Abstract
Akabane (AKA) virus is an arthropod-borne virus belonging to the Simbu group of the genus Bunyavirus. Neutralizing monoclonal antibodies (MAbs) against AKA virus were prepared, and the neutralizing epitopes of the virus were defined by competitive binding assay. Five distinct antigenic domains were identified and were designated A, B, C, D, and E. Domains A and C consisted of two epitopes each. It was demonstrated that seven neutralizing epitopes exist on the G1 glycoprotein of AKA virus. Dot immunobinding assays (DIAs) were performed with MAbs which recognize these seven neutralizing epitopes. The results were similar to those obtained by enzyme-linked immunosorbent assay. DIAs were performed using two Australian strains, one isolate from Taiwan, and isolates from Japan collected between the years 1959 and 1994, for a total of 63 isolates. The MAb response patterns were divided into five groups: the OBE-1 strain, the JaGAr39 strain, the Iriki strain, a group which consisted of features between those of the JaGAr39 strain and Iriki strain groups, and a group which did not belong to any of these patterns. The isolates which showed patterns similar to that of the JaGAr39 strain were found mostly among the isolates collected in 1974 and 1990, and isolates with patterns of MAb responses similar to the pattern of the Iriki strain were found mostly in the 1985 isolates. Those showing patterns in between were found mostly around 1977, 1987, and 1994. The results show that DIA can be used to effectively compare the antigenicities of AKA virus isolates within a few hours, even with lesser amounts of virus culture than is required for other assays.
Akabane (AKA) virus is an arthropod-borne virus belonging to the Simbu group of the genus Bunyavirus in the family Bunyaviridae. It causes epizootic and sporadic outbreaks of abortions, premature births, stillbirths, and congenital abnormalities characterized by arthrogryposis, hydranencephaly, or microanencephaly in cattle, sheep, and goats (7, 12, 14, 16, 17, 20, 22, 23). These outbreaks have been observed in Japan, Australia, Israel, and Turkey; moreover, serological evidence indicates that AKA virus may also be distributed in the Middle East, Asia, South-East Asia, and some African countries (8, 24–26). Recent studies using cross-neutralization tests with polyclonal antisera have revealed that antigenic variation exists in field isolates of AKA virus in Japan; in particular, the Iriki strain of AKA virus, isolated in 1984, shows low cross-reactivity with the JaGAr39 strain (prototype strain of AKA virus), which was isolated in 1959 (21). Also, a study using the neutralization and hemagglutination inhibition tests and enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies (MAbs) have revealed antigenic diversity among 21 isolates of AKA virus (1). However, these methods are very complicated in that they require antisera against each virus or virus purification.
In order to examine the antigenic changes of many viral isolates according to the year and location of collection, and to determine where these changes occur in relation to the host and vector, rapid and easier methods are needed to replace conventional ones. Therefore, MAbs against AKA virus were prepared to determine the neutralizing epitopes of the virus, and using MAbs against each epitope, dot immunobinding assays (DIAs) were performed to compare the antigenicities of 63 field-collected isolates of AKA virus.
MATERIALS AND METHODS
Propagation and purification of virus.
The OBE-1 strain of AKA virus, isolated from aborted fetuses (16), was used throughout this experiment. An inactivated AKA virus vaccine and a live vaccine derivative of the OBE-1 strain have been developed and are being used in Japan (13, 15). Virus isolates were propagated in hamster lung (HmLu-1) cells. The HmLu-1 cells were grown in Eagle’s minimal essential medium (Nissui Pharmaceutical Co., Tokyo, Japan) containing 10% tryptose phosphate broth (TPB) (Difco Laboratories, Detroit, Mich.) and 10% heat-inactivated calf serum. Medium without serum was used to propagate the virus isolates. The HmLu-1 cells were infected with the virus at a multiplicity of infection of 0.01 to 0.1 and were incubated at 37°C until they showed a complete cytopathic effect (CPE). Culture supernatant concentrated by 0.0025 M zinc acetate precipitation was layered onto a 20% sucrose gradient in an SW28 rotor (Beckman Instruments, Inc., Fullerton, Calif.) and centrifuged at 100,000 × g for 2 h. After the pellet was resuspended in NTE buffer (0.15 M NaCl, 0.01 M Tris-HCl [pH 7.4], 0.002 M EDTA), it was layered onto a continuous gradient of 10 to 70% (wt/vol) sucrose in an SW40 rotor (Beckman Instruments, Inc.) and centrifuged at 190,000 × g for 2 h, as described by Ide et al. (6). The virus band was collected and stored at −80°C until needed.
MAb production.
Adult female BALB/c mice were immunized intraperitoneally with 0.5 ml of a mixture of purified virus and an equal volume of Freund’s complete adjuvant. Four weeks later, a booster was inoculated with Freund’s incomplete adjuvant. Two weeks later, 0.5 ml of the purified virus preparation was inoculated intraperitoneally without adjuvant. Three days later, immune mouse spleen cells were fused with P3X63-Ag8-U1 myeloma cells at a ratio of 5:1 with 50% polyethylene glycol 4000 (Merck Darmstadt, Darmstadt, Germany). The fusion was carried out essentially by the method described by Köhler and Milstein (11) with a minor modification (1). Antibody-producing hybridomas were screened by an indirect ELISA, cloned by the micro-manipulation method, and stored in liquid nitrogen. Antibody-producing hybridomas were grown in RPMI 1640 (Nissui Pharmaceutical Co.) without serum, and their supernatant was used for immunoradioprecipitation, determination of antibody subtype, and comparison of the antigenicities of AKA virus isolates with ELISA. Some of the hybridomas were inoculated into the peritoneal cavities of BALB/c mice primed 2 to 3 weeks previously with 0.3 ml of pristane (2,6,10,14-tetramethylpentadecane) (3) for the neutralization test, competitive binding assay, and DIA.
Indirect ELISA.
Indirect ELISA was performed by the method of Akashi and Inaba (1). ELISA plates (Immulon 2; Dynatech Laboratories Inc., Chantilly, Va.) were coated overnight at 4°C with purified viral antigen diluted in carbonate-bicarbonate buffer (0.05 M, pH 9.6). Serial fourfold dilutions of MAbs were used for the first reaction. The optimal concentration of horseradish peroxidase-conjugated goat antibody against mouse immunoglobulins (Cappel, Organon Teknika Corp., West Chester, Pa.) was used for the second reaction. Substrate solution (0.1 M citric acid, 0.2 M Na2HPO4, 0.04% o-phenylenediamine dihydrochloride, 0.007% H2O2) was added to each well, the reaction was stopped with 3 N H2SO4, and the absorbance was measured by using an ELISA reader (S Jeia II, Sanko Junyaku Co., Tokyo, Japan) with a 492-nm filter. The ELISA titer was determined as the highest dilution showing an absorbance of over one-fifth of the absorbance of the appropriate concentration of the mouse serum immunized with OBE-1 strain.
Neutralization test.
Serial twofold dilutions of the MAbs were mixed with an equal volume of a diluted virus containing 200 50% tissue culture infective dose/0.1 ml. After a 1-h incubation at 37°C, 0.1 ml of each mixture was inoculated into cell cultures in flat-bottomed microplates (Sumitomo Bakelite Co., Tokyo, Japan), and the mixtures were incubated at 37°C in an atmosphere of 5% CO2 for 6 days. The antibody titer was determined as a reciprocal of the highest dilution which showed no CPE.
Determination of antibody subtype.
The subtypes of the MAbs were determined by using a mouse monoclonal subtyping kit (Zymed Laboratories, South San Francisco, Calif.) which detects immunoglobulin heavy and light chains.
Radioimmunoprecipitation.
Radioimmunoprecipitation was performed essentially by the method of Yamada et al. (28) with a slight modification. HmLu-1 cells were infected with AKA virus at a multiplicity of infection of 5 and were then metabolically radiolabeled with 10 μCi of l-[35S]methionine/ml for 10 h postinfection. The cells were lysed with lysis buffer (0.5% Nonidet P-40, 0.1 M NaCl, 0.1 M Tris-HCl [pH 8.0], 1 mM p-amidinophenyl methane sulfonyl fluoride, 10 μg of aprotinin/ml). After centrifugation of the lysates, the supernatants were incubated with MAb overnight at 4°C. The immune complexes were precipitated by adding 1/20 volume of protein G-Sepharose 4FF (Pharmacia, Uppsala, Sweden). The precipitates were washed three times with lysis buffer and analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and autoradiography.
Competitive binding assay.
MAbs were purified from ascites fluid by using a MAb G II affinity chromatography kit (Pharmacia). Conjugation of peroxidase to the MAbs was performed essentially as described by Tijssen and Kurstak (27).
The competitive binding assay was performed essentially by the method of Kimura-Kuroda and Yasui (9). Briefly, ELISA plates (Carboplate; Sumitomo Bakelite Co.), which had been preactivated with 4 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/ml at 30°C for 4 h, were coated overnight at 4°C with purified OBE-1 antigen diluted to the appropriate concentration in phosphate-buffered saline (PBS) (pH 5.8) and were washed three times with washing solution. Each competing antibody, which was diluted serially with PBS containing 0.5% Tween 20 and 0.5% ovalbumin, was added to the antigen-coated wells of ELISA plates. The plates were incubated for 3 h at 34°C and washed once. Peroxidase-conjugated MAb, at a predetermined dilution which gave an absorbance of between 0.5 and 0.8, was added, and the mixtures were incubated for 2 h at 34°C. The plates were washed eight times, and substrate solution was added. After a 40-min incubation at room temperature, the reaction was stopped with H2SO4, and the absorbance was measured by using an ELISA reader (S Jeia II) with a 492-nm filter. The percentage of competition was determined by the following formula (5): 100 × (OD without competitor − OD with competitor)/(OD without competitor − OD with homologous MAb) (OD with homologous MAb = 3,200 ELISA units).
Virus isolates.
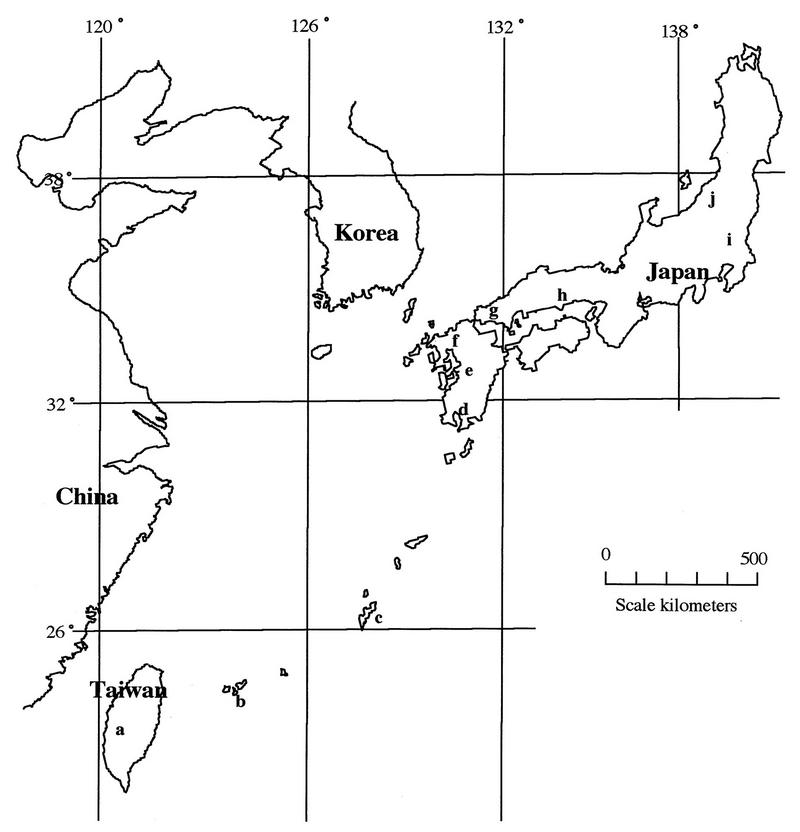
Strain designations, passage levels, origins, and locations and years of collection for the isolates used are listed in Table 1; the geographical distribution of the collection locations is shown in Fig. 1. The virus isolates were propagated in HmLu-1 cells with Eagle’s minimal essential medium without serum containing 10% TPB and were incubated at 37°C until they showed a CPE, and the resulting supernatant was used.
TABLE 1.
Characteristics of the isolates of Akabane virus used in this study
| Strain | Yr collected | Location
|
Patternb | Passage levelc | Origin | |
|---|---|---|---|---|---|---|
| Place name | On mapa | |||||
| JaGAr39 | 1959 | Gunma | i | 2 | ?+HL3 | Aedes vexans |
| R7949 | 1968 | Queensland Australia | 2 | Sm5HL3 | Culicoides brevitarsis | |
| B8935 | 1968 | Queensland Australia | 2 | Sm3HL4 | Culicoides brevitarsis | |
| SAB’74 | 1974 | Okayama | h | 2 | Sm3HL3 | Bovine fetus |
| NBE-8 | 1974 | Nigata | j | 2 | Sm6HL3 | Bovine fetus |
| NBE-9 | 1974 | Nigata | j | 2 | Sm3HL3 | Bovine fetus |
| OBE-1 | 1974 | Okayama | h | 1 | Sm1HL10 | Bovine fetus |
| KT3377 | 1977 | Kagoshima | d | 4 | Sm2HL4 | Bovine blood |
| M171 | 1979 | Ibaraki | i | 2 | Sm1HL3 | Culex triyaeniorhynchus |
| Iriki | 1984 | Kagoshima | d | 3 | Sm2BHK1HL10 | Calf cerebellum |
| KS-1/E/85 | 1985 | Kagoshima | d | 3 | HL6 | Bovine erythrocyte |
| KS-2/P/85 | 1985 | Kagoshima | d | 3 | HL7 | Bovine plasma |
| YG-1/E/85 | 1985 | Yamaguchi | g | 3 | HL8 | Bovine erythrocyte |
| YG-2/E/85 | 1985 | Yamaguchi | g | 3 | HL7 | Bovine erythrocyte |
| YG-3/E/85 | 1985 | Yamaguchi | g | 3 | HL8 | Bovine erythrocyte |
| YG-4/P/85 | 1985 | Yamaguchi | g | 3 | HL7 | Bovine plasma |
| YG-5/P/85 | 1985 | Yamaguchi | g | 3 | HL7 | Bovine plasma |
| YG-6/P/85 | 1985 | Yamaguchi | g | 3 | HL7 | Bovine plasma |
| ON-1/E/85 | 1985 | Okinawa (Okinawa Island) | c | 3 | HL8 | Bovine erythrocyte |
| ON-2/P/85 | 1985 | Okinawa (Okinawa Island) | c | 3 | HL6 | Bovine plasma |
| KSB-1/C/87 | 1987 | Kagoshima | d | 5 | HL6 | Culicoides oxystoma |
| KSB-2/C/87 | 1987 | Kagoshima | d | Other | HL7 | Culicoides oxystoma |
| KSB-3/P/87 | 1987 | Kagoshima | d | 5 | HL7 | Bovine plasma |
| KSB-4/P/87 | 1987 | Kagoshima | d | 5 | HL7 | Bovine plasma |
| KSB-5/P/87 | 1987 | Kagoshima | d | 5 | HL7 | Bovine plasma |
| KS-1/E/87 | 1987 | Kagoshima | d | 5 | BHK2HL3 | Bovine erythrocyte |
| OI-1/E/87 | 1987 | Oita | f | 5 | BHK2HL3 | Bovine erythrocyte |
| KM-1/P/87 | 1987 | Kumamoto | e | 2 | BHK2HL3 | Bovine plasma |
| NS-1/P/87 | 1987 | Nagasaki | f | 5 | BHK2HL3 | Bovine plasma |
| KSB-1/C/88 | 1988 | Kagoshima | d | 4 | HL5 | Culicoides oxystoma |
| YG-88-2 | 1988 | Yamaguchi | g | 4 | HL8 | Bovine erythrocyte |
| NS-88-1 | 1988 | Nagasaki | f | 1 | HL7 | Bovine erythrocyte |
| NS-88-2 | 1988 | Nagasaki | f | 4 | HL7 | Bovine erythrocyte |
| ON-89-2 | 1989 | Okinawa (Okinawa Island) | c | 4 | HL5 | Bovine erythrocyte |
| KSB-1/C/90 | 1990 | Kagoshima | d | 2 | HL6 | Culicoides oxystoma |
| KSB-2/C/90 | 1990 | Kagoshima | d | Other | HL5 | Culicoides jacobsoni |
| KSB-3/P/90 | 1990 | Kagoshima | d | 5 | HL5 | Bovine plasma |
| KSB-4/E/90 | 1990 | Kagoshima | d | 5 | HL6 | Bovine erythrocyte |
| KSB-5/P/90 | 1990 | Kagoshima | d | 5 | HL6 | Bovine plasma |
| KSB-6/E/90 | 1990 | Kagoshima | d | 1 | HL6 | Bovine erythrocyte |
| KSB-7/C/90 | 1990 | Kagoshima | d | 5 | HL6 | Culicoides oxystoma |
| KSB-8/C/90 | 1990 | Kagoshima | d | 5 | HL7 | Culicoides oxystoma |
| KS-90-2 | 1990 | Kagoshima | d | 2 | HL8 | Bovine plasma |
| KS-90-3 | 1990 | Kagoshima | d | 5 | HL9 | Bovine plasma |
| ON-3/E/90 | 1990 | Okinawa (Ishigaki Island) | b | 3 | HL8 | Bovine erythrocyte |
| ON-4/E/90 | 1990 | Okinawa (Ishigaki Island) | b | 3 | HL5 | Bovine erythrocyte |
| MZ-90-1 | 1990 | Miyazaki | d | 2 | Vero3HL4 | Culicoides spp. |
| MZ-90-2 | 1990 | Miyazaki | d | 2 | Vero3HL3 | Culicoides spp. |
| FO-90-3 | 1990 | Fukuoka | f | 2 | HL8 | Culicoides spp. |
| FO-90-4 | 1990 | Fukuoka | f | 4 | HL8 | Culicoides spp. |
| ON-1/E/91 | 1991 | Okinawa (Okinawa Island) | c | 2 | HL7 | Bovine erythrocyte |
| ON-2/P/91 | 1991 | Okinawa (Okinawa Island) | c | 2 | HL7 | Bovine plasma |
| ON-3/E/91 | 1991 | Okinawa (Ishigaki Island) | b | 2 | HL7 | Bovine erythrocyte |
| ON-4/P/91 | 1991 | Okinawa (Ishigaki Island) | b | 2 | HL7 | Bovine plasma |
| ON-1/P/93 | 1993 | Okinawa (Iriomote Island) | b | Other | HL6 | Bovine plasma |
| CY-77 | 1993 | Jiayi Taiwan | a | 3 | HL7 | Bovine erythrocyte |
| KSB-1/C/94 | 1994 | Kagoshima | d | 4 | HL5 | Culicoides oxystoma |
| KSB-2/C/94 | 1994 | Kagoshima | d | 4 | HL5 | Culicoides jacobsoni |
| KSB-3/P/94 | 1994 | Kagoshima | d | 4 | HL4 | Bovine plasma |
| KSB-4/P/94 | 1994 | Kagoshima | d | 4 | HL4 | Bovine plasma |
| ON-2/E/94 | 1994 | Okinawa (Ishigaki Island) | b | 2 | HL6 | Bovine erythrocyte |
| ON-3/P/94 | 1994 | Okinawa (Ishigaki Island) | b | 2 | HL5 | Bovine plasma |
| ON-4/P/94 | 1994 | Okinawa (Ishigaki Island) | b | 2 | HL5 | Bovine plasma |
Map in Fig. 1.
Pattern of the isolates of Akabane virus by DIA.
Letters indicate cell lines and numbers indicate number of passages. HL, HmLu-1 cells; Sm, suckling-mouse brain.
FIG. 1.
Locations for collection of virus isolates. See Table 1 for identification of areas denoted by letters.
DIA.
Milliblot Systems (Millipore Corp., Bedford, Mass.) was used as the blot, and Immobilon PVDF (pore size, 0.45 μm) (Millipore Corp.) was used as the membrane. For transcription, the supernatants of incubated virus isolates from undiluted samples and from serial 3-fold dilutions up to a 27-fold dilution were used, and Eagle’s minimal essential medium was used for the negative control.
The amount of supernatant used for each blotting was 250 μl, and the blots were sucked into the membrane at negative pressure. In the same manner, 200 μl of PBS was sucked into the membrane for washing. The membrane was immersed in Tris-buffered saline (TBS) (20 mM Tris-HCl [pH 7.5], 0.15 M NaCl), which included 8% skim milk for blocking. After that, the membrane was immersed in MAb (from the ascites fluid of a mouse), which was diluted 400-fold by TBS with 8% skim milk for 30 min, and then the membrane was washed once with TBS. Further, the membrane was reacted with horseradish peroxidase-conjugated goat antibody against mouse immunoglobulins (Cappel, Organon Teknika Corp.) which had been diluted by TBS with 4% skim milk, and then the membrane was washed with TBS three times. Color was developed with a substrate solution (0.016% H2O2, 0.027% 3,3′-diaminobenzidine tetrahydrochloride in TBS). Each absorbance was measured by using a Scanning Imager (Molecular Dynamics, Sunnyvale, Calif.).
Each isolate’s absorbance was divided by the absorbance of OBE-1 to determine the MAb reaction for each isolate relative to that for the OBE-1 strain. Furthermore, to correct for variance in viral density among the supernatants of the AKA virus isolates, the degree of reaction against each MAb is given as a percentage of the total MAb reactions in each isolate. The data were graphed, and response patterns were identified.
RESULTS
Characterization of MAbs.
Eleven MAbs which demonstrated neutralizing activity against Akabane virus OBE-1 strain reacted with G1 glycoprotein, and the neutralizing titer of the ascites fluid was strong, more than 256. The subtypes and neutralizing titers of the 11 neutralizing MAbs are shown in Table 2.
TABLE 2.
Characterization of neutralizing MAbsa
| MAb | Immunoglobulin G subtype | Neutralization titer |
|---|---|---|
| 4A1 | 2a | 4,096 |
| 5B6 | 1 | 4,096 |
| 6D9 | 2a | 4,096 |
| 11C4 | 2a | 256 |
| 12H6 | 1 | 512 |
| 13A9 | 2a | 4,096 |
| 13E4 | 2a | 4,096 |
| 13E7 | 3 | 4,096 |
| 13H9 | 2b | 256 |
| 15H6 | 2a | 2,048 |
| 19B4 | 1 | 1,024 |
All the MAbs recognized the G1 glycoprotein.
Competitive binding assay.
Ten neutralizing MAbs (all of the 11 except 13E7) were used to determine the topological relationship of the neutralizing epitopes. In each case, antigen binding of the conjugated MAbs was blocked efficiently by the homologous competitor MAb. Table 3 summarizes the results of the competitive binding assays.
TABLE 3.
Summary of competitive binding assays of neutralizing MAbsa
| Competitor MAb | Peroxidase-conjugated MAb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5B6 | 13E4 | 13H9 | 19B4 | 11C4 | 12H6 | 15H6 | 6D9 | 13A9 | 4A1 | |
| 5B6 | ++ | ++ | + | |||||||
| 13E4 | ++ | ++ | ++ | |||||||
| 13H9 | ++ | ++ | ++ | |||||||
| 19B4 | ++ | |||||||||
| 11C4 | ++ | ++ | ||||||||
| 12H6 | + | ++ | + | |||||||
| 15H6 | + | ++ | ||||||||
| 6D9 | ++ | ++ | ++ | |||||||
| 13A9 | ++ | ++ | ++ | |||||||
| 4A1 | ++ | ++ | ++ | |||||||
Antigenic domains A to E (left to right) are boxed. ++, >50% competition; +, 25 to 50% competition.
The competitor MAbs which showed approximately 100% competition with conjugated MAb 4A1 were 6D9, 13A9, and homologous 4A1. The other seven competitor MAbs showed an absence of competition, giving levels between 9 and −37% competition. The results of the competitive binding assays for conjugated MAbs 6D9 and 13A9 were almost similar to those for conjugated MAb 4A1. Reciprocal competitions were found among MAbs 4A1, 6D9, and 13A9; however, the binding of conjugate MAb 13A9 was inhibited by competitor MAb 12H6 at the low level of 31%.
The competitor MAbs 13E4 and 13H9 appeared to inhibit the binding of conjugated MAb 5B6 with the virus more effectively than did the homologous competitor MAb, at a level exceeding 100% competition. The other seven competitor MAbs showed an absence of competition, giving levels between 24 and −100% competition. The competitor MAbs 5B6 and 13H9 competed with conjugated MAb 13E4 at levels between 64 and 113%. The results of the competitive binding assay for conjugated MAb 13E4 were generally similar to those for conjugated MAb 5B6. Reciprocal competition was found between MAbs 5B6 and 13E4. The results of the competitive binding assay for conjugated MAb 13H9 were broadly similar to those for conjugated MAbs 5B6 and 13E4. However, the competitor, MAb 5B6, showed a low level of inhibition (less than 40%) of the binding of conjugated MAb 13H9.
Competitor MAb 11C4 appeared to inhibit the binding of conjugate MAb 12H6 more strongly than did homologous MAb 12H6, at a level of 182% competition. Competitor MAb 15H6 slightly inhibited conjugate MAb 12H6 at the low level of 30% competition. The other seven competitor MAbs demonstrated no competition, giving levels between 6 and −82%. However, the binding of conjugate MAb 11C4 was inhibited only by competitor MAb 12H6 at the low level of 25% competition, except for the homologous competitor MAb. Nonreciprocal competition was found between MAbs 11C4 and 12H6.
When 15H6 and 19B4 were used as conjugate MAbs, the only competitor MAbs showing any competition with them were the respective homologous MAbs. The other MAbs showed no competition, giving levels between 20 and −70%.
DIA.
In order to examine changes in the antigenicity of the virus, DIAs were performed with MAbs (13A9, 15H6, 12H6, 11C4, 19B4, 13H9, and 5B6) which recognized different neutralizing epitopes, as determined by competitive binding assays. DIAs were performed using virus culture supernatants of strain OBE-1 that were not incubated, that were incubated for 12 h at 37°C, and that were incubated for 24 h at 37°C to confirm the reproducibility of the DIA pattern. The results showed almost the same patterns. The results of tests with 63 isolates of virus at different levels of density, from no dilution to a ninefold dilution, showed that there was no difference in the patterns due to the density of the antigen. Seven strains of AKA virus, OBE-1, JaGAr39, Iriki, KS-1/E/85, KSB-2/C/87, KSB-3/P/90, and ON-1/P/93, were used to compare DIA absorbances of undiluted antigens and ELISA titers (Table 4). Similar results were obtained by the two assays. The reaction between MAb 13H9 and strain JaGAr39 was slight by ELISA but the DIA absorbance was high.
TABLE 4.
Comparison of ELISA and DIA
| MAb (epitope)a | Assayb | Assay result with Akabane virus isolateb
|
||||||
|---|---|---|---|---|---|---|---|---|
| OBE-1 | JaGAr39 | Iriki | KS-1/E/85 | KSB-2/C/87 | KSB-3/P/90 | ON-1/P/93 | ||
| 13A9 (E) | ELISA | 1,024 | <1 | <1 | <1 | <1 | <1 | <1 |
| DIA | 0.54 | 0.02 | 0.02 | 0.01 | 0.00 | 0.03 | 0.02 | |
| 15H6 (D) | ELISA | 1,024 | 256 | 16 | 1 | 64 | 16 | <1 |
| DIA | 0.54 | 0.68 | 0.10 | 0.05 | 0.51 | 0.36 | 0.03 | |
| 12H6 (C2) | ELISA | 1,024 | 256 | <1 | <1 | 4 | <1 | <1 |
| DIA | 0.38 | 0.52 | 0.14 | 0.05 | 0.13 | 0.19 | 0.06 | |
| 11C4 (C1) | ELISA | 1,024 | 256 | 256 | 16 | 16 | 64 | 4 |
| DIA | 0.54 | 0.65 | 0.49 | 0.13 | 0.22 | 0.46 | 0.28 | |
| 19B4 (B) | ELISA | 1,024 | 256 | 1,024 | 64 | 64 | 256 | 16 |
| DIA | 0.48 | 0.59 | 0.40 | 0.18 | 0.46 | 0.41 | 0.24 | |
| 13H9 (A2) | ELISA | 1,024 | <1 | 1,024 | 256 | 64 | 1,024 | <1 |
| DIA | 0.46 | 0.50 | 0.40 | 0.19 | 0.45 | 0.37 | 0.03 | |
| 5B6 (A1) | ELISA | 4,096 | 1,024 | 4,096 | 256 | 1,024 | 1,024 | 256 |
| DIA | 0.36 | 0.44 | 0.31 | 0.12 | 0.34 | 0.24 | 0.20 | |
Epitopes were determined by competitive binding assays.
ELISA results are given as titers. DIA results are absorbances.
The DIA results for 63 isolates of AKA virus were divided into patterns. Representative patterns are shown in Fig. 2; the isolates included in these patterns are listed in Table 1.
FIG. 2.
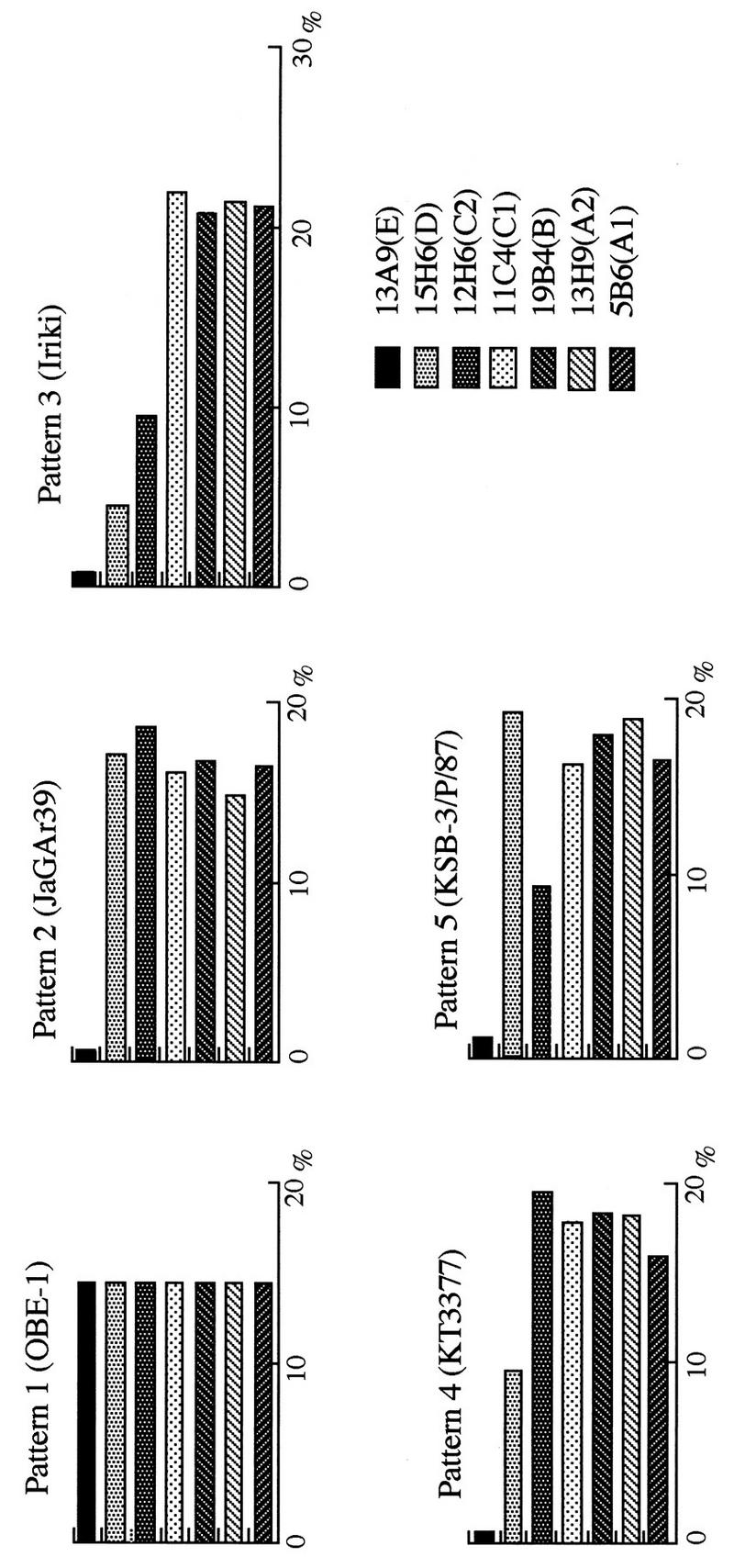
Representative patterns of Akabane virus isolates by DIA. The abscissa shows the degree of reaction against each MAb as a percentage of the total MAb reactions.
Pattern 1 shows a reaction with all the MAbs, and it was the only pattern that included a reaction with MAb 13A9. Pattern 2, illustrated by strain JaGAr39, is similar to pattern 1 except for the reaction with MAb 13A9. All strains were collected before 1979 (first nine strains in Table 1), except KT3377 (which showed pattern 4), which showed similar patterns but without regard to the year or location of the sample collections. Pattern 2 is represented by the strains collected in areas d and f in 1990, in areas b and c in 1991, and in area b in 1994 (Fig. 1 and Table 1). Pattern 3 is represented by Iriki, whose reactivity with MAbs 12H6, 13A9, and 15H6 was low. Although the reactivity with MAb 5B6 was a little scattered, this pattern was clearly distinguished from the others. All the isolates collected in 1984 and 1985 showed this pattern. The location of these isolates covered rather large amounts of areas c, d, and g. Isolates collected in area b in 1990 and strain CY-77, which was collected in Taiwan in 1993, also showed this pattern.
Pattern 4 is represented by KT3377, which demonstrated little reactivity with MAb 13A9 and low reactivity with MAb 15H6. This pattern was found among isolates collected in area d in 1977, in areas c, d, g, and f in 1988 to 1990, and in all the isolates collected in area d in 1994, though the reactivity of these isolates with MAb 5B6 was slightly dispersed. Pattern 5 is represented by KSB-3/P/87; this pattern showed the same reactivity with MAbs 13A9 and 12H6 as pattern 3, but like patterns 1 and 2, it revealed high reactivity with MAb 15H6. Although some of the isolates demonstrated low reactivity with MAb 5B6, this pattern was applied to almost all the isolates collected in areas d and f in 1987 and to many of the isolates collected in area d in 1990. Furthermore, ON-1/P/93, KSB-2/C/87, and KSB-2/C/90 did not belong to any of these patterns. The reaction pattern of ON-1/P/93 was similar to that of pattern 3 and showed low reactivity not only with MAbs 13A9 and 15H6 but also with MAb 12H6. In addition, ON-1/ P/93 showed low reactivity with MAb 13H9 and the reaction pattern of ON-1/P/93 was very different from that of OBE-1. KSB-2/C/87 and KSB-2/C/90 were collected in area d in 1987 and 1990, respectively, and their reactivities were similar to that of pattern 5. The former had low reactivity with MAb 11C4, and the latter had low reactivity with MAb 19B4.
DISCUSSION
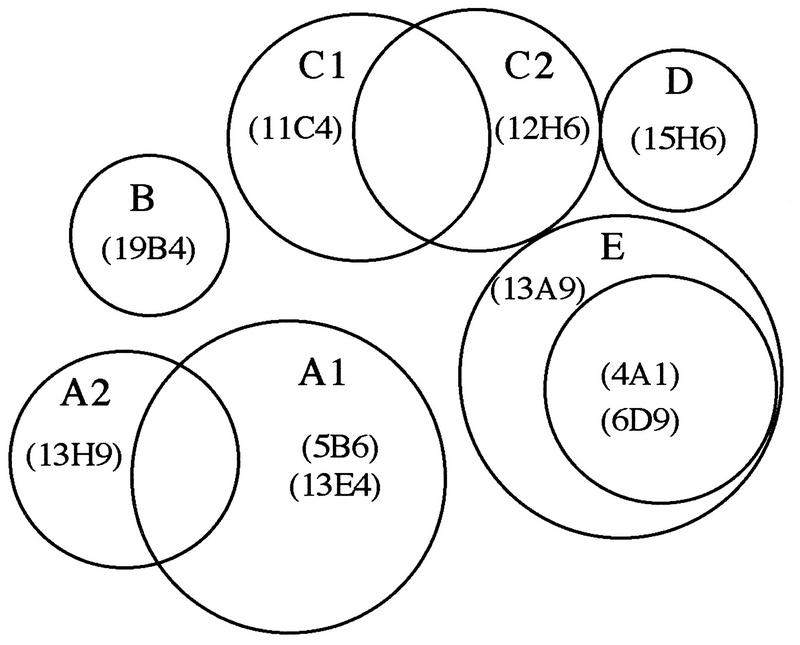
The results of the competitive binding assays using 10 neutralizing MAbs that reacted with the G1 glycoprotein of Akabane virus revealed the presence of five different antigenic domains: A, B, C, D, and E. Domains B and D comprised single epitopes recognized by MAbs 19B4 and 15H6, respectively. However, domain D might have been independent or slightly dependent on the epitope recognized by MAb 12H6. Domain E comprised epitopes recognized by MAbs 6D9, 13A9, and 4A1. However, it was suggested that the epitope recognized by MAb 13A9 was slightly dependent on the epitope recognized by MAb 12H6.
Domain A comprised epitopes recognized by MAbs 5B6, 13E4, and 13H9. However, MAb 13H9 recognized a slightly different epitope than did the other two MAbs. Consequently, domain A could be subdivided into A1 and A2. Domain C comprised two epitopes recognized by MAbs 11C4 and 12H6. However, there appeared to be nonreciprocal competition. Consequently, domain C was also subdivided into C1 and C2, like domain A. The proposed interrelationship among the epitopes on the G1 glycoprotein of AKA virus is shown in Fig. 3.
FIG. 3.
Proposed interrelationship of epitopes on G1 glycoprotein of Akabane virus. The number-letter combinations in parentheses are the MAbs which recognize epitopes of the antigenic domain A1 to E as shown. On antigenic domain E, MAbs 4A1 and 6D9 recognize the same restricted epitope within the epitope recognized by MAb 13A9.
It is useful to compare AKA virus isolates with MAbs which recognize different epitopes in order to analyze variations in antigenicity. However, it is necessary to compare a large number of isolates having many generations and covering large areas. For this purpose, conventional methods such as ELISA are quite complicated as they require virus purification and can handle only a limited number of isolates. DIA, on the other hand, can be used to compare patterns within a few hours and requires a smaller amount of virus culture. The ELISA and DIA results were compared for MAbs which recognized the neutralizing epitopes comprising domains A1, A2, B, C1, C2, D, and E (MAbs 5B6, 13H9, 19B4, 11C4, 12H6, 15H6, and 13A9, respectively). The reactivities of these MAbs were similar in both assays except for the reaction of MAb 13H9 with strain JaGAr39. It was speculated that the differences in reactivity originated from the fact that whereas ELISA uses purified virus as a coated antigen, DIA uses virus culture supernatant as a transferred antigen.
In order to examine antigenic changes among the 63 isolates of AKA virus by the reactivity of the MAbs to the epitopes several DIAs were performed, and many patterns of reactivity emerged. The reactivities to epitopes A1, A2, B, and C1 were similar in almost all the isolates; however, the reactivities to epitopes C2, D, and E were different. The collected isolates were divided primarily into five groups: OBE-1 strain (pattern 1), JaGAr39 strain (pattern 2), Iriki strain (pattern 3), a group which consisted of features in between those of the JaGAr39 and Iriki strains (patterns 4 and 5), and a group which did not belong to any of the five patterns. Only 3 of the 63 isolates showed the OBE-1 pattern, and the OBE-1 pattern was similar to the JaGAr39 pattern except for the reactivity to epitope E; it was assumed both that the group with the closest antigenicity to that of OBE-1 was the one that had the same features as the prototype strain, JaGAr39, and that the group with the most different antigenicity was the one which had features similar to Iriki. This process of reasoning suggests that epitopes C2, D, and E are the most mutable and that epitope B is the most conserved.
Isolates collected in the same area in the same year shared the same pattern. It is especially noteworthy that the isolates collected in the rather large areas of c, d, and g in 1984 and 1985 all showed identical patterns. It was presumed that the AKA virus, which retains the same antigenicity, was the prevailing virus in these large areas during those years. It was reported previously that cross-neutralization tests between the Iriki and JaGAr39 strains showed that the Iriki strain had low cross-reactivity with antiserum to JaGAr39 (20), and it was also shown that isolates collected in 1985 and isolate CY-77, collected in Taiwan in 1993, had similar reactivities to that of the Iriki strain (18, 19). These results are in agreement with the results of our DIAs.
On the other hand, reaction patterns of isolates collected in area b showed larger differences over the years of collection compared to those of other areas. From the pattern of the isolates collected in areas a and b, it was conjectured that viruses that are self-transmutable in different areas may communicate with each other through vectors across the oceans.
Comparison of the patterns found in areas d, e, and f, where the most isolates over many generations had been collected, revealed that isolates collected in 1984 and 1985 showed pattern 3, isolates obtained in 1987 showed mainly pattern 5, those gathered from 1988 to 1990 showed pattern 4, and those found in 1990 showed patterns 2, 4, and 5. Further, those collected in 1994 showed pattern 4. This reveals that even if the host-vector relationship in nature involves mutations (2, 4, 10), significant mutations do not take place within a short time or they do not occur in prevailing viruses. On the other hand, a previous report found that AKA virus isolates which were collected from Culicoides species at the same place over a 3-week period showed great antigenic variation (1). If it is possible for the virus to develop a variation within a short period of time, it would mutate frequently in order to escape the immunopressures of the vertebrate host. Yet such frequent mutation would be inconsistent with the fact that isolates from areas c, d, and g retained the same reactivity pattern between 1984 and 1985.
ACKNOWLEDGMENTS
We thank Tomomi Kubo for technical assistance.
This research was supported by grants received from the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Akashi H, Inaba Y. Antigenic diversity of Akabane virus detected by monoclonal antibodies. Virus Res. 1997;47:187–196. doi: 10.1016/s0168-1702(96)01415-3. [DOI] [PubMed] [Google Scholar]
- 2.Beaty B J, Bishop D H L. Bunyavirus-vector interactions. Virus Res. 1988;10:289–302. doi: 10.1016/0168-1702(88)90071-8. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur B R, Tsang P, Larose Y. Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods. 1984;71:265–272. doi: 10.1016/0022-1759(84)90073-5. [DOI] [PubMed] [Google Scholar]
- 4.Calisher C H. Evolutionary significance of the taxonomic data regarding bunyaviruses of the family Bunyaviridae. Intervirology. 1988;29:268–276. doi: 10.1159/000150055. [DOI] [PubMed] [Google Scholar]
- 5.Hughes G, Babiuk L A, van Drunen Littel-van den Hurk S. Functional and topographical analysis of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103:47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ide S, Baba K, Tsuchimoto K, Nagano H, Eiguchi Y, Yamagami T, Yamagishi H, Tanaka Y, Fujisaki Y, Hohdatsu T, Matumoto M. Detection of antibodies against Akabane virus in bovine sera by enzyme-linked immunosorbent assay. Vet Microbiol. 1989;20:275–280. doi: 10.1016/0378-1135(89)90051-5. [DOI] [PubMed] [Google Scholar]
- 7.Inaba Y, Kurogi H, Omori T. Akabane disease: epizootic abortion, premature birth, stillbirth and congenital arthrogryposis-hydranencephaly in cattle, sheep and goats caused by Akabane virus. Aust Vet J. 1975;51:584–585. doi: 10.1111/j.1751-0813.1975.tb09397.x. [DOI] [PubMed] [Google Scholar]
- 8.Inaba Y, Matumoto M. Akabane virus. In: Dinter Z, Morein B, editors. Virus infections of ruminants. Amsterdam, The Netherlands: Elsevier Science Publishers; 1990. pp. 467–480. [Google Scholar]
- 9.Kimura-Kuroda J, Yasui K. Topographical analysis of antigenic determinants on envelope glycoprotein V3 (E) of Japanese encephalitis virus, using monoclonal antibodies. J Virol. 1983;45:124–132. doi: 10.1128/jvi.45.1.124-132.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsford L. Antigenic variance. Curr Top Microbiol Immunol. 1991;169:181–216. doi: 10.1007/978-3-642-76018-1_7. [DOI] [PubMed] [Google Scholar]
- 11.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 12.Kurogi H, Inaba Y, Goto Y, Miura Y, Takahashi H, Sato K, Omori T, Matumoto M. Serologic evidence for the etiologic role of Akabane virus in epizootic abortion-arthrogryposis-hydranencephaly in cattle in Japan, 1972–1974. Arch Virol. 1975;47:71–83. doi: 10.1007/BF01315594. [DOI] [PubMed] [Google Scholar]
- 13.Kurogi H, Inaba Y, Takahashi E, Sato K, Akashi H, Satoda K, Omori T. An attenuated strain of Akabane virus: a candidate for live virus vaccine. Natl Inst Anim Health Q. 1979;19:12–22. [PubMed] [Google Scholar]
- 14.Kurogi H, Inaba Y, Takahashi E, Sato K, Goto Y, Omori T. Experimental infection of pregnant goats with Akabane virus. Natl Inst Anim Health Q. 1977;17:1–9. [PubMed] [Google Scholar]
- 15.Kurogi H, Inaba Y, Takahashi E, Sato K, Goto Y, Satoda K, Omori T, Hatakeyama H. Development of inactivated vaccine for Akabane disease. Natl Inst Anim Health Q. 1978;18:97–108. [PubMed] [Google Scholar]
- 16.Kurogi H, Inaba Y, Takahashi E, Sato K, Omori T, Miura Y, Goto Y, Fujiwara Y, Hatano Y, Kodama K, Fukuyama S, Sasaki N, Matumoto M. Epizootic congenital arthrogryposis-hydranencephaly syndrome in cattle: isolation of Akabane virus from affected fetuses. Arch Virol. 1976;51:67–74. doi: 10.1007/BF01317835. [DOI] [PubMed] [Google Scholar]
- 17.Kurogi H, Inaba Y, Takahashi E, Sato K, Satoda K, Goto Y, Omori T, Matumoto M. Congenital abnormalities in newborn calves after inoculation of pregnant cows with Akabane virus. Infect Immun. 1977;17:338–343. doi: 10.1128/iai.17.2.338-343.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, Y. S., Y. K. Liao, Y. Goto, K. Tsutsumi, K. Yoshida, and H. T. Sung. 1994. Unpublished data.
- 19.Matsuda, H., Y. Goto, Y. Miura, H. Ohzono, K. Tsutsumi, and T. Sugimura. 1993. Unpublished data.
- 20.Miura Y, Hayashi S, Ishihara T, Inaba Y, Omori T, Matumoto M. Neutralizing antibody against Akabane virus in precolostral sera from calves with congenital arthrogryposis-hydranencephaly syndrome. Arch Gesamte Virusforsch. 1974;46:377–380. doi: 10.1007/BF01240082. [DOI] [PubMed] [Google Scholar]
- 21.Miyazato S, Miura Y, Hase M, Kubo M, Goto Y, Kono Y. Encephalitis of cattle caused by Iriki isolate, a new strain belonging to Akabane virus. Jpn J Vet Sci. 1989;51:128–136. doi: 10.1292/jvms1939.51.128. [DOI] [PubMed] [Google Scholar]
- 22.Parsonson I M, Della-Porta A J, Snowdon W A. Congenital abnormalities in newborn lambs after infection of pregnant sheep with Akabane virus. Infect Immun. 1977;15:254–262. doi: 10.1128/iai.15.1.254-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsonson I M, Della-Porta A J, Snowdon W A, Murray M D. Congenital abnormalities in fetal lambs after inoculation of pregnant ewes with Akabane virus. Aust Vet J. 1975;51:585–586. doi: 10.1111/j.1751-0813.1975.tb09398.x. [DOI] [PubMed] [Google Scholar]
- 24.Parsonson I M, McPhee D A. Bunyavirus pathogenesis. In: Maramorosch K, Murphy F A, Shatkin A J, editors. Advances in virus research. Vol. 30. Orlando, Fla: Academic Press; 1985. pp. 279–316. [DOI] [PubMed] [Google Scholar]
- 25.Porterfield J S, Della-Porta A J. Bunyaviridae: infections and diagnosis. In: Kurstak E, Kurstak C, editors. Comparative diagnosis of viral diseases. IV. New York, N.Y: Academic Press; 1981. pp. 479–508. [Google Scholar]
- 26.Taylor W P, Mellor P S. The distribution of Akabane virus in the Middle East. Epidemiol Infect. 1994;113:175–185. doi: 10.1017/s0950268800051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tijssen P, Kurstak E. Highly efficient and simple methods for the preparation of oxidase and active peroxidase-antibody conjugates for enzyme immunoassay. Anal Biochem. 1984;136:451–457. doi: 10.1016/0003-2697(84)90243-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamada S, Imada T, Watanabe W, Honda Y, Nakajima-Iijima S, Shimizu Y, Sekikawa K. Nucleotide sequence and transcriptional mapping of the major capsid protein gene of pseudorabies virus. Virology. 1991;185:56–66. doi: 10.1016/0042-6822(91)90753-x. [DOI] [PubMed] [Google Scholar]