
Because of the background problems that can arise with the use of short peptides as antigens, a grid determined that a 1/1,000 dilution of both peptides (final concentration, 1 μg/ml) and sera was optimal for all ELISAs performed. Each assay was done in duplicate, and experiments were repeated at least three times. Controls consisted of the sera from healthy donors. After plates were coated, and after successive incubations with antibodies, peroxidase-labeled goat anti-human antibody (Sigma Chemical Co., St. Louis, Mo.), and substrate (ortho-phenylenediamine dihydrochloride tablets, Sigma), they were read for optical density at 490 nm.
Statistics.
ELISA absorbance data were transformed into Z scores (standard deviations from the mean of controls) based on the mean and standard deviation of the reference standard control sera included with every serum batch assayed. For each peptide studied, frequency distributions of the data from SS and SLE sera were compared with those from control sera for significant differences (P < 0.05) by the nonparametric Kolmogorov-Smirnov test. Within the control and significantly different experimental groups, individual sample values not contained within the 95% confidence limits for the median control value (23) were considered significantly different. The distribution of values between control and experimental groups was then evaluated for significance (P < 0.05) by chi-square analysis. Specificity and sensitivity were evaluated by the methods described by Anderson and Cockayne (1).
RESULTS
Western blots.
To provide a comparison with previous surveys, panels of patient sera were first reacted with commercially available Western blot strips for HIV-1. Blot data were scored as negative, plus-minus, and 1+ to 4+ on a visual basis by two independent observers. Those which were plus-minus or negative were considered to be negative. Representative samples are shown in Fig. 1. Nearly all significant reactivities were restricted to p24. Of the 38 control sera, 6 (16%) were positive (≥1+); of the 39 SS sera, 10 (27%) were positive; and of the 20 SLE sera, 11 (55%) were positive. The strongest reactions were most common in the SLE samples. Relative to previous surveys of such sera, the control group showed more frequent reactivity, perhaps reflecting an older median age than that of previous control populations. The SS group was similar in reactivity to that of earlier observations, while the SLE group was more frequently reactive. Thus, SLE was most reactive, SS was next most reactive, and controls were least reactive.
FIG. 1.
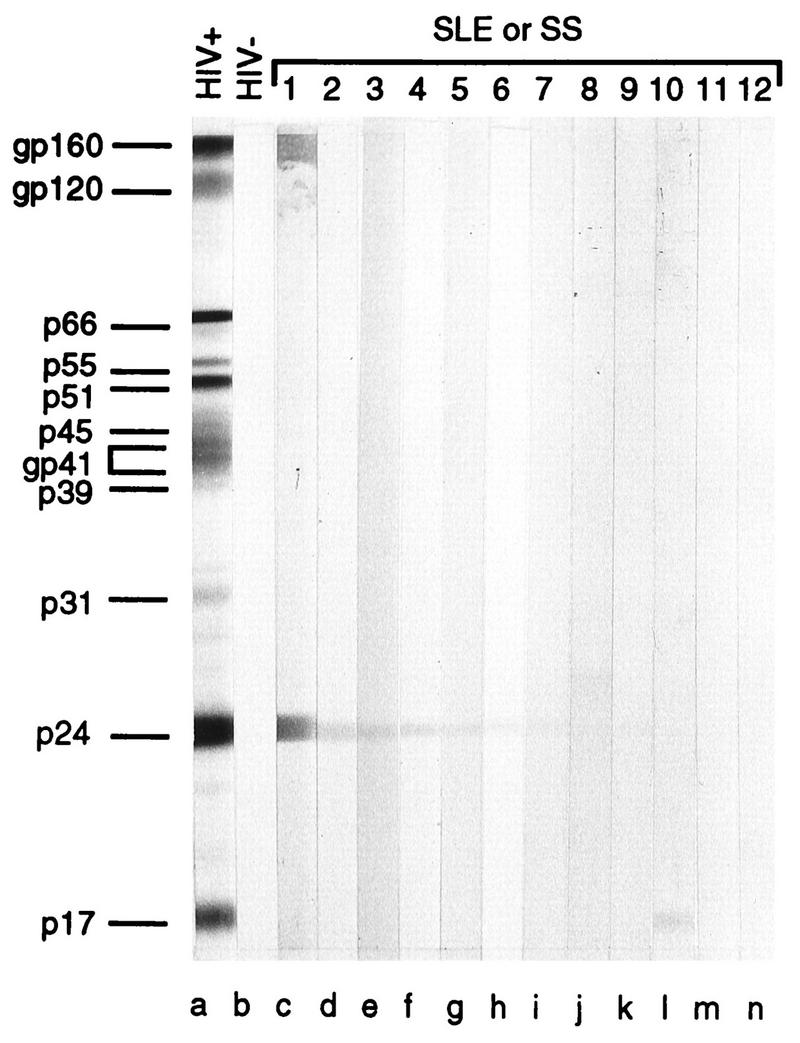
Immunoblot analysis of the reactions of antibodies in sera from persons with systemic autoimmune disease and control sera with HIV proteins on nitrocellulose strips. The sera were from a patient infected with HIV-1 (lane a), an HIV-seronegative blood donor (lane b), or patients with SLE syndrome (lanes c, d, g, i, l, and n) or Sjögren’s syndrome (lanes e, f, h, j, k, and m).
ELISA and statistics.
Figure 2 illustrates the reactivities of SS and SLE sera against each peptide, in relation to the 95% confidence level of control sera. By Kolmogorov-Smirnov analysis (two-sample test), sera from SS patients demonstrated a reactivity significantly different from that of controls (P < 0.05). Compared with controls, these sera had significantly increased reactivity to peptides F and H and significantly diminished reactivity to peptides A and P. Sera from SLE patients exhibited significantly increased reactivity to peptides E, H, and P compared with that of controls. Thus, SS and SLE patients express characteristic epitope specificity profiles for p24 peptides, the most striking finding being their contrasting responses to peptide P. SS, but not SLE, sera also showed increased reactivity to peptide F, whereas SLE, but not SS, sera showed increased reactivity to peptide E. These data are summarized in Table 2. The significant trends seen in patient groups suggested that there might be a specific set of peptide ELISA reactivities that could be exclusive for SS or SLE. No sera from SLE patients had diminished reactivity to peptide P, but incidence of peptide P hyporeactivity was 64% (25 of 39) and 32% (12 of 38) in SS and control groups, respectively. When analyzed on an individual basis, 72% of peptide P-hyporeactive SS sera were hyperreactive with at least one other peptide from the A, E, F, and H set, including 48% (12 of 25) specifically hyperreactive with peptide H and 48% (12 of 25) hyperreactive with peptide F. In control hyporeactors, 33% (4 of 12) of individual sera were also reactive to at least one other peptide. Thus, overall prevalence of peptide P hyporeactivity with associated hyperreactivity to at least one other peptide was 46% (18 of 39) in SS patients, 10.5% (4 of 38) in controls, and 0% in SLE patients.
FIG. 2.
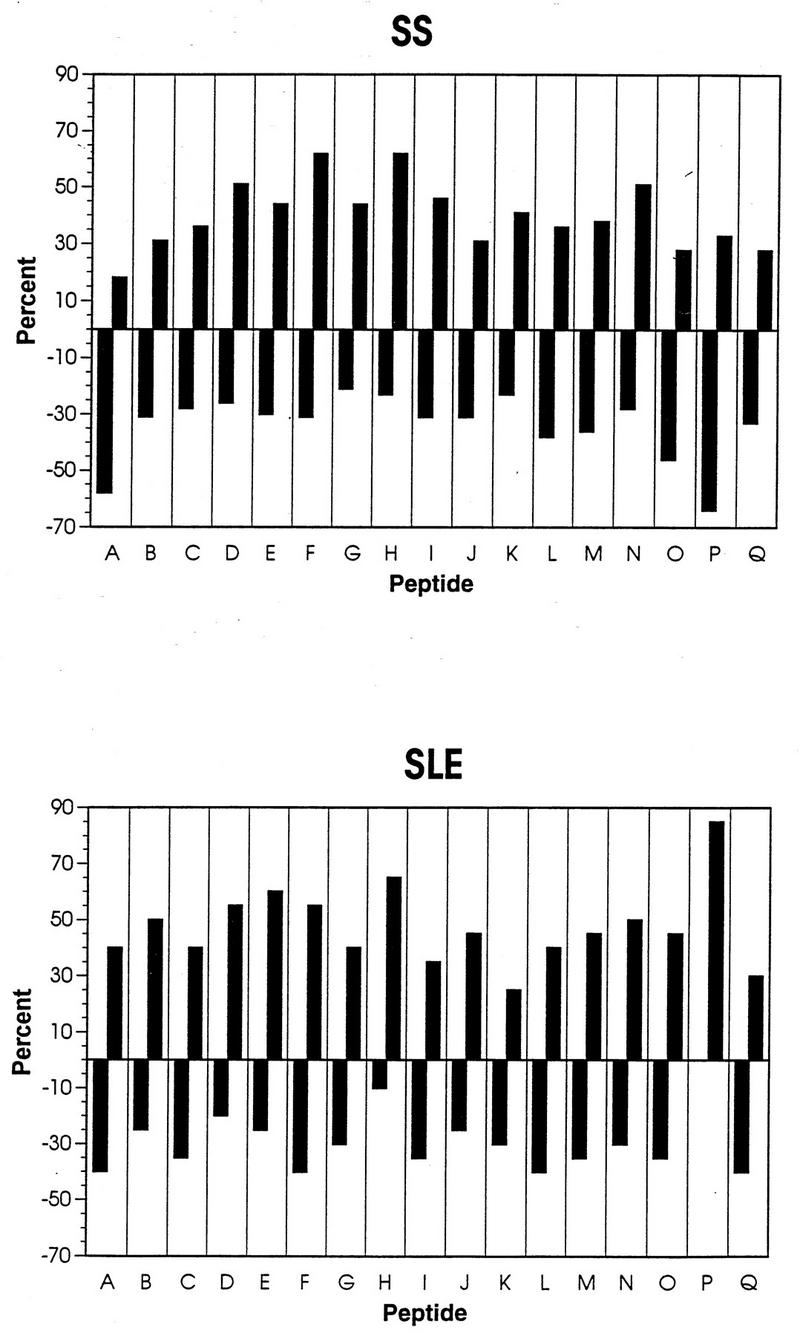
Percentage of sera from SS and SLE patients with reactions significantly different from those of control sera. Data for all 17 peptides are shown. Negative values are diminished and positive values are increased compared with controls.
TABLE 2.
Significantly increased (Up) or diminished (Down) reactivity of SS or SLE sera with p24 peptides
P < 0.05 (Kolmogorov-Smirnov).
P < 0.05 (chi-square).
In terms of sensitivity and specificity of the assays (1), utilizing peptide P hyporeactivity as the criterion to select for SS patients results in a screen that is moderately sensitive (64%) and specific (79.3%). Use of hyperreactivity to either peptide F or peptide H as a selective criterion yields a relatively low sensitivity of 30.8% for either peptide, with a specificity of 94.7% (F) or 89.5% (H). By combining diminished reactivity against peptide P with increased reactivity to peptide F and/or peptide H, sensitivity is increased to 41% and specificity remains high at 93.1%.
Correlation between ELISA and Western blotting.
All individual serum samples positive by Western blotting were reactive to one or more of the peptides tested by ELISA, but more sera were positive by ELISA than by Western blotting. Even if increased reactivity to the A, E, F, H, and P subset of peptides alone was evaluated, dual reactivity was present for 80, 88, and 100% of blot-positive control, SS, and SLE patients, respectively. These differences were not statistically significant by chi-square analysis (due to the low incidence of positive blots in controls). Thus, blot positivity is typically associated with ELISA reactivity to one or more p24-derived peptides.
These data correlate well with reactivity of this patient cohort with known autoantigens (21a). Of the sera presented here, 24 of 39 SS sera and 16 of the 20 SLE sera were also tested against autoantigens SSA, SSB, RNP, SM, and PO by ELISA or Western blotting (data not shown). Of the SS sera, 87.5% were positive for at least one of the autoantigens whereas 62.5% of the SLE sera were positive. Of the autoantigen-positive SS sera, 85.7% were negative for peptide P and 61.9% were negative for peptide A, the two peptides against which the SS sera showed significantly diminished reactivity in our statistical analysis. On the other hand, all autoantigen-positive SLE sera showed increased reactivity against peptide P. The SS sera again correlated well with the autoantigen data when reactions against peptides F (61.9% positive) and H (62.5% positive) are considered.
DISCUSSION
Previous Western blot data showed that about one-third of SS and SLE sera are positive against HIV-1 p24 (25, 26). That the patient sera never convert to “HIV-positive” by Western blotting suggests a relatedness but not an identical structure. Recent studies have revealed (i) a novel sequence related to type-B and type-D particles, but unrelated to human genomic DNA (16); and (ii) that particles isolated from salivary glands of SS patients are distinguishable from HIV-1 by hydrodynamic mobility and biochemical variances (17). We analyzed an independently derived bank of control, SS, and SLE sera against the HIV p24 protein and 17 peptides derived from it. Our Western blot analysis varied somewhat from the earlier work. Our samples showed that a comparable percentage of the SS sera were positive against p24 but that control and SLE sera had more positives than was previously observed. A possible reason for the difference from earlier serum panels was that the control population in this study was older (mean age, 53 years) to better match the age spectrum of the SS and SLE patients.
By ELISA, patient reactivities fell into syndrome-specific patterns to a large degree. When the pooled data for the commonly reacting peptides were examined, the differences between control and patient sera became statistically significant for peptides A, E, F, H, and P. One particularly unexpected finding was significant hyporeactivity to peptides P and A in SS patients (Table 2). The hyporeactivity seen with peptide P, coupled with hyperreactivities to peptides F and H, was a syndrome-specific (93.1%), if only moderately sensitive (41%), differential peptide result.
There was good general correlation between these ELISA results and reactivity to known autoantigens SSA, SSB, RNP, SM, and PO, a study to be reported in detail elsewhere (21a). When we correlated the pattern of peptide reactivity with previously published sequence similarities of p24 known autoantigens, we found that the two peptides that correspond to the 70-kDa snRNP spliceosome protein (11) reacted significantly with either SS (peptide F) or SLE (peptide E). However, only two of the seven RNP-reactive SS sera also reacted with either F or E, while four of six SLE RNP-reactive sera did so. Although the numbers are too small to draw definitive conclusions, they suggest that the reactivity of the SLE sera, but not that of the SS sera, may be related to the RNP molecule. Peptides G, K, and L, similar to Sm and SS-B (19), were not significantly reactive with SS or SLE sera. However, peptide P, with similarity to SSB-La (11), had significantly higher reactivity with SLE sera but significantly diminished reactivity with SS sera. When compared with the indeterminate HIV Western blots, all blot-positive sera were ELISA positive for at least one p24 peptide as well. The peptide-specific ELISA, however, more specifically delineates which of the peptides of the p24 molecule serve as reactive sites in SS patients.
The reactive peptides also correlate well with our current understanding of the structure of retroviral capsid proteins, typified by p24. A recent study by Gitti et al. (15) describes the structure of the amino-terminal core domain of the HIV-1 capsid protein. When our data are correlated with these nuclear magnetic resonance (NMR) data, several points of interest can be seen. Amino acids 1 to 13, which constitute almost all of peptide A (amino acids 1 to 16), form a beta hairpin with a three-residue (6 to 8) turn. The SS sera exhibited significantly low reactivity against this peptide. Peptide E, which reacts significantly with sera from SLE patients, corresponds to amino acids 61 to 76. Peptide F, reactive with sera from SS patients, corresponds to the overlapping amino acids 69 to 86. Both of these peptides are in the area designated Helix VI by the Gitti group (15). Peptide H, with which both SS and SLE sera reacted significantly, encompasses amino acids 101 to 111. On the Gitti et al. (15) NMR structure, amino acids 100 to 105 form Helix V, amino acids 106 to 109 form a turn linking Helices V and VI, and amino acids 110 to 119 form Helix VI. Therefore, this peptide encompasses one helix and part of another, in addition to one turn. Peptide P is beyond the Gitti et al. NMR structure but has been modeled by us (10) as in the area of a helix-turn-helix, which would reside at peptides 205 to 240. Therefore, all of the peptides with which the SS and SLE sera had significant reactions by the ELISA are at least part of one of these helical areas, whereas hyporeactivity with peptide A was associated with a partially disordered beta hairpin turn. It should be noted that all of these areas of the Gitti et al. structure with which our sera reacted, whether hypo- or hyperreactively, were restricted in location to the apex of the protein. Whether this region is selectively exposed in intact cross-reactive protein and thereby drives B-lymphocyte responses remains to be demonstrated.
In conclusion, we have found that a large percentage of 59 SS and SLE sera react significantly and specifically with only a few peptides derived from the sequence of HIV p24. While SS and SLE sera are distinguishable as groups, there is variance of peptide reactivity within each group. Because of this variance, sensitivity of detection is 64% for peptide P alone and 41% for peptide H; specificity, however, is raised to 93% for analyses combining P, H, and F. Therefore, by combining the reactivity profile of a serum which is significantly diminished against P and significantly increased against F and/or H, a highly specific analysis of SS results.
Our results with HIV ELISAs, as compared with immunoblotting, suggest that they are more sensitive and reveal differences not distinguished in blots. Peptide ELISAs are performed at relatively high molar levels, and as a consequence, antibody detection may be facilitated compared with detection by immunoblotting performed with whole proteins. Future work will focus specifically on optimizing ELISAs for peptides F, H, and P as possible diagnostic tools. Affinity and isotype of antibodies will be part of the ongoing studies.
ACKNOWLEDGMENT
This work was supported by grant number 5RO1 DE10862-03 from the National Institute of Dental Research.
REFERENCES
- 1.Anderson S C, Cockayne S. Clinical chemistry: concepts and applications. Philadelphia, Pa: W. B. Saunders Company; 1993. pp. 66–69. [Google Scholar]
- 2.Blomberg J, Nived O, Pipkorn R, Bengtsson A, Erlinge D, Sturfelt G. Increased antiretroviral antibody reactivity in sera from a defined population of patients with systemic lupus erythematosus. Correlation with autoantibodies and clinical manifestations. Arthritis Rheum. 1994;37:57–66. doi: 10.1002/art.1780370109. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg J, Vincic E, Jonsson C, Medstrand P, Pipkorn R. Identification of regions of HIV-1 p24 reactive with sera which give “indeterminate” results in electrophoretic immunoblots with the help of long synthetic peptides. AIDS Res Hum Retroviruses. 1990;6:1363–1372. doi: 10.1089/aid.1990.6.1363. [DOI] [PubMed] [Google Scholar]
- 4.Brookes S M, Pandolfino Y A, Mitchell T, Venables P, Shattles W, Clark D, Entwistle A, Maini R. The immune response to and expression of cross-reactive Gag sequences in autoimmune disease. Br J Rheumatol. 1992;31:735–742. doi: 10.1093/rheumatology/31.11.735. [DOI] [PubMed] [Google Scholar]
- 5.Brookes S M, Boyd M, Stephens R, Whitby D, Shattles W G, Veneables P J W, Schultz T, Maini R N. A novel retroviral sequence in Sjogren’s syndrome salivary gland biopsy co-cultures. Arthritis Rheum. 1992;35:s64. [Google Scholar]
- 6.Dang H, Dauphinee M J, Talal N, Garry R F, Seibold J R, Medsger T, Jr, Alexander S, Feghali C A. Serum antibody to retroviral gag proteins in systemic sclerosis. Arthritis Rheum. 1991;34:1336–1337. doi: 10.1002/art.1780341022. [DOI] [PubMed] [Google Scholar]
- 7.Douvas A, Takehana Y. Cross-reactivity between autoimmune anti-U1 snRNP antibodies and neutralizing epitopes on HIV-1 gp120/41. AIDS Res Hum Retroviruses. 1994;10:253–262. doi: 10.1089/aid.1994.10.253. [DOI] [PubMed] [Google Scholar]
- 8.Drabnick J J, Horning V L, Lennox J L, Coyne P E, Oster C N, Knight R D, Dillard T A, Fuller J J, Damato J J, Burke D S. A retrospective analysis of diseases associated with indeterminate HIV Western blot patterns. Mil Med. 1991;156:93–96. [PubMed] [Google Scholar]
- 9.Fraziano M, Montesano C, Lombardi V R, Sammarco I, De Pisa F, Mattei M, Valesini G, Pittoni V, Colizzi V. Epitope specificity of anti-HIV antibodies in human and murine autoimmune diseases. AIDS Res Hum Retroviruses. 1996;10:491–496. doi: 10.1089/aid.1996.12.491. [DOI] [PubMed] [Google Scholar]
- 10.Gallaher W R, Deas J E, Luo-Zhang H, Sander D, Garry R F. Reactivity of serum antibodies from Sjogren’s syndrome and lupus erythematosus patients with HIV capsid peptides. Arthritis Rheum. 1995;38:5378. [Google Scholar]
- 11.Garry R F. Extensive antigenic mimicry by retrovirus capsid proteins. AIDS Res Hum Retroviruses. 1990;6:1361–1362. doi: 10.1089/aid.1990.6.1361. [DOI] [PubMed] [Google Scholar]
- 12.Garry R F. New evidence for involvement of retroviruses in Sjogren’s syndrome and other autoimmune diseases. Arthritis Rheum. 1994;37:465–469. doi: 10.1002/art.1780370405. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 13.Garry R F, Fermin C D, Hart D J, Alexander S S, Donehower L A, Luo-Zhang H. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science. 1990;250:1127–1129. doi: 10.1126/science.1701273. [DOI] [PubMed] [Google Scholar]
- 14.Genesca J, Jett B W, Epstein J S, Shih J W-K, Hewlett I K, Alter H J. What do Western blot indeterminate patterns for human immunodeficiency virus mean in EIA-negative donors? Lancet. 1989;ii:1023–1025. doi: 10.1016/s0140-6736(89)91027-1. [DOI] [PubMed] [Google Scholar]
- 15.Gitti R K, Lee B M, Walker J, Summers M F, Yoo S, Sundquist W I. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996;273:231–235. doi: 10.1126/science.273.5272.231. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D J, Venables P J W, Weiss R, Boyd M T. A novel retrovirus sequence identified in humans. J Virol. 1997;71:2866–2872. doi: 10.1128/jvi.71.4.2866-2872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart D J, Luo H, Garry R F. Biochemical characterization of the reverse transcriptase of a human intracisternal A-type particle (HIAP) AIDS Res Hum Retroviruses. 1996;12:1367–1372. doi: 10.1089/aid.1996.12.1367. [DOI] [PubMed] [Google Scholar]
- 18.Kalden J R, Gay S. Retroviruses and autoimmune rheumatic diseases. Rev Clin Exp Immunol. 1994;98:1–5. doi: 10.1111/j.1365-2249.1994.tb06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meilof J F, Arentsen H, Kruize A A, Hene R J, Bylsma J W J, Van der Poel C L, Smeek R T J, Huisman J G. Sjogren’s syndrome and retroviral infection. Arthritis Rheum. 1992;35:1403–1404. doi: 10.1002/art.1780351133. [DOI] [PubMed] [Google Scholar]
- 20.Midthun K, Garrison L, Clements M L, Farzadegan H, Fernie B, Qukinn T. Frequency of indeterminate Western blot tests in healthy adults at low risk for human immunodeficiency virus infection. J Infect Dis. 1990;162:1379–1382. doi: 10.1093/infdis/162.6.1379. [DOI] [PubMed] [Google Scholar]
- 21.Ranki A, Kurki P, Riepponen S, Stephansson E. Antibodies to retroviral proteins in autoimmune connective tissue disease. Relation to clinical manifestations and ribonucleoprotein autoantibodies. Arthritis Rheum. 1992;35:1483–1491. doi: 10.1002/art.1780351212. [DOI] [PubMed] [Google Scholar]
- 21a.Sander, D. M. Unpublished data.
- 22.Sell S. Autoimmune diseases II—diffuse connective tissue (collagen-vascular, rheumatoid) diseases. In: Sell S, Berkower I, Max E E, editors. Immunology, immunopathology & immunity. Stamford, Conn: Appleton & Lange; 1996. pp. 551–587. [Google Scholar]
- 23.Siegel A F, Morgan C J. Statistics and data analysis. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 341–343. [Google Scholar]
- 24.Talal N. Sjogren’s syndrome and connective tissue disease with other immunologic disorders. In: Hollander J L, editor. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1972. pp. 849–865. [Google Scholar]
- 25.Talal N, Dauphinee M J, Dang H, Alexander S S, Hart D J, Garry R F. Detection of serum antibodies to retroviral proteins in patients with primary Sjogren’s syndrome (autoimmune exocrinopathy) Arthritis Rheum. 1990;33:774–781. doi: 10.1002/art.1780330603. [DOI] [PubMed] [Google Scholar]
- 26.Talal N, Garry R F, Alexander S S, Dauphinee M J, Ballester A, Takei M, Dang A. A conserved idiotype and antibodies to retroviral protein in systemic lupus erythematosus. J Clin Invest. 1990;85:1866–1871. doi: 10.1172/JCI114647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamano S, Saito J. Detection of retrovirus in salivary glands of patients with Sjogren’s syndrome. Nippon Rensho. 1995;53:2456–2460. [PubMed] [Google Scholar]


