Abstract
Five monoclonal antibodies (MAbs) were produced against the Streptococcus pneumoniae pneumococcal surface adhesin A (PsaA) 37-kDa common cell wall protein. These antibodies were used in a dot immunoblot and Western blot study of clinical isolates of S. pneumoniae to detect the presence of the protein. By both assays, the MAbs reacted with clinical isolates representing the 23 type-specific serotypes present in the licensed pneumococcal polysaccharide vaccine. Western blot analysis confirmed the presence of a protein migrating in the gel with a molecular mass of 37 kDa. An extension of the study by using dot immunoblot analysis that included an analysis of the 90 serotypes of S. pneumoniae showed that all five MAbs reacted with 89 of the 90 serotypes tested. MAb 1B6, the exception, did not react with S. pneumoniae serotype 16F. Dot immunoblot analysis of the MAbs with Enterococcus faecalis and viridans streptococci showed varied reactivity patterns, depending on the species. The MAbs against the 37-kDa antigen did not react with Escherichia coli, respiratory pathogens, or nonpathogens representing 22 genera and 29 species of bacteria. All five MAbs also reacted with five multidrug-resistant strains of S. pneumoniae. In summary, these MAbs may be useful for detection of pneumococcal antigen and may lead to the development of diagnostic assays for pneumococcal disease.
Streptococcus pneumoniae (pneumococcus) is a major bacterial pathogen that causes morbidity and mortality worldwide. Disease caused by pneumococcus is prevalent among the very young, the elderly, and immunocompromised persons. In the United States, pneumococcus accounts for an estimated 3,000 cases of meningitis, 500,000 cases of pneumonia, and 7 to 10 million cases of otitis media yearly, and in developing countries, it accounts for about 1.2 million deaths annually in children less than 5 years of age (6).
Currently, S. pneumoniae is composed of 90 serotypes, based on differences in their carbohydrate capsules (17). A licensed pneumococcal polysaccharide vaccine is composed of 23 different capsular serotypes representing from 85 to 90% of the serotypes that cause invasive pneumococcal disease in the United States (9). However, this vaccine is poorly immunogenic in children under 2 years of age (15), and efforts are focused on developing new second-generation polysaccharide-protein conjugate vaccines and third-generation common-protein vaccines.
Early detection and identification of S. pneumoniae bacteremia continues to be of primary importance to the clinician. Blood culture is the only widely accepted definitive method of pneumococcal diagnosis, but it is positive in only 20 to 25% of pneumonia cases (33). Therefore, investigators continue to search for rapid, sensitive, and specific diagnostic tests for pneumococcal infections. Numerous assays have been developed for diagnosis of pneumococcal infections, particularly meningitis, by techniques such as enzyme-linked immunosorbent assay (ELISA), counterimmunoelectrophoresis, and latex agglutination (1, 3, 19). However, the consensus among researchers is that these assays—especially those used to detect pneumococcal antigens in serum, urine, and sputum—lack the sensitivity and specificity to be useful in early, rapid diagnosis (11, 29, 47). Antigen detection of S. pneumoniae infections in cerebrospinal fluid aids in establishing the etiology of bacterial meningitis (43, 48). An ELISA for the measurement of antibody response to pneumolysin has also proved successful, but again the sensitivity and specificity of the assays need improvement (21). Currently, molecular techniques, such as PCR, have proved useful in the detection of S. pneumoniae isolated from normally sterile body sites (20). S. pneumoniae species-specific bacterial genes encoding autolysin or pneumolysin can be amplified in PCR and detect a small number of target bacteria (37). Although, this method appears promising, there is still the possibility of obtaining cross-reactions in the tests generated by contamination in the sample, and the sensitivity of the test in the field remains to be determined.
The emergence of antibiotic-resistant strains of S. pneumoniae (7, 8) has made definitive diagnosis of pneumococcal infections crucial. Drug-resistant pneumococcal strains were observed in Australia and South Africa in the 1970s (25) and spread rapidly during the 1980s throughout many regions of the world. In the United States, drug-resistant strains were relatively uncommon through the late 1980s (39). However, during the 1990s numerous drug-resistant strains of S. pneumoniae have been reported (45). Pneumococcal isolates that are penicillin resistant have emerged (14, 18) as well as isolates resistant to other antimicrobial drugs, including erythromycin, trimethoprim-sulfamethoxazole, and extended-spectrum cephalosporins.
In an earlier report, Russell et al. identified a 37-kDa pneumococcal surface adhesion protein (PsaA) (34). A monoclonal antibody (MAb) made against this protein reacted with the 23 type-specific serotypes comprising the licensed pneumococcal capsular polysaccharide vaccine (34). Subsequently, the gene encoding PsaA was cloned into Escherichia coli and its nucleotide sequence was determined (35). This sequence was later shown by PCR-restriction fragment length polymorphism analysis to be highly conserved among pneumococci (36). In addition, PsaA has been found to be a protective immunogen in mice (41). Efforts are ongoing to investigate this protein for its potential as a third-generation common-protein vaccine candidate. Investigators have shown that humans immunized intranasally with heat-killed, whole-cell inocula exhibited an increase in levels of salivary antibody to PsaA (2). To facilitate the investigation of the immunologic characteristics of the protein, define its epitopes, and determine the degree of its antigenic conservation, we evaluated five MAbs to PsaA. These MAbs were evaluated to determine the degree of epitope conservation among 90 pneumococcal serotypes. The production and characterization of these MAbs will aid in characterizing PsaA, will lead to their use in detecting pneumococcal antigen, and may lead to the development of diagnostic assays for pneumococcal disease.
MATERIALS AND METHODS
Bacterial strains.
Clinical isolates of 25 S. pneumoniae serotypes including the 23 capsular serotypes found in the licensed pneumococcal vaccine, heterologous streptococci, and eight serotypes including the six newly identified S. pneumoniae serotypes were obtained from the culture collection of the Streptococcal Reference Laboratory, Centers for Disease Control and Prevention (CDC), Atlanta, Ga. The 25 serotypes were random clinical isolates from patients from the United States with culture-confirmed S. pneumoniae infections. Twenty-three serotypes were blood isolates, one isolate was from pleural fluid, and the other was from cerebrospinal fluid. The serotypes are 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F, 6A, and 25F (the last two serotypes are not present in the licensed pneumococcal vaccine). Clinical isolates of heterologous streptococcal species included Enterococcus faecalis, Streptococcus oralis, Streptococcus mitior, Streptococcus gordonii, Streptococcus parasanguis, Streptococcus sanguis, Streptococcus mitis, and Streptococcus mutans. Serotypes 13 and 24B were provided along with the six newly identified S. pneumoniae serotypes, 10B, 10C, 11D, 12B, 25A, and 33D (17). The American Type Culture Collection (Rockville, Md.) provided serotypes 7A, 10F, 11F, 15F, 16F, 18F, 21, 24F, 27, 28F, 29, 15A, 31, 23F, 9A, 35F, 36, 37, 41F, 33C, 33A, 34, 33B, 18A, 40, 23A, 35A, 7B, 9L, 7C, 7F, 47F, 11C, 18B, 19B, 19C, 35C, 22A, 23B, 24A, 35B, 32A, 39, 33F, 38, 45, and 46. Serotypes 16A, 41A, 43, 11B, 15C, 17A, 28A, 42, 44, 48, 12A, and 47A were provided by Statens Seruminstitut (Copenhagen, Denmark).
The nonstreptococcal bacterial strains Enterobacter agglomerans, Enterobacter gergoviae, Enterobacter cloacae, and Serratia marcescens were provided by C. O’Hara of the Hospital Infections Laboratory at CDC. R. Weyant of the Special Pathogens Laboratory, CDC, provided the strains of Acinetobacter calcoaceticus, Neisseria flava, Neisseria subflava, Neisseria sicca, and Neisseria lactamica. T. Popovic of the Diphtheria Research Project Laboratory, CDC, provided Corynebacterium diphtheriae subsp. gravis and Corynebacterium diphtheriae subsp. mitis. Mycoplasma pneumoniae, Mycoplasma salivarium, and Mycoplasma orale were provided by L. Thacker of the Mycoplasma Laboratory, CDC. S. Skelton of the Respiratory Diseases Laboratory Section, CDC, provided Chlamydia pneumoniae. Mycobacterium tuberculosis was provided by R. Cooksey of the Diagnostic and Molecular Epidemiology Section, CDC. All other strains were obtained from the stock collection of the Immunochemistry and Molecular Biology Laboratory, CDC. These included Bacteroides fragilis, Bordetella pertussis, Bordetella bronchiseptica, Enterobacter aerogenes, E. coli, Fusobacterium nucleatum, Haemophilus influenzae, Klebsiella pneumoniae, Legionella micdadei, Legionella pneumophila, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus equisimilis, Streptococcus pyogenes, and group G streptococci.
Five drug-resistant isolates of S. pneumoniae (see Table 3) designated 97-018867, MD-9095, TS-80, TS-148, and TX-282, with various susceptibilities, were provided by F. Tenover of the Nosocomial Pathogens Laboratory Branch, Hospital Infections Program, CDC. Measurements of MICs for isolates were done in the Nosocomial Pathogens Laboratory according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines for the MIC test (see Table 3). Since evidence of penicillin resistance among pneumococci has increased (45), we used strains with varied patterns of penicillin resistance. Bacterial strain TS-80 was highly resistant (penicillin MIC, 4 μg/ml); strain TX-282 had resistance at the breakpoint (MIC, 2 μg/ml); strains TS-148 and 97-018867 had intermediate resistance (MICs, 1 μg/ml); and strain MD-9095 was not resistant to penicillin (MIC, 0.12 μg/ml).
TABLE 3.
Immunoreactivities of five anti-37-kDa MAbs to drug-resistant isolates of pneumococci and MICs for clinical isolatesa
| Isolate | Sero- type | MIC (μg/ml) ofb:
|
Reactivity of all 5 MAbs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PEN | CTX | SXT | TET | ERY | CLIN | CHL | |||
| MD-9095 | 19A | 0.12 | 0.06 | 0.12 | ≤0.5 | 8–32 | 0.5 | 2 | + |
| TS-80 | 9V | 4 | 4 | 4 | ≤0.5 | >32 | 0.5 | 2 | + |
| TS-148 | 6A | 1.0 | 0.5 | 4 | >16 | >32 | >32 | 4 | + |
| TX-282 | 6A | 2 | 1.0 | 8 | >16 | >32 | >32 | 4 | + |
| 97-018867 | 23F | 1.0 | 1.0 | 1.0 | 32 | >8 | >8 | 8 | + |
MICs were measured according to guidelines of the NCCLS, and breakpoint criteria used to define strains were based on the NCCLS MIC interpretive standards (31).
PEN, penicillin; ERY, erythromycin; CTX, cefotaxime; SXT, sulfamethoxazole-trimethoprim; CLIN, clindamycin; TET, tetracycline; CHL, chloramphenicol.
NCCLS MIC breakpoints are as follows. Penicillin: susceptible, ≤0.06 μg/ml; intermediate, 0.1 to 1.0 μg/ml; resistant, ≥2 μg/ml. Cefotaxime: susceptible, ≤0.5 μg/ml; intermediate, 1 μg/ml; resistant, ≥2 μg/ml. Trimethoprim-sulfamethoxazole: susceptible, ≤0.5/9.5 μg/ml; intermediate, 1/19 to 2/38 μg/ml; resistant, ≥4/76 μg/ml. Tetracycline: susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; resistant, ≥8 μg/ml. Erythromycin: susceptible, ≤0.25 μg/ml; intermediate, 0.5 μg/ml; resistant, ≥1 μg/ml. Clindamycin: susceptible, ≤0.25 μg/ml; intermediate, 0.5 μg/ml; resistant, ≥1 μg/ml. Chloramphenicol: susceptible, ≤4 μg/ml; resistant, ≥8 μg/ml.
Immunization of mice and production of hybridomas.
The MAbs were produced by the method of Kohler and Milstein (26) as modified by Zola and Brooks (50). The purified 37-kDa protein used for the immunization of mice was from S. pneumoniae serotype 22F. The protein was purified by procedures previously described (42). All MAbs were produced by immunizing with purified protein from Type 22F except MAb 1E7A3D7C2 (1E7). This MAb was produced by immunizing with a nonencapsulated strain of S. pneumoniae, R36A, and has been previously described (34). BALB/c mice were initially immunized intraperitoneally with 100 μg of purified protein at a final concentration of 180 μg/ml in a 1:1.5 emulsion of Freund’s incomplete adjuvant (Sigma, St. Louis, Mo.) and phosphate-buffered saline (PBS; pH 7.2). One month later, the mice were boosted with 110 μg of purified 37-kDa protein/ml without adjuvant. The fusion was performed by standard procedures (10). Spleen cells from two mice were fused with Sp 2/0-Ag14 myeloma cells (38). Sera from the immunized mice and tissue culture supernatant from hybridized cells were screened for reactivity by ELISA and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described below.
Determination of MAb isotypes.
Isotype determinations were made with the capture Mono-Ab ID EIA kit (Zymed Laboratories, Inc., San Francisco, Calif.) according to the manufacturer’s recommended procedure. Tissue culture supernatant fluids containing MAbs of known isotype were used as controls.
ELISA.
The screening of hybridoma culture supernatants was done by ELISA. Round-bottom microtitration plates (Costar, Cambridge, Mass.) were coated with 50 μl of the purified 37-kDa protein (1.25 μg/ml) diluted in 0.1 M carbonate buffer (pH 9.6) and were stored at 4°C overnight. Unbound antigen was removed, and the plates were blocked with 100 μl of 0.5% nonfat dry milk in PBS for 1 h at 37°C. The plates were washed five times with 0.15 M NaCl containing 0.05% Tween 20. Culture supernatants (50 μl) from the fusion plates were added to the microtitration plates, diluted in 0.5% gelatin–0.5% Tween 20–0.02% thimerosal in PBS (ELISA diluent), and incubated at 37°C for 1 h. The plates were washed as before, and 50 μl of goat anti-mouse immunoglobulin (Ig)–horseradish peroxidase conjugate (Bio-Rad Laboratories, Richmond, Calif.) diluted 1:1,000 in ELISA diluent was added to each well and incubated for 1 h at 37°C. The plates were washed as before, and 50 μl of 3,3′,5,5′-tetramethylbenzidine (0.1 mg/ml in 0.1 M sodium acetate–0.1 M citric acid [pH 5.7] with 0.005% hydrogen peroxide) was added to each well and incubated for 0.5 h at 37°C. The reaction was stopped by adding 50 μl of 4 M H2SO4, and the optical density was read on an ELISA reader (Dynatech Laboratories, Inc., Alexandria, Va.) at 450 nm. An optical density of >0.200 was considered positive. Hybridomas were expanded, and MAbs 1B6E12H9 (1B6), 4E9G9D3 (4E9), 6F6F9C8 (6F6), and 8G12G11B10 (8G12) were used for further analysis.
Production of polyclonal antibodies.
Polyclonal antibody was produced by the immunization of female rabbits with purified PsaA from S. pneumoniae serotype 22F. The protein was purified by procedures previously described (42). New Zealand White rabbits (Myrtles Rabbitry, Thompson Station, Tenn.) were initially immunized subcutaneously at 20 sites with approximately 0.1 ml of a suspension of equal volumes of Freund’s complete adjuvant (Sigma) and purified protein at a final concentration of 105 μg of protein/ml per animal. One month later the rabbits were injected intramuscularly in each hind leg with an equal volume of the previous antigen and Freund’s incomplete adjuvant (Sigma). A booster injection of the same mixture was given intramuscularly 2 weeks later. The animals were bled, and the sera were tested in a Western blot analysis (as described below) against purified PsaA and the whole-cell lysate.
Immunodot blot.
Immunodot blot analyses were done by a procedure of Pau et al. (32). Whole-cell preparations of S. pneumoniae or other appropriate organisms were used as antigens. Bacterial cultures were grown to an optical density of 0.9 at 540 nm. Four microliters of antigen was absorbed as a dot on nitrocellulose membrane (Schleicher and Schuell, Keene, N.H.). The blots were blocked at 0.5 h with casein-thimerosal buffer (CTB) (23). The blots were probed with rabbit polyclonal antibody (as described above) (1:1,000) and monoclonal ascites (1:500) or tissue culture supernatant (1:20) diluted in PBS, pH 7.2, containing 0.3% Tween 20 (PBS-Tween). The blots were incubated for 1 h at 25°C. After three washes for 5 min each with PBS-Tween, the blots were exposed to goat anti-mouse Ig–peroxidase conjugate (Bio-Rad Laboratories) for 1 h at 25°C. The blots were washed as before and exposed to a 4-chloro-1-naphthol–peroxidase substrate system (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 5 min. All reactions with visible dots (compared to positive control dots) were scored as positive. The enzyme reaction was stopped by rinsing with deionized water.
SDS-PAGE and Western blotting.
The procedure for SDS-PAGE was as described by Laemmli (28). Equal volumes of sample buffer (5% SDS–10% 2-mercaptoethanol–20% glycerol in 0.01 M Tris hydrochloride [pH 8.0]) and cell suspension containing 2.4 μg of protein per ml were mixed and heated at 100°C for 5 min. Low-molecular-mass protein standards (Bio-Rad Laboratories) were used according to the manufacturer’s instructions.
Immunoblots were done with the buffer system of Towbin et al. (44) as described previously. The transfer of protein to nitrocellulose membranes (Schleicher and Schuell) was performed at 100 V for 1 h with a miniblotter apparatus (Bio-Rad Laboratories). The sheets were washed three times for 5 min each with PBS-Tween and blocked at 30 min with CTB. All incubations, buffers, antibodies, conjugate dilutions, and substrates were the same as for the immunodot blot described above. All reactions with visible bands comigrating with the purified PsaA protein control band were scored as positive.
Absorption with MAb 1E7.
Ascites fluid of MAb 1E7 was diluted in CTB (1:500) and was absorbed with the purified 37-kDa protein for 3 h with gentle shaking. The absorbed serum was later added as a primary antibody in the development of a Western blot (described above) with S. aureus as an antigen and native 37-kDa protein as a control antigen.
RESULTS
Isotypes of MAbs.
Five MAbs were produced against native PsaA and were designated 1B6, 1E7, 4E9, 6F6, and 8G12. The isotype of three MAbs, 6F6, 8G12, and 4E9, was IgG1; that of 1B6 and 1E7 was IgG2b.
Immunodot blot and Western blot analysis of the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine.
Initial immunodot blot analyses were done to evaluate the reactivity of anti-37-kDa rabbit polyclonal antiserum and the five anti-37-kDa MAbs against the 23 capsular serotypes found in the 23-valent pneumococcal polysaccharide vaccine and two additional serotypes (6A and 25). Results of the immunodot blots indicated that all the MAbs as well as the anti-37-kDa rabbit polyclonal antibody reacted with all 23 capsular serotypes of S. pneumoniae and the two additional serotypes (data not shown).
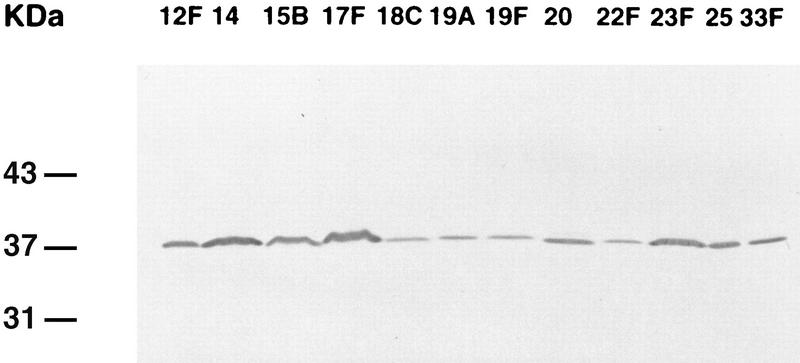
To confirm that the reactivity of the five MAbs was directed to a protein with a molecular mass of 37 kDa, and thus to PsaA, SDS-PAGE and Western blotting were done to examine the migration of PsaA from the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine. Western blot analysis of the anti-37-kDa rabbit polyclonal antiserum with whole-cell antigen preparations of the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine and the two additional serotypes (6A and 25) showed a band at 37 kDa (data not shown). Since the 37-kDa protein was shown with the anti-37-kDa rabbit polyclonal antiserum, the five anti-37-kDa MAbs were evaluated for reactivity to the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine and the two additional serotypes. Western blot analysis with all five anti-37-kDa MAbs showed a single immunoblot band at a molecular mass of 37 kDa. Figure 1 shows Western blots of the reactivity of MAb 4E9 to 11 of the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine and one additional capsular serotype (serotype 25). These patterns are representative of all five MAbs.
FIG. 1.
Immunoblot of anti-37-kDa MAb 4E9 to whole-cell antigen preparations of 11 of the 23 capsular serotypes in the licensed pneumococcal polysaccharide vaccine and one additional capsular serotype (serotype 25). The serotypes are indicated above each lane. MAb 4E9 revealed an antigen at 37 kDa in all serotypes tested.
Immunodot blot analysis of the remaining serotypes.
To ascertain the commonality of PsaA within the species, the remaining 65 S. pneumoniae serotypes were examined by immunodot blot analysis. Four of the five MAbs (1E7, 4E9, 6F6, and 8G12) reacted with all of the S. pneumoniae serotypes tested. The exception was MAb 1B6, which did not react with serotype 16F.
Western blot analysis of E. faecalis and viridans streptococci.
Since it has been shown that proteins with homology to PsaA are found among some viridans streptococci (27), the five MAbs and the polyclonal antiserum were tested for reactivity against E. faecalis and viridans streptococcal species. These strains were viridans streptococci and enterococci commonly isolated from human clinical specimens (22, 24). The strains included were E. faecalis, S. oralis, S. mitior, S. gordonii, S. mitis, S. sanguis, S. parasanguis, and S. mutans. E. faecalis and viridans streptococci reacted with the anti-37-kDa rabbit polyclonal antiserum, and all except S. mutans showed a band at 37 kDa (data not shown). Because of the reactivity of the rabbit polyclonal antiserum to E. faecalis and viridans streptococci, we evaluated the reactivities of the five anti-37-kDa MAbs to the organisms. Western blot analysis (Table 1) showed that none of the MAbs reacted with S. mutans. MAb 1E7 reacted with E. faecalis and all viridans streptococcal species tested except S. mutans. MAb 4E9 reacted with S. oralis, S. mitior, and S. parasanguis. MAb 6F6 reacted with S. oralis, S. mitior, and S. sanguis. MAb 1B6 reacted with S. mitior and S. sanguis. MAb 8G12 was the least cross-reactive, recognizing only S. mitior.
TABLE 1.
Immunoreactivities of anti-37-kDa MAbs and anti-37-kDa rabbit polyclonal antiserum to E. faecalis and viridans streptococci by Western blot analysisa
| Organism | MAb
|
37-kDa rabbit polyclonal antiserum | ||||
|---|---|---|---|---|---|---|
| 1B6 | 1E7 | 4E9 | 6F6 | 8G12 | ||
| E. faecalis | − | + | − | − | − | + |
| S. gordonii | − | + | − | − | − | + |
| S. mitior | + | + | + | + | + | + |
| S. mitis | − | + | + | − | − | + |
| S. oralis | − | + | + | + | − | + |
| S. mutans | − | − | − | − | − | − |
| S. parasanguis | − | + | + | − | − | + |
| S. sanguis | + | + | − | + | − | + |
Minus, negative reaction; plus, positive reaction.
Analysis of E. coli, respiratory pathogens, and nonpathogens.
Immunodot blot analysis was used to evaluate the reactivity of the MAbs with E. coli, respiratory pathogens, and nonpathogens representing 22 genera and 29 species. There was no reaction with 20 genera and 27 species of pathogens. However, by immunodot blot analysis all five anti-37-kDa MAbs reacted with S. equisimilis, group G streptococci, and S. aureus (Table 2). Western blot analysis with S. equisimilis and group G streptococci showed no reactivity with the MAbs (data not shown). Further analysis by Western blot with S. aureus showed reactivity with the MAbs by the presence of two single bands: one strong band at 50 kDa shown with all MAbs and an additional band at 30 kDa shown only with MAb 1E7. To further investigate the 50-kDa band, Western blot analysis was done with the five MAbs. The 50-kDa band was still observed with the five MAbs and a control antibody experiment using only goat anti-rabbit conjugate (data not shown). The 30-kDa band found only with MAb 1E7 was investigated by additional Western blot analyses with PsaA-absorbed sera to examine whether it was associated with PsaA. The antigens used were S. aureus and native PsaA as a control antigen. The primary antibody used in the development of the Western blot was MAb 1E7, which had been absorbed with native 37-kDa protein. The results were that the 30-kDa band was detected on the blot with S. aureus as the antigen and was not detected on the blot with PsaA as the control antigen (data not shown).
TABLE 2.
Immunoreactivities of five anti-37-kDa MAbs to E. coli, respiratory pathogens, and nonpathogens in immunodot blot analysisa
| Organism | MAb
|
||||
|---|---|---|---|---|---|
| 1B6 | 1E7 | 4E9 | 6F6 | 8G12 | |
| Acinetobacter calcoaceticus | − | − | − | − | − |
| Bacteroides fragilis | − | − | − | − | − |
| Bordetella bronchiseptica | − | − | − | − | − |
| Bordetella pertussis | − | − | − | − | − |
| Chylamydia pneumoniae | − | − | − | − | − |
| Corynebacterium diphtheriae | − | − | − | − | − |
| Enterobacter aerogenes | − | − | − | − | − |
| Enterobacter agglomerans | − | − | − | − | − |
| Enterobacter cloacae | − | − | − | − | − |
| Enterobacter gergoviae | − | − | − | − | − |
| Escherichia coli | − | − | − | − | − |
| Fusobacterium nucleatum | − | − | − | − | − |
| Haemophilus influenzae | − | − | − | − | − |
| Klebsiella pneumoniae | − | − | − | − | − |
| Legionella micdadei | − | − | − | − | − |
| Legionella pneumophila | − | − | − | − | − |
| Moraxella catarrhalis | − | − | − | − | − |
| Mycobacterium tuberculosis | − | − | − | − | − |
| Mycoplasma pneumoniae | − | − | − | − | − |
| Mycoplasma orale | − | − | − | − | − |
| Mycoplasma salivarium | − | − | − | − | − |
| Neisseria flava | − | − | − | − | − |
| Neisseria lactamica | − | − | − | − | − |
| Neisseria sicca | − | − | − | − | − |
| Neisseria subflava | − | − | − | − | − |
| Pseudomonas aeruginosa | − | − | − | − | − |
| Serratia marcescens | − | − | − | − | − |
| Staphylococcus aureusb | + | + | + | + | + |
| Streptococcus agalactiae | − | − | − | − | − |
| Streptococcus equisimilisb | + | + | + | + | + |
| Streptococcus pyogenes | − | − | − | − | − |
| Group G streptococcib | + | + | + | + | + |
Minus, negative reaction; plus, positive reaction.
After Western blot analysis with MAbs there was no reaction at 37 kDa.
Analysis of drug-resistant isolates of S. pneumoniae.
Since the function of PsaA and its role in antibiotic resistance are unknown, we investigated drug-resistant isolates of S. pneumoniae. We tested the five MAbs and the polyclonal antiserum against five clinical drug-resistant pneumococcal isolates with varied susceptibility patterns by immunodot blotting. The anti-37-kDa rabbit polyclonal antiserum and all five anti-37-kDa MAbs reacted with the isolates (Table 3).
DISCUSSION
In this study, we produced MAbs against the native 37-kDa protein of S. pneumoniae and evaluated them serologically for immunoreactivity against 90 pneumococcal serotypes, E. faecalis, viridans streptococci, E. coli, respiratory pathogens and nonpathogens, and drug-resistant streptococci. The specificities of the previously reported MAb 1E7 (34) and the four newly produced MAbs were determined by analyzing strains representing 90 of the pneumococcal capsular serotypes. The MAbs reacted in immunodot blot analysis and Western blot analysis with the 23 vaccine serotypes, which are known to cause approximately 85 to 90% of serious disease in the United States (9). Additionally, of the remaining 67 serotypes, 64 reacted with all five MAbs in an immunodot blot analysis. The reactivity of these MAbs with 89 of the 90 pneumococcal serotypes is an indication of the commonality of PsaA and demonstrates its presence throughout the species. Serotype 16F did not react with MAb 1B6 after testing with two different isolates. This negative reaction may be due to a conformational epitope of serotype 16F, a deletion or point mutation in the gene, or a posttranslational modification of the protein. Further studies are ongoing to explain the lack of reactivity of MAb 1B6 with S. pneumoniae serotype 16F.
Investigators have reported the presence of proteins sharing sequence similarity with PsaA in viridans streptococci and E. faecalis (27, 30). Many of these homologs, such as ScaA from S. gordonii, SsaB from S. sanguis, FimA from S. parasanguis, and EfaA from E. faecalis, have been cloned and sequenced. Comparison of their nucleotide sequences with that of PsaA showed similarities within the group that ranged from 57 to 82% (36). We examined these organisms by Western blotting and found that they contain proteins reactive with the MAbs described here. The fact that the MAbs had varied reactivity patterns among viridans streptococci and E. faecalis confirms that the nucleotide homologs have cross-reacting epitopes within the peptide sequences. In a recent study, anti-PsaA MAbs were used to map the immunogenic epitopes of PsaA. Amino acid sequences showed that the epitopes appear to be in the same region of PsaA, but there are differences in the binding sites of the MAbs (49). Additionally, the varied reactivity patterns of the MAbs with E. faecalis and viridans streptococci indicate that the anti-37-kDa MAbs are recognizing different epitopes (Table 1). MAb 8G12 is the least cross-reactive of the five MAbs. It reacted only with S. mitior, and therefore may be the most useful in the development of diagnostic assays.
Our MAbs were tested against E. coli, respiratory pathogens, and nonpathogens. These bacteria were tested because they may cross-react in a diagnostic assay and may hinder the detection of PsaA and thus increase the possibility of false-positive reactions. In an immunodot blot analysis, all five MAbs unexpectedly cross-reacted with S. equisimilis, streptococcus group G, and S. aureus. After Western blot analysis of the two organisms, there was no reaction at 37 kDa. The cross-reactivity found in the immunoblot with the S. equisimilis and group G streptococci may be nonspecific binding due to protein G, an Fc binding protein isolated from a group C streptococcus (5). The cross-reactivity found in the immunodot blot analysis of S. aureus can be postulated to be nonspecific binding of protein A. Protein A is a cell wall protein made by most strains of S. aureus which binds selectively to the Fc region of Ig molecules and has been found to have molecular masses between 29 and 63 kDa (40). However, the true molecular mass of native protein A is postulated to be 42 kDa (16). After Western blot analysis of S. aureus with the five MAbs, a single strong band was seen at approximately 50 kDa. Additionally, the 50-kDa band continued to be observed in a control experiment with only goat anti-rabbit conjugate, and was thus assumed to be nonspecific binding of protein A. The second (30-kDa) band found only with MAb 1E7 was further investigated by Western blot analysis with PsaA-absorbed MAb. Because the 30-kDa band remained on S. aureus immunoblots after addition of the PsaA-absorbed MAb 1E7, the protein at 30 kDa was determined not to be related to PsaA.
Investigators have shown evidence that penicillin resistance in S. pneumoniae results from alterations in the penicillin-binding proteins to which the antibiotic binds. These altered proteins arise through genetic transfer and recombination with other bacterial species (4, 13). Cundell et al. have suggested that transformation in S. pneumoniae may be aided by peptide permeases, a group of proteins that are involved in transport of materials into the cell (12). The role of PsaA has yet to be determined, but it has been hypothesized to be a permease. Therefore, it was of interest to examine PsaA of S. pneumoniae and to investigate whether the protein was altered in drug-resistant strains. The positive reactivities of the MAbs to the five drug-resistant isolates are noteworthy because all were positive regardless of the varied antibiotic susceptibilities of the strains. This implied that the PsaA which was expressed on the strains tested was similar if not identical to native PsaA. Thus, the MAbs appear promising for detection of PsaA antigens of drug-resistant as well as non-drug-resistant pneumococci. Further studies in our laboratory are ongoing to examine the role of PsaA.
The MAbs, particularly MAb 8G12, are likely candidates in the development of diagnostic assays for pneumococcal antigen detection. The MAbs may be a practical diagnostic reagent, because PsaA is expressed on 89 of 90 pneumococcal serotypes, most importantly the 23 vaccine serotypes which cause the majority of invasive pneumococcal disease (9, 15). The MAbs may be used alone or as a cocktail to detect antigen in an antigen-capture diagnostic assay. In the development of pneumococcal immunoassays, antigen diversity continues to be a problem (48). An immunoassay detecting PsaA will aid in solving this problem and at the same time increase the sensitivity of the assay, because our MAbs detected 89 immunologically distinct serotypes of S. pneumoniae. Because of the specificity of the MAbs, techniques such as counterimmunoelectrophoresis and latex agglutination may be improved. The MAbs may be used as standardized reagents in latex agglutination assays and provide sensitivities equivalent to, and perhaps better than, the commercially available kits that detect pneumococcal antigen, such as Wellcogen (19) and Directigen (46). Peptides specific for PsaA may be synthesized and evaluated by using the MAbs in diagnostic immunoassays. Furthermore, the monoclonals could also be used for epitope mapping on the PsaA antigen. PsaA, which is expressed on all serotypes of S. pneumoniae, may be a potential vaccine candidate for pneumococcal disease.
Our MAbs reacted with 89 of 90 serotypes of S. pneumoniae, which included the serotypes present in the licensed pneumococcal polysaccharide vaccine as well as drug-resistant pneumococcal isolates. The results of this study are favorable, and the MAbs appear to be good candidates for use in the development of pneumococcal diagnostic tests for detection of bacterial antigens.
ACKNOWLEDGMENTS
We gratefully thank Sandra Romero-Steiner at CDC for her advice in the compilation of the list of bacteria for specificity testing and Fred Tenover and Jana Swenson at CDC for the susceptibility testing of the drug-resistant isolates used in these studies.
This study was partially supported by the National Vaccine Program Office, CDC.
REFERENCES
- 1.Ajello G W, Bolan G A, Hayes P S, Lehman D, Montgomery J, Feeley J C, Perlino C A, Broome C V. Commercial latex agglutination tests for detection of Haemophilus influenzae type b and Streptococcus pneumoniae antigens in patients with bacteremic pneumonia. J Clin Microbiol. 1987;25:1388–1391. doi: 10.1128/jcm.25.8.1388-1391.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, P. W. Personal communication.
- 3.Anhalt J P, Yu P K W. Counterimmunoelectrophoresis of pneumococcal antigens: improved sensitivity for the detection of types VII and XIV. J Clin Microbiol. 1975;2:510–515. doi: 10.1128/jcm.2.6.510-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian R. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev Infect Dis. 1981;3:1–17. doi: 10.1093/clinids/3.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 5.Björck L, Kronvall G. Purification and some novel properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;133:969–973. [PubMed] [Google Scholar]
- 6.Breiman R F, Spika J S, Navarro V J, Darden P M, Darby C P. Pneumococcal bacteremia in Charleston County, South Carolina: a decade later. Arch Intern Med. 1990;150:1401–1405. [PubMed] [Google Scholar]
- 7.Breiman R F, Butler J C, Tenover F C, Elliot J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 8.Butler J C, Hofmann J, Cetron M S, Elliot J A, Facklam R R, Breiman R F the Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:4. [PubMed] [Google Scholar]
- 10.Clafin L, Williams K. Mouse myeloma-spleen cell hybrids: enhanced hybridization frequencies and rapid screening procedures. Curr Top Microbiol Immunol. 1978;81:107–109. doi: 10.1007/978-3-642-67448-8_16. [DOI] [PubMed] [Google Scholar]
- 11.Coonrod J D. Urine as an antigen reservoir for diagnosis of infectious diseases. Proceedings of the American Journal of Medicine Symposium, July 28, 1983. Am J Med. 1983;75:85–92. doi: 10.1016/0002-9343(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 12.Cundell D R, Pearce B J, Sandros J, Naughton A M, Masure H R. Bacterial permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect Immun. 1995;63:2493–2498. doi: 10.1128/iai.63.7.2493-2498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowson C G, Coffey T J, Kell C, Whiley R A. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol Microbiol. 1993;9:635–643. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 14.Duchin J, Breiman R F, Diamond A, Lipman H B, Block S L, Hedrick J A, Finger R, Elliot J A. High prevalence of multi-drug resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745–750. doi: 10.1097/00006454-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Facklam, R. R., and R. F. Breiman. 1991. Current trends in bacterial respiratory pathogens. Am. J. Med. 91(Suppl. 6A):3S–11S. [DOI] [PubMed]
- 16.Goding J W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- 17.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann J, Cetron M S, Farley M M, Baughman W S, Facklam R R, Elliot J A, Deaver K D, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 19.Ingram D L, Pearson A W, Occhiuti A R. Detection of bacterial antigens in body fluids with the Wellcogen Haemophilus influenzae b, Streptococcus pneumoniae, and Neisseria meningitidis (ACYW135) latex agglutination tests. J Clin Microbiol. 1983;18:1119–1121. doi: 10.1128/jcm.18.5.1119-1121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacman D, Zhang Y, Rydquist-White R M, Wadowsky J, Post J C, Ehrlich G D. Identification of a patient with Streptococcus pneumoniae bacteremia and meningitis by the polymerase chain reaction (PCR) Mol Cell Probes. 1995;9:157–160. doi: 10.1006/mcpr.1995.0026. [DOI] [PubMed] [Google Scholar]
- 21.Jalonen E, Paton J C, Koskela M, Kertula Y, Leinonen M. Measurement of antibody responses to pneumolysin—a promising method for the presumptive aetiological diagnosis of pneumococcal pneumonia. J Infect. 1989;19:127–134. doi: 10.1016/s0163-4453(89)91864-1. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura Y, Hou X-G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 23.Kenna J G, Major G N, Williams R S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985;85:409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- 24.Kilian M, Mikkelsen L, Hendrichsen J. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906) Int J Syst Bacteriol. 1989;39:471–484. [Google Scholar]
- 25.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 27.Kolenbrander P, Andersen R, Ganeshkumar N. Nucleotide sequence of Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect Immun. 1994;62:4469–4480. doi: 10.1128/iai.62.10.4469-4480.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lenthe-Eboa S, Brighouse G, Auckenyhaler R, Lew D, Zwahlen A, Lambert P H, Waldvogel F A. Comparison of immunological methods for diagnosis of pneumococcal pneumonia in biological fluids. Eur J Clin Microbiol. 1987;6:28–34. doi: 10.1007/BF02097186. [DOI] [PubMed] [Google Scholar]
- 30.Lowe A M, Lambert P A, Smith A W. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infect Immun. 1994;63:703–706. doi: 10.1128/iai.63.2.703-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 32.Pau C-P, Plikaytis B B, Carlone G M, Warner I M. Purification, partial characterization, and seroreactivity of a genuswide 60-kilodalton Legionella protein antigen. J Clin Microbiol. 1988;26:67–71. doi: 10.1128/jcm.26.1.67-71.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzi E, Masiero P, Oliva A. Evaluation of the invasive techniques for diagnosing bacterial respiratory infections. J Chemother. 1995;7:286–291. doi: 10.1179/joc.1995.7.4.286. [DOI] [PubMed] [Google Scholar]
- 34.Russell H, Tharpe J A, Wells D E, White E H, Johnson J E. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J Clin Microbiol. 1990;28:2191–2195. doi: 10.1128/jcm.28.10.2191-2195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampson J S, O’Connor S P, Stinson A R, Tharpe J A, Russell H. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson J S, Furlow Z, Whitney A M, Williams D, Facklam R, Carlone G M. Limited diversity of Streptococcus pneumoniae. Infect Immun. 1996;65:1967–1971. doi: 10.1128/iai.65.5.1967-1971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saruta K, Matsuna T, Sadayori H, Midori K, Kitahara S, Kanemoto S, Sakai O, Machida K. Rapid identification of Streptococcus pneumoniae by PCR amplification of ribosomal DNA spacer region. FEMS Microbiol Lett. 1995;132:165–170. doi: 10.1111/j.1574-6968.1995.tb07827.x. [DOI] [PubMed] [Google Scholar]
- 38.Schulman M, Wilde C D, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature (London) 1978;276:269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 39.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987: the Pneumococcal Surveillance Working Group. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 40.Sting R, Lauerman L, Blobel H. Isolations of protein A and protein G from the bacterial surface. Int J Med Microbiol. 1990;273:306–312. doi: 10.1016/s0934-8840(11)80433-0. [DOI] [PubMed] [Google Scholar]
- 41.Talkington D F, Brown B G, Tharpe J A, Koenig A, Russell H. Protection of mice against fatal challenge by immunization with pneumococcal surface adhesin A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 42.Tharpe J A, Russell H. Purification and seroreactivity of pneumococcal surface adhesin A (PsaA) Clin Diagn Lab Immunol. 1996;3:227–229. doi: 10.1128/cdli.3.2.227-229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilton R C, Dias F, Ryan R W. Comparative evaluation of three commercial products and counterimmunoelectrophoresis for the detection of antigens in cerebrospinal fluid. J Clin Microbiol. 1984;20:231–234. doi: 10.1128/jcm.20.2.231-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welby P L, Keller D S, Cromien J L, Tebas P, Storch G. Resistance to penicillin and non-β-lactam antibiotics of Streptococcus pneumoniae at a children’s hospital. Pediatr Infect Dis J. 1994;13:281–287. doi: 10.1097/00006454-199404000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Wellstood S. Evaluation of a latex test for rapid detection of pneumococcal antigens in sputum. Eur J Clin Microbiol Infect Dis. 1992;11:448–451. doi: 10.1007/BF01961861. [DOI] [PubMed] [Google Scholar]
- 47.Whitby M, Kritinsson K G, Brown M. Assessment of rapid methods of pneumococcal antigen detection in routine sputum bacteriology. J Clin Pathol. 1985;38:341–344. doi: 10.1136/jcp.38.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yolken R H, Davis D, Winkelstein J, Russell H, Sippel J E. Enzyme immunoassay for detection of pneumococcal antigen in cerebrospinal fluid. J Clin Microbiol. 1984;20:802–805. doi: 10.1128/jcm.20.4.802-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeiler J L, Sampson J S, Carlone G M, Ades E W, Westerink M A J. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. 1997. Epitope mapping of a species-specific 37-kDa lipoprotein present in all Streptococcus pneumoniae capsular serotypes, abstr. E-49; p. 248. [Google Scholar]
- 50.Zola H, Brooks D. Techniques for production and characterization of monoclonal hybridoma antibodies. In: Hurrell J G, editor. Monoclonal hybridoma antibodies: techniques and applications. Boca Raton, Fla: CRC Press, Inc.; 1982. pp. 1–57. [Google Scholar]